Abstract
The activity of the Insulin-like Growth Factors (IGFs) ligands elicited via their receptors and transduced by various intracellular signal pathways is modulated by the IGF Binding Proteins (IGFBPs). Among all the IGFBPs, IGFBP-2 has been implicated in the regulation of IGF activity in most tissue and organs. Besides binding to IGFs in the circulation these IGF-regulatory activities of IGFBP-2 involve interactions with components of the extracellular matrix, cell surface proteoglycans and integrin receptors. In addition to these local peri-cellular activities, IGFBP-2 exerts other key functions within the nucleus, where IGFBP-2 directly or indirectly promotes transcriptional activation of specific genes. All of these IGFBP-2 activities, intrinsic or dependent on IGFs, contribute to its functional roles in growth/development, metabolism and malignancy as evidenced by studies in IGFBP-2 animal models and also by many in vitro studies. Finally, preclinical studies have demonstrated that IGFBP-2 administration can be beneficial in improving metabolic responses (inhibition of adipogenesis and enhanced insulin sensitivity), while blockade of IGFBP-2 appears to be an effective approach to inhibiting tumour growth and metastasis.
Keywords: IGF system, IGFBP-2, Growth, Metabolism, Obesity, Diabetes, Leukaemia, Lung cancer, Colon cancer
The IGF system (Fig. 1)
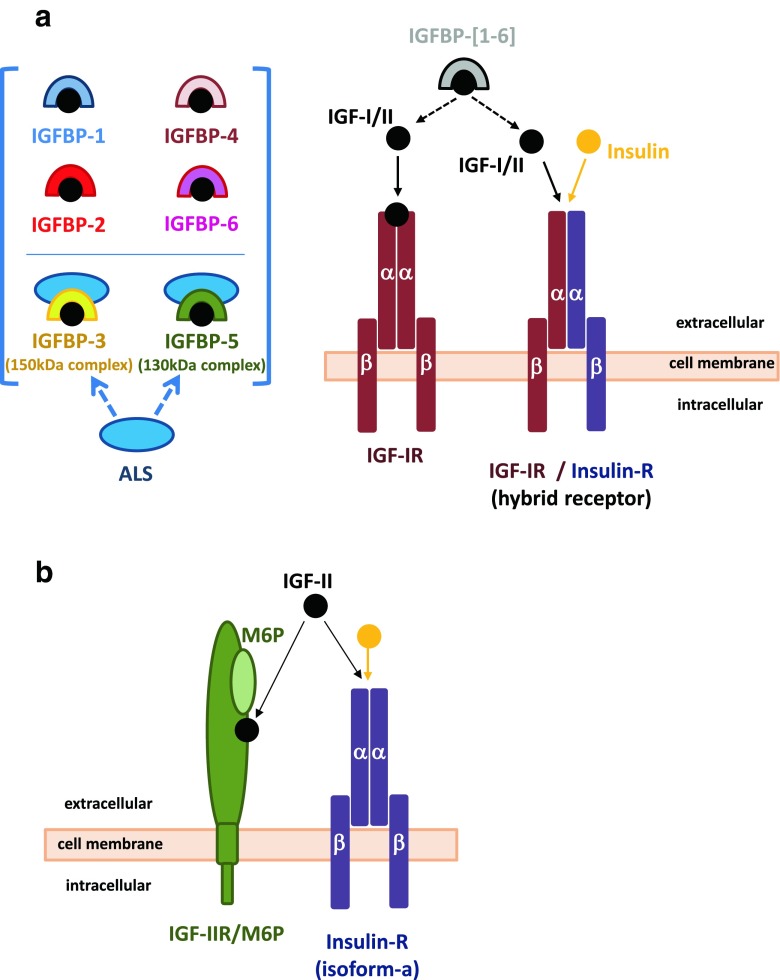
Fig. 1.
IGF System. The IGF system comprises of the IGF-I and IGF-II ligands, cell-membrane receptors, IGF1R and IGF-IIR/M6P (lower panel), insulin receptor isoform-a (IRa; lower panel) and IGF1R/IR hybrid receptors. Insulin is also included in these schematic cartoons to illustrate cross modulation of IRa and IGF1R/IR hybrid receptors by the three ligands. Six high affinity IGF binding proteins (IGFBPs; IGFBP-[1–6]) modulate IGF-I/II activities, with IGFBP-3 and IGFBP-5 also forming complexes with the acid labile subunit (ALS). IGFBPs in addition to modulating IGFs bioavailability to their receptors with involvement of specific IGFBP-proteases, possess intrinsic activities that are exerted via cell membrane receptors or via translocation into the cell and nuclear compartment (shown for IGFBP-2 in Fig. 3). These activities are exerted in the presence or absence of the IGF ligands (see also Fig. 3)
The insulin-like growth factors I and II (IGF-I and IGF-II) are growth-promoting peptides (Zapf and Froesch 1986; Daughaday and Rotwein 1989), that are expressed in most tissues from early in embryonic development, with IGF-II being highly expressed in utero and IGF-I prevalently expressed during postnatal life (Daughaday and Rotwein 1989; Han et al. 1987; Murphy et al. 1987).
The biological action of the IGFs are mainly mediated by the type I IGF-receptor (IGF1R) (Sepp-Lorenzino 1998) (Fig. 1a). The IGF1R receptor and insulin receptor (IR) are very similar in structure and function (Sepp-Lorenzino 1998), however, the insulin receptor mediates insulin specific metabolic functions, whereas the IGF-I receptor mediates IGF-I mitogenic and differentiation functions.
There are also various IGF-I receptor subtypes (Milazzo et al. 1992), which have one αins-βins IR dimer and one αI-βI IGF1R dimer (Moxham et al. 1989) and bind insulin as well as IGFs with relatively high affinity (Siddle et al. 1994) (Fig. 1a). IGF-II exerts insulin-like effects via the insulin and the IGF1R. However, IGF-II has great affinity for a distinct receptor, the IGF-II receptor (Sakano et al. 1991), which is identical to the cation-independent mannose-6 phosphate receptor (M6P) (Braulke 1999) (Fig. 1b).
There are six high affinity insulin-like growth factor binding proteins (IGFBPs) (Firth and Baxter 2002), which coordinate and regulate IGFs bioavailability and activity (Firth and Baxter 2002) (Fig. 1a). The IGFBPs bind IGF-I and IGF-II, but do not bind insulin. IGFBP-3 and IGFBP-5 can also form a ternary complex with IGF-I and acid-labile subunit (ALS) (Twigg et al. 2000) (Fig. 1a).
This review focuses on IGFBP-2 physiology and particularly discusses the role of IGFBP-2 in growth (bone), metabolism and cancer.
IGFBP-2 protein structure (Fig. 2)
Fig. 2.
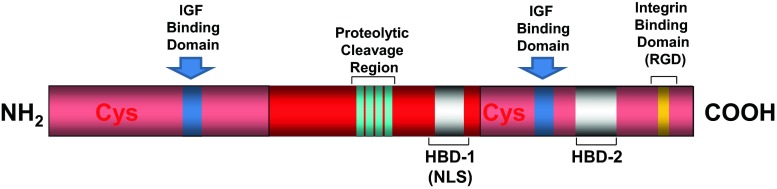
IGFBP-2 structure. Similarly to the other IGFBPs, mature IGFBP-2 protein has three structural regions: N-terminal cysteine-rich region (12 Cys); middle or link region; C-terminal cysteine-rich region (6 Cys). The cysteine-rich region of N- and C-terminal have IGF binding domains. A protease sensitive region is located within the link-region. A heparin binding domain (HBD-1) is located within the link-region. Another heparin binding domain (HBD-2) is located within the C-terminal regions. This region is also involved with integrin binding via the RGD domain
Similarly to the other IGFBPs (Firth and Baxter 2002), three structural regions can be identified in the IGFBP-2 mature protein: N-terminal cysteine-rich region; link region; C-terminal cysteine-rich region (Firth and Baxter 2002; Galea et al. 2012; Forbes et al. 1998), with the N- and C-terminal regions of IGFBP-2 involved in IGF-I/II binding (Ho and Baxter 1997a; Russo et al. 1999). IGFBP-2 is not glycosylated (Firth and Baxter 2002), and even though IGFBP-2 has a phosphorylation site (Graham et al. 2007), it is mainly found in a non phosphorylated form (Coverley and Baxter 1997).
-
i)
IGFBP-2 integrin binding domain (RGD). Similar to IGFBP-1, IGFBP-2 possesses an RGD sequence (Arg-Gly-Asp) at position 265–267 in human and 246–248 in rat (Firth and Baxter 2002) and other species (Daza et al. 2011). The IGFBP-2 RGD motif is functional and mediates IGFBP-2 association with cell membrane integrins (Song et al. 2003; Frommer et al. 2006; Pereira et al. 2004; Perks et al. 2007) including the integrins αVβ3 or α5β1 (Song et al. 2003; Frommer et al. 2006; Pereira et al. 2004; Perks et al. 2007; Foulstone et al. 2013) (Fig. 3, right side).
-
ii)
IGFBP-2 heparin binding domain (HBD). The middle region of IGFBP-2 presents a heparin binding domain (HBD) represented by the sequence 179PKKLRP184 in human IGFBP-2 mature protein. Using a site directed mutagenesis approach, we have demonstrated that the HBD motif is required for IGFBP-2 binding to components of the ECM, and IGFBP-2 mediated cell proliferation and migration (Russo et al. 2005) (Fig. 3, left side).
Recent work from Shen et al. (2012) demonstrated that the HBD motif in IGFBP-2, also reported as HBD1 (Fig. 2), mediates the binding of IGFBP-2 to the cell surface receptor protein tyrosine phosphatase β (RPTPβ) (Fig. 3 left), an event leading to inhibition of the RPTPβ phosphatase activity (Shen et al. 2012). An additional pH-dependent heparin-binding site, reported as HBD2 (Fig. 2), has been located within the carboxyl-terminal region and thyroglobulin type-1 domain of IGFBP-2 (Kuang et al. 2006).
-
iii)
IGFBP-2 nuclear localization signal (NLS) motif. Nuclear entry of IGFBP-2 (Besnard et al. 2001; Terrien et al. 2005; Miyako et al. 2009; Azar et al. 2011) has only been recently demonstrated by our laboratory, with the presence of a nuclear localization signal (NLS) (Azar et al. 2014). In contrast to IGFBP-3, −5, −6 (Schedlich et al. 2000; Iosef et al. 2008), which possess bi-partite NLS motifs in their C-terminal domains (Schedlich et al. 2000; Iosef et al. 2008), IGFBP-2 has a classic mono-partite NLS sequence (X-K-K/R-X-K/R-X), within its link/middle domain (Azar et al. 2014). This NLS domain is required for interaction with Importin-α (Chelsky et al. 1989) and corresponds exactly with the HBD1 sequence at 179PKKLRP184 (Russo et al. 2005; Azar et al. 2014) (Fig. 2).
Fig. 3.
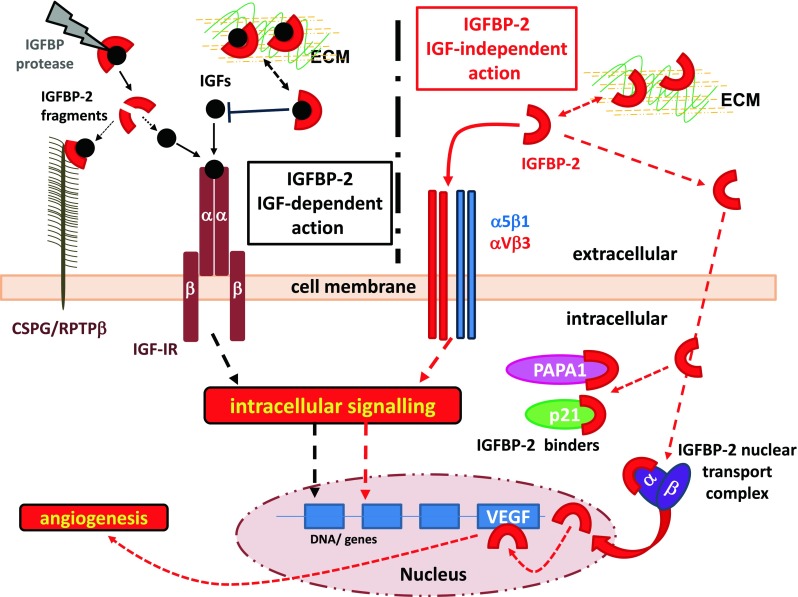
IGFBP-2 cellular activities, IGFBP-2 IGF-dependent action: IGFBP-2 modulates IGFs bioavailability to their receptors, with involvement of specific IGFBP-2 protease. IGFBP-2 also interacts with components of the extracellular matrix (ECM) and cell surface proteoglycan receptor (CSPG; RPTPβ), thus providing a peri-cellular reservoir of bioactive IGFs. IGFBP-2 IGF-independent action: IGFBP-2 also possesses intrinsic activities that are exerted via interaction with integrin receptors (αVβ3; α5β1) or via translocation into the cell and binding to specific binders (p21; PAPA1) or to nuclear transportation complexes (importin α and β) to enter the nuclear compartment. Within the nucleus IGFBP-2 directly or indirectly activates the expression of a number of genes involved tumourigenesis including angiogenesis. This IGFBP-2 nuclear activity might be also implicated in growth and metabolic action of IGFBP-2 as described in the main text. Although all these IGFBP-2 activities are likely to be exerted in the absence of the IGF ligands, as we suggest in this schematic cartoon, we cannot exclude the involvement of IGF-I/II in these IGFBP-2 intrinsic activities
IGFBP-2 - the knockout (KO) and transgenic (Tg) mice models
The IGFBP-2 KO mouse – effects on bone
Despite the widespread pattern of expression of IGFBP-2 during fetal development and the fact that IGFBP-2 exerts key endocrine, autocrine and paracrine functions such as transport and modulation of the IGFs in the pericellular space (Fig. 3 left), ablation of the IGFBP-2 gene has generated a phenotype surprisingly less dramatic than that initially predicted (Pintar et al. 1996; Wood et al. 2000). Initial analysis of IGFBP-2 null mice only identified alterations in spleen and liver size (Wood et al. 2000).
Critical clues about which tissue / organ might be affected by IGFBP2 gene ablation have come from studies regarding either IGFBP-2 expression in osteoblasts (Chen et al. 1993; Palermo et al. 2004; Wang et al. 1995), IGFBP-2 involvement in bone formation and density (Conover et al. 2002) or the growth of bones (Fisher et al. 2005).
In a follow up analysis of the Wood’s IGFBP-2 null mice (Wood et al. 2000) by DeMambro et al. (2008), it was demonstrated that these mice have gender specific changes in bone. DeMambro et al. (2008) reported that the IGFBP2 (−/−) male mice had shorter femora and were heavier than controls, while IGFBP2 (−/−) female mice had increased cortical thickness with a greater periosteal circumference compared with controls (DeMambro et al. 2008). Male IGFBP2(−/−) mice had instead reduced cortical bone area and reduction in the trabecular bone volume fraction due to thinner trabeculae than IGFBP2 (+/+) controls (DeMambro et al. 2008). While the cognate increase in the serum levels of IGF-I and liver expression of IGFBP-3 and IGFBP-5 (DeMambro et al. 2008) might have balanced the loss of circulating IGFBP-2, it appeared not to be effective in compensating the loss of local IGFBP-2 expression in bone.
The effects of IGFBP-2 ablation on bone have indirectly suggested that IGFBP-2 might play a key role in the regulation of bone turnover, and that this might be exerted via both IGF-dependent and IGF-independent mechanisms (Fig. 3). Investigation of these mechanisms by Kawai et al. (2011) then demonstrated that the HBD (Russo et al. 2005) of IGFBP-2 is a key determinant of IGFBP-2 action in bone and that IGFBP-2 functions are coordinated with IGF-I to stimulate growth and skeletal acquisition (Kawai et al. 2011). These findings were further supported in later work by DeMambro et al. (2012) where it was demonstrated that IGFBP-2 is a regulator of osteoclastogenesis and that the HBD (Russo et al. 2005) and the IGF-binding domains of IGFBP-2 (Fig. 2) are essential for the formation of fully differentiated and functional osteoclasts (DeMambro et al. 2012).
Furthermore, recent studies by Xi et al. (2014) have demonstrated that an IGFBP-2 based peptide containing the HBD1 sequence can rescue, presumably via interaction with RPTPβ, the differentiation and the expression of osteocalcin in calvarial osteoblast from the IGFBP-2 −/− mice. Although the IGFBP-2 −/− mouse model has greatly contributed to our understanding of the role IGFBP-2 in bone, the development of an animal model with osteoblast-specific deletion of IGFBP-2 should overcome some of the confounding intrinsic issues of Igfbp2 global deletion.
IGFBP-2 Tg mouse – effects on growth, metabolisms and bone
-
i)
Effects on growth. The IGFBP-2 transgenic mouse model developed by Hoeflich et al. (1999) has clearly demonstrated that IGFBP-2 is a negative regulator of postnatal growth in mice, an effect potentially mediated via reducing local IGF-I bioavailability. In these studies, the body weights of the IGFBP-2 Tg mice were substantially reduced, an effect attributable to the significant reduction in carcass weight, with absolute organ weights (except the spleen) not affected (Hoeflich et al. 1999). Similar effects were seen even when hemizygous CMV-IGFBP-2 transgenic mice were crossed with hemizygous PEPCK-bGH transgenic mice (with very high GH and IGF-I serum levels) (Hoeflich et al. 2001), thus supporting further the inhibitory role of IGFBP-2 in vivo.
Whether these effects are exclusively occurring as a result of IGFBP-2 sequestration of circulating and/or local IGF-I, or more likely involving both canonical and non-canonical actions of IGFBP-2 (Fig. 3 IGF-dependent and IGF-independent action), including IGFBP-2 interactions at the cell surface (Russo et al. 1995, 2005; Shen et al. 2012; Kawai et al. 2011; DeMambro et al. 2012) (Fig. 3) and intracellular / nuclear (Terrien et al. 2005; Miyako et al. 2009; Azar et al. 2011, 2014) (Fig. 3), is unclear. However, it is important to highlight that in both studies (Hoeflich et al. 1999, 2001), IGFBP-2 was constitutively over-expressed in most tissues resulting in very high local levels of IGFBP-2, such that overexpression of IGFBP-2 at supraphysiological levels may be different from more physiological alterations and therefore more profoundly affected local activities of IGFBP-2.
Further examination of these IGFBP-2 Tg mice (Rehfeldt et al. 2010) suggested that the reduced post-natal growth was also attributable to the observed reduced body lean protein, reduced water content and reduced muscle weight, although this was associated with increased fat percentage. Interestingly, the reduction in muscle weight was a result of smaller myofiber size, rather than reduced myofiber number, and in older mice, these myofibers showed elevated white glycolytic fibers and lactate dehydrogenase activity (Rehfeldt et al. 2010).
As seen in the studies by DeMambro (2008), most of the differences observed between transgenic and non-transgenic mice were more pronounced in males. These studies further support a functional role for IGFBP-2 in in vivo skeletal muscle growth and function, skeletal muscle metabolism as well as body growth and composition.
-
ii)
The effects on bone. Overexpression of IGFBP-2 also affects bone size and mass but not bone density (Eckstein et al. 2002), effects seen in either the presence or absence of growth hormone (GH) excess. These effects appear to be in contrast with those studies described by Conover et al. (2002), which demonstrated that administration of IGFBP-2 and IGF-II stimulated bone formation in rats.
Overexpression of IGFBP-2 in these mice (Eckstein et al. 2002) resembles, to some extent, the phenotype of the IGFBP2 −/− mice, while on the other hand the IGFBP-2 Tg mice bone phenotype is in part consistent with that describe for the IGF −/− mice.
These obvious discrepancies between the effects of IGFBP-2 overexpression in vivo (ie. increased fat mass, reduced skeletal muscle metabolism, body growth / composition and bone formation) and the effects of “add-back” or elevated circulating IGFBP-2 (e.g., inhibition of adipogenesis, anti-diabetic properties, bone formation and anorexia nervosa), suggest a potential dysregulation of normal physiological activities of IGFBP-2 in the IGFBP-2 Tg mice.
-
iii)
Effects on metabolism. In the above studies by Rehfeldt et al. (2010), the IGFBP-2 Tg mice also demonstrated a significant increase in fat proportion, which was confirmed by biochemical analysis of body composition indicating that the IGFBP-2 Tg mice contained a higher percentage of fat. Conversely, as mentioned above, these mice, showed lower percentages of water content and protein, but with ash percentage (bone) being equal to that of non-transgenic mice (Rehfeldt et al. 2010). Once again the differences in body fat and water content were more pronounced in males than in females.
In contrast are the findings from another study by Hoeflich et al. (1998) in non-transgenic mice selected for body weight (e.g., control [C] out-bred stock versus, high [H] or low [L] body weight) where only male L mice have significantly increased serum levels of IGFBP-2. The hepatic Igfbp2 mRNA levels were significantly increased in L mice, while they were decreased in H mice as compared to C mice. This increase in IGFBP-2 levels in the lower weight mice is consistent with the observation by Barrios et al. (2000), Hall et al. (1988) and Hotta et al. (2000) reporting elevated circulating levels of IGFBP-2 in patients with anorexia nervosa.
In agreement are the studies in human subjects by Wheatcroft et al. (2007a), Hedbacker et al. (2010), (Claudio et al. 2010a), Ballerini et al. (2004) and Frystyk et al. (1999), which report low circulating IGFBP-2 levels in obesity (Wheatcroft et al. 2007a; Hedbacker et al. 2010; Claudio et al. 2010a) and that administration of IGFBP-2 can prevent adipogenesis (Wheatcroft et al. 2007a) and has anti-diabetic properties (Hedbacker et al. 2010).
Modulation of IGFBP-2 levels
IGFBP-2 and PTEN cross-modulation
At the cellular level, the expression of IGFBP-2 is influenced by the functional status of the tumour suppressor gene phosphatase and tensin homolog (PTEN) (Levitt et al. 2005; Mehrian-Shai et al. 2007). Loss of function of PTEN, reported in 50 % of human cancers, is often cognate with high levels of IGFBP-2 expression (Levitt et al. 2005). On the other hand, induction of PTEN in cancer cells results in a substantial suppression of IGFBP-2 levels (Levitt et al. 2005). The involvement of PTEN in IGFBP-2 down-regulation, is also substantiated by chemical blockade of phosphatidylinositol 3 kinase (PI3K) / protein kinase B (Akt) (Levitt et al. 2005), (ie. mimicking PTEN activation) or that of the downstream signalling intermediate mammalian target of rapamycin (mTOR), both resulting in markedly reduced expression of IGFBP-2 (Mehrian-Shai et al. 2007; Martin and Baxter 2007).
A study by Perks et al. (2007) provides in vitro evidence for modulation of PTEN activity by IGFBP-2 via an integrin mediated mechanism. Subsequent work by Dean et al. (2014) in triple-negative breast cancer subjects, has reported that high levels of expression of IGFBP-2 are associated with loss of PTEN in these aggressive tumours, with this data suggesting that elevated IGFBP-2 is a potential prognostic marker for loss of PTEN in these tumours. It is thus reasonable to argue that activation of aggressive behavior in cancer cell might depend on the balance between IGFBP-2 and PTEN activation.
IGFBP-2 and p53
Another tumour suppressor gene involved in modulation of IGFBP-2 levels is p53 (Grimberg et al. 2006). As for IGFBP-3, IGFBP-2 mRNA is induced in a p53-dependent manner, with p53 shown to bind to an intronic regulative sequence of the IGFBP-2 gene (Grimberg et al. 2006). IGFBP-2 is a direct target of p53, and mediates p53 inhibition of IGF-I signalling (Grimberg et al. 2006), while the suppression of p53 results in reduction of IGFBP-2 expression, and activation ERK-1 in the presence of IGF-I (Grimberg et al. 2006). These findings suggested that the mitogenic activity of IGFs can be switched to growth inhibition and apoptosis via p53 activation of IGFBP-2.
IGFBP-2 and Ptch1
In a recent study by Villani et al. (2010) in basal cell carcinoma (BCC), it was demonstrated that the Hedgehog (Hh) signalling pathway, which is also implicated in many tumour types (e.g., brain, gastrointestinal, lung, breast and prostate cancers), plays a role in modulation of IGFBP-2 activities. These studies have shown that in the absence of Patched1 (Ptch1; trans-membrane receptor and negative regulator of Hh signalling), IGFBP-2 expression is enhanced and this led to the expansion of the epidermal basal cells in the proliferative layer of the skin, an event required to promote BCC development (Villani et al. 2010).
Thus Ptch1 inhibits epidermal progenitor cell expansion and basal cell carcinoma formation by limiting IGFBP-2 activities (e.g., IGFBP-2 mediated hair follicle progenitor cell progression into anagen) (Villani et al. 2010). Although synergism between the IGF1R and Hh signalling has been implicated in another cancer type (Rao et al. 2004), the expression and action of IGFBP-2 in BCC appears to be IGF-independent in nature (Villani et al. 2010) .
IGFBP-2 and HIF-1α
IGFBP-2 expression is also regulated by the transcription factor hypoxia-inducible factor 1 α (HIF-1α) (Feldser et al. 1999), the expression of which is in turn regulated by cellular O2 concentrations. Exposure to hypoxic conditions activates a number of adaptive cellular responses (Harris 2002; Semenza 1999). This is the case in solid tumours that become hypoxic because of tumour overgrowth and consequent aberrant development of new blood vessels within the tumour mass leading to inefficient local circulation (Harris 2002; Semenza 2003). Although hypoxia is toxic to both cancer cells and normal cells, cancer cells undergo genetic and adaptive changes that allow them to survive and even proliferate in a hypoxic environment (Harris 2002; Semenza 2003). These processes contribute to the malignant and aggressive phenotype (Poomthavorn et al. 2009). The upregulation of IGFBP-2 expression by HIF1α is therefore consistent with the crucial role of IGFBP-2 in tumourigenesis.
IGFBP-2 proteolysis
Providing another level of regulation of IGFBP-2 abundance and functionality is proteolysis of IGFBP-2. IGFBP-2 proteolysis has been reported under physiological conditions in vivo, in biological fluids (Matsumoto et al. 1996; Ishikawa et al. 1995), including human serum (Mark et al. 2005), human breast milk (Ho and Baxter 1997b), maternal serum during and post gestation (Giudice et al. 1990). Proteolysis or fragmentation of IGFBP-2 has also been exhaustively reported in many pathophysiological conditions, including nutritional deprivation or starvation (Pucilowska et al. 1993), inflammation (Brandt et al. 2011) and cancer (Russo et al. 1999; DeGraff et al. 2007).
Proteolysis of IGFBP-2 generates fragments of variable sizes (Fig. 3, left), depending on the cell line and species (Russo et al. 1999; Ishikawa et al. 1995; Ho and Baxter 1997b), including a small fragment of about 14–18 kD and a larger one of about 21–25 kDa. These fragments have been reported either not to bind or to have markedly reduced binding affinity for the IGFs (Russo et al. 1999; Ho and Baxter 1997b). We have described, in differentiating neuroblastoma cells (Russo et al. 2004) (1999), an IGFBP-2 fragment that binds IGF-I while it also associated to the cell surface (Fig. 3, left). This IGFBP-2 fragment most likely represents the carboxy-terminal portion of IGFBP-2 containing the HBD domain (Ishikawa et al. 1995; Ho and Baxter 1997b) (Fig. 3, left). A similar IGFBP-2 fragment was also detected peri-nuclearly in vivo in various tissues of the IGFBP-2 Tg mouse (Hoeflich et al. 2004). The role of this peri-nuclear IGFBP-2 fragment is yet to be determined.
In most cases, the activity of the IGFBP-2 proteases (e.g., MMPs, plasmin, calpain, kallikrein) can be activated or induced by hormones, cytokines and drugs (Russo et al. 2004; Si et al. 2007), but also modulated by protease inhibitors such as α2-macroglobulin (α2M), which enable the formation of an IGFBP-2/α2M complex resistant to proteolysis (Sunderic et al. 2013). Although formation of the IGFBP-2/IGF-I or -II complex might show an acquired resistance to proteolysis in some cases, a major function of IGFBP proteases is to cleave IGFBP and release IGFs from the IGFBP/IGF complex, an event likely to enhance local IGF action (Gerard et al. 2004; Qin et al. 2006). This has been exhaustively described in cancer and cancer cell models where proteolysis of IGFBP-2 might be regarded as an important post-translational event contributing to increased tumour aggressiveness.
IGFBP-2 cellular activities
IGFBP-2 action at the cell surface mediated via integrin receptors
Integrins represent a family of transmembrane α/β heterodimers receptors that mediate cell-cell and cell–extracellular matrix (ECM) interaction and play a key role in the regulation of cell cycle, cell shape and adhesion and cell motility (Ruoslahti 1996). Integrin interaction with the components of the ECM (e.g., fibronectin, vitronectin, laminin) occurs via a conserved integrin attachment recognition site containing the Arg-Gly-Asp (RGD) motif (Ruoslahti 1996). As mentioned earlier, this RGD motif is present in IGFBP-1 and IGFBP-2 (Fig. 2) and is required for their interactions with cell membrane integrin receptors.
The effects mediated by the interaction of IGFBP-2 with the αVβ3 or α5β1 integrins remain controversial. A study by Pereira and co-workers investigated the interaction of IGFBP-2 with αVβ3 integrin by over-expressing the αVβ3 in MCF-7 breast cancer cells (Pereira et al. 2004). It was demonstrated that αVβ3 integrin and IGFBP-2 act cooperatively in a negative regulatory manner to reduce tumour growth (MCF-7/αVβ3 cell xenografts in nude mice) and the migratory potential of breast cancer cells (Pereira et al. 2004).
In other studies by Schutt et al. (2004) and Frommer et al. (2006) investigating the effects of IGFBP-2 interaction with the α5β1 integrin in the breast cancer cell line Hs578T, it was shown that IGFBP-2 binding to integrin led to de-phosphorylation of the focal adhesion-kinase (FAK) and p42/44 MAP-kinases, reducing cell proliferation and activating pro-apoptotic events in Hs578T cells.
Conversely, studies by Mendes et al. (2010) demonstrated in glioma cells that the interaction of IGFBP-2 with the α5β1 integrin is required for IGFBP-2 induced motility of glioma cells and activation of invasion pathways, all events mediated via the RGD motif of IGFBP-2 and leading to tumour progression. Similarly, in work from Perks et al. (2007) in MCF-7 breast cancer cells and by Uzoh et al. (2011) in prostate cancer cells, an integrin-mediated pro-tumourigenic action of IGFBP-2, which occurs via modulation of PTEN has also been suggested.
More recently, Foulstone et al. (2013) demonstrated that IGFBP-2/α5β1-mediated PTEN suppression in MCF-7 breast cancer cells, resulted in protection of MCF-7 cells from chemotherapy induced cell death and this requires a functional oestrogen receptor-α (ER-α) (Foulstone et al. 2013). Silencing of IGFBP-2, leading to loss of the ER-α abolished cell response to estrogen while exogenous IGFBP-2, acting through the α5β1 integrin, increased ER-α (Foulstone et al. 2013). This is interesting because IGFBP-2 not only modulates IGFs and directly regulates PTEN via the α5β1 integrin, but also has a role in maintaining ER-α expression.
Consistent with this pro-tumourigenic action of IGFBP-2 exerted via integrin binding, is the work from Song et al. (2003), who used a yeast two-hybrid system and demonstrated in glioblastoma multiforme (GMB), that the invasion inhibitory protein-45 (IIp45) binds to IGFBP-2 via the thyroglobulin-RGD region of the C terminus of IGFBP-2. In these cells, IIp45 inhibited the expression of cell invasion-associated genes and invasion of GBM cells both in vitro and in xenograft models (Song et al. 2003). Their studies showed that IIp45 binding to IGFBP-2 prevented glioma cell invasion (Song et al. 2003), thus demonstrating that the IGFBP-2 interaction with integrins is required for an enhancing role for IGFBP-2 in GBM invasion.
A potential explanation for the observed integrin-mediated inhibitory activities of IGFBP-2, described less commonly, is that the cell lines used in those studies do not normally express αVβ3 integrin (Pereira et al. 2004), do not express IGFBP-2 and/or key components of the IGF system (Frommer et al. 2006; Schutt et al. 2004). It is therefore possible that the antagonistic and inhibitory activities of IGFBP-2 observed in these studies might reflect specific cell types and experimental conditions.
IGFBP-2 binding to ECM components and cell membrane proteoglycans
There is strong consensus that ECM bound IGFBP-2 contributes to modulation of IGF bioavailability in the peri-cellular space (Fig. 3 left), and the affinity of IGFs for ECM-bound IGFBP-2 is likely to be significantly reduced compared to that enabling the formation of the IGFBP-2/IGF complex in solution (Arai et al. 1996). Therefore ECM-bound IGFBP-2 acts as an active reservoir for the local IGFs, which in turn may also prevent IGF clearance from the peri-cellular space.
These peri-cellular or cell surface storage sites for bioactive IGFs, can be affected by IGFBP-2 proteases (Russo et al. 1999) (Ishikawa et al. 1995), which by cleaving IGFBP-2 augment local release of IGFs (Firth and Baxter 2002) (Fig. 3 left).
We have identified an IGFBP-2 fragment on the cell surface of neuroblastoma cells (Russo et al. 1999), and demonstrated that such IGFBP-2 fragment can hold IGF-I onto the cell surface (Fig. 3 left). Thus proteolysis of membrane-bound IGFBP-2 provides a mechanism for creating low affinity peri-receptor IGF-I binding sites in neuroblastoma cells (Russo et al. 1999), and most likely in other cancer cells.
Of interest is a study by Kiepe et al. (2008), which demonstrated that human haemo-filtrate derived IGFBP-2 fragments possess intrinsic mitogenic activities in growth plate chondrocytes. These IGFBP-2 fragments overlap the middle and carboxyl terminal region of IGFBP-2 (e.g., variably extending within the amino-acid stretch Gly104-Gln289 of the mature protein) and have a size comparable to that described for proteolytic fragments of IGFBP-2 (Kiepe et al. 2008). Interestingly, in the studies by Kiepe et al., it was shown that the mitogenic effects of these fragments were comparable to those seen with equimolar concentrations of IGF-I, although there was no IGF1R signalling associated with the activity of the haemo-filtrate derived IGFBP-2 fragments (Kiepe et al. 2008).
The biological activity of these IGFBP-2 fragments, described to occur independently of IGF-I, was associated with their ability to bind to the cell surface (Kiepe et al. 2008). Another interesting observation in these studies was that intact IGFBP-2 did not enhance chondrocyte proliferation (presumably by complexing with the IGFs), while partially reduced full-length IGFBP-2 (e.g., DTT-mediated disruption of the Cys-Cys bonds, leading to loss of IGF binding) stimulated chondrocyte proliferation to a comparable extent to that observed for the IGFBP-2 fragments (Kiepe et al. 2008). Although the putative cell membrane binding site was not identified in this study, activation of the mitogen-activated protein kinase (MAPK) pathway was shown to be involved in these events.
Of relevance to the findings of Kiepe et al., is the study by Kawai et al. (2011) demonstrating that an IGFBP-2 based HBD1 peptide (Russo et al. 2005; Azar et al. 2014) was able to produce skeletal rescue in the Igfbp2−/− mice (DeMambro et al. 2008), via activation of the IGF-I/Akt and β-catenin signalling pathways (Kawai et al. 2011). Kawai et al. demonstrated that the HBD1 of IGFBP-2 has IGF binding-independent biological activity in the growing skeleton (Kawai et al. 2011). These findings suggest, as we have described in another cell system (Russo et al. 2005; Azar et al. 2014), that the HBD1 motif present in the Kawai HBD peptide and within IGFBP-2 fragments (Russo et al. 1999, 2005; Kiepe et al. 2008), has a key functional role and is likely to be involved in recognition of a cell surface binding site. This putative IGFBP-2 receptor might be responsible for activation of specific intracellular signalling pathways as described by Kiepe et al. (2008) and Kawai et al. (2011).
In another study by Xi et al. (2013), peptides spanning the HBD1 or HBD2 motif (Fig. 2) were used to determine whether these domains were involved in the observed IGFBP-2-mediated resistance to high fat feeding and inhibition of preadipocyte differentiation (Wheatcroft et al. 2007a). The HBD2 motif containing peptide was more effective in mediating inhibition of adipogenesis in vitro and reduction of total fat mass and increase serum leptin in vivo in IGFBP-2−/− mice. The activity of the HBD2 peptide was comparable to that demonstrated by native full-length IGFBP-2 (Xi et al. 2013).
As mentioned earlier, studies by Shen et al. (2012) and DeMambro et al. (2012) have shown that IGFBP-2 in the presence or absence of IGFs, interacts with the cell surface receptor RPTPβ (Shen et al. 2012) (Fig. 3, left). The characterisation of this RPTPβ receptor was previously reported from work in the human brain by Shitara et al. (1994). It was demonstrated that RPTPβ was a chondroitin-sulfate proteoglycan with a molecular weight of about 350–500 kDa, and that the extracellular region of human RPTPβ contained a classic Fibronectin type III module (Shitara et al. 1994).
Shen et al. (2012) demonstrated that IGFBP-2 via its HBD (Russo et al. 2005), interacts with the cell surface receptor RPTPβ leading to dimerization and inactivation of this receptor, an event which in turn enhances PTEN tyrosine phosphorylation and inhibits PTEN activity. Interestingly, it was shown that an antibody against the Fibronectin motif (Fn3), prevented the interaction of IGFBP-2 with RPTPβ, suggesting that IGFBP-2 binds to RPTPβ via the Fn3 motif, previously described by Shitara et al. (1994).
Intracellular and intranuclear activities of IGFBP-2
IGFBP-2 is present within the cell (Terrien et al. 2005; Miyako et al. 2009; Hoeflich et al. 2004), where it interact with cell cycle inhibitor p21 or Pim-1-associated protein-1 (PAP-1)-associated protein-1 (PAPA-1), with both of these interactions affecting IGFBP-2 growth-promoting actions (Terrien et al. 2005; Miyako et al. 2009; Hoeflich et al. 2004) (Fig. 3, left). Recent work from our laboratory has provided strong biochemical evidence for IGFBP-2 nuclear localization in cancer cells (Azar et al. 2011, 2014). Nuclear translocation of IGFBP-2 is an active import process (Azar et al. 2011, 2014), mediated by importin-α and involves a nuclear localization signal (NLS) motif located within the link-region of human IGFBP-2 at 179P-K-K-L-R-PP185 (Azar et al. 2014) (Fig. 3, left). This NLS motif overlaps exactly with that of our previously described IGFBP-2 HBD motif (Russo et al. 2005) (Fig. 2), and this is also a common feature described for other IGFBPs (Firth and Baxter 2002). A functional role for the NLS motif of IGFBP-2 was then demonstrated via an NLS mutant form of IGFBP-2 (NLSmutIGFBP-2), which did not interact with importin-α and was not transported / translocated into the nucleus (Azar et al. 2014).
We have previously described that IGFBP-2 overexpression enhances neuroblastoma cell proliferation and migration (Russo et al. 2005), events suggesting that IGFBP-2 activates a pro-tumourigenic gene expression program in these cells. Overexpression of IGFBP-2 leads to induction of genes regulating cell proliferation, migration and invasion, while tumour suppressor genes are down-regulated (Azar et al. 2011, 2014).
Of these genes, vascular endothelial growth factor-A (VEGF-A) was markedly upregulated by the Wt-IGFBP-2, but not by the NLSmut-IGFBP-2 (Azar et al. 2014), suggesting a potential role for nuclear IGFBP-2 in transactivation of the VEGF gene (Fig. 3, bottom). This was confirmed via VEGF-A-promoter-luciferase assays. Furthermore, as we predicted the NLSmut-IGFBP-2 did not enter the nucleus, so failing to elicit luciferase activity. These findings provide strong support for IGFBP-2 as a transactivator of the VEGF-A gene (Fig. 3, bottom) and likely other growth promoting genes.
In further experiments, it was then demonstrated that IGFBP-2-mediated elevation of VEGF-A in neuroblastoma cells, led to activation of angiogenesis in vivo as determined by the chick embryo chorio-allantoic membrane (CAM) assay. No angiogenesis was seen in the presence of NLSmut-IGFBP-2.
Initiation of angiogenesis is an important early event in tumour progression, and the studies above demonstrate that IGFBP-2 enhances the expression of the angiogenic growth factor VEGF-A, an event that requires intracellular / nuclear localization of IGFBP-2. It is thus clear that nuclear IGFBP-2 directly or indirectly contributes to the development of a pro-metastatic milieu consistent with the enhancement of an aggressive cancer phenotype.
IGFBP-2 and metabolism
There is increasing evidence for IGFBP-2 regulation by metabolism and in metabolic disorders, while there is also evidence for IGFBP-2 contribution to regulation of normal metabolism (Sabin et al. 2011a). In obesity, circulating levels of IGFBP-2 are suppressed (Wheatcroft et al. 2007a; Hedbacker et al. 2010; Claudio et al. 2010a; Ballerini et al. 2004; Frystyk et al. 1999; Nam et al. 1997a), and low levels of IGFBP-2 are associated with Type 2 diabetes mellitus (T2DM) (Frystyk et al. 1999), and metabolic syndrome (Heald et al. 2006). On the other hand, IGFBP-2 overexpression protects against obesity and diabetes (Wheatcroft et al. 2007a; Hedbacker et al. 2010), via mechanisms involving IGFBP-2 mediated inhibition of adipogenesis and modulation of insulin sensitivity (Wheatcroft et al. 2007a; Hedbacker et al. 2010).
In a study in obese mice and men by Li and Picard (2010a), it was demonstrated that IGFBP-2 mRNA levels were significantly lower in visceral white adipose tissue (WAT) than in subcutaneous WAT. This differs from findings in individuals with anorexia nervosa, who have high IGFBP-2 levels and significantly reduced levels of total percentage fat and visceral adipose tissue (Krassas 2003; Counts et al. 1992). This suggests that IGFBP-2 gene might be modulated in a depot-specific fashion. Furthermore, studies from our laboratory (Sabin et al. 2011a), have demonstrated that increased consumption of dietary fat or sugar in early life differentially impact upon adiposity (increased) and insulin sensitivity (decreased). These changes were associated with alterations in IGFBP-2 mRNA expression in muscle and fat (Sabin et al. 2011a), with monounsaturated fatty acid affecting IGFBP-2 mRNA in in vitro cultures of skeletal myotubes (Sabin et al. 2011a).
A direct correlation between low circulating levels of IGFBP-2 and obesity has been described by Nam et al. (1997a) in obese males. IGFBP-2 levels were found to be suppressed and inversely related to levels of free IGF-I, which were elevated (Nam et al. 1997a) and positively correlated with elevated fasting serum insulin levels (Nam et al. 1997a). However, in obese subjects the levels of total IGF-I and those of IGFBP-3, despite suppressed GH levels, were not significantly different between obese and control subjects (Nam et al. 1997a). These findings suggest a potential role for IGFBP-2 in modulation of IGF-I bioavailability and action (e.g., abnormal low IGFBP-2 leading to abnormal elevated free IGF-I) and in turn, IGFBP-2 contribution to maintenance of metabolic function (Nam et al. 1997a).
A study by Frystyk et al. (1999), investigated potential alterations in the GH/ IGF axis following overnight fasting in matched groups of lean subjects, obese subjects with or without T2DM, and subjects with type 1 diabetes mellitus (T1DM). In obese subjects without T2DM, IGFBP-2 levels were reduced and they were further reduced in those patients that also had T2DM. Interestingly, IGFBP-2 was actually increased in patients with T1DM (Frystyk et al. 1999), consistent with findings by others in T1DM (Bereket et al. 1999) and diabetic retinopathy (Frystyk et al. 2003), suggesting that T1DM and T2DM have a different impact on IGFBP-2 levels, perhaps related to differences in insulin sensitivity.
Low circulating levels of IGFBP-2 have also been reported in lifestyle-related childhood obesity (with normal growth in spite of reduced GH secretion) (Ballerini et al. 2004) and pre-pubertal obese subjects (Street et al. 2013). In childhood obesity, the reduction in IGFBP-2 serum levels coupled with a lower ratio of IGFBP-2/IGF-I, suggest an increase of tissue IGF-I bioavailability which in turn might be contributing to the observed accelerated growth seen in the pre-pubertal years (Ballerini et al. 2004).
Furthermore, in intra-uterine growth retardation (IUGR) (Smerieri et al. 2011; Giudice et al. 1995), cord serum levels of IGFBP-2 are elevated and negatively correlate with both birth length and weight (Smerieri et al. 2011; Giudice et al. 1995). Conversely, circulating levels of IGFBP-2 are low in pre-pubertal and pubertal subjects that were born small for gestational age (IUGR/SGA) (Ko et al. 2012; de Kort et al. 2010). These changes in IGFBP-2 levels, were investigated by Ko et al. (2012), and de Kort et al. (2010); they assessed growth pattern in relation to insulin resistance, body composition and IGFBP-2 (potential modulator of insulin sensitivity) in individuals born small for gestational age (SGA;pre-pubertal and pubertal subjects). IGFBP-2 levels were inversely correlated with BMI, body fat mass, percentage body fat, insulin and leptin levels in both pre-pubertal and pubertal groups (Ko et al. 2012) (de Kort et al. 2010), while being positively correlated with insulin sensitivity (de Kort et al. 2010). In young adults who were born SGA, reduced serum IGFBP-2 levels also associated with increased cardiovascular risk markers (de Kort et al. 2010). These studies (Ko et al. 2012) (de Kort et al. 2010) therefore suggest that IGFBP-2 could be a potential marker for early recognition of insulin resistance, particularly in SGA children.
Increasing evidence has indicated that leptin, the product of the obesity gene (Ob), is a major metabolic regulator of circulating IGFBP-2 (Wheatcroft et al. 2007a; Hedbacker et al. 2010). Obesity is associated with leptin resistance and increased circulating levels of leptin (a regulator of energy balance produced from white adipose tissue) (Friedman and Halaas 1998). Studies in rodents have shown that leptin regulates hepatic IGFBP-2 expression (Hedbacker et al. 2010) and that IGFBP-2 levels are closely linked with body weight and glucose metabolism (Wheatcroft et al. 2007a; Sabin et al. 2011a). In recent studies from our laboratory (Yau et al. 2014a), we demonstrate that leptin increases skeletal muscle IGFBP-2 by directly activating leptin signalling pathways in the periphery, as well as activating central mechanisms which communicate to peripheral tissues via the sympathetic nervous system (SNS) through sympathetic activation of β-adrenergic receptors (Yau et al. 2014a). We also showed, in sheep, that central infusion of leptin improves glucose tolerance and reduces circulating insulin levels in vivo, while silencing of IGFBP-2 in human skeletal muscle reduces insulin signalling, as well as leptin- and insulin-stimulated glucose uptake (Yau et al. 2014a). The direct and indirect regulation of skeletal muscle IGFBP-2 by leptin, mediated via STAT-3 and PI3K/AKT signalling pathways, suggest that leptin appears to regulate peripheral insulin sensitivity via IGFBP-2 (Yau et al. 2014a). These findings also suggest that leptin may improve insulin sensitivity by directly and indirectly regulating peripheral IGFBP-2 expression.
As mentioned earlier, low circulating levels of IGFBP-2 are associated with adiposity (Sabin et al. 2011a, b; Nam et al. 1997b; Ballerini et al. 2004; Claudio et al. 2010b) and particularly with visceral adiposity rather than subcutaneous adiposity (Li and Picard 2010b). Conversely, high IGFBP-2 levels as seen by overexpression of IGFBP-2 in mice appears to prevent diet induced obesity and insulin resistance, with IGFBP-2 also preventing murine adipocytes differentiation in vitro (Wheatcroft et al. 2007b). Interestingly, recent studies by Xi et al. (2013) have shown that IGFBP-2-based peptides containing the HBD motifs, efficiently inhibited preadipocyte differentiation in male mice, suggesting that IGFBP-2 cell surface interactions via these motifs might be implicated in the modulation of adipogenesis.
Recent studies from our laboratory (Yau et al. 2014b), investigating the effects of IGFBP-2 on visceral versus subcutaneous adipocytes, have demonstrated that exogenously added human IGFBP-2 significantly reduced adipo/lipogenesis in visceral adipocytes, but not in subcutaneous adipocytes. In fact, silencing IGFBP-2 resulted in exaggerated adipo/lipogenesis in visceral adipocytes, but not in subcutaneous adipocytes, an effect ablated when IGFBP-2 was added-back.
In agreement with Xi et al. (2013) studies with peptides containing the HBD motifs, our HBD-1 mutant IGFBP-2 had reduced inhibitory effects on adipogenesis, when compared with wild-type IGFBP-2 (Yau et al. 2014b). Wild-type IGFBP-2 increased phosphorylation of focal adhesion kinase (FAK) and decreased phosphatase and tensin homolog (PTEN) levels, suggestive of integrin-mediated signalling (Yau et al. 2014b). Blockade of this signalling, using Echistatin, completely negated the effects of IGFBP-2 on visceral adipo/lipogenesis (Yau et al. 2014b). These studies have thus provided strong evidence that IGFBP-2 inhibits both adipogenesis and lipogenesis in visceral, but not subcutaneous, adipocytes (Yau et al. 2014b). This depot-specific impairment appears to be independent of IGF-I and involves cell-surface association of IGFBP-2 and activation of integrin signalling pathways (Yau et al. 2014b).
IGFBP-2 and cancer
IGFBP-2 is one of the most commonly and abundantly expressed IGFBPs in a broad range of human cancers, often reaching the high abundance levels seen in fetal life (Reeve et al. 1992a). IGFBP-2 expression positively correlates with tumour aggressiveness and the expression of known tumour markers (Tennant et al. 1996; Wex et al. 1998). The role of IGFBP-2 in glioma (Wang et al. 2003; Fukushima and Kataoka 2007; Becher et al. 2008; Moore et al. 2009; Fukushima et al. 2007), prostate (Ho and Baxter 1997a; Heinlein and Chang 2004; Karantanos et al. 2013; Kimura et al. 1996; Cohen et al. 1993; Degraff et al. 2009), breast (Perks et al. 2007; Foulstone et al. 2013; Martin and Baxter 2007; Wang et al. 2008; Juncker-Jensen et al. 2006; Sohn et al. 2013; Mireuta et al. 2010) and ovarian (Karasik et al. 1994; Kanety et al. 1996; Wang et al. 2006; Lee et al. 2005) cancers has been exhaustively discussed and therefore we wish to focus on other major malignancies in which the role of IGFBP-2 remains still unclear.
Leukaemia
Leukaemias are cancer of the white blood cells (lymphoid or myeloid; acute and chronic), which begin in the bone marrow, with four major types including acute lymphocytic leukaemia (ALL) (Inaba et al. 2013; Lo Nigro 2013; Carroll and Raetz 2012; Rivera et al. 1994), chronic lymphocytic leukaemia (CLL) (Rodríguez-Vicente et al. 2013; Shanshal and Haddad 2012; Hillmen 2011), acute myeloid leukaemia (AML) (Estey 2013; Puumala et al. 2013; Gamis et al. 2013) and chronic myeloid leukaemia (CML) (Jabbour and Kantarjian 2012; Maru 2012; Zhang and Rowley 2011).
A number of studies have associated IGFBP-2 levels to the proliferation of lymphoblasts (Barrios et al. 2000; Wex et al. 1998; Mohnike et al. 1996; Dawczynski et al. 2003), and high levels of IGFBP-2 have been associated with or predicted adverse outcomes in childhood leukaemia (Barrios et al. 2000; Wex et al. 1998; Elmlinger et al. 1996; Attard-Montalto et al. 1998; Vorwerk et al. 2005; Dawczynski et al. 2006, 2008; Hattori et al. 2006; Kuhnl et al. 2011; Kitszel and Krawczuk-Rybak 2007).
IGFBP-2 appears to be differentially expressed between leukaemic B- and T-lymphocyte cell lines (Elmlinger et al. 1996), with the latter showing higher levels of IGFBP-2 mRNA (Elmlinger et al. 1996, 1998). The levels of IGFBP-2 secreted by these cells are regulated by the IGFs, mostly IGF-II, via pathways involving activation of the IGF1R (Elmlinger et al. 1998).
In a study by Mohnike et al. (1996) it was shown that in children with ALL the levels of IGF-I, −II and IGFBP-3 are significantly decreased, while the levels of IGFBP-2 are markedly elevated (+4.0/−0.45 to +7.4 SDS). Similar regulation of IGF system components in ALL patients, including the increase in IGFBP-2 levels, was also reported by Wex et al. (1998). Interestingly, these studies showed that the high serum levels of IGFBP-2 correlated with the detection rate of IGFBP-2 mRNA in the tumour cells. During chemotherapy, IGFBP-2 mRNA levels decreased from 72 % at diagnosis to 35 % post-chemotherapy. These finding suggest that the increased serum levels of IGFBP-2, measured in children suffering from ALL, mainly originated from the tumour clone itself (Wex et al. 1998).
Studies by Dawczynski et al. (2003) report steady and significant increases in IGFBP-2 levels in patients with either ALL or AML following hematological stem cell transplantation (HSCT) (Dawczynski et al. 2003). This observed elevation in serum IGFBP-2, in childhood AML after HSCT, was associated with a high relapse risk (Dawczynski et al. 2006). In fact, most AML-patients with evidence of relapse showed a continuous increase of IGFBP-2 during 100 days follow-up. After HSCT, as above, IGFBP-2 concentrations were significantly higher in patients with relapse than in children without relapse, and patients with IGFBP-2 concentrations up to 4.5 SDS were more likely to relapse and demonstrate poorer clinical outcome (Dawczynski et al. 2006).
In summary, there is a strong consensus that serum and mRNA levels of IGFBP-2 are independent risk factors for the prediction of chemo-resistance and relapse in ALL and AML patients (Dawczynski et al. 2006, 2008; Hattori et al. 2006; Kuhnl et al. 2011; Kitszel and Krawczuk-Rybak 2007). It could be speculated that molecular therapies targeting the IGF pathway, and in particularly IGFBP-2, might assist with the management of these difficult to cure malignancies.
Lung cancer
Lung cancers are classified according to the type of cell affected and include the most common non-small cell lung cancer (NSCLC), also classified as squamous cell carcinoma, small cell lung cancer (SCLC) and mesothelioma. IGFBP-2 is consistently expressed in small cell lung cancer (SCLC), non-small cell lung cancer (NSCLC) cell lines and primary NSCLC tumours (Jaques et al. 1992; Reeve et al. 1992b; Hu et al. 2014) (Reeve et al. 1993), while the pattern of expression for all the other IGFBPs differ significantly between cell lines and primary tumours.
Interesting, studies from Reeve and co-workers in SCLC and NSCLC (Reeve et al. 1993), showed that the secreted IGFBP-2 can also associate with the cell surface of SCLC cells, but not with that of the NSCLC cells. As a result of this, when the SCLC and NSCLC cells were exposed to iodinated IGF-I or -II, most of the IGF binding was found associated with cell surface bound IGFBP-2 in the SCLC cells, while it was associated with the IGF1R in the NSCLC cells (Reeve et al. 1993). These findings suggest for the first time that in addition to the canonical regulatory activities of soluble IGFBP-2, cell surface associated IGFBP-2 might also play a key role in regulating IGF responsiveness in these type of tumours (Reeve et al. 1993).
A later report from Dong et al. (2002) provided evidence for soluble IGFBP-2 inhibiting DNA synthesis in Mv1 mink lung epithelial cells (CCL64), an event that was attenuated in the presence of IGF-I. As shown by Reeve et al. in SCLC cells (Reeve et al. 1993), Dong and co-workers also showed that IGFBP-2 was associated with the CCL64 cell surface and that this association was blunted in the presence of IGF-I (Dong et al. 2002). The use of IGF analogs, with selective high binding affinity for IGFBPs or for the IGF1R, indicated that the observed IGFBP-2-mediated inhibition of DNA synthesis in CCL64 cells only occurs when IGFBP-2 binds to the cell surface of CCL64 cells (Dong et al. 2002), where it presumably activates specific signalling.
SCLC cells express high levels of IGFBP-2 and this expression appears to be mediated via NeuroD, a neuroendocrine cell-specific transcription factor (Yazawa et al. 2009). SCLC is the most aggressive in vivo lung cancer, whereas in vitro growth of SCLC is paradoxically slow as compared with that of NSCLC (Yazawa et al. 2009). A further observation by Yazawa et al., that might provide an explanation for this intriguing behavior of SCLC cell in vitro, is that the IGFBP-2 promoter is rarely methylated in tumour samples, whereas it is hyper-methylated in most adenocarcinomas and squamous cell carcinoma lines. It is thus likely that epigenetic alterations at the IGFBP-2 promoter are responsible for the striking differences in IGFBP-2 expression between SCLC and NSCLC in vivo and growth of SCLC and NSCLC cells in vitro.
Further studies in lung adenocarcinoma cells suggest that the elevated expression of IGFBP-2 enhances aggressive behavior of cancer cells via inhibition of apoptosis (Migita et al. 2010). This was shown to occur via an IGFBP-2 mediated decrease of pro-caspase-3 levels, supported by the observation that intracellular IGFBP-2 and procaspase-3 are expressed in a mutually exclusive manner (Migita et al. 2010).
The dramatic increase in circulating levels of IGFBP-2 observed in many cancers, suggests that IGFBP-2 might be considered as a tumour antigen and as such, it could activate an IGFBP-2 tumour-specific immunity. This appears to be the case in lung cancer where serum levels of IGFBP-2 autoantibody are significantly elevated (Zhang et al. 2013). Whether the high circulating levels of IGFBP-2 (Lee et al. 1999; Guo et al. 2013) and anti-IGFBP-2 autoantibodies are appropriate diagnostic biomarkers for lung cancer (Zhang et al. 2013) remains to be determined.
Colon cancer
Colon or colorectal cancer (CRC) originates in the mucosal surface of the large bowel, where dysregulated cell growth can lead to pre-cancerous formations such as intestinal polyposis including hyperplastic or inflammatory polyps or serrate adenomas (Moser et al. 1992). Common sites of CRC development are the sigmoid colon (25 %), rectum (21 %), caecum (20 %), rectosigmoid junction (20 %), followed by transverse colon (15 %) and the ascending colon (10 %).
A large proportion (80 %) of CRCs carries a mutation in the tumour suppressor gene APC (Adenomatous Polyposis Coli; e.g., Familial Adenomatosus Polyposis). This mutation produces a truncated and inactive APC protein, an event leading to dysregulated cell growth and oncogenic transformation (Burgess 1998) (Rowan et al. 2000). Mutations in the APC gene affect regulation and degradation of β-catenin, a protein with multiple functions including regulation of cell-cell adhesion and gene transcription (Valenta et al. 2012). Overexpression and/or cytoplasm accumulation of β-catenin is often associated with colorectal carcinomas, which might lead to its translocation into the nucleus where β-catenin acts as a proto-oncogene modulating the expression of a number genes (Valenta et al. 2012), including up-regulation of IGFBP-2 gene (Naishiro et al. 2005).
Although the IGF system is significantly expressed in CRC, the role of IGFBP-2 in CRC is unclear with substantial discrepancies between the in vivo (human and animal models) and in vitro data suggesting either tumour promoting or tumour inhibiting activity for IGFBP-2.
IGFBP-2 is overexpressed in colorectal carcinomas (Naishiro et al. 2005; Ben-Shmuel et al. 2013; Renehan et al. 2000) and its mRNA is reported to be increased in sporadic colorectal cancers (Mishra et al. 1998).
In the study by Mishra et al. (1998), it was shown by in situ hybridization and IGF binding studies that the mRNA for IGFBP-2 and its protein product were located in malignant cells in the colon cancer tissue and not in the surrounding stromal cells. Furthermore, a study by Ben-Shmuel et al. (2012) provided evidence for a role for IGFBP-2 in promotion of colon cancer progression, an event under the transcriptional control of the NF-κB signalling pathway. In these studies (Ben-Shmuel et al. 2012), IGFBP-2 was highly expressed throughout human CRC tissue samples and co-localized with the phosphorylated p65 subunit of NF-κB (Ben-Shmuel et al. 2012). These findings suggest a potential autocrine action for IGFBP-2 and point to a key role for IGFBP-2 in progression (Naishiro et al. 2005; Ben-Shmuel et al. 2012) and metastatic processes in CRC.
Interestingly, serum IGFBP-2 levels are elevated in CRC patients (el Atiq et al. 1994) and appears to be associated with increased risk of mortality (Liou et al. 2010). A study by Miraki-Moud et al. (2001) in patients with acromegaly, which are at increased risk of developing colorectal carcinoma, reported significantly increased IGFBP-2 serum levels, and strong immunoreactivity for IGFBP-2 in malignant colonic epithelium compared to benign epithelium.
Conversely, some in vitro work shows that IGFBP-2 inhibits proliferation of IGF-responsive colon carcinoma cell lines (Hoflich et al. 1998; Corkins et al. 1995). A study by Michell et al. (1997) in normal and tumour colonic tissue, demonstrated that IGFBP-2 mRNA is expressed in all normal and cancer cells, with IGFBP-2 only proteolysed in colon cancer tissue. Proteolysis of IGFBP-2, most likely, would result in increased local levels of IGF, thus conferring a growth advantage for the colonic tumour cells as described by (Si et al. 2007) in the human colon cancer cell line HT29.
An inhibitory role for IGFBP-2 in CRC was also demonstrated by Diehl et al. (2009), where IGFBP-2 Tg mice with high levels of IGFBP-2 both systemically and locally in the intestine, were subjected to chemically induced colorectal carcinogenesis. Although the tumour incidence in the Tg and Wt control animal was comparable, the volume of the adenomas in the IGFBP-2 Tg was dramatically reduced and IGFBP-2 serum levels negatively correlated with tumour volume (Diehl et al. 2009). In these studies it was also demonstrated that the reduced volume of the adenomas in the IGFBP-2 Tg mice was consistent with reduced number of cells positive for the proliferation marker Ki67. However, it could be argued that the persistent extreme overabundance of IGFBP-2 in these animals, might significantly account for the inhibitory effects of IGFBP-2 and reduced tumour volume.
Conclusive remarks
In this review, we have described and discussed the multifunctional roles of IGFBP-2 in health and disease, which include both IGFBP-2 IGF-dependent and IGF-independent activities The effects of IGFBP-2 presence or absence in normal development and physiology, and the experimental evidence derived from IGFBP-2 overexpression or down regulation, suggest IGFBP-2 as a crucial molecule that could be used to enhance health and target disease.
Acknowledgments
This work was supported by the National Health and Medical Research Council (NHMRC) of Australia (Project Grant, # 1008062) awarded to GAW and VCR. MAS is supported through a National Health and Medical Research Council Professional Training Fellowship (APP1012201). SWY is a recipient of an Australian Postgraduate Award scholarship. We also wish to acknowledge the generous support from the Murdoch Childrens Research Institute and the Royal Children’s Hospital Foundation to GAW, MAS and VCR. We also like to thank the Victorian Government Operational Infrastructure Support Program.
Disclosure statement
This work was supported by the National Health and Medical Research Council (NHMRC) of Australia (Project Grant, # 1008062) awarded to GAW, VCR, and by the Victorian Government Operational Infrastructure Support Program. SWY is a recipient of an Australian Postgraduate Award scholarship.
Footnotes
S. W. Yau and W. J. Azar contributed equally to this work.
References
- Zapf J, Froesch ER. Insulin-like growth factors/somatomedins: structure, secretion, biological actions and physiological role. Horm Res. 1986;24:121–130. doi: 10.1159/000180551. [DOI] [PubMed] [Google Scholar]
- Daughaday WH, Rotwein P. Insulin-like growth factors I and II. Peptide messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev. 1989;10:68–91. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- Han VK, D’Ercole AJ, Lund PK. Cellular localization of somatomedin (insulin-like growth factor) messenger RNA in the human fetus. Science. 1987;236:193–197. doi: 10.1126/science.3563497. [DOI] [PubMed] [Google Scholar]
- Murphy LJ, Bell GI, Friesen HG. Tissue distribution of insulin-like growth factor I and II messenger ribonucleic acid in the adult rat. Endocrinology. 1987;120:1279–1282. doi: 10.1210/endo-120-4-1279. [DOI] [PubMed] [Google Scholar]
- Sepp-Lorenzino L. Structure and function of the insulin-like growth factor I receptor. Breast Cancer Res Treat. 1998;47:235–253. doi: 10.1023/a:1005955017615. [DOI] [PubMed] [Google Scholar]
- Milazzo G, Yip CC, Maddux BA, Vigneri R, Goldfine ID. High-affinity insulin binding to an atypical insulin-like growth factor-I receptor in human breast cancer cells. J Clin Investig. 1992;89:899–908. doi: 10.1172/JCI115670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxham CP, Duronio V, Jacobs S. Insulin-like growth factor I receptor beta-subunit heterogeneity. Evidence for hybrid tetramers composed of insulin- like growth factor I and insulin receptor heterodimers. J Biol Chem. 1989;264:13238–13244. [PubMed] [Google Scholar]
- Siddle K, Soos MA, Field CE, Nave BT. Hybrid and atypical insulin/insulin-like growth factor I receptors. Horm Res. 1994;41(Suppl 2):56–64. doi: 10.1159/000183962. [DOI] [PubMed] [Google Scholar]
- Sakano K, Enjoh T, Numata F, Fujiwara H, Marumoto Y, Higashihashi N, Sato Y, Perdue JF, Fujita-Yamaguchi Y. The design, expression, and characterization of human insulin- like growth factor II (IGF-II) mutants specific for either the IGF-II/cation-independent mannose 6-phosphate receptor or IGF-I receptor. J Biol Chem. 1991;266:20626–20635. [PubMed] [Google Scholar]
- Braulke T. Type-2 IGF receptor: a multi-ligand binding protein. Horm Metab Res. 1999;31:242–246. doi: 10.1055/s-2007-978725. [DOI] [PubMed] [Google Scholar]
- Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- Twigg SM, Kiefer MC, Zapf J, Baxter RC. A central domain binding site in insulin-like growth factor binding protein-5 for the acid-labile subunit. Endocrinology. 2000;141:454–457. doi: 10.1210/endo.141.1.7375. [DOI] [PubMed] [Google Scholar]
- Galea CA, Mobli M, McNeil KA, Mulhern TD, Wallace JC, King GF, Forbes BE, Norton RS. Insulin-like growth factor binding protein-2: NMR analysis and structural characterization of the N-terminal domain. Biochimie. 2012;94:608–616. doi: 10.1016/j.biochi.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Forbes BE, Turner D, Hodge SJ, McNeil KA, Forsberg G, Wallace JC. Localization of an insulin-like growth factor (IGF) binding site of bovine IGF binding protein-2 using disulfide mapping and deletion mutation analysis of the C-terminal domain. J Biol Chem. 1998;273:4647–4652. doi: 10.1074/jbc.273.8.4647. [DOI] [PubMed] [Google Scholar]
- Ho PJ, Baxter RC. Insulin-like growth factor-binding protein-2 in patients with prostate carcinoma and benign prostatic hyperplasia. Clin Endocrinol. 1997;46:333–342. [PubMed] [Google Scholar]
- Russo VC, Rekaris G, Baker NL, Bach LA, Werther GA. Basic fibroblast growth factor induces proteolysis of secreted and cell membrane-associated insulin-like growth factor binding protein-2 in human neuroblastoma cells. Endocrinology. 1999;140:3082–3090. doi: 10.1210/endo.140.7.6771. [DOI] [PubMed] [Google Scholar]
- Graham ME, Kilby DM, Firth SM, Robinson PJ, Baxter RC. The in vivo phosphorylation and glycosylation of human insulin-like growth factor-binding protein-5. Mol Cell Proteomics. 2007;6:1392–1405. doi: 10.1074/mcp.M700027-MCP200. [DOI] [PubMed] [Google Scholar]
- Coverley JA, Baxter RC. Phosphorylation of insulin-like growth factor binding proteins. Mol Cell Endocrinol. 1997;128:1–5. doi: 10.1016/s0303-7207(97)04032-x. [DOI] [PubMed] [Google Scholar]
- Daza DO, Sundström G, Bergqvist CA, Duan C, Larhammar D. Evolution of the insulin-like growth factor binding protein (IGFBP) family. Endocrinology. 2011;152:2278–2289. doi: 10.1210/en.2011-0047. [DOI] [PubMed] [Google Scholar]
- Song SW, Fuller GN, Khan A, Kong S, Shen W, Taylor E, Ramdas L, Lang FF, Zhang W. IIp45, an insulin-like growth factor binding protein 2 (IGFBP-2) binding protein, antagonizes IGFBP-2 stimulation of glioma cell invasion. Proc Natl Acad Sci U S A. 2003;100:13970–13975. doi: 10.1073/pnas.2332186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer KW, Reichenmiller K, Schutt BS, Hoeflich A, Ranke MB, Dodt G, Elmlinger MW. IGF-independent effects of IGFBP-2 on the human breast cancer cell line Hs578T. J Mol Endocrinol. 2006;37:13–23. doi: 10.1677/jme.1.01955. [DOI] [PubMed] [Google Scholar]
- Pereira JJ, Meyer T, Docherty SE, Reid HH, Marshall J, Thompson EW, Rossjohn J, Price JT. Bimolecular interaction of insulin-like growth factor (IGF) binding protein-2 with alphavbeta3 negatively modulates IGF-I-mediated migration and tumor growth. Cancer Res. 2004;64:977–984. doi: 10.1158/0008-5472.can-03-3056. [DOI] [PubMed] [Google Scholar]
- Perks CM, Vernon EG, Rosendahl AH, Tonge D, Holly JM. IGF-II and IGFBP-2 differentially regulate PTEN in human breast cancer cells. Oncogene. 2007;26:5966–5972. doi: 10.1038/sj.onc.1210397. [DOI] [PubMed] [Google Scholar]
- Foulstone EJ, Zeng L, Perks CM, Holly JM (2013) Insulin-like growth factor binding protein 2 (IGFBP-2) promotes growth and survival of breast epithelial cells: novel regulation of the estrogen receptor. Endocrinology [DOI] [PubMed]
- Russo VC, Schutt BS, Andaloro E, Ymer SI, Hoeflich A, Ranke MB, Bach LA, Werther GA. Insulin-like growth factor binding protein-2 binding to extracellular matrix plays a critical role in neuroblastoma cell proliferation, migration, and invasion. Endocrinology. 2005;146:4445–4455. doi: 10.1210/en.2005-0467. [DOI] [PubMed] [Google Scholar]
- Shen X, Xi G, Maile LA, Wai C, Rosen CJ, Clemmons DR. Insulin-like growth factor binding protein-2 functions coordinately with receptor protein tyrosine phosphatase beta and the IGF-I receptor to regulate IGF-I-stimulated signaling. Mol Cell Biol. 2012;32:4116–4130. doi: 10.1128/MCB.01011-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang Z, Yao S, Keizer DW, Wang CC, Bach LA, Forbes BE, Wallace JC, Norton RS. Structure, dynamics and heparin binding of the C-terminal domain of insulin-like growth factor-binding protein-2 (IGFBP-2) J Mol Biol. 2006;364:690–704. doi: 10.1016/j.jmb.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Besnard V, Corroyer S, Trugnan G, Chadelat K, Nabeyrat E, Cazals V, Clement A. Distinct patterns of insulin-like growth factor binding protein (IGFBP)-2 and IGFBP-3 expression in oxidant exposed lung epithelial cells. Biochim Biophys Acta (BBA) Mol Cell Res. 2001;1538:47–58. doi: 10.1016/s0167-4889(00)00136-1. [DOI] [PubMed] [Google Scholar]
- Terrien X, Bonvin E, Corroyer S, Tabary O, Clement A, Henrion Caude A. Intracellular colocalization and interaction of IGF-binding protein-2 with the cyclin-dependent kinase inhibitor p21CIP1/WAF1 during growth inhibition. Biochem J. 2005;392:457–465. doi: 10.1042/BJ20050517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyako K, Cobb LJ, Francis M, Huang A, Peng B, Pintar JE, Ariga H, Cohen P. PAPA-1 Is a nuclear binding partner of IGFBP-2 and modulates its growth-promoting actions. Mol Endocrinol. 2009;23:169–175. doi: 10.1210/me.2008-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar WJ, Azar SH, Higgins S, Hu JF, Hoffman AR, Newgreen DF, Werther GA, Russo VC. IGFBP-2 enhances VEGF gene promoter activity and consequent promotion of angiogenesis by neuroblastoma cells. Endocrinology. 2011;152:3332–3342. doi: 10.1210/en.2011-1121. [DOI] [PubMed] [Google Scholar]
- Azar WJ, Zivkovic S, Werther GA, Russo VC. IGFBP-2 nuclear translocation is mediated by a functional NLS sequence and is essential for its pro-tumorigenic actions in cancer cells. Oncogene. 2014;33:578–588. doi: 10.1038/onc.2012.630. [DOI] [PubMed] [Google Scholar]
- Schedlich LJ, Le Page SL, Firth SM, Briggs LJ, Jans DA, Baxter RC. Nuclear import of insulin-like growth factor-binding protein-3 and -5 is mediated by the importin beta subunit. J Biol Chem. 2000;275:23462–23470. doi: 10.1074/jbc.M002208200. [DOI] [PubMed] [Google Scholar]
- Iosef C, Gkourasas T, Jia CY, Li SS, Han VK. A functional nuclear localization signal in insulin-like growth factor binding protein-6 mediates its nuclear import. Endocrinology. 2008;149:1214–1226. doi: 10.1210/en.2007-0959. [DOI] [PubMed] [Google Scholar]
- Chelsky D, Ralph R, Jonak G. Sequence requirements for synthetic peptide-mediated translocation to the nucleus. Mol Cell Biol. 1989;9:2487–2492. doi: 10.1128/mcb.9.6.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintar JE, Cerro JA, Wood TL. Genetic approaches to the function of insulin-like growth factor- binding proteins during rodent development. Horm Res. 1996;45:172–177. doi: 10.1159/000184782. [DOI] [PubMed] [Google Scholar]
- Wood TL, Rogler LE, Czick ME, Schuller AG, Pintar JE. Selective alterations in organ sizes in mice with a targeted disruption of the insulin-like growth factor binding protein-2 gene. Mol Endocrinol. 2000;14:1472–1482. doi: 10.1210/mend.14.9.0517. [DOI] [PubMed] [Google Scholar]
- Chen TL, Chang LY, DiGregorio DA, Perlman AJ, Huang YF. Growth factor modulation of insulin-like growth factor-binding proteins in rat osteoblast-like cells. Endocrinology. 1993;133:1382–1389. doi: 10.1210/endo.133.3.7689954. [DOI] [PubMed] [Google Scholar]
- Palermo C, Manduca P, Gazzerro E, Foppiani L, Segat D, Barreca A. Potentiating role of IGFBP-2 on IGF-II-stimulated alkaline phosphatase activity in differentiating osteoblasts. Am J Physiol Endocrinol Metab. 2004;286:E648–E657. doi: 10.1152/ajpendo.00049.2003. [DOI] [PubMed] [Google Scholar]
- Wang E, Wang J, Chin E, Zhou J, Bondy CA. Cellular patterns of insulin-like growth factor system gene expression in murine chondrogenesis and osteogenesis. Endocrinology. 1995;136:2741–2751. doi: 10.1210/endo.136.6.7750499. [DOI] [PubMed] [Google Scholar]
- Conover CA, Johnstone EW, Turner RT, Evans GL, John Ballard F, Doran PM, Khosla S. Subcutaneous administration of insulin-like growth factor (IGF)-II/IGF binding protein-2 complex stimulates bone formation and prevents loss of bone mineral density in a rat model of disuse osteoporosis. Growth Hormon IGF Res. 2002;12:178–183. doi: 10.1016/s1096-6374(02)00044-8. [DOI] [PubMed] [Google Scholar]
- Fisher MC, Meyer C, Garber G, Dealy CN. Role of IGFBP2, IGF-I and IGF-II in regulating long bone growth. Bone. 2005;37:741–750. doi: 10.1016/j.bone.2005.07.024. [DOI] [PubMed] [Google Scholar]
- DeMambro VE, Clemmons DR, Horton LG, Bouxsein ML, Wood TL, Beamer WG, Canalis E, Rosen CJ. Gender-specific changes in bone turnover and skeletal architecture in Igfbp-2-null mice. Endocrinology. 2008;149:2051–2061. doi: 10.1210/en.2007-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Breggia AC, DeMambro VE, Shen X, Canalis E, Bouxsein ML, Beamer WG, Clemmons DR, Rosen CJ. The heparin-binding domain of IGFBP-2 has IGF binding-independent biologic activity in the growing skeleton. J Biol Chem. 2011;286:14670–14680. doi: 10.1074/jbc.M110.193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMambro VE, Maile L, Wai C, Kawai M, Cascella T, Rosen CJ, Clemmons D. Insulin-like growth factor-binding protein-2 is required for osteoclast differentiation. J Bone Miner Res. 2012;27:390–400. doi: 10.1002/jbmr.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi G, Wai C, DeMambro V, Rosen CJ, Clemmons DR. IGFBP-2 directly stimulates osteoblast differentiation. J Bone Miner Res. 2014;29:2427–2438. doi: 10.1002/jbmr.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeflich A, Wu M, Mohan S, Foll J, Wanke R, Froehlich T, Arnold GJ, Lahm H, Kolb HJ, Wolf E. Overexpression of insulin-like growth factor-binding protein-2 in transgenic mice reduces postnatal body weight gain. Endocrinology. 1999;140:5488–5496. doi: 10.1210/endo.140.12.7169. [DOI] [PubMed] [Google Scholar]
- Hoeflich A, Nedbal S, Blum WF, Erhard M, Lahm H, Brem G, Kolb HJ, Wanke R, Wolf E. Growth inhibition in giant growth hormone transgenic mice by overexpression of insulin-like growth factor-binding protein-2. Endocrinology. 2001;142:1889–1898. doi: 10.1210/endo.142.5.8149. [DOI] [PubMed] [Google Scholar]
- Russo VC, Bach LA, Werther GA. Cell membrane association of insulin-like growth factor binding protein-2 (IGFBP-2) in the rat brain olfactory bulb. Prog Growth Factor Res. 1995;6:329–336. doi: 10.1016/0955-2235(95)00018-6. [DOI] [PubMed] [Google Scholar]
- Rehfeldt C, Renne U, Sawitzky M, Binder G, Hoeflich A. Increased fat mass, decreased myofiber size, and a shift to glycolytic muscle metabolism in adolescent male transgenic mice overexpressing IGFBP-2. Am J Physiol Endocrinol Metab. 2010;299:E287–E298. doi: 10.1152/ajpendo.00492.2009. [DOI] [PubMed] [Google Scholar]
- Eckstein F, Pavicic T, Nedbal S, Schmidt C, Wehr U, Rambeck W, Wolf E, Hoeflich A. Insulin-like growth factor-binding protein-2 (IGFBP-2) overexpression negatively regulates bone size and mass, but not density, in the absence and presence of growth hormone/IGF-I excess in transgenic mice. Anat Embryol (Berlin) 2002;206:139–148. doi: 10.1007/s00429-002-0282-5. [DOI] [PubMed] [Google Scholar]
- Hoeflich A, Schmidt P, Foll J, Rottmann O, Weber MM, Kolb HJ, Pirchner F, Wolf E. Altered growth of mice divergently selected for body weight is associated with complex changes in the growth hormone/insulin-like growth factor system. Growth Hormon IGF Res. 1998;8:113–123. doi: 10.1016/s1096-6374(98)80101-9. [DOI] [PubMed] [Google Scholar]
- Barrios V, Buno M, Pozo J, Munoz MT, Argente J. Insulin-like growth factor-binding protein-2 levels in pediatric patients with growth hormone deficiency, eating disorders and acute lymphoblastic leukemia. Horm Res. 2000;53:221–227. doi: 10.1159/000023571. [DOI] [PubMed] [Google Scholar]
- Hall K, Lundin G, Povoa G. Serum levels of the low molecular weight form of insulin-like growth factor binding protein in healthy subjects and patients with growth hormone deficiency, acromegaly and anorexia nervosa. Acta Endocrinol (Copenh) 1988;118:321–326. doi: 10.1530/acta.0.1180321. [DOI] [PubMed] [Google Scholar]
- Hotta M, Fukuda I, Sato K, Hizuka N, Shibasaki T, Takano K. The relationship between bone turnover and body weight, serum insulin-like growth factor (IGF) I, and serum IGF-binding protein levels in patients with anorexia nervosa. J Clin Endocrinol Metab. 2000;85:200–206. doi: 10.1210/jcem.85.1.6321. [DOI] [PubMed] [Google Scholar]
- Wheatcroft SB, Kearney MT, Shah AM, Ezzat VA, Miell JR, Modo M, Williams SCR, Cawthorn WP, Medina-Gomez G, Vidal-Puig A, Sethi JK, Crossey PA. IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes. 2007;56:285–294. doi: 10.2337/db06-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedbacker K, Birsoy K, Wysocki RW, Asilmaz E, Ahima RS, Farooqi IS, Friedman JM. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 2010;11:11–22. doi: 10.1016/j.cmet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Claudio M, Benjamim F, Riccardo B, Massimiliano C, Francesco B, Luciano C. Adipocytes IGFBP-2 expression in prepubertal obese children. Obesity (Silver Spring) 2010;18:2055–2057. doi: 10.1038/oby.2010.7. [DOI] [PubMed] [Google Scholar]
- Ballerini MG, Ropelato MG, Domene HM, Pennisi P, Heinrich JJ, Jasper HG. Differential impact of simple childhood obesity on the components of the growth hormone-insulin-like growth factor (IGF)-IGF binding proteins axis. J Pediatr Endocrinol Metab. 2004;17:749–757. doi: 10.1515/jpem.2004.17.5.749. [DOI] [PubMed] [Google Scholar]
- Frystyk J, Skjaerbaek C, Vestbo E, Fisker S, Orskov H. Circulating levels of free insulin-like growth factors in obese subjects: the impact of type 2 diabetes. Diabetes Metab Res Rev. 1999;15:314–322. doi: 10.1002/(sici)1520-7560(199909/10)15:5<314::aid-dmrr56>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Levitt RJ, Georgescu M-M, Pollak M. PTEN-induction in U251 glioma cells decreases the expression of insulin-like growth factor binding protein-2. Biochem Biophys Res Commun. 2005;336:1056–1061. doi: 10.1016/j.bbrc.2005.08.229. [DOI] [PubMed] [Google Scholar]
- Mehrian-Shai R, Chen CD, Shi T, Horvath S, Nelson SF, Reichardt JK, Sawyers CL. Insulin growth factor-binding protein 2 is a candidate biomarker for PTEN status and PI3K/Akt pathway activation in glioblastoma and prostate cancer. Proc Natl Acad Sci U S A. 2007;104:5563–5568. doi: 10.1073/pnas.0609139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Baxter RC. Expression of insulin-like growth factor binding protein-2 by MCF-7 breast cancer cells is regulated through the phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin pathway. Endocrinology. 2007;148:2532–2541. doi: 10.1210/en.2006-1335. [DOI] [PubMed] [Google Scholar]
- Dean SJR, Perks CM, Holly JMP, Bhoo-Pathy N, Looi L-M, Mohammed NAT, Mun K-S, Teo S-H, Koobotse MO, Yip C-H, Rhodes A. Loss of PTEN expression is associated with IGFBP2 expression, younger age, and late stage in triple-negative breast cancer. Am J Clin Pathol. 2014;141:323–333. doi: 10.1309/AJCPR11DEAYPTUSL. [DOI] [PubMed] [Google Scholar]
- Grimberg A, Coleman CM, Shi Z, Burns TF, MacLachlan TK, Wang W, El-Deiry WS. Insulin-like growth factor factor binding protein-2 is a novel mediator of p53 inhibition of insulin-like growth factor signaling. Cancer Biol Ther. 2006;5:1408–1414. doi: 10.4161/cbt.5.10.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani RM, Adolphe C, Palmer J, Waters MJ, Wainwright BJ. Patched1 inhibits epidermal progenitor cell expansion and basal cell carcinoma formation by limiting Igfbp2 activity. Cancer Prev Res. 2010;3:1222–1234. doi: 10.1158/1940-6207.CAPR-10-0082. [DOI] [PubMed] [Google Scholar]
- Rao G, Pedone CA, Valle LD, Reiss K, Holland EC, Fults DW. Sonic hedgehog and insulin-like growth factor signaling synergize to induce medulloblastoma formation from nestin-expressing neural progenitors in mice. Oncogene. 2004;23:6156–6162. doi: 10.1038/sj.onc.1207818. [DOI] [PubMed] [Google Scholar]
- Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999;59:3915–3918. [PubMed] [Google Scholar]
- Harris AL. Hypoxia–a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Poomthavorn P, Wong SHX, Higgins S, Werther GA, Russo VC. Activation of a prometastatic gene expression program in hypoxic neuroblastoma cells. Endocr Relat Cancer. 2009;16:991–1004. doi: 10.1677/ERC-08-0340. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Gargosky SE, Iwasaki K, Rosenfeld RG. Identification and characterization of insulin-like growth factors (IGFs), IGF-binding proteins (IGFBPs), and IGFBP proteases in human synovial fluid. J Clin Endocrinol Metab. 1996;81:150–155. doi: 10.1210/jcem.81.1.8550744. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Ohe Y, Tatemoto K. Synthesis and secretion of insulin-like growth factor (IGF)-II and IGF binding protein-2 by cultivated brain meningeal cells. Brain Res. 1995;697:122–129. doi: 10.1016/0006-8993(95)00798-u. [DOI] [PubMed] [Google Scholar]
- Mark S, Kübler B, Höning S, Oesterreicher S, John H, Braulke T, Forssmann W-G, Ständker L. Diversity of human insulin-like growth factor (IGF) binding protein-2 fragments in plasma: primary structure, IGF-binding properties, and disulfide bonding pattern†. Biochemistry. 2005;44:3644–3652. doi: 10.1021/bi0478401. [DOI] [PubMed] [Google Scholar]
- Ho PJ, Baxter RC. Characterization of truncated insulin-like growth factor-binding protein-2 in human milk. Endocrinology. 1997;138:3811–3818. doi: 10.1210/endo.138.9.5415. [DOI] [PubMed] [Google Scholar]
- Giudice LC, Farrell EM, Pham H, Lamson G, Rosenfeld RG. Insulin-like growth factor binding proteins in maternal serum throughout gestation and in the puerperium: effects of a pregnancy-associated serum protease activity. J Clin Endocrinol Metab. 1990;71:806–816. doi: 10.1210/jcem-71-4-806. [DOI] [PubMed] [Google Scholar]
- Pucilowska JB, Davenport ML, Kabir I, Clemmons DR, Thissen JP, Butler T, Underwood LE. The effect of dietary protein supplementation on insulin-like growth factors (IGFs) and IGF-binding proteins in children with shigellosis. J Clin Endocrinol Metab. 1993;77:1516–1521. doi: 10.1210/jcem.77.6.7505287. [DOI] [PubMed] [Google Scholar]
- Brandt K, Lundell K, Brismar K. Neutrophil-derived azurocidin cleaves insulin-like growth factor-binding protein-1, −2 and −4. Growth Hormon IGF Res. 2011;21:167–173. doi: 10.1016/j.ghir.2011.04.003. [DOI] [PubMed] [Google Scholar]
- DeGraff DJ, Malik M, Chen Q, Miyako K, Rejto L, Aguiar AA, Bancroft DRE, Cohen P, Sikes RA. Hormonal regulation of IGFBP-2 proteolysis is attenuated with progression to androgen insensitivity in the LNCaP progression model. J Cell Physiol. 2007;213:261–268. doi: 10.1002/jcp.21123. [DOI] [PubMed] [Google Scholar]
- Russo VC, Andaloro E, Fornaro SA, Najdovska S, Newgreen DF, Bach LA, Werther GA. Fibroblast growth factor-2 over-rides insulin-like growth factor-I induced proliferation and cell survival in human neuroblastoma cells. J Cell Physiol. 2004;199:371–380. doi: 10.1002/jcp.10416. [DOI] [PubMed] [Google Scholar]
- Hoeflich A, Reisinger R, Schuett BS, Elmlinger MW, Russo VC, Vargas GA, Jehle PM, Lahm H, Renner-Muller I, Wolf E. Peri/nuclear localization of intact insulin-like growth factor binding protein-2 and a distinct carboxyl-terminal IGFBP-2 fragment in vivo. Biochem Biophys Res Commun. 2004;324:705–710. doi: 10.1016/j.bbrc.2004.09.111. [DOI] [PubMed] [Google Scholar]
- Si M, Nakamura M, Yano K, Ishii G, Hasebe T, Endoh Y, Sangai T, Maeda H, Shi-chuang Z, Chiba T, Ochiai A. Matrix metalloproteinase-7 triggers the matricrine action of insulin-like growth factor-II via proteinase activity on insulin-like growth factor binding protein 2 in the extracellular matrix. Cancer Sci. 2007;98:685–691. doi: 10.1111/j.1349-7006.2007.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderic M, Miljus G, Nedic O. Interaction of insulin-like growth factor-binding protein 2 with alpha2-macroglobulin in the circulation. Protein J. 2013;32:138–142. doi: 10.1007/s10930-013-9471-8. [DOI] [PubMed] [Google Scholar]
- Gerard N, Delpuech T, Oxvig C, Overgaard M, Monget P. Proteolytic degradation of IGF-binding protein (IGFBP)-2 in equine ovarian follicles: involvement of pregnancy-associated plasma protein-A (PAPP-A) and association with dominant but not subordinated follicles. J Endocrinol. 2004;182:457–466. doi: 10.1677/joe.0.1820457. [DOI] [PubMed] [Google Scholar]
- Qin X, Wergedal JE, Rehage M, Tran K, Newton J, Lam P, Baylink DJ, Mohan S. Pregnancy-associated plasma protein-A increases osteoblast proliferation in vitro and bone formation in vivo. Endocrinology. 2006;147:5653–5661. doi: 10.1210/en.2006-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- Schutt B, Langkamp M, Rauschnabel U, Ranke M, Elmlinger M. Integrin-mediated action of insulin-like growth factor binding protein-2 in tumor cells. J Mol Endocrinol. 2004;32:859–868. doi: 10.1677/jme.0.0320859. [DOI] [PubMed] [Google Scholar]
- Mendes KN, Wang GK, Fuller GN, Zhang W. JNK mediates insulin-like growth factor binding protein 2/integrin alpha5-dependent glioma cell migration. Int J Oncol. 2010;37:143–153. doi: 10.3892/ijo_00000662. [DOI] [PubMed] [Google Scholar]
- Uzoh CC, Holly JM, Biernacka KM, Persad RA, Bahl A, Gillatt D, Perks CM. Insulin-like growth factor-binding protein-2 promotes prostate cancer cell growth via IGF-dependent or -independent mechanisms and reduces the efficacy of docetaxel. Br J Cancer. 2011;104:1587–1593. doi: 10.1038/bjc.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T, Clarke J, Parker A, Busby W, Jr, Nam T, Clemmons DR. Substitution of specific amino acids in insulin-like growth factor (IGF) binding protein 5 alters heparin binding and its change in affinity for IGF-I response to heparin. J Biol Chem. 1996;271:6099–6106. doi: 10.1074/jbc.271.11.6099. [DOI] [PubMed] [Google Scholar]
- Kiepe D, Van Der Pas A, Ciarmatori S, Standker L, Schutt B, Hoeflich A, Hugel U, Oh J, Tonshoff B. Defined carboxy-terminal fragments of insulin-like growth factor (IGF) binding protein-2 exert similar mitogenic activity on cultured rat growth plate chondrocytes as IGF-I. Endocrinology. 2008;149:4901–4911. doi: 10.1210/en.2007-1395. [DOI] [PubMed] [Google Scholar]
- Xi G, Solum MA, Wai C, Maile LA, Rosen CJ, Clemmons DR. The heparin-binding domains of IGFBP-2 mediate its inhibitory effect on preadipocyte differentiation and fat development in male mice. Endocrinology. 2013;154:4146–4157. doi: 10.1210/en.2013-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitara K, Yamada H, Watanabe K, Shimonaka M, Yamaguchi Y. Brain-specific receptor-type protein-tyrosine phosphatase RPTP beta is a chondroitin sulfate proteoglycan in vivo. J Biol Chem. 1994;269:20189–20193. [PubMed] [Google Scholar]
- Sabin MA, Russo VC, Azar WJ, Yau SW, Kiess W, Werther GA. IGFBP-2 at the interface of growth and metabolism–implications for childhood obesity. Pediatr Endocrinol Rev. 2011;8:382–393. [PubMed] [Google Scholar]
- Nam SY, Lee EJ, Kim KR, Cha BS, Song YD, Lim SK, Lee HC, Huh KB. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord. 1997;21:355–359. doi: 10.1038/sj.ijo.0800412. [DOI] [PubMed] [Google Scholar]
- Heald AH, Kaushal K, Siddals KW, Rudenski AS, Anderson SG, Gibson JM. Insulin-like growth factor binding protein-2 (IGFBP-2) is a marker for the metabolic syndrome. Exp Clin Endocrinol Diabetes. 2006;114:371–376. doi: 10.1055/s-2006-924320. [DOI] [PubMed] [Google Scholar]
- Li Z, Picard F. Modulation of IGFBP2 mRNA expression in white adipose tissue upon aging and obesity. Horm Metab Res. 2010;42:787–791. doi: 10.1055/s-0030-1262854. [DOI] [PubMed] [Google Scholar]
- Krassas GE. Endocrine abnormalities in Anorexia Nervosa. Pediatr Endocrinol Rev. 2003;1:46–54. [PubMed] [Google Scholar]
- Counts DR, Gwirtsman H, Carlsson LM, Lesem M, Cutler GB. The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. J Clin Endocrinol Metab. 1992;75:762–767. doi: 10.1210/jcem.75.3.1381372. [DOI] [PubMed] [Google Scholar]
- Bereket A, Lang CH, Wilson TA. Alterations in the growth hormone-insulin-like growth factor axis in insulin dependent diabetes mellitus. Horm Metab Res. 1999;31:172–181. doi: 10.1055/s-2007-978716. [DOI] [PubMed] [Google Scholar]
- Frystyk J, Bek T, Flyvbjerg A, Skjærbæk C, Ørskov H. The relationship between the circulating IGF system and the presence of retinopathy in Type 1 diabetic patients. Diabet Med. 2003;20:269–276. doi: 10.1046/j.1464-5491.2003.00921.x. [DOI] [PubMed] [Google Scholar]
- Street ME, Smerieri A, Montanini L, Predieri B, Iughetti L, Valenzise M, De Luca F, Vigone M, Weber G, Maghnie M, Bernasconi S. Interactions among pro-inflammatory cytokines, IGF system and thyroid function in pre-pubertal obese subjects. J Biol Regul Homeost Agents. 2013;27:259–266. [PubMed] [Google Scholar]
- Smerieri A, Petraroli M, Ziveri MA, Volta C, Bernasconi S, Street ME. Effects of cord serum insulin, IGF-II, IGFBP-2, IL-6 and cortisol concentrations on human birth weight and length: pilot study. PLoS One. 2011;6:e29562. doi: 10.1371/journal.pone.0029562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice LC, de Zegher F, Gargosky SE, Dsupin BA, de las Fuentes L, Crystal RA, Hintz RL, Rosenfeld RG. Insulin-like growth factors and their binding proteins in the term and preterm human fetus and neonate with normal and extremes of intrauterine growth. J Clin Endocrinol Metab. 1995;80:1548–1555. doi: 10.1210/jcem.80.5.7538146. [DOI] [PubMed] [Google Scholar]
- Ko JM, Park HK, Yang S, Hwang IT. Influence of catch-up growth on IGFBP-2 levels and association between IGFBP-2 and cardiovascular risk factors in Korean children born SGA. Endocr J. 2012;59:725–733. doi: 10.1507/endocrj.ej12-0080. [DOI] [PubMed] [Google Scholar]
- de Kort SW, van Doorn J, van de Sande AG, Leunissen RW, Hokken-Koelega AC. Serum insulin-like growth factor-binding protein-2 levels and metabolic and cardiovascular risk factors in young adults and children born small for gestational age. J Clin Endocrinol Metab. 2010;95:864–871. doi: 10.1210/jc.2009-1508. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Yau SW, Henry BA, Russo VC, McConell GK, Clarke IJ, Werther GA, Sabin MA. Leptin enhances insulin sensitivity by direct and sympathetic nervous system regulation of muscle Igfbp-2 expression - evidence from non-rodent models. Endocrinology. 2014;155:2133–2143. doi: 10.1210/en.2013-2099. [DOI] [PubMed] [Google Scholar]
- Sabin MA, Yau SW, Russo VC, Clarke IJ, Dunshea FR, Chau J, Cox M, Werther GA. Dietary monounsaturated fat in early life regulates IGFBP2: implications for fat mass accretion and insulin sensitivity. Obesity. 2011;19:2374–2381. doi: 10.1038/oby.2011.55. [DOI] [PubMed] [Google Scholar]
- Nam S, Lee E, Kim K, Cha B, Song Y, Lim S. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord. 1997;21:355–359. doi: 10.1038/sj.ijo.0800412. [DOI] [PubMed] [Google Scholar]
- Claudio M, Benjamim F, Riccardo B, Massimiliano C, Francesco B, Luciano C. Adipocytes IGFBP-2 expression in prepubertal obese children. Obesity. 2010;18:2055–2057. doi: 10.1038/oby.2010.7. [DOI] [PubMed] [Google Scholar]
- Li Z, Picard F (2010) Modulation of IGFBP2 mRNA expression in white adipose tissue upon aging and obesity. Horm Metab Res:787–791 [DOI] [PubMed]
- Wheatcroft S, Kearney M, Shah A, Ezzat V, Miell J, Modo M. IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes. 2007;56:284–294. doi: 10.2337/db06-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau SW, Russo VC, Clarke IJ, Dunshea FR, Werther GA, Sabin MA. IGFBP-2 inhibits adipogenesis and lipogenesis in human visceral, but not subcutaneous, adipocytes. Int J Obes. 2014 doi: 10.1038/ijo.2014.192. [DOI] [PubMed] [Google Scholar]
- Reeve JG, Kirby LB, Brinkman A, Hughes SA, Schwander J, Bleehen NM. Insulin-like growth-factor-binding protein gene expression and protein production by human tumour cell lines. Int J Cancer. 1992;51:818–821. doi: 10.1002/ijc.2910510525. [DOI] [PubMed] [Google Scholar]
- Tennant MK, Thrasher JB, Twomey PA, Birnbaum RS, Plymate SR. Insulin-like growth factor-binding protein-2 and −3 expression in benign human prostate epithelium, prostate intraepithelial neoplasia, and adenocarcinoma of the prostate. J Clin Endocrinol Metab. 1996;81:411–420. doi: 10.1210/jcem.81.1.8550786. [DOI] [PubMed] [Google Scholar]
- Wex H, Vorwerk P, Mohnike K, Bretschneider D, Kluba U, Aumann V, Blum WF, Mittler U. Elevated serum levels of IGFBP-2 found in children suffering from acute leukaemia is accompanied by the occurrence of IGFBP-2 mRNA in the tumour clone. Br J Cancer. 1998;78:515–520. doi: 10.1038/bjc.1998.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Shen W, Huang H, Hu L, Ramdas L, Zhou YH, Liao WS, Fuller GN, Zhang W. Insulin-like growth factor binding protein 2 enhances glioblastoma invasion by activating invasion-enhancing genes. Cancer Res. 2003;63:4315–4321. [PubMed] [Google Scholar]
- Fukushima T, Kataoka H. Roles of insulin-like growth factor binding protein-2 (IGFBP-2) in glioblastoma. Anticancer Res. 2007;27:3685–3692. [PubMed] [Google Scholar]
- Becher OJ, Peterson KM, Khatua S, Santi MR, MacDonald TJ. IGFBP2 is overexpressed by pediatric malignant astrocytomas and induces the repair enzyme DNA-PK. J Child Neurol. 2008;23:1205–1213. doi: 10.1177/0883073808321766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LM, Holmes KM, Smith SM, Wu Y, Tchougounova E, Uhrbom L, Sawaya R, Bruner JM, Fuller GN, Zhang W. IGFBP2 is a candidate biomarker for Ink4a-Arf status and a therapeutic target for high-grade gliomas. Proc Natl Acad Sci U S A. 2009;106:16675–16679. doi: 10.1073/pnas.0900807106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima T, Tezuka T, Shimomura T, Nakano S, Kataoka H. Silencing of insulin-like growth factor-binding protein-2 in human glioblastoma cells reduces both invasiveness and expression of progression-associated gene CD24. J Biol Chem. 2007;282:18634–18644. doi: 10.1074/jbc.M609567200. [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013;32:5501–5511. doi: 10.1038/onc.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura G, Kasuya J, Giannini S, Honda Y, Mohan S, Kawachi M, Akimoto M, Fujita-Yamaguchi Y. Insulin-like growth factor (IGF) system components in human prostatic cancer cell-lines: LNCaP, DU145, and PC-3 cells. Intl J Urol : Off J Jpn Urol Assoc. 1996;3:39–46. doi: 10.1111/j.1442-2042.1996.tb00628.x. [DOI] [PubMed] [Google Scholar]
- Cohen P, Peehl DM, Stamey TA, Wilson KF, Clemmons DR, Rosenfeld RG. Elevated levels of insulin-like growth factor-binding protein-2 in the serum of prostate cancer patients. J Clin Endocrinol Metab. 1993;76:1031–1035. doi: 10.1210/jcem.76.4.7682560. [DOI] [PubMed] [Google Scholar]
- Degraff DJ, Aguiar AA, Sikes RA. Disease evidence for IGFBP-2 as a key player in prostate cancer progression and development of osteosclerotic lesions. Am J Transl Res. 2009;1:115–130. [PMC free article] [PubMed] [Google Scholar]
- Wang H, Arun BK, Wang H, Fuller GN, Zhang W, Middleton LP, Sahin AA. IGFBP2 and IGFBP5 overexpression correlates with the lymph node metastasis in T1 breast carcinomas. Breast J. 2008;14:261–267. doi: 10.1111/j.1524-4741.2008.00572.x. [DOI] [PubMed] [Google Scholar]
- Juncker-Jensen A, Lykkesfeldt AE, Worm J, Ralfkiær U, Espelund U, Jepsen JS. Insulin-like growth factor binding protein 2 is a marker for antiestrogen resistant human breast cancer cell lines but is not a major growth regulator. Growth Hormon IGF Res. 2006;16:224–239. doi: 10.1016/j.ghir.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Sohn J, Do KA, Liu S, Chen H, Mills GB, Hortobagyi GN, Meric-Bernstam F, Gonzalez-Angulo AM. Functional proteomics characterization of residual triple-negative breast cancer after standard neoadjuvant chemotherapy. Ann Oncol. 2013;24:2522–2526. doi: 10.1093/annonc/mdt248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mireuta M, Darnel A, Pollak M. IGFBP-2 expression in MCF-7 cells is regulated by the PI3K/AKT/mTOR pathway through Sp1-induced increase in transcription. Growth Factors. 2010;28:243–255. doi: 10.3109/08977191003745472. [DOI] [PubMed] [Google Scholar]
- Karasik A, Menczer J, Pariente C, Kanety H. Insulin-like growth factor-I (IGF-I) and IGF-binding protein-2 are increased in cyst fluids of epithelial ovarian cancer. J Clin Endocrinol Metab. 1994;78:271–276. doi: 10.1210/jcem.78.2.7508947. [DOI] [PubMed] [Google Scholar]
- Kanety H, Kattan M, Goldberg I, Kopolovic J, Ravia J, Menczer J, Karasik A. Increased insulin-like growth factor binding protein-2 (IGFBP-2) gene expression and protein production lead to high IGFBP-2 content in malignant ovarian cyst fluid. Br J Cancer. 1996;73:1069–1073. doi: 10.1038/bjc.1996.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Rosen DG, Wang H, Fuller GN, Zhang W, Liu J. Insulin-like growth factor-binding protein 2 and 5 are differentially regulated in ovarian cancer of different histologic types. Mod Pathol. 2006;19:1149–1156. doi: 10.1038/modpathol.3800637. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Mircean C, Shmulevich I, Wang H, Liu J, Niemisto A, Kavanagh JJ, Lee JH, Zhang W. Insulin-like growth factor binding protein 2 promotes ovarian cancer cell invasion. Mol Cancer. 2005;4:7. doi: 10.1186/1476-4598-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943–1955. doi: 10.1016/S0140-6736(12)62187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Nigro L. Biology of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2013;35:245–252. doi: 10.1097/MPH.0b013e31828f8746. [DOI] [PubMed] [Google Scholar]
- Carroll WL, Raetz EA. Clinical and laboratory biology of childhood acute lymphoblastic leukemia. J Pediatr. 2012;160:10–18. doi: 10.1016/j.jpeds.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Rivera GK, Crist WM, Sallan SE (1994) Biology and therapy of childhood acute lymphoblastic leukemia. Revista de investigacion clinica; organo del Hospital de Enfermedades de la Nutricion Suppl: 26–33 [PubMed]
- Rodríguez-Vicente AE, Díaz MG, Hernández-Rivas JM. Chronic lymphocytic leukemia: a clinical and molecular heterogenous disease. Cancer Genet. 2013;206:49–62. doi: 10.1016/j.cancergen.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Shanshal M, Haddad RY. Chronic lymphocytic leukemia. Dis Mon. 2012;58:153–167. doi: 10.1016/j.disamonth.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Hillmen P. Using the biology of chronic lymphocytic leukemia to choose treatment. ASH Educ Program Book. 2011;2011:104–109. doi: 10.1182/asheducation-2011.1.104. [DOI] [PubMed] [Google Scholar]
- Estey EH. Acute myeloid leukemia: 2013 update on risk-stratification and management. Am J Hematol. 2013;88:317–327. doi: 10.1002/ajh.23404. [DOI] [PubMed] [Google Scholar]
- Puumala SE, Ross JA, Aplenc R, Spector LG. Epidemiology of childhood acute myeloid leukemia. Pediatr Blood Cancer. 2013;60:728–733. doi: 10.1002/pbc.24464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamis AS, Alonzo TA, Perentesis JP, Meshinchi S, COGAMLC Children’s Oncology Group’s 2013 blueprint for research: Acute myeloid leukemia. Pediatr Blood Cancer. 2013;60:964–971. doi: 10.1002/pbc.24432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2012 update on diagnosis, monitoring, and management. Am J Hematol. 2012;87:1037–1045. doi: 10.1002/ajh.23282. [DOI] [PubMed] [Google Scholar]
- Maru Y. Molecular biology of chronic myeloid leukemia. Cancer Sci. 2012;103:1601–1610. doi: 10.1111/j.1349-7006.2012.02346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Rowley JD. Chronic myeloid leukemia: current perspectives. Clin Lab Med. 2011;31:687–698. doi: 10.1016/j.cll.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Mohnike KL, Kluba U, Mittler U, Aumann V, Vorwerk P, Blum WF. Serum levels of insulin-like growth factor-I, −II and insulin- like growth factor binding proteins −2 and −3 in children with acute lymphoblastic leukaemia. Eur J Pediatr. 1996;155:81–86. doi: 10.1007/BF02075755. [DOI] [PubMed] [Google Scholar]
- Dawczynski K, Kauf E, Zintl F. Changes of serum growth factors (IGF-I,-II and IGFBP-2,-3) prior to and after stem cell transplantation in children with acute leukemia. Bone Marrow Transplant. 2003;32:411–415. doi: 10.1038/sj.bmt.1704149. [DOI] [PubMed] [Google Scholar]
- Elmlinger MW, Wimmer K, Biemer E, Blum WF, Ranke MB, Dannecker GE. Insulin-like growth factor binding protein 2 is differentially expressed in leukaemic B- and T-cell lines. Growth Regul. 1996;6:152–157. [PubMed] [Google Scholar]
- Attard-Montalto SP, Camacho-Hübner C, Cotterill AM, D’Souza-Li L, Daley S, Bartlett K, Halliday D, Eden OB. Changes in protein turnover, IGF-I and IGF binding proteins in children with cancer. Acta Paediatr. 1998;87:54–60. doi: 10.1080/08035259850157877. [DOI] [PubMed] [Google Scholar]
- Vorwerk P, Mohnike K, Wex H, Rohl FW, Zimmermann M, Blum WF, Mittler U. Insulin-like growth factor binding protein-2 at diagnosis of childhood acute lymphoblastic leukemia and the prediction of relapse risk. J Clin Endocrinol Metab. 2005;90:3022–3027. doi: 10.1210/jc.2004-0461. [DOI] [PubMed] [Google Scholar]
- Dawczynski K, Kauf E, Schlenvoigt D, Gruhn B, Fuchs D, Zintl F. Elevated serum insulin-like growth factor binding protein-2 is associated with a high relapse risk after hematopoietic stem cell transplantation in childhood AML. Bone Marrow Transplant. 2006;37:589–594. doi: 10.1038/sj.bmt.1705281. [DOI] [PubMed] [Google Scholar]
- Hattori H, Matsuzaki A, Suminoe A, Koga Y, Tashiro K, Hara T. Identification of novel genes with prognostic value in childhood leukemia using cDNA microarray and quantitative RT-PCR. Pediatr Hematol Oncol. 2006;23:115–127. doi: 10.1080/08880010500457780. [DOI] [PubMed] [Google Scholar]
- Dawczynski K, Steinbach D, Wittig S, Pfaffendorf N, Kauf E, Zintl F. Expression of components of the IGF axis in childhood acute myelogenous leukemia. Pediatr Blood Cancer. 2008;50:24–28. doi: 10.1002/pbc.21294. [DOI] [PubMed] [Google Scholar]
- Kuhnl A, Kaiser M, Neumann M, Fransecky L, Heesch S, Radmacher M, Marcucci G, Bloomfield CD, Hofmann WK, Thiel E, Baldus CD. High expression of IGFBP2 is associated with chemoresistance in adult acute myeloid leukemia. Leuk Res. 2011;35:1585–1590. doi: 10.1016/j.leukres.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitszel A, Krawczuk-Rybak M. Are elevated serum levels of IGFBP-2 after intensive chemotherapy of childhood acute lymphoblastic leukemia a risk factor of relapse? Adv Med Sci. 2007;52:147–153. [PubMed] [Google Scholar]
- Elmlinger M, Sanatani M, Bell M, Dannecker G, Ranke M. Elevated insulin-like growth factor (IGF) binding protein (IGFBP)-2 and IGFBP-4 expression of leukemic T-cells is affected by autocrine/paracrine IGF-II action but not by IGF type I receptor expression. Eur J Endocrinol. 1998;138:337–343. doi: 10.1530/eje.0.1380337. [DOI] [PubMed] [Google Scholar]
- Jaques G, Kiefer P, Schoneberger HJ, Wegmann B, Kaiser U, Brandscheid D, Havemann K. Differential expression of insulin-like growth factor binding proteins in human non-small cell lung cancer cell lines. Eur J Cancer. 1992;28A:1899–1904. doi: 10.1016/0959-8049(92)90032-w. [DOI] [PubMed] [Google Scholar]
- Reeve JG, Brinkman A, Hughes S, Mitchell J, Schwander J, Bleehen NM. Expression of Insulinlike Growth Factor (IGF) and IGF-binding protein genes in human lung tumor cell lines. J Natl Cancer Inst. 1992;84:628–634. doi: 10.1093/jnci/84.8.628. [DOI] [PubMed] [Google Scholar]
- Hu Q, Huang L, Kuang X, Zhang H, Ling G, Chen X, Li K, Deng Z, Zhou J. Is insulin-like growth factor binding protein 2 associated with metastasis in lung cancer? Clin Exp Metastasis. 2014;31:535–541. doi: 10.1007/s10585-014-9647-4. [DOI] [PubMed] [Google Scholar]
- Reeve JG, Morgan J, Schwander J, Bleehen NM. Role for membrane and secreted insulin-like growth factor- binding protein-2 in the regulation of insulin-like growth factor action in lung tumors. Cancer Res. 1993;53:4680–4685. [PubMed] [Google Scholar]
- Dong F, Wu HB, Hong J, Rechler MM. Insulin-like growth factor binding protein-2 mediates the inhibition of DNA synthesis by transforming growth factor-beta in mink lung epithelial cells. J Cell Physiol. 2002;190:63–73. doi: 10.1002/jcp.10034. [DOI] [PubMed] [Google Scholar]
- Lee DY, Kim SJ, Lee YC. Serum insulin-like growth factor (IGF)-I and IGF-binding proteins in lung cancer patients. J Korean Med Sci. 1999;14:401–404. doi: 10.3346/jkms.1999.14.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Lu H, Gao W, Wang L, Lu K, Wu S, Pataer A, Huang M, El-Zein R, Lin T, Roth JA, Mehran R, Hofstetter W, Swisher SG, Wu X, Fang B. Insulin-like growth factor binding protein-2 level is increased in blood of lung cancer patients and associated with poor survival. PLoS One. 2013;8:e74973. doi: 10.1371/journal.pone.0074973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa T, Sato H, Shimoyamada H, Okudela K, Woo T, Tajiri M, Ogura T, Ogawa N, Suzuki T, Mitsui H, Ishii J, Miyata C, Sakaeda M, Goto K, Kashiwagi K, Masuda M, Takahashi T, Kitamura H. Neuroendocrine cancer-specific up-regulating mechanism of insulin-like growth factor binding protein-2 in small cell lung cancer. Am J Pathol. 2009;175:976–987. doi: 10.2353/ajpath.2009.081004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migita T, Narita T, Asaka R, Miyagi E, Nagano H, Nomura K, Matsuura M, Satoh Y, Okumura S, Nakagawa K, Seimiya H, Ishikawa Y. Role of insulin-like growth factor binding protein 2 in lung adenocarcinoma: IGF-independent antiapoptotic effect via caspase-3. Am J Pathol. 2010;176:1756–1766. doi: 10.2353/ajpath.2010.090500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ying X, Han S, Wang J, Zhou X, Bai E, Zhang J, Zhu Q. Autoantibodies against insulin-like growth factorbinding protein-2 as a serological biomarker in the diagnosis of lung cancer. Int J Oncol. 2013;42:93–100. doi: 10.3892/ijo.2012.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser AR, Dove WF, Roth KA, Gordon JI. The Min (multiple intestinal neoplasia) mutation: its effect on gut epithelial cell differentiation and interaction with a modifier system. J Cell Biol. 1992;116:1517–1526. doi: 10.1083/jcb.116.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess AW. Growth control mechanisms in normal and transformed intestinal cells. Philos Trans R Soc Lond Ser B Biol Sci. 1998;353:903–909. doi: 10.1098/rstb.1998.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan AJ, Lamlum H, Ilyas M, Wheeler J, Straub J, Papadopoulou A, Bicknell D, Bodmer WF, Tomlinson IPM. APC mutations in sporadic colorectal tumors: A mutational “hotspot” and interdependence of the “two hits”. Proc Natl Acad Sci U S A. 2000;97:3352–3357. doi: 10.1073/pnas.97.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naishiro Y, Yamada T, Idogawa M, Honda K, Takada M, Kondo T, Imai K, Hirohashi S (2005) Morphological and transcriptional responses of untransformed intestinal epithelial cells to an oncogenic [beta]-catenin protein. 24:3141–3153 [DOI] [PubMed]
- Ben-Shmuel A, Shvab A, Gavert N, Brabletz T, Ben-Ze’ev A. Global analysis of L1-transcriptomes identified IGFBP-2 as a target of ezrin and NF-[kappa]B signaling that promotes colon cancer progression. Oncogene. 2013;32:3220–3230. doi: 10.1038/onc.2012.340. [DOI] [PubMed] [Google Scholar]
- Renehan AG, Painter JE, O’Halloran D, Atkin WS, Potten CS, O’Dwyer ST, Shalet SM. Circulating insulin-like growth factor II and colorectal adenomas. J Clin Endocrinol Metab. 2000;85:3402–3408. doi: 10.1210/jcem.85.9.6770. [DOI] [PubMed] [Google Scholar]
- Mishra L, Bass B, Ooi BS, Sidawy A, Korman L. Role of insulin-like growth factor-I (IGF-I) receptor, IGF-I, and IGF binding protein-2 in human colorectal cancers. Growth Horm IGF Res. 1998;8:473–479. doi: 10.1016/s1096-6374(98)80300-6. [DOI] [PubMed] [Google Scholar]
- el Atiq F, Garrouste F, Remacle-Bonnet M, Sastre B, Pommier G. Alterations in serum levels of insulin-like growth factors and insulin-like growth-factor-binding proteins in patients with colorectal cancer. Int J Cancer. 1994;57:491–497. doi: 10.1002/ijc.2910570409. [DOI] [PubMed] [Google Scholar]
- Liou J-M, Shun C-T, Liang J-T, Chiu H-M, Chen M-J, Chen CC, Wang H-P, Wu M-S, Lin J-T. Plasma insulin-like growth factor-binding protein-2 levels as diagnostic and prognostic biomarker of colorectal cancer. J Clin Endocrinol Metab. 2010;95:1717–1725. doi: 10.1210/jc.2009-2668. [DOI] [PubMed] [Google Scholar]
- Miraki-Moud F, Jenkins PJ, Fairclough PD, Jordan S, Bustin SA, Jones AM, Lowe DG, Monson JP, Grossman AB, Besser GM, Camacho-Hubner C. Increased levels of insulin-like growth factor binding protein-2 in sera and tumours from patients with colonic neoplasia with and without acromegaly. Clin Endocrinol (Oxford) 2001;54:499–508. doi: 10.1046/j.1365-2265.2001.01221.x. [DOI] [PubMed] [Google Scholar]
- Ben-Shmuel A, Shvab A, Gavert N, Brabletz T, Ben-Ze’ev A (2012) Global analysis of L1-transcriptomes identified IGFBP-2 as a target of ezrin and NF-[kappa]B signaling that promotes colon cancer progression. Oncogene [DOI] [PubMed]
- Hoflich A, Lahm H, Blum W, Kolb H, Wolf E. Insulin-like growth factor-binding protein-2 inhibits proliferation of human embryonic kidney fibroblasts and of IGF- responsive colon carcinoma cell lines. FEBS Lett. 1998;434:329–334. doi: 10.1016/s0014-5793(98)01011-4. [DOI] [PubMed] [Google Scholar]
- Corkins MR, Vanderhoof JA, Slentz DH, MacDonald RG, Park JH. Growth stimulation by transfection of intestinal epithelial cells with an antisense insulin-like growth factor binding protein-2 construct. Biochem Biophys Res Commun. 1995;211:707–713. doi: 10.1006/bbrc.1995.1870. [DOI] [PubMed] [Google Scholar]
- Michell NP, Langman MJ, Eggo MC. Insulin-like growth factors and their binding proteins in human colonocytes: preferential degradation of insulin-like growth factor binding protein 2 in colonic cancers. Br J Cancer. 1997;76:60–66. doi: 10.1038/bjc.1997.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl D, Hessel E, Oesterle D, Renner-Muller I, Elmlinger M, Langhammer M, Gottlicher M, Wolf E, Lahm H, Hoeflich A. IGFBP-2 overexpression reduces the appearance of dysplastic aberrant crypt foci and inhibits growth of adenomas in chemically induced colorectal carcinogenesis. Int J Cancer. 2009;124:2220–2225. doi: 10.1002/ijc.24193. [DOI] [PubMed] [Google Scholar]