Abstract
Fetal growth restriction (FGR) increases the risk of perinatal complications and predisposes the infant to developing metabolic, cardiovascular, and neurological diseases in childhood and adulthood. The pathophysiology underlying FGR remains poorly understood and there is no specific treatment available. Biomarkers for early detection are also lacking. The insulin-like growth factor (IGF) system is an important regulator of fetal growth. IGF-I is the primary regulator of fetal growth, and fetal circulating levels of IGF-I are decreased in FGR. IGF-I activity is influenced by a family of IGF binding proteins (IGFBPs), which bind to IGF-I and decrease its bioavailability. During fetal development the predominant IGF-I binding protein in fetal circulation is IGFBP-1, which is primarily secreted by the fetal liver. IGFBP-1 binds IGF-I and thereby inhibits its bioactivity. Fetal circulating levels of IGF-I are decreased and concentrations of IGFBP-1 are increased in FGR. Phosphorylation of human IGFBP-1 at specific sites markedly increases its binding affinity for IGF-I, further limiting IGF-I bioactivity. Recent experimental evidence suggests that IGFBP-1 phosphorylation is markedly increased in the circulation of FGR fetuses suggesting an important role of IGFBP-1 phosphorylation in the regulation of fetal growth. Understanding of the significance of site-specific IGFBP-1 phosphorylation and how it is regulated to contribute to fetal growth will be an important step in designing strategies for preventing, managing, and/or treating FGR. Furthermore, IGFBP-1 hyperphosphorylation at unique sites may serve as a valuable biomarker for FGR.
Keywords: IGFBP-1 phosphorylation, IGF-I bioavailability, IGFBP-1 phosphorylation sites, Fetal growth restriction (FGR)
Introduction
Fetal growth is hindered by many disparate influences including an inadequate intrauterine environment due mainly to a lack of nutrient and oxygen supply to the fetus. In 8–10 % of all pregnancies, oxygen and nutrient delivery to the fetus are impaired, leading to FGR. Although the mechanisms underlying restricted fetal growth are yet to be fully established, alterations in the fetal IGF axis have been implicated in the pathophysiology of FGR (Baschat and Hecher. 2004). IGFs (IGF-I and IGF-II) synthesised by the fetal liver are secreted into fetal circulation and tissue fluids. The IGFs play a direct role in cell growth, differentiation, and metabolism (Murphy et al. 2006), and IGF bioavailability is regulated by six binding proteins (IGFBP-1−6) (Clemmons. 1997; Coverley and Baxter. 1997). IGF-I is a key regulator of fetal growth (Liu et al. 1993, 2003). Although fetal circulating IGF-II levels are higher throughout gestation, only free circulating IGF-I concentrations have consistently been shown to be reduced in FGR (Lassarre et al. 1991; Cianfarani et al. 1998). A 40 % decrease in fetal weight observed in IGF-I knockout mice with increased neonatal death provides a dramatic illustration of the critical role of IGF-I in normal fetal growth (Liu et al. 1993; Baker et al. 1993).
IGF-I primarily exists in circulation bound to IGFBPs, and the endocrine responses of IGF-I are primarily mediated by binding to the IGF1 receptor (IGF1R). Binding of IGF-I to IGF1R initiates secondary messenger pathways which stimulates protein translation, proliferation and antiapoptotic activity in most cells (Baxter. 2000). IGFBPs regulate the distribution of IGFs between the extracellular compartment and binding sites at the cell surface and control IGF bioactivity. IGFBP-1, which is primarily secreted from the liver (Han et al. 1996b; Tapanainen et al. 1994) is a major regulator of IGF-I bioavailability in the fetus (Chard. 1994). Fetal circulating levels of IGFBP-1 are elevated in FGR (Giudice et al. 1995). Three of the six IGFBPs, IGFBP-1, IGFBP-3 and IGFBP-5 are phosphorylated. The significance of IGFBP phosphorylation in the regulation of IGFBP function has been previously reviewed (Coverley and Baxter. 1997). Phosphorylation of only IGFBP-1 enhances both its affinity for IGF-I (Jones et al. 1993a) and its capacity to inhibit IGF-I actions (Dolcini et al. 2009; Firth and Baxter. 2002; Koistinen et al. 1990; Busby et al. 1988; Yu et al. 1998) providing more effective means of regulation for IGF-bioavailability relative to IGFBP-1 concentration alone. Despite a vast amount of literature describing the significance of IGFBP-1 phosphorylation in human pregnancy (Iwashita et al. 1998; Westwood et al. 1994; Gibson et al. 2001; Westwood 1999; Koistinen et al. 1993b; Bhatia et al. 2002; Fowler et al. 1999; Westwood et al. 1997; Gibson et al. 1999; Loukovaara et al. 2005; Abu Shehab et al. 2009, 2010, 2013, 2014; Martina et al. 1997), the biological/clinical role of IGFBP-1 phosphorylation in FGR or in normal pregnancies remains to be established. This review highlights the importance of, and the molecular mechanisms regulating IGFBP-1 phosphorylation in FGR that are vital in studying the underlying pathophysiology and/or the potential role of IGFBP-1 phosphorylation as a biomarker for FGR.
IGFBP-1 in normal and FGR pregnancies
IGFBP-1 is a secretory product of the adult and fetal liver and decidualized endometrial stromal cells and is the most predominant IGFBP in amniotic fluid (Han et al. 1996b; Tapanainen et al. 1994; Westwood et al. 1994; Martina et al. 1997; Fang et al. 2004). IGFBP-1 is also the major regulator of IGF-I-mediated cell growth in catabolic states such as fasting, malnutrition, and hypoxia (Orlando-Mathur et al. 1996; Popovici et al. 2001). It has been demonstrated that umbilical cord blood levels of IGF-I are decreased and concentrations of IGFBP-1 are increased in human FGR (Lassarre et al. 1991; Chard. 1994) implicating IGFBP-1 as a key inhibitor of IGF action and mediator of fetal growth. Cord blood levels of IGFBP-1 examined by Ong et.al in a cohort of 199 normal term singleton pregnancies were inversely related to infant size at birth (Ong et al. 2000). Indeed, overexpression of hepatic IGFBP-1 in transgenic mice resulted in an 18 % reduction in birthweight, suggesting a causal relationship between increased fetal circulating IGFBP-1 and FGR (Watson et al. 2006).
In the maternal compartment, the placenta (decidua) is a major site of maternal IGFBP-1 synthesis and the major source for maternal IGFBP-1 (Martina et al. 1997; Fang et al. 2004). The role of decidual IGFBP-1 at the maternal fetal interface is, however, unclear. It is generally believed that a lack of increase in uteroplacental blood flow is an early event in the development of FGR, which may limit oxygen and nutrient delivery to the fetus via the placenta. IGFBP-1 levels were elevated in placental (decidual) tissues of mothers who gave birth to FGR infants (Street et al. 2006). Elevated levels of IGFBP-1 were also detected in amniotic fluid, but there is conflicting evidence regarding the association of these levels with birth weight (Bhatia et al. 2002; Tisi et al. 2005; Ben Lagha et al. 2006).
Most (Holmes et al. 1997; Olausson et al. 2010; Hills et al. 1996) but not all (Bhatia et al. 2002; Qiu et al. 2005; Sifakis et al. 2012) studies show that FGR is associated with increased maternal serum IGFBP-1 levels. Maternal IGF-I is a powerful positive regulator of placental function and growth, therefore alterations in maternal IGF-I and IGFBP-1 levels may alter IGF-I bioavailability in pregnancies complicated by FGR and directly contribute to restricted fetal growth. Maternal serum IGF-I concentrations are decreased in mothers delivering FGR babies and an inverse correlation is demonstrated between maternal IGFBP-1 and birth weight (Holmes et al. 1997; Wang et al. 1991). The predictive role of maternal serum IGFBP-1 is still unclear (Bhatia et al. 2002; Sifakis et al. 2012; Qiu et al. 2005). Also, whether IGFBP-1 of fetal (liver) or maternal (decidual) origin is more important (Giudice. 2002), or both fetal and maternal IGFBP-1 are equally involved in sequestering fetal derived IGF-I is unclear. Importantly, while all forms of IGFBP-1 can sequester IGF-I, the predominant increase in IGF-I affinity occurs due to an increase in IGFBP-1 phosphorylation. IGF-I binding is more profoundly affected by the sites and degree of IGFBP-1 phosphorylation than by increases in total IGFBP-1 (Seferovic et al. 2009).
IGFBP-1 phosphorylation
The sites of IGFBP-1 phosphorylation
All IGFBPs contain structured N- and C- terminal domains and an unstructured central flexible linker domain. The structures of the N- and C- terminal domains of the IGFBPs have been extensively studied by nuclear magnetic resonance (Sitar et al. 2006; Siwanowicz et al. 2005) mass spectrometry (Neumann and Bach. 1999) and mutation analysis (Imai et al. 2000; Buckway et al. 2001; Yan et al. 2004). The greatest contribution of these studies to our understanding of IGFBP function relates to the organization of the disulfide bonds formed by the highly conserved cysteine residues in both the N- and C- terminals which together form an important IGF binding pocket (Chelius et al. 2001). All six IGFBPs have similar IGF binding determinants located in both the N- and C-domains and both of these domains are required for high-affinity IGF binding (Sitar et al. 2006). Each IGFBP also has distinguishing features which may contribute to the diversity in their function and tissue expression. The linker domains of all IGFBPs are unstructured and are the least conserved domains between IGFBPs (Sitar et al. 2006). The linker domains contain sites for post-translational modification and proteolytic cleavage. However, direct binding of IGFs to linker region has not been demonstrated. Phosphorylation in linker region has been well established on IGFBP-1, IGFBP-3 and IGFBP-5. It has been shown that phosphorylation of IGFBP-3 by nuclear DNA-PK greatly decreases or abolishes affinity for IGF-I (Schedlich et al. 2003). Phosphorylation of IGFBP-1 on the other hand enhances its binding affinity for IGF-I by as much as 6- to 10- fold (Westwood. 1999; Jones et al. 1993a) as well as its capacity to inhibit IGF-I actions (Dolcini et al. 2009; Firth and Baxter. 2002; Koistinen et al. 1990; Busby et al. 1988; Yu et al. 1998). It was reported that phosphorylation affected IGFBP-5 binding to heparin but no distinct changes in IGF affinity were recorded (Graham et al. 2007). In addition, although the affinity of phosphorylated IGFBP-1 for IGF-I is higher compared to non-phosphorylated IGFBP-1, no discernible effect on its affinity for IGF-II has been reported (Westwood et al. 1994). Moreover, Sakai et.al. demonstrated physiological differences between the phosphorylation state of human and rat IGFBP-1. Mouse kinases were unable to phosphorylate human IGFBP-1 and the phosphorylation of rodent IGFBP-1 did not seem to affect its affinity for IGF-I (Sakai et al. 2001) further suggesting that human IGFBP-1 phosphorylation is unique in its ability to inhibit IGF-I actions.
Phosphoamino acid analysis of 32P labeled IGFBP-1 purified from endometrial stromal cells as well as transfected CHO cells (Jones et al. 1993a, b) demonstrated that IGFBP-1 is phosphorylated on serine residues. Jones et al. recognized Ser101 as the main IGFBP-1 phosphorylation site in CHO cells, while Ser169 and Ser119 were considered minor and S95 and Ser98 as non-phosphorylated residues (Jones et al. 1993b). The major site of phosphorylation was Ser101 as it accounted for 70 % of total IGFBP-1 phosphorylation, while Ser169 and Ser119 accounted for only 25 % and 5 % of phosphorylation, respectively. The physiological consequence of Ser101 phosphorylation was confirmed by site-directed mutagenesis (Jones et al. 1993b), where up to a 3-fold reduction in IGFBP-1 binding affinity to IGF-I was observed. In addition, binding studies using IGFBP-1 isolated from HepG2 cells showed 6-fold higher binding affinity for IGF-I compared to dephosphorylated IGFBP-1 (Jones et al. 1991).
Using mass spectrometry, phosphorylation at five serine residues (Ser95, 98, 101, 119 and 169) has now been confirmed (Dolcini et al. 2009; Abu Shehab et al. 2009, 2010, 2013; Seferovic et al. 2009; Temporini et al. 2008; Nissum et al. 2009). As shown in Table 1, in addition to Ser101, phosphorylation at Ser98, Ser119 and Ser169, has been validated to cause varied responses to IGF-I action using mutagenesis (Abu Shehab et al. 2013). The effects of Ser119 were most prominent and similar to Ser101. It has been speculated that Ser119 phosphorylation possibly elicits conformational changes in the IGF-I/IGFBP-1 complex to enhance its affinity for IGF-I. It was proposed that relatively weaker effects with Ser169 and Ser98 appear to act in a coordinated fashion and require synergistic interactions to result in the high affinity interaction, which needs further investigation. Further, the structures of any intact IGFBPs or an intact IGFBP:IGF complex have not been solved as yet. Although no experimental data are available, molecular modeling of the IGFBP-1:IGF-I complex based on the known structure of IGF-I in complex with IGFBPs indicates that residues Ser98, Ser169, and the previously recognized Ser101 are on relatively mobile regions of the IGFBP-1 sequence. These residues are close to structured regions and are predicted to be involved in IGF-I binding (Abu Shehab et al. 2010). To date no structural data have been published to identify the interactions of any of the IGFBP-1 phosphorylation sites with the IGF binding pocket in order to demonstrate how certain phosphorylated residues alter IGFBP-1 affinity for IGF-I. Therefore the mode of interaction between specific phosphorylated residues and IGF-I nonetheless remains unclear.
Table 1.
Changes in IGF-I affinity and biological effects of site-specific IGFBP-1 mutations
| Phospho-site | Source | Mutation | Changes in phos levels | IGF-I affinity (BIAcore) | Biological effects* | Reference |
|---|---|---|---|---|---|---|
| S98 | CHO CM** | S98A | NA*** | 42-fold lower | 2-fold higher | Abu Shehab et al. 2013 |
| S101 | CHO CM | S101A | 70 % lower | 3-fold lower | Jones et al. 1993a, b | |
| S101A | NA lower | 90-fold lower | 2.5-fold higher | Abu Shehab et al. 2013 | ||
| S119 | CHO CM | S119A | 5 % lower | NA | Jones et al. 1993a, b | |
| S119A | NA | 100-fold lower | 2.5-fold higher | Abu Shehab et al. 2013 | ||
| S169 | CHO CM | S169A | NA | NA | 25 % lower | Jones et al. 1993a, b |
| S169A | NA | 34-fold lower | 2-fold higher | Abu Shehab et al. 2013 |
Employing receptor-stimulation assay utilizing IGF-1R-overexpressing P6 cells it was shown that relative to WT, S98A and S169A mutants did not inhibit receptor autophosphorylation. S119A on the other hand, greatly stimulated the receptor. The data with S119A matched S101A. In summary, phosphorylation at S98 and S169 resulted in milder changes. Most dramatic inhibitory effects were due to phosphorylation at S119 suggesting enhanced affinity for IGF-I may be through stabilization of the IGF-IGFBP-1 complex possibly involving other phosphorylation sites
*IGF-I receptor autophosphorylation assay
**Chinese Hamster Ovary Cell Media
***NA = (data) Not Available
It is expected that possibly the most important contribution of structural studies will be to enable the understanding of synergistic interactions through phosphorylation at doubly or multiply phosphorylated residues which may result in the very high affinity of IGFBP-1 for IGF-I. Since a defined role for IGFBP-1 phosphorylation in FGR has yet to be demonstrated, structural information would provide a more conclusive role of IGFBP-1 phosphorylation as compared to IGFBP-1 concentration in the pathophysiology of FGR. Structural data will also be valuable in screening (in silico and/or through binding assays) for small molecule inhibitors that could prevent binding of IGF-1 to IGFBP-1 as a future therapeutic strategy based on enhancing IGF-I activity particularly in FGR.
IGFBP-1 phosphorylation in human FGR
Considering that IGFBP-1 phosphorylation alters IGF-I bioavailability, the association of IGFBP-1 phosphorylation in fetal and maternal serum as well as in amniotic fluid with birth weight has been investigated in human pregnancies, but with mixed results (Iwashita et al. 1998; Westwood et al. 1994, 1997; Gibson et al. 1999, 2001; Westwood. 1999; Koistinen et al. 1993a, b; Bhatia et al. 2002; Fowler et al. 1999; Loukovaara et al. 2005; Abu Shehab et al. 2009, 2010, 2013, 2014). It has been shown that the highly phosphorylated IGFBP-1 isolated from human plasma has ~10 fold higher binding affinity for IGF-I compared to the non-phosphorylated recombinant protein (Westwood et al. 1997). Under basal conditions, human decidualized endometrium produces both non- and phosphorylated IGFBP-1 (Gibson et al. 2001). Varying proportions of phosphorylated and non-phosphorylated IGFBP-1 isoforms have been detected in human amniotic fluid as well as maternal and fetal serum during pregnancy (Westwood et al. 1994; Gibson et al. 2001; Westwood. 1999; Koistinen et al. 1993a, b). It was shown that the ratio of high (hp) to low level phosphorylated (lp) IGFBP-1 was significantly increased in type 1 diabetic maternal serum, being negatively associated with birth size (i.e., lower phosphorylation correlated with larger babies). Conversely, in non-diabetic pregnancies, total and less phosphorylated IGFBP-1 were elevated in maternal serum of the low birth weight group (Gibson et al. 1999). Similarly, in diabetic pregnancies Loukovaara et al. detected suppression of highly phosphorylated (hp) forms with higher affinity for IGF-I than low phosphorylated (lp) forms which were linked with stimulated fetal growth (Loukovaara et al. 2005). These studies highlight the regulatory role of variable IGFBP-1 phosphorylation in amniotic fluid as well in maternal and fetal plasma in the pathophysiology of FGR.
Evidence for IGFBP-1 phosphoisoforms mentioned above (Gibson et al. 1999; Loukovaara et al. 2005) along with other studies using clinical samples has mainly been based on ratios of non- or low- to highly-phosphorylated IGFBP-1 determined utilizing monoclonal antibodies (Mab 6303 and Mab 6305) with two different immunoassays or non-denaturing gels combined with radio ligand blotting. Additionally, using amniotic fluid samples, 2-D PAGE (Weber et al. 1999) was combined with western bloting using a polyclonal antibody highly specific to IGFBP-1 as described by Nissum et al. which revealed an increase in IGFBP-1 phosphorylation in FGR pregnancies vs. controls (Nissum et al. 2009). However, due to a variety of limitations such as differential antibody recognitions, requiring conventional purification, and the lack of ability to identify the sites of IGFBP-1 phosphorylation, establishing a direct link between altered IGFBP-1 phosphorylation and fetal growth has been technically challenging. Phosphoproteomic approaches were necessary to establish required links of site-specific phosphorylation of IGFBP-1 with FGR.
Site-specific IGFBP-1 phosphorylation: known or predicted consequences
Assessment of IGFBP-1 phosphorylation in FGR was later performed using an ELISA that accounted for serine phosphorylated IGFBP-1 levels (Khosravi et al. 2007). A 16-fold increase in phosphorylation at serine residues was detected in the amniotic fluid from FGR compared to control pregnancies (Abu Shehab et al. 2010). The role of amniotic fluid IGFBP-1 in FGR is currently not known. Amniotic fluid IGFBP-1 is expected to be secreted by maternal decidua. Studies of cellular localization with placental tissues have shown that the IGFBP-1 peptide is localized in the basal plate decidua (BPD) at the maternal-fetal interface (Han et al. 1996a). However, there are no data that demonstrate IGFBP-1 phosphorylation status (sites and degree) in placenta (BPD) from women with FGR or from normal pregnancies that would clarify the decidual contribution to circulating or amniotic fluid IGFBP-1 in FGR.
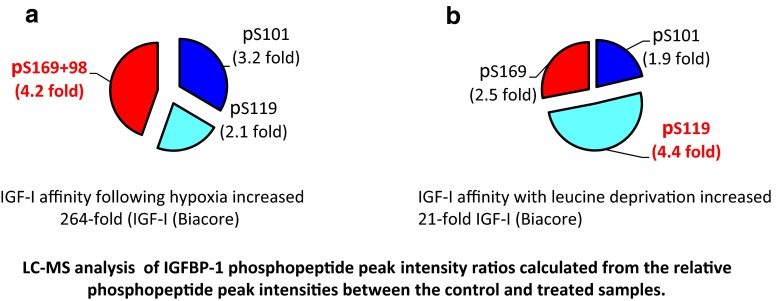
To identify the sites of IGFBP-1 phosphorylation, HepG2 cells were treated to mimic FGR conditions in vitro (Seferovic et al. 2009). Using LC-MS/MS, a greater degree of phosphorylation of IGFBP-1 in leucine deprivation at Ser119 (4.5-fold) led to 30-fold higher affinity for IGF-I. Hyperphosphorylation at Ser169 combined with Ser98 in a doubly phosphorylated peptide led to a 4-fold increase due to hypoxia but increased IGF-I affinity ~264-fold and inhibited IGF-I-stimulated cell growth. The level of phosphorylation increased at Ser101 which was common to both treatments. These data put forth a new concept that although the fold increase in IGFBP-1 phosphorylation between the two triggers was not as different, phosphorylation at Ser169 combined with Ser98 in hypoxia could more profoundly modulate IGF-I bioavailability than Ser119 alone (Fig. 1a, b), thus highlighting potential synergistic effects between the two phosphosites.
Fig. 1.
Using a cell culture model for hypoxia and nutritional deprivation, distinct variability in the sites of phosphorylation of IGFBP-1 and differences in IGF-I affinity were reported. It was shown that residues Ser101/119/169 were common to both stress stimuli. a There was a significant Increase in IGFBP-1 phosphorylation (at Ser169 + Ser98) that was associated with 264 -fold higher IGF-I affinity in hypoxia. b Under leucine deprivation a 21- fold increase in IGFBP-1 phosphorylation at Ser119 correlated with 21 × higher affinity for IGF-I (Seferovic et al. 2009.)
LC-MS analysis was also performed using amniotic fluid samples (Fig. 2a). It was demonstrated that IGFBP-1 phosphorylation sites were common in both FGR and controls. However, there was ~7-fold higher phosphopeptide intensity for Ser101 and of a doubly phosphorylated Ser98/Ser101 phosphorylation in FGR relative to controls, while a 23-fold higher intensity was recorded for Ser169 (Abu Shehab et al. 2010). Additionally, the kinetics of IGF binding using BIAcore showed ~5-fold greater association rate between IGFBP-1 and IGF-I (summarized in Table 1) and increased IGF-I affinity which correlated with reduced fetal growth in FGR (Fig. 2b). These data provided stronger support for a link between site-specific phosphorylation and FGR. Phospho Ser119 enriched using free flow electrophoresis (FFE) was linked with highest affinity of IGFBP-1 for IGF-1 (Nissum et al. 2009). Shown in Fig. 3 is the linear structure of IGFBP-1 that summarizes distinctive effects on increased IGFBP-1 phosphorylation at specific sites triggered by two differing catabolic conditions in vitro and in FGR in vivo. These studies provide novel evidence for sensitive regulation of IGF-I bioavailability in response to individual stress stimuli mediated by altered phosphorylation of IGFBP-1 that needs to be tested in FGR. These studies also suggest that potentially multiple kinases and diverse mechanisms must be involved mediating these effects.
Fig. 2.
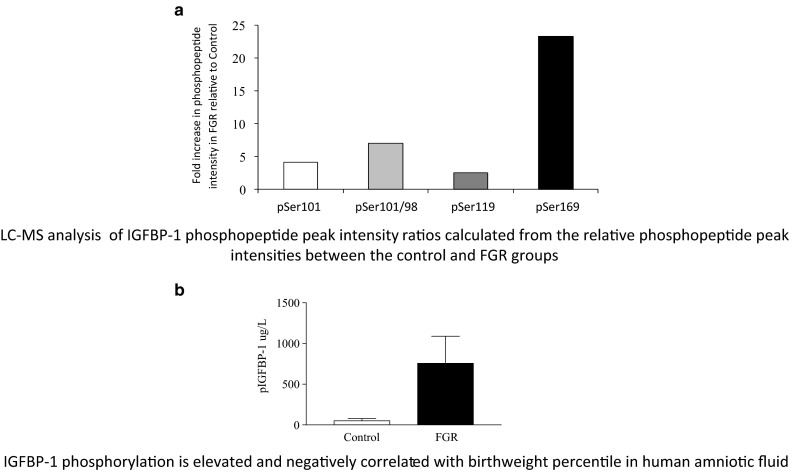
Functional impact of the site-specific phosphorylation of IGFBP-1 was clearly demonstrated in human FGR, a condition characterized by various degrees of hypoxia and decreased nutrient availability. It was indicated that higher IGFBP-1 phosphorylation at Ser98/101 but most notably, at Ser169 correlated with lower birth weight. (Abu Shehab et al. 2010)
Fig. 3.
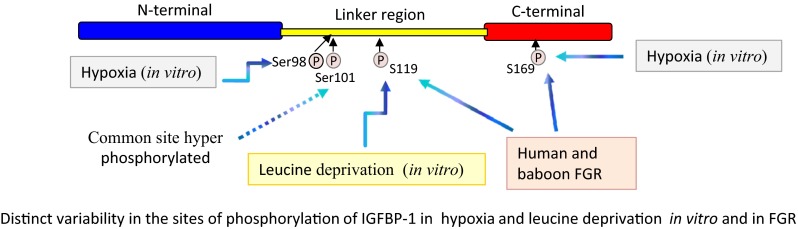
Distinct variability in the sites of phosphorylation of IGFBP-1 in two different conditions of stress. Residues Ser101/119/169 were common to both stress stimuli, but there were clear differences in IGFBP-1 phosphorylation patterns between hypoxia and leucine deprivation. In particular, a dramatic increase in phosphorylation at Ser169 as well as at a new site Ser98 was observed in hypoxia. However, the most significant change in leucine deprivation was a marked increase in phosphorylation at Ser119. These findings provide novel evidence of sensitive regulation of IGF-I bioavailability in response to individual stress stimuli mediated by altered hyperphosphorylation of IGFBP-1 (Seferovic et al. 2009; Abu Shehab et al. 2010, 2014)
More recently, using three different phospho-site specific IGFBP-1 antibodies, increases in phosphorylation at Ser101, Ser119, and Ser169 were demonstrated in human umbilical cord plasma of FGR fetuses (Abu Shehab et al. 2014). Phosphorylation at these three residues was also consistently increased in fetal liver from a baboon FGR model of maternal nutrient restriction (MNR), further signifying the role of IGFBP-1 hyperphosphorylation in FGR in vivo (Abu Shehab et al. 2014). A summary of IGFBP-1 phosphorylation sites and their effects on IGF-I affinity in biological samples is described in Table 2.
Table 2.
Relative IGFBP-1 phosphorylation and IGF-I affinity in samples from control and FGR pregnancies
| Phospho-sites | Source | Biological Samples | Fold increase in relative phosphopeptide intensities FGR vs. Control | Fold increase in IGF-I affinity FGR vs. Control (BIAcore) | Mode of detection | Ref |
|---|---|---|---|---|---|---|
| S95 | Human | AF* | NA** | NA | MS | Dolcini et al. 2009 |
| S98/S101 | Human | AF | 7-fold | NA | LC-MS*** | Abu Shehab et al. 2010 |
| S101 | Baboon | Fetal liver | 1.4-fold | NA | WB# | Abu Shehab et al. 2014 |
| Human | AF | 4.5-fold | 2-fold | LC-MS*** | Abu Shehab et al. 2010 | |
| Cord blood | 2-fold | NA | WB# | Abu Shehab et al. 2014 | ||
| S119 | Baboon | Fetal liver | 1.5-fold | NA | WB# | Abu Shehab et al. 2014 |
| Human | AF | 2.5-fold | 2-fold | LC-MS*** | Abu Shehab et al. 2010 | |
| Cord blood | 1.5-fold | NA | WB# | Abu Shehab et al. 2014 | ||
| S169 | Baboon | Fetal liver | 2-fold | NA | WB# | Abu Shehab et al. 2014 |
| Human | AF | 23-fold | 2-fold | LC-MS*** | Abu Shehab et al. 2010 | |
| Human | Cord blood | 1.8-fold | NA | Abu Shehab et al. 2014 |
*Amniotic Fluid
**NA = (data) Not Available
*** Relative phosphopeptide intensities (LC-MS) between FGR and controls
#Phospho-site specific IGFBP-1 antibodies
Although successful, the strategy using phosphosite-specific antibodies has limitations. First it requires the production of highly specific antibodies to confirm identity of the sites. Second, comparisons to determine which different sites are involved is biased towards the analysis of known phosphorylation sites; consequently, the identification of new sites and/or synergistically interacting multiple sites is not possible. Alternate proteomic methods to identify changes in the phosphorylation sites are required such as stable isotopic labelling of amino acids in cell culture (SILAC) and Multiple Reaction Monitoring-Mass Spectrometry (MRM-MS) for differential profiling. These sensitive and quantitative approaches will be more apt to link IGFBP-1 phosphorylation sites and degree of phosphorylation with FGR etiology. Further structural-functional studies will be needed together with structural data in order to provide an in-depth understanding of how complex synergistic interactions result in more precise and predominant changes in IGF-I bioavailability and increased inhibition of IGF-I bioactivity.
Protein kinases that phosphorylate IGFBP-1
Given that IGFBP-1 is phosphorylated on multiple serine residues (Ser95, 98, 101, 119 and 169) (Dolcini et al. 2009; Abu Shehab et al. 2009, 2010, 2013; Seferovic et al. 2009; Temporini et al. 2008; Nissum et al. 2009; Jones et al. 1993b), phosphorylation is likely to be modulated by serine/ threonine kinases. IGFBP-1 has consensus sequences for protein kinase A (PKA), protein kinase C (PKC), cAMP-dependent protein kinase and protein kinase CK1 and CK2 (Ankrapp et al. 1996; Frost and Tseng. 1991; Koistinen et al. 1993a, b). CK2 modulates IGF-I activity in vitro based on the finding that it phosphorylates IGFBP-1 in HepG2 (Ankrapp et al. 1996) and decidual cells (Frost and Tseng. 1991). CK2 is a constitutively active, ubiquitous, and highly pleiotropic kinase that may phosphorylate >300 proteins in vitro which are yet to be identified as true substrates (St-Denis and Litchfield. 2009). Whether IGFBP-1 is a bona fide substrate for CK2 and whether CK2 is a key kinase involved in regulation of IGFBP-1 phosphorylation in FGR has not been directly investigated. More recently, to determine the role of CK2, HepG2 cells were treated with the selective CK2 inhibitor 4,5,6,7-tetrabromobenzotriazole (TBB) (Abu Shehab et al. 2014). As expected, TBB significantly decreased CK2 activity with a concomitant decrease in IGFBP-1 phosphorylation. Considering that the effects of TBB could be nonspecific, an alternate RNAi knockdown approach was used to validate these findings. CK2 exists in tetrameric structures with two catalytic (α or α’) and two regulatory (β) subunits that differ in their substrate specificity (Olsten and Litchfield. 2004; Janeczko et al. 2011). Therefore to confirm the specificity for CK2 actions, siRNA silencing was performed targeting individual CK2 subunits as well the CK2 holoenzyme (α or α’ and β subunits combined) (Abu Shehab et al. 2014) to establish CK2 specificity. Furthermore to determine whether CK2 can phosphorylate IGFBP-1 in vivo, the fetal liver from a well established baboon FGR model was tested also demonstrated higher CK2 activity with an increase in IGFBP-1 phosphorylation in FGR, supporting that CK2 can phosphorylate IGFBP-1 in vivo (Abu Shehab et al. 2014). Since phosphorylation was assessed using phosphosite-specific IGFBP-1 antibodies (Ser101, Ser119 and Ser169), no additional CK2 targeted sites could be identified.
Additionally, it is currently unclear if IGFBP-1 phosphorylation occurs before, after or concomitantly with its secretion. CK2 is localized in multiple intracellular compartments (Faust et al. 2001). Importantly, CK2 can also phosphorylate extra-cellular proteins (Bohana-Kashtan et al. 2005). Given that IGFBP-1 is a secretory protein, it is likely that CK2 acts on it as an ectokinase, phosphorylating IGFBP-1 concomitantly with or following its secretion. In addition, multiple kinases, such as PKA, can phosphorylate IGFBP-1 in vitro (Ankrapp et al. 1996; Frost and Tseng. 1991; Koistinen et al. 1993a, b). Whether IGFBP-1 is a preferred physiological substrate for these kinases has not been tested. It is expected that distinct kinases may be involved under varied conditions in FGR, allowing a large degree of combinatorial regulation. Therefore, knowing which residues are phosphorylated during particular response is essential in understanding the mechanistic regulation by specific kinases in FGR.
Phosphatases that regulate IGFBP-1 dephosphorylation
Dephosphorylated IGFBP-1 has lower affinity for IGF-I and shows stimulatory effects on IGF-I activity; thus, dephosphorylation of IGFBP-1 is a potential mechanism for potentiating IGF-I action (Westwood et al. 1997). It is suggested that the variable phosphorylated isoforms in amniotic fluid are a result of dephosphorylation by placental alkaline phosphatase (ALP) (Westwood. 1999). Dephosphorylation of IGFBP-1 by ALP results in isoforms with reduced affinity to bind IGF-I in vitro (Gibson et al. 2001; Solomon et al. 2014). Gibson et al. proposed paracrine modulation of IGFBP-1 at the maternal-fetal interface via dephosphorylation combined with proteolysis of IGFBP-1 as a mean of enhanced IGF-I bioavailability in regulating fetal and maternal tissue growth (Gibson et al. 2001; Solomon et al. 2014). ALP isoforms with aberrant glycosylation display reduced dephosphorylation activity in association with cellular stresses, such as nutrient deprivation. It is conceivable that regulation of ALP activity might constitute an alternative mechanistic link with hyperphosphorylated IGFBP-1 in cellular stress such as in FGR. Both in vitro and in vivo studies are necessary to elucidate the complex molecular pathways that may be involved in IGFBP-1 phosphorylation via kinases and/or phosphatases to better understand the pathophysiology of FGR.
Models of FGR
The fetal liver is the primary source of fetal IGFBP-1 during pregnancy (Watson et al. 2006; Tapanainen et al. 1997). HepG2 cells have been commonly used as a model for human fetal hepatocytes (Wilkening et al. 2003; Kelly and Darlington. 1989; Maruyama et al. 2007). In addition, due to limited human fetal liver availability, fetal primary hepatocytes have served as a physiologically relevant in vitro model to elucidate mechanisms regulating IGFBP-1 phosphorylation in FGR (Abu Shehab et al. 2014; Li et al. 2013; Eghbali-Fatourechi et al. 1994).
Animal models to study in vivo consequences of IGFBP-1 phosphorylation are not yet available. Previous in vivo studies with animal models of FGR have been focused on IGFBP-1 mRNA and/or fetal circulating IGFBP-1 (Woodall et al. 1996; Tapanainen et al. 1994) but IGFBP-1 phosphorylation in fetal liver has never been studied in vivo. IGFBP-1 is conserved in mouse (Sakai et al. 2001) and rodent models for studying the significance of IGFBP-1 exist, but they are insufficient to understand the importance of IGFBP-1 phosphorylation in regulating IGF bioavailability. Human IGFBP-1 could not be phosphorylated at Ser101 by mouse protein kinase due to specific recognition based on the region surrounding the phosphorylated residue of corresponding mouse IGFBP-1 residue and Ser169 is not conserved in mouse IGFBP-1 (Sakai et al. 2001). Although Ser119 residue in human IGFBP-1 is conserved in mouse and rat (Ser132), it was considered a minor site in human IGFBP-1 (Sakai et al. 2001). A transgenic model for overexpression of IGFBP-1 has been developed before (Watson et al. 2006), nevertheless much higher complexity is expected in developing a model for IGFBP-1 phosphorylation due to site-specific effects on IGF-I bioavailability as well as phenotypic differences between the species. This illustrates the need for alternative models.
A well established and internationally recognized baboon model of FGR is available based on 30 % global maternal nutrient restriction (MNR). The model reduces fetal circulating levels of essential amino acids resulting in structural and functional changes in fetal organs. Baboon MNR typically results in ~15 % decrease in fetal weight at GD 165 days gestation (0.9 gestation), which is similar to pre-term (34–37 weeks) humans (Schlabritz-Loutsevitch et al. 2007; Antonow-Schlorke et al. 2011). Despite no significant effect on fetal growth at mid (0.5) gestation, IGFBP-1 mRNA and protein expression were increased in the liver at this gestational age (Li et al. 2009). A marked increase in IGFBP-1 phosphorylation in baboon fetal liver from MNR induced FGR has been reported recently (Abu Shehab et al. 2014). The MNR model can be highly suitable for studying the gestational age based changes in IGFBP-1 phosphorylation from early to late pregnancy. Knowing if IGFBP-1 phosphorylation increases prior to presentation of FGR in baboon MNR would suggest a causal effect of IGFBP-1 modification on reduced fetal growth in utero. The well established baboon model that is closely related to human FGR provides strong justification to study experimentally-induced reproducible MNR, in order to understand the causative link between changes in fetal circulating IGFBP-1 phosphorylation and FGR.
The inhibitory effects of IGFBP-1 phosphorylation in FGR
Human IGFBP-1 phosphorylation alters IGF-I actions such as inhibiting IGF-I-stimulated DNA synthesis and wound healing in experimental animals (Firth and Baxter. 2002). Phosphorylated IGFBP-1 has shown inhibition of IGF-I-induced DNA synthesis in smooth muscle cells (Busby et al. 1988) and in fetal skin fibroblasts (Koistinen et al. 1990). Additionally, highly phosphorylated IGFBP-1 has also been shown to inhibit IGF-I-stimulated cell proliferation, amino acid transport and apoptosis in various cell types (Yu et al. 1998). Furthermore, IGFBP-1 is susceptible to proteolytic cleavage which is prevented by its phosphorylation (Gibson et al. 2001), possibly by inducing structural modifications or charge repulsion. The acute inhibitory effects of IGFBP-1 phosphorylation on the IGF-I-mediated activities may significantly contribute to restricted fetal growth.
Regulation of IGFBP-1 phosphorylation in FGR
Differential measurement of non- and highly phosphorylated IGFBP-1 forms in maternal serum during gestation suggests that phosphorylation status is under hormonal control (Kabir-Salmani et al. 2005; Westwood et al. 1995). In addition to endocrine factors, IGFBP-1 is regulated by a variety of catabolic conditions. Regulation and function of IGFBP-1 has been well documented (Lee et al. 1993). For example, protein restriction rapidly induces expression of IGFBP-1. The depletion of a single amino acid such as leucine (or arginine/cysteine or all essential amino acids combined) is sufficient to induce IGFBP-1 expression in vitro (Averous et al. 2005). Another catabolic condition regulating IGFBP-1 expression in FGR is hypoxic stress. The Igfbp1 gene has a consensus sequence for the hypoxia response element (HRE)-1 that causes significant induction of IGFBP-1 (Popovici et al. 2001; Tazuke et al. 1998; Kajimura et al. 2006). In zebrafish, for example, up-regulation of IGFBP-1 gene and protein expression by hypoxia inhibits embryonic growth and causes developmental delays in several vital organs, as well as IGF-stimulated embryonic cell proliferation in vitro. It is likely that conditions such as hypoxia and leucine deprivation that trigger IGFBP-1 hyperphosphorylation (Seferovic et al. 2009) could minimize IGFBP-1 proteolysis increasing its half-life. Moreover, IGFBP-1 secretion is inhibited by insulin and is stimulated by cAMP and glucocorticoids (Orlowski et al. 1990; Powell et al. 1993; Conover et al. 1996) but their effects on IGFBP-1 phosphorylation and the biological and physiological relevance of this regulation is not known. Given IGFBP-1’s importance to IGF-I signaling, understanding the factors that affect IGFBP-1 phosphorylation and IGF-I actions will provide a significant link between environmental events that alter IGF signaling and promote FGR.
Signalling pathway (s) involved in IGFBP-1 phosphorylation in FGR
Since conditions consistent with placental insufficiency lead to IGFBP-1 hyperphosphorylation, hypoxia and nutrient-sensing mechanisms may be involved for example, unfolded protein response (UPR) (Koumenis and Wouters. 2006), the hypoxia-inducible factor (HIF)-1 (Kajimura et al. 2006) and mechanistic target of rapamycin (mTOR) signaling (Sengupta et al. 2010). Placental mTOR signaling is markedly decreased in human FGR (Jansson et al. 1998). The inhibitory effect of insulin on Igfbp-1 gene expression is dependent on mTOR signaling (Patel et al. 2002). Like IGFBP-1, mTOR is subject to dynamic control by hypoxia and leucine deprivation although mTOR elicits opposite effect compared to IGFBP-1 on cell growth (Laplante and Sabatini. 2009; Foster and Fingar. 2010). mTOR inhibition has been mechanistically linked to increased secretion and IGFBP-1 phosphorylation in fetal liver cells and in the fetal liver mTOR regulates IGFBP-1 phosphorylation mediated by protein kinase CK2 (Abu Shehab et al. 2014) (Fig. 4). Whether mTOR is the link in hypoxia and leucine deprivation-induced IGFBP-1 phosphorylation needs to be understood. Elucidation of additional molecular mechanisms involved in regulation of IGFBP-1 phosphorylation via specific kinases or phosphatases are necessary to provide new fundamental knowledge about FGR pathophysiology.
Fig. 4.
Role of IGFBP-1 phosphorylation in regulation of IGF-I bioavailability in FGR. It is proposed that hypoxia and nutritional deprivation-induced IGFBP-1 phosphorylation is mediated by inhibition of mTOR signaling and stimulation of CK2 activity in fetal liver resulting in reduced IGF-1 bioavailability and fetal growth in FGR. (Abu Shehab et al. 2014)
Clinical relevance
FGR remains an important pregnancy complication with adverse short and long-term consequences. Studies exploring the mechanisms underlying FGR, the kinases and phosphatases involved in IGFBP-1 phosphorylation, and the consequences in the context of particular signaling pathways will provide valuable information to design strategies to prevent or treat FGR. Identifying infants with FGR prenatally rather than perinatally significantly improves perinatal outcomes (Geerts et al. 1996; Lindqvist and Molin. 2005). Biomarker detection is therefore important for the prediction of FGR in early pregnancy for better management and prevention of long term consequences (Barker. 2006). Increased phosphorylation of IGFBP-1 at specific site(s) could be a useful predictive indicator of FGR. This may also provide options for interventions such as leucine supplementation, which may be attempted in baboon model and, if successful in promoting fetal growth though experimental treatment, could be a clinically useful non-invasive approach in utero to stimulate growth in very small human fetuses. Furthermore, it may be valuable to identify key fetal circulating phosphoisoforms of IGFBP-1 that exert most bio-inhibitory effects on IGF-I bioavailability. This knowledge would help in designing small molecule inhibitors to displace IGF-I from phosphorylated IGFBP-1 in order to promote growth. However, reduced IGF-I bioavailability in the fetus may be an important adaptive mechanism to restrict growth for survival. Administration of excess IGF-I in vivo may be detrimental to the fetus and the small molecule inhibitor-strategy may not be suitable for FGR. However, it is possible that the release of IGF-I from the IGF:IGFBP-1 complex does not have similar adverse effects as external IGF-I administration. It will be important to consider a strategy which will involve IGF-I displacement aimed at increasing IGF-I bioavailability together with leucine supplementation (combined with other key nutrients) to prevent the detrimental effects of administration of IGF-I alone. An understanding of the mechanisms that produce the FGR phenotype and further phenotypic changes brought about by re-introduction of nutrients or growth factors such as leucine/other key constituents and IGF-I should provide novel directions for improving the outcome of FGR fetuses. This new knowledge may be critical in developing IGFBP-1 phosphoisoforms as biochemical markers for the assessment of fetal and postnatal growth.
Concluding comments
Our understanding of IGF-1 bioavailability in modulation of fetal growth in FGR via phosphorylation of IGFBP-1 has made little progress from the time when Ser101 phosphorylation was demonstrated to regulate IGF-I affinity (Jones et al. 1993a). However, as highlighted in this review, it is in the interactions of multiple IGFBP-1 phosphorylation sites at a synergistic level that may provide the most predominant effects on IGF-I bioavailability and regulation of fetal growth. Along with the recent discovery linking mTOR with CK2 and IGF-I bioavailability in FGR (Abu Shehab et al. 2014), other molecular pathways sensing nutrient and metabolic signals need be evaluated for their roles in FGR both in vitro and in vivo. Knowledge of the intracellular signaling systems involved may reveal a more predominant means for decreasing IGF-I bioavailability such as in severe FGR (<3rd percentile fetus). Such mechanistic responses and adaptations facilitating hypoxia and lack of nutrient tolerance during fetal development might also clarify whether IGFBP-1 phosphorylation-mediated mechanisms are adaptive or pathological in nature.
Research integrating cell culture systems and a primate model together with human clinical samples is expected to demonstrate the significance of IGFBP-1 phosphorylation in FGR and may bring knowledge from the bench to the bedside. In addition, IGFBP-1 phosphorylation has the potential to impact on areas beyond the regulation of fetal growth, such as follow up postnatal growth of FGR babies and/or identifying women who may have a preterm delivery (Kwek et al. 2004).
Many fundamental questions remain with regard to the involvement of IGFBP-I phosphorylation in modulation of IGF-I actions. Despite the recent advances in IGFBP cell physiology, we do not yet have a structural model for the interaction of IGFBP-1 phosphorylation sites with IGF-I domains. At the protein kinase level, the identity and function of specific kinases regulating IGFBP-1 phosphorylation in FGR other than CK2 as well as the role of phosphatases remain to be elucidated. Having recognized IGF-I/IGFBP-1 phosphorylation as a likely mechanism underlying FGR, it will be equally important to determine its role in normal fetal growth by finding answers to these questions.
Acknowledgments
This work was supported by grants from the National Institute of Health (HD078313 and HD071306), the Lawson and the Children’s Health Research Institutes.
References
- Abu Shehab M, Inoue S, Han VK, Gupta MB. Site specific phosphorylation of insulin-like growth factor binding protein-1 (IGFBP-1) for evaluating clinical relevancy in fetal growth restriction. J Proteome Res. 2009;8:5325–5335. doi: 10.1021/pr900633x. [DOI] [PubMed] [Google Scholar]
- Abu Shehab M, Khosravi J, Han VK, Shilton BH, Gupta MB. Site-specific IGFBP-1 hyper-phosphorylation in fetal growth restriction: clinical and functional relevance. J Proteome Res. 2010;9:1873–1881. doi: 10.1021/pr900987n. [DOI] [PubMed] [Google Scholar]
- Abu Shehab M, Iosef C, Wildgruber R, Sardana G, Gupta MB. Phosphorylation of IGFBP-1 at discrete sites elicits variable effects on IGF-I receptor autophosphorylation. Endocrinology. 2013;154:1130–1143. doi: 10.1210/en.2012-1962. [DOI] [PubMed] [Google Scholar]
- Abu Shehab M, Damerill I, Shen T, Rosario FJ, Nijland M, Nathanielsz PW, Kamat A, Jansson T, Gupta MB. Liver mTOR controls IGF-I bioavailability by regulation of protein kinase CK2 and IGFBP-1 phosphorylation in fetal growth restriction. Endocrinology. 2014;155:1327–1339. doi: 10.1210/en.2013-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankrapp DP, Jones JI, Clemmons DR. Characterization of insulin-like growth factor binding protein-1 kinases from human hepatoma cells. J Cell Biochem. 1996;60:387–399. doi: 10.1002/(sici)1097-4644(19960301)60:3<387::aid-jcb10>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Antonow-Schlorke I, Schwab M, Cox LA, Li C, Stuchlik K, Witte OW, Nathanielsz PW, McDonald TJ. Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. Proc Natl Acad Sci U S A. 2011;108:3011–3016. doi: 10.1073/pnas.1009838108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averous J, Maurin AC, Bruhat A, Jousse C, Arliguie C, Fafournoux P. Induction of IGFBP-1 expression by amino acid deprivation of HepG2 human hepatoma cells involves both a transcriptional activation and an mRNA stabilization due to its 3′UTR. FEBS Lett. 2005;579:2609–2614. doi: 10.1016/j.febslet.2005.03.077. [DOI] [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49:270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- Baschat AA, Hecher K. Fetal growth restriction due to placental disease. Semin Perinatol. 2004;28:67–80. doi: 10.1053/j.semperi.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab. 2000;278:E967–76. doi: 10.1152/ajpendo.2000.278.6.E967. [DOI] [PubMed] [Google Scholar]
- Ben Lagha N, Seurin D, Le Bouc Y, Binoux M, Berdal A, Menuelle P, Babajko S. Insulin-like growth factor binding protein (IGFBP-1) involvement in intrauterine growth retardation: study on IGFBP-1 overexpressing transgenic mice. Endocrinology. 2006;147:4730–4737. doi: 10.1210/en.2006-0171. [DOI] [PubMed] [Google Scholar]
- Bhatia S, Faessen GH, Carland G, Balise RL, Gargosky SE, Druzin M, El-Sayed Y, Wilson DM, Giudice LC. A longitudinal analysis of maternal serum insulin-like growth factor I (IGF-I) and total and nonphosphorylated IGF-binding protein-1 in human pregnancies complicated by intrauterine growth restriction. J Clin Endocrinol Metab. 2002;87:1864–1870. doi: 10.1210/jcem.87.4.8418. [DOI] [PubMed] [Google Scholar]
- Bohana-Kashtan O, Pinna LA, Fishelson Z. Extracellular phosphorylation of C9 by protein kinase CK2 regulates complement-mediated lysis. Eur J Immunol. 2005;35:1939–1948. doi: 10.1002/eji.200425716. [DOI] [PubMed] [Google Scholar]
- Buckway CK, Wilson EM, Ahlsen M, Bang P, Oh Y, Rosenfeld RG. Mutation of three critical amino acids of the N-terminal domain of IGF-binding protein-3 essential for high affinity IGF binding. J Clin Endocrinol Metab. 2001;86:4943–4950. doi: 10.1210/jcem.86.10.7936. [DOI] [PubMed] [Google Scholar]
- Busby WH, Jr, Klapper DG, Clemmons DR. Purification of a 31,000-dalton insulin-like growth factor binding protein from human amniotic fluid. Isolation of two forms with different biologic actions. J Biol Chem. 1988;263:14203–14210. [PubMed] [Google Scholar]
- Chard T. Insulin-like growth factors and their binding proteins in normal and abnormal human fetal growth. Growth Regul. 1994;4:91–100. [PubMed] [Google Scholar]
- Chelius D, Baldwin MA, Lu X, Spencer EM. Expression, purification and characterization of the structure and disulfide linkages of insulin-like growth factor binding protein-4. J Endocrinol. 2001;168:283–296. doi: 10.1677/joe.0.1680283. [DOI] [PubMed] [Google Scholar]
- Cianfarani S, Germani D, Rossi P, Rossi L, Germani A, Ossicini C, Zuppa A, Argiro G, Holly JM, Branca F. Intrauterine growth retardation: evidence for the activation of the insulin-like growth factor (IGF)-related growth-promoting machinery and the presence of a cation-independent IGF binding protein-3 proteolytic activity by two months of life. Pediatr Res. 1998;44:374–380. doi: 10.1203/00006450-199809000-00018. [DOI] [PubMed] [Google Scholar]
- Clemmons DR. Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev. 1997;8:45–62. doi: 10.1016/s1359-6101(96)00053-6. [DOI] [PubMed] [Google Scholar]
- Conover CA, Lee PD, Riggs BL, Powell DR. Insulin-like growth factor-binding protein-1 expression in cultured human bone cells: regulation by insulin and glucocorticoid. Endocrinology. 1996;137:3295–3301. doi: 10.1210/endo.137.8.8754754. [DOI] [PubMed] [Google Scholar]
- Coverley JA, Baxter RC. Phosphorylation of insulin-like growth factor binding proteins. Mol Cell Endocrinol. 1997;128:1–5. doi: 10.1016/s0303-7207(97)04032-x. [DOI] [PubMed] [Google Scholar]
- Dolcini L, Sala A, Campagnoli M, Labo S, Valli M, Visai L, Minchiotti L, Monaco HL, Galliano M. Identification of the amniotic fluid insulin-like growth factor binding protein-1 phosphorylation sites and propensity to proteolysis of the isoforms. FEBS J. 2009;276:6033–6046. doi: 10.1111/j.1742-4658.2009.07318.x. [DOI] [PubMed] [Google Scholar]
- Eghbali-Fatourechi G, Conover CA, Sieck GC, Gores GJ, Fitzpatrick LA. Secretion of insulin-like growth factor binding protein-1 from individual hepatocytes. Res Commun Mol Pathol Pharmacol. 1994;85:243–259. [PubMed] [Google Scholar]
- Fang Q, Wang YX, Zhou Y. Insulin-like growth factor binding protein 1 and human embryonic development during 6–10 gestational weeks. Chin Med J (Engl) 2004;117:488–491. [PubMed] [Google Scholar]
- Faust M, Jung M, Gunther J, Zimmermann R, Montenarh M. Localization of individual subunits of protein kinase CK2 to the endoplasmic reticulum and to the Golgi apparatus. Mol Cell Biochem. 2001;227:73–80. [PubMed] [Google Scholar]
- Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem. 2010;285:14071–14077. doi: 10.1074/jbc.R109.094003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler D, Albaiges G, Lees C, Jones J, Nicolaides K, Miell J. The role of insulin-like growth factor binding protein-1 phosphoisoforms in pregnancies with impaired placental function identified by doppler ultrasound. Hum Reprod. 1999;14:2881–2885. doi: 10.1093/humrep/14.11.2881. [DOI] [PubMed] [Google Scholar]
- Frost RA, Tseng L. Insulin-like growth factor-binding protein-1 is phosphorylated by cultured human endometrial stromal cells and multiple protein kinases in vitro. J Biol Chem. 1991;266:18082–18088. [PubMed] [Google Scholar]
- Geerts LT, Brand EJ, Theron GB. Routine obstetric ultrasound examinations in South Africa: cost and effect on perinatal outcome–a prospective randomised controlled trial. Br J Obstet Gynaecol. 1996;103:501–507. doi: 10.1111/j.1471-0528.1996.tb09796.x. [DOI] [PubMed] [Google Scholar]
- Gibson JM, Westwood M, Lauszus FF, Klebe JG, Flyvbjerg A, White A. Phosphorylated insulin-like growth factor binding protein 1 is increased in pregnant diabetic subjects. Diabetes. 1999;48:321–326. doi: 10.2337/diabetes.48.2.321. [DOI] [PubMed] [Google Scholar]
- Gibson JM, Aplin JD, White A, Westwood M. Regulation of IGF bioavailability in pregnancy. Mol Hum Reprod. 2001;7:79–87. doi: 10.1093/molehr/7.1.79. [DOI] [PubMed] [Google Scholar]
- Giudice LC. Maternal-fetal conflict–lessons from a transgene. J Clin Invest. 2002;110:307–309. doi: 10.1172/JCI16389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice LC, de Zegher F, Gargosky SE, Dsupin BA, de las Fuentes L, Crystal RA, Hintz RL, Rosenfeld RG. Insulin-like growth factors and their binding proteins in the term and preterm human fetus and neonate with normal and extremes of intrauterine growth. J Clin Endocrinol Metab. 1995;80:1548–1555. doi: 10.1210/jcem.80.5.7538146. [DOI] [PubMed] [Google Scholar]
- Graham ME, Kilby DM, Firth SM, Robinson PJ, Baxter RC. The in vivo phosphorylation and glycosylation of human insulin-like growth factor-binding protein-5. Mol Cell Proteomics. 2007;6:1392–1405. doi: 10.1074/mcp.M700027-MCP200. [DOI] [PubMed] [Google Scholar]
- Han VK, Bassett N, Walton J, Challis JR. The expression of insulin-like growth factor (IGF) and IGF-binding protein (IGFBP) genes in the human placenta and membranes: evidence for IGF-IGFBP interactions at the feto-maternal interface. J Clin Endocrinol Metab. 1996;81:2680–2693. doi: 10.1210/jcem.81.7.8675597. [DOI] [PubMed] [Google Scholar]
- Han VK, Matsell DG, Delhanty PJ, Hill DJ, Shimasaki S, Nygard K. IGF-binding protein mRNAs in the human fetus: tissue and cellular distribution of developmental expression. Horm Res. 1996;45:160–166. doi: 10.1159/000184780. [DOI] [PubMed] [Google Scholar]
- Hills FA, English J, Chard T. Circulating levels of IGF-I and IGF-binding protein-1 throughout pregnancy: relation to birthweight and maternal weight. J Endocrinol. 1996;148:303–309. doi: 10.1677/joe.0.1480303. [DOI] [PubMed] [Google Scholar]
- Holmes R, Montemagno R, Jones J, Preece M, Rodeck C, Soothill P. Fetal and maternal plasma insulin-like growth factors and binding proteins in pregnancies with appropriate or retarded fetal growth. Early Hum Dev. 1997;49:7–17. doi: 10.1016/s0378-3782(97)01867-7. [DOI] [PubMed] [Google Scholar]
- Imai Y, Moralez A, Andag U, Clarke JB, Busby WH, Jr, Clemmons DR. Substitutions for hydrophobic amino acids in the N-terminal domains of IGFBP-3 and −5 markedly reduce IGF-I binding and alter their biologic actions. J Biol Chem. 2000;275:18188–18194. doi: 10.1074/jbc.M000070200. [DOI] [PubMed] [Google Scholar]
- Iwashita M, Sakai K, Kudo Y, Takeda Y. Phosphoisoforms of insulin-like growth factor binding protein-1 in appropriate-for-gestational-age and small-for-gestational-age fetuses. Growth Horm IGF Res. 1998;8:487–493. doi: 10.1016/s1096-6374(98)80302-x. [DOI] [PubMed] [Google Scholar]
- Janeczko M, Maslyk M, Szyszka R, Baier A. Interactions between subunits of protein kinase CK2 and their protein substrates influences its sensitivity to specific inhibitors. Mol Cell Biochem. 2011;356:121–126. doi: 10.1007/s11010-011-0951-x. [DOI] [PubMed] [Google Scholar]
- Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res. 1998;44:532–537. doi: 10.1203/00006450-199810000-00011. [DOI] [PubMed] [Google Scholar]
- Jones JI, D’Ercole AJ, Camacho-Hubner C, Clemmons DR. Phosphorylation of insulin-like growth factor (IGF)-binding protein 1 in cell culture and in vivo: effects on affinity for IGF-I. Proc Natl Acad Sci U S A. 1991;88:7481–7485. doi: 10.1073/pnas.88.17.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JI, Busby WH, Jr, Wright G, Smith CE, Kimack NM, Clemmons DR. Identification of the sites of phosphorylation in insulin-like growth factor binding protein-1. Regulation of its affinity by phosphorylation of serine 101. J Biol Chem. 1993;268(2):1125–31. [PubMed] [Google Scholar]
- Jones JI, Busby WH, Jr, Wright G, Clemmons DR. Human IGFBP-1 is phosphorylated on 3 serine residues: effects of site-directed mutagenesis of the major phosphoserine. Growth Regul. 1993;3:37–40. [PubMed] [Google Scholar]
- Kabir-Salmani M, Shimizu Y, Sakai K, Iwashita M. Posttranslational modifications of decidual IGFBP-1 by steroid hormones in vitro. Mol Hum Reprod. 2005;11:667–671. doi: 10.1093/molehr/gah222. [DOI] [PubMed] [Google Scholar]
- Kajimura S, Aida K, Duan C. Understanding hypoxia-induced gene expression in early development: in vitro and in vivo analysis of hypoxia-inducible factor 1-regulated zebra fish insulin-like growth factor binding protein 1 gene expression. Mol Cell Biol. 2006;26:1142–1155. doi: 10.1128/MCB.26.3.1142-1155.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JH, Darlington GJ. Modulation of the liver specific phenotype in the human hepatoblastoma line Hep G2. In Vitro Cell Dev Biol. 1989;25:217–222. doi: 10.1007/BF02626182. [DOI] [PubMed] [Google Scholar]
- Khosravi J, Krishna RG, Bodani U, Diamandi A, Khaja N, Kalra B, Kumar A. Immunoassay of serine-phosphorylated isoform of insulin-like growth factor (IGF) binding protein (IGFBP)-1. Clin Biochem. 2007;40:86–93. doi: 10.1016/j.clinbiochem.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Koistinen R, Itkonen O, Selenius P, Seppala M. Insulin-like growth factor-binding protein-1 inhibits binding of IGF-I on fetal skin fibroblasts but stimulates their DNA synthesis. Biochem Biophys Res Commun. 1990;173:408–415. doi: 10.1016/s0006-291x(05)81073-3. [DOI] [PubMed] [Google Scholar]
- Koistinen R, Angervo M, Leinonen P, Seppala M. Phosphorylation of insulin-like growth factor-binding protein-1 from different sources. Growth Regul. 1993;3:34–37. [PubMed] [Google Scholar]
- Koistinen R, Angervo M, Leinonen P, Hakala T, Seppala M. Phosphorylation of insulin-like growth factor-binding protein-1 increases in human amniotic fluid and decidua from early to late pregnancy. Clin Chim Acta. 1993;215:189–199. doi: 10.1016/0009-8981(93)90125-n. [DOI] [PubMed] [Google Scholar]
- Koumenis C, Wouters BG. “Translating” tumor hypoxia: unfolded protein response (UPR)-dependent and UPR-independent pathways. Mol Cancer Res. 2006;4:423–436. doi: 10.1158/1541-7786.MCR-06-0150. [DOI] [PubMed] [Google Scholar]
- Kwek K, Khi C, Ting HS, Yeo GS. Evaluation of a bedside test for phosphorylated insulin-like growth factor binding protein-1 in preterm labour. Ann Acad Med Singap. 2004;33:780–783. [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassarre C, Hardouin S, Daffos F, Forestier F, Frankenne F, Binoux M. Serum insulin-like growth factors and insulin-like growth factor binding proteins in the human fetus. Relationships with growth in normal subjects and in subjects with intrauterine growth retardation. Pediatr Res. 1991;29:219–225. doi: 10.1203/00006450-199103000-00001. [DOI] [PubMed] [Google Scholar]
- Lee PD, Conover CA, Powell DR. Regulation and function of insulin-like growth factor-binding protein-1. Proc Soc Exp Biol Med. 1993;204:4–29. doi: 10.3181/00379727-204-43630. [DOI] [PubMed] [Google Scholar]
- Li C, Schlabritz-Loutsevitch NE, Hubbard GB, Han V, Nygard K, Cox LA, McDonald TJ, Nathanielsz PW. Effects of maternal global nutrient restriction on fetal baboon hepatic insulin-like growth factor system genes and gene products. Endocrinology. 2009;150:4634–4642. doi: 10.1210/en.2008-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Shu ZJ, Lee S, Gupta MB, Jansson T, Nathanielsz PW, Kamat A. Effects of maternal nutrient restriction, intrauterine growth restriction, and glucocorticoid exposure on phosphoenolpyruvate carboxykinase-1 expression in fetal baboon hepatocytes in vitro. J Med Primatol. 2013;42:211–219. doi: 10.1111/jmp.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist PG, Molin J. Does antenatal identification of small-for-gestational age fetuses significantly improve their outcome? Ultrasound Obstet Gynecol. 2005;25:258–264. doi: 10.1002/uog.1806. [DOI] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Liu Z, Panousis C, Smyth FE, Murphy R, Wirth V, Cartwright G, Johns TG, Scott AM. Generation of anti-idiotype antibodies for application in clinical immunotherapy laboratory analyses. Hybrid Hybridomics. 2003;22:219–228. doi: 10.1089/153685903322328947. [DOI] [PubMed] [Google Scholar]
- Loukovaara M, Leinonen P, Teramo K, Nurminen E, Andersson S, Rutanen EM. Effect of maternal diabetes on phosphorylation of insulin-like growth factor binding protein-1 in cord serum. Diabet Med. 2005;22:434–439. doi: 10.1111/j.1464-5491.2005.01430.x. [DOI] [PubMed] [Google Scholar]
- Martina NA, Kim E, Chitkara U, Wathen NC, Chard T, Giudice LC. Gestational age-dependent expression of insulin-like growth factor-binding protein-1 (IGFBP-1) phosphoisoforms in human extraembryonic cavities, maternal serum, and decidua suggests decidua as the primary source of IGFBP-1 in these fluids during early pregnancy. J Clin Endocrinol Metab. 1997;82:1894–1898. doi: 10.1210/jcem.82.6.3974. [DOI] [PubMed] [Google Scholar]
- Maruyama M, Matsunaga T, Harada E, Ohmori S. Comparison of basal gene expression and induction of CYP3As in HepG2 and human fetal liver cells. Biol Pharm Bull. 2007;30:2091–2097. doi: 10.1248/bpb.30.2091. [DOI] [PubMed] [Google Scholar]
- Murphy VE, Smith R, Giles WB, Clifton VL. Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocr Rev. 2006;27:141–169. doi: 10.1210/er.2005-0011. [DOI] [PubMed] [Google Scholar]
- Neumann GM, Bach LA. The N-terminal disulfide linkages of human insulin-like growth factor-binding protein-6 (hIGFBP-6) and hIGFBP-1 are different as determined by mass spectrometry. J Biol Chem. 1999;274:14587–14594. doi: 10.1074/jbc.274.21.14587. [DOI] [PubMed] [Google Scholar]
- Nissum M, Abu Shehab M, Sukop U, Khosravi JM, Wildgruber R, Eckerskorn C, Han VK, Gupta MB. Functional and complementary phosphorylation state attributes of human insulin-like growth factor-binding protein-1 (IGFBP-1) isoforms resolved by free flow electrophoresis. Mol Cell Proteomics. 2009;8:1424–1435. doi: 10.1074/mcp.M800571-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson H, Lof M, Brismar K, Forsum E, Sohlstrom A. Maternal serum concentrations of insulin-like growth factor (IGF)-I and IGF binding protein-1 before and during pregnancy in relation to maternal body weight and composition and infant birth weight. Br J Nutr. 2010;104:842–848. doi: 10.1017/S0007114510001224. [DOI] [PubMed] [Google Scholar]
- Olsten ME, Litchfield DW. Order or chaos? An evaluation of the regulation of protein kinase CK2. Biochem Cell Biol. 2004;82:681–693. doi: 10.1139/o04-116. [DOI] [PubMed] [Google Scholar]
- Ong K, Kratzsch J, Kiess W, Costello M, Scott C, Dunger D. Size at birth and cord blood levels of insulin, insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-1 (IGFBP-1), IGFBP-3, and the soluble IGF-II/mannose-6-phosphate receptor in term human infants. The ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. J Clin Endocrinol Metab. 2000;85:4266–4269. doi: 10.1210/jcem.85.11.6998. [DOI] [PubMed] [Google Scholar]
- Orlando-Mathur CE, Bechberger JF, Goldberg GS, Naus CC, Kidder GM, Kennedy TG. Rat endometrial stromal cells express the gap junction genes connexins 26 and 43 and form functional gap junctions during in vitro decidualization. Biol Reprod. 1996;54:905–913. doi: 10.1095/biolreprod54.4.905. [DOI] [PubMed] [Google Scholar]
- Orlowski CC, Ooi GT, Rechler MM. Dexamethasone stimulates transcription of the insulin-like growth factor-binding protein-1 gene in H4-II-E rat hepatoma cells. Mol Endocrinol. 1990;4:1592–1599. doi: 10.1210/mend-4-10-1592. [DOI] [PubMed] [Google Scholar]
- Patel S, Lochhead PA, Rena G, Fumagalli S, Pende M, Kozma SC, Thomas G, Sutherland C. Insulin regulation of insulin-like growth factor-binding protein-1 gene expression is dependent on the mammalian target of rapamycin, but independent of ribosomal S6 kinase activity. J Biol Chem. 2002;277:9889–9895. doi: 10.1074/jbc.M109870200. [DOI] [PubMed] [Google Scholar]
- Popovici RM, Lu M, Bhatia S, Faessen GH, Giaccia AJ, Giudice LC. Hypoxia regulates insulin-like growth factor-binding protein 1 in human fetal hepatocytes in primary culture: suggestive molecular mechanisms for in utero fetal growth restriction caused by uteroplacental insufficiency. J Clin Endocrinol Metab. 2001;86:2653–2659. doi: 10.1210/jcem.86.6.7526. [DOI] [PubMed] [Google Scholar]
- Powell D, Lee PD, DePaolis LA, Morris SL, Suwanichkul A. Dexamethasone stimulates expression of insulin-like growth factor binding protein-1 in HEP G2 human hepatoma cells. Growth Regul. 1993;3:11–13. [PubMed] [Google Scholar]
- Qiu C, Vadachkoria S, Meryman L, Frederick IO, Williams MA. Maternal plasma concentrations of IGF-1, IGFBP-1, and C-peptide in early pregnancy and subsequent risk of gestational diabetes mellitus. Am J Obstet Gynecol. 2005;193:1691–1697. doi: 10.1016/j.ajog.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Sakai K, D’Ercole AJ, Murphy LJ, Clemmons DR. Physiological differences in insulin-like growth factor binding protein-1 (IGFBP-1) phosphorylation in IGFBP-1 transgenic mice. Diabetes. 2001;50:32–38. doi: 10.2337/diabetes.50.1.32. [DOI] [PubMed] [Google Scholar]
- Schedlich LJ, Nilsen T, John AP, Jans DA, Baxter RC. Phosphorylation of insulin-like growth factor binding protein-3 by deoxyribonucleic acid-dependent protein kinase reduces ligand binding and enhances nuclear accumulation. Endocrinology. 2003;144:1984–1993. doi: 10.1210/en.2002-220798. [DOI] [PubMed] [Google Scholar]
- Schlabritz-Loutsevitch NE, Dudley CJ, Gomez JJ, Nevill CH, Smith BK, Jenkins SL, McDonald TJ, Bartlett TQ, Nathanielsz PW, Nijland MJ. Metabolic adjustments to moderate maternal nutrient restriction. Br J Nutr. 2007;98:276–284. doi: 10.1017/S0007114507700727. [DOI] [PubMed] [Google Scholar]
- Seferovic MD, Ali R, Kamei H, Liu S, Khosravi JM, Nazarian S, Han VK, Duan C, Gupta MB. Hypoxia and leucine deprivation induce human insulin-like growth factor binding protein-1 hyperphosphorylation and increase its biological activity. Endocrinology. 2009;150:220–231. doi: 10.1210/en.2008-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifakis S, Akolekar R, Kappou D, Mantas N, Nicolaides KH. Maternal serum placental growth hormone at 11–13 weeks’ gestation in pregnancies delivering small for gestational age neonates. J Matern Fetal Neonatal Med. 2012;25:1796–1799. doi: 10.3109/14767058.2012.663834. [DOI] [PubMed] [Google Scholar]
- Sitar T, Popowicz GM, Siwanowicz I, Huber R, Holak TA. Structural basis for the inhibition of insulin-like growth factors by insulin-like growth factor-binding proteins. Proc Natl Acad Sci U S A. 2006;103:13028–13033. doi: 10.1073/pnas.0605652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwanowicz I, Popowicz GM, Wisniewska M, Huber R, Kuenkele KP, Lang K, Engh RA, Holak TA. Structural basis for the regulation of insulin-like growth factors by IGF binding proteins. Structure. 2005;13:155–167. doi: 10.1016/j.str.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Solomon AL, Siddals KW, Baker PN, Gibson JM, Aplin JD, Westwood M. Placental alkaline phosphatase de-phosphorylates insulin-like growth factor (IGF)-binding protein-1. Placenta. 2014;35:520–522. doi: 10.1016/j.placenta.2014.04.014. [DOI] [PubMed] [Google Scholar]
- St-Denis NA, Litchfield DW. Protein kinase CK2 in health and disease: From birth to death: the role of protein kinase CK2 in the regulation of cell proliferation and survival. Cell Mol Life Sci. 2009;66:1817–1829. doi: 10.1007/s00018-009-9150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street ME, Seghini P, Fieni S, Ziveri MA, Volta C, Martorana D, Viani I, Gramellini D, Bernasconi S. Changes in interleukin-6 and IGF system and their relationships in placenta and cord blood in newborns with fetal growth restriction compared with controls. Eur J Endocrinol. 2006;155:567–574. doi: 10.1530/eje.1.02251. [DOI] [PubMed] [Google Scholar]
- Tapanainen PJ, Bang P, Wilson K, Unterman TG, Vreman HJ, Rosenfeld RG. Maternal hypoxia as a model for intrauterine growth retardation: effects on insulin-like growth factors and their binding proteins. Pediatr Res. 1994;36:152–158. doi: 10.1203/00006450-199408000-00004. [DOI] [PubMed] [Google Scholar]
- Tapanainen PJ, Bang P, Muller HL, Wilson K, Rosenfeld RG. Hypoxia-induced changes in insulin-like growth factors and their binding proteins in pregnant rats. Horm Res. 1997;48:227–234. doi: 10.1159/000185520. [DOI] [PubMed] [Google Scholar]
- Tazuke SI, Mazure NM, Sugawara J, Carland G, Faessen GH, Suen LF, Irwin JC, Powell DR, Giaccia AJ, Giudice LC. Hypoxia stimulates insulin-like growth factor binding protein 1 (IGFBP-1) gene expression in HepG2 cells: a possible model for IGFBP-1 expression in fetal hypoxia. Proc Natl Acad Sci U S A. 1998;95:10188–10193. doi: 10.1073/pnas.95.17.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temporini C, Dolcini L, Abee A, Calleri E, Galliano M, Caccialanza G, Massolini G. Development of an integrated chromatographic system for on-line digestion and characterization of phosphorylated proteins. J Chromatogr A. 2008;1183:65–75. doi: 10.1016/j.chroma.2007.12.091. [DOI] [PubMed] [Google Scholar]
- Tisi DK, Liu XJ, Wykes LJ, Skinner CD, Koski KG. Insulin-like growth factor II and binding proteins 1 and 3 from second trimester human amniotic fluid are associated with infant birth weight. J Nutr. 2005;135:1667–1672. doi: 10.1093/jn/135.7.1667. [DOI] [PubMed] [Google Scholar]
- Wang HS, Lim J, English J, Irvine L, Chard T. The concentration of insulin-like growth factor-I and insulin-like growth factor-binding protein-1 in human umbilical cord serum at delivery: relation to fetal weight. J Endocrinol. 1991;129:459–464. doi: 10.1677/joe.0.1290459. [DOI] [PubMed] [Google Scholar]
- Watson CS, Bialek P, Anzo M, Khosravi J, Yee SP, Han VK. Elevated circulating insulin-like growth factor binding protein-1 is sufficient to cause fetal growth restriction. Endocrinology. 2006;147:1175–1186. doi: 10.1210/en.2005-0606. [DOI] [PubMed] [Google Scholar]
- Weber MM, Spottl G, Gossl C, Engelhardt D. Characterization of human insulin-like growth factor-binding proteins by two-dimensional polyacrylamide gel electrophoresis and Western ligand blot analysis. J Clin Endocrinol Metab. 1999;84:1679–1684. doi: 10.1210/jcem.84.5.5686. [DOI] [PubMed] [Google Scholar]
- Westwood M. Role of insulin-like growth factor binding protein 1 in human pregnancy. Rev Reprod. 1999;4:160–167. doi: 10.1530/ror.0.0040160. [DOI] [PubMed] [Google Scholar]
- Westwood M, Gibson JM, Davies AJ, Young RJ, White A. The phosphorylation pattern of insulin-like growth factor-binding protein-1 in normal plasma is different from that in amniotic fluid and changes during pregnancy. J Clin Endocrinol Metab. 1994;79:1735–1741. doi: 10.1210/jcem.79.6.7527409. [DOI] [PubMed] [Google Scholar]
- Westwood M, Gibson JM, Williams AC, Clayton PE, Hamberg O, Flyvbjerg A, White A. Hormonal regulation of circulating insulin-like growth factor-binding protein-1 phosphorylation status. J Clin Endocrinol Metab. 1995;80:3520–3527. doi: 10.1210/jcem.80.12.8530593. [DOI] [PubMed] [Google Scholar]
- Westwood M, Gibson JM, White A. Purification and characterization of the insulin-like growth factor-binding protein-1 phosphoform found in normal plasma. Endocrinology. 1997;138:1130–1136. doi: 10.1210/endo.138.3.5020. [DOI] [PubMed] [Google Scholar]
- Wilkening S, Stahl F, Bader A. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab Dispos. 2003;31:1035–1042. doi: 10.1124/dmd.31.8.1035. [DOI] [PubMed] [Google Scholar]
- Woodall SM, Breier BH, Johnston BM, Gluckman PD. A model of intrauterine growth retardation caused by chronic maternal undernutrition in the rat: effects on the somatotrophic axis and postnatal growth. J Endocrinol. 1996;150:231–242. doi: 10.1677/joe.0.1500231. [DOI] [PubMed] [Google Scholar]
- Yan X, Forbes BE, McNeil KA, Baxter RC, Firth SM. Role of N- and C-terminal residues of insulin-like growth factor (IGF)-binding protein-3 in regulating IGF complex formation and receptor activation. J Biol Chem. 2004;279:53232–53240. doi: 10.1074/jbc.M409345200. [DOI] [PubMed] [Google Scholar]
- Yu J, Iwashita M, Kudo Y, Takeda Y. Phosphorylated insulin-like growth factor (IGF)-binding protein-1 (IGFBP-1) inhibits while non-phosphorylated IGFBP-1 stimulates IGF-I-induced amino acid uptake by cultured trophoblast cells. Growth Horm IGF Res. 1998;8:65–70. doi: 10.1016/s1096-6374(98)80323-7. [DOI] [PubMed] [Google Scholar]