Abstract
Epigenetics refers to heritable changes in gene expression that are independent of alterations in DNA sequence. It is now accepted that disruption of epigenetic mechanisms plays a key role in the pathogenesis of cancer: culminating in altered gene function and malignant cellular transformation. DNA methylation and histone modifications are the most widely studied changes but non-coding RNAs such as miRNAs are also considered part of the epigenetic machinery. The insulin-like growth factor (IGF) axis is composed of two ligands, IGF-I and –II, their receptors and six high affinity IGF binding proteins (IGFBPs). The IGF axis plays a key role in cancer development and progression. As IGFBP genes have consistently been identified among the most common to be aberrantly altered in tumours, this review will focus on epigenetic regulation of IGFBP-3 in cancer for which the majority of evidence has been obtained.
Keywords: IGFBP-3, DNA methylation, Cancer
Epigenetic modifications
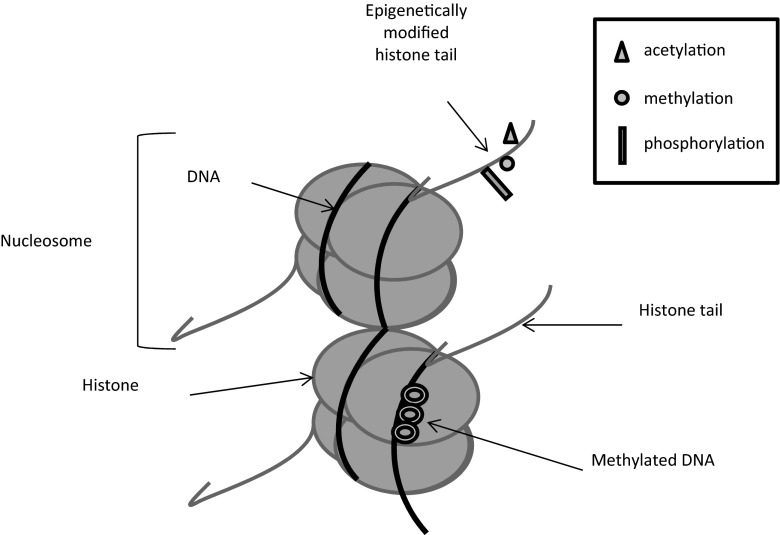
Epigenetic mechanisms are required for normal development in mammals that enables cells to respond and adapt dynamically to changes in their environment. Unfortunately disruption of epigenetic mechanisms plays a key role in the pathogenesis of cancer: culminating in altered gene function and malignant cellular transformation. The particular advantage with regard to epigenetic changes is that they are potentially reversible, in contrast to irreversible genetic mutations. This has huge implications relating to the prevention, diagnosis, and treatment of cancer and other diseases: and therefore represent potential targets for therapy. The two epigenetic modifications that have been most studied are: chemical modifications to the cytosine residues of DNA (DNA methylation) and modifications to histone proteins associated with DNA (histone modifications) (Jenuwein and Allis 2001; Lo and Weksberg 2014) (Fig. 1).
Fig. 1.
Different types of epigenetic modifications. Histones can undergo phosphorylation ( ) methylation (
) methylation ( ) and acetylation (
) and acetylation ( ) amongst other modifications that are involved in chromatin remodelling and transcriptional regulation. DNA molecules are methylated which inhibits gene expression
) amongst other modifications that are involved in chromatin remodelling and transcriptional regulation. DNA molecules are methylated which inhibits gene expression
DNA can be modified by methylation of cytosine bases by enzymes called DNA methyl-transferases (DNMTs): DNMT1, DNMT2 and DNMT3 families. Methylated DNA predominantly inhibits gene expression, as it attracts methyl cytosine binding proteins that promote chromatin condensation into transcriptionally repressive conformations (Miranda and Jones 2007). Genomic DNA is packaged around histones to form a complex called chromatin. A unit of chromatin, known as a nucleosome, is composed of 146 base pairs of DNA wrapped around an octamer of four core histones (H2A, H2B, H3, and H4). With amino-terminal tails extending from the globular region of the histones, they also undergo a number of different covalent modifications, including methylation, acetylation, phosphorylation, ubiquitination, and sumoylation (Marino-Ramirez et al. 2005). Histone modifications can have different outcomes that are dependent upon the type and the location of the modification on the histone and in contrast to DNA methylation can be activating or inhibiting. The most widely reported histone modifications are acetylation and methylation (Lakshmaiah et al. 2014). Changes in the acetylation of histones are mediated by enzymes called histone acetyl transferases (HATs) and histone deacetylases (HDACs). Acetylation of the lysine residues of histones enables an open chromatin structure, whereas deacetylation induces a closed chromatin conformation that results in the inhibition of transcription (Grunstein 1997). Histones are not the only proteins that interact with DNA in chromatin. RNAs especially non-coding RNAs such as miRNAs are known to play several roles in the control of chromatin structure (Yang et al. 1839). MiRNAs are approximately 22 nucleotide non-coding RNAs, that regulate gene expression through posttranscriptional silencing of target genes. Sequence-specific base pairing of miRNAs with 3’ untranslated regions of target messenger RNA within the RNA-induced silencing complex culminates in the degradation of target messenger RNA or the inhibition of translation (He and Hannon 2004). MiRNAs are expressed in a tissue-specific manner and control a wide array of biological processes including cell proliferation, apoptosis and differentiation. Zhang B (2007) elegantly reviewed the impact of miRNAs in the pathogenesis of cancer with examples of how they can act as oncogenes or tumour suppressor genes: like normal genes miRNAs can also be subject to epigenetic regulation or affect epigenetic mechanisms (Zhang and Pan 2007) (Zhang et al. 2007).
The insulin-like growth factor (IGF) axis
The IGF axis is composed of two ligands IGF-I and IGF-II, their receptors and a family of six high affinity IGF binding proteins (IGFBPs). The cellular effects of the IGFs are mediated by a number of cell surface receptors including the type I and type II receptors, the insulin receptor and insulin receptor-IGF-I receptor hybrids. Both IGF-I and IGF-II can mediate their actions on cell growth and survival via the IGF-IR, which is a transmembrane, tyrosine kinase that is structurally and functionally homologous to the insulin receptor. IGF-I can act via the insulin receptor but only at supra-physiological doses (LeRoith et al. 1995). IGF-II can bind with high affinity to the IGF-II receptor/mannose-6-phosphate receptor, a non-tyrosine kinase receptor considered to play an important role in the clearance and degradation of IGF-II. There are six high affinity IGFBPs that all have greater affinity for binding to the IGFs, than the IGF-IR, and can modulate IGF actions in many cell types. The IGFBPs slow the clearance of the IGFs, particularly IGFBP-3 and IGFBP-5, which also bind to a glycoprotein called the acid labile subunit (ALS) forming ternary complexes which have very long half-lives in the circulation. It is these complexes that maintain very high concentrations of IGFs in the body. IGFBP-3 is the main IGFBP found in human serum and has the potential to either inhibit or enhance IGF actions in many cell types: IGFBP-3 can restrict tumour growth and progression by limiting IGF-mitogenic and cell survival actions. The actions of many anti-proliferative agents appear to operate, at least in part, via up-regulation of endogenous IGFBP-3 produced by the tumour cells including the tumour suppressor gene, p53 (Buckbinder et al. 1995), transforming growth factor-beta (TGF-beta) (McCaig et al. 2002a), retinoids (Gucev et al. 1996), vitamin D (Colston et al. 1998), the anti-estrogen, Tamoxifen (Huynh and Pollak 1994) and the fatty acid, butyrate (Collard et al. 2003).
Accumulating evidence indicates that most of the IGFBPs can also act in an intrinsic manner, independent of IGF-binding, affecting various aspects of cell function in both a positive and negative manner. There are a number of theories as to the mechanisms underlying the intrinsic actions of IGFBP-3 and these have been extensively reviewed recently (see (Baxter 2014; Johnson and Firth 2014)).
Structure of the IGFBP-3 gene
(Cubbage et al. 1990) characterized the IGFBP-3 gene and promoter. The authors reported that the IGFBP-3 gene spans approximately 8.9 kb of chromosomal DNA that contains five exons (exon 1 is 535 base pairs (bp) long, exon 2: spans 227 bp, exon 3: spans 120 bp, exon 4 141 bp and exon 5 1482 bp) and four introns. The IGFBP-3 gene has a classic CpG island that is 850 bp in length and is made up of exon 1 and 250 bp of DNA immediately 5’ to exon 1 enabling it to be amenable to epigenetic modifications. Cubbage et al. further delineated that the IGFBP-3 promoter contains a single cap site, a TATA box, an upstream promoter element and each is located in a CpG island that spans the promoter region and first exon (Cubbage et al. 1990).
Evidence for epigenetic alteration of IGFBP-3 in cancer
The IGF axis plays a key role in cancer development and progression. IGFBP genes have consistently been identified among the most common to be aberrantly altered in tumours and this review will focus on epigenetic regulation of IGFBP-3 in cancer, the IGFBP for which the majority of evidence has been obtained. The most studied cancers in relation to epigenetic modulation of IGFBP-3 are lung and liver but there are additional reports in other cancers including ovarian, melanoma, breast, bladder, prostate and gastric.
Lung cancer: patient samples
Chang et al. in 2002 performed one of the first studies investigating the association of DNA methylation of the IGFBP-3 promoter in relation to non-small-cell lung cancer (NSCLC) prognosis. They identified 83 patients with pathological stage 1 NSCLC who had undergone curative surgery. Using bisulfite modification followed by methylation-specific PCR (MSP) they found that patients with a hyper-methylated IGFBP-3 promoter, compared with those without, exhibited a reduced 5 year disease-specific survival rate (53.1 % compared to 86.1 %), a reduced disease-free survival rate (36.5 % compared to 76.2 %) in addition to a reduction in overall survival rate (38.9 % versus 64 %) (Chang et al. 2002). In 2004 the same researchers also assessed the methylation status of IGFBP-3 in tumour tissue at different stages. With stage 1: 16 of the 23 specimens were positive, for stage 2: 7 of the 9 were positive, for stage 3: 8 of 11 and for stage 4: 6 out of 6 were positive for methylated IGFBP-3. These data suggested that more advanced disease was associated with increased incidence of IGFBP-3 promoter methylation (Chang et al. 2004). (Pernia et al. 2014) using 40 NSCLC patients who were treated with chemotherapy with or without radiotherapy determined that patients with an unmethylated IGFBP-3 promoter would do better with chemotherapy alone as opposed to the combination therapy (Pernia et al. 2014).
Cell line studies
In 2004 (Chang et al. 2004) performed further investigations using NSCLC cell lines representing different stages of the disease and also using tumour tissue to confirm if hyper-methylation of the IGFBP-3 promoter did culminate in a loss of IGFBP-3 abundance. They found that IGFBP-3 was methylated in 7 of 13 cell lines examined and of these 7 the level of IGFBP-3 mRNA and protein only correlated to the level of DNA methylation in some instances. The authors treated the 7 cell lines that contained a methylated IGFBP-3 promoter with a de-methylating agent called 5’-aza-2’-deoxycytidine (5’-aza-dC), AZA. They observed that IGFBP-3 re-expression only occurred in 4 of the 7 cell lines and they concluded that many mechanisms may be involved in regulating IGFBP-3 in these cell lines. Cisplatin-based chemotherapy is the mainstay treatment for NSCLC: unfortunately as with other types of cancers, cells can develop drug resistance. Drug resistance against cisplatin has in part been attributed to epigenetic silencing of genes necessary to enable chemo-sensitivity. In 2010 Ibanez de Caceres et al. performed a microarray expression screening to compare the genetic profile in cisplatin-resistant with cisplatin sensitive cell lines following epigenetic manipulation in an attempt to identify key genes that might be involved (Ibanez de Caceres I et al. 2010). The authors found that the promoter of only one gene, IGFBP-3 was specifically methylated in cisplatin-resistant cells and IGFBP-3 was re-expressed upon treatment of these cells with AZA. Other genes were modified indirectly in an epigenetic manner. Furthermore silencing IGFBP-3 in the cisplatin-sensitive cells induced chemo-resistance suggesting that IGFBP-3 mediated cisplatin-induced chemo-sensitivity (Ibanez de Caceres I et al. 2010). Furthermore in 2014 the same group reported that radiation had the potential to sensitise cisplatin-resistant cell lines via IGFBP-3 demethylation (Pernia et al. 2014). A tobacco carcinogen called NNK, a chemical inducer of lung cancer, was administered to normal lung epithelial cells and tumorigenic derivatives: it increased their growth and this was mediated by down-regulating IGFBP-3 via increased DNA methylation. Conversely over-expressing IGFBP-3 in one of the NNK chemically–induced cancer cells resulted in cell death, suggesting that IGFBP-3 acts as a tumour suppressor and so is down-regulated by DNA methylation to promote cancer progression (Harada et al. 2013).
Liver cancer: patient samples
Using RT-PCR and Northern blot analysis Hanafusa 2002 et al. concluded that 9 out of 12 human hepatocellular carcinoma (HCC) samples exhibited reduced IGFBP-3 expression. Following bisulfite-PCR-restriction analysis on the promoter region of IGFBP-3 in the same samples, they identified that reduced IGFBP-3 expression in 4 of the 12 samples was the result of DNA hypermethylation (Hanafusa et al. 2002). In 2012 Regel et al. studied the role of IGFBP-3 methylation in hepatoblastoma (HB) and embryonal liver tumour that occurs in early childhood. They found that IGFBP-3 mRNA levels are reduced in HB cases compared to normal liver controls in 26 out of 36 samples. They also detected reduced levels of IGFBP-3 in 6 out of 9 pediatric HCC cases (Regel et al. 2012).
Cell line studies
Of the HCC cell lines: HEPG2, HEP3B, PLC/PRF/5, HLE and HuH-7, only HEPG2, HEP3B and HuH-7 (Hanafusa et al. 2002) showed reduced expression of IGFBP-3. Treatment with AZA caused re-expression of IGFBP-3 in HEPG2 cells in which the IGFBP-3 promoter was methylated, but not in HuH-7 cells that expressed unmethylated IGFBP-3. One of the major regulators of IGFBP-3 is the tumour suppressor gene p53: IGFBP-3 possesses 11 p53 consensus binding sequences upstream of the IGFBP-3 promoter (Hanafusa et al. 2002). In 2005 Hanafusa et al. showed that 4 of these 11 p53 consensus sequences were critical for p53-induced IGFBP-3 production and that hyper-methylation of these sequences was a mechanism in these cells for suppressing p53-induced IGFBP-3 production (Hanafusa et al. 2005). Current evidence suggests that hepatitis B virus (HBx) protein plays a key role in the pathogenesis of HBv-induced HCC by promoting the progression through the cell cycle for example by binding to and inhibiting the expression of p53 tumour suppressor genes (Kew 2011). Park et al. (Park et al. 2007) investigated the impact of transfecting The Chang Liver (CHL), HEPG2 and HuH-7 HCC cells with an HB-x-expressing construct. They found that IGFBP-3 expression was reduced in each of these cell lines and IGFBP-3 was re-expressed upon treatment with AZA, suggesting that HBx caused IGFBP-3 down-regulation through DNA methylation. They showed further that this was mediated by the DNA methyltransferases (DNMT) DNMT3A1 and DNMT3A2 (Park et al. 2007). In 2012 Regel et al. found that IGFBP-3 was completely methylated in all 4 cell lines examined: HuH6, HepT3, HepT1, HepG2, HuH-7 and following AZA treatment was re-expressed. They also used a histone deacetylase inhibitor Trichostatin A, which similarly induced re-expression of IGFBP-3 to the same degree (Regel et al. 2012). The involvement of histone deacetylation in repressing IGFBP-3 production was similarly observed by (Lin et al. 2011), where using HepG2 cells, an HDAC inhibitor (MS-275), induced IGFBP-3 expression by increasing the binding of acetylated histone H3 to IGFBP-3 promoter sequences. Both of these studies suggest that in HCC cell lines suppression of IGFBP-3 occurs by both DNA methylation and histone deacetylation (Lin et al. 2011).
Other cancers
Ovarian cancer: patient samples
Wiley et al. (2006) assessed the methylation status of 6 key genes thought to be important in the pathogenesis of ovarian cancer (p16, breast cancer 1 BRCA1, IGFBP-3, glutathione S-transferase pi1 (GSTP1) and ERα and human MutL homologue (hMLH1)) in ovarian tumours using methylation-specific PCR (MS-PCR) but were unable to identify a ‘methylator phenotype’. However, they did observe that the frequency of methylation of IGFBP-3 was much higher in the 215 malignant tumours (44 %) compared to the 19 non-malignant (26 %) (Wiley et al. 2006a). They further determined that this was associated with disease progression and death and more marked in patients with early stage disease. The authors suggest that IGFBP-3 promoter methylation status may be a useful prognostic marker for disease progression and death in early-stage ovarian cancers (Wiley et al. 2006b).
In 2009 Torng et al. found that among 40 ovarian endometriod cancer (OEC) samples higher IGFBP-3 methylation was associated with reduced abundance of IGFBP-3 and higher histological grade. Cox regression analysis revealed that low IGFBP-3 expression was associated with lower progression free survival and lower overall survival (Torng et al. 2009).
Skin cancer: patient samples
(Dar et al. 2010) identified significant methylation observed in primary malignant melanoma samples (n = 36) compared to the benign nevi (n = 26) (Dar et al. 2010). Oy et al. (Oy et al. 2010) performed methylation-specific real time PCR (MSP) on 5 melanoma patient samples that were IHC positive for IGFBP-3 and 6 that were negative. IGFBP-3 methylation was observed in 2 of the 5 positive and in 5 of the 6 negative suggesting that IGFBP-3 methylation is one mechanism that exists in malignant melanoma cell lines and in patient samples (Oy et al. 2010).
Cell line studies
Early work performed by (Fraga et al. 2004) used cell lines from the mouse skin multistage tumour progression model in addition to 15 primary mouse skin tumours. Using the cell lines they identified IGFBP-3 as a methylation-positive gene in this mouse model. Furthermore, this result was validated in mouse tissue and the frequency of methylation appeared to increase with tumour stage: the promoter of the IGFBP-3 gene was unmethylated in five papilloma samples but was methylated in 2 of 5 (40 %) squamous carcinoma and in 4 of 5 (80 %) spindle carcinomas (Fraga et al. 2004). These data from a mouse model were later confirmed in a number of human melanoma cell lines. (Dar et al. 2010) determined that there was low expression of IGFBP-3 in a range of melanoma cell lines (WM3211, DO4, WM1205, MaMel71, MaMel144a1 and C8161.9) in comparison to normal melanocytes. Treating the cells with AZA induced re-expression of IGFBP-3 in most of the cell lines and subsequent ChIP analysis revealed that this was accompanied by an enrichment of acetylated histones H3, H4 and H3 di- and tri-methylated lysine 4 close to the IGFBP-3 transcription start site. Bisulfite-modified PCR analysis confirmed that IGFBP-3 was methylated in most of the cell lines (Dar et al. 2010). To determine a potential role of IGFBP-3 in melanoma, IGFBP-3 was over-expressed in cell lines: this resulted in growth inhibition, apoptosis, reduced colony formation and invasion (Dar et al. 2010). Another study found similar findings: Oy et al. (Oy et al. 2010) examined The Wistar Melanoma cell lines and the FEMX-1 and LOX cell lines. Seven of the 10 cell lines expressed negligible IGFBP-3 and DNA methylation of the IGFBP-3 promoter was responsible in approximately half of cases (Oy et al. 2010).
Urological cancers: patient samples
In relation to renal cancer, Ibanez de Caceres et al. (2006) examined the methylation status of the IGFBP-3 gene in tumour samples (32 primary renal tumours) and observed that the IGFBP-3 promoter was methylated in 37 % of tumour DNA (Ibanez de Caceres I et al. 2006). In contrast, a study that selected samples from 90 patients with clear cell carcinoma and 20 normal kidney samples, did not observe that hyper-methylation of IGFBP-3 was a common event (Christoph et al. 2006a).
Christoph et al. (2006) performed MSP on tissue samples obtained from 110 patients with bladder tumours and 20 patients with no urological cancers. Hyper-methylation of the IGFBP-3 promoter in tumour samples was common (66 %) but not in the normal samples. In addition, IGFBP-3 methylation levels enabled the differentiation between tumours with higher risk of recurrence from lower risk tumours (Christoph et al. 2006b), (Ohashi et al. 2012) investigated the methylation status of the IGFBP-3 promoter in 80 samples of biliary tract carcinomas and found that it was hyper-methylated in 41 % of cases (Ohashi et al. 2012) . Perry et al. as developed an in silico strategy to globally identify targets of promoter hyper-methylation in prostate cancer. One target IGFBP-3 was found to be methylated in 49/79 primary prostate adenocarcinoma samples, and in 7/14 adjacent pre-invasive high-grade prostatic intraepithelial neoplasia (PIN). However, it was only observed in 5/37 benign prostatic hyperplasia and in 0/39 histologically normal adjacent prostate tissue (Perry et al. 2007).
Cell line studies
Ibanez de Caceres et al. (2006) using the renal cancer cell lines 786–0, ACHN, HRC51 and HRC59 determined that methylation of IGFBP-3 was only observed in cancer cell lines as opposed to normal cell DNA.
Breast cancer: cell line studies
In relation to another hormone responsive cancer, breast cancer, in vitro data indicated that AZA increased the expression of IGFBP-3 in a range of breast cancer cell lines (MCF-7, T47D, HS578T & MDA-MB-231) and promoted growth inhibition and apoptosis. Silencing AZA-induced IGFBP-3 indicated that IGFBP-3 played a key role in the actions of AZA in these breast cancer cell lines (Zeng et al. 2013).
Gastric cancer: patient samples
(Gigek et al. 2010) assessed the methylation status of IGFBP-3 in 94 adenocarcinoma samples and in 43 normal gastric mucosa samples. The methylation frequency between the cancer and normal samples was similar 95.7 and 97.7 % respectively so this was not a viable method of distinguishing between the sample types. Interestingly the methylation status of IGFBP-3 did not relate to the tissue levels of IGFBP-3. Immunohistochemistry on 54 gastric cancer and 20 normal samples revealed that IGFBP-3 abundance was significantly greater in the tumour than the normal samples and that this assessment of IGFBP-3 may be more relevant and informative as a marker of malignancy (Gigek et al. 2010).
Targeting
There are many classes of drugs that inhibit DNA methylation; 5-Azacytidine and Zebularine that are cytidine analogs and integrate into the DNA (Momparler 2005; Santi et al. 1984). There are also anti-sense oligonucleotides that have been developed such as MG98 that binds to the 3’ untranslated region of DNMT1 which blocks transcription of the gene (Amato 2007), in addition to agents designed to inhibit histone acetylases (HATs) and deacetylases (HDACi). These drugs are designed to have a global effect: as opposed to specifically targeting one molecule, such as IGFBP-3 and so attempts to optimise current agents and develop new ones is ongoing particularly in terms of trying to increase specificity and reduce toxicity. In an effort to overcome these issues these agents are being tested in combination with established cancer drugs such as chemotherapeutics (see review (Heerboth et al. 2014)). Overall it appears that in the majority of cancers IGFBP-3 promoter methylation and/or de-acetylation is associated with disease progression as its re-expression as demonstrated from in vitro data suggest that IGFBP-3 predominantly acts to inhibit growth, induce apoptosis and reduce migration and invasion. Therefore, epigenetic manipulation of IGFBP-3 may be a potential way forward in the management of certain cancers: however, the ability to achieve this effectively is not straightforward for a number of reasons. IGFBP-3 has both IGF-dependent and independent actions and its epigenetic re-expression may be of potential benefit by inhibiting tumour growth both by intrinsic IGF-independent actions and by blocking the mitogenic and survival actions of IGFs. However both in vivo and in vitro data provide evidence that IGFBP-3 can exert both positive and negative effects on cell function. Using breast cancer as an example, in vivo studies suggest that high levels of IGFBP-3 are associated with a poor prognosis, whereas the epidemiology studies relating to circulating concentrations have reported both positive and inverse associations of IGFBP-3 with breast cancer (see review (Perks and Holly 2008)). In vitro studies have reported that breast cancer cells can respond both in a positive or negative manner to IGFBP-3, which appears dependent upon changes in the extracellular matrix (ECM) (McCaig et al. 2002b; Butt et al. 2004). It is well established that the ECM to which breast cancer cells are exposed changes with tumour progression, with more advanced tumours seeing more fibronectin, which is associated with a poor prognosis (Ioachim et al. 2002). In vitro studies have shown that increased exposure to fibronectin versus laminin or collagen switches IGFBP-3 from acting as a growth inhibitor and inducer of apoptosis to a survival factor and growth promoter (McCaig et al. 2002b). These studies indicate that for breast cancer at least an understanding of the stage of tumour progression and hence the tumour microenvironment is of criticial importance to ensure that epigenetic reactivation of IGFBP-3 would actually be beneficial and negate tumour growth and survival. A further level of complexity is faced when considering tumour heterogeneity that greatly influences the efficacy of cancer treatments. Considerable variation exists between tumours (inter-tumoural heterogeneity) in different individuals, even those of similar phenotype in the same tissue: in addition to this however it has gradually become clear that there is also considerable heterogeneity within individual tumours and heterogeneity between the primary tumour and metastatic lesions within any cancer patient (see review (Holly et al. 2013)). Breast cancers can be present for many years prior to diagnosis and the normal mutation rate combined with acquired genetic and epigenetic instability result in inevitable intra-tumoural heterogeneity; subsequent treatments and dissemination to secondary sites with differing local environments than add differential selection pressures to the multiple clones. Massively parallel sequencing of breast cancers have confirmed that primary tumours can be comprised of multiple genetically diverse clones and that metastatic lesions may differ from the primary in their genetic repertoire (Ng et al. 2015) As a consequence, even if re-expression of IGFBP-3 were effective in treating the majority of a tumour, if a sub-clone existed that was resistant to IGFBP-3 or on which IGFBP-3 had positive effects then these cells may have the capacity to repopulate the tumour and this could then result in a more resistant and aggressive cancer. Such issues of intra-tumoural heterogeneity explain why many targeted therapies only provide months of respite before resistance develops; but this is of especial concern for IGFBP-3 that in some contexts could actually promote a more aggressive behaviour of some cells.
Summary
DNA methylation of IGFBP-3 may be a potential marker of disease stage and the potential of the tumour to exhibit a more aggressive phenotype. Furthermore, the frequency of IGFBP-3 promoter methylation is significantly lower in normal tissue compared with tumour samples which may support the notion that methylation of the IGFBP-3 promoter could be a potential marker of malignancy. Epigenetic manipulation of IGFBP-3 may be a potential way forward in the management of certain cancers.
References
- Amato RJ. Inhibition of DNA methylation by antisense oligonucleotide MG98 as cancer therapy. Clin Genitourin Cancer. 2007;5:422–6. doi: 10.3816/CGC.2007.n.029. [DOI] [PubMed] [Google Scholar]
- Baxter RC. IGF binding proteins in cancer: mechanistic and clinical insights. Nat Rev Cancer. 2014;14:329–41. doi: 10.1038/nrc3720. [DOI] [PubMed] [Google Scholar]
- Buckbinder L, Talbott R, Velasco-Miguel S, Takenaka I, Faha B, Seizinger BR, et al. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature. 1995;377:646–9. doi: 10.1038/377646a0. [DOI] [PubMed] [Google Scholar]
- Butt AJ, Martin JL, Dickson KA, McDougall F, Firth SM, Baxter RC. Insulin-like growth factor binding protein-3 expression is associated with growth stimulation of T47D human breast cancer cells: the role of altered epidermal growth factor signaling. J. Clin. Endocrinol. Metab. 2004;89:1950–6. doi: 10.1210/jc.2003-030914. [DOI] [PubMed] [Google Scholar]
- Chang YS, Wang L, Liu D, Mao L, Hong WK, Khuri FR, et al. Correlation between insulin-like growth factor-binding protein-3 promoter methylation and prognosis of patients with stage I non-small cell lung cancer. Clin Cancer Res. 2002;8:3669–75. [PubMed] [Google Scholar]
- Chang YS, Wang L, Suh YA, Mao L, Karpen SJ, Khuri FR, et al. Mechanisms underlying lack of insulin-like growth factor-binding protein-3 expression in non-small-cell lung cancer. Oncogene. 2004;23:6569–80. doi: 10.1038/sj.onc.1207882. [DOI] [PubMed] [Google Scholar]
- Christoph F, Weikert S, Kempkensteffen C, Krause H, Schostak M, Kollermann J, et al. Promoter hypermethylation profile of kidney cancer with new proapoptotic p53 target genes and clinical implications. Clin Cancer Res. 2006;12:5040–6. doi: 10.1158/1078-0432.CCR-06-0144. [DOI] [PubMed] [Google Scholar]
- Christoph F, Weikert S, Kempkensteffen C, Krause H, Schostak M, Miller K, et al. Regularly methylated novel pro-apoptotic genes associated with recurrence in transitional cell carcinoma of the bladder. Int J Cancer. 2006;119:1396–402. doi: 10.1002/ijc.21971. [DOI] [PubMed] [Google Scholar]
- Collard TJ, Guy M, Butt AJ, Perks CM, Holly JM, Paraskeva C, et al. Transcriptional upregulation of the insulin-like growth factor binding protein IGFBP-3 by sodium butyrate increases IGF-independent apoptosis in human colonic adenoma-derived epithelial cells. Carcinogenesis. 2003;24:393–401. doi: 10.1093/carcin/24.3.393. [DOI] [PubMed] [Google Scholar]
- Colston KW, Perks CM, Xie SP, Holly JM. Growth inhibition of both MCF-7 and Hs578T human breast cancer cell lines by vitamin D analogues is associated with increased expression of insulin-like growth factor binding protein-3. J Mol Endocrinol. 1998;20:157–62. doi: 10.1677/jme.0.0200157. [DOI] [PubMed] [Google Scholar]
- Cubbage ML, Suwanichkul A, Powell DR. Insulin-like growth factor binding protein-3. organization of the human chromosomal gene and demonstration of promoter activity. J Biol Chem. 1990;265:12642–9. [PubMed] [Google Scholar]
- Dar AA, Majid S, Nosrati M, de Semir D, Federman S, Kashani-Sabet M. Functional modulation of IGF-binding protein-3 expression in melanoma. J. Investig. Dermatol. 2010;130:2071–9. doi: 10.1038/jid.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga MF, Herranz M, Espada J, Ballestar E, Paz MF, Ropero S, et al. A mouse skin multistage carcinogenesis model reflects the aberrant DNA methylation patterns of human tumors. Cancer Res. 2004;64:5527–34. doi: 10.1158/0008-5472.CAN-03-4061. [DOI] [PubMed] [Google Scholar]
- Gigek CO, Leal MF, Lisboa LC, Silva PN, Chen ES, Lima EM, et al. Insulin-like growth factor binding protein-3 gene methylation and protein expression in gastric adenocarcinoma. Growth Hormon. IGF Res. 2010;20:234–8. doi: 10.1016/j.ghir.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–52. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- Gucev ZS, Oh Y, Kelley KM, Rosenfeld RG. Insulin-like growth factor binding protein 3 mediates retinoic acid- and transforming growth factor beta2-induced growth inhibition in human breast cancer cells. Cancer Res. 1996;56:1545–50. [PubMed] [Google Scholar]
- Hanafusa T, Yumoto Y, Nouso K, Nakatsukasa H, Onishi T, Fujikawa T, et al. Reduced expression of insulin-like growth factor binding protein-3 and its promoter hypermethylation in human hepatocellular carcinoma. Cancer Lett. 2002;176:149–58. doi: 10.1016/S0304-3835(01)00736-4. [DOI] [PubMed] [Google Scholar]
- Hanafusa T, Shinji T, Shiraha H, Nouso K, Iwasaki Y, Yumoto E, et al. Functional promoter upstream p53 regulatory sequence of IGFBP3 that is silenced by tumor specific methylation. BMC Cancer. 2005;5:9. doi: 10.1186/1471-2407-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A, Jogie-Brahim S, Oh Y. Tobacco specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone suppresses a newly identified anti-tumor IGFBP-3/IGFBP-3R system in lung cancer cells. Lung Cancer. 2013;80:270–7. doi: 10.1016/j.lungcan.2013.02.016. [DOI] [PubMed] [Google Scholar]
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–31. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Heerboth S, Lapinska K, Snyder N, Leary M, Rollinson S, Sarkar S. Use of epigenetic drugs in disease: an overview. Genetics & Epigen. 2014;6:9–19. doi: 10.4137/GEG.S12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly JM, Zeng L, Perks CM. Epithelial cancers in the post-genomic era: should we reconsider our lifestyle? Cancer Metastasis Rev. 2013;32(3–4):673–705. doi: 10.1007/s10555-013-9445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh H, Pollak M. Uterotrophic actions of estradiol and tamoxifen are associated with inhibition of uterine insulin-like growth factor binding protein 3 gene expression. Cancer Res. 1994;54:3115–9. [PubMed] [Google Scholar]
- Ibanez de Caceres I, Dulaimi E, Hoffman AM, Al-Saleem T, Uzzo RG, Cairns P. Identification of novel target genes by an epigenetic reactivation screen of renal cancer. Cancer Res. 2006;66:5021–8. doi: 10.1158/0008-5472.CAN-05-3365. [DOI] [PubMed] [Google Scholar]
- Ibanez de Caceres I, Cortes-Sempere M, Moratilla C, Machado-Pinilla R, Rodriguez-Fanjul V, Manguan-Garcia C, et al. IGFBP-3 hypermethylation-derived deficiency mediates cisplatin resistance in non-small-cell lung cancer. Oncogene. 2010;29:1681–90. doi: 10.1038/onc.2009.454. [DOI] [PubMed] [Google Scholar]
- Ioachim E, Charchanti A, Briasoulis E, Karavasilis V, Tsanou H, Arvanitis DL, et al. Immunohistochemical expression of extracellular matrix components tenascin, fibronectin, collagen type IV and laminin in breast cancer: their prognostic value and role in tumour invasion and progression. Eur J Cancer. 2002;38:2362–70. doi: 10.1016/S0959-8049(02)00210-1. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–80. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Firth SM. IGFBP-3: A cell fate pivot in cancer and disease. Growth Hormon. IGF Res. 2014;24(5):164–73. doi: 10.1016/j.ghir.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Kew MC. Hepatitis B, virus x protein in the pathogenesis of hepatitis B virus-induced hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26(Suppl 1):144–52. doi: 10.1111/j.1440-1746.2010.06546.x. [DOI] [PubMed] [Google Scholar]
- Lakshmaiah KC, Jacob LA, Aparna S, Lokanatha D, Saldanha SC. Epigenetic therapy of cancer with histone deacetylase inhibitors. J. Cancer Res. Ther. 2014;10:469–78. doi: 10.4103/0973-1482.137937. [DOI] [PubMed] [Google Scholar]
- LeRoith D, Werner H, Beitner-Johnson D, Roberts CT., Jr Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143–63. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- Lin WH, Martin JL, Marsh DJ, Jack MM, Baxter RC. Involvement of insulin-like growth factor-binding protein-3 in the effects of histone deacetylase inhibitor MS-275 in hepatoma cells. J Biol Chem. 2011;286:29540–7. doi: 10.1074/jbc.M111.263111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo R, Weksberg R. Biological and biochemical modulation of DNA methylation. Epigenomics. 2014;6:593–602. doi: 10.2217/epi.14.49. [DOI] [PubMed] [Google Scholar]
- Marino-Ramirez L, Kann MG, Shoemaker BA, Landsman D. Histone structure and nucleosome stability. Expert Rev. Proteomics. 2005;2:719–29. doi: 10.1586/14789450.2.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaig C, Fowler CA, Laurence NJ, Lai T, Savage PB, Holly JM, et al. Differential interactions between IGFBP-3 and transforming growth factor-beta (TGF-beta) in normal vs cancerous breast epithelial cells. Br J Cancer. 2002;86:1963–9. doi: 10.1038/sj.bjc.6600355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaig C, Perks CM, Holly JM. Intrinsic actions of IGFBP-3 and IGFBP-5 on Hs578T breast cancer epithelial cells: inhibition or accentuation of attachment and survival is dependent upon the presence of fibronectin. J Cell Sci. 2002;115:4293–303. doi: 10.1242/jcs.00097. [DOI] [PubMed] [Google Scholar]
- Miranda TB, Jones PA. DNA methylation: the nuts and bolts of repression. J Cell Physiol. 2007;213:384–90. doi: 10.1002/jcp.21224. [DOI] [PubMed] [Google Scholar]
- Momparler RL. Epigenetic therapy of cancer with 5-aza-2’-deoxycytidine (decitabine) Semin Oncol. 2005;32:443–51. doi: 10.1053/j.seminoncol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Ng CK, Schultheis AM, Bidard FC, Weigelt B, Reis-Filho JS (2015) Breast cancer genomics from microarrays to massively parallel sequencing: paradigms and new insights. Journal of the National Cancer Institute. 107:(5) [DOI] [PubMed]
- Ohashi H, Adachi Y, Yamamoto H, Taniguchi H, Nosho K, Suzuki H, et al. Insulin-like growth factor receptor expression is associated with aggressive phenotypes and has therapeutic activity in biliary tract cancers. Cancer Sci. 2012;103:252–61. doi: 10.1111/j.1349-7006.2011.02138.x. [DOI] [PubMed] [Google Scholar]
- Oy GF, Slipicevic A, Davidson B, Solberg Faye R, Maelandsmo GM, Florenes VA. Biological effects induced by insulin-like growth factor binding protein 3 (IGFBP-3) in malignant melanoma. Int J Cancer. 2010;126:350–61. doi: 10.1002/ijc.24727. [DOI] [PubMed] [Google Scholar]
- Park IY, Sohn BH, Yu E, Suh DJ, Chung YH, Lee JH, et al. Aberrant epigenetic modifications in hepatocarcinogenesis induced by hepatitis B virus X protein. Gastroenterology. 2007;132:1476–94. doi: 10.1053/j.gastro.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Perks CM, Holly JM. IGF binding proteins (IGFBPs) and regulation of breast cancer biology. J Mammary Gland Biol Neoplasia. 2008;13:455–69. doi: 10.1007/s10911-008-9106-4. [DOI] [PubMed] [Google Scholar]
- Pernia O, Belda-Iniesta C, Pulido V, Cortes-Sempere M, Rodriguez C, Vera O, et al. Methylation status of IGFBP-3 as a useful clinical tool for deciding on a concomitant radiotherapy. Epigen Off J DNA Methylation Soc. 2014;9:1446–53. doi: 10.4161/15592294.2014.971626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AS, Loftus B, Moroose R, Lynch TH, Hollywood D, Watson RW, et al. In silico mining identifies IGFBP3 as a novel target of methylation in prostate cancer. Br J Cancer. 2007;96:1587–94. doi: 10.1038/sj.bjc.6603767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regel I, Eichenmuller M, Joppien S, Liebl J, Haberle B, Muller-Hocker J, et al. IGFBP3 impedes aggressive growth of pediatric liver cancer and is epigenetically silenced in vascular invasive and metastatic tumors. Mol Cancer. 2012;11:9. doi: 10.1186/1476-4598-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi DV, Norment A, Garrett CE. Covalent bond formation between a DNA-cytosine methyltransferase and DNA containing 5-azacytosine. Proc Natl Acad Sci U S A. 1984;81:6993–7. doi: 10.1073/pnas.81.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torng PL, Lin CW, Chan MW, Yang HW, Huang SC, Lin CT. Promoter methylation of IGFBP-3 and p53 expression in ovarian endometrioid carcinoma. Mol Cancer. 2009;8:120. doi: 10.1186/1476-4598-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley A, Katsaros D, Chen H, de la Rigault Longrais IA, Beeghly A, Puopolo M, et al. Aberrant promoter methylation of multiple genes in malignant ovarian tumors and in ovarian tumors with low malignant potential. Cancer. 2006;107:299–308. doi: 10.1002/cncr.21992. [DOI] [PubMed] [Google Scholar]
- Wiley A, Katsaros D, Fracchioli S, Yu H. Methylation of the insulin-like growth factor binding protein-3 gene and prognosis of epithelial ovarian cancer. Int J Gynecol Cancer. 2006;16:210–8. doi: 10.1111/j.1525-1438.2006.00299.x. [DOI] [PubMed] [Google Scholar]
- Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 1839;2014:1097–109. doi: 10.1016/j.bbagrm.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Zeng L, Jarrett C, Brown K, Gillespie KM, Holly JM, Perks CM. Insulin-like growth factor binding protein-3 (IGFBP-3) plays a role in the anti-tumorigenic effects of 5-Aza-2’-deoxycytidine (AZA) in breast cancer cells. Exp Cell Res. 2013;319:2282–95. doi: 10.1016/j.yexcr.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wang Q, Pan X. MicroRNAs and their regulatory roles in animals and plants. J Cell Physiol. 2007;210:279–89. doi: 10.1002/jcp.20869. [DOI] [PubMed] [Google Scholar]