Abstract
Botulinum Neurotoxin type D (BoNT/D) causes periodic outbreaks of botulism in cattle and horses, but is rarely associated with human botulism. Previous studies have shown that humans responded poorly to peripheral injection of up to 10 U of BoNT/D. Isolated human pyramidalis muscle preparations were resistant to BoNT/D, whereas isolated human intercostal muscle preparations responded to BoNT/D similarly as to other BoNT serotypes. In vitro data indicate that BoNT/D does not cleave human VAMP1 efficiently, and differential expression of the VAMP 1 and 2 isoforms may be responsible for the above observations. Here we examined sensitivity of cultured human neurons derived from human induced pluripotent stem cells to BoNT/D. Our data indicate that BoNT/D can enter and cleave VAMP 2 in human neurons, but at significantly lower efficiency than other BoNT serotypes. In addition, BoNT/D had a short duration of action in the cultured neurons, similar to that of BoNT/E. In vivo analyses indicated a slower time to death in mice, as well as a later onset and shorter duration of action than BoNT/A1. Finally, examination of BoNT/D activity in various rodent and human cell models resulted in dramatic differences in sensitivity, indicating a unique cell entry mechanism of BoNT/D.
1. Introduction
Botulinum Neurotoxin type D (BoNT/D) has been mainly associated with botulism in cattle and horses, at times leading to large outbreaks in cattle herds with a mortality rate above 80 % and large associated economic losses (1). Botulism in cattle caused by BoNT/D has been reported mostly in South Africa, Europe and Canada, but also in USA and Israel (1). Studies investigating the epidemiology of BoNT/D-producing Group III C. botulinum in the environment (1-3) showed periodic isolation from cattle feeds, animal remains and cows' gastrointestinal contents. This indicates potentially widespread presence of BoNT/D producing C. botulinum in the environment, as is the case with other C. botulinum strains, and thus potential exposure of humans to this toxin. However, BoNT/D very rarely causes human botulism. In fact, there is only one published report of BoNT/D identified in naturally occurring human botulism (4). This report described a mild botulism outbreak in Moundou (Tchad) in January 1958 involving 8 people who ate raw ham. Two of them ate very little and had no symptoms, four had very mild early botulism symptoms accompanied by extensive diarrhea and vomiting, and recovered completely the following day. One person had these symptoms for 10 days but required no specialized treatment, and one person had symptoms for 1 month with recovery beginning at 2 ½ weeks after the ham consumption. Treatment of this last patient with anti-BoNT/A and /B antitoxin did not affect the symptoms, and analysis of extracts from the contaminated ham as well as cultures grown from the ham showed that only anti-BoNT/D serum and not anti-A, B, C, or E sera protected mice against botulism symptoms, confirming that the outbreak was caused by BoNT/D (4). A subsequent study isolated C. botulinum strain 1873, which produces BoNT/D, from the contaminated ham (5).
Botulinum neurotoxins have been classified into seven immunologically distinct serotypes (A-G) (6). BoNTs are the causative agent of human and animal botulism, which is characterized by flaccid paralysis that may last for several weeks or months and up to a year, depending on the serotype and dose (9). The toxins exert their pathology by selectively entering neuronal cells and cleaving soluble N-ethylmaleimide-sensitive-factor attachment protein receptor (SNARE) proteins (10-12), which are VAMP1 and VAMP2 in the case of BoNT/D (12). Due to the high potency of botulinum neurotoxins combined with the lack of efficacious countermeasures, particularly after entry into neuronal cells, BoNTs are considered a potential bioterrorism threat (13, 14). On the other hand, BoNT serotype A (and to a lesser extent B), are being used as powerful pharmaceuticals to treat a variety of disorders (15). Since BoNT/A, B, E, and F cause almost all naturally occurring human botulism cases, countermeasure efforts as well as novel pharmaceuticals usually focus on these four serotypes. However, it is unknown whether humans would be susceptible to a bioterrorist attack using BoNT/D or derivatives thereof, and whether BoNT/D may present an alternative potential biopharmaceutical.
Scientific evidence on the susceptibility of humans to BoNT/D is unclear. A recent in vitro study showed that the BoNT/D light chain (LC) efficiently cleaved neuronal human VAMP 2 but inefficiently cleaved the other neuronal human VAMP isoform, VAMP 1 (16). Inefficient and slow proteolysis of rat VAMP1 by BoNT/D has also been reported (17, 18). This may be the reason underlying the recent observation in an experimental electrophysiology study in humans that up to 10 mouse LD50 Units of BoNT/D was poorly effective in inducing local paralysis in humans after injection into the extensor digitalis brevis muscle (19). In addition, a study using isolated human pyramidalis muscle indicated that the neuromuscular junction in this human muscle preparation was resistant to BoNT/D (20). However, in another study, human intercostal muscle was found to be similarly sensitive to BoNT/D as to BoNT/A, B, and E (21). The human botulism outbreak attributed to BoNT/D also indicates that humans can be susceptible to intoxication by BoNT/D (4), although the symptoms appear to be much milder than in botulism caused by BoNT/A or/B.
In this study we examined the activity of BoNT/D in human neurons and analyzed the onset and duration of action in mice. Our data indicate that BoNT/D can enter and cleave VAMP 2 in human neurons, but at significantly lower efficiency than BoNT/A1, and that it has a short duration of action similar to that of BoNT/E. In addition, dramatically differential sensitivity of various neuronal cell populations and a slow time to death in mice indicate potentially distinct pharmacological properties of this toxin compared to BoNT/A1.
2. Materials and Methods
All the work described in this manuscript was approved by the University of Wisconsin-Madison Institutional Biosafety Committee. All animal experiments were approved by and conducted according to guidelines by the University of Wisconsin Animal Care and Use Committee.
2.1 Botulinum Neurotoxins
BoNT/A1 was isolated from C. botulinum strain Hall A hyper as previously described (22). The specific activity in mice was determined to be 1.25 × 108 mouse LD50 Units (U) / mg. BoNT/D toxin was isolated from C. botulinum strain D 1873 using a method similar to that used in BoNT/A purification with the following modifications. The ammonium sulfate precipitated material from DEAE chromatography at pH 5.5 was collected by centrifugation and resuspended in 20 mM NaOAc buffer pH 6.0. The sample was applied to a p-aminobenzyl 1-thio-β-D-galactopyranoside agarose affinity column (pABTG agarose) that tightly binds the hemagglutinin of the toxin complex (23). The toxin was eluted from the column by addition of 20 mM Tris buffer pH 8.0 containing 0.15 M NaCl. Specific activity in mice was determined to be 1.15 × 108 mouse LD50 Units / mg. BoNT/B1 was isolated from C. botulinum strain as previously described (24), and the specific activity in mice was determined to be 3.13 × 108 mouse LD50 Units (U) / mg.
2.2 Neuronal cell models
Several neuronal cell models were used in this study. The iCell Neurons and media were purchased from Cellular Dynamics Inc. (Madison, W1) and seeded and maintained according to company instructions. HIP Neurons and media were provided by Globalstem (Gaithersburg, MD) and were prepared as previously described (25). Primary rat (strain Sprague Dawley) and mouse (strain C57/BL6 or ICR, as indicated) spinal cord cells, hippocampal cells, or cortical cells were all prepared as described previously (26, 27) and seeded into 0.01% poly-L-ornithine (SIGMA) and 8.3 μg/cm2 matrigel (BD Biosciences) coated 96-well Techno Plastic Products (TPP) plates at a density of 50,000 cells/well. The cells were allowed to mature for at least 18 days before use unless otherwise noted. The primary cells were seeded and maintained in serum free culture medium (Neurobasal® medium supplemented with 2% B27, 2 mM glutamax, and 100 units/mL penicillin/streptomycin (all from Invitrogen)).
2.3 Cell-based assays
The cells were exposed to serial dilutions of BoNT/D in 50 μL of the respective media for 48 h. The extracellular solution containing BoNT/D was removed, cells were lysed in 1X lithium dodecyl sulfate (LDS) sample buffer (Invitrogen) and analyzed by Western immunoblot for VAMP2 cleavage as previously described using a monoclonal anti-VAMP2 antibody from Synaptic Systems (Göttingen, Germany) (27, 28). VAMP2 and syntaxin bands were quantified by densitometry using a Foto/Analyst FX system and TotalLab Quant software (Fotodyne). Data plots and EC50 values (four parameters – variable slope) were generated using PRISM 6 software, and statistical significance was determined by PRISM 6 software using an Extra-sum-of-squares F-test with an α-value of 0.05.
For the duration of action assay, iCell Neurons were exposed to 800 mouse LD50 Units (U) of BoNT/D in 50 μL of modified culture media containing 56 mM KCl and 2.2 mM CaCl2 (cell stimulation media) for 10 min., followed by complete removal of the toxin, washing of the cells two times with 300 μL of culture media, and further incubation of the cells in culture media at 37°C in a CO2 incubator. Four replicate samples were harvested every 2-3 days, and analyzed for VAMP2 cleavage as above.
To determine onset of action in human neurons, iCell Neurons were exposed to 10,000 U of BoNT/D in 50 μL of either culture media or cell stimulation media for 0-8 h, and triplicate samples were harvested every hour for analysis of VAMP2 cleavage. To further analyze activity dependent uptake, iCell Neurons were exposed to serial dilutions of BoNT/D in 50 μL of either cell stimulation media or culture media for 10 min., followed by complete removal of the toxin, washing of the cells two times with 300 μL of culture media, and further incubation of the cells in culture media at 37°C in a CO2 incubator for 16 h.
2.4 In vivo analysis of BoNT/D activities in mice
Groups of 5 female ICR mice (weight between 18-22 g) were injected intraperitoneally with 100 or 1,000 U of BoNT/D, BoNT/B1 or BoNT/A1 in 0.5 ml of GelPhos buffer (30 mM sodium phosphate [pH 6.3] and 0.2% gelatin). Mice were observed for typical botulism symptoms including ruffled fur, wasped waist, paralysis, difficulty breathing, and spasticity just before death, and the time of death was noted. The statistical significance of the time to death was evaluated using a paired, two-tailed t-test in Excel.
To determine motor-neuron deficiency after local injection, groups of 5 female ICR mice were injected into the gastrocnemius muscle with 1.5, 2, or 2.5 U of BoNT/D or 0.25, 0.5, or 0.75 U of BoNT/A1 in 10 μl of GelPhos buffer using an insulin syringe. Motorneuron deficiency was evaluated by Rotarod analysis using an accelerating cycle (4-40 rpm over 5 min). Mice were analyzed on a daily basis until able to complete the full 5 minute cycle.
3. Results
3.1 BoNT/D is moderately active in human neurons
In order to determine whether BoNT/D was able to enter human neurons and be catalytically active inside the neuronal cell cytosol, two different human neuronal cell models were exposed to serial dilutions of BoNT/D. Both are neuronal populations derived from human induced pluripotent stem cells. One (iCell Neurons) was purchased as already differentiated cells and consists of a mixture of predominantly glutamatergic and GABAergic neurons, whereas the other (HIP Neurons) was a population of neurons differentiated from a neural stem cell line and consisted of a mixture of predominantly cholinergic, GABAergic and glutamatergic neurons (25). Both human neuronal cell models were approximately equally sensitive to BoNT/D, with EC50 values of about 50 U (Figure 1). Compared to BoNT/A1, this is more than 150 times lower sensitivity in iCell Neurons and about 25 times reduced sensitivity in HIP Neurons (28). Cleavage of VAMP-1 could not be evaluated due to low expression in these cells, but has been examined in detail in another recent study (29).
Figure 1.
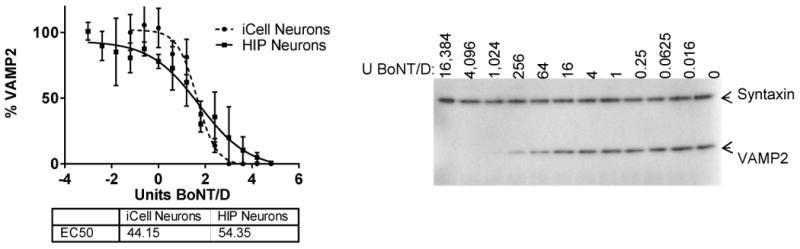
Activity of BoNT/D in human stem cell derived neurons. ICell Neurons (CDI) or HIP Neurons (Globalstem) were exposed to serial dilutions of BoNT/D for 48 h, and cell lysates were analyzed for the percentage of VAMP2 in relation to syntaxin by Western blot and densitometry. EC50 values were determined using PRISM 6 software. Quantitative data from triplicates for each cell line are shown in the graph, and an example of a Western blot in iCell Neurons is shown to the right.
3.2 BoNT/D has a similar specific activity in mice as BoNT/A1, but causes slower death
In order to determine whether high levels of BoNT/D toxin cause similar symptoms in mice as BoNT/A1 and BoNT/B1, mice were injected intraperitoneally with 100 U or 1,000 U of BoNT/D or BoNT/A1, or BoNT/B1. All groups of mice exhibited the typical botulism symptoms of ruffled fur, wasped waist, increasing paralysis, labored breathing, and spasticity just prior to death. However, symptoms in the mice injected with BoNT/D appeared milder except for the spasticity prior to death, which was at least as pronounced as in the mice injected with BoNT/A1. There was a significant delay in time to death in mice injected with BoNT/D and BoNT/B1 versus mice injected with BoNT/A1. The mice injected with 1,000 U and 100 U BoNT/A1 died on average within 146 min and 220 min, respectively, whereas the mice injected with BoNT/D or BoNT/B1 survived for 193 (D) and 209 (B1) min, and 304 (D) and 301 (B1) min, respectively (Table 1). This delay in death also was observed in the specific titer determination, in that the mice injected with BoNT/D died on days 2 and 3 post injection, whereas the mice injected with BoNT/A1 died within the first two days. These data indicate potential distinct pharmacological properties of BoNT/D compared to BoNT/A1 in the ICR mouse strain and greater similarity with BoNT/B1, which also cleaves VAMP1 and 2 and causes milder botulism than BoNT/A1. A recent study has identified a VAMP1 polymorphism (M/148) in the BoNT/D cleavage site in the commonly used Sprague Dawley rat strain, which affects BoNT/D sensitivity (29). Even though no such polymorphism is known for ICR mice or suggested by the data, such or similar variation cannot be entirely excluded in the outbred ICR mouse strain at this point.
Table 1.
| time to death (min) | average | standard deviation |
|---|---|---|
| BoNT/A1, 1000 U | 146.3 | 19.6 |
| BoNT/B1, 1000 U | 209 * | 20.0 |
| BoNT /D, 1000 U | 193.2 * | 18.9 |
| BoNT /A1, 100 U | 220.5 | 32.7 |
| BoNT/B1, 100 U | 301.3 * | 22.9 |
| BoNT /D, 100 U | 304.4 * | 22.3 |
significantly different from A1, p<0.02
3.3 BoNT/D has a slower onset of action and faster recovery than BoNT/A1
In order to determine duration of action of BoNT/D in human neurons, the iCell Neurons were exposed to 800 U (∼ 1 nM) of BoNT/D for 10 min in cell stimulation media (culture media modified to contain 56 mM KCl and 2.2 mM CaCl2), followed by toxin removal, washing of the cells, and further incubation of the neurons in culture media. Analysis of cell lysates by Western blot showed that VAMP2 cleavage reached a maximum at 2 days post toxin exposure, and recovery of VAMP2 started at 1 week post exposure and proceeded steadily until VAMP2 was completely restored at about 3 weeks post toxin exposure (Figure 2a). This indicates a relatively short duration of action of this toxin in human neurons similar to that of BoNT/E.
Figure 2.
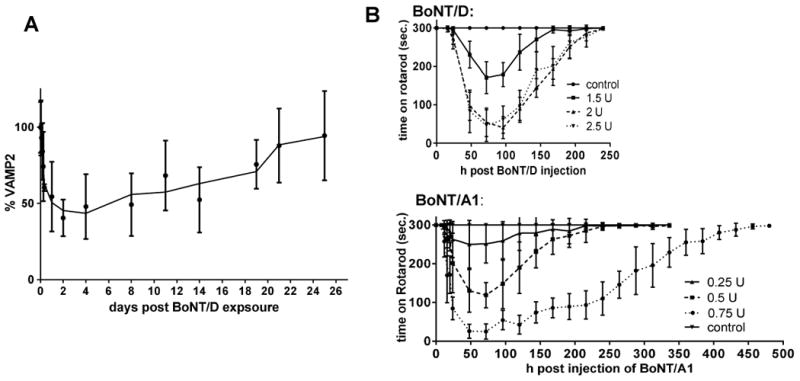
Onset and Duration of BoNT/D activity. A: To determine recovery of VAMP2 in hiPSC derived neurons after exposure to BoNT/D, iCell Neurons were exposed to 800 U of BoNT/D for 48 h, followed by complete removal of the toxin and further incubation of the exposed cells in culture media. Cells were harvested at the indicated time points, and the percentage of VAMP2 in relation to syntaxin was determined by Western blot and densitometry.
B: The onset and duration of motor-neuron deficiency after local injection into the gastrocnemius muscle in mice was determined by Rotarod analysis. The time the mice remained on an accelerating rotarod (4-40 rpm over 5 min) is shown in relation to the time after toxin injection.
Onset and duration of action of BoNT/D was also assessed in vivo by measuring motoneuron deficiency as determined by Rotarod analysis after local injection of BoNT/D into the gastrocnemius muscle of mice. Significantly greater amounts of BoNT/D were required as compared to BoNT/A1 to achieve a similar level of motor neuron deficiency as evidenced by an inability of the mice to remain on an accelerating Rotarod (Figure 2 b). While 0.75 U of BoNT/A1 resulted in an about 90 % reduction in time mice could remain on the Rotarod, 2 U of BoNT/D were required to see the same effect. In addition, recovery was faster for all mice injected with BoNT/D. At 10 days post- injection, all mice injected with BoNT/D were able to remain on the Rotarod for the entire 5 min (Figure 2 b), whereas mice injected with BoNT/A1 having the same severity of motor neuron deficiency required over 14 days for recovery (Figure 2 b). Since about 3 times more BoNT/D was required to observe the same motor-neuron deficiency as observed with BoNT/A1 and recovery from BoNT poisoning is dose-dependent, these data demonstrate a significantly faster recovery from BoNT/D as compared to BoNT/Al This indicates a lower efficiency of BoNT/D in causing motor-neuron deficiency after local injection in mice, and faster recovery. It is interesting to note that with BoNT/A1, there was a clear and gradual dose-dependent increase in motor-neuron deficiency and recovery time, whereas with BoNT/D there was only a difference between 1.5 and 2 U, but no difference between 2 and 2.5 U. This may be due to distinct pharmacologic distribution and properties of the two toxins. It should also be noted that the Rotarod analysis assesses overall motor neuron deficiency rather than local paralysis. In fact, it appeared that the time remaining on the Rotarod correlated more with overall well-being of the mice than with local paralysis in the injected leg.
3.4 BoNT/D enters depolarized neurons more efficiently than resting neurons
Cell entry of BoNT/D into human neurons was also investigated by exposing iCell Neurons to 10,000 U of BoNT/D in either culture media or cell stimulation media for 10 min, followed by toxin removal and cell washing, and further incubation of cells in culture media. Cells were harvested at 1-8 h post toxin exposure, and VAMP2 cleavage analyzed by Western blot. Significantly faster and more efficient VAMP2 cleavage was observed with cell stimulation media (Figure 3a). This was further confirmed by exposing the human neurons to serial dilutions of BoNT/D for 10 min in either cell stimulation media or in culture media, followed by toxin removal and further incubation in culture media for 16 h to allow for VAMP2 cleavage. Dose dependent VAMP 2 cleavage was observed in both, depolarized and not depolarized cells, but was about 3-4-fold more efficient in depolarized cells (Figure 3 b). This indicates that chemical depolarization of the neurons enhanced BoNT/D uptake.
Figure 3.
Activity dependence of BoNT/D uptake into hiPSC derived Neurons. A: iCell Neurons were exposed to 10,000 U of BoNT/D at 37°C in either culture media (CM) or cell stimulation media (CSM) containing 56 mM KCl and 2.2 mM CaCl2. Cells were harvested every hour at from 0 to 8 h, and cell lysates analyzed for VAMP2 in relation to syntaxin by Western blot and densitometry. B: iCell Neurons were exposed to serial dilution of BoNT/D in either culture media (CM) or cell stimulation media (CSM) containing 56 mM KCl and 2.2 mM CaCl2 for 10 min. at 37°C. All toxin was removed, cells washed, and incubated further in culture media. Cells were harvested after 16 h, and cell lysates analyzed for VAMP2 in relation to syntaxin by Western blot and densitometry. The graphs depict quantitative data from triplicate samples, respectively.
3.5 BoNT/D activity in different primary rodent neuronal cultures varies
BoNT/D activity in primary rodent neuronal cell models has previously been described (19). In order to determine the activity of BoNT/D in primary rodent cell models relative to that in human cell models, primary rat (E15) and mouse (E14, from BL6 or ICR mice) spinal cord cells, as well as mouse cortical and hippocampal neurons were exposed to serial dilutions of the BoNT/D for 48 h. While no cleavage was observed in rat or mouse primary spinal cord cells even at concentrations as high as 16,000 U, sensitive cleavage was detected in the mouse cortical neurons, with an EC50 of about 7 U (Figure 4 a). However, VAMP2 cleavage in this culture did not reach 100 % as in the human cell models. Primary mouse hippocampal neurons appeared similarly sensitive (data not shown). Since previous publications have indicated cleavage of VAMP2 in primary rodent spinal cord cells, the effect of the gestational age of the pups and mouse strains used to prepare primary mouse spinal cord cells were examined. First, primary mouse spinal cord neurons were prepared from E13 or E14 mouse pups of either BL6/C57 or ICR mice and exposed to serial dilutions of BoNT/D in parallel. The VAMP2 in cells derived from E13 pups of either mouse strain was cleaved by BoNT/D with EC50 values of around 2 U, whereas no cleavage was observed in the cells prepared from E14 pups of either strain. BoNT/D exposure of primary spinal cord cells derived from E11 and E12 mouse pups (ICR) resulted in a similar cleavage pattern with an EC50 of about 2 U (Figure 4 b). In all cases, VAMP2 cleavage was not complete and some residual VAMP2 was detected even at toxin concentrations as high as 16,000 U / well. In contrast, primary mouse spinal cord cells from E11-E14 pups were all sensitive to BoNT/A1, with SNAP-25 being cleaved with EC50 values of about 0.1 U as previously described (25). Interestingly, when the primary mouse spinal cord cells derived from E12 ICR mouse pups were examined for BoNT/D cleavage after remaining in culture for 5 weeks, VAMP2 cleavage was significantly reduced.
Figure 4.
Sensitivity of different neuronal cell models to BoNT/D. The different neuronal cell models were exposed to serial dilutions of BoNT/D for 48 h, and cell lysates were analyzed by Western blot and densitometry for the amount of VAMP2 in relation to syntaxin. The graphs show the average values and standard deviations of triplicate samples for each, and the Western blot images show representative immunoblot data. A: Sensitivity of hiPSC derived neurons (iCell Neurons), primary rat spinal cord cells (RSC cells), and mouse cortical neurons are shown. B: Sensitivity of primary mouse spinal cord cells (strain ICR) harvested from E11, E12, E13, or E14 pups are shown.
4. Discussion
BoNT/D is not considered to be a cause of human botulism, and this was supported by a trial study in humans which indicated a poor electrophysiological response to up to 10 U of BoNT/D injected in the EDB muscle (19) and studies that indicate that human VAMP1 is less sensitive to cleavage by BoNT/D than VAMP2 (16-18, 29). Another study has demonstrated that at high concentrations of BoNT/D, human intercostal muscle preparations are sensitive to BoNT/D (21). Here, we show that in vitro cultured human neurons are susceptible to BoNT/D, but sensitivity is about 25-100 times lower than to BoNT/A1 (Figure 1). All of these data together suggest that humans might be susceptible to BoNT/D intoxication if exposed to large enough quantities, but that potency of BoNT/D in humans is significantly lower than for BoNT/A, B, E, or F. The single human botulism outbreak described for BoNT/D supports this hypothesis (4). The absence of other reports of human botulism caused by BoNT/D may be due to several factors including non-reporting or misdiagnosis of mild cases, low occurrence of intoxication, differential sensitivity of human motor-neurons to BoNT/D due to a VAMP-1 polymorphism (29), poor intestinal absorption, and possibly low occurrence of toxigenic strains producing BoNT/D in the environment, as well as low levels of BoNT/D production by toxigenic strains.
The results of this study indicate that the VAMP 2 in cultured human neurons completely recovers from BoNT/D induced cleavage within 2 weeks, with recovery beginning at 3 days post intoxication (Figure 2 a). This is further supported by in vivo experiments showing recovery of motor-neuron deficiency in mice within 10 days after local injection with BoNT/D in the gastrocnemius muscle, compared to 2-3 weeks required for full recovery after injection with BoNT/A1 (Figure 2 b). Thus, recovery from BoNT/D induced botulism in humans would be expected to be relatively fast. This is also supported by the only case report of BoNT/D induced human botulism, in which the most severe case started to recover after 2 ½ weeks, and the other affected individuals recovered within days (4). In addition, the report also described the disease as ‘subacute nonlethal botulism with no need for respiratory support and with the atypical symptoms of diarrhea and vomiting playing a larger role’, which was confirmed experimentally in guinea pigs (4). In agreement with this observation of distinct symptoms caused by BoNT/D, the in vivo data presented here indicated longer time to death after intraperitoneal injection of mice with 100 and 1,000 Units of BoNT/D than BoNT/A1, and similar to BoNT/B1. The hallmark symptoms of ruffled fur, wasped abdomen, and difficulty breathing were milder, whereas spasticity just prior to death, as is usually observed with high doses of BoNT/A1, was pronounced.
It is interesting to speculate why large animals such as cattle and horses can readily develop botulism caused by BoNT/D, whereas it is rare or absent in humans. Several reasons might account for that, including preferential growth of BoNT/D producing Clostridia in animal feed, ingestions of larger amounts of contaminated foods (and BoNT/D) by animals, potential colonization of animal's digestive tracts with toxin producing Clostridia, potential poor absorption of BoNT/D from the human gastrointestinal tract, and certainly VAMP1 polymorphism (29), and as our data indicate, potentially variable cell entry.
Human VAMP1 differs from the VAMP1 of most other mammalian species by one amino acid (I48 instead of M48) (29). It has been shown that VAMP-1 is the predominant and almost exclusive VAMP isoform in the neuromuscular junction in rats (30). Diaphragms from rats containing the I48 polymorphism had an about 2-fold prolonged half-paralysis time in the hemidiaphragm assay if exposed to BoNT/D than diaphragms from rats homozygous for the M48-VAMP1 (29), and in vitro assays have shown that the human VAMP1 is less susceptible to cleavage by BoNT/D than the VAMP 2 isoform (17). This less efficient proteolysis of human VAMP1 by BoNT/D certainly contributes to variable neuronal susceptibility to BoNT/D and milder or sublethal symptoms of type D botulism in humans.
The dependence of BoNT/D cell entry into human neurons on membrane depolarization (Figure 3) is consistent with previous data showing that membrane depolarization enhanced cell entry of the BoNT/D receptor binding domain (31). This indicates that BoNT/D may predominantly enter active neurons in vivo, which may further contribute to differential pharmacological properties from other BoNTs. Further research into this topic will be of great interest with regards to the potential use of BoNT/D or domains of BoNT/D as unique and new pharmaceuticals. The results obtained using cultured primary rodent spinal cord and cortical cells further support that culture conditions affect neuronal susceptibility to BoNT/D (Figure 4). Although the mechanism underlying these observations is currently unknown, the data suggests a potentially unique distribution of BoNT/D to certain subpopulations of neurons. BoNT/D has been shown to contain dual ganglioside binding pockets that are essential for cell entry, and to bind to b-series gangliosides similar to TeNT (31 -33). Others have hypothesized that SV2 is involved as a protein receptor in BoNT/D cell entry (34). While gangliosides and SV2 are widely expressed on neurons, the composition of gangliosides and SV2 isoforms displayed on the cell membrane differs on different neuronal subpopulations (35-38), such that preference for certain gangliosides and SV2 isoforms may cause preferential cell entry (19). Further research is needed to elucidate the cell entry mechanism of BoNT/D and in vivo distribution. In addition, the large variation in BoNT/D sensitivity observed for the different cell models and within different laboratories highlights the importance of recognizing the limitations of cell models when analyzing novel toxins. While cultured neurons from various sources can provide defined and species specific models for research purposes that would not be possible in vivo, data derived from such models must be interpreted within the context of the model and further studies.
Recent efforts based largely on sequencing have identified a multitude of BoNT subtypes for most serotypes. An analysis of sequences published in the NCBI databank related to BoNT/D revealed that the complete sequences of four different strains for BoNT/D have been deposited and have 96-100 % sequence identity to that of strain 1873. In addition, a myriad of chimeric toxins consisting of combinations of BoNT/D and C1 have been deposited, with sequence identities of around 65-85% for the entire protein, but high homology for the regions homologous to BoNT/D. As toxin serotypes are determined by neutralization with monovalent antibodies to the corresponding serotype, many of the chimeric toxins are described either as BoNT/D or BoNT/C1. It is feasible that the chimeric toxins and possibly full length BoNT/D subtypes have unique cell entry and in vivo intoxication characteristics that differ from those described here. It should be noted that the published amino acid sequences of BoNT/D from strain 1873, which was used in this study and was isolated from the only reported type D botulism case (5), and CB-16, which was used in the human injection trial(19), are 100% identical.
In summary, we show that cultured human neurons are susceptible to BoNT/D (strain 1873), but at a lower sensitivity than to BoNT/A1 (150-fold and 25-fold in the two tested models). The data suggest that humans may be susceptible to botulism caused by BoNT/D if exposed to sufficient quantities of toxin, although the botulism would b362 e expected to be mild and of relatively short duration, and possibly with distinct symptoms. Nevertheless, based on the presented data, BoNT/D and potential engineered derivatives should not be discounted as a potential human pathogen and counter-terrorism efforts should include BoNT/D. Atoxic BoNT/D or BoNT/A have previously been suggested as a specific neuronal drug delivery platform (39), and the data presented here suggest that these two proteins may display a preference for distinct neuronal subpopulations in vivo. In addition, further research is required to determine whether the unique properties of BoNT/D render this toxin a potential candidate as a novel bio-therapeutic either directly or as a chimera with another BoNT serotype.
Supplementary Material
The sensitivity of cultured human neurons to botulinum neurotoxin D was examined.
BoNT/ D enters human neurons 20-100 times less efficiently than BoNT/ A.
BoNT/D has a relatively short duration of action in human neurons (∼3 weeks).
BoNT/D has a slower onset and shorter duration of action in mice than BoNT/A.
Cultured rodent neurons have varying sensitivity to BoNT/D.
Ethical Statement.
All the work described in this manuscript was approved by the University of Wisconsin-Madison Institutional Biosafety Committee. All animal experiments were approved by and conducted according to guidelines by the University of Wisconsin Animal Care and Use Committee.
Acknowledgments
The authors wish to thank Dr. Cesare Montecucco and Dr. Ornella Rossetto for their intellectual input into this project and thorough review of the manuscript. Special thanks to Dr. Michel Popoff for providing valuable background information on the isolation of C. botulinum strain 1873. This study was supported by the NIH/NIAID Pacific Southwest Regional Center of Excellence grant U54 AI065359 and Great Lakes Regional Center of Excellence U54AI057153, as well as by the National Institute of Allergy and Infectious Diseases Award Number R01 AI093504.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lindstrom M, Myllykoski J, Sivela S, Korkeala H. Clostridium botulinum in cattle and dairy products. Crit Rev Food Sci Nutr. 2010;50:281–304. doi: 10.1080/10408390802544405. [DOI] [PubMed] [Google Scholar]
- 2.Rodloff AC, Kruger M. Chronic Clostridium botulinum infections in farmers. Anaerobe. 2012;18:226–228. doi: 10.1016/j.anaerobe.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Kruger M, Grosse-Herrenthey A, Schrodl W, Gerlach A, Rodloff A. Visceral botulism at dairy farms in Schleswig Holstein, Germany: prevalence of Clostridium botulinum in feces of cows, in animal feeds, in feces of the farmers, and in house dust. Anaerobe. 2012;18:221–223. doi: 10.1016/j.anaerobe.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Demarchi J, Mourgues C, Orio J, Prevot AR. Existence of type D botulism in man. Bulletin de l'Academie nationale de medecine. 1958;142:580–582. [PubMed] [Google Scholar]
- 5.Prevot AR, Sillioc R. Activation of botulinum D toxinogenesis by Bacillus licheniformis. Ann Inst Pasteur (Paris) 1959;96:630–632. [PubMed] [Google Scholar]
- 6.Gimenez DF, Gimenez JA. The typing of botulinal neurotoxins. International journal of food microbiology. 1995;27:1–9. doi: 10.1016/0168-1605(94)00144-u. [DOI] [PubMed] [Google Scholar]
- 7.Dover N, Barash JR, Hill KK, Xie G, Arnon SS. Molecular characterization of a novel botulinum neurotoxin type h gene. J Infect Dis. 2014;209:192–202. doi: 10.1093/infdis/jit450. [DOI] [PubMed] [Google Scholar]
- 8.Barash JR, Arnon SS. A Novel Strain of Clostridium botulinum That Produces Type B and Type H Botulinum Toxins. J Infect Dis. 2014;209:183–191. doi: 10.1093/infdis/jit449. [DOI] [PubMed] [Google Scholar]
- 9.Johnson EA, Montecucco C. Chapter 11 Botulism. In: Andrew GE, editor. Handbook of Clinical Neurology. Vol. 91. Elsevier; 2008. pp. 333–368. [DOI] [PubMed] [Google Scholar]
- 10.Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiological Reviews. 2000;80:717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- 11.Schiavo G, Rossetto O, Tonello F, Montecucco C. Intracellular targets and metalloprotease activity of tetanus and botulism neurotoxins. Current topics in microbiology and immunology. 1995;195:257–274. doi: 10.1007/978-3-642-85173-5_12. [DOI] [PubMed] [Google Scholar]
- 12.Rossetto O, Pirazzini M, Montecucco C. Botulinum neurotoxins: genetic, structural and mechanistic insights. Nat Rev Microbiol. 2014;12:535–549. doi: 10.1038/nrmicro3295. [DOI] [PubMed] [Google Scholar]
- 13.Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, Lillibridge S, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Swerdlow DL, Tonat K, Working Group on Civilian B. Botulinum toxin as a biological weapon: medical and public health management. JAMA : the journal of the American Medical Association. 2001;285:1059–1070. doi: 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- 14.Kman NE, Nelson RN. Infectious agents of bioterrorism: a review for emergency physicians. Emergency medicine clinics of North America. 2008;26:517–547. x–xi. doi: 10.1016/j.emc.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Dressler D. Curr Opin Microbiol. Vol. 15. England: 2012. Clinical applications of botulinum toxin; pp. 325–336. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto H, Ida T, Tsutsuki H, Mori M, Matsumoto T, Kohda T, Mukamoto M, Goshima N, Kozaki S, Ihara H. Specificity of botulinum protease for human VAMP family proteins. Microbiol Immunol. 2012;56:245–253. doi: 10.1111/j.1348-0421.2012.00434.x. [DOI] [PubMed] [Google Scholar]
- 17.Yamasaki S, Baumeister A, Binz T, Blasi J, Link E, Cornille F, Roques B, Fykse EM, Sudhof TC, Jahn R. Cleavage of members of the synaptobrevin/VAMP family by types D and F botulinal neurotoxins and tetanus toxin. The Journal of biological chemistry. 1994;269:12764–12772. [PubMed] [Google Scholar]
- 18.Pellizzari R, Mason S, Shone CC, Montecucco C. The interaction of synaptic vesicle-associated membrane protein/synaptobrevin with botulinum neurotoxins D and F. FEBS Lett. 1997;409:339–342. doi: 10.1016/s0014-5793(97)00482-1. [DOI] [PubMed] [Google Scholar]
- 19.Eleopra R, Montecucco C, Devigili G, Lettieri C, Rinaldo S, Verriello L, Pirazzini M, Caccin P, Rossetto O. Botulinum neurotoxin serotype D is poorly effective in humans: An in vivo electrophysiological study. Clin Neurophysiol. 2013;124:999–1004. doi: 10.1016/j.clinph.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Coffield JA, Bakry N, Zhang RD, Carlson J, Gomella LG, Simpson LL. In vitro characterization of botulinum toxin types A, C and D action on human tissues: combined electrophysiologic, pharmacologic and molecular biologic approaches. J Pharmacol Exp Ther. 1997;280:1489–1498. [PubMed] [Google Scholar]
- 21.Anderson J, Williams PT, Katos AM, Krasna M, Burrows W, Hilmas CJ. CHAPTER 30 - Botulinum Toxin. In: Ramesh CG, editor. Handbook of Toxicology of Chemical Warfare Agents. Academic Press; San Diego: 2009. pp. 407–432. [Google Scholar]
- 22.Malizio CJ, Goodnough MC, Johnson EA. Purification of Clostridium botulinum type A neurotoxin. Methods in molecular biology (Clifton, NJ) 2000;145:27–39. doi: 10.1385/1-59259-052-7:27. [DOI] [PubMed] [Google Scholar]
- 23.Moberg LI, Sugiyama H. Affinity chromatography purification of type A botulinum neurotoxin from crystalline toxic complex. Appl Environ Microbiol. 1978;35:878–880. doi: 10.1128/aem.35.5.878-880.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prabakaran S, Tepp W, DasGupta BR. Botulinum neurotoxin types B and E: purification, limited proteolysis by endoproteinase Glu-C and pepsin, and comparison of their identified cleaved sites relative to the three-dimensional structure of type A neurotoxin. Toxicon : official journal of the International Society on Toxinology. 2001;39:1515–1531. doi: 10.1016/s0041-0101(01)00124-6. [DOI] [PubMed] [Google Scholar]
- 25.Whitemarsh RC, Tepp WH, Bradshaw M, Lin G, Pier CL, Scherf JM, Johnson EA, Pellett S. Characterization of Botulinum Neurotoxin A Subtypes 1 Through 5 by Investigation of Activities in Mice, Neuronal Cell Cultures, and In Vitro. Infect Immun. 2013;81:3894–3902. doi: 10.1128/IAI.00536-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pellett S, Tepp WH, Toth SI, Johnson EA. Comparison of the primary rat spinal cord cell (RSC) assay and the mouse bioassay for botulinum neurotoxin type A potency determination. Journal of pharmacological and toxicological methods. 2010;61:304–310. doi: 10.1016/j.vascn.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Pellett S, Tepp WH, Clancy CM, Borodic GE, Johnson EA. A neuronal cell-based botulinum neurotoxin assay for highly sensitive and specific detection of neutralizing serum antibodies. FEBS letters. 2007;581:4803–4808. doi: 10.1016/j.febslet.2007.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitemarsh RC, Strathman MJ, Chase LG, Stankewicz C, Tepp WH, Johnson EA, Pellett S. Novel application of human neurons derived from induced pluripotent stem cells for highly sensitive botulinum neurotoxin detection. Toxicological sciences : an official journal of the Society of Toxicology. 2012;126:426–435. doi: 10.1093/toxsci/kfr354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng L, Adler M, Demogines A, Borrell A, Liu H, Tao L, Tepp WH, Zhang SC, Johnson EA, Sawyer SL, Dong M. Widespread sequence variations in VAMP1 across vertebrates suggest a potential selective pressure from botulinum neurotoxins. PLoS Pathog. 2014;10:e1004177. doi: 10.1371/journal.ppat.1004177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li JY, Edelmann L, Jahn R, Dahlstrom A. Axonal transport and distribution of synaptobrevin I and II in the rat peripheral nervous system. J Neurosci. 1996;16:137–147. doi: 10.1523/JNEUROSCI.16-01-00137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroken AR, Karalewitz AP, Fu Z, Kim JJ, Barbieri JT. Novel ganglioside-mediated entry of botulinum neurotoxin serotype D into neurons. J Biol Chem. 2011;286:26828–26837. doi: 10.1074/jbc.M111.254086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strotmeier J, Lee K, Volker AK, Mahrhold S, Zong Y, Zeiser J, Zhou J, Pich A, Bigalke H, Binz T, Rummel A, Jin R. Botulinum neurotoxin serotype D attacks neurons via two carbohydrate-binding sites in a ganglioside-dependent manner. The Biochemical journal. 2010;431:207–216. doi: 10.1042/BJ20101042. [DOI] [PubMed] [Google Scholar]
- 33.Rummel A. Double receptor anchorage of botulinum neurotoxins accounts for their exquisite neurospecificity. Curr Top Microbiol Immunol. 2013;364:61–90. doi: 10.1007/978-3-642-33570-9_4. [DOI] [PubMed] [Google Scholar]
- 34.Peng L, Tepp WH, Johnson EA, Dong M. Botulinum Neurotoxin D Uses Synaptic Vesicle Protein SV2 and Gangliosides as Receptors. PLoS pathogens. 2011;7:e1002008. doi: 10.1371/journal.ppat.1002008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnaar RL, Gerardy-Schahn R, Hildebrandt H. Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol Rev. 2014;94:461–518. doi: 10.1152/physrev.00033.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bajjalieh SM, Frantz GD, Weimann JM, McConnell SK, Scheller RH. Differential expression of synaptic vesicle protein 2 (SV2) isoforms. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:5223–5235. doi: 10.1523/JNEUROSCI.14-09-05223.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogawa-Goto K, Funamoto N, Abe T, Nagashima K. Different ceramide compositions of gangliosides between human motor and sensory nerves. J Neurochem. 1990;55:1486–1493. doi: 10.1111/j.1471-4159.1990.tb04930.x. [DOI] [PubMed] [Google Scholar]
- 38.Gong Y, Tagawa Y, Lunn MP, Laroy W, Heffer-Lauc M, Li CY, Griffin JW, Schnaar RL, Sheikh KA. Localization of major gangliosides in the PNS: implications for immune neuropathies. Brain. 2002;125:2491–2506. doi: 10.1093/brain/awf258. [DOI] [PubMed] [Google Scholar]
- 39.Bade S, Rummel A, Reisinger C, Karnath T, Ahnert-Hilger G, Bigalke H, Binz T. Botulinum neurotoxin type D enables cytosolic delivery of enzymatically active cargo proteins to neurones via unfolded translocation intermediates. J Neurochem. 2004;91:1461–1472. doi: 10.1111/j.1471-4159.2004.02844.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


