Abstract
IGFBP-6 is an O-linked glycoprotein that preferentially binds IGF-II over IGF-I. It is a relatively selective inhibitor of IGF-II actions including proliferation, survival and differentiation of a wide range of cells. IGFBP-6 has recently been shown to have a number of IGF-independent actions, including promotion of apoptosis in some cells and inhibition of angiogenesis. IGFBP-6 also induces migration of tumour cells including rhabdomyosarcomas by an IGF-independent mechanism. This chemotactic effect is mediated by MAP kinases. IGFBP-6 binds to prohibitin-2 on the cell surface and the latter is required for IGFBP-6-induced migration by a mechanism that is independent of MAP kinases. IGFBP-6 may enter the nucleus and modulate cell survival and differentiation. IGFBP-6 expression is decreased in a number of cancer cells and it has been postulated to act as a tumour suppressor. IGFBP-6 expression is increased in a smaller number of cancers, which may reflect a compensatory mechanism to control IGF-II actions or IGF-independent actions. The relative balance of IGF-dependent and IGF-independent actions of IGFBP-6 in vivo together with the related question regarding the roles of IGFBP-6 binding to IGF and non-IGF ligands are keys to understanding the physiological role of this protein.
Keywords: Insulin like growth factor, Insulin like growth factor binding protein-6, Migration, Proliferation, Survival, Structure
Introduction
The family of six high affinity insulin-like growth factor binding proteins (IGFBPs) is a major regulator of IGF actions. In addition, most IGFBPs have been reported to have IGF-independent actions. IGFBP-6, the focus of this article, is distinctive for its ~50-fold binding preference for IGF-II over IGF-I and relatively specific inhibition of IGF-II actions (reviewed in (Bach 1999, 2005)). Recent studies indicate that IGFBP-6 also inhibits angiogenesis and promotes cell migration by IGF-independent mechanisms, and enters the nucleus where it modulates differentiation and survival (Bach, et al. 2013).
Structure
IGFBPs 1–6 share a three-domain structure (Bach, et al. 2005). The N-domain contains a highly conserved high-affinity IGF binding subdomain that has two disulphide linkages stabilising a globular structure containing a three-stranded anti-parallel β-sheet (Kalus, et al. 1998). The N-terminal subdomains of IGFBPs 1–5 contain a conserved GCGCC motif and a ladder-like structure stabilised by four disulphide bonds (Sitar, et al. 2006). IGFBP-6 differs from other IGFBPs as it lacks this cysteine-rich motif and only has three disulphide bonds (Neumann and Bach 1999). Further, a peptide based on this subdomain is predominantly extended with minimal secondary structure (Chandrashekaran, et al. 2007).
The C-domains of IGFBPs 1–6 share sequence homology and a common structure. Each contains three homologous disulphide linkages, a conserved CWCV sequence and a thyroglobulin type 1 fold comprising an α -helix followed by a loop, a three-stranded antiparallel β-sheet incorporating a second loop, and finally a disulphide-bonded flexible third loop (Bach et al. 2005; Headey, et al. 2004a). The C-domain binds IGFs through a largely hydrophobic surface involving the α-helix, the first β-strand, and the first and second loops. Many IGF-independent actions of IGFBPs are mediated by interaction of the C-domain with other proteins or glycosaminoglycans (Bach et al. 2005).
In contrast to their N- and C-domains, the linker domains of the six IGFBPs have no sequence homology. They vary in length and are sites of post-translational modifications including glycosylation, phosphorylation and proteolysis. They do not directly bind IGFs but may contribute by optimising orientation of the N- and C-domains around the IGF molecule. IGFBP-6 is O-glycosylated within this domain, which has no effect on IGF binding but modulates its stability and localisation (Bach, et al. 1992; Marinaro, et al. 2000; Neumann, et al. 1998; Shalamanova, et al. 2008). IGFBP-6 may also be phosphorylated and sulphated although the effects of these modifications on its properties are unknown (Shalamanova et al. 2008).
Specific limited proteolysis of individual IGFBPs is a potential mechanism by which complexed IGFs are released for binding to IGF receptors. Indeed, there are a number of IGFBP-6 proteases, including a cathepsin-D-like acid protease (Marinaro, et al. 1999), a neutral serine protease (Shalamanova, et al. 2001), and MMP-7 (Nakamura, et al. 2005). IGFBP-6 is also a substrate for a number of other MMPs, including MMP-2, which may modulate its anti-angiogenic properties (Dean, et al. 2007), and MMP-9 and MMP-12, which may regulate its capacity to inhibit myelination (Larsen, et al. 2006).
IGFBP-6 is highly conserved across species, and human IGFBP-6 shares 70–85 % sequence identity with rat, mouse, bovine and pig IGFBP-6 (Fig. 1). In zebrafish, the duplicated IGFBP6 genes have distinct temporal and spatial expression profiles (Wang, et al. 2009). They are 52 % identical to each other and 35–37 % identical with human IGFBP-6. Both gene products retain important N-domain residues that are essential for IGF binding, as well as C-domains containing six conserved cysteines and the CWCV motif, together with most of the amino acids involved in IGF binding. This suggests that zebrafish IGFBPs bind IGFs with high affinity, although this has not been empirically tested. IGFBP-6 genes have been detected in several other fish species but not birds or opossum (Daza, et al. 2011).
Fig. 1.
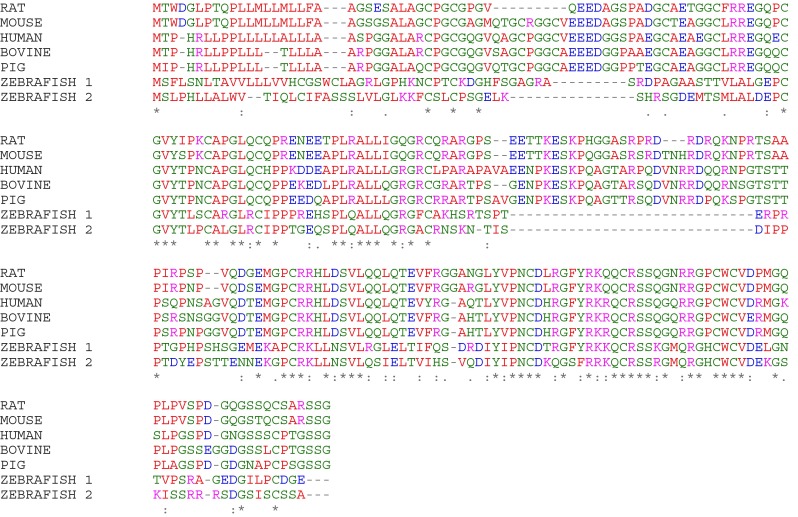
Sequence alignment of IGFBP-6 from rat, mouse, human, cow, pig and zebrafish (2 gene products). Small residues are shown in red, acidic residues in blue, basic residues in magenta, and others in green. * fully conserved residue; : strongly conserved residue with similar properties; . weakly conserved residue. Alignment was performed using Clustal Omega v 1.2.1, EMBL-EBI (www.ebi.ac.uk) (Sievers, et al. 2011)
Regulation
Serum levels of IGFBP-6 increase gradually with age and are higher in men than in women (Baxter and Saunders 1992; Van Doorn, et al. 1999), but there are conflicting studies of the direct effects of sex steroids on IGFBP-6 expression in different tissues (Bach et al. 2013). Serum levels are decreased during pregnancy, and increased in renal failure and in patients with the rare condition of non-islet cell tumour hypoglycaemia. In different cells, IGFBP-6 is regulated by factors including cAMP, IGFs, retinoic acid, vitamin D, p53 and glucocorticoids in vitro (Bach et al. 2013).
In desmoid tumours, IGFBP6 transcription was significantly downregulated by β–catenin, an important component of the Wnt signaling pathway (Denys, et al. 2004). In cells from these tumours, transforming growth factor (TGF)-β increased β-catenin and both of these independently inhibited IGFBP6 promoter activity (Amini Nik, et al. 2007). In contrast, TGF-β increased IGFBP6 expression in fibroblasts (Ong, et al. 2009), suggesting that its effect is cell-type specific.
The hedgehog (Hh) pathway is critical for development, and stimulates both IGF-II and IGFBP-6 expression (Ingram, et al. 2002; Yoon, et al. 2002). Specifically, sonic hedgehog increased IGFBP-6 expression during fetal prostate development (Lipinski, et al. 2005). IGFBP-6 levels were higher in prostate cancer-associated fibroblasts than in normal prostate fibroblasts, and levels were regulated by Hh signalling (Wilkinson, et al. 2013). In pancreatic cancer cells, inhibition of Hh signalling decreased IGFBP-6 and Bcl2 expression and increased apoptosis, and it was suggested that IGFBP-6 may thereby modulate survival of these cells (Xu, et al. 2009).
Functions of IGFBP-6
IGF-II inhibition
The principal function of IGFBP-6 is inhibiting IGF-II actions, which has been demonstrated in many cell lines (Bach 1999, 2005). IGFBP-6 inhibited IGF-II-induced cell proliferation, differentiation, migration and survival, but, in contrast, has little or no effect on IGF-I actions, at least in part due to its lower binding affinity.
IGFBP-6 inhibits growth of IGF-II-dependent cancer cells in vivo. IGFBP-6 inhibited the growth of neuroblastoma cell xenografts in vivo (Grellier, et al. 1998). IGFBP-6 also inhibited anchorage-dependent and -independent proliferation and survival of rhabdomyosarcoma cells in vitro, and IGF-II addition partially overcame these effects (Gallicchio, et al. 2001). Xenografts of rhabdomyosarcoma clones overexpressing IGFBP-6 were ~80 % smaller than control clones after 18 days (Gallicchio et al. 2001), and treatment with CCI-779, a rapamycin analogue, additively delayed tumour formation in IGFBP-6-overexpressing xenografts (Gallicchio, et al. 2003).
IGF-independent actions
Although a number of studies have shown that IGFBP-6 decreases cell proliferation and survival by inhibiting IGF-II actions (Bach 1999, 2005), there is increasing evidence that IGFBPs, including IGFBP-6, also have IGF-independent actions. A recent study suggested that IGFBP-6 inhibited fibroblast proliferation by both IGF-dependent and –independent mechanisms (Raykha, et al. 2013). Additionally, IGFBP-6-induced apoptosis appeared to be IGF-dependent (Hale, et al. 2000) and IGF-independent (Iosef, et al. 2008; Sueoka, et al. 2000b) in different cell lines.
Intracellular actions
A series of studies in osteoblasts suggested that IGFBP-6 has intracrine actions by interacting with LIM mineralization protein-1 (LMP-1), a protein that shuttles between the cytoskeleton and nucleus (Strohbach, et al. 2008; Yan, et al. 2001). Intracrine IGFBP-6 inhibited differentiation of these cells by binding LMP-1 and modulating shuttling, and coexpression of LMP-1 prevented inhibition of the promoter for type I procollagen, a differentiation marker, by IGFBP-6.
Nuclear actions
IGFBPs may enter the nucleus and modulate cellular actions by interacting with transcription factors. IGFBP-6 inhibited vitamin D and liothyronine-mediated differentiation of osteoblasts by interacting with their cognate nuclear receptors (Cui, et al. 2011; Qiu, et al. 2012). IGFBP-6 entered the nucleus in rhabdomyosarcoma cells via a C-domain nuclear localisation sequence that interacted with importin-α, and deletion of the sequence prevented IGFBP-6-mediated apoptosis (Iosef et al. 2008). Nuclear localisation of IGFBP-6 was not altered by the presence of IGF-II (Iosef et al. 2008). IGFBP-6 interacted with and regulated the availability of Ku80, a DNA repair and stability protein, and it was postulated that IGFBP-6 may modulate cell survival by perturbating DNA repair (Iosef, et al. 2010). IGFBP-6 acted as a tumour suppressor in nasopharyngeal cancer cells via its role as a transcription factor that directly bound the EGR-1 promoter and modulated its expression (Kuo, et al. 2010).
Senescence
Senescent cells remain metabolically active but are unresponsive to growth stimuli due to cell cycle arrest. Senescence is associated with ageing and atherosclerosis, and may be induced by processes including inflammation and oxidative stress. In contrast, it has been also linked to tumour suppression. The IGF system, including IGFBP-6, has been implicated in this process. Senescence induced by hydrogen peroxide or in physiological oxygen conditions increased IGFBP-6 levels in fibroblasts (Coppe, et al. 2010; Xie, et al. 2005) and doxorubicin-induced senescence in colon cancer cells was also associated with increased IGFBP-6 levels (Chang, et al. 2002). Additionally, serum IGFBP-6 levels were higher in ageing mice (Xie et al. 2005) and humans (Micutkova, et al. 2011). One study suggested that IGFBP-6 inhibits senescence, but it also showed that IGFBP-6 increased cell proliferation and survival (Micutkova et al. 2011), which contradicts essentially all other studies of this protein. Additionally. other IGFBPs (IGFBP-3 and IGFBP-5) promote senescence, so the role of IGFBP-6 clearly requires further study.
Inhibition of angiogenesis
Angiogenesis, the formation of new capillaries from existing blood vessels, is a physiological process that is important for tissue repair following injury. It also plays key roles in cancer, both by being essential for ongoing solid tumour growth (Potente, et al. 2011; Roodink and Leenders 2010) and by enhancing metastasis through ingress of cancer cells into abnormal, tumour-related blood vessels (Valastyan and Weinberg 2011). Hypoxia is a major regulator of angiogenesis via hypoxia-inducible transcriptional factors (HIFs). Vascular endothelial growth factor (VEGF) pathways are the major mediators of angiogenesis, but IGFs also play a role (Carmeliet and Jain 2000) by stimulating HIF-1α expression (Hoeben, et al. 2004), and inducing VEGF synthesis (Hoeben et al. 2004; Stearns, et al. 2005; Warren, et al. 1996) via HIF-1-dependent and -independent pathways (Slomiany and Rosenzweig 2006). The human IGFBP6 promoter contains multiple hypoxia response elements and HIF-1 ancillary sequences, and IGFBP-6 expression was upregulated by hypoxia in endothelial cells via HIF-1α (Zhang, et al. 2012).
IGFBP-6 overexpression inhibited angiogenesis in rhabdomyosarcoma xenografts and zebrafish embryos, and prolonged hypoxia increased IGFBP-6 expression via HIF-1α (Zhang et al. 2012). These findings suggest that IGFBP-6 contributes to a negative feedback mechanism limiting hypoxia-induced angiogenesis (Messmer-Blust, et al. 2009). IGFBP-6-induced inhibition of tube formation by human umbilical vein endothelial cells, an in vitro model of angiogenesis, was IGF-independent (Zhang et al. 2012). SEMA3B is a tumour suppressor that has VEGF-dependent and independent actions, and it increased IGFBP-6 expression in lung cancer cells (Koyama, et al. 2008). IGFBP-6 mediated the antiproliferative effect of SEMA3B, but its effect on angiogenesis was not studied (Koyama et al. 2008). Vasohibin-2, an angiogenic factor that promoted breast cancer cell proliferation, also increased IGFBP-6 expression, but the functional role of IGFBP-6 was not explored (Min et al. 2014).
Cell migration
IGFBP-6 promoted migration of two rhabdomyosarcoma cell lines and a colon cancer cell line by IGF-independent mechanisms (Fu, et al. 2007, 2010). It was further shown that rhabdomyosarcoma migration was due to chemotaxis rather than chemokinesis (Fu et al. 2010).
MAP kinase pathways are implicated in cell migration and invasion (Huang, et al. 2004). IGFBP-6 increased p38 MAP kinase phosphorylation in one rhabdomyosarcoma cell line, and inhibition of p38 MAP kinase prevented IGFBP-6-induced migration (Fu et al. 2007). In contrast, IGFBP-6 increased phosphorylation of ERK and JNK1 but not p38 MAP kinase in another rhabdomyosarcoma cell line (Fu et al. 2010). In both cell lines, cross-talk between the MAP kinase pathways was involved in the migratory response (Fu et al. 2007, 2010). Interestingly, JNK activation increased IGFBP-6 expression in oral cancer cells (Cacalano, et al. 2008), suggesting the possibility of a positive feedback loop between IGFBP-6 and JNK activation in these cells. We recently found that IGFBP-6 had opposing effects on migration of two ovarian cancer cell lines (Yang and Bach 2015). Further, this difference was observed despite similar MAP kinase pathway activation in both cell lines, indicating that other pathways are also involved in the migratory response.
Prohibitin-2
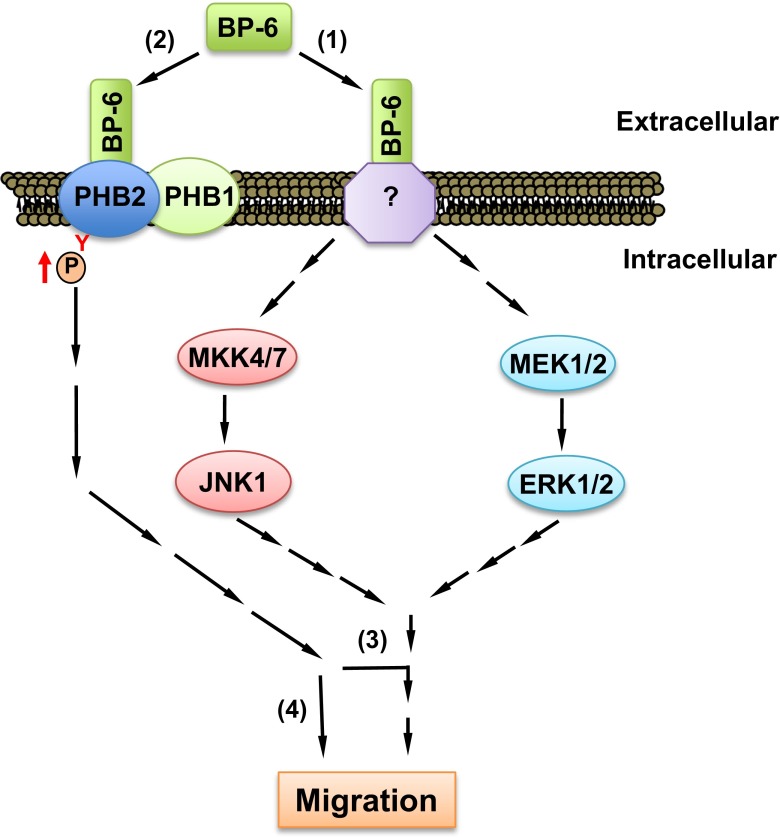
One of the major recent challenges in understanding IGFBP biology has been identification of cell surface receptors that mediate their IGF-independent actions. A proteomic approach was used to identify rhabdomyosarcoma cell membrane proteins that may mediate the promigratory effect of IGFBP-6 (Fu et al. 2013). Prohibitin-2 (PHB2), a single-span membrane protein, was found to bind IGFBP-6 by multiple techniques. The binding affinity for this interaction was in the nanomolar range via the C-domain but not the N-domain of IGFBP-6. IGFBP-6 increased tyrosine phosphorylation of cell membrane PHB2, although the effect was indirect as IGFBP-6 has no kinase activity. PHB2 knockdown completely abolished IGFBP-6-mediated rhabdomyosarcoma cell migration, but it had no effect on MAP kinase activation, suggesting that PHB2 either acts as a downstream effector of these pathways or acts via independent pathways (Fig. 2). These results indicate that PHB2 plays a key role in IGF-independent inhibition of cancer cell migration by IGFBP-6.
Fig. 2.
Potential roles of prohibitin-2 (PHB2) in IGFBP-6-induced rhabdomyosarcoma cell migration. IGFBP-6 binds to (1) an unknown cell surface receptor, leading to MAP kinase pathway activation. (2) IGFBP-6 also binds to PHB2 on the cell surface and indirectly increases its tyrosine phosphorylation. PHB2 is essential for IGFBP-6-induced migration either by (3) acting downstream of MAP kinases, or (4) regulating migration independently of MAP kinase activation. This figure and research were originally published in J Biol Chem. Fu P, Yang Z, Bach LA. Insulin-like growth factor binding protein-6 (IGFBP-6)-induced rhabdomyosarcoma cell migration is modulated by binding to prohibitin-2. J Biol Chem. 2013; 288: 29890–900. © the American Society for Biochemistry and Molecular Biology
The biology of prohibitins (PHB1 and −2) suggest a number of ways in which they might mediate actions of IGFBP-6. They are highly conserved eukaryotic proteins that contain a SPFH or PHB domain that is important for membrane association (Mishra et al. 2006). Although most studies emphasise their role in mitochondria, prohibitins are also found on plasma membranes and in nuclei. PHB1 and −2 form high molecular weight, functionally active ring complexes in mitochondria and plasma membranes. PHBs modulate mitochondrial function by acting as chaperones for newly synthesised enzymes and/or acting as scaffolding proteins (Osman et al. 2009). Nuclear prohibitins modulate transcription, but the functions of plasma membrane prohibitin complexes are not well understood. PHB1 and −2 are expressed in a range of cancers, including colon cancer, and are found on plasma membranes of colon cancer cells in vitro (Mengwasser et al. 2004). It has been suggested that prohibitins also act as chaperones at the cell surface (Sievers, et al. 2010).
Information about the specific functions of PHB2, which is also known as ‘repressor of estrogen receptor activity’ (REA) or ‘B-cell receptor-associated protein’ (BAP37), is limited. PHB2 knockout resulted in early embryonic lethality (Park et al. 2005), while PHB2 deletion impaired mouse embryonic fibroblast proliferation and increased their susceptibility to apoptotic stimuli (Merkwirth et al. 2008). In breast cancer cells, nuclear PHB2 repressed the activity of estrogen receptor-α (Kim et al. 2009).
Although prohibitins were initially identified as antiproliferative genes, their actions are cell- and context-specific. Thus, PHB1 or −2 deficiency shortened the lifespan of wild-type C. elegans, but lengthened it in diapause mutants (involving insulin/IGF and TGF-β pathways) or with dietary restriction (Artal-Sanz and Tavernarakis 2009). Further, knockdown of PHB1 or −2 decreased HeLa cancer cell proliferation and adhesion to extracellular matrix proteins (Sievers et al. 2010).
As mentioned above, IGFBP-6 induced phosphorylation of PHB2 (Fu et al. 2013). Phosphorylation of prohibitins modulated their effects on cell signalling and function (Mishra et al. 2010). Insulin phosphorylated PHB1 at Tyr114, which is conserved in PHB2, resulting in SHP1 recruitment and decreased AKT phosphorylation (Ande, et al. 2009).
Plasma membrane prohibitins have been implicated in PI3 kinase/AKT and Ras/MAPK/ERK signaling (Chowdhury, et al. 2014; Mishra et al. 2010). For example, PHB1 bound c-Raf and was required for its membrane targeting and activation, leading to cell migration (Rajalingam, et al. 2005). PHB2 but not PHB1 bound AKT2, and it reciprocally regulated AKT2 levels in myoblasts (Heron-Milhavet, et al. 2008). PHBs therefore have a broad range of biological roles and the consequences of the IGFBP-6/PHB2 interaction are worthy of further study.
IGFBP-6 and cancer
The role of IGFBP-6 has been investigated in many cancers, which may be especially relevant since IGF-II is frequently an autocrine cancer growth factor (Bach et al. 2013). In most studies, IGFBP-6 expression was lower in malignant cells than in normal cells, which is consistent with the idea that it acts as an inhibitor of tumorigenic processes, including those driven by excess IGF-II activity. However, a smaller number of studies have shown the opposite, which may represents a compensatory response to increased IGF-II activity or may reflect IGF-independent actions of IGFBP-6. A number of brief examples in diifferent cancers follow.
Adrenocortical cancer
A number of recent studies have shown increased expression of IGFBP-6 in adrenocortical tumours. IGFBP6 was one of several IGF system genes that was higher in human adrenocortical carcinomas than in adenomas (Velazquez-Fernandez, et al. 2005). Increased IGFBP6 expression and gene hypomethylation were also seen in adrenocortical neoplasms induced by gonadectomy in mice (Schillebeeckx, et al. 2014).
Breast cancer
IGFBP-6 was expressed in estrogen-receptor negative and positive breast carcinoma cell lines (Figueroa, et al. 1993; Martin, et al. 1995; Sheikh, et al. 1993) and was also expressed at low levels in breast cancer specimens (Figueroa et al. 1993). The IGF system is implicated in the development of resistance to HER2 inhibitors, which is an important clinical problem in breast cancer. IGFBP-6 expression was higher in resistant breast cancer cells than in sensitive cells, but its functional consequences were not studied (Oliveras-Ferraros, et al. 2010).
Colon cancer
IGF2 was the most highly overexpressed gene in colorectal tumours (Zhang, et al. 1997). IGFBP-6 may be anti-tumorigenic for colon cancer as evidenced by lower levels in a metastatic than a non-metastatic cell line (Futschik, et al. 2002), and in a multidrug-resistant than in a sensitive colon cancer cell line (Fan, et al. 2004). Exogenous IGFBP-6 inhibited IGF-II-induced proliferation and adhesion of colon cancer cells (Leng, et al. 2001), and IGFBP-6 expression was increased in colon cancer cells by (n-3) fatty acids and retinoic acid, both of which inhibit proliferation (Kim, et al. 2000, 2001). Coculture of colon cancer cells with normal colonic epithelial cells decreased IGFBP-6 secretion, and IGFBP-6 expression was dramatically decreased in normal colonic epithelial cells undergoing epithelial-mesenchymal transdifferentiation, a process that is implicated in tumorigenesis (Zeng, et al. 2013).
Gastric cancer
Epigenetic silencing may contribute to cancer development. The IGFBP6 promoter was abnormally methylated in a quarter of 152 gastric cancers, and hypermethylation was associated with decreased IGFBP-6 expression in cancer cell lines (Jee, et al. 2009). Conversely, an inhibitor of DNA methylation increased IGFBP-6 expression in immortalized fibroblasts (Kulaeva, et al. 2003).
Head and neck cancer
IGFBP-6 expression was lower in metastatic head and neck squamous cell carcinomas than in primary disease (Liu, et al. 2008). IGFBP-6 was down-regulated in nasopharyngeal cancer cells and it acted as a tumour suppressor by its effects on transcription factor EGR-1 expression (Kuo et al. 2010). In oral carcinoma cells, IGFBP-6 decreased migration but not proliferation (Liu et al. 2008), whereas apoptosis and IGFBP-6 expression were both increased by JNK activation and NF-κB inhibition (Cacalano et al. 2008).
Lung cancer
IGFBP-6 appears to have an inhibitory role in lung cancer. Although commonly expressed in human lung cancers (Wegmann, et al. 1993), its expression was lower in cancers than in normal lung in mice (Yao, et al. 2002). IGFBP-6 inhibited proliferation of human bronchial epithelial cells (Sueoka, et al. 2000a) and increased apoptosis of non-small cell lung cancer cells (Sueoka et al. 2000b). In lung cancer cells, IGFBP-6 levels were increased by the tumour suppressor SEMA3B, which may mediate its antiproliferative effect (Koyama et al. 2008). IGFBP-6 levels were also increased by the tumour suppressor p53 (Kannan, et al. 2001).
Neuroblastoma
Neuroblastoma is an IGF-II-dependent tumour that is commonest in children (Toretsky and Helman 1996). N-myc oncogene overexpression, which is associated with poor prognosis, decreased IGFBP-6 levels (Chambery, et al. 1999), whereas FGF-2, which stimulates neuronal differentiation, increased IGFBP-6 expression (Russo, et al. 2004). IGFBP-6 has functional effects in neuroblastoma cells. Constitutive IGFBP-6 overexpression inhibited neuroblastoma xenograft growth and promoted apoptosis in vivo (Grellier, et al. 2002; Grellier et al. 1998). IGFBP-6 infusion also delayed neuroblastoma xenograft growth in vivo, probably by inhibiting IGF-II actions (Seurin, et al. 2002).
Ovarian cancer
IGF-II mRNA levels were >300-fold higher in cancer tissues than in normal ovary, and IGF-II levels predicted poor survival in advanced stage ovarian cancer (Sayer, et al. 2005). IGFBP-6 was detected in 38 of 41 ovarian cancers (Walker, et al. 2007), but a microarray study showed that IGFBP-6 mRNA levels were lower in ovarian cancer tissue than in non-cancerous tissue (Bahrani-Mostafavi, et al. 2008). Our recent finding that IGFBP-6 had opposing effects on migration of two ovarian cancer cell lines suggests heterogeneous responsiveness in this cancer (Yang and Bach 2015).
Prostate cancer
IGFBP-6 and other IGF system components were upregulated during prostate epithelial cell differentiation (Massoner et al. 2011), whereas expression of IGFBP-6 and other IGFBPs was progressively lower in more tumorigenic transformed human prostate epithelial cells (Plymate, et al. 1996). Vitamin D, which inhibits proliferation of prostate carcinoma cells, increased IGFBP-6 levels (Drivdahl et al. 1995). In androgen-independent prostate cancer cells, IGFBP-6 may mediate the antiproliferative effects of diethylstilbestrol (Koike et al. 2005).
Rhabdomyosarcoma
IGF-II is an autocrine growth factor in rhabdomyosarcoma, which is a tumour of childhood and adolescence (Foulstone et al. 2005; Toretsky and Helman 1996). In animal models, IGF-II was required for development and malignant behaviour of rhabdomyosarcoma (Hahn, et al. 2000) and it increased angiogenesis (Wang et al. 1998). IGFBP-6 inhibited anchorage-dependent and –independent proliferation and survival of rhabdomyosarcoma cells in vitro; these effects appeared to be at least partly IGF-dependent (Fu et al. 2007; Gallicchio et al. 2001). Intranuclear actions were also implicated in apoptosis induced by IGFBP-6 (Iosef et al. 2008, 2010). As described above, IGFBP-6 overexpression inhibited rhabdomyosarcoma xenograft growth and angiogenesis in vivo (Fu et al. 2013; Gallicchio et al. 2001; Zhang et al. 2012), and IGFBP-6 promotes rhabdomyosarcoma cell migration via pathways dependent on PHB2 and MAP kinase signalling (Fu et al. 2007, 2010, 2013).
Clinical relevance of serum IGFBP-6 levels
A number of studies have investigated circulating IGFBP-6 levels in various diseases. As mentioned above, a small study showed that serum IGFBP-6 levels were lower in women with breast cancer than in those with benign breast disease (Kaulsay et al. 1999). However, two studies of serum IGFBP-6 levels in women with ovarian cancer had opposing results (Gunawardana et al. 2009; Lin et al. 2009). Higher plasma IGFBP-6 levels were associated with good prognosis in patients with malignant glioma (Lin et al. 2013), whereas serum IGFBP-6 levels were lower in patients with prostate cancer than those with benign prostatic hypertrophy (Xu et al. 2014). Plasma IGFBP-6 levels were also significantly lower in patients with hepatocellular cancer than in those with hepatitis (Sun et al. 2008).
With regard to non-malignant disease, serum IGFBP-6 levels were substantially higher in children with chronic renal failure, which may contribute to decreased growth in these patients (Powell et al. 1997). Serum IGFBP-6 levels were higher in patients with type 1 diabetes and its complications, although there was substantial overlap with control subjects (Lu et al. 2012). IGFBP-6 was also recently proposed as a candidate serum biomarker of proliferative vitreoretinopathy (Yu et al. 2014).
Many of these studies are limited by small sample size and results have not been validated in independent cohorts or by different laboratories. There is also considerable overlap in levels between normal and disease states in many of these studies. Further studies are therefore required to determine a clear clinical indication for measurement of plasma IGFBP-6 levels.
Conclusions and future directions
IGFBP-6 clearly has a physiological role as an inhibitor of IGF-II actions, and recent evidence indicates that it also has IGF-independent actions including inhibition of angiogenesis. This combination of properties would appear to be ideal for a cancer inhibitor. However, promotion of migration is generally held to be pro-tumorigenic, and the association with PHB2 binding and MAP kinase pathway activation provides some direction in furthering our understanding of this action.
There are a number of important questions regarding IGFBP-6 that remain unanswered. One of these is the structural basis for the IGF-II binding preference of IGFBP-6. The N-domain high affinity IGF binding subdomain is highly conserved between the IGFBPs, making it unlikely to contribute to this preference. Studies of isolated N- and C-domains of IGFBP-6 suggested that the C-domain of IGFBP-6 is responsible for its IGF-II binding preference (Headey et al. 2004b), but structural studies did not reveal a single key C-domain determinant for this preference (Headey et al. 2004a). A peptide based on the N-terminal subdomain of IGFBP-6 preferentially bound IGF-II, but its affinity for both IGFs was very low (Chandrashekaran et al. 2007). It is possible that both N- and C- domains cooperatively contribute to the binding preference since the binding sites for the N-terminal subdomain and C-domain of IGFBP-6 on IGF-II are adjacent.
Regarding IGF-independent actions, the mechanism whereby IGFBP-6 inhibits angiogenesis requires further study. IGFBP-6 inhibited basal and VEGF-induced angiogenesis in vitro as well as angiogenesis during development and xenograft growth in vivo (Zhang et al. 2012). The underlying molecular interactions as well as the relative roles of IGFBP-6 in endothelial and tumour cells are worthy of study. The role of PHB2 in IGFBP-6-induced cancer cell migration also requires further study at the molecular and cellular level. For example, the effects of IGFBP-6 binding and phosphorylation of PHB2 on PHB1/PHB2 ring complex formation and intracellular localisation are of interest. The molecular mechanisms whereby IGFBP-6 increases PHB2 phosphorylation and the consequent pathway leading to cell migration are also important questions. In this respect, the dissociation between IGFBP-6-induced migratory responses and MAP kinase pathway activation in some circumstances (Fu et al. 2013) (Yang and Bach 2015) indicates that other pathways are involved.
The relative balance of IGF-dependent and IGF-independent actions of IGFBP-6 in vivo is clearly a key question in understanding the physiological role of this protein. Related to this, the roles of non-IGF binding partners such as PHB2 on IGFBP-6 actions such as proliferation, survival, angiogenesis and migration are also important. Further studies of the structural determinants of binding of IGFBP-6 to IGF-II, PHB2 and other key molecules implicated in the angiogenic and migratory responses could lead to the design of IGFBP-6 mutants with specific binding characteristics that will assist in answering these critical questions.
Acknowledgements
The author’s work described in this review was funded by grants from the National Health and Medical Research Council of Australia, Australian Research Council, Cancer Council Victoria, Alfred Research Trust, Austin Hospital Medical Research Foundation and Melbourne Research Grants Scheme.
Abbreviations
- BAP
B-cell receptor-associated protein
- cAMP
cyclic AMP
- Hh
hedgehog
- HIF
hypoxia-inducible factor
- IGF
insulin-like growth factor
- IGFBP
IGF binding protein
- LMP
LIM mineralization protein
- MAP kinase
mitogen activated protein kinase
- MMP
matrix metalloprotease
- NF-κB
nuclear factor-κB
- PHB
prohibitin
- REA
repressor of estrogen receptor activity
- RMS
rhabdomyosarcoma
- SEMA
semaphorin
- TGF
transforming growth factor
- VEGF
vascular endothelial growth factor
References
- Amini Nik S, Ebrahim RP, Van Dam K, Cassiman JJ, Tejpar S. TGF-beta modulates beta-catenin stability and signaling in mesenchymal proliferations. Exp Cell Res. 2007;313:2887–2895. doi: 10.1016/j.yexcr.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Ande SR, Gu Y, Nyomba BL, Mishra S. Insulin induced phosphorylation of prohibitin at tyrosine 114 recruits Shp1. Biochim Biophys Acta. 2009;1793:1372–1378. doi: 10.1016/j.bbamcr.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Artal-Sanz M, Tavernarakis N. Prohibitin couples diapause signalling to mitochondrial metabolism during ageing in C. elegans. Nature. 2009;461:793–797. doi: 10.1038/nature08466. [DOI] [PubMed] [Google Scholar]
- Bach LA. Insulin-like growth factor binding protein-6: the “forgotten” binding protein? Horm Metab Res. 1999;31:226–234. doi: 10.1055/s-2007-978723. [DOI] [PubMed] [Google Scholar]
- Bach LA. IGFBP-6 five years on; not so ‘forgotten’? Growth Horm IGF Res. 2005;15:185–192. doi: 10.1016/j.ghir.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Bach LA, Thotakura NR, Rechler MM. Human insulin-like growth factor binding protein-6 is O-glycosylated. Biochem Biophys Res Commun. 1992;186:301–307. doi: 10.1016/s0006-291x(05)80807-1. [DOI] [PubMed] [Google Scholar]
- Bach LA, Headey SJ, Norton RS. IGF-binding proteins - the pieces are failing into place. Trends Endocrinol Metab. 2005;16:228–234. doi: 10.1016/j.tem.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Bach LA, Fu P, Yang Z. Insulin-like growth factor-binding protein-6 and cancer. Clin Sci. 2013;124:215–229. doi: 10.1042/CS20120343. [DOI] [PubMed] [Google Scholar]
- Bahrani-Mostafavi Z, Tickle TL, Zhang J, Bennett KE, Vachris JC, Spencer MD, Mostafavi MT, Tait DL. Correlation analysis of HOX, ERBB and IGFBP family gene expression in ovarian cancer. Cancer Invest. 2008;26:990–998. doi: 10.1080/07357900802074349. [DOI] [PubMed] [Google Scholar]
- Baxter RC, Saunders H. Radioimmunoassay of insulin-like growth factor-binding protein-6 in human serum and other body fluids. J Endocrinol. 1992;134:133–139. doi: 10.1677/joe.0.1340133. [DOI] [PubMed] [Google Scholar]
- Cacalano N, Le D, Paranjpe A, M-y W, Fernandez A, Evazyan T, Park N-H, Jewett A. Regulation of IGFBP6 gene and protein is mediated by the inverse expression and function of c-jun N-terminal kinase (JNK) and NFκB in a model of oral tumor cells. Apoptosis. 2008;13:1439–1449. doi: 10.1007/s10495-008-0270-1. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Chambery D, Mohseni-Zadeh S, de Galle B, Babajko S. N-myc regulation of type I insulin-like growth factor receptor in a human neuroblastoma cell. Cancer Res. 1999;59:2898–2902. [PubMed] [Google Scholar]
- Chandrashekaran IR, Yao SG, Wang CC, Bansal PS, Alewood PF, Forbes BE, Wallace JC, Bach LA, Norton RS. The N-terminal subdomain of insulin-like growth factor (IGF) binding protein 6. Structure and interaction with IGFs. Biochemistry. 2007;46:3065–3074. doi: 10.1021/bi0619876. [DOI] [PubMed] [Google Scholar]
- Chang BD, Swift ME, Shen M, Fang J, Broude EV, Roninson IB. Molecular determinants of terminal growth arrest induced in tumor cells by a chemotherapeutic agent. Proc Natl Acad Sci U S A. 2002;99:389–394. doi: 10.1073/pnas.012602599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury I, Thompson WE, Thomas K. Prohibitins role in cellular survival through Ras-Raf-MEK-ERK pathway. J Cell Physiol. 2014;229:998–1004. doi: 10.1002/jcp.24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe J-P, Patil CK, Rodier F, Krtolica A, Beausejour CM, Parrinello S, Hodgson JG, Chin K, Desprez P-Y, Campisi J. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS One. 2010;5:e9188. doi: 10.1371/journal.pone.0009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Ma C, Qiu J, Ma X, Wang X, Chen H, Huang B. A novel interaction between insulin-like growth factor binding protein-6 and the vitamin D receptor inhibits the role of vitamin D3 in osteoblast differentiation. Mol Cell Endocrinol. 2011;338:84–92. doi: 10.1016/j.mce.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Daza DO, Sundstrom G, Bergqvist CA, Duan C, Larhammar D. Evolution of the insulin-like growth factor binding protein (IGFBP) family. Endocrinology. 2011;152:2278–2289. doi: 10.1210/en.2011-0047. [DOI] [PubMed] [Google Scholar]
- Dean RA, Butler GS, Hamma-Kourbali Y, Delbe J, Brigstock DR, Courty J, Overall CM. Identification of candidate angiogenic inhibitors processed by matrix metalloproteinase 2 (MMP-2) in cell-based proteomic screens: disruption of vascular endothelial growth factor (VEGF)/heparin affin regulatory peptide (pleiotrophin) and VEGF/connective tissue growth factor angiogenic inhibitory complexes by MMP-2 proteolysis. Mol Cell Biol. 2007;27:8454–8465. doi: 10.1128/MCB.00821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denys H, Jadidizadeh A, Amini Nik S, Van Dam K, Aerts S, Alman BA, Cassiman JJ, Tejpar S. Identification of IGFBP-6 as a significantly downregulated gene by beta-catenin in desmoid tumors. Oncogene. 2004;23:654–664. doi: 10.1038/sj.onc.1207160. [DOI] [PubMed] [Google Scholar]
- Drivdahl RH, Loop SM, Andress DL, Ostenson RC. IGF-binding proteins in human prostate: expression and regulation by 1,25-dihydroxyvitamin D3. Prostate. 1995;26:72–79. doi: 10.1002/pros.2990260203. [DOI] [PubMed] [Google Scholar]
- Fan CW, Chan CC, Chao CCK, Fan HA, Sheu DL, Chan EC. Expression patterns of cell cycle and apoptosis-related genes in a multidrug-resistant human colon carcinoma cell line. Scand J Gastroenterol. 2004;39:464–469. doi: 10.1080/00365520310008809. [DOI] [PubMed] [Google Scholar]
- Figueroa JA, Jackson JG, McGuire WL, Krywicki RF, Yee D. Expression of insulin-like growth factor binding proteins in human breast cancer correlates with estrogen receptor status. J Cell Biochem. 1993;52:196–205. doi: 10.1002/jcb.240520211. [DOI] [PubMed] [Google Scholar]
- Foulstone E, Prince S, Zaccheo O, Burns JL, Harper J, Jacobs C, Church D, Hassan AB. Insulin-like growth factor ligands, receptors, and binding proteins in cancer. J Pathol. 2005;205:145–153. doi: 10.1002/path.1712. [DOI] [PubMed] [Google Scholar]
- Fu P, Thompson JA, Bach LA. Promotion of cancer cell migration: an insulin-like growth factor (IGF)-independent action of IGF-binding protein-6. J Biol Chem. 2007;282:22298–22306. doi: 10.1074/jbc.M703066200. [DOI] [PubMed] [Google Scholar]
- Fu P, Liang GJ, Khot SS, Phan R, Bach LA. Cross-talk between MAP kinase pathways is involved in IGF-independent, IGFBP-6-induced Rh30 rhabdomyosarcoma cell migration. J Cell Physiol. 2010;224:636–643. doi: 10.1002/jcp.22156. [DOI] [PubMed] [Google Scholar]
- Fu P, Yang Z, Bach LA. Prohibitin-2 binding modulates insulin-like growth factor binding protein-6 (IGFBP-6)-induced rhabdomyosarcoma cell migration. J Biol Chem. 2013;288:29890–29900. doi: 10.1074/jbc.M113.510826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futschik M, Jeffs A, Pattison S, Kasabov N, Sullivan M, Merrie A, Reeve A. Gene expression profiling of metastatic and nonmetastatic colorectal cancer cell lines. Genome Lett. 2002;1:26–34. [Google Scholar]
- Gallicchio MA, Kneen M, Hall C, Scott AM, Bach LA. Overexpression of insulin-like growth factor binding protein-6 inhibits rhabdomyosarcoma growth in vivo. Int J Cancer. 2001;94:645–651. doi: 10.1002/ijc.1519. [DOI] [PubMed] [Google Scholar]
- Gallicchio MA, van Sinderen M, Bach LA. Insulin-like growth factor binding protein-6 and CCI-779, an ester analogue of rapamycin, additively inhibit rhabdomyosarcoma growth. Horm Metab Res. 2003;35:822–827. doi: 10.1055/s-2004-814153. [DOI] [PubMed] [Google Scholar]
- Grellier P, Degalle B, Babajko S. Expression of insulin-like growth factor-binding protein 6 complementary DNA alters neuroblastoma cell growth. Cancer Res. 1998;58:1670–1676. [PubMed] [Google Scholar]
- Grellier P, Berrebi D, Peuchmaur M, Babajko S. The IGF system in neuroblastoma xenografts: focus on IGF-binding protein-6. J Endocrinol. 2002;172:467–476. doi: 10.1677/joe.0.1720467. [DOI] [PubMed] [Google Scholar]
- Gunawardana CG, Kuk C, Smith CR, Batruch I, Soosaipillai A, Diamandis EP. Comprehensive analysis of conditioned media from ovarian cancer cell lines identifies novel candidate markers of epithelial ovarian cancer. J Proteome Res. 2009;8:4705–4713. doi: 10.1021/pr900411g. [DOI] [PubMed] [Google Scholar]
- Hahn H, Wojnowski L, Specht K, Kappler R, Calzada-Wack J, Potter D, Zimmer A, Muller U, Samson E, Quintanilla-Martinez L. Patched target igf2 is indispensable for the formation of medulloblastoma and rhabdomyosarcoma. J Biol Chem. 2000;275:28341–28344. doi: 10.1074/jbc.C000352200. [DOI] [PubMed] [Google Scholar]
- Hale K, Murray AW, Cosgrove LJ, Bach LA, Hartfield PJ. Prevention of apoptosis by insulin-like growth factor (IGF)-I and IGF-II is differentially attenuated by IGF-binding proteins in PC12 cells. Neurosci Res Commun. 2000;27:75–83. [Google Scholar]
- Headey SJ, Keizer DW, Yao S, Brasier G, Kantharidis P, Bach LA, Norton RS. C-terminal domain of insulin-like growth factor (IGF) binding protein-6: structure and interaction with IGF-II. Mol Endocrinol. 2004;18:2740–2750. doi: 10.1210/me.2004-0248. [DOI] [PubMed] [Google Scholar]
- Headey SJ, Leeding KS, Norton RS, Bach LA. Contributions of the N- and C-domains of IGFBP-6 to IGF binding and inhibition of IGF actions. J Mol Endocrinol. 2004;33:377–386. doi: 10.1677/jme.1.01547. [DOI] [PubMed] [Google Scholar]
- Heron-Milhavet L, Mamaeva D, Rochat A, Lamb NJ, Fernandez A. Akt2 is implicated in skeletal muscle differentiation and specifically binds Prohibitin2/REA. J Cell Physiol. 2008;214:158–165. doi: 10.1002/jcp.21177. [DOI] [PubMed] [Google Scholar]
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci. 2004;117:4619–4628. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- Ingram WJ, Wicking CA, Grimmond SM, Forrest AR, Wainwright BJ. Novel genes regulated by Sonic Hedgehog in pluripotent mesenchymal cells. Oncogene. 2002;21:8196–8205. doi: 10.1038/sj.onc.1205975. [DOI] [PubMed] [Google Scholar]
- Iosef C, Gkourasas T, Jia CYH, Li SSC, Han VKM. A functional nuclear localization signal in insulin-like growth factor binding protein-6 mediates its nuclear import. Endocrinology. 2008;149:1214–1226. doi: 10.1210/en.2007-0959. [DOI] [PubMed] [Google Scholar]
- Iosef C, Vilk G, Gkourasas T, Lee K, Chen P, Fu P, Bach L, Lajoie G, Gupta M, Li S, et al. Insulin-like growth factor binding protein 6 (IGFBP-6) interacts with DNA-end binding protein Ku80 to regulate cell fate. Cell Signal. 2010;22:1033–1043. doi: 10.1016/j.cellsig.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Jee CD, Kim MA, Jung EJ, Kim J, Kim WH. Identification of genes epigenetically silenced by CpG methylation in human gastric carcinoma. Eur J Cancer. 2009;45:1282–1293. doi: 10.1016/j.ejca.2008.12.027. [DOI] [PubMed] [Google Scholar]
- Kalus W, Zweckstetter M, Renner C, Sanchez Y, Georgescu J, Crol M, Demuth D, Schumacher R, Dony C, Lang K, et al. Structure of the IGF-binding domain of the insulin-like growth factor-binding protein-5 (IGFBP-5): implications for IGF and IGF-I receptor interactions. Embo J. 1998;17:6558–6572. doi: 10.1093/emboj/17.22.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan K, Amariglio N, Rechavi G, Jakob-Hirsch J, Kela I, Kaminski N, Getz G, Domany E, Givol D. DNA microarrays identification of primary and secondary target genes regulated by p53. Oncogene. 2001;20:2225–2234. doi: 10.1038/sj.onc.1204319. [DOI] [PubMed] [Google Scholar]
- Kaulsay KK, Ng EH, Ji CY, Ho GH, Aw TC, Lee KO. Serum IGF-binding protein-6 and prostate specific antigen in breast cancer. Eur J Endocrinol. 1999;140:164–168. doi: 10.1530/eje.0.1400164. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Kim WY, Ha YL, Bach LA, Park JHY. Inhibition of Caco-2 cell proliferation by (n-3) fatty acids: possible mediation by increased secretion of insulin-like growth factor binding protein-6. Nutrition Res. 2000;20:1409–1421. [Google Scholar]
- Kim EJ, Kang Y-H, Schaffer BS, Bach LA, MacDonald RG, Park JHY. Inhibition of Caco-2 cell proliferation by all-trans retinoic acid (tRA): role of insulin-like growth factor binding protein-6. J Cell Physiol. 2001;190:92–100. doi: 10.1002/jcp.10045. [DOI] [PubMed] [Google Scholar]
- Kim J-W, Akiyama M, Park J-H, Lin M-L, Shimo A, Ueki T, Daigo Y, Tsunoda T, Nishidate T, Nakamura Y, et al. Activation of an estrogen/estrogen receptor signaling by BIG3 through its inhibitory effect on nuclear transport of PHB2/REA in breast cancer. Cancer Sci. 2009;100:1468–1478. doi: 10.1111/j.1349-7006.2009.01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Ito K, Takezawa Y, Oyama T, Yamanaka H, Suzuki K. Insulin-like growth factor binding protein-6 inhibits prostate cancer cell proliferation: implication for anticancer effect of diethylstilbestrol in hormone refractory prostate cancer. Br J Cancer. 2005;92:1538–1544. doi: 10.1038/sj.bjc.6602520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama N, Zhang J, Huqun MH, Tanaka T, Su X, Hagiwara K. Identification of IGFBP-6 as an effector of the tumor suppressor activity of SEMA3B. Oncogene. 2008;27:6581–6589. doi: 10.1038/onc.2008.263. [DOI] [PubMed] [Google Scholar]
- Kulaeva OI, Draghici S, Tang L, Kraniak JM, Land SJ, Tainsky MA. Epigenetic silencing of multiple interferon pathway genes after cellular immortalization. Oncogene. 2003;22:4118–4127. doi: 10.1038/sj.onc.1206594. [DOI] [PubMed] [Google Scholar]
- Kuo Y-S, Tang Y-B, Lu T-Y, Wu H-C, Lin C-T. IGFBP-6 plays a role as an oncosuppressor gene in NPC pathogenesis through regulating EGR-1 expression. J Pathol. 2010;222:299–309. doi: 10.1002/path.2735. [DOI] [PubMed] [Google Scholar]
- Larsen PH, DaSilva AG, Conant K, Yong VW. Myelin formation during development of the CNS is delayed in matrix metalloproteinase-9 and −12 null mice. J Neurosci. 2006;26:2207–2214. doi: 10.1523/JNEUROSCI.1880-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng SL, Leeding KS, Whitehead RH, Bach LA. Insulin-like growth factor (IGF)-binding protein-6 inhibits IGF-II-induced but not basal proliferation and adhesion of LIM 1215 colon cancer cells. Mol Cell Endocrinol. 2001;174:121–127. doi: 10.1016/s0303-7207(00)00444-5. [DOI] [PubMed] [Google Scholar]
- Lin BY, White JT, Wu J, Lele S, Old LJ, Hood L, Odunsi K. Deep depletion of abundant serum proteins reveals low-abundant proteins as potential biomarkers for human ovarian cancer. Proteomics Clin Appl. 2009;3:853–861. doi: 10.1002/prca.200800141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Zhang G, Zhang J, Gao G, Li M, Chen Y, Wang J, Li G, Song SW, Qiu X, et al. A panel of four cytokines predicts the prognosis of patients with malignant gliomas. J Neurooncol. 2013;114:199–208. doi: 10.1007/s11060-013-1171-x. [DOI] [PubMed] [Google Scholar]
- Lipinski RJ, Cook CH, Barnett DH, Gipp JJ, Peterson RE, Bushman W. Sonic hedgehog signaling regulates the expression of insulin-like growth factor binding protein-6 during fetal prostate development. Dev Dyn. 2005;233:829–836. doi: 10.1002/dvdy.20414. [DOI] [PubMed] [Google Scholar]
- Liu CJ, Li TY, Kuo LT, Cheng HW, Chu TH, Chang KW, Lin SC. Differential gene expression signature between primary and metastatic head and neck squamous cell carcinoma. J Pathol. 2008;214:489–497. doi: 10.1002/path.2306. [DOI] [PubMed] [Google Scholar]
- Lu S, Purohit S, Sharma A, Zhi W, He M, Wang Y, Li C-J, She J-X. Serum insulin-like growth factor binding protein 6 (IGFBP6) is increased in patients with type 1 diabetes and its complications. Int J Clin Exp Med. 2012;5:229–237. [PMC free article] [PubMed] [Google Scholar]
- Marinaro JA, Hendrich EC, Leeding KS, Bach LA. HaCaT human keratinocytes express IGF-II, IGFBP-6 and an acid-activated protease with activity against IGFBP-6. Am J Physiol. 1999;276:E542–E548. doi: 10.1152/ajpendo.1999.276.3.E536. [DOI] [PubMed] [Google Scholar]
- Marinaro JA, Neumann GM, Russo VC, Leeding KS, Bach LA. O-glycosylation of insulin-like growth factor (IGF) binding protein-6 maintains high IGF-II binding affinity by decreasing binding to glycosaminoglycans and susceptibility to proteolysis. Eur J Biochem. 2000;267:5378–5386. doi: 10.1046/j.1432-1327.2000.01575.x. [DOI] [PubMed] [Google Scholar]
- Martin JL, Coverley JA, Pattison ST, Baxter RC. Insulin-like growth factor-binding protein-3 production by MCF-7 breast cancer cells: stimulation by retinoic acid and cyclic adenosine monophosphate and differential effects of estradiol. Endocrinology. 1995;136:1219–1226. doi: 10.1210/endo.136.3.7532580. [DOI] [PubMed] [Google Scholar]
- Massoner P, Ladurner Rennau M, Heidegger I, Kloss-Brandstatter A, Summerer M, Reichhart E, Schafer G, Klocker H. Expression of the IGF axis is decreased in local prostate cancer but enhanced after benign prostate epithelial differentiation and TGF-beta treatment. Am J Pathol. 2011;179:2905–2919. doi: 10.1016/j.ajpath.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengwasser J, Piau A, Schlag P, Sleeman JP. Differential immunization identifies PHB1/PHB2 as blood-borne tumor antigens. Oncogene. 2004;23:7430–7435. doi: 10.1038/sj.onc.1207987. [DOI] [PubMed] [Google Scholar]
- Merkwirth C, Dargazanli S, Tatsuta T, Geimer S, Lower B, Wunderlich FT, von Kleist-Retzow J-C, Waisman A, Westermann B, Langer T. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev. 2008;22:476–488. doi: 10.1101/gad.460708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer-Blust A, An X, Li J. Hypoxia-regulated angiogenic inhibitors. Trends Cardiovasc Med. 2009;19:252–256. doi: 10.1016/j.tcm.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micutkova L, Diener T, Li C, Rogowska-Wrzesinska A, Mueck C, Huetter E, Weinberger B, Grubeck-Loebenstein B, Roepstorff P, Zeng R, et al. Insulin-like growth factor binding protein-6 delays replicative senescence of human fibroblasts. Mech Ageing Dev. 2011;132:468–479. doi: 10.1016/j.mad.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min T, Liu X, Han B, Ge Q, Li Z, Zipeng L, Wei J, Song G, Cai B, Lv N, et al. Vasohibin-2 promotes proliferation in human breast cancer cells via upregulation of fibroblast growth factor-2 and growth/differentiation factor-15 expression. Mol Med Rep. 2014;10:663–669. doi: 10.3892/mmr.2014.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Murphy LC, Murphy LJ. The prohibitins: emerging roles in diverse functions. J Cell Mol Med. 2006;10:353–363. doi: 10.1111/j.1582-4934.2006.tb00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Ande SR, Nyomba BLG. The role of prohibitin in cell signaling. FEBS J. 2010;277:3937–3946. doi: 10.1111/j.1742-4658.2010.07809.x. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Miyamoto S, Maeda H, Ishii G, Hasebe T, Chiba T, Asaka M, Ochiai A. Matrix metalloproteinase-7 degrades all insulin-like growth factor binding proteins and facilitates insulin-like growth factor bioavailability. Biochem Biophys Res Commun. 2005;333:1011–1016. doi: 10.1016/j.bbrc.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Neumann GM, Bach LA. The N-terminal disulfide linkages of human insulin-like growth factor binding protein-6 (hIGFBP-6) and hIGFBP-1 are different as determined by mass spectrometry. J Biol Chem. 1999;274:14587–14594. doi: 10.1074/jbc.274.21.14587. [DOI] [PubMed] [Google Scholar]
- Neumann GM, Marinaro JA, Bach LA. Identification of O-glycosylation sites and partial characterization of carbohydrate structure and disulfide linkages of human insulin-like growth factor binding protein 6. Biochemistry. 1998;37:6572–6585. doi: 10.1021/bi972894e. [DOI] [PubMed] [Google Scholar]
- Oliveras-Ferraros C, Vazquez-Martin A, Martin-Castilló B, Pérez-Martínez MC, Cufí S, Del Barco S, Bernado L, Brunet J, López-Bonet E, Menendez JA. Pathway-focused proteomic signatures in HER2-overexpressing breast cancer with a basal-like phenotype: New insights into de novo resistance to trastuzumab (Herceptin) Int J Oncol. 2010;37:669–678. doi: 10.3892/ijo_00000716. [DOI] [PubMed] [Google Scholar]
- Ong VH, Carulli MT, Xu S, Khan K, Lindahl G, Abraham DJ, Denton CP. Cross-talk between MCP-3 and TGFbeta promotes fibroblast collagen biosynthesis. Exp Cell Res. 2009;315:151–161. doi: 10.1016/j.yexcr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Osman C, Merkwirth C, Langer T. Prohibitins and the functional compartmentalization of mitochondrial membranes. J Cell Sci. 2009;122:3823–3830. doi: 10.1242/jcs.037655. [DOI] [PubMed] [Google Scholar]
- Park SE, Xu J, Frolova A, Liao L, O’Malley BW, Katzenellenbogen BS. Genetic deletion of the repressor of estrogen receptor activity (REA) enhances the response to estrogen in target tissues in vivo. Mol Cell Biol. 2005;25:1989–1999. doi: 10.1128/MCB.25.5.1989-1999.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plymate SR, Tennant M, Birngaum RS, Thrasher JB, Chatta G, Ware JL. The effect on the insulin-like growth factor system in human prostate epithelial cells of immortalization and transformation by simian virus-40 T antigen. J Clin Endocrinol Metab. 1996;81:3709–3716. doi: 10.1210/jcem.81.10.8855827. [DOI] [PubMed] [Google Scholar]
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Powell DR, Liu F, Baker BK, Hintz RL, Durham SK, Brewer ED, Frane JW, Tonshoff B, Mehls O, Wingen AM, et al. Insulin-like growth factor-binding protein-6 levels are elevated in serum of children with chronic renal failure - a report of the Southwest Pediatric Nephrology Study Group. J Clin Endocrinol Metab. 1997;82:2978–2984. doi: 10.1210/jcem.82.9.4215. [DOI] [PubMed] [Google Scholar]
- Qiu J, Ma XL, Wang X, Chen H, Huang BR. Insulin-like growth factor binding protein-6 interacts with the thyroid hormone receptor alpha1 and modulates the thyroid hormone-response in osteoblastic differentiation. Mol Cell Biochem. 2012;361:197–208. doi: 10.1007/s11010-011-1104-y. [DOI] [PubMed] [Google Scholar]
- Rajalingam K, Wunder C, Brinkmann V, Churin Y, Hekman M, Sievers C, Rapp UR, Rudel T. Prohibitin is required for Ras-induced Raf-MEK-ERK activation and epithelial cell migration. Nat Cell Biol. 2005;7:837–843. doi: 10.1038/ncb1283. [DOI] [PubMed] [Google Scholar]
- Raykha C, Crawford J, Gan BS, Fu P, Bach LA, O’Gorman DB. IGF-II and IGFBP-6 regulate cellular contractility and proliferation in Dupuytren’s disease. Biochim Biophys Acta. 2013;1832:1511–1519. doi: 10.1016/j.bbadis.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Roodink I, Leenders WPJ. Targeted therapies of cancer: Angiogenesis inhibition seems not enough. Cancer Lett. 2010;299:1–10. doi: 10.1016/j.canlet.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Russo VC, Andaloro E, Fornaro SA, Najdovska S, Newgreen DF, Bach LA, Werther GA. Fibroblast growth factor-2 over-rides insulin-like growth factor-I induced proliferation and cell survival in human neuroblastoma cells. J Cell Physiol. 2004;199:371–380. doi: 10.1002/jcp.10416. [DOI] [PubMed] [Google Scholar]
- Sayer RA, Lancaster JM, Pittman J, Gray J, Whitaker R, Marks JR, Berchuck A. High insulin-like growth factor-2 (IGF-2) gene expression is an independent predictor of poor survival for patients with advanced stage serous epithelial ovarian cancer. Gynecol Oncol. 2005;96:355–361. doi: 10.1016/j.ygyno.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Schillebeeckx M, Pihlajoki M, Gretzinger E, Yang W, Thol F, Hiller T, Lobs AK, Rohrig T, Schrade A, Cochran R, et al. Novel markers of gonadectomy-induced adrenocortical neoplasia in the mouse and ferret. Mol Cell Endocrinol. 2015;399:122–30. doi: 10.1016/j.mce.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seurin D, Lassarre C, Blenvenu G, Babajko S. Insulin-like growth factor binding protein-6 inhibits neuroblastoma and cell proliferation and tumour development. Eur J Cancer. 2002;38:2058–2065. doi: 10.1016/s0959-8049(02)00240-x. [DOI] [PubMed] [Google Scholar]
- Shalamanova L, Kubler B, Scharf JG, Braulke T. MDCK cells secrete neutral proteases cleaving insulin-like growth factor-binding protein-2 to-6. Am J Physiol - End ocrinol Metab. 2001;281:E1221–E1229. doi: 10.1152/ajpendo.2001.281.6.E1221. [DOI] [PubMed] [Google Scholar]
- Shalamanova L, Kubler B, Storch S, Scharf JG, Braulke T. Multiple post-translational modifications of mouse insulin-like growth factor binding protein-6 expressed in epithelial Madin-Darby canine kidney cells. Mol Cell Endocrinol. 2008;295:18–23. doi: 10.1016/j.mce.2008.08.034. [DOI] [PubMed] [Google Scholar]
- Sheikh MS, Shao ZM, Hussain A, Clemmons DR, Chen JC, Roberts CT, Jr, LeRoith D, Fontana JA. Regulation of insulin-like growth factor-binding-protein-1, 2, 3, 4, 5, and 6: synthesis, secretion, and gene expression in estrogen receptor-negative human breast carcinoma cells. J Cell Physiol. 1993;155:556–567. doi: 10.1002/jcp.1041550314. [DOI] [PubMed] [Google Scholar]
- Sievers C, Billig G, Gottschalk K, Rudel T. Prohibitins are required for cancer cell proliferation and adhesion. PLoS One. 2010;5:e12735. doi: 10.1371/journal.pone.0012735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitar T, Popowicz GM, Siwanowicz I, Huber R, Holak TA. Structural basis for the inhibition of insulin-like growth factors by insulin-like growth factor-binding proteins. Proc Natl Acad Sci U S A. 2006;103:13028–13033. doi: 10.1073/pnas.0605652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany MG, Rosenzweig SA. Hypoxia-inducible factor-1-dependent and -independent regulation of insulin-like growth factor-1-stimulated vascular endothelial growth factor secretion. J Pharmacol Exp Ther. 2006;318:666–675. doi: 10.1124/jpet.106.104158. [DOI] [PubMed] [Google Scholar]
- Stearns M, Tran J, Francis MK, Zhang H, Sell C. Activated ras enhances insulin-like growth factor I induction of vascular endothelial growth factor in prostate epithelial cells. Cancer Res. 2005;65:2085–2088. doi: 10.1158/0008-5472.CAN-04-4100. [DOI] [PubMed] [Google Scholar]
- Strohbach C, Kleinman S, Linkhart T, Amaar Y, Chen S-T, Mohan S, Strong D. Potential involvement of the interaction between insulin-like growth factor binding protein (IGFBP)-6 and LIM mineralization protein (LMP)-1 in regulating osteoblast differentiation. J Cell Biochem. 2008;104:1890–1905. doi: 10.1002/jcb.21761. [DOI] [PubMed] [Google Scholar]
- Sueoka N, Lee HY, Walsh GL, Fang B, Ji L, Roth JA, LaPushin R, Hong WK, Cohen P, Kurie JM. Insulin-like growth factor binding protein-6 inhibits the growth of human bronchial epithelial cells and increases in abundance with all- trans-retinoic acid treatment. Am J Respir Cell Mol Biol. 2000;23:297–303. doi: 10.1165/ajrcmb.23.3.4013. [DOI] [PubMed] [Google Scholar]
- Sueoka N, Lee HY, Wiehle S, Cristiano RJ, Fang B, Ji L, Roth JA, Hong WK, Cohen P, Kurie JM. Insulin-like growth factor binding protein-6 activates programmed cell death in non-small cell lung cancer cells. Oncogene. 2000;19:4432–4436. doi: 10.1038/sj.onc.1203813. [DOI] [PubMed] [Google Scholar]
- Sun H, Chua MS, Yang D, Tsalenko A, Peter BJ, So S. Antibody arrays identify potential diagnostic markers of hepatocellular carcinoma. Biomark Insights. 2008;3:1–18. doi: 10.4137/bmi.s595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toretsky JA, Helman LJ. Involvement of IGF-II in human cancer. J Endocrinol. 1996;149:367–372. doi: 10.1677/joe.0.1490367. [DOI] [PubMed] [Google Scholar]
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doorn J, Ringeling AM, Shmueli SS, Kuijpers MC, Hokken-Koelega ACS, van Buul-Offers SC, Jansen M. Circulating levels of human insulin-like growth factor binding protein-6 (IGFBP-6) in health and disease as determined by radioimmunoassay. Clin Endocrinol. 1999;50:601–609. doi: 10.1046/j.1365-2265.1999.00694.x. [DOI] [PubMed] [Google Scholar]
- Velazquez-Fernandez D, Laurell C, Geli J, Hoog A, Odeberg J, Kjellman M, Lundeberg J, Hamberger B, Nilsson P, Backdahl M. Expression profiling of adrenocortical neoplasms suggests a molecular signature of malignancy. Surgery. 2005;138:1087–1094. doi: 10.1016/j.surg.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Walker G, MacLeod K, Williams ARW, Cameron DA, Smyth JF, Langdon SP. Insulin-like growth factor binding proteins IGFBP3, IGFBP4, and IGFBP5 predict endocrine responsiveness in patients with ovarian cancer. Clin Cancer Res. 2007;13:1438–1444. doi: 10.1158/1078-0432.CCR-06-2245. [DOI] [PubMed] [Google Scholar]
- Wang W, Kumar P, Wang WZ, Epstein J, Helman L, Moore JV, Kumar S. Insulin-like growth factor II and PAX3-FKHR cooperate in the oncogenesis of rhabdomyosarcoma. Cancer Res. 1998;58:4426–4433. [PubMed] [Google Scholar]
- Wang X, Lu L, Li Y, Li M, Chen C, Feng Q, Zhang C, Duan C. Molecular and functional characterization of two distinct IGF binding protein-6 genes in zebrafish. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1348–1357. doi: 10.1152/ajpregu.90969.2008. [DOI] [PubMed] [Google Scholar]
- Warren RS, Yuan H, Matli MR, Ferrara N, Donner DB. Induction of vascular endothelial growth factor by insulin-like growth factor 1 in colorectal carcinoma. J Biol Chem. 1996;271:29483–29488. doi: 10.1074/jbc.271.46.29483. [DOI] [PubMed] [Google Scholar]
- Wegmann B, Schoeneberger H-J, Kiefer PE, Jaques G, Brandscheid D, Havemann K. Molecular cloning of IGFBP-5 from SCLC cell lines and expression of IGFBP-4, IGFBP-5 and IGFBP-6 in lung cancer cell lines and primary tumours. Eur J Cancer. 1993;29A:1578–1584. doi: 10.1016/0959-8049(93)90298-t. [DOI] [PubMed] [Google Scholar]
- Wilkinson SE, Furic L, Buchanan G, Larsson O, Pedersen J, Frydenberg M, Risbridger GP, Taylor RA. Hedgehog signaling is active in human prostate cancer stroma and regulates proliferation and differentiation of adjacent epithelium. Prostate. 2013;213:1810–23. doi: 10.1002/pros.22720. [DOI] [PubMed] [Google Scholar]
- Xie LF, Tsaprailis G, Chen QM. Proteomic identification of insulin-like growth factor-binding protein-6 induced by sublethal H2O2 stress from human diploid fibroblasts. Mol Cell Proteomics. 2005;4:1273–1283. doi: 10.1074/mcp.M500032-MCP200. [DOI] [PubMed] [Google Scholar]
- Xu XF, Guo CY, Liu J, Yang WJ, Xia YJ, Xu L, Yu YC, Wang XP. Gli1 maintains cell survival by up-regulating IGFBP6 and Bcl-2 through promoter regions in parallel manner in pancreatic cancer cells. J Carcinog. 2009;8:13. doi: 10.4103/1477-3163.55429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhang L, S-k S, Zhang X. CC Chemokine ligand 18 and IGF-binding protein 6 as potential serum biomarkers for prostate cancer. Tohoku J Exp Med. 2014;233:25–31. doi: 10.1620/tjem.233.25. [DOI] [PubMed] [Google Scholar]
- Yan T, Wergedal J, Zhou Y, Mohan S, Baylink DJ, Strong DD. Inhibition of human osteoblast marker gene expression by retinoids is mediated in part by insulin-like growth factor binding protein-6. GH IGF Res. 2001;11:368–377. doi: 10.1054/ghir.2001.0249. [DOI] [PubMed] [Google Scholar]
- Yang Z, Bach LA (2015) Differential effects of insulin-like growth factor binding protein-6 (IGFBP-6) on migration of Two ovarian cancer cell lines. Front Endocrinol 5 [DOI] [PMC free article] [PubMed]
- Yao R, Wang Y, Lubet RA, You M. Differentially expressed genes associated with mouse lung tumor progression. Oncogene. 2002;21:5814–5821. doi: 10.1038/sj.onc.1205422. [DOI] [PubMed] [Google Scholar]
- Yoon JW, Kita Y, Frank DJ, Majewski RR, Konicek BA, Nobrega MA, Jacob H, Walterhouse D, Iannaccone P. Gene expression profiling leads to identification of GLI1-binding elements in target genes and a role for multiple downstream pathways in GLI1-induced cell transformation. J Biol Chem. 2002;277:5548–5555. doi: 10.1074/jbc.M105708200. [DOI] [PubMed] [Google Scholar]
- Yu J, Peng R, Chen H, Cui C, Ba J, Wang F. Kininogen 1 and insulin-like growth factor binding protein 6: candidate serum biomarkers of proliferative vitreoretinopathy. Clin Exp Optom. 2014;97:72–79. doi: 10.1111/cxo.12088. [DOI] [PubMed] [Google Scholar]
- Zeng X, Yang P, Chen B, Jin X, Liu Y, Zhao X, Liang S. Quantitative secretome analysis reveals the interactions between epithelia and tumor cells by in vitro modulating colon cancer microenvironment. J Proteomics. 2013;89:51–70. doi: 10.1016/j.jprot.2013.05.032. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, Vogelstein B, Kinzler KW. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- Zhang C, Lu L, Li Y, Wang X, Zhou J, Liu Y, Fu P, Gallicchio MA, Bach LA, Duan C. IGF binding protein-6 expression in vascular endothelial cells is induced by hypoxia and plays a negative role in tumor angiogenesis. Int J Cancer. 2012;130:2003–2012. doi: 10.1002/ijc.26201. [DOI] [PMC free article] [PubMed] [Google Scholar]