Abstract
Background
Timely and accurate diagnosis of influenza remains a challenge, but is critical for patients who may benefit from antiviral therapy. This study determined the test characteristics of provider diagnosis of influenza, final ED electronic medical record (EMR) diagnosis of influenza, and influenza like illness (ILI) in patients recommended to receive antiviral treatment according to Centers for Disease Control and Prevention (CDC) guidelines. Additionally, we evaluated the compliance with CDC antiviral guidelines.
Methods
A prospective cohort of adults presenting to a tertiary care ED with an acute respiratory illness who met CDC criteria for recommended antiviral treatment were enrolled and tested for influenza. A clinical diagnosis of influenza was assessed by asking the clinician: “Do you think this patient has influenza?” ILI was defined according to current CDC criteria.
Results
In this cohort of 270 subjects, 42 (16%; 95% CI 11-20%) had influenza. Clinician diagnosis had a sensitivity of 36% (95% CI 22-52%) and specificity of 78% (95%CI 72-83%); EMR final ED diagnosis had a sensitivity of 26% (95% CI 14-42%) and specificity of 97% (95% CI 94-99%); ILI had a sensitivity of 31% (95% CI 18-47%) and specificity of 88% (95% CI 83-92%). Only 15 (36%) influenza positive patients received antiviral treatment.
Conclusion
Clinician diagnosis, final ED EMR diagnosis and ILI have low sensitivity for diagnosing influenza, and there is overall poor compliance with CDC antiviral treatment recommendations. Improved methods of influenza diagnosis are needed to help guide management in the clinical setting.
Keywords: Influenza, Diagnosis, Emergency Department
INTRODUCTION
Each year influenza causes significant morbidity and mortality including over 200,000 hospitalizations and 3,000-49,000 deaths in the United States alone [1,2]. Fortunately, timely antiviral treatment can decrease morbidity and mortality in patients with, or at increased risk of influenza-related complications, and is recommended in these populations by the Centers for Disease Control and Prevention (CDC), World Health Organization (WHO), and Infectious Disease Society of America (IDSA) [3-5]. Specifically, the CDC recommends antiviral treatment for patients with a severe or complicated course, those requiring hospitalization, and those at high risk of complications, including patients at the extremes of age (<5 years old, >65 years old), residing in a chronic care facility, or with specific chronic medical conditions, immunosuppression, pregnancy, morbid obesity, or Native American heritage [3]. Antiviral therapy is most effective when given close to the time of symptom onset, therefore rapid diagnosis and treatment of individuals with influenza and existing or increased risk of complications is essential [6-9]. This is especially critical in the emergency department (ED) where increasing numbers of patients with influenza and other respiratory infections first seek medical care. Moreover, given the limited number of effective antiviral options, and concerns of increasing antiviral resistance, antiviral treatment must also be targeted to those with influenza, who will benefit most from treatment. Hence, accurate and timely diagnosis of influenza virus infections is key to providing targeted antiviral treatment.
Diagnosing influenza remains a challenge, especially in the ED where short, episodic visits, leave emergency clinicians to make diagnostic and treatment decisions with limited, insufficient information. The current gold standard influenza test, real-time polymerase chain reaction (rt-PCR) typically requires several hours for results, and the more rapid antigen based influenza tests have poor to moderate sensitivity reducing their capability to rule out influenza. Highly sensitive, rapid, random-access PCR-based tests are increasingly available but have not yet been widely adopted, particularly in the ED setting [10].
Given the lack of access to rapid, highly accurate tests, ED clinicians often diagnose influenza based on clinical signs and symptoms. Although many studies have attempted to validate the use of clinical signs and symptoms to diagnose influenza, findings indicate overall poor sensitivity and specificity. The CDC created the case definition of influenza-like illness (ILI) as fever equal to or greater than 37.8 Celsius with either cough or sore throat; however, sensitivity of ILI for influenza is only 55-69% [11,12]. Further, two meta-analyses confirmed that there is no combination of signs and symptoms which have adequate sensitivity to make informed clinical decisions regarding influenza treatment [13,14]. Clinician judgment, combining clinical symptoms, physical exam and laboratory findings, does not out-perform basic clinical symptoms. A previous ED-based study demonstrated that clinician judgment had a poor sensitivity of only 29% (95% CI 18-43%) [15]. However, this study was done in an otherwise healthy population and excluded subjects with pneumonia, immunosuppression, and many other factors that would place them at high risk of influenza-related complications. Hence, in the current ED practice environment, accurate diagnosis of influenza remains a challenge.
Timely diagnosis of influenza in the ED is critical to initiate time sensitive antiviral treatment in patients with or at increased risk of influenza-related complications. However, poor access to sensitive and rapid diagnostic tests, and non-specific clinical symptoms make influenza diagnosis a challenge for ED providers. Previous estimates of the diagnostic accuracy of clinician judgment and ILI are based on healthy individuals, and do not consider the high risk population in whom diagnosis and subsequent treatment is most critical. This study evaluates the sensitivity and specificity of physician diagnosis in high risk individuals who would be recommended to receive antiviral treatment according to CDC guidelines. Additionally, this study evaluates the sensitivity and specificity of a documented electronic medical record (EMR) final ED diagnosis of influenza, and the CDC’s definition of ILI. Lastly, we evaluate current compliance with the CDC’s antiviral treatment recommendations.
METHODS
Study design
This was a prospective observational cohort study to determine the sensitivity and specificity of clinician diagnosis compared to PCR testing for influenza in adult ED patients with an acute respiratory illness who met CDC criteria for recommended influenza antiviral treatment. The study was conducted at an urban, university-affiliated tertiary care ED with an ED volume of over 60,000 annual patient visits. The ED site for this study is staffed by approximately 50 attending physicians, 10 midlevel providers, 48 emergency medicine residents, and various residents from other specialties. The ED site has no internal departmental policy regarding antiviral use. This study was approved by the XXX Institutional Review Board.
Study Population
All adult patients presenting to the ED between December, 2012 and March, 2013 during study enrollment hours, with a documented chief complaint of fever or any respiratory-related symptom, were screened by trained, dedicated study coordinators. Study enrollment hours were Monday through Friday from 9am to 5pm during the month of December, and from 9am to 11pm during January, February, and March. Study coordinators determined eligibility of potential subjects by evaluating the following inclusion criteria: 1) 18 years of age or older; 2) patient verbal report of symptoms of an acute respiratory tract infection defined as new symptoms of cough, sinus pain, nasal congestion, rhinorrhea, sore throat, shortness of breath, or fever which developed over the previous 2 weeks; and 3) one or more of the following CDC indications for influenza treatment: hospital or observation admission, potential influenza related complications (i.e. pneumonia), age 65 years old or older, chronic pulmonary disease, cardiovascular disease (except hypertension alone), renal disease, hepatic disease, hematologic disease, metabolic disorders, neurologic and neurodevelopment conditions, immunosuppression (including that caused by medications or HIV infection), pregnant or less than two weeks postpartum, American Indians or Alaska natives, morbid obesity (body mass index ≥40), or resident of a chronic-care facility. Patients were excluded if they had prior diagnosis of influenza within the previous 2 weeks, did not speak English, were unable to provide informed consent, or were unable to provide follow-up contact information.
Study Protocol
Consenting subjects were asked to complete a written structured questionnaire regarding basic demographics, current symptoms, and past medical history including influenza vaccination for the current season. A nasopharyngeal swab was collected from each patient, placed in viral transport media (MicroTest M4RT, Remel, Lenexa, KS), aliquoted, frozen to minus 70 degrees, and stored for subsequent influenza testing with a PCR assay (Prodesse ProFlu +, Hologic Gen-probe Incorporated, San Diego, CA). ED providers were blinded from the influenza PCR test results.
In order to obtain the clinician diagnosis, the ED provider for each subject was asked to respond “Yes” or “No” to the following question: “Do you think this patient has influenza?”. Study coordinators were instructed to pose this question to providers as close to the time of subject disposition from the ED as possible but prior to the result of provider-requested rapid influenza testing (antigen detection by fluorescence microscopy).
Following the ED visit, data were extracted from the hospital’s electronic medical record (EMR), which included both ED and inpatient documentation. Data were entered into a standardized, closed entry, Microsoft Excel (Microsoft Corporation, Redmond, Washington) database and included initial ED vital signs, ED laboratory and culture data, ED radiologic findings, ED management, disposition from the ED, and EMR final ED diagnoses.
Measurements
Presence or absence of influenza-like illness (ILI) was based upon the CDC criteria for ILI of a fever equal to or greater than 37.8 Celsius with either cough or sore throat [11]. ILI criteria were collected using the initial temperature measured at ED triage, and symptoms (e.g. cough or sore throat) reported by the patient upon direct questioning during the enrollment questionnaire conducted during the ED visit. The EMR final ED diagnosis of influenza was based on the final ED diagnoses recorded in the EMR at the time of ED disposition. Antibiotic and antiviral administration was recorded as either none, administered in the ED, or discharged with a prescription. A subject was considered to have received ED antiviral or antibiotic treatment if they were administered an antiviral or antibiotic in the ED, or discharged from the ED with an antiviral or antibiotic prescription.
Data Analysis
A power calculation was performed to ensure a 95% confidence interval of the sensitivity of provider decision making of +/−15%. Assuming an overall influenza prevalence of 20%, a provider sensitivity of 40%, and a 10% sample error rate, 303 total subjects with 275 subjects undergoing full analysis, would be needed to provide a sufficiently narrow confidence interval for the estimate of sensitivity of provider diagnosis. Data were analyzed using basic descriptive statistics including proportions and percentages for dichotomous variables, median and interquartile range for continuous data, sensitivity, specificity, and likelihood ratios. Chi squared was used for comparison of proportions. Data were analyzed using Stata Statistical Software: Release 11 (Stata Corp LP, 2009. College Station, TX).
RESULTS
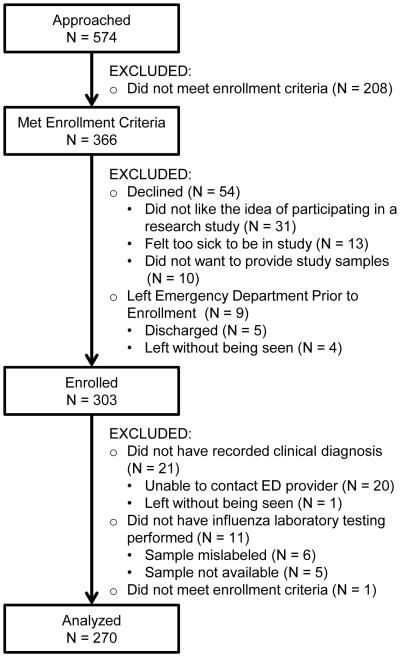
Of the 366 subjects who met enrollment criteria, 303 (83%) were enrolled in the study and 270 (89%) of those enrolled were included in the final analysis. Figure 1 details the enrollment process. Each subject included in the analysis had a clinical diagnosis for influenza which was provided by the attending physician for 114 (42%) subjects, resident physician for 145 (54%) subjects, or midlevel provider for 11 (4%) subjects.
Figure 1.
Diagram Of Enrolled For Subjects Presenting To An Inner-city Emergency Department During The 2012-2013 Influenza Season With An Acute Respiratory Illness And Criteria To Indicate Antiviral Treatment According To Centers For Disease Control And Prevention Guidelines
Table 1 displays the basic demographics of the study subjects, as well as indications for potential antiviral treatment according to the CDC recommendations. The most common CDC indications for antiviral treatment were chronic pulmonary disease (64%), hospital admission (43%), chronic metabolic disease such as diabetes mellitus (30%), and immunosuppression (26%). One third of the subjects (88) presented with symptoms for less than 48 hours.
Table 1.
Characteristics Of Enrolled Subjects Presenting To An Inner-city Emergency Department During Influenza Season 2012-2013 With An Acute Respiratory Illness And Criteria To Indicate Antiviral Treatment According To Centers For Disease Control And Prevention Guidelines
| All n (column %) |
Influenza Positive n (column %) |
Influenza negative n (column %) |
|
|---|---|---|---|
| Number of Subjects | 270 | 42 | 228 |
|
| |||
| Age (Years) * | 50 (38-58) | 43.5 (32-55) | 50.5 (39.5-58) |
| Male Gender | 110 (41%) | 15 (36%) | 95 (42%) |
| Race | |||
| African American | 220 (81%) | 34 (81%) | 186 (82%) |
| White | 41 (15%) | 5 (12%) | 36 (16%) |
| Other | 5 (1.9%) | 1 (2.4%) | 4 (1.8%) |
| CDC Guidelines (indication for antiviral treatment) | |||
| Hospital admission | 117 (43%) | 16 (38%) | 101 (44%) |
| Complications/Pneumonia | 32 (12%) | 4 (9.5%) | 28 (12%) |
| Age 65 or greater | 37 (14%) | 5 (12%) | 32 (14%) |
| Chronic Disease | |||
| Pulmonary | 172 (64%) | 17 (40%) | 155 (68%) |
| Cardiovascular | 62 (23%) | 5 (12%) | 57 (25%) |
| Renal | 31 (11%) | 6 (14%) | 25 (11%) |
| Hematologic | 22 (8.1%) | 4 (9.5%) | 18 (7.9%) |
| Metabolic | 82 (30%) | 11 (26%) | 71 (31%) |
| Neurologic | 26 (9.6%) | 7 (17%) | 19 (8.3%) |
| Immunosuppression | 71 (26%) | 13 (31%) | 58 (25%) |
| Pregnancy | 1 (0.4%) | 1 (2.4%) | 0 (0%) |
| Morbid Obesity | 23 (8.5%) | 3 (7.1%) | 20 (8.8%) |
| Resides in Nursing Home | 6 (2.2%) | 0 (0%) | 6 (2.6%) |
| Native American | 0 (0%) | 0 (0%) | 0 (0%) |
| Influenza Vaccination | 146 (54%) | 15 (36%) | 131 (57%) |
| Symptoms less than 48 hours | 88 (33%) | 13 (31%) | 75 (33%) |
CDC – Centers for Disease Control and Prevention
Age is listed as median and interquartile range
Of the 270 subjects analyzed, 42 (16%, 95% CI 11%-20%) had influenza according to PCR testing. Of subjects with confirmed influenza, 27 (64%) had Influenza A and 15 (36%) had Influenza B. Table 2 displays the number of influenza positive and negative subjects who were diagnosed with influenza according to a clinical diagnosis, an EMR recorded final ED diagnosis, and the classic clinical symptoms of ILI. Clinical diagnosis had a sensitivity of 36% (95%CI 22-52%) and a specificity of 78% (95% CI 72-83%). For patients who presented within 48 hours of symptom onset, the sensitivity of clinician diagnosis was 39% (95%CI 14-69%). Only 18 patients had an EMR recorded diagnosis of influenza, which had a sensitivity of 26% (95% CI 14-42%) and specificity of 97% (95% CI 94-99%). Of the 42 patients who were influenza positive, the EMR recorded final ED diagnoses were: influenza (11), viral syndrome (4), congestive heart failure (4), pneumonia (3), bronchitis (3), upper respiratory tract infection (3), fever (3), asthma exacerbation (3), chronic obstructive pulmonary disease exacerbation (2), cough (1), chest pain (1), pain (1), renal insufficiency (1), thrombocytopenia (1), and no diagnosis recorded (1). ILI had an overall sensitivity of 31% (95%CI 18-47%), and a specificity of 88% (95%CI 83-92%). Table 3 further outlines the sensitivity, specificity and likelihood ratios for these diagnostic methods.
Table 2.
Emergency Department (ED) Diagnosis And Treatment Of Influenza In A High Risk Population
| All n (column %) |
Influenza Positive n (column %) |
Influenza Negative n (column %) |
|
|---|---|---|---|
|
| |||
| Number of Subjects | 270 | 42 | 228 |
| Diagnosis | |||
| Clinician diagnosis of influenza | 65 (24%) | 15 (36%) | 50 (22%) |
| Final ED diagnosis of influenza | 18 (6.6%) | 11 (26%) | 7 (3.1%) |
| Influenza-like Illness | 40 (15%) | 13 (31%) | 27 (12%) |
| ED treatment | |||
| Antiviral given in ED | 31 (11%) | 11 (26%) | 20 (8.8%) |
| Antiviral prescription only | 9 (3.3%) | 4 (9.5%) | 5 (2.2%) |
| Any ED antiviral treatment | 40 (15%) | 15 (36%) | 25 (11%) |
| Any ED antibiotic treatment | 123 (46%) | 22 (52%) | 101 (44%) |
Table 3.
Sensitivity, Specificity And Likelihood Ratios Of Emergency Department Clinician Diagnosis And Influenza Like Illness (ILI)
|
Overall
n=270 |
Symptom onset
< 48 hours n=88 |
Symptom
onset > 48 hours n=182 |
|
|---|---|---|---|
|
| |||
| Influenza Prevalence | 16% (11-20%) | 15% (8-24%) | 16% (11-22%) |
| Clinician Diagnosis | |||
| Sensitivity | 36% (22-52%) | 39% (14-69%) | 35% (18-54%) |
| Specificity | 78% (72-83%) | 83% (72-90%) | 76% (68-82%) |
| Positive Likelihood Ratio | 1.63 (1.01-2.62) | 2.22 (0.95-5.17) | 1.43 (0.80-2.53) |
| Negative Likelihood Ratio | 0.82 (0.65-1.04) | 0.74 (0.48-1.12) | 0.86 (0.65-1.14) |
| EMR Final ED diagnosis | |||
| Sensitivity | 26% (14-42%) | 31% (9.1-61%) | 24% (10-44%) |
| Specificity | 97% (94-99%) | 79% (91-100%) | 97% (93-99%) |
| Positive Likelihood Ratio | 8.53 (3.51-20.7) | 11.5 (2.35-56.7) | 7.39 (2.52-21.7) |
| Negative Likelihood Ratio | 0.76 (0.64-0.91) | 0.71 (0.49-1.02) | 0.78 (0.64-0.97) |
| Influenza-like Illness | |||
| Sensitivity | 31% (18-47%) | 46% (19-75%) | 24% (10-43%) |
| Specificity | 88% (83-92%) | 88% (78-94%) | 88% (82-93%) |
| Positive Likelihood Ratio | 2.61 (1.47-4.64) | 3.85 (1.65-8.99) | 2.05 (0.94-4.46) |
| Negative Likelihood Ratio | 0.78 (0.64-0.96) | 0.61 (0.37-1.02) | 0.86 (0.70-1.06) |
In this population of patients recommended to receive antiviral treatment according to CDC guidelines, only 15 (36%) subjects with PCR-confirmed influenza received antiviral treatment from the ED, with 11 (26%) starting the medication while still in the ED. Interestingly, 22 (52%) of subjects with PCR-confirmed influenza received antibiotic treatment from the ED, 16 of whom were admitted to the hospital and 4 of whom had an infiltrate on chest X-ray and a corresponding diagnosis of pneumonia. Influenza positive patients admitted to the hospital were no more likely to receive an antiviral than those who were discharged (44% versus 31%, p = 0.394). However, influenza positive patients admitted to the hospital were more likely to receive an antibiotic than those who were discharged (88% versus 31%, p < 0.001). Among the 13 patients who had influenza and symptoms for less than 48 hours, only 6 (46%) received ED antiviral treatment, 3 (23%) of whom initiated treatment while still in the ED.
DISCUSSION
This study evaluated the sensitivity and specificity of ED clinician diagnosis of influenza in adult ED patients recommended to receive antiviral treatment according to CDC guidelines. Overall, clinical diagnosis of influenza by ED clinicians had poor sensitivity and specificity. The low sensitivity (36%; 95% CI 22-52%) is similar to that reported previously by Stein et al (29%) in an otherwise healthy population [15]. Unlike Stein and colleagues; however, the sensitivity of clinical diagnosis did not improve in the subset of patients who presented with less than 48 hours of symptoms. The specificity for clinician diagnosis in this study (78%; 95% CI 72-83%) was lower than that found in previous reports (92%; 95% CI 87-95%). The reduced specificity is likely related to the relatively higher level of medical complexity of our study population. The population in this study included those with current pneumonia or a history of pulmonary disease, which may have led to an increase in false positive influenza diagnoses.
Similar to the clinical diagnosis reported by the clinician when directly asked, the final ED diagnosis of influenza, as recorded in the EMR, had poor sensitivity 26% (95% CI 14-42%), but had high specificity (97%; 95% CI 94-99%). While likely that provider’s clinical diagnosis may differ somewhat from that recorded in the EMR, this finding suggest that using an EMR diagnosis of influenza in research or clinical efforts does not accurately reflect a patients true influenza status. Additionally, alternate diagnoses recorded in the EMR (e.g. congestive heart failure, asthma exacerbation, chronic obstructive, pulmonary disease) in influenza positive patients suggest that providers may overlook influenza as an important secondary diagnosis, especially in patients with other pulmonary conditions.
Clinicians may rely on CDC’s definition of ILI when considering the diagnosis of influenza in clinical practice. Although previous studies have demonstrated that the CDCs definition of ILI has a sensitivity of 55-69% in a broad population, our evaluation found a substantially lower sensitivity (31%; 95% CI 18-47%) [12]. The decreased sensitivity of ILI is likely related to the patient population which included several patient groups that may not be able to mount an appropriate immune response or fever (e.g. immunosuppressed, elderly). This is consistent with previous findings that the sensitivity of symptoms such as cough and fever for diagnosing influenza are decreased in elderly patients (30%) compared to the larger population (64%) [13,16,17]. Thus, in those recommended to receive antiviral treatment in whom diagnosis is most essential, the classic symptoms of ILI are less reliable. This finding has important implications for clinical diagnosis and outcomes.
This study was conducted during the 2012-2013 influenza season which was a predominantly H3N2 influenza season of moderate severity and duration, for which XXX had a similar course to the remainder of the US. The 2012-2013 season began approximately 4 weeks earlier, and lasted slightly longer than the typical US influenza season. Deviations in the influenza seasons timings may have impacted the provider’s clinical diagnosis of influenza. However, influenza season often varies in time of onset, severity, and strain, and providers have access to multiple forms of influenza surveillance to keep them up-to-date. Other than the early onset, the 2012-2013 season was a typical influenza season such that the prevalence of influenza or general severity of illness should not have impacted providers decision making compared to previous seasons. It remains unclear how integration of influenza surveillance, and an understanding of the timing, changing prevalence, and severity of an individual influenza season impacts provider’s clinical diagnosis, especially during atypical seasons.
Previous work has demonstrated poor compliance with CDC recommendations regarding antiviral treatment. Hsieh et al. found that only 50% of ED patients with a final ED diagnosis of influenza, who met CDC criteria for recommended antiviral treatment, actually received it [18]. As this evaluation demonstrates, the final EMR diagnosis of influenza is a poor proxy for actual influenza. Thus, the actual compliance with CDC recommendations is likely to be even lower than previous estimates, because those estimates do not take into account patients who had influenza, but were not diagnosed due to the poor sensitivity of clinicians’ diagnosis. This is confirmed by the current study, which demonstrates that only 36% of subjects with laboratory confirmed influenza, from a population of those recommended to receive antiviral treatment according to CDC guidelines, received antiviral treatment. In fact, a patient with influenza was more likely to receive antibiotic (52%) than antiviral (36%) treatment. Using hospital admission as a proxy of severity of illness, it appear that more severely ill patients (those admitted to the hospital) with influenza are more likely to receive antibiotics, but not more likely to receive antivirals than those who are less ill (i.e. not admitted to the hospital). This emphasizes provider’s impulse to begin empiric antibiotics in ill patients, but not to empirically initiate antivirals. There is substantial debate on the effectiveness of antiviral treatment initiated greater than 48 hours after symptom onset, especially in the hospitalized population, which represents 43% of this cohort. However, limiting our analysis to the 33% of this patient population who did present within 48 hours of symptom onset, still only 46% received antiviral treatment.
In addition to undertreating subjects with influenza, this study demonstrated antiviral overtreatment in patients without influenza, which raises concerns of increasing antiviral resistance. Eleven percent of patients who did not have influenza received antiviral treatment. Due to the lack of rapid accurate influenza testing, the CDC recommends initiating antiviral treatment for all patients with existing or increased risk of influenza related complications in whom influenza is suspected, regardless of influenza testing [3,19]. These recommendations are based on the assumption that the prolonged time to result of accurate conventional or batched molecular diagnostic tests will significantly delay antiviral treatment, which is most effective when given close to the time of symptom onset. Rapid antigen based tests have poor sensitivity requiring additional testing if negative, also potentially increasing the time to antiviral treatment. Though these recommendations are well founded, they result in extensive overtreatment.
Both over and under treatment could be substantially improved by integrating highly sensitive, rapid, random access molecular diagnostic tests into ED clinical care. Currently, several rapid random-access molecular influenza diagnostics are FDA approved for use in the US. Test turn-around times of these assays range from 1-3 hours, and the test can be run on an individual rather than batched basis, further reducing total test turn-around time. Thus, rapid molecular tests are a viable option for improving influenza diagnosis and treatment in the ED. The incredibly poor sensitivity of clinical diagnosis coupled with low rate of antiviral treatment suggests a direct need for improved rapid influenza diagnostics to improve both the accuracy of influenza diagnosis in the ED and to inform antiviral treatment decisions. However, the specific clinical utility of integrating rapid molecular influenza testing remains unknown.
This study was performed at a single academic medical center, thus potentially limiting its generalizability to other geographic areas and practices. As an academic center, medical care is often conducted in resident/attending teams, and approximately half of our clinical diagnoses came from resident physicians, who have less clinical experience thus potentially impacting our outcome of clinician diagnosis. However, the resident and attending work together to evaluate the patient and discuss the diagnosis and management. Though a resident may have less clinical experience, their clinical diagnosis is informed by discussions and impressions of the entire clinical management team, and benefits from the attending’s extended clinical experience. Additionally, our results are consistent with previous literature demonstrating poor sensitivity of physician diagnosis of influenza.
As with any prospective study there exists the possibility of selection bias in enrollment. In order to minimize this we used broad inclusion criteria so as not to exclude individuals with atypical symptoms of influenza. Despite the use of these broad criteria, we may have missed patents presenting with atypical or asymptomatic influenza. Additionally, the subjects enrolled may differ from those presenting outside of our study enrollment hours. Finally, some eligible patients declined study participation or left prior to enrollment, which may affect our final enrolled population.
Another potential limitation is the method of obtaining the clinician diagnosis. This study sought to determine the accuracy of clinician diagnosis of influenza in the absence of ancillary influenza testing. ED clinicians were queried prior to the result of routine (i.e., provider requested) influenza testing if it was performed. We obtained ED clinician diagnosis as close as possible to final patient disposition so the clinician would have the full benefit of ancillary tests such as basic laboratory tests and diagnostic imaging. It is possible that the clinician obtained additional clinical information (e.g. new fever, and/or radiographic or other laboratory testing) after giving their study-related clinical diagnosis, which may have affected their ultimate diagnosis. Notably however, the EMR diagnosis of influenza, which was recorded after clinicians had all available information, had similar or even lower sensitivity (26%; 95% CI 14-42%) than the reported clinician diagnosis (36%; 95% CI 22-52%).
CONCLUSIONS
Overall, this study evaluated the diagnosis and treatment of influenza in adult ED patients who met CDC criteria for recommended antiviral treatment. In this target population, ED clinician diagnosis, final ED EMR diagnosis of influenza, and the classic CDC definition of ILI have poor sensitivity for influenza. ED management of influenza demonstrates both under-treatment, in those with confirmed influenza (36%), and overtreatment in those without influenza (11%). Clinician’s inability to appropriately administer antivirals is likely related to the underlying challenges of accurate diagnosis. Integrating new highly-sensitive rapid diagnostic tests for influenza could improve accuracy of both diagnosis and treatment of influenza in the ED.
ACKNOWLEDGEMENTS
The authors thank XXX for performing the influenza testing for this study.
Funding: This work was supported by the Johns Hopkins Clinical Research Scholars Program through the National Institutes of Health [5KL2RR025006] and Cepheid (Sunnyvale, CA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous Presentations: This work has been presented as an oral presentation at the Society of Emergency Medicine Annual Meeting on May 15th, 2014 in Dallas, TX.
REFERENCES
- [1].Thompson WWS, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–40. doi: 10.1001/jama.292.11.1333. D.K. [DOI] [PubMed] [Google Scholar]
- [2].Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- [3].Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki T. Centers for Disease Control and Prevention (CDC). Antiviral agents for the treatment and chemoprophylaxis of influenza --- recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Surveill Summ. 2011;60:1–24. [PubMed] [Google Scholar]
- [4].Harper SA, Bradley JS, Englund JA, et al. Seasonal influenza in adults and children--diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1003–32. doi: 10.1086/604670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].WHO recommendations on the use of rapid testing for influenza diagnosis 2005 (Accessed March 12, 2013, at http://www.who.int/influenza/resources/documents/rapid_testing/en/index.html.)
- [6].Hayden FG, Osterhaus AD, Treanor JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. N Engl J Med 1997. 1997;337:874–80. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- [7].Monto AS, Fleming DM, Henry D, et al. Efficacy and safety of the neuraminidase inhibitor zanamivirin the treatment of influenza A and B virus infections. J Infect Dis. 1999;180:254–61. doi: 10.1086/314904. [DOI] [PubMed] [Google Scholar]
- [8].Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet. 2000;355:1845–50. doi: 10.1016/s0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- [9].Heinonen S, Silvennoinen H, Lehtinen P, et al. Early oseltamivir treatment of influenza in children 1-3 years of age: a randomized controlled trial. Clin Infect Dis. 2010;51:887–94. doi: 10.1086/656408. [DOI] [PubMed] [Google Scholar]
- [10].Mahony JB, Petrich A, Smieja M. Molecular diagnosis of respiratory virus infections. Crit Rev Clin Lab Sci. 2011;48:217–49. doi: 10.3109/10408363.2011.640976. [DOI] [PubMed] [Google Scholar]
- [11].Overview of Influenza Surveillance in the United States. 2013 (Accessed November 21, 2013, at http://www.cdc.gov/flu/pdf/weekly/overview.pdf.)
- [12].Ong AK, Chen MI, Lin L, et al. Improving the clinical diagnosis of influenza--a comparative analysis of new influenza A (H1N1) case. PLoS One. 2009;4:e8453. doi: 10.1371/journal.pone.0008453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? JAMA. 2005;293:987–97. doi: 10.1001/jama.293.8.987. [DOI] [PubMed] [Google Scholar]
- [14].Ebell MH, White LL, Casault T. A systematic review of the history and physical examination to diagnose influenza. J Am Board Fam Pract. 2004;17:1–5. doi: 10.3122/jabfm.17.1.1. [DOI] [PubMed] [Google Scholar]
- [15].Stein J, Louie J, Flanders S, et al. Performance characteristics of clinical diagnosis, a clinical decision rule, and a rapid influenza test in the detection of influenza infection in a community sample of adults. Ann Emerg Med. 2005;46:412–9. doi: 10.1016/j.annemergmed.2005.05.020. [DOI] [PubMed] [Google Scholar]
- [16].Govaert TM, Dinant GJ, Aretz K, Knottnerus JA. The predictive value of influenza symptomatology in elderly people. Fam Pract. 1998;15:16–22. doi: 10.1093/fampra/15.1.16. [DOI] [PubMed] [Google Scholar]
- [17].Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Archives of internal medicine. 2000;160:3243–7. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- [18].Hsieh Y-H, Kelen GD, Dugas AF, Chen K-F, Rothman RE. Emergency Physicians' Adherence to Center for Disease Control and Prevention Guidance During the 2009 Influenza A H1N1 Pandemic. West J Emerg Med. 2013;14:191–9. doi: 10.5811/westjem.2012.11.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Uyeki T. Diagnostic Testing for 2009 Pandemic Influenza A (H1N1) Virus Infection in Hospitalized Patients. N Engl J Med. 2009;361:e114. doi: 10.1056/NEJMopv0911052. [DOI] [PubMed] [Google Scholar]