SUMMARY
Chromatin immunoprecipitation (ChIP) serves as a central experimental technique in epigenetics research, yet there are serious drawbacks: it is a relative measurement, which untethered to any external scale obscures fair comparison amongst experiments; it employs antibody reagents that have differing affinities and specificities for target epitopes that vary in abundance; and it is frequently not reproducible. To address these problems, we developed Internal Standard Calibrated ChIP (ICeChIP), wherein a native chromatin sample is spiked with nucleosomes reconstituted from recombinant and semisynthetic histones on barcoded DNA prior to immunoprecipitation. ICeChIP measures local histone modification densities on a biologically meaningful scale, enabling unbiased trans-experimental comparisons and reveals unique insight into the nature of bivalent domains. This technology provides in situ assessment of the immunoprecipitation step, accommodating for many experimental pitfalls, as well as providing a critical examination of untested assumptions inherent to conventional ChIP.
INTRODUCTION
The mechanisms by which histone modifications act are sensitive to their localization and density (Lauberth et al., 2013; Vermeulen et al., 2007; Voigt et al., 2012; Yuan et al., 2012); therefore, simultaneous measurement of both of these values is paramount in deciphering how they function. Present technology provides either absolute global amounts by mass spectrometry or relative local levels from a chromatin immunoprecipitation (ChIP) experiment, but no direct measurement of histone modification density in a locus-specific manner.
ChIP uses affinity capture from a pool of fragmented chromatin “input” to enrich fragments that bear the epitope of interest. The captured material is then analyzed by qPCR or next generation sequencing and compared to negative controls to assess the relative enrichment afforded by the immunoprecipitation.
Although ChIP has made tremendous inroads into understanding the patterns and functions of histone posttranslational modifications and variants (Barski et al., 2007; Bernstein et al., 2006; Gifford et al., 2013; Guenther et al., 2007; Mikkelsen et al., 2007; Xie et al., 2013), it has a number of serious drawbacks. The greatest source of experimental error is the frequently poor affinity, specificity and reproducibility of the antibodies employed to capture desired epitopes (either histone modifications, variants or transcription factors)(Bock et al., 2011; Egelhofer et al., 2011; Fuchs et al., 2011). Indeed, up to 80% of several hundred commercial antibodies fail stringent quality controls (Egelhofer et al., 2011; Landt et al., 2012). Even different lots of the same commercial antibody can vary in affinity for target by up to 20-fold (Hattori et al., 2013) and display marked specificity differences (Nishikori et al., 2012). At present, there are no measures of antibody specificity within ChIP experiments available, leading to substantial uncertainty in data evaluation.
In addition, differences in epitope abundance (Leroy et al., 2013; Young et al., 2009), experimenter handling (Marinov et al., 2014), as well as differential amplification prior to next generation sequencer loading (Zhang and Pugh, 2011) render unbiased ChIP-based comparisons problematic. Because ChIP data are expressed on a relative scale that is dependent on the precise experimental conditions, normalization ultimately requires assumptions that may not be warranted (Bin Liu et al., 2013; Liang and Keles, 2012), or the bulk of experimental information must be sacrificed to permit comparisons (Zhang et al., 2008). There are few widely applied ChIP-seq quality controls, yet in the worst cases, ChIP is not reproducible (Egelhofer et al., 2011; Landt et al., 2012; Marinov et al., 2014).
To address these problems, we developed Internal Standard Calibrated ChIP (ICeChIP), a method that provides the first local measurement of histone modifications on a biologically meaningful scale. Here, we spike in semisynthetic nucleosomes as internal standards to calibrate native ChIP-seq experiments, revealing the average amount of a given histone modification present at any locus. The internal standard consists of reconstituted nucleosomes bearing a given mark (or a series of marks) on a library of DNAs composed of a constant strong nucleosome positioning sequence that is flanked by a variable “barcode” that encodes each member’s concentration (Figure 1). After immunoprecipitation with modification-directed antibodies followed by sequencing, the tag counts from the semisynthetic nucleosome DNA ladder(s) serve as an internal-standard calibration curve to measure the IP enrichment. From the IP enrichment of the internal standard, we can quantify amounts of histone modifications with the positional accuracy of native ChIP-seq in a genome-wide data set. Herein we demonstrate that ICeChIP measurements of histone modification density are precise, accurate, and reproducible, rendering ChIP robust to handling differences and preserving quantitative relationships between samples. Unlike other attempts to calibrate ChIP with chromatin from other organisms (Bonhoure et al., 2014; Orlando et al., 2014), our method measures actual amounts of marks using defined standards and can provide in situ evaluation of the specificity of the immunoprecipitation and a means of data correction. ICeChIP also enables direct and unbiased comparison amongst experiments: we present quantitative analyses of patterns of histone marks that reinterprets the nature of bivalent domains (Bernstein et al., 2006; Mikkelsen et al., 2007)), and suggest a correlation between the mark symmetry of promoter nucleosomes and transcriptional output.
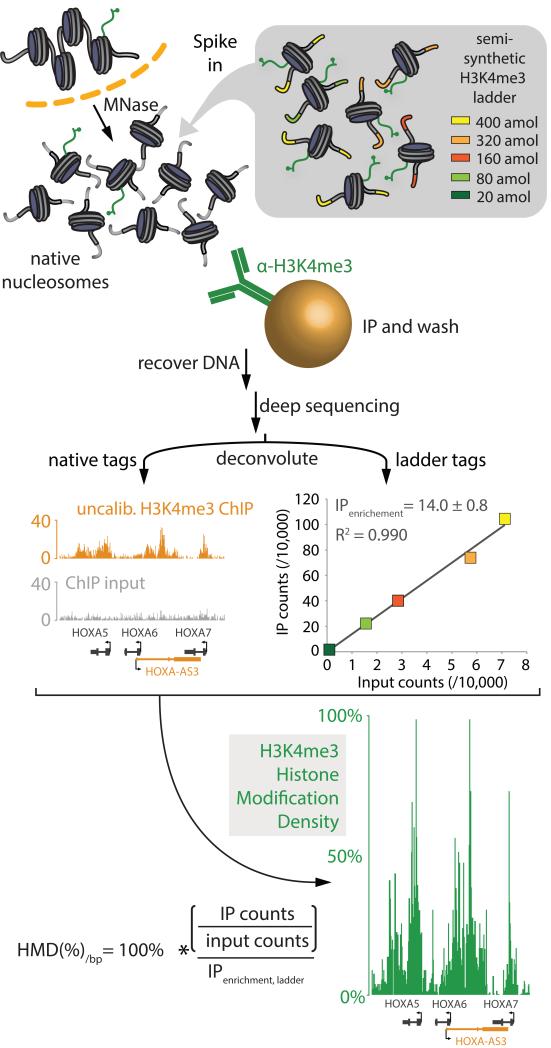
Figure 1. Scheme of internal standard calibrated ChIP (ICeChIP).
Diagram of the ICeChIP-seq experiment wherein the native pool of mononucleosomes released by in nucleo digestion with micrococcal nuclease is spiked with a semisynthetic nucleosome ladder bearing the histone modification H3K4me3, in defined concentrations (encoded by each unique DNA barcode) as an internal standard. Chromatin immunoprecipitation followed by Illumina sequencing (ChIP-seq) is then performed and resulting tag counts for the added semisynthetic nucleosomes constitute a calibration curve to derive histone modification density for the native nucleosomes genome-wide. ICeChIP can also be used to calibrate ChIP mated to quantitative PCR (qPCR) with locus-specific primers as in Figure S1.
RESULTS
Engineering nucleosome internal standards for ICeChIP
To normalize chromatin immunoprecipitation to a biologically meaningful scale, we adapted the analytical chemistry concept of calibration by defined internal standards. We spiked-in reconstituted nucleosomes bearing a posttranslational modification that precisely resembles its native mononucleosomal counterpart isolated by micrococcal nuclease fragmentation in conventional native ChIP (Brand et al., 2008) (Figure 1). In ICeChIP, such nucleosomal internal standards take the form of a “ladder” or concentration series of the same modified nucleosome, distinct only in short barcoded sequences that encode the relative concentration of each ladder member to construct a calibration curve (Figure 1, 2A).
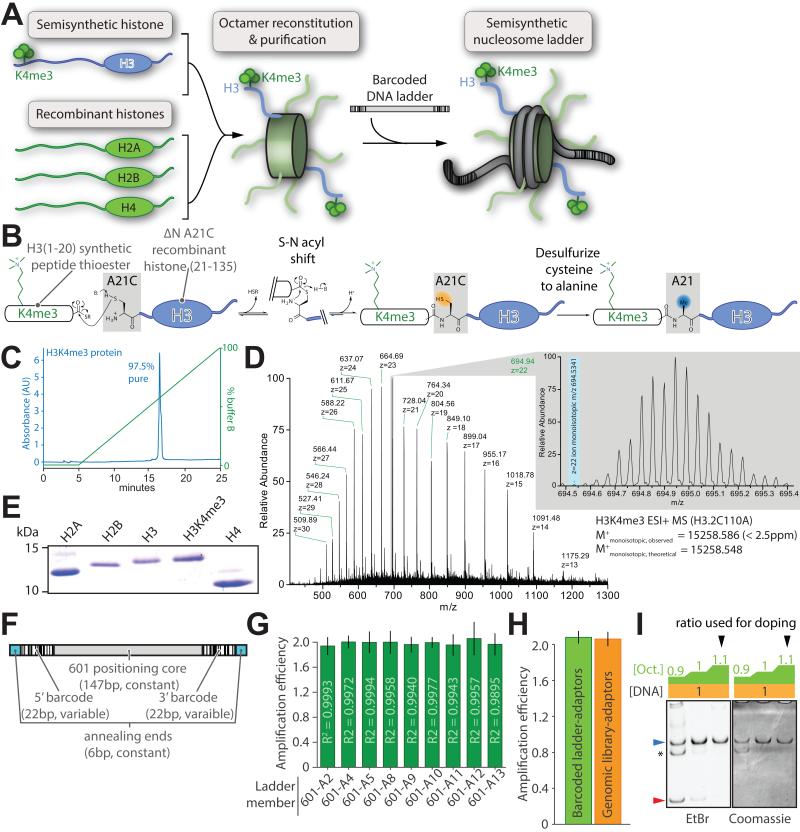
Figure 2. Design and preparation of barcoded semisynthetic nucleosomes.
(A) Schematic depiction of the reconstitution of a semisynthetic H3K4me3 nucleosome ladder: histone octamers, produced by refolding equimolar core histones from recombinant and semisynthetic sources, are purified then mixed with equal amounts of barcoded ladder DNA. (B) Semisynthesis by native chemical ligation from a K4me3-modified peptide thioester and the recombinantly produced remainder of the H3 protein bearing an N-terminal engineered cysteine. Subsequent desulfurization of this cysteine recapitulates the native alanine at this position. Characterization of the purified H3K4me3 semisynthetic histone via (C) analytical C18-HPLC and (D) electrospray mass spectrometry. (E) Representative Coomassie-stained SDS-PAGE gel of the recombinant human core histones and semisynthetic H3K4me3 used in reconstitutions. (F) Schematic representation of barcoded nucleosome positioning DNA sequences based on the 601 positioning nucleosome sequence. (G) Amplification per cycle of barcoded ladder DNA is measured with qPCR utilizing a 2× serial dilution series fit by linear regression (R2 of the fit displayed in each bar). (H) Amplification per cycle of all barcoded DNA ladder members versus native genomic DNA fragments after ligation of sequencing adaptors. (I) A representative reconstitution of internal standard nucleosome where octamer is titrated (wedge: blue, nucleosome; red, free DNA; asterisk: uncharacterized complex). All error bars are 95% CI. See also Figure S2.
As a starting point, we chose histone 3 lysine 4 trimethylation (H3K4me3), an important hallmark of transcriptional initiation (Guenther et al., 2007; Lauberth et al., 2013; Ruthenburg et al., 2007a; Santos-Rosa et al., 2002; Schübeler et al., 2004; Vermeulen et al., 2007) for which highly specific antibodies are available (Nishikori et al., 2012). To faithfully represent the native epitope (Chen et al., 2014; Munari et al., 2012; Seeliger et al., 2012) we constructed H3K4me3 by native chemical ligation followed by radical initiated desulfurization (Chiang et al., 2009; Muir, 2002; Shogren-Knaak et al., 2003)(Figure 2B). Although H3K4me3 has been previously semi-synthesized (Bartke et al., 2010; He et al., 2003; Ruthenburg et al., 2011), we employed a traceless H3K4me3 preparation here, mitigating concern about differences from its natural counterpart. Characterization of the purified H3K4me3 product by HPLC and mass spectrometry revealed high homogeneity (Figure 2C,D & S2A-D). We expressed and purified recombinant histones using conventional methods to complement this H3K4me3 species in histone octamer reconstitution (Figure 2A,E, S2E).
The second component of our nucleosomal internal standard is a set of barcoded DNA species readily distinguished from genomic sequences that will stably associate with histone octamer upon reconstitution. We initially constructed a nine member DNA library composed of a constant “601” nucleosome-positioning sequence (Lowary and Widom, 1998) and variable flanking barcodes sequences selected to be both unique and devoid of PCR amplification artifacts relative to random DNA (Figure 2F). Barcode sequences were designed to be substantially different from the human, mouse, fly and yeast genomes so that deconvolution of the internal standard ladder from genomic DNA sequences is robust to four or more base-calling errors in paired-end sequencing. Candidate barcodes were appended in pairs flanking the 601-core and further selected for clean single-band PCR product formation with high and equal amplification efficiency (Figure 2G, S2F-H; Table S1,S2). As all analytical readouts entail PCR, we examined whether our ladder DNA displays any amplification bias relative to genomic DNA and found no detectable differences (Figure 2H). We prepared the ICeChIP nucleosome ladder by gradient dialysis of histone octamer with a concentration series of different barcoded DNAs in a single tube (Luger et al., 1999; Ruthenburg et al., 2011) (Figure 2A,I). For accurate calibration, each member must reconstitute into nucleosomes cleanly and close to quantitatively (Figure 2I) over a broad range of individual ladder member DNA concentrations (Figure S2I).
ICeChIP-seq quantifies histone marks with locus precision
We performed ICeChIP-seq by doping a nucleosome internal standard bearing the H3K4me3 mark into digested genomic chromatin prior to immunoprecipitation. Here we present ICeChIP-seq data for E14 mouse embryonic stem cells and human embryonic kidney 293 cells (Figure 3, S3A-C). We found that subtle improvements to the Dilworth protocol for native ChIP (Brand et al., 2008) maximized recovery of chromatin (>80% by qPCR) affording at least 95% pure mononucleosomes (Figure S3D-G), and thereby minimized euchromatin bias (Figure S3H). This native nucleosome population was then spiked with the internal standard ladder and subjected to hydroxyapatite chromatography purification (Figure S3D) prior to immunoprecipitation. We quantified the number of nuclei prior to MNase digestion in order to stage our nucleosome ladder range around the genome copies present so that our ladder concentration is representative of a given native nucleosome. With miniscule quantities of ladder added (the whole set typically represents 0.0001-0.002% of total nucleosomes in the input, Table S3), we do not appreciably undercut our sequencing depth, nor perturb native nucleosome capture (Figure S3I). We subjected both the immunoprecipitated material and doped-input to Illumina sequencing; reads from the ladder and native nucleosomes were deconvoluted by alignment to the appropriate genome assembly concatenated with the internal standard DNA sequences.
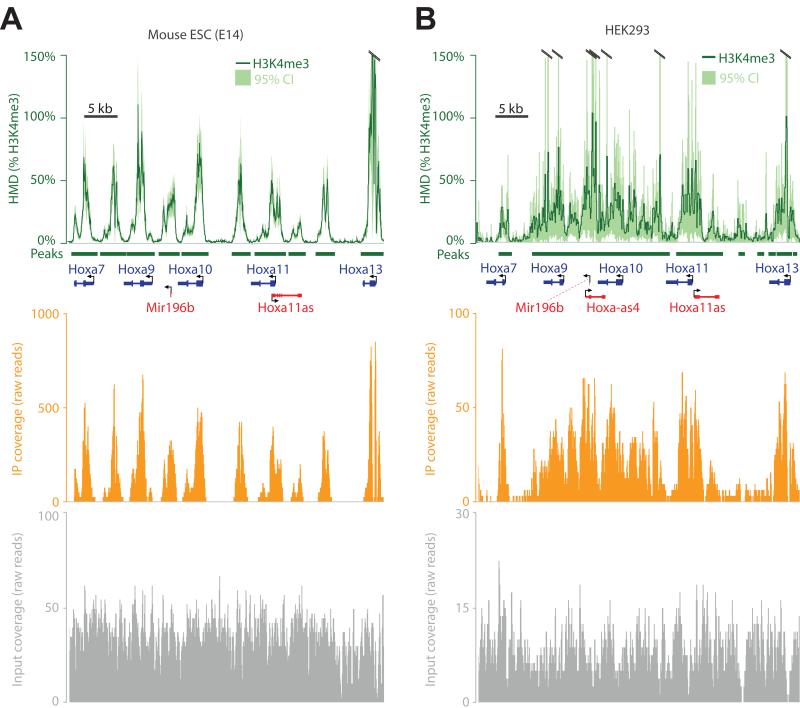
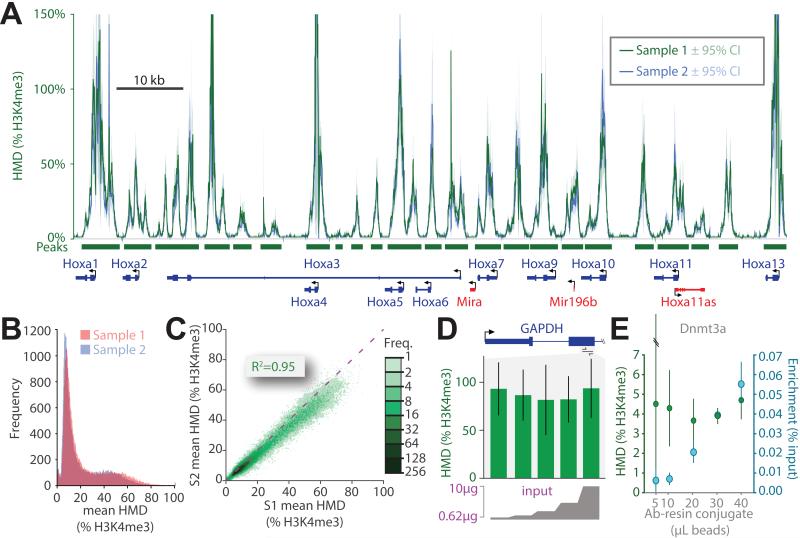
Figure 3. H3K4me3 modification density measured from mouse and human cells.
(A) ICeChIP coupled to Illumina paired-end sequencing reveals H3K4me3 modification density contoured over 25 bp intervals (HMD, dark green; 95% confidence interval, light green) at the Hoxa gene cluster in the E14 mESC line. Orange and grey tracks depict H3K4me3 ChIP and input raw read counts on the same chromosomal coordinate, respectively; MACS-called peaks (dark green bars). (B) The H3K4me3 ICeChIP-seq at the HOXA cluster from the HEK293 cell line read out by single-end Illumina HiSeq colored as in panel A. The same amount of input (10 μg) chromatin and antibody were used in both experiments. See also Figure S3.
As opposed to conventional ChIP, where the peak heights lack direct biological meaning, ICeChIP is able to calculate histone modification density (HMD%): the actual percentage of a mark’s epitope present on a given chromosomal interval, with the sub-nucleosomal resolution proper to native ChIP-seq. With a good antibody, HMD% typically spans 0-100% but is not restricted to be in this range (Figure 3). In ICeChIP-seq, the ratio of internal standard reads in the IP and input is a direct measure of IP enrichment, a value applied to the ratio of aligned native IP/input reads computed over a 25 base pair intervals, genome wide (Figure 1, S1).
As a representative region of H3K4me3 enrichment, we present part of the HOXA/Hoxa gene clusters in mouse and human cells (Figure 3). At this sequencing depth, significantly enriched peaks range in HMD between as little as 1% to over 100%. The error estimates tend to spike asymptotically near the dyad of high-occupancy nucleosomes as number of reads from these regions are low. Greater input sequencing depth and integrating HMD over longer intervals reduces the magnitude of the error, discernable by comparing Figure 3A versus 3B (error ∝ 1/√depth, Table S3). Importantly, these data are within a physically plausible range — apparent modification density rarely exceeds 100% within experimental error. In particular, of 60,530 called peaks in the mESC H3K4me3 dataset (MACS2, p < 10−20), 18,300 of these have an HMD/bp value that exceeds 100% at any point within the peak, yet only 1627 have an HMD/bp value where the lower bound of the 95% confidence interval is greater than 100%. Nonetheless, we undertook a more careful appraisal of this method’s validity in measuring histone modification density.
Validation of ICeChIP-seq: precision and accuracy
The behavior of internal standards in the course of performing the ICeChIP-seq measurements in Figure 3 affords a direct assessment of precision— susceptibility of the HMD measurement to random error on a given genome interval. Linear regression of the observed relative abundances of each ladder member in the IP versus the input for our ICeChIP directed against H3K4me3 in HEK293 cells revealed a marked correlation with a slope of 1.02 ± 0.02 and an R2 of 0.998 (Figure 4A, S4A). Additional independent experiments demonstrated similarly striking linearity, suggesting that each ladder member displays equivalent IP-enrichment independent of concentration and sampling error in the ranges examined (Figure S4A-C, S6B-F). To our knowledge, these experiments represent the first demonstration that there can be a linear relationship between the amount of epitope present and corresponding ChIP-signal intensity. This linearity exists through a useful working range as we have staged the concentration series of nucleosomal internal standards in approximately the same range of the number of nuclei in the experiment. Ladder linearity is a requirement for using scalar factor correction in ICeChIP. In practice, we routinely examine linearity prior to applying ICeChIP scaling; in over 50 experiments we have not encountered any deviation that would necessitate revisiting the ChIP conditions.
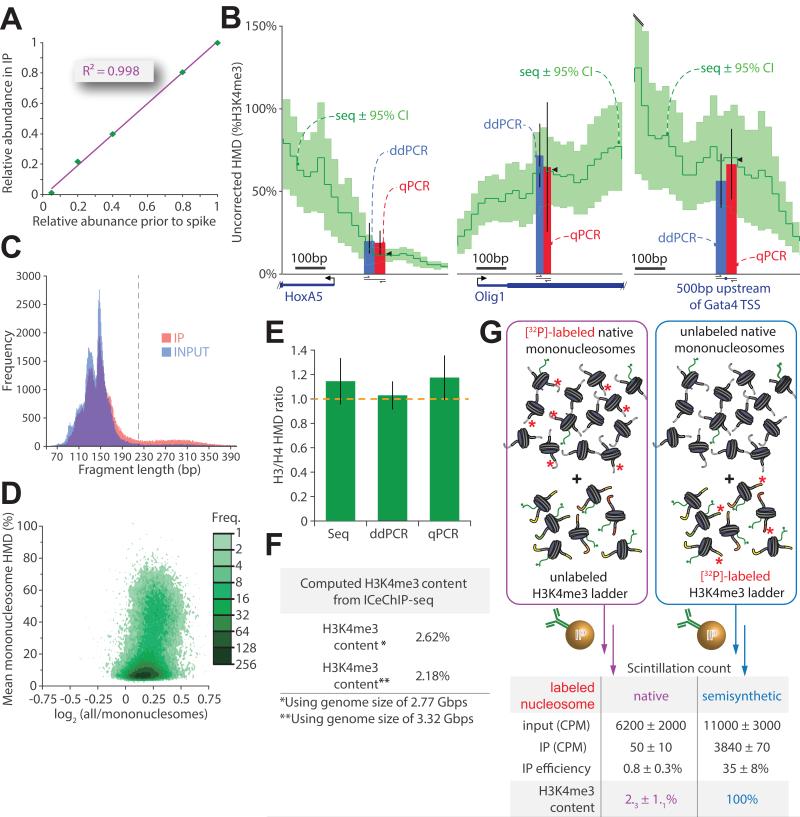
Figure 4. A critical examination of ICeChIP: precision and accuracy.
(A) The relative abundance for each barcode in the H3K4me3-directed IP is plotted against it’s abundance in the input (measured by densitometry in triplicate prior to nucleosome reconstitution and spiking). (B) ICeChIP-seq compared to ddPCR and qPCR in mESCs: uncorrected H3K4me3 Histone Modification Density (HMD, dark green; 95% confidence interval, light green) contoured over 25 bp intervals. Blue and red bars represent HMD (%H3K4me3) measured by ddPCR and qPCR, respectively, positioned over the indicated amplicon; black wedges are mean seq HMD over the amplicon. (C) Histogram of chromatin fragment length distributions for mESC input and IP for 105 randomly selected paired-end reads each dataset; upper bound chosen for mononucleosome filter indicated in grey dashed line. (D) Avidity bias measured by HMD averaged over peaks called with MACS (p<10−20) on mESC dataset filtered for mononucleosomes expressed as a function of the log2 of ratio of HMD signal averaged over peaks for all nucleosomes over HMD signal averaged over peaks for mononucleosomes only. (E) The accuracy of ICeChIP-seq assessed by comparison of H3 to H4 HMD measurement techniques by measuring the ratio of histone H3 to histone H4 densities at three discrete loci. (F) Global H3K4me3 abundance in HEK293 cells calculated by averaging the ICeChIP-derived HMD over the assembled and theoretical genome sizes. (G) Total H3K4me3 content for the HEK293 measured by the recovery of radioactive genomic DNA post-H3K4me3 IP, corrected with the IP efficiency measured in parallel experiments with radiolabeled semisynthetic nucleosomes in the presence of unlabeled native chromatin (both performed in triplicate). All error bars are 95%CI. See also Figure S4.
The accuracy of ICeChIP is more challenging to evaluate owing to lack of comparable analyses. Initially, we compared HMD computed from Illumina sequencing to other quantitative DNA counting methods. Digital droplet PCR (ddPCR) and quantitative PCR (qPCR) rely on amplicons defined by specific primer sets, so that ICeChIP-seq HMD average over the chromosomal interval of the amplicon should be comparable (Figure 4B). Yet these direct comparisons are complicated by a 5.7-fold enrichment of DNA fragments larger than mononucleosomes in our IP relative to input detected by paired-end sequencing, leading to ~16% inflation of apparent HMD (Figure 4C-D, S4E). We refer to this overrepresentation as an “oligonucleosome avidity bias”, which we believe stems from a higher valence of epitope per DNA fragment. We typically filter raw paired-end sequencing data to remove larger DNA fragments (Table S3, Figure S4G), but for the purposes of comparison to qPCR and ddPCR, which cannot distinguish between mononucleosome and oligonucleosome-derived signal without stringent size selection, we present the uncorrected and HMD signal (Figure 4B, S4F). With this analysis, the three methods of measurement were identical within experimental error at six arbitrarily chosen genomic loci in mESCs representing different apparent HMDs. Additionally, we performed ICeChIP with antibodies for histones H3 and H4 and found the expected ~2:2 ratio in nucleosomes to be indistinguishable for all three measurement modalities (Figure 4E). This congruence suggests that either the ICeChIP is accurate or harbors systematic error independent of the method of DNA quantification.
As an orthogonal measure of accuracy, we integrated the average H3K4me3 density for the whole genome from ICeChIP (Figure 4F) and compared this to global levels of H3K4me3 assessed by external-standardized ChIP with radiolabeled nucleosomes (Figure 4G). Values from these measurements are indistinguishable and generally concordant with H3K4me3 abundance measurements made by mass spectrometry (Binda et al., 2010; Leroy et al., 2013). Taken together, these data attest to the accuracy of ICeChIP.
Validation of ICeChIP-seq: reproducibility and robustness
To examine the consistency of ICeChIP upon replication we repeated the H3K4me3 ICeChIP-seq in mESCs and observed tight coupling of HMD tracks (Figure 5A). Correspondingly, mean HMD values for each biological replicate at called peaks are highly correlated (R2 = 0.95) and distributed within the estimated error (Figure 5B,C), with a slight deviation from a slope of unity suggesting a modest systematic skew. A frequency plot of HMD values at equivalent peak loci also indicates modest variation (Figure S5A).
Figure 5. ICeChIP is highly reproducible and more robust to experimental differences.
(A) The superposition of HMD derived from two biological replicates (green and blue) of the H3K4me3 ICeChIP-seq in mESCs displayed at the Hoxa gene cluster. HMD for both replicates averaged over 25bp intervals and displayed with 95%CI. (B) Frequency histogram of the mean mononucleosome HMD values averaged over called peaks (p<10−20) for biological replicates. (C) Scatter plot comparison of the two samples (S1 and S2) via plotting the mean mononucleosome HMD (%H3K4me3) for called peaks; the dashed purple line indicates a slope of 1. (D) HMD measurements (%H3K4me3) are independent of input loaded into immunoprecipitation over a large interval: doped HEK293 native chromatin input (0.625-10 μg, 2 fold concentration series) analyzed by ICeChIP-qPCR at within GAPDH with 50 μL of antibody-resin conjugate. (E) Measurement of IP-enrichment and HMD (% H3K4me3) at the DNMT3a locus by ICeChIP-qPCR in mESCs with fixed 10 μg of chromatin input as a function of antibody-resin conjugate amount. See also Figure S5.
Variation of IP enrichment as a consequence of different experimental handling conditions is a major complicating factor in the reproducibility of conventional ChIP (Marinov et al., 2014). By tethering the output of each experiment to a defined internal standard, HMD measured by ICeChIP should be more tolerant of experimental variation. We simulated experimental handling disparities by manipulating the ratio of input relative to resin-immobilized antibody. In a linear staging regime (Figure S5B), we examined H3K4me3 HMD via ICeChIP-qPCR and found that it is independent of the amount of input (Figure 5D), traceable to relatively uniform IP enrichment of H3K4me3 at the GAPDH locus (Figure S5C). However, for a fixed amount of input, altering the amount of resin-immobilized antibody used in the immunoprecipitation produced a range of IP efficiencies greater than 6-fold, yet H3K4me3 density computed from these experiments for the Dnmt3A and Hoxa9 loci was identical within experimental error (Figure 5E, S5D). Nevertheless, the experimenter should empirically establish the ideal operating regime for a given antibody relative to the amount of input used. Additionally, radical alteration of binding and wash conditions during ICeChIP-seq can afford very similar HMD measurements (Figure S5E,F). Finally, we titrated down the input quantity to examine the performance of ICeChIP near the limits of low-cell number protocols, and found it to perform stably down to the input equivalent to ~400 cells (Figure S6H). Collectively, these data indicate that while IP enrichment may vary as a function of the experimental conditions, HMD remains relatively stable and highly reproducible.
Multiple ladder ICeChIP measures IP specificity in situ
Apparent ChIP signal is an admixture of on-target capture, off-target capture of related epitopes (for example other marks of the same type) and non-specific adhesion of nucleosomes to the antibody resin; however, the relative proportions of ChIP signal attributable to each of these sources is unknown. ICeChIP performed with several different types of internal standards should be able to measure all three of these possible sources of apparent ChIP signal, thereby determining the true signal and error. Therefore, we queried mESC nucleosomes doped with three types internal standards, H3K4me3, H3K36me3, and unmodified nucleosomes reconstituted on distinguishable DNA species (two nucleosomes of each type) by ICeChIP-qPCR (Figure 6A). H3K36me3 was chosen as it bears a trimethyllysine embedded in a different sequence context and modest off-target affinity of this antibody for H3K36me3 has been previously observed in peptide arrays (Bock et al., 2011). By inspection of our internal standards, we barely detected enrichment of H3K36me3 (2.8 ± 0.4) beyond the unmodified nucleosome background (1.9 ± 0.2). Compared to the robust on target signal (81 ± 10), there is a 30-fold apparent specificity, suggesting that off-target binding of the antibody to H3K36me3-bearing nucleosomes is a negligible contributor to apparent H3K4me3 density under these conditions.
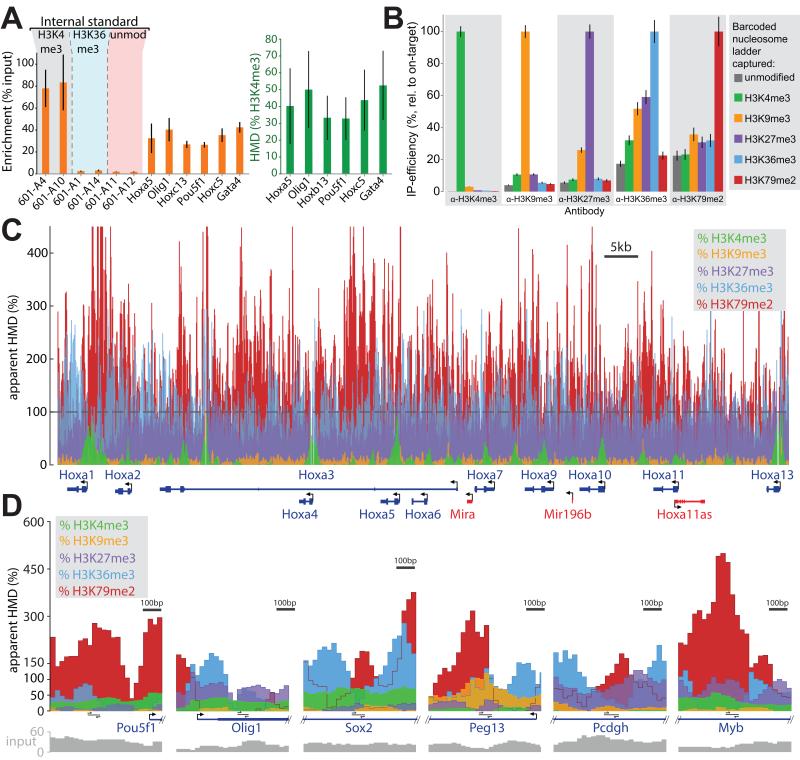
Figure 6. ICeChIP with multiple internal standards reveals the specificity of the IP in situ.
(A) Multiple internal standards (H3K4me3, H3K36me3, and unmodified nucleosomes) within an ICeChIP-qPCR experiment permit in situ assessment of antibody specificity. All error bars in this figure represent 95%CI. (B) A comparison of IP specificity: six different barcoded nucleosome ladders (unmodified, H3K4me3, H3K9me3, H3K27me3, H3K36me3, H3K79me2) are simultaneously doped into mESC input and subjected to capture with the indicated antibodies. Data are presented as relative IP-efficiency, normalized to the on-target ladder capture. (C) Apparent HMD for indicated marks at the Hoxa gene cluster. For H3K4me3, H3K9me3 and H3K27me3 ICeChIP, the background binding is sufficiently low to permit direct error correction and comparisons, whereas the poor specificity of the H3K36me3 and H3K79me2 antibodies combined with their target’s relative scarcity lead to more noise than signal manifested as massive inflation. (D) HMD presented at additional loci as in Figure 6C binned into 25 bp intervals. See also Figure S6.
To establish a more comprehensive set of internal standards, we constructed a number of modified histones encompassing many of the most well-studied di- and tri-methyllysines in histone H3 (Chen et al., 2014) and engineered a much larger set of barcoded DNA templates. Specifically, we engineered 100 second-generation templates and reconstituted them into H3K4me3-bearing nucleosomes in two parallel ICeChIP experiments, spiking them in either before or after MNase digestion of mESC nuclei (Figure S6A). The unique barcoded templates that passed this stringent test were then used to create six discrete ladders for unmodified, H3K4me3, H3K9me3, H3K27me3, H3K36me3 and H3K79me2 nucleosomes. The combined mixture of these ladders was doped into a single pool of mESC nuclei and subjected to micrococcal nuclease digestion, followed by hydroxyapatite purification, and then the pool of largely mononucleosomes was probed with the best antibodies available for each of these marks.
Sequencing of each ICeChIP afforded a direct in situ assessment of antibody specificity by comparison of on- versus off-target internal standard capture (Figure 6B). The H3K4me3 antibody proved highly specific when challenged with these other nucleosomal internal standards (H3K9me3 is equivalent to 3% of the on-target capture), whereas the H3K9me3 and H3K27me3 antibodies were slightly less specific, with mutual cross-recognition representing 10% and 26% of the on-target signal, respectively (as both marks reside within in an “ARKS” motif). Surprisingly, the most widely used antibodies for H3K36me3 and H3K79me2 were quite promiscuous. The modest apparent selectivity is especially problematic for these two marks, as they are far less abundant than most of the off-target marks that their antibodies also capture. Mass spectrometry measurements from the same cell line report H3K36me3 and H3K79me2 to account for 2.5% and 0.5% of all H3, whereas off-target marks H3K9me3 and H3K27me3 are an order of magnitude more abundant (Voigt et al., 2012). Thus, the modest fold-specificities are more than offset by the fold-abundance differences in the opposite direction.
The off-target capture appears to be linear with respect to the amount of nucleosomal epitope for every antibody examined (Figure S6B-F), suggesting that while antibody specificity may vary, the background for a given antibody is deterministic and proportional to the amount of the off-target species present in the input. Thus, application of the internal standard as a scalar is valid when the internal standard is linear and the background binding is modest and measurable. Specific HMD signal for a given mark can be corrected by solving a set of linear equations (see supplemental methods). However, this treatment only accounts for the marks we have represented as internal standards— can we detect background binding to entities beyond this set? Such sources of background are conflated into apparent HMD and lead to inflation of the measurement beyond what our off-target internal standards can account for. Despite higher apparent H3K36me3 and H3K79me2 modification densities at loci previously reported to be enriched in these marks (Figure 6D, panels 3 and 6), measurements with these antibodies represent more noise than signal in our experiments (Figure 6C,D S6G, S6I). Conversely, HMD values for H3K4me3, H3K9me3 and H3K27me3 display minimal inflation and are correctable for off-target binding, so that we may quantitatively compare the amounts of these three marks genome wide.
Comparative analysis of histone modification density patterns
With accurate measurements of actual histone amounts from H3K4me3, H3K9me3 and H3K27me3 ICeChIP-seq from diploid cells, we can make a statistical case for nucleosomal co-occupancy when the sum of HMDs for two marks exceeds 100%. This interpretation applies to two different marks as well as one versus two copies of a given mark within a nucleosome, termed “asymmetrically” and “symmetrically” modified nucleosomes, respectively (Voigt et al., 2012). Plotting heatmaps for H3K4me3 and H3K27me3 modification density reveals several broad classes of genes with different patterns of these two modifications (Figure 7A). Surprisingly, the levels of H3K27me3 are only modestly reduced at genes that are highly expressed, as exemplified by metabolic/housekeeping genes (Figure 7A,C S7A,C,D), whereas the highest HMDs for this mark are present at a subset of early development genes (Figure 7A,C,E, S7A,B,D). Other repressed late development genes from classes that are silent in mESCs, such as neurological and immune system processes (58 and 62% H3K27me3), are significantly less enriched in H3K27me3 (p < 10−56, 10−19, respectively) than repressed cell-differentiation genes (70% H3K27me3, Figure S7A). Developmental genes in Drosophila S2 cells also populate the highest H3K27me3 average HMD bin (Figure S7D).
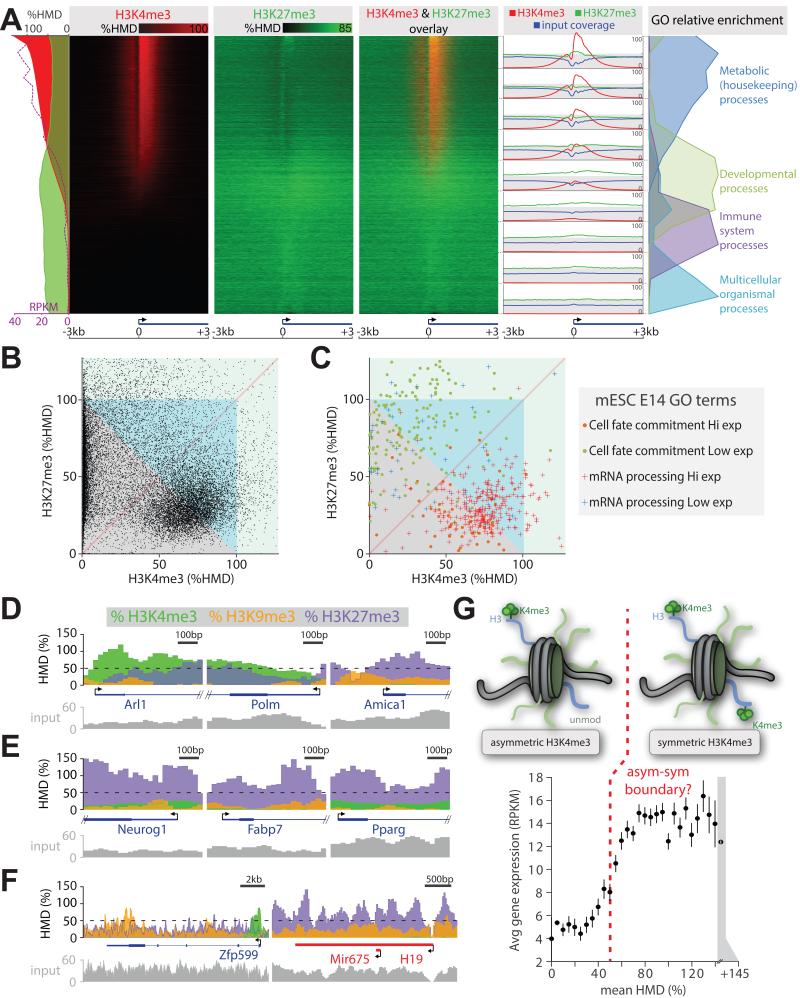
Figure 7. Comparison of histone modification amounts measured with locus precision.
(A) Heatmap presentation of H3K4me3 and H3K27me3 amounts measured by ICeChIP from mESCs at all coding TSSs, sorted by decreasing H3K4me3 modification density; average HMDs and expression level from (Xiao et al., 2012), or input coverage is plotted along the left edge; relative GO term enrichment or average HMD and input coverage for the 9 indicated blocks is depicted on the right. (B) Scatter plot of H3K4me3 versus H3K27me3 HMD for the first 400bp of all mESC genes. (C) HMDs as in panel B, displayed for cell fate determination or mRNA processing genes grouped into high expression (RPKM > 5) and low expression categories (RPKM ≤ 5). Zoomed views of selected loci associated with: (D) metabolic/housekeeping (Arl1, Polm), immunity (Amica1); (E) developmental genes; and (F) zinc finger (Zfp599) and imprinted (H19) genes. (G) Binned average gene expression (RPKM) ± SEM as the function of binned mean H3K4me3 HMD averaged over TSS ± 200bp. See also Figure S7.
The H3K4me3 mark promotes transcriptional initiation via several known mechanisms (Guenther et al., 2007; Lauberth et al., 2013; Ruthenburg et al., 2007a; Santos-Rosa et al., 2002; Schübeler et al., 2004; Vermeulen et al., 2007), and we observe this mark to approach 100% at the most actively transcribed genes (Figure 7A). A priori, HMD might be construed to be uninformative for examining correlations to gene expression, because the scaling should not matter for this analysis. Yet, when H3K4me3 is examined on a biologically meaningful scale, binned mRNA abundance reveals an intriguing sigmoidal dependence on average apparent HMD at the corresponding TSSs (Figure 7G, S7E-H). Assuming accurate measurement of H3K4me3 density, the inflection point of this curve (~50% HMD) lies approximately at the statistical boundary between, on average, asymmetrically- versus symmetrically-modified nucleosomes over both alleles. Could the lower HMD population simply represent a broader spatial distribution of the H3K4me3 beyond the TSS, suggested to reduce transcriptional variation (Benayoun et al., 2014)? Close examination reveals quite the opposite-- mean peak HMD values positively correlate with peak span in mice (Figure S7H); again this distribution is bimodal suggesting larger modification domains have higher average HMD values consistent with symmetric modification.
Our measurement of actual histone amounts by ICeChIP allows us to reinterpret bivalency as broken into two gross classes: where either H3K27me3 or H3K4me3 predominates. The canonical bivalent genes in mESCs (Bernstein et al., 2006; Mikkelsen et al., 2007) of early development, exemplified by cell fate commitment genes (Figure S7B), bear 70-100% H3K27me3 and 1-60% H3K4me3 density near the TSS of largely transcriptionally silent loci (Figure 7A,C,E). Many of these genes appear to cross the statistical threshold of 100% total content of these two marks (Figure 7A,C,E, S7A-C); however, quite a few do not due to lower H3K4me3 levels than one might expect based on uncalibrated ChIP (Bernstein et al., 2006; Gifford et al., 2013; Mikkelsen et al., 2007; Xie et al., 2013). The second class that appears to be formally bivalent has not been previously observed—a cohort of highly transcriptionally active genes with near maximal H3K4me3 approaching 100% at the TSS, and H3K27me3 hovering between 10-40% (Figure 7A,C S7C). Because H3K27me3 is ubiquitous and H3K4me3 is present at the TSSs of ~70-80% of genes where the densities are especially elevated as a function of transcriptional initiation (Guenther et al., 2007), this sort of bivalency is also to a large extent, pervasive (Figure 7A,C,D panel 1,2). We note that our mESC culture conditions with 2i likely underrepresent bivalency that occurs in serum culture, as it has been previously reported that 2i yields a more homogenous population in terms of morphology and pluripotency gene expression at the expense of apparent H3K27me3 levels at bivalent genes (Marks et al., 2012). Under our conditions, most active genes straddle the statistical boundary between demonstrably and possibly bivalent in a cluster centered around ~30% H3K27me3 and ~80% H3K4me3 (Figure 7B).
H3K9me3 decorates the 3′ exons of certain actively transcribed zinc-finger transcription factors (Blahnik et al., 2011)—we now find that this mark can approach 100% HMD at these sites (Figure 7F). Several imprinted loci where one allele is silenced via DNA methylation, which promotes H3K9me3 installation, bear this modification just below 50% H3K9me3 consistent with saturating demarcation of one allele (Figure 7F).
DISCUSSION
We have devised ICeChIP, a method that uses a series of semisynthetic nucleosomes as internal standards to calibrate ChIP so that: 1.) data is expressed on an precise, accurate, reproducible and biologically meaningful scale of histone modification density, rather than an arbitrary and experimental condition-biased relative scale; 2.) this calibrated scale, when applied from experiment-to-experiment enables legitimate and unbiased quantitative comparisons between experiments; and 3.) IP specificity within a given ChIP experiment is measurable, and serves as an in situ metric of whether the experiment actually worked.
Internal standard-normalization of ChIP
ICeChIP is a relative internal standard calibration of ChIP, wherein the proportion of each “spiked-in” ladder member in the IP versus input is used to compute the IP efficiency of the standard, then applied to the native ChIP data. Using a relative calculation of histone modification density largely circumvents biases due to incomplete genome extraction or nucleosome stability during input preparation (Brand et al., 2008; Henikoff et al., 2009), or variable adapter ligation efficiency and PCR amplification artifacts (Park, 2009). For example, a nucleosomal DNA fragment that is amplified poorly in PCR due to extreme AT- or GC-rich composition (Aird et al., 2011), should display similar deficits in both the immunoprecipitated and input libraries effectively canceling each other. The artifacts we observe for barcode 601-A1 illustrate this point: although the relative abundance of this member falls below the expected amount following PCR amplification in library preparation or in qPCR measurement (Figure S4D), the proportion of reads in the IP versus input lies directly on the calibration curves for multiple independent experiments (Figure S4A-C).
Any internal standard accommodating for sporadic or systematic handling losses in the procedure preserves quantitative relationships between samples, thereby enabling better trans-experiment comparisons. Recently, Pol III ChIP-seq from mouse cells under different experimental conditions was normalized by adding internal standard chromatin isolated from human cells (Bonhoure et al., 2014). Similarly, H3K79me2 ChIP from human cell lines treated with a specific Dot1L inhibitor was recently calibrated with Drosophila chromatin (Orlando et al., 2014). Although these approaches have value in making comparative measurements, the internal controls are not well defined. Such methods are suitable for comparisons within a given lab using the same batch of dopant, but are likely not portable to measurements made by others as differences in chromatin “spike-in” material preparation are likely to alter the apparent readout. By virtue of measurement on a biologically meaningful and theoretically constant scale with reagents that are homogeneous, defined and storable, ICeChIP has no such limitations. More importantly, only ICeChIP with combinations of different defined internal standards permits the experimenter to assess whether their IP was specific for the desired epitope by direct comparison to potential off-target standards as well as assessment of apparent HMD inflation.
Without internal standards, most normalization choices could be considered forms of experimenter bias (Orlando et al., 2014). To compare effects of two different experimental conditions at certain loci by ChIP-seq, one must assume that the changes are local rather than global, typically by normalizing the largest peaks elsewhere in two ChIP-seq datasets to be equivalent (Bardet et al., 2012). Yet this treatment is biased by assumptions— which peaks are held to be invariant or that the global amount of epitope is largely unchanged— whose validity is uncertain to the experimenter. If the effects of an experimental perturbation are global (i.e., there are substantially fewer epitopes attached to chromatin per genome as a consequence of a perturbation), then it is difficult to detect, as the condition with reduced bound epitope will likely be inflated by extra amplification in library preparation so that equivalent amounts of DNA may be loaded into the sequencer (Zhang and Pugh, 2011). Accommodating for these problems so that quantitative comparisons can be made is another notable benefit of our method or any other use of internal standards (Bonhoure et al., 2014; Orlando et al., 2014).
In situ measurement of specificity and signal correction as quality controls
In conventional ChIP analysis, the specificities of a given antibody are assumed to be perfect; however, in other assays many of these very same “ChIP grade” antibodies display substantial off-target binding (Bock et al., 2011; Egelhofer et al., 2011; Fuchs et al., 2011; Hattori et al., 2013; Nishikori et al., 2012). To comprehensively address ChIP specificity within a given experiment, we embarked on multi-ladder ICeChIP experiments comprising a host of related H3 tri- and di-methyllysine nucleosomal internal standards. Not only can we assess the specificity of ChIP in situ, but we can correct for known off-target binding when it is modest (Figure 6). The application of a correction matrix can accommodate not merely for off target effects of a given antibody, but uses all other IPs performed from the same input and their attendant specificities to correct the signal.
ChIP-seq as currently practiced, particularly for histone modifications, is lacking in meaningful quality controls. Peak calling is often necessary to establish statistically significant enrichment over input signal (Rozowsky et al., 2009; Zhang et al., 2008). Yet compression of the data rich dimension into a peak-versus-no-peak binary discards the majority of experimental information and does not take IP specificity into account. A recent meta-analysis of nearly 1000 transcription factor ChIP experiments, using peak clustering and comparisons to negative controls as the central metrics, revealed 45% of published transcription factor ChIP to be of poor or intermediate quality (Marinov et al., 2014). Similar analyses for histone modifications would be problematic for most histone modifications and variants, due to broader distributions of peaks. Other quality control approaches measure enrichment, but similarly have no way of assessing what is actually being enriched (Diaz et al., 2012). We envision that ICeChIP with multiple standards could serve as a critical needed quality control for ChIP data. To facilitate widespread adoption, we are willing to distribute our internal standard ladder reagents until they become commercially available.
Limitations and extensions
As ICeChIP measures the modification percentage of total histone present over a given genomic interval, it is relatively insensitive to nucleosome density. However nucleosome free regions have very high HMD uncertainty, as far fewer reads are present in the input and corresponding IP. Far deeper sequencing in combination with a general-H3 ICeChIP could more accurately resolve the modification patterns at these sparse-nucleosome regions. More broadly, the stability of nucleosomes, particularly those composed of histone variants (Jin and Felsenfeld, 2007), may be quite variable, leading to underrepresentation in ChIP input, and potentially differential integrity during the IP as compared to the standard. The native input preparation that we employ also sacrifices positional accuracy in favor of higher signal to noise in the measurement. These issues could be addressed by calibration of cross-linked ChIP via defined nucleosomal internal standards, though such experiments are potentially complicated by differential epitope masking of the native sample relative to the ladder.
Additional modifications proximal to the target modification have been demonstrated to impair antibody binding in some cases (Bock et al., 2011; Fuchs et al., 2011), presenting a problem for measuring this mark by any type of ChIP. We envision future implementations of ICeChIP with biologically relevant combinatorial patterns of modifications represented as internal standards, coupled with the development of combination-selective affinity reagents (Fischle et al., 2005) will provide a resolution to this problem.
Bivalent domains recast
In stem and progenitor cells, the chromatin states demarcating genes that are poised to be rapidly activated or fully repressed early along a potential differentiation axis are thought to bear both transcriptionally activating and repressive histone marks, H3K4me3 and H3K27me3, respectively. Chromatin with this paradoxical spatial apposition of chromatin marks has been termed a “bivalent” (Bernstein et al., 2006). However, the precise molecular nature of bivalency has proven to be elusive. There is no evidence that these marks co-exist on the same histone tail from mass spectrometry (Voigt et al., 2012; Young et al., 2009). Perhaps the best evidence for nucleosomal co-occurrence of these two marks derives from direct MS of an H3K4me3-immunoprecipitation from a pool of least 90% mononucleosomes: ~15% of this captured material also had H3K27me3 (Voigt et al., 2012). However, it is not clear from this experiment where such nucleosomes might reside in the genome.
The bulk of evidence for the existence of bivalency at certain developmental loci is derived from overlapping ChIP-chip or -seq from regions of chromatin that have large peaks for both marks (Bernstein et al., 2006; Gifford et al., 2013; Mikkelsen et al., 2007; Xie et al., 2013). Yet comparison of two ChIP signals on arbitrary scales that are the product of differential antibody affinity and mark abundance cannot unequivocally demonstrate coexistence of these marks at the same allele. More direct examination by sequential ChIP with antibodies for each of the two marks has been used at several loci (Bernstein et al., 2006; Seenundun et al., 2010; Voigt et al., 2012). Although these experiments may suggest that these two marks may reside within a single nucleosome, they do not comment on the frequency of this co-occurrence, nor rigorously exclude fragments of chromatin that are larger than one nucleosome (we note a surprisingly high oligonucleosomal avidity bias in ChIP, Figure 4C,D). The remaining ambiguity presented by the divide between the IP-MS measurements of bivalency abundance without location and the ChIP measurements of potential location without clear evidence of bivalency motivated our attempts to quantify each mark’s abundance at relevant loci.
With ICeChIP we can now make a statistical argument for the existence of bivalency’s bona fide existence at ~35% of all genes in mESCs. Our measurements of HMDs for each of these two marks at promoters suggest that bivalency bifurcates into two classes with reciprocal mark ascendancy. Many developmental loci previously implicated as bivalent appear to be so in our analysis, although the amount of H3K27me3 decorating them is far higher (approaching 100%) than any other class of silent genes, and there are only modest amounts of H3K4me3 present. Surprisingly, highly active genes bear ~30% repressive H3K27me3 mark, and sufficient H3K4me3 (approaching 100%) to qualify as bivalent (Figure 7A). That such genes were not previously suspected of bivalency is likely due to the way two ChIP-seq tracks were normalized (Gifford et al., 2013; Mikkelsen et al., 2007; Xie et al., 2013). In essence, normalization of sequencing reads by depth for two very different abundance marks with very different distributions effectively suppresses apparent signal for H3K27me3 (abundant and everywhere), while inflating H3K4me3 (~10-fold less abundant, relatively defined peaks). We suspect that differential antibody capture efficiency, unequal amplification and sequencer loading were also key contributors to this skewing (Zhang and Pugh, 2011). Our reinterpretation of bivalency hinges on HMD measurements that reflect the actual amounts of H3K27me3 and H3K4me3 and bring the integrated amounts closer in line with the MS measured global mark abundances.
We note that bivalent co-existence that occurs on a single allele or smaller sub-population of cells, though both may exist, would not meet the statistical HMD threshold used here. For example, one allele could be fully bivalent with one of each mark per nucleosome, and the other allele could have only one H3K27me3 mark per nucleosome with no H3K4me3, and the apparent HMD from the conflation of the homologous pair would be 25% H3K4me3 and 50% H3K27me3—shy of the statistical boundary. Thus, our data likely underestimate the actual amount of bivalency. Clearly, more direct quantitative measures of bivalent mark co-existence by sequential ICeChIP with a host of internal standards including bivalent nucleosomes is required to conclusively resolve the molecular nature of bivalency and to quantify it accurately across the genome.
Nucleosomal symmetry of marks and variants
Global measurements by IP-mass spectrometry of nucleosomes suggest that the distribution of individual marks in each histone is non-random (Voigt et al., 2012). Here we measure mark symmetry as a function of genomic location. The sensitive part of the curve described by plotting binned TSS-proximal H3K4me3 HMD versus mean RNA expression for the corresponding set of genes encompasses the apparent transition from on average asymmetric to symmetric H3K4me3-modified nucleosomes (Figure 7G). In diploid cells, the predominance of bi-allelic gene expression is crucial to this interpretation of symmetry-- only 5-10% of autosomal genes are subject to mono-allelic expression control (Gimelbrant et al., 2007). Our data implies that there may be distinct mechanisms and roles for regulating this mark’s symmetry to fine-tune transcriptional initiation. In the simplest mechanism, the symmetric H3K4me3 modification decorating highly transcribed gene TSSs could prevent encroachment of PRC2-mediated repression. In support of this model, PRC2 has been demonstrated to be inactive in vitro on nucleosomes bearing symmetric marks linked to transcriptional activation, such as H3K4me3 and H3K36me3 (Schmitges et al., 2011), but active on asymmetric H3K4me3-bearing nucleosomes (Voigt et al., 2012). Another intriguing possibility is that the symmetry increases the valence to potentiate the residence time of binding partners such as Taf3 (Vermeulen et al., 2007) to promote transcription (Lauberth et al., 2013), or perhaps even recruits distinct binding partner complexes that specifically recognize both tails simultaneously (Canzio et al., 2011; Ruthenburg et al., 2011; 2007b). Indeed, the hint that H3K4me3 symmetry may be of functional importance warrants more direct study with ICeChIP using discrete internal standards for both symmetry states, as asymmetric nucleosomes might display different enrichment in the IP. More broadly, the rotational symmetry of the nucleosome core particle structure itself tempts speculation that there may be important biologic principles remaining to be discovered in the assortment and spatial disposition of histone marks and variants within individual nucleosomes. We envision ICeChIP will be an important tool for delving into this problem of sub-nucleosomal chromatin variation.
EXPERIMENTAL PROCEDURES
The details of recombinant histone production, histone semisynthesis, barcode DNA preparation, nucleosome reconstitution, ICeChIP, radioactive ChIP, multiple internal standard experiments and variation of ChIP conditions are available in the Extended Experimental Procedures. In brief, recombinant histones were refolded into octamers with semisynthetic histones prepared via expressed protein ligation; purified octamer was mixed with equimolar barcoded DNA ladder; these nucleosomes were then doped into chromatin (either prior to, or after MNase digests), and native ChIP was performed according to established protocols (Brand et al., 2008; Ruthenburg et al., 2011).
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Peter Faber and Erika Hanson of the University of Chicago Functional Genomics Core for Illumina sequencing and the Rod Davis of the UIC proteomics core for assistance with mass spectrometry. We are grateful to Lucia Rothman-Denes for helpful comments on the manuscript. This work was supported by awards to A.J.R from the NIH (R21HG007426), the Chicago Biotechnology Consortium with support from The Searle Funds at The Chicago Community Trust and the Ellison Foundation. A.T.G. is supported by a University of Chicago Dean’s International Student Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ACCESSION NUMBERS
The ICeChIP reported in this paper have been deposited to the Gene Expression Omnibus (GEO) with the accession number GSE60378.
REFERENCES
- Aird D, Ross MG, Chen W-S, Danielsson M, Fennell T, Russ C, Jaffe DB, Nusbaum C, Gnirke A. Analyzing and minimizing PCR amplification bias in Illumina sequencing libraries. Genome Biol. 2011;12:R18. doi: 10.1186/gb-2011-12-2-r18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardet AF, He Q, Zeitlinger J, Stark A. A computational pipeline for comparative ChIP-seq analyses. Nature Protocols. 2012;7:45–61. doi: 10.1038/nprot.2011.420. [DOI] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh T-Y, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Bartke T, Vermeulen M, Xhemalce B, Robson SC, Mann M, Kouzarides T. Nucleosome-interacting proteins regulated by DNA and histone methylation. Cell. 2010;143:470–484. doi: 10.1016/j.cell.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benayoun BA, Pollina EA, Ucar D, Mahmoudi S, Karra K, Wong ED, Devarajan K, Daugherty AC, Kundaje AB, Mancini E, et al. doi: 10.1016/j.cell.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Bin Liu, Yi J, SV A, Lan X, Ma Y, Huang TH, Leone G, Jin VX. QChIPat: a quantitative method to identify distinct binding patterns for two biological ChIP-seq samples in different experimental conditions. BMC Genomics. 2013;14:S3. doi: 10.1186/1471-2164-14-S8-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda O, Leroy G, Bua DJ, Garcia BA, Gozani O, Richard S. Trimethylation of histone H3 lysine 4 impairs methylation of histone H3 lysine 9: regulation of lysine methyltransferases by physical interaction with their substrates. Epigenetics. 2010;5:767–775. doi: 10.4161/epi.5.8.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blahnik KR, Dou L, Echipare L, Iyengar S, O’Geen H, Sanchez E, Zhao Y, Marra MA, Hirst M, Costello JF, et al. Characterization of the contradictory chromatin signatures at the 3′ exons of zinc finger genes. PLoS ONE. 2011;6:e17121. doi: 10.1371/journal.pone.0017121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock I, Dhayalan A, Kudithipudi S, Brandt O, Rathert P, Jeltsch A. Detailed specificity analysis of antibodies binding to modified histone tails with peptide arrays. Epigenetics. 2011;6:256–263. doi: 10.4161/epi.6.2.13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhoure N, Bounova G, Bernasconi D, Praz V, Lammers F, Canella D, Willis IM, Herr W, Hernandez N, Delorenzi M, et al. Quantifying ChIP-seq data: a spiking method providing an internal reference for sample-to-sample normalization. Genome Res. 2014 doi: 10.1101/gr.168260.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Rampalli S, Chaturvedi C-P, Dilworth FJ. Analysis of epigenetic modifications of chromatin at specific gene loci by native chromatin immunoprecipitation of nucleosomes isolated using hydroxyapatite chromatography. Nature Protocols. 2008;3:398–409. doi: 10.1038/nprot.2008.8. [DOI] [PubMed] [Google Scholar]
- Canzio D, Chang EY, Shankar S, Kuchenbecker KM, Simon MD, Madhani HD, Narlikar GJ, Al-Sady B. Chromodomain-mediated oligomerization of HP1 suggests a nucleosome-bridging mechanism for heterochromatin assembly. Mol Cell. 2011;41:67–81. doi: 10.1016/j.molcel.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Grzybowski AT, Ruthenburg AJ. Traceless semisynthesis of a set of histone 3 species bearing specific lysine methylation marks. Chembiochem. 2014;15:2071–2075. doi: 10.1002/cbic.201402313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang KP, Jensen MS, McGinty RK, Muir TW. A semisynthetic strategy to generate phosphorylated and acetylated histone H2B. Chembiochem. 2009;10:2182–2187. doi: 10.1002/cbic.200900238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A, Nellore A, Song JS. CHANCE: comprehensive software for quality control and validation of ChIP-seq data. Genome Biol. 2012;13:R98. doi: 10.1186/gb-2012-13-10-r98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelhofer TA, Minoda A, Klugman S, Lee K, Kolasinska-Zwierz P, Alekseyenko AA, Cheung M-S, Day DS, Gadel S, Gorchakov AA, et al. An assessment of histone-modification antibody quality. Nat Struct Mol Biol. 2011;18:91–93. doi: 10.1038/nsmb.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischle W, Tseng BS, Dormann HL, Ueberheide BM, Garcia BA, Shabanowitz J, Hunt DF, Funabiki H, Allis CD. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature. 2005;438:1116–1122. doi: 10.1038/nature04219. [DOI] [PubMed] [Google Scholar]
- Fuchs SM, Krajewski K, Baker RW, Miller VL, Strahl BD. Influence of combinatorial histone modifications on antibody and effector protein recognition. Curr Biol. 2011;21:53–58. doi: 10.1016/j.cub.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford CA, Ziller MJ, Gu H, Trapnell C, Donaghey J, Tsankov A, Shalek AK, Kelley DR, Shishkin AA, Issner R, et al. Transcriptional and epigenetic dynamics during specification of human embryonic stem cells. Cell. 2013;153:1149–1163. doi: 10.1016/j.cell.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimelbrant A, Hutchinson JN, Thompson BR, Chess A. Widespread monoallelic expression on human autosomes. Science. 2007;318:1136–1140. doi: 10.1126/science.1148910. [DOI] [PubMed] [Google Scholar]
- Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;130:77–88. doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Taft JM, Swist KM, Luo H, Witt H, Slattery M, Koide A, Ruthenburg AJ, Krajewski K, Strahl BD, et al. Recombinant antibodies to histone post-translational modifications. Nat Methods. 2013;10:992–995. doi: 10.1038/nmeth.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Bauman D, Davis JS, Loyola A, Nishioka K, Gronlund JL, Reinberg D, Meng F, Kelleher N, McCafferty DG. Facile synthesis of site-specifically acetylated and methylated histone proteins: reagents for evaluation of the histone code hypothesis. Proc Natl Acad Sci USA. 2003;100:12033–12038. doi: 10.1073/pnas.2035256100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S, Henikoff JG, Sakai A, Loeb GB, Ahmad K. Genome-wide profiling of salt fractions maps physical properties of chromatin. Genome Res. 2009;19:460–469. doi: 10.1101/gr.087619.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Felsenfeld G. Nucleosome stability mediated by histone variants H3.3 and H2A.Z. Genes & Development. 2007;21:1519–1529. doi: 10.1101/gad.1547707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landt SG, Marinov GK, Kundaje A, Kheradpour P, Pauli F, Batzoglou S, Bernstein BE, Bickel P, Brown JB, Cayting P, et al. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res. 2012;22:1813–1831. doi: 10.1101/gr.136184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauberth SM, Nakayama T, Wu X, Ferris AL, Tang Z, Hughes SH, Roeder RG. H3K4me3 Interactions with TAF3 Regulate Preinitiation Complex Assembly and Selective Gene Activation. Cell. 2013;152:1021–1036. doi: 10.1016/j.cell.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy G, Dimaggio PA, Chan EY, Zee BM, Blanco MA, Bryant B, Flaniken IZ, Liu S, Kang Y, Trojer P, et al. A quantitative atlas of histone modification signatures from human cancer cells. Epigenetics Chromatin. 2013;6:20. doi: 10.1186/1756-8935-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K, Keles S. Normalization of ChIP-seq data with control. BMC Bioinformatics. 2012;13:199. doi: 10.1186/1471-2105-13-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowary PT, Widom J. New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J Mol Biol. 1998;276:19–42. doi: 10.1006/jmbi.1997.1494. [DOI] [PubMed] [Google Scholar]
- Luger K, Rechsteiner TJ, Richmond TJ. Preparation of nucleosome core particle from recombinant histones. Meth Enzymol. 1999;304:3–19. doi: 10.1016/s0076-6879(99)04003-3. [DOI] [PubMed] [Google Scholar]
- Marinov GK, Kundaje A, Park PJ, Wold BJ. Large-scale quality analysis of published ChIP-seq data. G3 (Bethesda) 2014;4:209–223. doi: 10.1534/g3.113.008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks H, Kalkan T, Menafra R, Denissov S, Jones K, Hofemeister H, Nichols J, Kranz A, Stewart AF, Smith A, et al. The Transcriptional and Epigenomic Foundations of Ground State Pluripotency. Cell. 2012;149:590–604. doi: 10.1016/j.cell.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim T-K, Koche RP, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir TW. Semisynthesis of proteins by expressed protein ligation. Annu Rev Biochem. 2002;72:249–289. doi: 10.1146/annurev.biochem.72.121801.161900. [DOI] [PubMed] [Google Scholar]
- Munari F, Soeroes S, Zenn HM, Schomburg A, Kost N, Schröder S, Klingberg R, Rezaei-Ghaleh N, Stützer A, Gelato KA, et al. Methylation of lysine 9 in histone H3 directs alternative modes of highly dynamic interaction of heterochromatin protein hHP1β with the nucleosome. J Biol Chem. 2012;287:33756–33765. doi: 10.1074/jbc.M112.390849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikori S, Hattori T, Fuchs SM, Yasui N, Wojcik J, Koide A, Strahl BD, Koide S. Broad ranges of affinity and specificity of anti-histone antibodies revealed by a quantitative peptide immunoprecipitation assay. J Mol Biol. 2012;424:391–399. doi: 10.1016/j.jmb.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando DA, Chen MW, Brown VE, Solanki S, Choi YJ, Olson ER, Fritz CC, Bradner JE, Guenther MG. Quantitative ChIP-Seq Normalization Reveals Global Modulation of the Epigenome. Cell Rep. 2014;9:1163–1170. doi: 10.1016/j.celrep.2014.10.018. [DOI] [PubMed] [Google Scholar]
- Park PJ. ChIP-seq: advantages and challenges of a maturing technology. Nat Rev Genet. 2009;10:669–680. doi: 10.1038/nrg2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozowsky J, Euskirchen G, Auerbach RK, Zhang ZD, Gibson T, Bjornson R, Carriero N, Snyder M, Gerstein MB. PeakSeq enables systematic scoring of ChIP-seq experiments relative to controls. Nat Biotechnol. 2009;27:66–75. doi: 10.1038/nbt.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthenburg AJ, Allis CD, Wysocka J. Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol Cell. 2007a;25:15–30. doi: 10.1016/j.molcel.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Ruthenburg AJ, Li H, Milne TA, Dewell S, McGinty RK, Yuen M, Ueberheide B, Dou Y, Muir TW, Patel DJ, et al. Recognition of a Mononucleosomal Histone Modification Pattern by BPTF via Multivalent Interactions. Cell. 2011;145:692–706. doi: 10.1016/j.cell.2011.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthenburg AJ, Li H, Patel DJ, Allis CD. Multivalent engagement of chromatin modifications by linked binding modules. Nat Rev Mol Cell Biol. 2007b;8:983–994. doi: 10.1038/nrm2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NCT, Schreiber SL, Mellor J, Kouzarides T. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419:407–411. doi: 10.1038/nature01080. [DOI] [PubMed] [Google Scholar]
- Schmitges FW, Prusty AB, Faty M, Stützer A, Lingaraju GM, Aiwazian J, Sack R, Hess D, Li L, Zhou S, et al. Histone methylation by PRC2 is inhibited by active chromatin marks. Mol Cell. 2011;42:330–341. doi: 10.1016/j.molcel.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Schübeler D, MacAlpine DM, Scalzo D, Wirbelauer C, Kooperberg C, van Leeuwen F, Gottschling DE, O’Neill LP, Turner BM, Delrow J, et al. The histone modification pattern of active genes revealed through genome-wide chromatin analysis of a higher eukaryote. Genes & Development. 2004;18:1263–1271. doi: 10.1101/gad.1198204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeliger D, Soeroes S, Klingberg R, Schwarzer D, Grubmüller H, Fischle W. Quantitative assessment of protein interaction with methyl-lysine analogues by hybrid computational and experimental approaches. ACS Chem. Biol. 2012;7:150–154. doi: 10.1021/cb200363r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seenundun S, Rampalli S, Liu Q-C, Aziz A, Palii C, Hong S, Blais A, Brand M, Ge K, Dilworth FJ. UTX mediates demethylation of H3K27me3 at muscle-specific genes during myogenesis. Embo J. 2010;29:1401–1411. doi: 10.1038/emboj.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shogren-Knaak MA, Fry CJ, Peterson CL. A native peptide ligation strategy for deciphering nucleosomal histone modifications. J Biol Chem. 2003;278:15744–15748. doi: 10.1074/jbc.M301445200. [DOI] [PubMed] [Google Scholar]
- Vermeulen M, Mulder KW, Denissov S, Pijnappel WWMP, van Schaik FMA, Varier RA, Baltissen MPA, tunnenberg HG, Mann M, Timmers HTM. Selective anchoring of TFIID to nucleosomes by trimethylation of histone H3 lysine 4. Cell. 2007;131:58–69. doi: 10.1016/j.cell.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Voigt P, Leroy G, Drury WJ, III, Zee BM, Son J, Beck DB, Young NL, Garcia BA, Reinberg D. Asymmetrically modified nucleosomes. Cell. 2012;151:181–193. doi: 10.1016/j.cell.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Xie D, Cao X, Yu P, Xing X, Chen C-C, Musselman M, Xie M, West FD, Lewin HA, et al. Comparative epigenomic annotation of regulatory DNA. Cell. 2012;149:1381–1392. doi: 10.1016/j.cell.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Schultz MD, Lister R, Hou Z, Rajagopal N, Ray P, Whitaker JW, Tian S, Hawkins RD, Leung D, et al. Epigenomic analysis of multilineage differentiation of human embryonic stem cells. Cell. 2013;153:1134–1148. doi: 10.1016/j.cell.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young NL, Dimaggio PA, Plazas-Mayorca MD, Baliban RC, Floudas CA, Garcia BA. High throughput characterization of combinatorial histone codes. Mol Cell Proteomics. 2009;8:2266–2284. doi: 10.1074/mcp.M900238-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Wu T, Fu H, Dai C, Wu H, Liu N, Li X, Xu M, Zhang Z, Niu T, et al. Dense chromatin activates Polycomb repressive complex 2 to regulate H3 lysine 27 methylation. Science. 2012;337:971–975. doi: 10.1126/science.1225237. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nussbaum C, Myers RM, Brown M, Li W, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Pugh BF. High-resolution genome-wide mapping of the primary structure of chromatin. Cell. 2011;144:175–186. doi: 10.1016/j.cell.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.