Abstract
Bladder cancer is a highly recurrent disease in need of novel, durable treatment strategies. This study assessed the ability of an intravesical immunotherapy composed of a coformulation of the biopolymer chitosan with interleukin-12 (CS/IL-12) to induce systemic adaptive tumor-specific immunity. Intravesical CS/IL-12 immunotherapy was used to treat established orthotopic MB49 and MBT-2 bladder tumors. All mice receiving intravesical CS/IL-12 immunotherapy experienced high cure rates of orthotopic disease. To investigate the durability and extent of the resultant adaptive immune response, cured mice were rechallenged both locally (intravesically) and distally. Cured mice rejected 100 % of intravesical tumor rechallenges and 50–100 % of distant subcutaneous rechallenges in a tumor-specific manner. The ability of splenocytes from cured mice to lyse targets in a tumor-specific manner was assessed in vitro, revealing that lytic activity of splenocytes from cured mice was robust and tumor specific. Protective immunity was durable, lasting for at least 18 months after immunotherapy. In an advanced bladder cancer model, intravesical CS/IL-12 immunotherapy controlled simultaneous orthotopic and subcutaneous tumors in 70 % of treated mice. Intravesical CS/IL-12 immunotherapy creates a robust and durable tumor-specific adaptive immune response against bladder cancer. The specificity, durability, and potential of this therapy to treat both superficial and advanced disease are deserving of consideration for clinical translation.
Keywords: Intravesical immunotherapy, Bladder cancer, Interleukin-12, Chitosan
Introduction
Worldwide annual incidence of bladder cancer is approximately 356,000 with a prevalence estimated to be 2.7 million [1, 2]. The majority (~70 %) of new cases present as non-muscle invasive bladder cancer (NMIBC). For patients with intermediate-to-high-risk NMIBC, the standard of care for over 30 years has been intravesical immunotherapy with Mycobacterium bovis bacillus Calmette–Guerin (BCG) [3]. Since its pioneering use by Morales et al. in 1976, BCG has proven more effective than any single chemotherapeutic agent at preventing recurrence of high-grade NMIBC [4, 5].
Despite its success, BCG immunotherapy retains several limitations. Five percent of patients treated with BCG exhibit serious side effects including sepsis and allergic reactions [6, 7]. Furthermore, of the 50–75 % of patients who completely respond to BCG, 30–50 % experience a recurrence within 5 years [8, 9]. These relapses are likely due to the inability of BCG to induce a tumor-specific T cell response [5]. Thus, strategies that limit recurrence of high-grade NMIBC and improve patient survival by promoting protective antitumor immunity are needed.
We have previously shown that the coformulation of interleukin-12 (IL-12) with a viscous solution of the biopolymer chitosan (CS/IL-12) was superior to both BCG and IL-12 alone against orthotopic bladder tumors [10]. Chitosan, as detailed below, is a mucoadhesive delivery vehicle that has no antitumor activity by itself [10]. The subject of many preclinical and clinical studies, IL-12 is a powerful cytokine that induces a TH1-polarized immune response while engaging both the innate and adaptive branches of immunity [11–15]. Chitosan is an abundant, natural polysaccharide with a broad range of applications and an established safety profile in humans [16–19].
Chitosan is particularly well suited for delivery within the bladder due to its potential to enhance three aspects of delivery. First, chitosan’s polycationic nature facilitates adhesion to negatively charged mucosal surfaces. Second, chitosan solution is viscous which may reduce the amount of therapeutic agent washed away during voiding of the bladder. Third, chitosan may enhance bladder wall absorption by opening gap junctions between epithelial cells [18].
Our previous study showed that 4 weekly intravesical treatments with CS/IL-12 (5 μg) eliminated 80–100 % of orthotopic bladder tumors in mice, while all mice treated similarly with BCG succumbed to the disease within 60 days [10]. Only 37 % of tumor-bearing mice treated with IL-12 alone survived long term. Similar to other preclinical results involving intravesical IL-12, 100 % of cured mice were completely protected from intravesical rechallenge [20, 21]. Intravesical CS/IL-12 induced brisk tumor infiltration by CD3+ T cells and F4/80+ macrophages. Within the T cell compartment, CD8+ infiltrates outnumbered CD4+ infiltrates. Overall, immunohistochemistry data suggested that CD8+ T cells and macrophages are potential effector cells mediating the regression of bladder tumors. However, despite the involvement of T lymphocytes and the induction of protective immunity, the study stopped short of confirming the induction of adaptive immunity.
Therefore, the primary goal of the present study was to determine whether intravesical CS/IL-12 can induce adaptive tumor-specific immunity. A secondary goal was to explore the efficacy of CS/IL-12 immunotherapy at lower doses and in multiple preclinical models.
Materials and methods
Animals, materials, and cell lines
Female C57BL/6J and C3H/HeJ mice, 6–12 weeks old, were obtained from the Jackson Laboratory. Mice were housed and maintained under pathogen-free conditions in microisolator cages. The Institutional Animal Care and Use Committee at the University of Arkansas approved all experimental procedures. Animal care complied with the recommendations of The Guide for Care and Use of Laboratory Animals (National Research Council).
Dulbecco’s modified Eagle’s medium (DMEM), RPMI 1640, Dulbecco’s phosphate-buffered saline (PBS), and fetal bovine serum (FBS) were obtained from HyClone Laboratories. Chitosan glutamate 200–600 kDa, 75–90 % deacetylated (Protosan G 213), was purchased from Novamatrix. Recombinant murine IL-12 was purchased from PeproTech.
The C57BL/6 syngeneic transitional cell carcinoma MB49 was kindly provided by Dr. Jeffrey Schlom, Laboratory of Tumor Immunology and Biology, National Cancer Institute. The C3H syngeneic transitional cell carcinoma MBT-2 was kindly provided by Dr. Yi Luo, Department of Urology, University of Iowa. The C57BL/6 syngeneic melanoma B16-F10 was purchased from the American Type Culture Collection. MB49 and B16-F10 cells were maintained in DMEM, while MBT-2 cells were maintained in RPMI 1640. Both media were supplemented with 10 % FBS, 2 mM l-glutamine, and 1 % penicillin/streptomycin.
Tumor implantation and therapy
Orthotopic bladder tumors were generated via intravesical instillation of either 75,000 MB49 cells or 100,000 MBT-2 cells following a poly-l-lysine wash as described previously [10]. Intravesical tumor burden was monitored by development of hematuria as well as palpation of tumors.
Intravesical treatments were administered via the same catheterization technique. Chitosan glutamate was added to PBS at a concentration of 10 mg/ml before addition of IL-12. For IL-12 alone, the cytokine was diluted in PBS. Treatments were allowed to dwell for 30–45 min.
All subcutaneous implantations were administered in the right flank. Subcutaneous tumor width and length were measured using calipers, and the tumor volume was calculated as Volume = 0.5 * Length * Width2. Mice were euthanized when moribund or when tumor volume reached 2000 mm3.
Cytotoxicity T lymphocyte (CTL) assay
Splenocytes from mice that had been cured of orthotopic MB49 tumors were cultured with irradiated (25 Gy) MB49 cells. After one week, lymphocytes were collected on a histopaque gradient and quantified. Cytotoxic activity of recovered lymphocytes against MB49 and B16 targets was measured via the CytoTox-Glo assay (Promega). Spontaneous lysis of tumor cells cultured without lymphocytes as well as complete lysis of tumor cells exposed to a lysis reagent served as controls. The percentage of specific lysis was calculated as follows:
Results
Treatment of MBT-2 with intravesical CS/IL-12
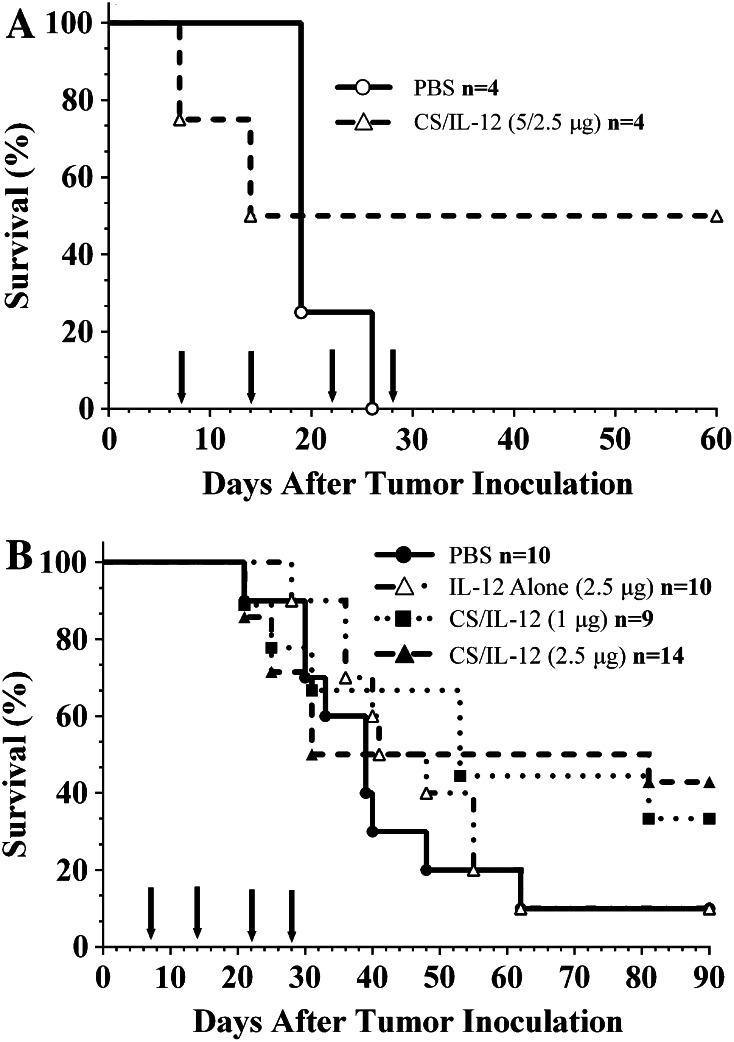
Previous studies exploring intravesical CS/IL-12 immunotherapy to treat orthotopic bladder cancer focused only on the MB49 tumor model [10]. In a preliminary experiment to explore the efficacy of CS/IL-12 in an additional tumor model, C3H mice bearing orthotopic MBT-2 were treated weekly starting 7 days post-implantation. Mice were randomized to receive either PBS or CS/IL-12 intravesically. Initial attempts to treat MBT-2 with CS/IL-12 (5 μg) resulted in two early treatment-related deaths (Fig. 1a) characterized by mice emerging from anesthesia in a moribund state, i.e., hunched and slow with ruffled fur. This timeline of toxicity is consistent with reported accounts of IL-12 toxicity in which mice typically succumbed 2 days after multiple IL-12 administrations [22]. Consequently, the final two treatments were reduced to CS/IL-12 (2.5 μg), resulting in complete tumor elimination, as evidenced by clear urine and no palpable tumors, in the remaining mice. The overall survival for this pilot experiment was 50 % (2/4).
Fig. 1.
Intravesical CS/IL-12 immunotherapy is effective against orthotopic MBT-2 tumors. C3H/HeJ mice were inoculated with 100,000 MBT-2 cells intravesically. a Mice were treated intravesically with either PBS (open circle, n = 4) or CS/IL-12 (open triangle, n = 4) every 7 days starting 7 days post-inoculation (arrows indicate treatments). The first two treatments contained 5 μg IL-12; however, the final two treatments were reduced to 2.5 μg to reduce toxicity. b MBT-2 tumor-bearing mice were treated intravesically with PBS (filled circle, n = 10), IL-12 alone (open triangle, n = 10), CS/IL-12 (1 μg) (filled square, n = 9), or CS/IL-12 (2.5 μg) (filled triangle, n = 14) weekly for four consecutive weeks starting 7 days post-implantation (arrows indicate treatments). Mice were monitored for hematuria and survival. Survival curves were not statistically different (P > 0.05 via log-rank test)
In a subsequent experiment (Fig. 1b), the antitumor activities of IL-12 alone (2.5 μg), CS/IL-12 (2.5 μg), and CS/IL-12 (1 μg) were explored. IL-12 alone was no more effective than PBS with one of 10 mice in both groups surviving long term; the surviving mice in both groups did not exhibit hematuria at any point in the experiment. CS/IL-12 (1 μg) treatments resulted in two of nine mice surviving long term, while mice treated with CS/IL-12 (2.5 μg) experienced a long-term survival rate of 43 % (6 of 14). Despite clear differences in long-term survivorship, log-rank analysis did not reveal statistically significant differences in median survival times of any of the treatment groups. The experiment was monitored until all mice either were euthanized due to tumor progression or had no evidence of disease.
Lower dose CS/IL-12 therapy of orthotopic MB49
To determine whether CS/IL-12 immunotherapy is effective at a lower doses, intravesical CS/IL-12 (1 μg) was used to treat orthotopic MB49 tumors (Fig. 2). The 1-μg dose was chosen as it had been shown to be effective at hindering bladder tumors (Fig. 1b), and we hypothesized that an accelerated schedule of twice weekly instead of once weekly would be more effective against the more sensitive MB49 model. Treatment was begun upon onset of hematuria. All control mice receiving PBS succumbed to disease with a median survival of 22 days, while intravesical treatment with IL-12 alone resulted in a 52-day median survival. Intravesical immunotherapy with CS/IL-12 (1 μg) eradicated 100 % of orthotopic MB49 tumors with all treated mice surviving long term (>70 days). Differences in survival among the three treatment groups were significant (P < 0.05).
Fig. 2.
Intravesical CS/IL-12 (1 μg) therapy eradicates orthotopic MB49 tumors. Mice were implanted intravesically with 75,000 MB49 cells. Upon onset of hematuria, mice were treated (arrows) every 3 or 4 days with PBS (filled circle, n = 9), IL-12 alone (1 μg) (filled square, n = 10), or CS/IL-12 (1 μg) (filled triangle, n = 8). Mice were monitored for hematuria and survival. Asterisks indicate a significant difference between the survival curves (P < 0.05 via log-rank test)
Rechallenge of cured mice and in vitro cytotoxicity studies
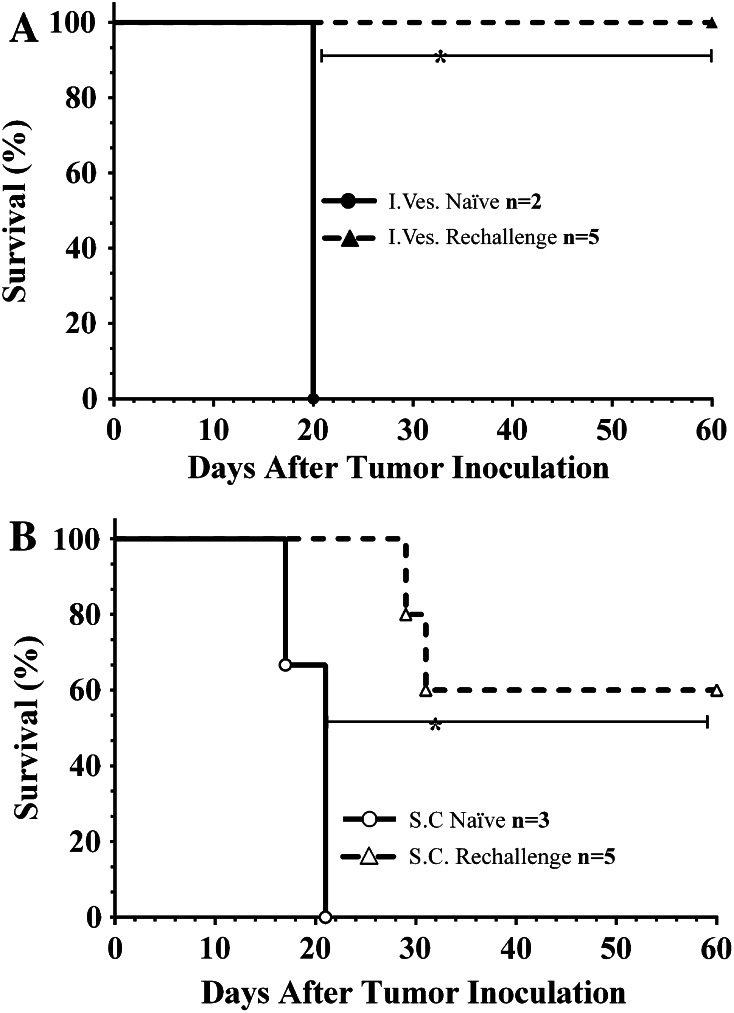
C3H mice completely eliminating orthotopic MBT-2 tumors were rechallenged with MBT-2 intravesically (Fig. 3a). All cured mice rejected intravesical rechallenge (n = 5) with no evidence of tumor development, i.e., hematuria. Naïve control mice (two of two) developed tumors indicated by severe hematuria, a palpable mass, and a moribund state within 20 days post-implantation. Cured mice previously rejecting intravesical rechallenge were then inoculated subcutaneously with MBT-2 in the right flank (Fig. 3b). Three of five mice rejected the subcutaneous rechallenge, while three of three naïve mice developed tumors. In both rechallenge experiments, the difference in survival between naïve and cured mice was significant (P < 0.05). All mice that rejected rechallenge survived long term (>90 days post-inoculation).
Fig. 3.
Survival of cured C3H/HeJ mice following both local and distant rechallenge. a Both naïve (filled circle, n = 2) and mice previously cured (filled triangle, n = 5) of orthotopic MBT-2 using intravesical CS/IL-12 were rechallenged intravesically with 100,000 cells 6 weeks after their last treatment. b Mice rejecting orthotopic rechallenge (open triangle, n = 5) were then rechallenged in the right flank with 100,000 MBT-2 cells subcutaneously 8 weeks after their last CS/IL-12 administration. Naive mice (open circle, n = 3) were also challenged subcutaneously with the same number of cells. Asterisks indicate a significant difference between the survival curves (P < 0.05 via log-rank test)
Previous studies demonstrated that C57BL/6 mice previously cured of orthotopic MB49 using CS/IL-12 also reject intravesical rechallenge [10]. To investigate the extent and specificity of this antitumor immunity, cured mice were inoculated subcutaneously in the right flank with either MB49 or an unrelated tumor, B16-F10 melanoma (Fig. 4a). All of the previously cured mice receiving B16 cells developed lesions (n = 8), while all cured mice given MB49 cells rejected rechallenge (n = 13). Naïve mice quickly developed either B16 or MB49 tumors.
Fig. 4.
Cured mice exhibit both local and systemic tumor-specific immunity. a Tumor incidence of cured C57BL/6 mice rechallenged at a distant site. C57BL/6 mice previously cured of orthotopic MB49 using intravesical CS/IL-12 were rechallenged subcutaneously at the right flank with either 300,000 MB49 cells (open triangle, n = 18) or 300,000 B16-F10 cells (filled triangle, n = 8). Naïve mice were also implanted with either MB49 or B16-F10 tumor cells as positive controls. The incidence of tumor appearance was monitored. b In vitro cytotoxicity reveals tumor-specific lysis. Splenocytes isolated from mice cured of orthotopic MB49 via intravesical CS/IL-12 immunotherapy were stimulated for 1 week with irradiated MB49 cells before being allowed to lyse either MB49 (filled triangle, n = 3) or B16-F10 (open triangle, n = 3) tumor cells. The percent of tumor cells lysed by collected lymphocytes was measured after overnight incubation at increasing effector:tumor ratios. Results for three separate experiments are shown. The difference between MB49 and B16 lysis was significant (P < 0.05 via two-tailed t test) within each experiment. Error bars represent standard deviation of triplicate measurements
CTL assays demonstrated that lymphocytes derived from the spleens of cured mice lysed MB49 targets much more efficiently than B16 targets (Fig. 4b). Specifically, at an effector:tumor ratio of 50:1, lymphocytes from three cured mice lysed 41.1, 49.3, and 51.8 % of MB49 targets but only 9.8, 20.3, and 20.2 % of B16 targets, respectively. The difference between MB49 and B16 lysis was significant (P < 0.05 via two-tailed t test) within each experiment.
In a separate experiment to evaluate the durability of antitumor immunity, mice that received their last CS/IL-12 treatment 9, 10, or 18 months prior were rechallenged with MB49 cells intravesically. All (eight of eight) of these mice rejected the orthotopic rechallenge (Table 1). Those same mice also completely rejected a subcutaneous rechallenge.
Table 1.
Local and systemic durability of tumor-specific immunity generated via CS/IL-12 immunotherapy
| Time since last CS/IL-12 treatment | Intravesical rechallenge | Subcutaneous rechallenge |
|---|---|---|
| 9 Months | 3/3 | 3/3 |
| 10 Months | 3/2 | 3/2 |
| 18 Months | 3/3 | 3/3 |
| Naïve | 0/3 | 0/3 |
All mice previously had orthotopic MB49 tumors that were eradicated following intravesical CS/IL-12 immunotherapy. Tumor challenge consisted of 75,000 MB49 cells administered intravesically or 300,000 MB49 cells injected in the right flank. Naïve mice served as positive controls. Mice were monitored for tumor development and hematuria. Overall survival was recorded
Treatment of distant lesions with intravesical therapy
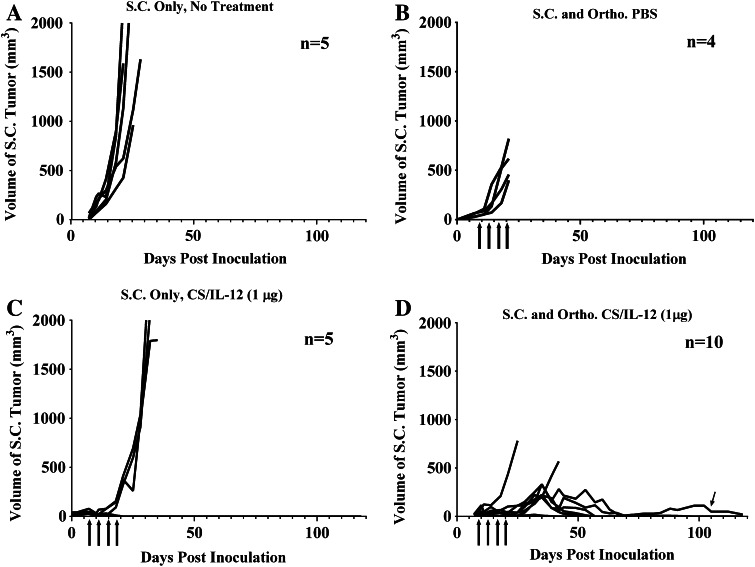
The studies above demonstrated that intravesical CS/IL-12 can induce systemic, adaptive antitumor immunity. Therefore, to determine whether intravesical CS/IL-12 can control both orthotopic and distant disease simultaneously, naïve mice were divided into four groups (Fig. 5) and given MB49 either subcutaneously only (A and C) or both subcutaneously and orthotopically (B and D). Mice were treated intravesically with either PBS (B) or CS/IL-12 (1 μg) (C and D). A cohort of mice bearing subcutaneous MB49 tumors were not treated (A) to serve as a negative control.
Fig. 5.
Intravesical CS/IL-12 immunotherapy of orthotopic bladder tumors is effective against distant lesions. C57BL/6 mice were implanted with MB49 either subcutaneously (a, c) or both subcutaneously and orthotopically (b, d). Mice in groups C (n = 5) and D (n = 10) received intravesical treatments of CS/IL-12 (1 μg) twice per week starting 7 days after implantation. Group B (n = 4) received intravesical PBS. Group A (n = 5) served as a negative control and did not receive any treatment. Tumor volume, hematuria, and overall survival were monitored. Arrows indicate treatments
Subcutaneous tumors in untreated mice grew rapidly, and all mice were euthanized by day 30 (A). Mice with both intravesical and subcutaneous tumor burdens that received PBS (B) exhibited a slower subcutaneous growth rate compared to mice in group A, but were all moribund and euthanized by day 21 due to dual high tumor burdens. Mice bearing only subcutaneous tumors, but treated intravesically with CS/IL-12 (C) experienced slowed tumor growth during treatments likely due to systemic leakage of IL-12. Our previous paper demonstrated that intravesical CS/IL-12 does result in measurable serum IL-12 levels [10]. It has also been established that systemic IL-12 can induce subcutaneous MB49 regression [23]. Once the treatments ceased, three of five mice exhibited exponential tumor growth and succumbed within 20 days after the last treatment. The other two mice survived and remained tumor-free long term.
The final group (D) bearing both orthotopic and subcutaneous tumor burdens was treated intravesically with CS/IL-12. One of ten mice initially failed to reject its primary orthotopic tumor and succumbed within 25 days after implantation. The remaining nine mice exhibited suppressed subcutaneous tumor growth both during and after treatment. Seven of those nine mice completely eradicated both their subcutaneous and orthotopic burdens. One of the nine mice eradicated its orthotopic tumor but did not completely eradicate its subcutaneous tumor. That mouse, indicated by an arrow, maintained a small tumor over a timescale of more than 4 months with no other ill effects. One of the seven mice that eliminated both tumors eventually succumbed to a recurrence of orthotopic disease. Overall, over the 4-month experiment, seven of ten either eradicated or controlled both their orthotopic and subcutaneous tumors following intravesical CS/IL-12 immunotherapy.
Discussion
The most significant finding of this study is the demonstration of tumor-specific adaptive immunity following intravesical CS/IL-12 immunotherapy. Our previous study showed that CS/IL-12-treated mice were protected from orthotopic bladder cancer rechallenge [10]. In addition, immunohistochemistry studies demonstrated high levels of macrophage and T cell infiltrates during and after treatment. Thus, while adaptive immunity was implied, our previous data did not provide a causal link between an adaptive immune response and protective immunity. The present study provides that link in three ways. First, the in vitro cytotoxicity assays demonstrated that lymphocytes from cured mice lyse targets in a tumor-specific manner (Fig. 4b). Second, the ability of cured mice to reject MB49 cells but not B16 cells implanted at a distant site demonstrates not only a tumor-specific but also a systemic, adaptive immune response (Fig. 4a). Third, mice rechallenged long after their last treatment successfully rejected both intravesical and subcutaneous inoculations (Table 1). Our previous study showed that immune infiltrates were returning to normal levels by 6 months post-treatment [10]. Thus, rejection of orthotopic and subcutaneous tumors at 9, 10, and 18 months post-CS/IL-12 immunotherapy when the immune infiltration has subsided demonstrates a long-lived effector-memory response. These data combine to show that this rejection is not a local, mucosal phenomena due to high levels of non-specific residual infiltrates, but instead a full-fledged adaptive, tumor-specific immune response that extends to the systemic circulation.
To our knowledge, this is the first demonstration of an intravesical-to-systemic transfer of immunity. This finding could have pertinent implications for the treatment of advanced bladder cancers. Our preclinical model (Fig. 5) demonstrates that intravesical treatment with CS/IL-12 has the potential to actively engage and direct the immune system toward distant malignancies. A significant fraction of new bladder cancer cases (~30 %) initially present as muscle invasive disease with or without distant metastases [3]. Standard treatments for these patients include radiation therapy and life-altering radical cystectomy. Even after removal of the bladder, approximately half of patients already have metastases that are associated with poor outcomes [24]. The dual-burden model explored here is not a perfect representation of true metastatic disease. Nonetheless, the ability of CS/IL-12 to eliminate both orthotopic and distant tumors is promising and could lead to an alternative treatment for regionally advanced or metastatic bladder cancers.
One of the goals of this study was to investigate the efficacy of CS/IL-12 against an additional tumor model. The MB49 model is the most commonly used model of murine bladder cancer even though it was initially derived from male mice and bears the Hy antigen, which is foreign in female mice [25, 26]. However, female mice are commonly used in orthotopic bladder tumor models due to their less tortuous urethras. Despite the presence of the Hy antigen, MB49 develops aggressive, rapid, and multifocal tumors in female mice. Untreated mice usually succumb within 30 days of implantation. This aggressiveness is likely due to the ability of MB49 to recruit immune cells producing immunosuppressive IL-10 as described previously [27].
The second most utilized murine bladder tumor model, MBT-2 cell line, was derived from female mice and is not known to bear any foreign antigens [26]. The MBT-2 model offers the additional challenge of being syngeneic to C3H mice, which are reportedly more sensitive to IL-12-induced toxicities [20, 28]. The results of our study demonstrate that intravesical CS/IL-12 immunotherapy effectively eradicated six of 14, or 43 %, of orthotopic MBT-2 tumors (Fig. 1b) and protected mice from local and distant tumor rechallenges (Fig. 3). Thus, intravesical CS/IL-12 immunotherapy is effective in diverse bladder tumor models. Though the 43 % cure rate was not as efficient as seen in the MB49 model, better outcomes are likely given an optimized treatment regimen. To this end, planned studies will continue to investigate optimal delivery strategies for CS/IL-12, especially in terms of dose, time of installation, times between treatments, number of treatments, and therapeutic volume.
It is also interesting to note that the median survival of MBT-2-bearing mice treated with CS/IL-12 did not increase in a statistically significant manner despite a clear increase in the percentage of long-term survivors (Fig. 1b). Survival curves seem to indicate that there are responders, which can be cured, and non-responders, which progress as if untreated, within CS/IL-12 treatment cohort. We believe this phenomenon is due to the unique growth kinetics of MBT-2 tumors. This tumor line takes longer to establish than MB49, but once it does establish, tumor growth is exponential and mice succumb within days. Ongoing studies are investigating more aggressive dosing schedules to try to stay on top of the MBT-2 tumor and alter the responder/non-responder balance.
The final major finding from this study is the durability of antitumor immunity generated via CS/IL-12 immunotherapy (Table 1). This has potential clinical impact in terms of reducing the cost and increasing the compliance associated with NMIBC bladder cancer treatment. The high rate of recurrence following BCG immunotherapy necessitates frequent monitoring of the patient and repeated treatments. Current American Urological Association guidelines recommend a cystoscopy every 3–6 months. As such, bladder cancer has the highest domestic cost per patient ($96,000–$187,000) of all cancers, with a total burden exceeding $4 billion annually [29]. Additionally, only 40 % of patients fully comply with the recommended guidelines [29, 30]. In addition to eliminating bladder cancer, the durable and specific immunity induced by intravesical CS/IL-12 immunotherapy could lead to fewer maintenance treatments, reduce the number of monitoring appointments, and subsequently increase patient compliance while decreasing per-patient costs.
Acknowledgments
This work was supported by National Institutes of Health Grants K22CA131567, R15CA176649, and R01CA172631 to D.A. Zaharoff.
Conflict of interest
None.
Abbreviations
- BCG
Mycobacterium bovis bacillus Calmette–Guerin
- CS/IL-12
Coformulation of chitosan and IL-12
- CTL
Cytotoxic T lymphocyte
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
Fetal bovine serum
- IL-12
Interleukin-12
- NMIBC
Non-muscle invasive bladder cancer
- PBS
Dulbecco’s phosphate-buffered saline
References
- 1.Parkin DM. The global burden of urinary bladder cancer. Scand J Urol Nephrol. 2008;42(Suppl 218):12–20. doi: 10.1080/03008880802285032. [DOI] [PubMed] [Google Scholar]
- 2.Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27:289–293. doi: 10.1007/s00345-009-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–249. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 4.Morales A, Eidinger D, Bruce A. Intracavitary Bacillus Calmette–Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 5.Alexandroff AB, Jackson AM, O’Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353:1689–1694. doi: 10.1016/S0140-6736(98)07422-4. [DOI] [PubMed] [Google Scholar]
- 6.Anastasiadis A, de Reijke TM. Best practice in the treatment of nonmuscle invasive bladder cancer. Ther Adv Urol. 2012;4:13–32. doi: 10.1177/1756287211431976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamm DL, Van der Meijden P, Morales A, Brosman SA, Catalona WJ, Herr HW, Soloway MS, Steg A, Debruyne F. Incidence and treatment of complications of bacillus Calmette–Guerin intravesical therapy in superficial bladder cancer. J Urol. 1992;147:596–600. doi: 10.1016/s0022-5347(17)37316-0. [DOI] [PubMed] [Google Scholar]
- 8.Malmstrom PU, Wijkstrom H, Lundholm C, Wester K, Busch C, Norlen BJ. 5-Year followup of a randomized prospective study comparing mitomycin C and bacillus Calmette–Guerin in patients with superficial bladder carcinoma. J Urol. 1999;161:1124–1127. doi: 10.1016/S0022-5347(01)61607-0. [DOI] [PubMed] [Google Scholar]
- 9.Askeland EJ, Newton MR, O’Donnell MA, Luo Y. Bladder cancer immunotherapy: BCG and beyond. Adv Urol. 2012;2012:181987. doi: 10.1155/2012/181987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaharoff DA, Hoffman BS, Hooper HB, Benjamin CJ, Jr, Khurana KK, Hance KW, Rogers CJ, Pinto PA, Schlom J, Greiner JW. Intravesical immunotherapy of superficial bladder cancer with chitosan/interleukin-12. Cancer Res. 2009;69:6192–6199. doi: 10.1158/0008-5472.CAN-09-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 12.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. doi: 10.1016/S1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Zaharoff DA. Role of chitosan co-formulation in enhancing interleukin-12 delivery and antitumor activity. Biomaterials. 2013;34:3828–3836. doi: 10.1016/j.biomaterials.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss JM, Subleski JJ, Wigginton JM, Wiltrout RH. Immunotherapy of cancer by IL-12-based cytokine combinations. Expert Opin Biol Ther. 2007;7(11):1705–1721. doi: 10.1517/14712598.7.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, Anichini A. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 16.McNeela EA, Jabbal-Gill I, Illum L, Pizza M, Rappuoli R, Podda A, Lewis DJ, Mills KH. Intranasal immunization with genetically detoxified diphtheria toxin induces T cell responses in humans: enhancement of Th2 responses and toxin-neutralizing antibodies by formulation with chitosan. Vaccine. 2004;22:909–914. doi: 10.1016/j.vaccine.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Wedmore I, McManus JG, Pusateri AE, Holcomb JB. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J Trauma. 2006;60(3):655–658. doi: 10.1097/01.ta.0000199392.91772.44. [DOI] [PubMed] [Google Scholar]
- 18.Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm Res. 1998;15:1326–1331. doi: 10.1023/A:1011929016601. [DOI] [PubMed] [Google Scholar]
- 19.Arai K, Kinumaki T, Fujita T. Toxicity of chitosan. Bull Tokai Region Fish Res Lab. 1968;56:89–94. [Google Scholar]
- 20.O’Donnell MA, Luo Y, Hunter SE, Chen X, Hayes LL, Clinton SK. Interleukin-12 immunotherapy of murine transitional cell carcinoma of the bladder: dose dependent tumor eradication and generation of protective immunity. J Urol. 2004;171:1330–1335. doi: 10.1097/01.ju.0000109742.88380.a2. [DOI] [PubMed] [Google Scholar]
- 21.Horinaga M, Harsch KM, Fukuyama R, Heston W, Larchian W. Intravesical interleukin-12 gene therapy in an orthotopic bladder cancer model. Urology. 2005;66:461–466. doi: 10.1016/j.urology.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 22.Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, Sosman JA, Dutcher JP, Vogelzang NJ, Ryan JL. Effects of single-dose interleukin-12 exposure on interleukin-12—associated toxicity and interferon-γ production. Blood. 1997;90:2541–2548. [PubMed] [Google Scholar]
- 23.Hunter SE, Waldburger KE, Thibodeaux DK, Schaub RG, Goldman SJ, Leonard JP. Immunoregulation by interleukin-12 in MB49. 1 tumor-bearing mice: cellular and cytokine-mediated effector mechanisms. Eur J Immunol. 1997;27:3438–3446. doi: 10.1002/eji.1830271244. [DOI] [PubMed] [Google Scholar]
- 24.Herr HW, Dotan Z, Donat SM, Bajorin DF. Defining optimal therapy for muscle invasive bladder cancer. J Urol. 2007;177:437–443. doi: 10.1016/j.juro.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Summerhayes I, Franks L. Effects of donor age on neoplastic transformation of adult mouse bladder epithelium in vitro. J Natl Cancer Inst. 1979;62:1017–1023. [PubMed] [Google Scholar]
- 26.Loskog A, Ninalga C, Hedlund T, Alimohammadi M, Malmstrom PU, Totterman TH. Optimization of the MB49 mouse bladder cancer model for adenoviral gene therapy. Lab Anim. 2005;39:384–393. doi: 10.1258/002367705774286475. [DOI] [PubMed] [Google Scholar]
- 27.Halak BK, Maguire HC, Lattime EC. Tumor-induced interleukin-10 inhibits type 1 immune responses directed at a tumor antigen as well as a non-tumor antigen present at the tumor site. Cancer Res. 1999;59:911–917. [PubMed] [Google Scholar]
- 28.Dekernion JB, Soloway MS, Persky L. Chemotherapy of experimental transitional-cell carcinoma. Urology. 1974;4:63–68. doi: 10.1016/0090-4295(74)90110-1. [DOI] [PubMed] [Google Scholar]
- 29.Chamie K, Saigal CS, Lai J, Hanley JM, Setodji CM, Konety BR, Litwin MS. Compliance with guidelines for patients with bladder cancer. Cancer. 2011;117:5392–5401. doi: 10.1002/cncr.26198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrag D, Hsieh LJ, Rabbani F, Bach PB, Herr H, Begg CB. Adherence to surveillance among patients with superficial bladder cancer. J Natl Cancer Inst. 2003;95:588–597. doi: 10.1093/jnci/95.8.588. [DOI] [PubMed] [Google Scholar]