Abstract
Cystoid macular edema is a condition that involves the macula, caused by an accumulation of extracellular fluid in the macular region with secondary formation of multiple cystic spaces. This condition is provoked by a variety of pathological conditions such as intraocular inflammation, central or branch retinal vein occlusion, diabetic retinopathy and most commonly following cataract extraction, hereditary retinal dystrophies, and topical or systemic assumption of drugs. Niacin is a vitamin preparation usually used for the treatment of lipid disorders. The treatment with niacin, alone or in combination with other lipid-lowering agents, significantly reduces total mortality and coronary events and slows down the progression of and induces the regression of coronary atherosclerosis. Several cases of niacin-induced cystoid macular edema have been reported with different dosages.
Key Words: Cystoid Macular Edema, Niacin Maculopathy, Nicotinic Acid, Side Effects
INTRODUCTION
Cystoid macular edema (CME) refers to swelling of the central part of the retina. The macula is responsible for detailed central vision. When the macula experiences swelling (edema), central vision is reduced. CME represents a common pathologic sequel of the retina and occurs in a variety of pathological conditions such as intraocular inflammation, central or branch retinal vein occlusion, diabetic retinopathy and most commonly following cataract extraction, hereditary retinal dystrophies, and topical or systemic assumption of drugs (1–2).
Several cases of niacin induced CME have been reported (3–6). The aim of this review is to show the use of niacin and its correlation between administration of niacin and CME.
CME: ETIOPATHOGENESIS
CME is a disorder that involves the central retina, caused by an accumulation of extracellular fluid in the macular region with secondary formation of multiple cystic spaces (7). Histological studies show that radially orientated cystoid spaces consisting of ophthalmoscopically clear fluid are often clinically detectable in the macula area. These cysts seem to be areas of the retina in which the cells have been displaced (1). It is the result of cystic accumulation of extracellular intraretinal fluid in the outer plexiform and inner nuclear layers of the retina (8).
The exact pathogenesis of CME remains uncertain. CME following disruption of the blood–retinal barrier (BRB) (9). When the BRB is damaged, fluid accumulates within the retina both intra- and extracellularly (10). Müller cells are thought to play an important role in acting as metabolic pumps that keep the macula dehydrated. However, intracellular fluid accumulation in the Müller cells may also occur in CME and further reduce macular retinal function. Vitreous traction may also play a part in the development of CME (11). Macular edema is the most frequent and severe complications of pars planitis, HLA-B27-associated acute anterior uveitis, sarcoidosis, birdshot retinochoroidopathy, Behcet’s syndrome, toxoplasmosis, Eales’ disease, idiopathic vitritis, Vogt-Koyanagi-Harada syndrome, and scleritis (12–17). CME can follow cataract surgery in the Irvine–Gass syndrome (18), because after cataract surgery, inflammatory mediators from the anterior chamber diffuse posteriorly into the vitreous, causing leakage from the retinal vasculature (19–20).
CME is the consequence of diabetic retinopathy (21). The edema in the macular area occurs secondary to an abnormal permeability of the capillaries surrounding the macula (failure of inner retinal blood barrier), and in turn to a failure in the outer retinal barrier (formed by the retinal pigmented epithelium) (22–24). Retinal vein obstructions (RVO) represent another cause of CME. Increased rigidity of a crossing artery because an atherosclerotic process has been suggested to cause compression of the underlying vein, provoking turbulent blood flow, endothelial damage, and thrombus formation (25). Likewise, a common vitreous adhesion at the obstruction site has also been reported, suggesting a possible role of vitreovascular traction in the etiology of some cases of BRVO (26–27). Atherosclerosis is a chronic low-grade inflammatory disorder and inflammation within the vascular wall contributes to the development of CME (28–29). Due to BRB breakdown secondary to damage at the tight junctions of endothelial cells, fluid diffusion from the occluded veins into the tissue can lead to CME (30). In addition, through such mechanisms, inflammatory responses and vascular dysfunction can all interact to cause retinal ischemia, which induces the expression of VEGF and others inflammatory agents (31), which participate in a complex chain of events that has yet to be fully defined (32–34).
NIACIN: DEFINITION AND RULES
Niacin (nicotinic acid) is a vitamin preparation usually used for the treatment of lipid disorders (35). Niacin favorably affects apolipoprotein (apo) B–containing lipoproteins (very-low-density lipoprotein [VLDL], low-density lipoprotein [LDL], lipoprotein[a]) and increases apo A-I–containing lipoproteins (high-density lipoprotein [HDL]). There are new data on how niacin affects triglycerides (TG), vascular anti-inflammatory events, a particular niacin receptor in adipocytes and immune cells, and the characterization of a niacin transport system in liver and intestinal cells that is dependent on acidic pH, temperature, energy, Ca2+-calmodulin-mediated pathways, but transport is sodium independent (36–42). Niacin directly and noncompetitively inhibits hepatocyte diacylglycerol acyltransferase–2, a key enzyme in TG synthesis that results in accelerated intracellular hepatic apo B degradation. Niacin reduces apolipoprotein C-III levels by inhibition of peroxisome-proliferator-activated receptor gamma co-activator 1b (PGC-1b), allowing greater apoE-driven clearance of triglyceride-rich lipoproteins (43).
Niacin is available in a variety of formulations: immediate-release (IR), slow-release (SR), extended-release (ER) (44). Studies have demonstrated that ER provides lipid-modifying efficacy equivalent to that of IR niacin, but with less flushing, while avoiding the hepatotoxicity of other long-acting niacins (45–46). However, the use of niacin in patients with diabetes has been discouraged because high doses can worsen glycemic control (47). The discovery that niacin reduced free fatty acids (FFA) lead to suggestions that its primary effect was on peripheral lipolysis and that all other actions were secondary. This was reinforced by the discovery of the HM74⁄ GPR109A cyclic-G protein-coupled receptor in adipose tissue (48) and niacin is a specific ligand for this receptor which mediates the flushing response in dendritic cells and macrophages (49–50). Treatment with niacin, alone or in combination with other lipid-lowering agents, significantly reduces total mortality and coronary events and holds back the progression of and induces the regression of coronary atherosclerosis (37,51–52).
Niacin improves endothelial function (53–54) and reduces progression of carotid intima-media thickness (cIMT) (55) and also had this effect in trials on top of statin-optimised LDL-C when used at a low dose of 1 g ⁄ day in the Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER)-2 (& 3) trials (52,56).
SYSTEMIC SIDE EFFECTS OF NIACIN
The problem with niacin is the high incidence of flushing (57). The flushing is cutaneous and usually restricted to the face and chest and is also associated with a burning sensation and often generalized pruritus. It lasts 20–30 min and habituates with exposure declining in both severity and frequency with time (58–59).
Niacin-induced flushing appears to be due to the subcutaneous release of PGD2, which is mediated by niacin’s action as a pharmacologic ligand for the adipocyte and macrophage G protein–coupled nicotinic acid receptor GPR109A and appears to involve the formation of vasodilatory prostanoids (60–61). Epidermal Langerhans cells are essential mediators of the flushing response (62–65). Niacin also has adverse effects on some aspects of the metabolic syndrome: it is weight neutral and delivers a small blood pressure reduction (66) but it can increase glucose and HbA1c (67–69).
This effect is often temporary (70) but in 5–10% patients with diabetes it can raise HbA1c by 0.5–1.0% even if the alteration of hypoglycaemic therapy occurs (71). Clinical studies suggest that the niacin can raise the plasma levels of uric acid and reduce the glucose tolerance. Dry skin and cutaneous hyperpigmentation (acanthosis nigricans) are also commonly reported (72).
NIACIN AND OCULAR EFFECTS
Many authors reported ocular side effect after therapy with niacin. Fraunfelder et al. (5) reported that 3 g or more per day of nicotinic acid, could cause blurred vision, eyelid edema, toxic amblyopia, proptosis, loss of eyelashes or eyebrow, superficial punctate keratitis and CME. Some cases of blurred vision were reported in the literature (73), and 18 cases were reported to the National Registry or the FDA spontaneous reporting system. In these cases, the average dose of niacin was 1.5–2 g per day, with a duration of therapy varying from 6 weeks to 1 year. Follow-up reported complete resolution of visual symptoms after discontinuing niacin. Dry eye was explained because this vitamin may be secreted and concentrated in human tears, thereby irritating an already dry eye. Niacin can cause ocular signs and symptoms reversible dose related, so if the patient wishes to continue this therapy, it may be feasible to reduce the dose of the drug (6). Cases of niacin related maculopathy there is a 10:1 male: female ratio. Most cases were in their third to fifth decade of life and were being treated with an average dose of 3–6 g of niacin per day. The etiology of niacin’s effect on the macula is unknown, A first hypothesis suggests that, in patients with vascular or inflammatory diseases, niacin induces the release of prostaglandins, causing the blood-retinal barrier compromise with extracellular accumulation of fluid in many cystic spaces. This theory is not entirely accepted for the absence of fluorescein leakage (74).
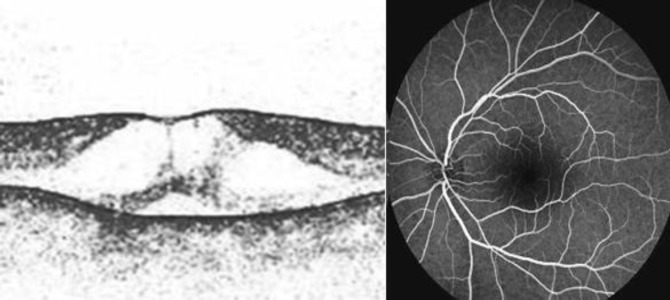
The second and the most reliable hypothesis supports that the niacin has a direct toxic effect on Müller cells, without disruption of the blood-retinal-barrier. Alterations in cellular metabolism cause intracellular fluid increase and swelling of these cells, with secondary formation of cysts between the glial spaces. After the discontinuation of niacin therapy, there is a complete regeneration of the Müller cells and their normal function (75–76). Reports from the literature (77) suggest that the onset of maculopathy ranges from 1 to 36 months after initiation of relatively high-dose therapy (3 g or more daily). There have also been reports of this condition with lower dosages (1.5 g daily) (3). This patient had increased the dosage of niacin to 3 g daily nearly 2 months before documentation of CME. In a previous case report a very low dose of niacin was administered (18 mg) and niacin maculopathy appears 4 weeks later with a complete resolution after discontinuation of drug, confirming the relationship between drug administration and macular edema appearance. In this patient, funduscopic examination showed bilateral macular edema, but no signs of diabetic or hypertensive retinopathy excluding the presence of other factors that may contribute to the capillary dysfunction associated with edema onset (78). At funduscopic examination, the foveomacular area had a peculiar aspect: the foveola takes on a bright yellow hue, similar to an exudate. Cysts primarily involve the foveal area, and they are smaller than those seen in post-surgical or inflammatory CME (3). FA and OCT are useful to confirm the diagnosis. FA showed the absence of fluorescein leakage or vascular alteration, whereas OCT confirms the presence of cystic hyporeflective spaces in the outer plexiform and inner nuclear layers. The cystoid spaces were more numerous and larger in the outer plexiform layer compared with the inner nuclear layer. Indeed previous studies reported that the fluor-angiographic pattern is atypical (79–81) (Fig. 1).
Figure 1.
On the left, there is an image of Stratus OCT 2 weeks after the start of niacin treatment. On the right fluorescein angiography of the same patient after 3 weeks after the beginning of niacin treatment with the typical absence of leakage also in later phases
Gass described reversible bilateral CME with no angiographic fluorescein leakage in 3 patients taking doses of niacin greater than 1.5 g daily to treat hyperlipidemia (6). Although Gass described transmitted hyper-fluorescence with a cartwheel pattern in the fluorescein angiograms of his patients, later reports described typical fluorescence in similarly affected patients (3). In all these cases, symptoms resolved over 4 to 8 weeks following discontinuation of niacin. First-line treatment for CME of toxic origin, such as that induced by niacin, is the removal of the offending agent (77). In milder cases of maculopathy, symptoms have resolved within a few days of drug discontinuation.
CONCLUSION
It was shown that the incidence of niacin maculopathy has been estimated to occur in 0.67% of patients being treated for hyperlipidemia. Studies have shown that even small doses of niacin cause CME. Patients with ocular symptoms such as blurred vision, decrease in visual acuity and metamorphopsia, should be immediately go to an ophthalmologist and stop the assumption of nicotinic acid before the onset of maculopathy. Niacin should be used with caution and under medical monitoring or periodic examination. Further studies would be desirable to investigate the safety dose, pathophysiologic mechanisms or predisposing risk factors and the possible interaction with others agents that may cause niacin maculopathy.
DISCLOSURE
The authors report no conflicts of interest in this work.
References
- 1.Rotsos TG, Moschos MM. Cystoid macular edema. Clin Ophthalmol. 2008 Dec;2(4):919–30. doi: 10.2147/opth.s4033. PMID: 19668445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanski JJ. Oftalmologia clinica. Masson: Elsevier; 2008. pp. 650–53. [Google Scholar]
- 3.Millay RH, Klein KL, Illingsworth DR. Niacin maculopathy. Ophthalmology. 1988 Jul;95(7):930–6. doi: 10.1016/s0161-6420(88)33073-3. PMID: 3174043. [DOI] [PubMed] [Google Scholar]
- 4.Callanan D, Blodi BA, Martin DF. Macular edema associated with nicotinic acid (niacin) [letter] JAMA. 1998 Jun ;279(21) doi: 10.1001/jama.279.21.1702-b. PMID: 9624021. [DOI] [PubMed] [Google Scholar]
- 5.Fraunfelder FW, Fraunfelder FT, Illingsworth DR. Adverse ocular effects associated with niacin therapy. Br J Ophthalmol. 1995 Jan;79(1):54–6. doi: 10.1136/bjo.79.1.54. PMID: 7880795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gass JDM. Nicotinic acid maculopathy. Am J Ophthalmol. 1973 Oct;76(4):500–10. doi: 10.1016/0002-9394(73)90738-1. PMID: 4743805. [DOI] [PubMed] [Google Scholar]
- 7.Neu F. Les oedèmes maculaires cystoides (OMC) Bull Soc belge Ophtalmol. 2007;304:71–6. PMID: 17718230. [PubMed] [Google Scholar]
- 8.Quinn CJ. Cystoid macular edema. Optom Clin. 1996;5(1):111–30. [PubMed] [Google Scholar]
- 9.Cunha-Vaz JG, Travassos A. Breakdown of the blood-retinal barriers and cystoid macular edema. Surv Ophthalmol. 1984 May;28:485–92. doi: 10.1016/0039-6257(84)90230-3. PMID: 6379947. [DOI] [PubMed] [Google Scholar]
- 10.Yanoff M, Fine BS, Brucker AJ, Eagle RC Jr. Pathology of human cystoid macular edema. Surv Ophthalmol. 1984 May;28:505–11. doi: 10.1016/0039-6257(84)90233-9. PMID: 6463850. [DOI] [PubMed] [Google Scholar]
- 11.Hirokawa H, Takahashi M, Trempe CL. Vitreous changes in peripheral uveitis. Arch Ophthalmol. 1985 Nov;103(11):1704–7. doi: 10.1001/archopht.1985.01050110098035. PMID: 4062638. [DOI] [PubMed] [Google Scholar]
- 12.Henderly DE, Genstler AJ, Rao NA, Smith RE. Pars planitis. Trans Ophthalmol Soc U K. 1986;105:227–32. PMID: 3467497. [PubMed] [Google Scholar]
- 13.Cassoux N, Fardeau C, Lehoang P. Ocular manifestations of Behçet’s disease. Ann Med Interne (Paris) 1999 Nov;150(7):529–34. PMID: 10637668. [PubMed] [Google Scholar]
- 14.Dana MR, Merayo-Lloves J, Schaumberg DA, Foster CS. Prognosticators for visual outcome in sarcoid uveitis. Ophthalmology. 1996 Nov;103(11):1846–53. doi: 10.1016/s0161-6420(96)30417-x. PMID : 8942880. [DOI] [PubMed] [Google Scholar]
- 15.Dodds EM, Lowder CY, Meisler DM. Posterior segment inflammation in HLA-B27+ acute anterior uveitis: clinical characteristics. Ocul Immunol Inflamm. 1999 Jun;7(2):85–92. doi: 10.1076/ocii.7.2.85.4015. PMID: 10420203. [DOI] [PubMed] [Google Scholar]
- 16.Helm CJ, Holland GN, Webster RG, Maloney RK, Mondino BJ. Combination intravenous ceftazidime and aminoglycosides in the treatment of pseudomonal scleritis. Ophthalmology. 1997 May;104(5):838–43. doi: 10.1016/s0161-6420(97)30225-5. PMID: 9160031. [DOI] [PubMed] [Google Scholar]
- 17.Schlaegel TF Jr, Weber JC. The macula in ocular toxoplasmosis. Arch Ophthalmol. 1984 May;102(5):697–8. doi: 10.1001/archopht.1984.01040030553014. PMID: 6721755. [DOI] [PubMed] [Google Scholar]
- 18.Irvine AR, Bresky R, Crowder BM, Forster RK, Hunter DM, Kulvin SM. Macular edema after cataract extraction. Ann Ophthalmol. 1971 Nov;3(11):1234–5. PMID: 5163800. [PubMed] [Google Scholar]
- 19.Schalnus RW, Ohrloff C, Magone T. The aqueous humor-vitreous body barrier and the blood-aqueous humor barrier after YAG laser capsulotomy in capsular sac vs ciliary sulcus fixation of the intraocular lens. Ophthalmologe. 1995 Jun;92(3):289–92. PMID: 7655200. [PubMed] [Google Scholar]
- 20.Ohrloff C, Schalnus R, Rothe R, Spitznas M. Role of the posterior capsule in the aqueous–vitreous barrier in aphakic and pseudophakic eyes. J Cataract Refract Surg. 1990 Mar;16(2):198–201. doi: 10.1016/s0886-3350(13)80730-4. PMID: 2329477. [DOI] [PubMed] [Google Scholar]
- 21.Romero-Aroca P, Fernández-Balart J, Baget-Bernaldiz M, Martinez-Salcedo I, Méndez-Marín I, Salvat-Serra M, Buil-Calvo JA. Changes in the diabetic retinopathy epidemiology after 14 years in a population of Type 1 and 2 diabetic patients after the new diabetes mellitus diagnosis criteria and a more strict control of the patients. J Diabetes Complications. 2009 Jul-Aug;23(4):229–38. doi: 10.1016/j.jdiacomp.2008.02.012. PMID: 18439844. [DOI] [PubMed] [Google Scholar]
- 22.Hikichi T, Fujio N, Akiba J, Azuma Y, Takahashi M, Yoshida A. Association between the short-term natural history of diabetic macular edema and the vitreomacular relationship in type II diabete mellitus. Ophthalmology. 1997 Mar;104(3):473–8. doi: 10.1016/s0161-6420(97)30289-9. PMID: 9082275. [DOI] [PubMed] [Google Scholar]
- 23.Early photocoagulation for diabetic retinopathy ETDRS report number 9 Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991 May;98(5):766–85. PMID: 2062512. [PubMed] [Google Scholar]
- 24.Bresnick GH. Diabetic maculopathy A critical review highlighting diffuse macular edema. Ophthalmology. 1983 Nov;90(11):1301–17. doi: 10.1016/s0161-6420(83)34388-8. PMID: 6664669. [DOI] [PubMed] [Google Scholar]
- 25.Fekrat S, Finkelstein D C D, Regillo G C, Flynn Jr. Venous occlusive disease. Vitreoretinal Disease: The Essentials. 1999;9:117–132. [Google Scholar]
- 26.Ascaso FJ, Huerva V. Vitreoretinal traction in impending branch retinal vein occlusion: a pathogenetic role? Thrombosis and Haemostasis. 2012 Aug;108(2):208–9. doi: 10.1160/TH12-03-0190. PMID: 22688432. [DOI] [PubMed] [Google Scholar]
- 27.Ascaso FJ, Padgett E, Núñez E, Villén L, Grzybowski A, Cristóbal JA. Branch retinal vein occlusion and vitreovascular traction: a preliminary spectral domain OCT case–control study. Graefes Arch Clin Exp Ophthalmol. 2014 Mar;252(3):375–81. doi: 10.1007/s00417-013-2463-8. PMID: 25147879. [DOI] [PubMed] [Google Scholar]
- 28.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999 Oct;19(10):2364–7. doi: 10.1161/01.atv.19.10.2364. PMID: 10521365. [DOI] [PubMed] [Google Scholar]
- 29.Pinderski LJ, Fischbein MP, Subbanagounder G, Fishbein MC, Kubo N, Cheroutre H, Curtiss LK, Berliner JA, Boisvert WA. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient mice by altering lymphocyte and macrophage phenotypes. Circ Res. 2002 May;90(10):1064–71. doi: 10.1161/01.res.0000018941.10726.fa. PMID: 12039795. [DOI] [PubMed] [Google Scholar]
- 30.Silva RM, Faria de Abreu JR, Cunha-Vaz JG. Graefes. Arch Clin Exp Ophthalmol. 1995 Nov;233(11):721–6. doi: 10.1007/BF00164677. PMID: 8566831. [DOI] [PubMed] [Google Scholar]
- 31.Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol. 1995 Dec;113(12):1538–44. doi: 10.1001/archopht.1995.01100120068012. PMID: 7487623. [DOI] [PubMed] [Google Scholar]
- 32.Vinores SA, Youssri AI, Luna JD, Chen YS, Bhargave S, Vinores MA, Schoenfeld CL, Peng B, Chan CC, LaRochelle W, Green WR, Campochiaro PA. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol. 1997 Jan;12(1):99–109. PMID: 9046048. [PubMed] [Google Scholar]
- 33.Joussen AM, Smyth N, Niessen C. Pathophysiology of diabetic macular edema. Dev Ophthalmol. 2007;39:1–12. doi: 10.1159/000098495. PMID: 17245075. [DOI] [PubMed] [Google Scholar]
- 34.Pasqualetti G, Danesi R, Del Tacca M, Bocci G. Vascular endothelial growth factor pharmacogenetics: a new perspective for anti-angiogenic therapy. Pharmacogenomics. 2007 Jan;8(1):49–66. doi: 10.2217/14622416.8.1.49. PMID: 17187509. [DOI] [PubMed] [Google Scholar]
- 35.Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol. 2008 Apr ;101(8A):20B–26B. doi: 10.1016/j.amjcard.2008.02.029. PMID: 18375237. [DOI] [PubMed] [Google Scholar]
- 36.Altschul R, Hoffer A, Stephen JD. Influence of nicotinic acid on serum cholesterol in man. Arch Biochem Biophys. 1955 Feb;54(2):558–9. doi: 10.1016/0003-9861(55)90070-9. PMID: 14350806. [DOI] [PubMed] [Google Scholar]
- 37.Meyers CD, Kamanna VS, Kashyap ML. Niacin therapy in atherosclerosis. Curr Opin Lipidol. 2004 Dec;15(6):659–65. doi: 10.1097/00041433-200412000-00006. PMID: 15529025. [DOI] [PubMed] [Google Scholar]
- 38.Carlson LA. Nicotinic acid: the broad-spectrum lipid drug A 50th anniversary review. J Intern Med. 2005 Aug;258(2):94–114. doi: 10.1111/j.1365-2796.2005.01528.x. PMID: 16018787. [DOI] [PubMed] [Google Scholar]
- 39.Ganji SH, Zhang L-H, Kamanna VS, Kashyap ML. Effect of niacin on lipoproteins and atherosclerosis. Future Lipidol. 2006;1:549–57. [Google Scholar]
- 40.Morgan JM, Capuzzi DM, Baksh RI, Intenzo C, Carey CM, Reese D, Walker K. Effects of extended-release niacin on lipoprotein subclass distribution. Am J Cardiol. 2003 Jun ;91(12):1432–6. doi: 10.1016/s0002-9149(03)00394-1. PMID: 12804729. [DOI] [PubMed] [Google Scholar]
- 41.Zambon A, Hokanson JE, Brown BG, Brunzell JD. Evidence for a new pathophysiological mechanism for coronary artery disease regression: hepatic lipase-mediated changes in LDL density. Circulation. 1999 Apr ;99(15):1959–64. doi: 10.1161/01.cir.99.15.1959. PMID: 10208998. [DOI] [PubMed] [Google Scholar]
- 42.Said HM, Nabokina SM, Balamurgan K, Mohammed ZM, Urbina C, Kashyap ML. Mechanism of nicotinic acid transport in human liver cells: studies with HepG2 cells and primary hepatocytes. Am J Physiol Cell Physiol. 2007 Dec;293(6):C1773–8. doi: 10.1152/ajpcell.00409.2007. PMID: 17928533. [DOI] [PubMed] [Google Scholar]
- 43.Hernandez C, Molusky M, Li Y, Li S, Lin JD. Regulation of hepatic ApoC3 expression by PGC-1beta mediates hypolipidemic effect of nicotinic acid. Cell Metab. 2010 Oct ;12(4):411–9. doi: 10.1016/j.cmet.2010.09.001. PMID: 20889132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capuzzi DM, Guyton JR, Morgan JM, Goldberg AC, Kreisberg RA, Brusco OA, Brody J. Efficacy and safety of an extended-release niacin (Niaspan): a long-term study. Am J Cardiol. 1998 Dec;82(12A):74U–81U. doi: 10.1016/s0002-9149(98)00731-0. PMID: 9915666. [DOI] [PubMed] [Google Scholar]
- 45.Morgan JM, Capuzzi DM, Guyton JR. A new, extended-release niacin (Niaspan): efficacy, tolerability, and safety in hypercholesterolemic patients. Am J Cardiol. 1998 Dec ;82(12A):29U–34U. doi: 10.1016/s0002-9149(98)00732-2. PMID: 9915660. [DOI] [PubMed] [Google Scholar]
- 46.Guyton JR, Blazing MA, Hagar J, Kashyap ML, Knopp RH, McKenney JM, Nash DT, Nash SD. Extended-release niacin vs gemfibrozil for the treatment of low levels of high-density lipoprotein cholesterol: Niaspan-Gemfibrozil Study Group. Arch Intern Med. 2000 Apr;160(8):1177–84. doi: 10.1001/archinte.160.8.1177. PMID: 10789612. [DOI] [PubMed] [Google Scholar]
- 47.Grundy SM, Vega GL, McGovern ME, Tulloch BR, Kendall DM, Fitz-Patrick D, Ganda OP, Rosenson RS, Buse JB, Robertson DD, Sheehan JP. Efficacy, Safety, and Tolerability of Once-Daily Niacin for the Treatment of Dyslipidemia Associated With Type 2 Diabetes. Arch Intern Med. 2002 Jul ;162(14):1568–76. doi: 10.1001/archinte.162.14.1568. PMID: 12123399. [DOI] [PubMed] [Google Scholar]
- 48.Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, Offermanns S. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med. 2003 Mar;9(3):352–5. doi: 10.1038/nm824. PMID: 12563315. [DOI] [PubMed] [Google Scholar]
- 49.Benyo Z, Gille A, Bennett CL, Clausen BE, Offermanns S. Nicotinic acid-induced flushing is mediated by activation of epidermal langerhans cells. Mol Pharmacol. 2006 Dec;70(6):1844–9. doi: 10.1124/mol.106.030833. PMID: 17008386. [DOI] [PubMed] [Google Scholar]
- 50.Knowles HJ, te Poele RH, Workman P, Harris AL. Niacin induces PPARgamma expression and transcriptional activation in macrophages via HM74 and HM74a-mediated induction of prostaglandin synthesis pathways. Biochem Pharmacol. 2006 Feb;71(5):646–56. doi: 10.1016/j.bcp.2005.11.019. PMID: 16386710. [DOI] [PubMed] [Google Scholar]
- 51.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001 Nov;345(22):1583–92. doi: 10.1056/NEJMoa011090. PMID: 11757504. [DOI] [PubMed] [Google Scholar]
- 52.Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial biology for the investigation of the treatment effects of reducing cholesterol (ARBITER)2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004 Dec ;110(23):3512–7. doi: 10.1161/01.CIR.0000148955.19792.8D. PMID: 15537681. [DOI] [PubMed] [Google Scholar]
- 53.Thoenes M, Oguchi A, Nagamia S, Vaccari CS, Hammoud R, Umpierrez GE, Khan BV. The effects of extended-release niacin on carotid intimal media thickness, endothelial function and inflammatory markers in patients with the metabolic syndrome. Int J Clin Pract. 2007 Nov;61(11):1942–8. doi: 10.1111/j.1742-1241.2007.01597.x. PMID: 17935553. [DOI] [PubMed] [Google Scholar]
- 54.Warnholtz A, Wild P, Ostad MA, Elsner V, Stieber F, Schinzel R, Walter U, Peetz D, Lackner K, Blankenberg S, Munzel T. Effects of oral niacin on endothelial dysfunction in patients with coronary artery disease: results of the randomized, double-blind, placebo-controlled INEF study. Atherosclerosis. 2009 May;204(1):216–21. doi: 10.1016/j.atherosclerosis.2008.08.003. PMID: 18822413. [DOI] [PubMed] [Google Scholar]
- 55.Hiukka A, Westerbacka J, Leinonen ES, Watanabe H, Wiklund O, Hulten LM, Salonen JT, Tuomainen TP, Yki-Järvinen H, Keech AC, Taskinen MR. Long-term effects of fenofibrate on carotid intima-media thickness and augmentation index in subjects with type 2 diabetes mellitus. J Am Coll Cardiol. 2008 Dec ;52(25):2190–7. doi: 10.1016/j.jacc.2008.09.049. PMID: 19095138. [DOI] [PubMed] [Google Scholar]
- 56.Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin. 2006 Nov;22(11):2243–50. doi: 10.1185/030079906x148508. PMID: 17076985. [DOI] [PubMed] [Google Scholar]
- 57.Oberwittler H, Baccara-Dinet M. Clinical evidence for use of acetyl salicylic acid in control of flushing related to nicotinic acid treatment. Int J Clin Pract. 2006 Jun;60(6):707–15. doi: 10.1111/j.1368-5031.2006.00957.x. PMID: 16805757. [DOI] [PubMed] [Google Scholar]
- 58.Guyton JR, Bays HE. Safety considerations with niacin therapy. Am J Cardiol. 2007 Mar ;99(6A):22C–31C. doi: 10.1016/j.amjcard.2006.11.018. Epub 2006 Nov 28. Review. PMID: 17368274. [DOI] [PubMed] [Google Scholar]
- 59.Birjmohun RS, Kastelein JJ, Poldermans D, Stroes ES, Hostalek U, Assmann G. Safety and tolerability of prolonged-release nicotinic acid in statin-treated patients. Curr Med Res Opin. 2007 Jul;23(7):1707–13. doi: 10.1185/030079907x199682. PMID: 17588301. [DOI] [PubMed] [Google Scholar]
- 60.Benyó Z, Gille A, Bennett CL, Clausen BE, Offermanns S. Nicotinic acid-induced flushing is mediated by activation of epidermal Langerhans cells. Mol Pharmacol. 2006 Dec;70(6):1844–9. doi: 10.1124/mol.106.030833. PMID: 17008386. [DOI] [PubMed] [Google Scholar]
- 61.Guyton JR. Niacin in cardiovascular prevention: mechanisms, efficacy, and safety. Curr Opin Lipidol. 2007 Aug;18(4):415–20. doi: 10.1097/MOL.0b013e3282364add. PMID: 17620858. [DOI] [PubMed] [Google Scholar]
- 62.McKenney JM, Proctor JD, Harris S, Chinchili VM. A comparison of the efficacy and toxic effects of sustained- vs immediate-release niacin in hypercholesterolemic patients. JAMA. 1994 Mar;271(9):672–7. PMID: 8309029. [PubMed] [Google Scholar]
- 63.Stern RH, Freeman D, Spence JD. Differences in metabolism of time-release and unmodified nicotinic acid: explanation of the differences in hypolipidemic action? Metabolism. 1992 Aug;41(8):879–81. doi: 10.1016/0026-0495(92)90170-f. PMID: 1640866. [DOI] [PubMed] [Google Scholar]
- 64.Stern RH. The role of nicotinic acid metabolites in flushing and hepatotoxicity. J Clin Lipidol. 2007 Jul;1(3):191–3. doi: 10.1016/j.jacl.2007.04.003. PMID: 21291680. [DOI] [PubMed] [Google Scholar]
- 65.Cheng K, Wu TJ, Wu KK, Sturino C, Metters K, Gottesdiener K, Wright SD, Wang Z, O'Neill G, Lai E, Waters MG. Antagonism of the prostaglandin D2 receptor 1 suppresses nicotinic acid-induced vasodilation in mice and humans. Proc Natl Acad Sci U S A. 2006 Apr ;103(17):6682–7. doi: 10.1073/pnas.0601574103. PMID: 16617107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bays HE, Maccubbin D, Meehan AG, Kuznetsova O, Mitchel YB, Paolini JF. Blood pressure-lowering effects of extended-release niacin alone and extended-release niacin ⁄ laropiprant combination: a post hoc analysis of a 24-week, placebo-controlled trial in dyslipidemic patients. Clin Ther. 2009 Jan;31(1):115–22. doi: 10.1016/j.clinthera.2009.01.010. PMID: 19243712. [DOI] [PubMed] [Google Scholar]
- 67.Tenenbaum A, Motro M, Fisman EZ, Adler Y, Shemesh J, Tanne D, Leor J, Boyko V, Schwammenthal E, Behar S. Effect of bezafibrate on incidence of type 2 diabetes mellitus in obese patients. Eur Heart J. 2005 Oct;26(19):2032–8. doi: 10.1093/eurheartj/ehi310. PMID: 15872029. [DOI] [PubMed] [Google Scholar]
- 68.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010 Feb ;375(9716):735–42. doi: 10.1016/S0140-6736(09)61965-6. PMID: 20167359. [DOI] [PubMed] [Google Scholar]
- 69.Vogt A, Kassner U, Hostalek U, Steinhagen-Thiessen E. Evaluation of the safety and tolerability of prolonged-release nicotinic acid in a usual care setting: the NAUTILUS study. Curr Med Res Opin. 2006 Feb;22(2):417–25. doi: 10.1185/030079906x89766. PMID: 16466614. [DOI] [PubMed] [Google Scholar]
- 70.Elam MB, Hunninghake DB, Davis KB, Garg R, Johnson C, Egan D, Kostis JB, Sheps DS, Brinton EA. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT study: a randomized trial Arterial Disease Multiple Intervention Trial. JAMA. 2000 Sep ;284(10):1263–70. doi: 10.1001/jama.284.10.1263. PMID: 10979113. [DOI] [PubMed] [Google Scholar]
- 71.Lukasova M, Hanson J, Tunaru S, Offermanns S. Nicotinic acid (niacin): new lipid-independent mechanisms of action and therapeutic potentials. Trends Pharmacol Sci. 2011 Dec;32(12):700–7. doi: 10.1016/j.tips.2011.08.002. PMID: 21944259. [DOI] [PubMed] [Google Scholar]
- 72.Capuzzi DM, Morgan JM, Brusco OA Jr, Intenzo CM. Niacin dosing: relationship to benefits and adverse effects. Curr Atheroscler Rep. 2000 Jan;2(1):64–71. doi: 10.1007/s11883-000-0096-y. PMID: 11122726. [DOI] [PubMed] [Google Scholar]
- 73.Harris JL. Toxic amblyopia associated with administration nicotinic acid. Am J Ophthalmol. 1963 Jan;55:133–4. doi: 10.1016/0002-9394(63)91658-1. PMID: 13952933. [DOI] [PubMed] [Google Scholar]
- 74.Devaney DM. Maculopathy induced by nicotinic acid. Clinical Eye and Vision Care. 1998;10(2):67–71. [Google Scholar]
- 75.Jampol LM. Niacin maculopathy Author's reply RH Millay. Ophthalmology. 1988 Dec;95(12):1704–5. doi: 10.1016/s0161-6420(88)32955-6. PMID: 3231439. [DOI] [PubMed] [Google Scholar]
- 76.Karakashian S, Bayliff CD. Niacin-Induced Cystoid Macular Edema. The Canadian Journal of Hospital Pharmacy. 2001;54(1):35–6. [Google Scholar]
- 77.Fraunfelder FW, Fraunfelder FT, Illingsworth DR. Adverse ocular effects associated with niacin therapy. Br J Ophthalmol. 1995 Jan;79(1):54–6. doi: 10.1136/bjo.79.1.54. PMID: 7880795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Domanico D, Carnevale C, Fragiotta S, Verboschi F, Altimari S, Vingolo EM. Cystoid macular edema induced by low doses of nicotinic Acid. Case Rep Ophthalmol Med. 2013;2013 doi: 10.1155/2013/713061. PMID: 23662229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.NM Bressler. Cystoid macular edema from niacin typically is not accompanied by fluorescein leakage on angiography. Am J Ophthalmol. 2005 May;139(5) doi: 10.1016/j.ajo.2004.11.040. PMID: 15860328. [DOI] [PubMed] [Google Scholar]
- 80.Dajani HM, Lauer AK. Optical coherence tomography findings in niacin maculopathy. Can J Ophthalmol. 2006 Apr;41(2):197–200. doi: 10.1139/I06-008. PMID: 16767207. [DOI] [PubMed] [Google Scholar]
- 81.Spirn MJ, Warren FA, Guyer DR, Klancnik JM, Spaide RF. Optical coherence tomography findings in nicotinic acid maculopathy. Am J Ophthalmol. 2003 Jun;135(6):913–4. doi: 10.1016/s0002-9394(02)02296-1. PMID: 12788145. [DOI] [PubMed] [Google Scholar]