Abstract
Genetic male sterility (GMS) in cotton mediated by two homozygous recessive genes, ms5ms5 and ms6ms6, is expressed as non-dehiscent anthers and unviable pollen grains. Sequence analysis on ms5 and ms6 loci in Gossypium hirsutum was conducted to reveal genomic variation at these two loci between GMS and wild-type G. hirsutum inbred lines, and sequence polymorphism linked to ms5 on A12 and ms6 on D12 was revealed. A haplotype marker set that consisted of four SNPs targeting both ms5 and ms6 gene regions was developed and validated for association with GMS in cotton. Predictability of GMS phenotype by this haplotype SNP set was over 99 %. GMS haplotype marker set can serve as a high-throughput molecular breeding tool to select GMS individuals and improve hybrid production efficiency.
Keywords: Genetic male sterility (GMS), Hybrid cotton, Molecular markers, Haplotype, Phenotype prediction
Introduction
Genetic male sterility (GMS) in cotton occurs in a form of non-dehiscent anthers and unviable pollens when reproductive development process fails due to possible loss of nuclear gene functions required for pollen development. Unlike cytoplasmic male sterility, genes related to GMS phenotypes can be easily transferred to various genetic backgrounds by routine crossing practice and allow the trait to be inherited at full penetrance in successive generations. There have been 19 different GMS genes, Ms1 to Ms19, identified in tetraploid cotton species (summarized by Chen et al. 2009), and are classified by genetic characterization and multigenic nature such as single dominant gene (Ms4), single recessive gene (ms2) and paired recessive genes (ms5 and ms6). Single recessive genes ms1 and ms3 reportedly conferred partial genetic male sterility (Justus and Leinweber 1960; Justus et al. 1963). In case of ms3, it produced fertile flowers more often in greenhouse compared with field condition (Justus et al. 1963). In contrast, GMS conferred by a single recessive gene, ms2, and paired recessive genes, ms5 and ms6, conferred stable male sterility (Richmond and Kohel 1961). Because GMS maintainers need to be fertile, recessive genetic mechanisms with complete male sterility especially mediated by ms5 and ms6 have been widely used in hybrid cotton production (Basu 1996).
Cytoplasm male sterility (CMS) technology is also available to produce hybrid cotton. The first cytoplasmic male sterility was developed by transferring diploid A2 genome of Gossypium arboreum to cytoplasm of Gossypium anomalum carrying diploid B1 genome. Male sterile plants were produced when CMS inducer was pollinated by pollens of G. arboreum, and fertility was restored with G. anomalum pollen (Meyer and Meyer 1965; Meyer 1969). Because of incomplete or unstable male sterility and undesirable traits in CMS lines generated by G. arboreum and G. anomalum cytoplasm system, alternative CMS system called CMSD-2 was created by transferring genomes of commercial cotton (G. hirsutum) to Gossypium harknessii cytoplasm (Meyer 1973, 1975). Restorer of CMSD-2 system was created by transferring a restorer gene, Rf1, in G. harknessii to G. hirsutum genome (Meyer 1975). Cytoplasm of Gossypium trilobum carrying diploid D8 genome was also used to develop CMS system known as CMS-D8 (Stewart 1992), and Rf2 in D8 genome of G. trilobum was found to restore fertility (Zhang and Stewart 2001). Rf1 functioning sporophytically was found to be nonallelic to Rf2 which functions gametophytically, and these two genes were linked on chromosome LGD08 which is same to D05 (Zhang and Stewart 2001, 2004; Liu et al. 2003; Feng et al. 2005; Wang et al. 2007; Yin et al. 2006). In spite of high male sterility frequency close to 100 % by CMS, broader application of CMS to hybrid production has been limited due to narrow genetic choices of CMS and restorer line combination and cytoplasmic effect on potential yield drag and CMS stability (Meyer 1969; Meyer and Meyer 1965; Bhale and Bhat 1990; Schnable and Wise 1998).
The sources of ms5 and ms6 genes were speculated to be G. tomentosum or progeny from an interspecific cross with G. hirsutum. A nectariless F3 individual from a cross of Stoneville 20 × nectariless G. tomentosum was crossed with a paternal parent, Empire WR. From this cross, a nectariless F3 individual was crossed with Gregg. An F2 individual with nectariless phenotype was crossed to Lankart 57 and a single male sterile individual with nectariless phenotype was identified in their F2 progenies (Weaver 1968). Recessive genetic inheritance of GMS trait was confirmed by backcrossing fertile progenies from the sterile individual against paternal ancestors. Assuming that there has been no spontaneous mutation occurring during crossing and population advancement, emergence of male sterility within F3 progenies from interspecific cross between G. hirsutum and G. tomentosum might be due to interaction between two genes originated from two different tetraploid species. The recessive allele of either ms5 or ms6 originated from G. tomentosum might have interacted with the recessive allele that already existed in G. hirsutum to express male sterility. Although unlikely, the chance of spontaneous mutation at any stage throughout the interspecific and intraspecific crosses cannot be ruled out and can be considered as a possible source of GMS until disproven.
Genetic map positions of GMS genes were revealed by linkage mapping using SSR markers (Chen et al. 2009). Two Chinese GMS source lines, Lang-A carrying a recessive gene, ms15, and Zhongkang-A carrying two recessive genes, ms5 and ms6, were crossed to Hai7124 carrying homozygous dominant alleles of both ms5 and ms6 first. Their F1 progenies were backcrossed to sterile parents to generate mapping populations in which only one of the two genes was segregating. Authors mapped each gene independently to chromosomes 12 (ms5 and ms15) and 26 (ms6) and claimed that ms5 and ms15 are two different genes on chromosome 12 with a distance of 6 cM between loci. Rhyne (1991) reportedly mapped ms8 and ms9 on chromosome 12 and 26, respectively, as well. It is uncertain whether they are related to ms5 and ms6 as a test cross between these two genetic sources has not been attempted.
Although molecular and biochemical mechanisms of male sterility caused by ms5ms5 and ms6ms6 are not fully understood, analogous studies conducted in other species could be used to predict potential mechanism(s) that cause pollen development failure in cotton. In Arabidopsis, causes of failure in male gamete development were classified into four cases: (1) non-functioning stamen resulting in deformed anthers, (2) failure in microsporogenesis, (3) lack of pollen-coating agent, tryphine, and (4) non-dehiscent anthers (Okada and Shimura 1994). Observation of stamen and pollens from male sterile flowers indicated that all four cases appear to contribute to the sterility phenotype in cotton. Additional molecular mechanisms related to pollen development and mutant phenotypes due to genetic mutations on metabolic pathways are well characterized in model species (reviewed by Wilson and Zhang 2009; Okada and Shimura 1994; Chaudhury 1993). Genes of other functions but which are still associated with pollen development in Arabidopsis have been well characterized at the molecular level which include the following: MS1, a PHD-finger family of transcription factor related to microsporogenesis (Wilson et al. 2001; Ito et al. 2007), MYB transcription factor related to dehiscence of pollen and pollen wall development (Steiner-Lange et al. 2003; Preston et al. 2004), MS2, elongation/condensation complex presumably related to pollen wall formation (Aarts et al. 1997), AtPTEN, tumor suppressor homolog related to pollen cell death after mitosis (Gupta et al. 2002). With in-depth whole-genome sequence data available, reverse genetic approaches can be used to further identify candidate genes associated with genetic male sterility in cotton. To characterize the genetic and genomic characteristics of the GMS in cotton, we conducted genomic analysis at ms5 and ms6 loci. Our study revealed genomic variation at both loci and also within candidate genes possibly associated with genetic male sterility in cotton. By SNP markers designed to detect genomic variation at both loci, a haplotype for GMS and predictability of GMS phenotype by SNP haplotyping were determined.
Materials and methods
Genetic characterization of GMS
Segregating populations for genetic study of GMS were generated from a cross between DPGh98018 and DPGh04651. DPGh98018 was used as a full fertile male parent, and DPGh04651 was used as a female parent presumably carrying multiple recessive GMS genes. Multiple recessive genetic inheritance patterns of GMS in DPGh04651 were observed within progenies generated by multiple generations of selfing in nursery in Scott, Mississippi during 2004–2005 season (data not provided). Male sterility of each plant was determined by monitoring presence of non-dehiscent anther in five flowers at anthesis over a month. In addition, flower abortion was also observed to confirm male sterility. Plants were scored as fertile if at least one dehiscent anther was visible. The same method was applied to phenotype all genetic materials used in our study. Based on inheritance patterns of GMS within progenies of DPGh04651, two recessive genes presumably ms5 and ms6 were speculated to confer GMS in DPGh04651. All genetic materials used in our study were prepared based on GMS mechanism by paired ms5 and ms6 genes. Sibcrossing between fertile and sterile F2 progenies from a cross between DPGh98018 and DPGh04651 was made to produce sibcross F1 progenies in the greenhouse in 2004. Sibcross F1 progenies were planted in greenhouse, and male sterility was phenotyped by each sibcrossing event. Fertile F1 progenies were advanced to sibcross F2 populations by selfing. Segregation pattern of male sterility was monitored in each sibcross F2 population independently, and sibcross F2 populations segregating for male sterility were selected for further genetic study.
SNP discovery by amplicon sequencing of ms5 on A12 and ms6 on D12
Two recessive GMS gene, ms5 and ms6, were previously mapped to chromosomes 12 (A12) and 26 (D12), respectively, using SSR markers (Chen et al. 2009). The primer sequences of SSR markers linked to ms5 and ms6 were mapped to diploid D genome sequence of G. raimondii (JGI v2.0, annot v2.1, www.cottongen.org) by in silico comparative mapping approach. The diploid D genome sequence between 19,114,325 bp and 21,205,044 bp was used to discover genomic polymorphism linked to ms5 and ms6. PCR assays to produce sequencing templates were designed using Primer3 (http://frodo.wi.mit.edu/primer3/) with default parameters. G. hirsutum lines including TM-1, DPGh98018 (male fertile) and DPGh04651 (GMS donor) and diploid A (G. arboreum, PI629477) and D (Gossypium raimondii, PI530898) as subgenomic references were used for amplicon PCR. To demonstrate homoeology between two loci on chromosome 12 and 26, respectively, chromosome nomenclature of A12 and D12 was used instead.
PCR for sequencing and SNP genotyping
PCR to amplify sequencing template was prepared using Platinum® Taq DNA Polymerase High Fidelity manufactured by Life Technologies (Grand Island, NY) following the manufacturer’s manual. The PCR was conducted by initial denaturation at 95 °C for 5 min followed by 35 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 30 s, and extension at 72 °C for 2 min and a final extension at 72 °C for 5 min. Sequencing of PCR amplicons was performed using 3730XL DNA Analyzer (Life Technologies, Grand Island, NY), and sequence analysis and SNP discovery were conducted using CLC Bio Genomics Workbench software (Aarhus, Denmark). All genomic polymorphisms identified by amplicon sequence analysis were converted to TaqMan® assays for further SNP validation and SNP genotyping. DNA extraction was done by a protocol developed by Dellaporta et al. (1983).
PCR of 5 µl total volume for SNP assay validation and SNP genotyping was conducted using GTXpress™ Master Mix for SNP genotyping assay manufactured by Life Technologies following the manufacturer’s manual (Life Technologies, Grand Island, NY). Each PCR included two PCR primers (final concentration of 900 nmol for each primer), two TaqMan® MGB probes with NFQ labeled by FAM or VIC dye (final concentration of 250 nmol for each probe) and 4 ng genomic DNA. The PCR was conducted using GeneAmp® PCR System 9700 (Life Technologies, Grand Island, NY) by initial denaturation at 95 °C for 10 min followed by 40 cycles of denaturation at 95 °C for 15 s, annealing and extension at 60 °C for 60 s. SNP data collection and analysis were conducted by ViiA™ 7 Software v1.2.1. (Life Technologies, Grand Island, NY).
Genetic linkage of SNPs to ms5 and ms6
F3 populations were generated by combining 14 different sibcross F2 populations originated from a cross between DPGh98018 and DPGh04651. Genetic linkage between SNPs and chromosome assignment of linked markers to A12 or D12 were estimated by genetic distance calculated using R-Genetics (http://cran.r-project.org/web/packages/genetics/genetics.pdf) among newly developed markers and also to the chromosome-specific markers assigned to A12 and D12 (Cho et al. 2014).
Fine mapping of both ms5 and ms6 was carried out by association analysis using a population of 1044 inbred lines representing 899 Monsanto germplasm lines of diverse genetic backgrounds consisting of 880 male fertile and 164 male sterile individuals. Diverse genetic materials were generated by unstructured random mating using male sterile progenies originated from DPGh04651 and diverse Monsanto germplasm lines. Male sterile phenotypes for each plant were binary coded as 1 for male fertile and 0 for male sterile and used in association analysis. Each plant was also genotyped by SNP markers putatively linked to ms5 and ms6, and two-step analysis was performed to identify haplotype marker set predicting male sterility. First, logistic regression was performed using binary sterility outcome with corresponding marker genotypes as predictors on the assumption of monogenic recessive model. As a second step, markers showing linkage to ms5 or ms6 loci were subjected to logistic regression analysis assessing relationships of alleles between two loci in association with the male sterility.
Haplotype SNPs associated with GMS were also tested on Lankart 57 (PI528822), Gregg (PI529094), Empire WR (PI529224) and G. tomentosum (PI 530723) which were reportedly served as donors of recessive ms5 and ms6 alleles (Weaver 1968), and the origins of recessive ms5 and ms6 alleles were estimated based on haplotype SNP patterns in each test material.
Results
Genetic characterization of ms5 and ms6 genes using mapping populations
Three independent sibcrossings between three different pairs of fertile and sterile F2 siblings produced sibcross F1 progenies, and fertile-to-sterile phenotype ratios within these sibcross F1 populations were close to 1:1 (Table 1). Based on 1:1 phenotype ratio within these small populations of sibling crosses, genotypes of fertile parent plants used in these crosses were speculated to be Ms5ms5ms6ms6 or ms5ms5Ms6ms6, while sterile progenies were homozygous recessive for both loci. Fertile progenies from these sibcrosses were advanced to F2 populations by selfing, and male sterility phenotypes were monitored. Segregation patterns of phenotypes in F2 populations were either 1:3 sterile to fertile indicating that a single gene was segregating or 1:15 sterile to fertile indicating that both genes were segregating in each F2 population (Table 1).
Table 1.
Segregation of genetic male sterility in F1 of F3 sibling crosses and their F2 progenies
| Generation | Fertile frequency | Sterile frequency | Expected ratio (F:S) | Chi-square probability |
|---|---|---|---|---|
| Fa1 | 11 | 19 | 1:1 | 0.144 |
| Fa1 | 1 | 2 | 1:1 | 0.564 |
| Fa1 | 3 | 0 | 1:1 | 0.083 |
| Fb2 | 51 | 27 | 3:1 | 0.05 |
| Fb2 | 26 | 16 | 3:1 | 0.05 |
| Fb2 | 25 | 11 | 3:1 | 0.441 |
| Fb2 | 76 | 18 | 3:1 | 0.19 |
| Fb2 | 45 | 20 | 3:1 | 0.283 |
| Fb2 | 399 | 35 | 15:1 | 0.118 |
aSibcross F1 progenies from a cross between fertile and sterile siblings selected from F3 segregating populations
bF2 progenies from F1 by selfing
Sequencing to discover SNPs for ms5 and ms6
Whole-genome sequence of G. raimondii D genome (JGI v2.0, annot v2.1, www.cottongen.org) was used as a reference to nominate target regions for discovery of SNPs. Presumably about 0.5-Mbp-long sequences were subjected to amplicon sequencing. Sequence polymorphism was identified in forms of single-nucleotide polymorphism or single- or multiple-nucleotide deletion among the test materials which included male fertile and male sterile inbred lines on diploid A and D genome species. Among all genomic polymorphism identified within target regions covered by amplicon sequences, 23 polymorphic sequences qualified for PCR assay design were subjected to further analysis.
Genetic linkage of SNPs to ms5 and ms6
SNPs were tested for linkage to ms5 on A12 and ms6 on D12 using F3 populations originated from 14 different sibcross F2 populations segregating for male sterility. Polymorphisms of SNP markers corresponding to all 23 sequence polymorphisms identified by amplicon sequencing were tested in F3 populations, and genetic distances between SNPs and linkage to ms5 and ms6 were calculated based on genetic linkage to chromosome-specific SNPs. All 23 markers segregated in F3 sibcross populations, and all were assigned to ms5 and ms6 loci. Annotation of the putative genes carrying SNPs linked to ms5 and ms6 and their in silico comparative map positions on chromosome 08 on G. raimondii D genome (D5) are summarized in Table 2 and Fig. 1. Further analysis to associate each marker to the trait was conducted by the association mapping approach.
Table 2.
Summary of amplicon sequences used to identify genomic polymorphism at ms5 on A12 and ms6 on D12 and their in silico comparative map positions on D5 genome of Gossypium raimondii
| Chromosome | Sequence annotation | Position on chromosome 08 of D5 genome syntenic to A12/D12 (base pairs)a | Polymorphism |
|---|---|---|---|
| A12 | Cytochrome p450 | 36601681—36601890 | C/T |
| A12 | Cytochrome p450 | 36601598—36601781 | A/G |
| A12 | Cytochrome p450 | 36600316—36600453 | A/G |
| A12 | SINA protein family (zinc finger) | 36517055—36517264 | A/G |
| A12 | Ubiquitin family | 36513917—36513713 | A/G |
| A12 | Eukaryotic translation initiation factor 3 subunit | 36487438—36486837 | C/T |
| A12 | PHD-zinc-finger-like domain | 36448804—36448648 | A/G |
| A12 | PHD-zinc-finger-like domain | 36448539—36448899 | C/T |
| A12 | PHD-zinc-finger-like domain | 36444422—36444671 | C/T |
| A12 | Telomere stability and silencing | 36437252—36437058 | C/T |
| A12 | Telomere stability and silencing | 36436860—36436639 | C/T |
| A12 | Kinetochore centromere component | 36404821—36405030 | C/G |
| A12 | Mitochondrial carrier-like protein | 36180644—36180843 | G/T |
| D12 | Homeobox-associated leucine zipper | 36829091—36829251 | C/T |
| D12 | ORDML family protein | 36823054—36823710 | C/G |
| D12 | AP2(ERF domain) | 36670553—36670417 | C/G |
| D12 | Cytochrome P450 | 36600741—36600580 | A/G |
| D12 | Cytochrome P450 | 36600741—36600580 | A/G |
| D12 | Aspartic proteinase | 36590566—36590776 | A/G |
| D12 | PHD-zinc-finger-like domain | 36443655—36443785 | G/C |
| D12 | Cyclic nucleotide-gated ion channel | 36432550—36432377 | C/T |
| D12 | Cyclic nucleotide-gated ion channel | 36432269—36432083 | C/G |
| D12 | Cyclic nucleotide-gated ion channel | 36429252—36429459 | C/G |
aPhysical position of amplicon sequences on whole genome of Gossypium raimondii (JGI assembly v2.0, www.cottongen.org)
Fig. 1.
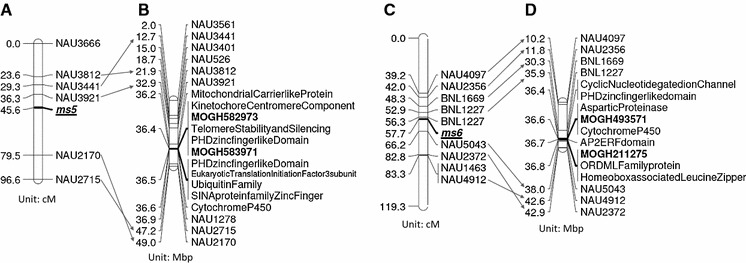
In silico comparative mapping of molecular markers linked to ms5 and ms6 to chromosome 08 of G. raimondii D genome (D5). Maps a and c were recreated from the publication by Chen et al. (2009) describing map positions of ms5 and ms6 on chromosome 12 (A12) and 26 (D12), respectively. Maps b and d are summary of in silico comparative map positions of SSRs and newly developed SNPs linked to ms5 and ms6 on chromosome 08 of D5 genome. In silico map positions of SSRs on D5 genome are available in CottonGen (http://www.cottongen.org/gb/gbrowse/JGI_221_Dgenome/). Gene names on maps b and d represent annotation of the template sequences used to develop SNPs
Association mapping to ms5 and ms6 loci
Monsanto germplasm lines of diverse genetic backgrounds consisted of 880 male fertile and 164 male sterile inbred lines were genotyped by all 23 SNPs assigned to ms5 and ms6. Male sterility phenotypes were collected from all association test materials and the phenotype data were categorized in binary format as either male sterile or male fertile. Using logistic regression model, all possible SNP combinations between two loci were tested to explain male sterility within diverse test materials. The recessive ms5 allele was explained best by TT allele on MOGH583971 and TT allele on MOGH582973, and the recessive ms6 allele was explained best by CC allele on MOGH211275 and GG allele on MOGH493571. When these two haplotypes for recessive alleles of ms5 and ms6 were combined, phenotype predictability in the diverse test panel was 99.6 % (Table 3). By putative gene annotation, these markers are located on PHD-zinc-finger-like domain protein on A12 and AP2 gene on D12. Detailed SNP information for TaqMan genotyping is summarized in Table 4.
Table 3.
Probability of male sterility occurrence using genotype combinations of MONGH583971 (TT) and MONGH582973 (TT) associated with ms5ms5 and MONGH211275 (CC) and MONGH493571 (GG) associated with ms6ms6
| Frequency (count) | Individual with male sterile haplotype on ms5 and ms6 | Individual with male fertile haplotype on ms5 and ms6 | p value | Probability |
|---|---|---|---|---|
| Male sterile individual | 109 | 17 | 2.00E−16 | 99.60 % |
| Male fertile individual | 1 | 769 |
Table 4.
Haplotype SNP set designed to predict genetic male sterility in Gossypium hirsutum conferred by paired recessive genes, ms5 on A12 and ms6 on D12
| Chromosome | SNP name | Polymorphisma | TaqMan SNP assay oligo sequenceb |
|---|---|---|---|
| (5′→3′) | |||
| A12 | MOGH583971 | C/T(ms5) | F: AGAACATTGATTATTACTGCCCCGAAT |
| R: GGCTCTCTTTTTGCTAAGCATGATT | |||
| P-FAM: CTTGGACTTCACTTTAC | |||
| P-VIC: CTTGGACTTCGCTTTAC | |||
| A12 | MOGH582973 | C/T(ms5) | F: CCGGTTCGGTTTATAAGAGAAGATCTG |
| R: AAATGTCTTTCTCTTCCTCGGAACC | |||
| P-FAM: CTCTCACTCAATACTC | |||
| P-VIC: ACTCTCACTCGATACTC | |||
| D12 | MOGH211275 | C(ms6)/G | F: AGCTAATGCTCTCTGACGGAACTA |
| R: ACAAAAGAGTAGATTTTAGTGTACCGTGTATTT | |||
| P-FAM: TGTGCGTAATTCATGATC | |||
| P-VIC: TTGTGCGTAATTGATGATC | |||
| D12 | MOGH493571 | A/G(ms6) | F: CTCTCGGGCCTGAACGA |
| R: GCTTTTACATCCCGGCTAAGACA | |||
| P-FAM: TGGGACGACGTTGAA | |||
| P-VIC: ATTATGGGACGATGTTGAA |
aSingle-nucleotide polymorphism linked to ms5 and ms6 was indicated by “ms5 or ms6” designation
bF, R, P-FAM and P-VIC represent forward primer, reverse primer and two probes labeled by FAM and VIC dyes, respectively. Oligo sequences for SNP markers and template sequences of ms5 and ms6 are available at CottonGen (http://www.cottongen.org/search/markers)
Origin of ms5 and ms6
Lankart 57 (PI528822), Gregg (PI529094), Empire WR (PI529224) and G. tomentosum were tested for the presence of ms5 and ms6 alleles using the haplotype markers described above. Based on haplotype patterns of the samples tested, G. tomentosum indicated the presence of ms5 and ms6 and Lankart 57 indicated the presence of ms6. All other varieties tested showed no presence of the ms5 and ms6 haplotypes. From these results, it is probable that the ms5 was transmitted from G. tomentosum. However, since none of the F2 populations occurring before the crossing with Lankart 57 showed male sterility (Weaver 1968), it is unlikely that the ms6 allele from G. tomentosum was causative for male sterility. Therefore, the Lankart 57 ms6 gene is most likely responsible for male sterility in one of its F2 progenies in combination with ms5 from G. tomentosum. It is also possible for the ms5 allele of Lankart 57 to have differential phenotypic expression when compared to the G. tomentosum allele.
Discussion
Map positions of ms5 and ms6 were defined by Chen and his colleagues in 2009 using higher density SSR markers tested on backcrossing populations. By testing molecular marker patterns in Ms5ms5Ms6ms6 of male fertile and ms5ms5ms6ms6 of male sterile progenies, the authors were able to identify markers explaining dominant and recessive alleles of each gene. Markers found to be associated with the genes were further tested for genetic distance against other existing molecular markers. Using populations segregating for only one of the two genes with the second fixed to recessive homozygote is another effective approach beyond synthetic backcrossing population method used by Chen and his colleagues. However, the single gene segregating population approach will require tedious monitoring of progeny phenotypes in each family and can be very difficult to determine which gene is segregating in each population. In our study, we scanned entire genetic regions neighboring ms5 and ms6 and discovered markers polymorphic in diverse genetic backgrounds. All newly developed SNPs were quickly mapped to ms5 and ms6 by in silico comparative mapping using D diploid genome sequence and by linkage analysis using randomly segregating F3 populations. Based on known genetic locations and distances between markers, all possible pairwise combinations of markers between two different chromosomes were tested for association with male sterility using logistic regression modeling. As a result, two haplotypes consisting of 2 SNPs linked to ms5 and 2 SNPs linked to ms6 were identified that explained male fertility and sterility with 99 % accuracy.
Two GMS genes, ms5 and ms6, were expected to be homoeologs and possibly duplicated through polyploidization. However, we found haplotype markers representing ms5 and ms6 located about 150 Kbp apart from one another on the diploid D genome sequence. If genomic variation exist only on one of two homoeologs, then it is still possible to identify polymorphic markers unique to one homoeologous chromosome. Additional genomic polymorphisms at flanking regions from the other homoeologous chromosome can be used as flanking markers and allowing the two haplotypes high phenotypic prediction accuracy.
From the in silico comparative mapping of template sequences of the SNPs to diploid D genome, genomic regions targeted by male sterility haplotypes appeared to be very narrow and contained a limited number of genes. Based on putative gene annotation, PHD-zinc-finger-like domain protein on A12 and AP2 (ERF domain) on D12 were contained between markers defining the haplotypes. ArabidopsisMALE STERILITY1 gene was found to be PHD-zinc-finger-like domain protein which plays a critical role in pollen development (Wilson et al. 2001; Ito et al. 2007). Recessive alleles created by EMS mutation conferred male sterility in Arabidopsis only when it is homozygous recessive. Pollens of ms1 mutant with homozygous recessive alleles failed developing viable pollen (Wilson et al. 2001). The function of APETALA2 (AP2) as a negative regulator of AGAMOUS has been well established using Arabidopsis flower development mutants. Stamen, ovule and other floral organ development was significantly prohibited in the ap2 mutant (Jofuku et al. 1994). Further investigation is needed to prove functional association of any of these genes to male sterility in cotton.
The initial ms5 and ms6 study hypothesized the origin of the alleles from Gregg and Lankart 57. The reasoning is that following the identification of the first sterile individual, backcrossing was performed with Gregg and Lankart 57, each producing F2 segregating populations with 3:1 male-fertile-to-male-sterile ratio. Our study with haplotype markers tested on Lankart 57, Gregg, Empire WR and G. tomentosum showed that recessive ms5 allele was present only in G. tomentosum and that recessive ms6 allele was present in G. tomentosum and Lankart 57. This indicates that ms5 allele in male sterile F2 progeny was likely transmitted from G. tomentosum. However, due to absence of male sterile progenies in the F2 populations occurring before the crossing with Lankart 57, it is likely that ms6 allele causative for male sterility is found in Lankart 57 and not G. tomentosum. Although genotype test materials we used might not be identical to the ones used in the study by Weaver in 1968, the results from our study were able to explain potential sources of genetic male sterility in cotton.
In conclusion, a haplotype SNP marker set that consisted of four SNPs, two linked to ms5 on A12 and two linked to ms6 on D12 in G. hirsutum was developed. This haplotype marker set was able to predict male sterile phenotype at the rate of 99 % accuracy within our diverse germplasm test set. Each haplotype SNP marker can differentiate zygosity of ms5 and ms6 loci and can be used to predict GMS phenotype in G. hirsutum of diverse genetic background at any generations including inbred parents and progenies segregating randomly. With reliability as molecular markers and tight linkage to ms5 and ms6, our GMS haplotype SNP marker set can serve as a high-throughput molecular breeding tool to select GMS individuals to be used as hybrid parents and also to confirm purity of hybrids produced through larger scale commercial seed production. This new molecular breeding tool should be able to eliminate tedious hand emasculation and fertile plant removal after flower phenotyping in the field and improve genetic purity and quality of hybrid production.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Xuehui Feng, Don Keim and Humphrey Wanjugi have contributed equally to this work.
References
- Aarts MG, Hodge R, Kalantidis K, Florack D, Wilson ZA, Mulligan BJ, Stiekema WJ, Scott R, Pereira A. The Arabidopsis MALE STERILITY 2 protein shares similarity with reductases in elongation/condensation complexes. Plant J. 1997;12(3):615–623. doi: 10.1046/j.1365-313X.1997.d01-8.x. [DOI] [PubMed] [Google Scholar]
- Basu AK. Current genetic research in cotton in India. Genetic. 1996;97:279–290. doi: 10.1007/BF00055314. [DOI] [Google Scholar]
- Bhale NL, Bhat MG. Investigations on exploitation of heterosis in cotton (Gossypium hirsutum L.) using male sterility. Ind. J Genet. 1990;50(1):37–44. [Google Scholar]
- Chaudhury AM. Nuclear genes controlling male fertility. Plant Cell. 1993;5(10):1277–1283. doi: 10.1105/tpc.5.10.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Ding Y, Guo W, Zhang T. Molecular mapping of genic male-sterile genes ms15, ms5 and ms6 in tetraploid cotton. Plant Breed. 2009;128:193–198. doi: 10.1111/j.1439-0523.2008.01562.x. [DOI] [Google Scholar]
- Cho S, Yanai G, Schwarz J, Curtis A, Butruille M. Genome-wide SNP marker panel applicable to cotton genetic diversity test. Proc Int Cotton Genome Initiat Conf. 2014;2(1):p11. [Google Scholar]
- Dellaporta S, Wood J, Hicks J. A plant DNA mini preparation: version II. Plant Mol Biol Rep. 1983;1(4):19–21. doi: 10.1007/BF02712670. [DOI] [Google Scholar]
- Feng CD, Stewart JM, Zhang JF. STS markers linked to the Rf1 fertility restorer gene of cotton. Theor Appl Genet. 2005;110(2):237–243. doi: 10.1007/s00122-004-1817-3. [DOI] [PubMed] [Google Scholar]
- Gupta R, Ting JT, Sokolov LN, Johnson SA, Luan S. A tumor suppressor homolog, AtPTEN1, is essential for pollen development in Arabidopsis. Plant Cell. 2002;14:2495–2507. doi: 10.1105/tpc.005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Nagata N, Yoshiba Y, Ohme-Takagi M, Ma H, Shinozaki K. Arabidopsis MALE STERILITY1 encodes a PHD-type transcription factor and regulates pollen and tapetum development. Plant Cell. 2007;19(11):3549–3562. doi: 10.1105/tpc.107.054536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofuku KD, den Boer BG, Montagu MV, Okamuro JK. Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell. 1994;6(9):1211–1225. doi: 10.1105/tpc.6.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justus NJ, Leinweber CL. A heritable partial male-sterile character in cotton. J Hered. 1960;51(4):191–192. [Google Scholar]
- Justus NJ, Meyer JR, Roux JB. A partially male-sterile character in upland cotton. Crop Sci. 1963;3(5):428–429. doi: 10.2135/cropsci1963.0011183X000300050018x. [DOI] [Google Scholar]
- Liu L, Guo W, Zhu X, Zhang T. Inheritance and fine mapping of fertility restoration for cytoplasmic male sterility in Gossypium hirsutum L. Theor Appl Genet. 2003;106(3):461–469. doi: 10.1007/s00122-002-1084-0. [DOI] [PubMed] [Google Scholar]
- Meyer VG. Some effects of genes, cytoplasm, and environment on male sterility of cotton (Gossypium) Crop Sci. 1969;9:237–242. doi: 10.2135/cropsci1969.0011183X000900020039x. [DOI] [Google Scholar]
- Meyer VG (1973) Fertility restorer genes for cytoplasmic male-sterility from Gossypium harknessii. In: Proc Beltwide Cotton Prod Res Conf, p 65
- Meyer VG. Male sterility from Gossypium harknessii. J Hered. 1975;66:23–27. [Google Scholar]
- Meyer VG, Meyer JR. Cytoplasmically controlled male sterility in cotton. Crop Sci. 1965;5:444–448. doi: 10.2135/cropsci1965.0011183X000500050021x. [DOI] [Google Scholar]
- Okada K, Shimura Y. Genetic analyses of signaling in flower development using Arabidopsis. Plant Mol Biol. 1994;26(5):1357–1377. doi: 10.1007/BF00016480. [DOI] [PubMed] [Google Scholar]
- Preston J, Wheeler J, Heazlewood J, Li SF, Parish RW. AtMYB32 is required for normal pollen development in Arabidopsis thaliana. Plant J. 2004;40(6):979–995. doi: 10.1111/j.1365-313X.2004.02280.x. [DOI] [PubMed] [Google Scholar]
- Rhyne CL (1991) Male-sterile ms5ms5ms6ms6 and ms8ms8ms9ms9. In: Proc Beltwide Cotton Prod Res Conf, pp 532–533
- Richmond TR, Kohel RJ. Analysis of a completely male-sterile character in American upland cotton. Crop Sci. 1961;1(6):397–401. doi: 10.2135/cropsci1961.0011183X000100060005x. [DOI] [Google Scholar]
- Schnable PS, Wise RP. The molecular basis of cytoplasmic male sterility and fertility restoration. Trends Plant Sci. 1998;3(5):175–180. doi: 10.1016/S1360-1385(98)01235-7. [DOI] [Google Scholar]
- Steiner-Lange S, Unte US, Eckstein L, Yang C, Wilson ZA, Schmelzer E, Dekker K, Saedler H. Disruption of Arabidopsis thaliana MYB26 results in male sterility due to non-dehiscent anthers. Plant J. 2003;34(4):519–528. doi: 10.1046/j.1365-313X.2003.01745.x. [DOI] [PubMed] [Google Scholar]
- Stewart JM (1992). A new cytoplasmic male sterile and restorer for cotton. In: Proc Beltwide Cotton Prod Res Conf, p 610
- Wang F, Stewart JM, Zhang J. Molecular markers linked to the Rf2 fertility restorer gene in cotton. Genome. 2007;50(9):818–824. doi: 10.1139/G07-061. [DOI] [PubMed] [Google Scholar]
- Weaver JB. Analysis of a genetic double recessive completely male-sterile cotton. Crop Sci. 1968;8(5):597–600. doi: 10.2135/cropsci1968.0011183X000800050027x. [DOI] [Google Scholar]
- Wilson ZA, Zhang DB. From Arabidopsis to rice: pathways in pollen development. J Exp Bot. 2009;60(5):1479–1492. doi: 10.1093/jxb/erp095. [DOI] [PubMed] [Google Scholar]
- Wilson ZA, Morroll SM, Dawson J, Swarup R, Tighe PJ. The Arabidopsis MALE STERILITY1 (MS1) gene is a transcriptional regulator of male gametogenesis, with homology to the PHD-finger family of transcription factors. Plant J. 2001;28(1):27–39. doi: 10.1046/j.1365-313X.2001.01125.x. [DOI] [PubMed] [Google Scholar]
- Yin J, Guo W, Yang L, Liu L, Zhang T. Physical mapping of the Rf1 fertility-restoring gene to a 100 kb region in cotton. Theor Appl Genet. 2006;112(7):1318–1325. doi: 10.1007/s00122-006-0234-1. [DOI] [PubMed] [Google Scholar]
- Zhang JF, Stewart JM. Inheritance and genetic relationship of the D8 and D2-2 restorer genes for cotton cytoplasmic male sterility. Crop Sci. 2001;41(2):289–294. doi: 10.2135/cropsci2001.412289x. [DOI] [Google Scholar]
- Zhang JF, Stewart JM. Identification of molecular markers linked to the fertility restorer genes for CMS-D8 in cotton (Gossypium hirsutum L.) Crop Sci. 2004;44(4):1209–1217. doi: 10.2135/cropsci2004.1209. [DOI] [Google Scholar]


