Abstract
Purpose
To determine the relationship between delayed patchy choroidal filling and morphologic and functional outcomes among eyes treated with ranibizumab or bevacizumab.
Design
Cohort study.
Methods
Comparison of Age-related Macular Degeneration Treatment Trials participants were randomly assigned to ranibizumab or bevacizumab on a monthly or as needed schedule. Presence of delayed patchy choroidal filling and morphologic and functional outcomes were evaluated among eyes with gradeable fluorescein angiography at baseline (N=973) and at one year (N=860) eyes.
Results
Delayed filling was present in 75 (7.7%) of 973 eyes at baseline. Eyes with incident delayed filling at one year (23 (2.9%) of 798) showed a mean decrease of 1.7 letters in visual acuity, whereas eyes without incident delayed filling had a mean improvement of 8.1 letters (Δ=−9.8 [−15.8, −3.9], p<0.01). Eyes with incident delayed filling had a larger increase in mean total lesion area of choroidal neovascularization (3.00 mm2) than eyes without incident delayed filling (0.56 mm2, Δ=2.4 [0.4, 4.4], p=0.02). The proportion with incident delayed filling at one year was similar among eyes treated with ranibizumab (10 (2.4%) of 413) or bevacizumab (13 (3.3%) of 385, p=0.53) and among eyes treated monthly (12 (3.1%) of 388) or as needed (11 (2.7%) of 410, p=0.83).
Conclusions
Delayed patchy choroidal filling was uncommon at baseline. Although only a small percentage of eyes developed delayed filling during the first year of anti-vascular endothelial growth factor treatment, these eyes had worse visual acuity and a larger increase in total lesion area of choroidal neovascularization.
INTRODUCTION
The pathogenesis of age-related macular degeneration is believed to be a multi-factorial process involving genetic predisposition, inflammatory mediators, oxidative stress, and hypoxia-induced angiogenesis. While the interplay of these factors still remains elusive, increasing evidence points towards an underlying ischemic process as a major contributing factor to age-related macular degeneration (AMD) pathogenesis. Abnormalities of the choroidal circulation are associated with the development of choroidal neovascularization (CNV) in patients with AMD by a process that may involve ischemia, hypoxia, and resultant vascular endothelial growth factor (VEGF) production1–9.
Choroidal circulation abnormalities, as displayed by fluorescein and indocyanine green angiography10–12, color Doppler flowmetry13–16, laser Doppler flowmetry17,18, pulsatile ocular blood flow19,20 and histopathological analyses21,22, have been associated with progression of age-related macular degeneration. The choroidal circulation plays a vital role in both the provision of oxygen and nutrients to the outer retina, as well as removal of metabolic waste products. Impairment of these functions may contribute to accumulation of Bruch’s membrane deposits, retinal atrophy, and choroidal neovascularization.
The results from several previous studies have suggested a role for decreased choroidal blood flow in the development of CNV in AMD23. Decreased choroidal blood flow is associated with many AMD risk factors including extent of drusen, retinal hyper-pigmentary changes, hypertension, and hyperopia24,25. Furthermore, in a longitudinal study of choroidal blood flow in AMD patients, Metelitsina et al. reported that patients who developed CNV during the study had lower choroidal blood flow at baseline than those who did not develop CNV26. In addition, choroidal blood flow decreased prior to CNV formation, suggesting a role for ischemia in the development of CNV. Finally, patients with lower choroidal blood flow at baseline were three times more likely to experience decreases in visual acuity during the study.
A different line of evidence pointing to a role of decreased choroidal blood flow and ischemia in the pathogenesis of CNV can be derived from reports suggesting that in exudative AMD patients, there is a tendency for CNV to form in proximity to angiographically documented watershed areas27–29 where blood flow may be diminished. In addition, Stefansson et al. recently noted features of AMD that may contribute to abnormal retinal oxygen metabolism and potentially ischemia, including the presence of confluent drusen, retinal elevation, retinal edema, and vitreo-retinal adhesion9.
Delayed filling of the choroidal lobules during the transit phase of fluorescein angiography, or delayed patchy choroidal filling, may be related to decreased choroidal circulation, as seen in choroidal ischemia secondary to vascular diseases30–32. In this study, we investigated the association of delayed patchy choroidal filling with morphological and functional outcomes following anti-VEGF treatment in participants of the Comparison of Age-related Macular Degeneration Treatments Trials (CATT).
MATERIALS AND METHODS
Institutional review board approval was obtained from the University of Pennsylvania and all participating CATT clinical centers. Each participant provided written informed consent that was HIPAA-compliant before entry into the study. Details regarding the methodology of the CATT study, an interventional double masked trial, have been reported previously and can also be reviewed at ClinicalTrials.gov (NCT00593450)33. Below is a description of the methodology involved in this cohort study within CATT pertaining to the fluorescein angiography evaluation of choroidal filling in study participants.
Study Participants
A total of 1185 participants from 43 clinical centers in the U.S. were enrolled in CATT between 2008 and 2009. After written consent was obtained, study participants were randomized to one of the four treatment groups: (1) ranibizumab monthly, (2) bevacizumab monthly, (3) ranibizumab pro re nata (PRN), and (4) bevacizumab PRN. Inclusion criteria for the study included the presence of active CNV, sub-foveal involvement by CNV or sequelae of CNV, fibrosis < 50% of total lesion area, visual acuity (VA) 20/25–20/320, age ≥ 50 yrs, and at least one drusen (>63µ) in either eye or late AMD in fellow eye. Exclusion criteria included previous treatment for CNV in the study eye, other progressive retinal disease likely to compromise visual acuity, as well as any contraindications to injections with ranibizumab or bevacizumab.
Study Procedures
During the initial visit to the participating centers, several baseline characteristics were recorded including age, gender, race, smoking status, systolic and diastolic blood pressures, use of anti-hypertensive medications. Visual acuity was measured in both eyes after refraction, using the Electronic Visual Acuity Tester following the protocol used in the Diabetic Retinopathy Clinical Research Network34. Systemic hypertension was defined as a systolic blood pressure of 160 mmHg, diastolic blood pressure of 95 mmHg, or current use of anti-hypertensive medications.
Trained photographers followed a standard protocol to obtain color fundus photographs and fluorescein angiograms prior to initiation of anti-VEGF therapy and at one year of follow up. All photographs were digital with the exception of one site that carried out film-based imaging.
Graders at the CATT Fundus Photograph Reading Center reviewed color photographs and fluorescein angiograms of study eyes (one study eye per subject) and recorded the presence or absence of CNV, CNV lesion type (i.e. predominantly classic, minimally classic, occult only, cannot grade/no lesion), and retinal angiomatous proliferation. The total CNV lesion area was measured at baseline by taking into consideration the area of CNV plus any associated hemorrhage, scarring, blocked fluorescence or pigment epithelial detachment associated with neovascularization. At follow-up, areas of geographic atrophy in areas previously occupied by the CNV lesion were included in the measurement to the total CNV lesion area. Graders at the CATT Optical Coherence Tomography (OCT) Reading Center reviewed images from time-domain optical coherence tomography and recorded the presence of intra-retinal fluid, sub-retinal fluid, or sub-retinal pigment epithelial fluid. Total retinal thickness in microns was measured at the foveal center based on the average of six fast macular OCT scans.
For the evaluation of the choroidal filling pattern, two trained readers (DYG, CPO) in the CATT Photograph Reading Center initially determined whether delayed patchy choroidal filling was present at baseline and one year after anti-VEGF treatment. Non-physiologic delayed patchy choroidal filling was deemed present if at least half a disc diameter of patchy choroidal filling was present beyond the early venous transit phase. Early venous transit phase was defined as lamellar filling with fluorescein that comprised less than half of the diameter of the larger retinal venules. At least two clear transit frames were required in order to evaluate a series. In equivocal cases, a third senior grader (JEG) adjudicated. Photos from the first 5% of patients were graded by both trained readers to assess inter-grader agreement with respect to presence of delayed patchy choroidal filling (Yes/No/Can’t Grade, n=118 photos, 94.1% agreement, kappa=0.89 [95% CI 0.82, 0.97]), and subsequent to the determination of acceptable agreement, all patient photos were randomly assigned to only one grader. Only the 1118 eyes that had angiography at both baseline and one year were graded for delayed patchy choroidal filling. All assessments were performed without access to demographic data, treatment allocation and clinical outcomes.
Data Analysis
The comparisons between groups of eyes based on presence of delayed patchy choroidal filling were performed for baseline characteristics and morphologic and functional outcomes at one year using Fisher’s exact test for proportions and the independent t-test for means. Similar comparisons were made between groups of eyes based on development of delayed patchy choroidal filling within one year. McNemar’s test was used for comparisons of delayed patchy choroidal filling in the same eyes over time. All data analyses were performed using Statistical Analysis Software (version 9.3, SAS Inc., Cary, NC).
RESULTS
Baseline Characteristics of Study Participants
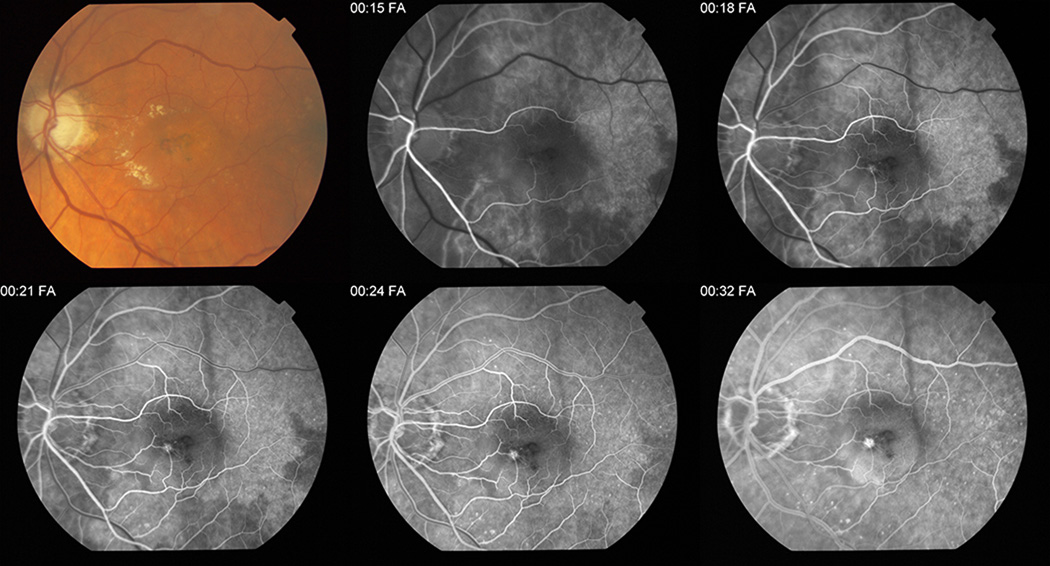
Among the 1118 graded eyes, 973 (87.0%) were gradable for delayed patchy choroidal filling, and 75 (7.7%) of these had evidence of delayed patchy choroidal filling at baseline. Figure 1 shows a typical case of delayed patchy choroidal filling.
Figure 1. Typical example of delayed patchy choroidal filling on fluorescein angiography in a Comparison of Age-related Treatment Trials participant.
This figure represents a typical example of delayed patchy choroidal filling on fluorescein angiography of the left eye of a Comparison of Age-Related Treatment Trials study patient. The first image is a color fundus photograph of the left eye showing exudates and pigmentary changes in the macula. At 15 seconds in the arterial phase, several well-demarcated areas of relatively hypofluorescent patchy choroidal filling are visible in all quadrants of the macula. At 21 seconds, these patchy areas persist in the venous phase and are considered abnormally delayed. A choroidal neovascular membrane is evident. Residual mild patchiness can be seen at 24 seconds and it is not until the last frame of this figure that the delayed patchy choroidal filling resolves.
The mean age was 79.3 years in patients with baseline delayed patchy choroidal filling and 78.8 years in patients with normal baseline choroidal filling; this difference was not statistically significant (Δ=0.5 [−1.3, 2.2], p=0.62; Table 1). The mean baseline visual acuity was 62.1 letters in those with baseline delayed patchy choroidal filling and 61.3 letters in those without baseline delayed patchy choroidal filling; this difference was not significant (Δ=0.8 [−1.3, 2.2], p=0.61). There were no statistically significant differences in gender, race, smoking status, hypertension, CNV area, CNV lesion type, or retinal angiomatous proliferation (RAP) lesion type and OCT total retinal thickness between patients with and those without baseline delayed patchy choroidal filling.
Table 1.
Comparison of Baseline Characteristics Between Study Eyes With Versus Without Presence of Delayed Patchy Choroidal Filling at Baseline in the Comparison of Age-related Macular Degeneration Treatment Trials
| Baseline Delayed Patchy Choroidal Filling | |||||
|---|---|---|---|---|---|
| No (n=898) |
Yes (n=75) |
Difference*: Yes versus No (95% CI) |
p-value | ||
| Age (years) | Mean (95% CI) | 78.8 (78.3, 79.3) | 79.3 (77.6, 81.0) | +0.5 (−1.3, 2.2) | 0.62 |
| Sex | Male | 344 (38.3%) | 29 (38.7%) | −0.4% (−11.8%, 11.1%) | 1.00 |
| Female | 554 (61.7%) | 46 (61.3%) | |||
| Race | White | 886 (98.7%) | 74 (98.7%) | 0.0% (−2.7%, 2.7%) | 1.00 |
| Other | 12 (1.3%) | 1 (1.3%) | |||
| Smoking | Never | 384 (42.8%) | 26 (34.7%) | +7.2% (−0.1%, 15.7%) | 0.09 |
| Past | 435 (48.4%) | 37 (49.3%) | |||
| Current | 79 (8.8%) | 12 (16.0%) | |||
| Diastolic BP (mmHg) | Mean (95% CI) | 75.5 (74.9, 76.2) | 74.0 (71.8, 76.2) | −1.5 (−0.8, 3.9) | 0.20 |
| Systolic BP (mmHg) | Mean (95% CI) | 135 (134, 136) | 134 (130, 138) | −1.0 (−3.2, 5.2) | 0.65 |
| Hypertension | No | 267 (29.7%) | 28 (37.3%) | −7.6% (−18.9%, 3.7%) | 0.19 |
| Yes | 631 (70.3%) | 47 (62.7%) | |||
| Visual Acuity (letters) | Mean (95% CI) | 61.3 (60.4, 62.1) | 62.1 (59.1, 65.1) | +0.8 (−2.3, 3.9) | 0.61 |
| Area of CNV (mm2) | Mean (95% CI) | 4.4 (4.1, 4.7) | 5.3 (4.1, 6.5) | +0.9 (−0.2, 2.0) | 0.10 |
| Total area of CNV lesion (mm2) | Mean (95% CI) | 5.8 (5.5, 6.2) | 6.1 (4.8, 7.3) | +0.2 (−1.1, 1.5) | 0.71 |
| Lesion type | Predominantly classic | 204 (22.7%) | 15 (20.0%) | −6.0% (−17.8%, 5.7%) | 0.24 |
| Minimally classic | 144 (16.0%) | 19 (25.3%) | |||
| Occult only | 533 (59.4%) | 40 (53.3%) | |||
| Can't grade/No lesion | 17 (1.9%) | 1 (1.3%) | |||
| RAP Lesion | None/Questionable | 786 (88.7%) | 64 (87.7%) | +1.0% (−6.8%, 8.9%) | 0.70 |
| Yes | 100 (11.3%) | 9 (12.3%) | |||
| OCT total retinal thickness (microns) | Mean (95% CI) | 456 (444, 468) | 440 (405, 476) | −15.1 (−57.8, 27.6) | 0.49 |
BP=Blood pressure, CNV=Choroidal neovascularization, CI=Confidence Interval
RAP= Retinal angiomatous proliferation, OCT=Optical coherence tomography
For categorical variables, difference is listed for only one level and is listed next to the selected level.
When we compared the one year outcomes between eyes without delayed patchy choroidal filling at baseline and eyes with delayed patchy choroidal filling at baseline, we also found no statistically significant differences in visual acuity, total area of CNV lesion, presence of fluorescein leakage, total OCT retinal thickness, presence of OCT fluid, presence of scar and geographic atrophy (Table 2).
Table 2.
Comparison of One-year Outcomes Between Study Eyes With Versus Without Presence of Delayed Patchy Choroidal Filling at Baseline in the Comparison of Age-related Macular Degeneration Treatments Trials
| Baseline Delayed Patchy Choroidal Filling | ||||||
|---|---|---|---|---|---|---|
| No (n=898) |
Yes (n=75) |
Difference: Yes versus No (95% CI) |
p-value | |||
| Visual acuity (letters) | 1 year | Mean (95% CI) | 69.0 (67.8, 70.1) | 70.4 (66.3, 74.5) | +1.4 (−2.9, 5.7) | 0.52 |
| Change from baseline | Mean (95% CI) | 7.6 (6.6, 8.5) | 8.5 (5.3, 11.7) | +0.9 (−2.7, 4.6) | 0.61 | |
| Total area of CNV lesion (mm2) | 1 year | Mean (95% CI) | 6.6 (6.1, 7.0) | 6.5 (5.1, 7.8) | −0.1 (−1.8, 1.5) | 0.90 |
| Change from baseline | Mean (95% CI) | 0.75 (0.37, 1.12) | 0.25 (−0.79, 1.28) | −0.5 (−1.9, 0.9) | 0.47 | |
| FA leakage at 1year | n (%) | 386 (45.6%) | 31 (47.0%) | +1.3% (−11.2%, 13.8%) | 0.90 | |
| OCT total retinal thickness (microns) | 1 year | Mean (95% CI) | 287 (278, 296) | 279 (249, 309) | −8.3 (−42.1, 25.5) | 0.63 |
| Change from baseline | Mean (95% CI) | −168 (−180, −156) | −169 (−209, −129) | −1.1 (−45.4, 43.2) | 0.96 | |
| OCT fluid at 1 yr | n (%) | 587 (69.9%) | 45 (69.2%) | −0.7% (−12.3%, 11.0%) | 0.89 | |
| Fibrotic scar at 1 yr | n (%) | 165 (19.9%) | 11 (16.7%) | −3.2% (−12.6%, 6.2%) | 0.63 | |
| Any scar at 1 yr | n (%) | 273 (32.9%) | 20 (30.3%) | −2.5% (−14.1%, 9.0%) | 0.79 | |
| GA at 1 yr | n (%) | 45 (5.7%) | 5 (8.3%) | +2.7% (−4.5%, 9.8%) | 0.39 | |
CI=Confidence interval, CNV=Choroidal neovascularization, GA=Geographic Atrophy
FA=Fluorescein angiogram, OCT=Optical coherence tomography
Among the 75 eyes with delayed patchy choroidal filling at baseline, delayed patchy choroidal filling was observed after the early venous phase in 8 (11%), after the late venous phase in 56 (75%), and past the transit phase entirely in 11 (15%). There was no significant difference in visual acuity between these eyes with different degrees of delayed patchy choroidal filling (p=0.23, data not shown).
Changes in Ocular Characteristics Compared to Baseline
Among the 973 eyes gradable at baseline, 860 (88.4%) were gradable for delayed patchy choroidal filling at one year. Participants were divided into four descriptive groups based on the delayed patchy choroidal filling status at baseline and at one year: no delayed patchy choroidal filling at baseline or at one year (775, 90.1%), delayed patchy choroidal filling present at baseline and one year (51, 5.9%), no delayed patchy choroidal filling at baseline but development of delayed patchy choroidal filling at one year (incident cases at one year, 23, 2.7%), and delayed patchy choroidal filling present at baseline but not at one year (disappeared cases, 11, 1.3%). The patchy status comparison at baseline and at one year indicates that there were significantly more incident cases than disappeared cases (p=0.04). The mean change (95% confidence interval) in visual acuity over one year was −1.7 (−8.7, 5.2) letters in those with incident delayed patchy choroidal filling and +8.1 (7.1, 9.1) in patients with normal choroidal filling at both baseline and one year; this difference was statistically significant (Δ=−9.8 [−15.8, −3.9], p<0.01; Table 3). Incident delayed patchy choroidal filling cases at one year also displayed a larger increase in mean total CNV lesion area (Δ=2.4 [0.4, 4.4], p=0.02) and a smaller but not significant decrease in OCT total retinal thickness (Δ=44.2 [−28.1, 116.5], p=0.23).
Table 3.
Comparison of One-Year Outcomes Between Study Eyes That Do Versus Do Not Develop Incident Delayed Patchy Choroidal Filling in the Comparison of Age-related Macular Degeneration Treatment Trials
| Incident Delayed Patchy Choroidal Filling | ||||||
|---|---|---|---|---|---|---|
| No (n=775) |
Yes (n=23) |
Difference: Yes versus No (95% CI) |
p-value | |||
| Visual acuity (letters) | Baseline | Mean (95% CI) | 61.3 (60.4, 62.2) | 64.8 (59.8, 69.9) | +3.5 (−1.9, 8.9) | 0.20 |
| 1 year | Mean (95% CI) | 69.4 (68.2, 70.6) | 63.1 (52.9, 73.2) | −6.3 (−13.5, 0.9) | 0.08 | |
| Change from baseline | Mean (95% CI) | 8.1 (7.1, 9.1) | −1.7 (−8.7, 5.2) | −9.8 (−15.8, −3.9) | <0.01 | |
| Total area of CNV lesion (mm2) | Baseline | Mean (95% CI) | 5.6 (5.2, 6.0) | 6.8 (3.9, 9.7) | +1.2 (−1.0, 3.4) | 0.29 |
| 1 year | Mean (95% CI) | 6.2 (5.8, 6.7) | 9.8 (6.0, 13.5) | +3.5 (1.1, 6.0) | <0.01 | |
| Change from baseline | Mean (95% CI) | 0.56 (0.22, 0.91) | 3.00 (0.61, 5.40) | +2.4 (0.4, 4.4) | 0.02 | |
| FA leakage at 1year | n (%) | 355 (46.0%) | 11 (47.8%) | +1.8% (−18.9%, 22.5%) | 1.00 | |
| OCT total retinal thickness (microns) | Baseline | Mean (95% CI) | 454 (441, 466) | 394 (338, 450) | −59.5 (−133.5, 14.4) | 0.11 |
| 1 year | Mean (95% CI) | 286 (276, 295) | 271 (220, 322) | −14.9 (−70.2, 40.4) | 0.60 | |
| Change from baseline | Mean (95% CI) | −168 (−180, −155) | −123 (−167, −80) | +44.2 (−28.1, 116.5) | 0.23 | |
| OCT fluid at 1 yr | n (%) | 527 (69.9%) | 16 (69.6%) | +0.3% (−19.4%, 18.8%) | 1.00 | |
| Fibrotic scar at 1 yr | n (%) | 143 (19.1%) | 6 (27.3%) | +8.1% (−10.7%, 27.0%) | 0.41 | |
| Any scar at 1 yr | n (%) | 244 (32.7%) | 7 (31.8%) | −0.8% (−20.6%, 18.9%) | 1.00 | |
| GA at 1 yr | n (%) | 40 (5.6%) | 2 (8.7%) | +3.1% (−8.6%, 14.7%) | 0.38 | |
CI=Confidence interval, CNV=Choroidal neovascularization, GA=Geographic Atrophy
FA=Fluorescein angiogram, OCT=Optical coherence tomography
At one year, there were no statistically significant differences in fluorescein leakage, presence of intra−/sub- retinal/sub-retinal pigment epithelium (RPE) fluid, or development of scar or geographic atrophy between the eyes that developed delayed patchy choroidal filling at one year and the eyes that did not have delayed patchy choroidal filling at baseline and one year.
Among the 23 incident delayed patchy choroidal filling eyes at one year, delayed patchy choroidal filling was observed only through the early venous phase in 4 (17%), through late venous in 14 (61%), and past the transit phase entirely in 5 (22%). There was no significant difference in visual acuity between these eyes with varying degrees of delayed patchy choroidal filling (p=0.36, data not shown).
Anti-VEGF Treatment and Delayed Patchy Choroidal Filling
The incidence of delayed patchy choroidal filling was similar in eyes treated with ranibizumab (10/413, 2.4%) and with bevacizumab (13/385, 3.4%, Δ=1.0% [−1.1%, 3.3%], p=0.53) and in eyes treated monthly (12/388, 3.1%) and PRN (11/410, 2.7%, Δ=0.4% [−1.9%, 2.7%], p=0.83).
DISCUSSION
The presence of delayed patchy choroidal filling was a relatively rare finding, observed at baseline in less than 8% of participants. In comparison to baseline, there was a small, but statistically significant increase in delayed patchy choroidal filling after one year of treatment, suggesting a more delayed choroidal filling time at one year. Whether this is an effect of the anti-VEGF treatment or an effect of aging, which is known to be associated with decreased choroidal blood flow35, cannot be answered in our study because of the lack of a control group. There were 11 patients that had delayed patchy choroidal filling at baseline but did not have it at one year. This may be due to fluctuations in choroidal filling that may be related to blood pressure changes and heart rate changes or other physiologic parameters that may influence choroidal blood flow. In addition this phenomenon may be due to variations in the determination of delayed patchy choroidal filling.
We found no statistically significant differences in vision and anatomical outcomes at baseline between eyes with and eyes without delayed patchy choroidal filling at baseline. Eyes that developed delayed patchy choroidal filling after one year in the study, however, had significantly worse visual acuity and larger increases in total CNV lesion area than those that did not develop this feature during the study.
Evidence of retinal vasoconstriction following ranibizumab therapy has been reported by Papadopoulou36 in a study performed in 11 AMD eyes that received monthly intravitreal injections of ranibizumab for three consecutive months. This study showed significant vasoconstriction of retinal arterioles ranging from 8.1 ± 3.2 % at 30 days after the first injection, to 11.5 ± 4.4% at 30 days after the second injection and 17.6 ± 7.4% at 30 days after the third injection. An additional preliminary report by Fontaine O et al. also suggested the presence of arteriolar vasoconstriction of about 5% after bevacizumab treatment in 16 patients with neovascular AMD37.
Although there are no reports on the effect of anti-VEGF therapy on the choroidal vessel diameter, it is possible that the vasoconstriction reported in the retina may also occur in the choroidal vasculature. A recent report of Yamazaki T et al.38 showing significant decreases in subfoveal choroidal thickness measured by OCT following anti-VEGF intravitreal injections supports this hypothesis. Vasoconstriction of both retinal and choroidal vessels could result in a decreased perfusion of the macula with deleterious consequences. Although there is evidence that anti-VEGF drugs cause vasoconstriction, reduction of choroidal blood flow as determined by incident delayed patchy choroidal filling was rare in this study with a rate of 2.7%36,37.
Our results showing worse visual acuity and larger increases in total CNV lesion area in eyes with incident delayed patchy choroidal filling at one year suggest that a decreased macular circulation may have a role in the lack of improvement in visual acuity following anti-VEGF therapy in some patients.
We found no significant differences in the incidence of delayed patchy choroidal filling between ranibizumab or bevacizumab treatment (difference of 1.0% [95% CI −1.1%, 3.3%], p=0.53) and between the monthly and PRN treatment schedules (difference of 0.4% [95% CI −1.9%, 2.7%], p=0.83). Our number of cases with incident patchy delayed choroidal filling is rather small and a larger population may be needed to reach any strong conclusions regarding this point.
Our study did not reveal a statistically significant association between baseline delayed patchy choroidal filling and systemic hypertension or smoking status. However, there was a marginally significant difference in smoking (p=0.09), with a higher percent of current smoking in the delayed patchy choroidal filling group (16.0% vs. 8.8%, Table 1). The small number of cases with delayed patchy choroidal filling may not allow us to make any strong conclusion regarding this point. Additionally, it is possible that antihypertensive treatment may affect the relationship between delayed patchy choroidal filling and blood pressure.
In summary, our results suggest that the presence of delayed patchy choroidal filling is rare at baseline, present in less than 8% of participants. Interestingly, the 23 (2.9%) incident cases of delayed patchy choroidal filling at one year displayed worse visual acuity and larger increases in total CNV lesion area suggesting that decreased perfusion could have a role in explaining poor visual outcomes in patients treated with anti-VEGF therapy.
ACKNOWLEDGEMENTS
Funding/Support (including none): The Comparison of Age-Related Treatment Trials (CATT, ClinicalTrials.gov, NCT00593450) is supported by NEI/NIH, DHHS grants: U10 EY017823; U10 EY017825; U10 EY017826; U10 EY017828
Other Acknowledgments: None
Footnotes
Online Material: None
Financial Disclosures: None
- Conception and design: DYG, JEG, MP, GSY, MM, EB
- Analysis and interpretation: DYG, JEG, MP, GSY, MM, EB, SLF
- Writing the article: DYG, JEG, MP, GSY, MM, EB
- Critical revision of the article: JEG, MP, GSY, MM, EB, SLF
- Final approval of the article: JEG, MP, GSY, MM, EB, SLF
- Data collection: DYG, CPO
- Provision of materials, patients, or resources: JEG, MM, SLF
- Statistical expertise: MP, GSY, MM
- Obtaining funding: JEG, MM, SLF
- Literature search: DYG, JEG
- Administrative, technical, or logistical support: JEG, MM, SLF
REFERENCES
- 1.Boltz A, Luksch A, Wimpissinger B, et al. Choroidal blood flow and progression of age-related macular degeneration in the fellow eye in patients with unilateral choroidal neovascularization. Invest Ophthalmol Vis Sci. 2010;51(8):4220–4225. doi: 10.1167/iovs.09-4968. [DOI] [PubMed] [Google Scholar]
- 2.Chen SJ, Cheng CY, Lee AF, et al. Pulsatile ocular blood flow in asymmetric exudative age related macular degeneration. Br J Ophthalmol. 2001;85(12):1411–1415. doi: 10.1136/bjo.85.12.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayashi K, de Laey JJ. Indocyanine green angiography of choroidal neovascular membranes. Ophthalmologica. 1985;190(1):30–39. doi: 10.1159/000309489. [DOI] [PubMed] [Google Scholar]
- 4.Mendrinos E, Pournaras CJ. Topographic variation of the choroidal watershed zone and its relationship to neovascularization in patients with age-related macular degeneration. Acta Ophthalmol. 2009;87(3):290–296. doi: 10.1111/j.1755-3768.2008.01247.x. [DOI] [PubMed] [Google Scholar]
- 5.Metelitsina TI, Grunwald JE, DuPont JC, et al. Foveolar choroidal circulation and choroidal neovascularization in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49(1):358–363. doi: 10.1167/iovs.07-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mori F. Pulsatile ocular blood flow and choroidal blood flow in age-related macular degeneration. Nihon Ganka Gakkai Zasshi. 2003;107(11):674–677. [PubMed] [Google Scholar]
- 7.Ross RD, Barofsky JM, Cohen G, et al. Presumed macular choroidal watershed vascular filling, choroidal neovascularization, and systemic vascular disease in patients with age-related macular degeneration. Am J Ophthalmol. 1998;125(1):71–80. doi: 10.1016/s0002-9394(99)80237-2. [DOI] [PubMed] [Google Scholar]
- 8.Schmetterer L, Kruger A, Findl O, et al. Topical fundus pulsation measurements in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1998;236(3):160–163. doi: 10.1007/s004170050058. [DOI] [PubMed] [Google Scholar]
- 9.Stefánsson E, Geirsdóttir A, Sigurdsson H. Metabolic physiology in age related macular degeneration. Prog Retin Eye Res. 2011;30(1):72–80. doi: 10.1016/j.preteyeres.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg MF, Dhaliwal RS, Olk RJ. Indocyanine green angiography patterns of zones of relative decreased choroidal blood flow in patients with exudative age-related macular degeneration. Ophthalmic Surg Lasers. 1998;29(5):385–390. [PubMed] [Google Scholar]
- 11.Pauleikhoff D, Spital G, Radermacher M, et al. A fluorescein and indocyanine green angiographic study of choriocapillaris in age-related macular disease. Arch Ophthalmol. 1999;117(10):1353–1358. doi: 10.1001/archopht.117.10.1353. [DOI] [PubMed] [Google Scholar]
- 12.Piguet B, Palmvang IB, Chisholm IH, et al. Evolution of age-related macular degeneration with choroidal perfusion abnormality. Am J Ophthalmol. 1992;113(6):657–663. doi: 10.1016/s0002-9394(14)74790-7. [DOI] [PubMed] [Google Scholar]
- 13.Ciulla TA, Harris A, Chung HS, et al. Color Doppler imaging discloses reduced ocular blood flow velocities in nonexudative age-related macular degeneration. Am J Ophthalmol. 1999;128(1):75–80. doi: 10.1016/s0002-9394(99)00061-6. [DOI] [PubMed] [Google Scholar]
- 14.Friedman E, Krupsky S, Lane AM, et al. Ocular blood flow velocity in age-related macular degeneration. Ophthalmology. 1995;102(4):640–646. doi: 10.1016/s0161-6420(95)30974-8. [DOI] [PubMed] [Google Scholar]
- 15.Hosal BM, Karakoç G, Gürsel E, et al. Color Doppler imaging of the retrobulbar circulation in age-related macular degeneration. Eur J Ophthalmol. 1998;8(4):234–238. doi: 10.1177/112067219800800406. [DOI] [PubMed] [Google Scholar]
- 16.Uretmen O, Akkin C, Erakgün T, et al. Color Doppler imaging of choroidal circulation in patients with asymmetric age-related macular degeneration. Ophthalmologica. 2003;217(2):137–142. doi: 10.1159/000068559. [DOI] [PubMed] [Google Scholar]
- 17.Boltz A, Luksch A, Wimpissinger B, et al. Choroidal blood flow and progression of age-related macular degeneration in the fellow eye in patients with unilateral choroidal neovascularization. Invest Ophthalmol Vis Sci. 2010;51(8):4220–4225. doi: 10.1167/iovs.09-4968. [DOI] [PubMed] [Google Scholar]
- 18.Pournaras CJ, Logean E, Riva CE, et al. Regulation of subfoveal choroidal blood flow in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47(4):1581–1586. doi: 10.1167/iovs.05-0434. [DOI] [PubMed] [Google Scholar]
- 19.Chen SJ, Cheng CY, Lee AF, et al. Pulsatile ocular blood flow in asymmetric exudative age related macular degeneration. Br J Ophthalmol. 2001;85(12):1411–1415. doi: 10.1136/bjo.85.12.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori F, Konno S, Hikichi T, et al. Pulsatile ocular blood flow study: decreases in exudative age related macular degeneration. Br J Ophthalmol. 2001;85(5):531–533. doi: 10.1136/bjo.85.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman E. The pathogenesis of age-related macular degeneration. Am J Ophthalmol. 2008;146(3):348–349. doi: 10.1016/j.ajo.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 22.McLeod DS, Grebe R, Bhutto I, et al. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50(10):4982–4991. doi: 10.1167/iovs.09-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grunwald JE, Hariprasad SM, DuPont J, et al. Foveolar choroidal blood flow in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1998;39(2):385–390. [PubMed] [Google Scholar]
- 24.Berenberg TL, Metelitsina TI, Madow B, et al. The association between drusen extent and foveolar choroidal blood flow in age-related macular degeneration. Retina. 2012;32(1):25–31. doi: 10.1097/IAE.0b013e3182150483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu W, Grunwald JE, Metelitsina TI, et al. Risk factors for choroidal neovascularization in AMD are associated with decreased foveolar choroidal circulation. Am. J. Ophthalmol. 2010;150(1):40–47. doi: 10.1016/j.ajo.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metelitsina TI, Grunwald JE, DuPont JC, et al. Foveolar choroidal blood flow and choroidal neovascularization in age related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2008;49(1):358–363. doi: 10.1167/iovs.07-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg MF, Dhaliwal RS, Olk RJ. Indocyanine green angiography patterns of zones of relative decreased choroidal blood flow in patients with exudative age-related macular degeneration. Ophthalmic Surg Lasers. 1998;29(5):385–390. [PubMed] [Google Scholar]
- 28.Hayashi K, de Laey JJ. Indocyanine green angiography of choroidal neovascular membranes. Ophthalmologica. 1985;190(1):30–39. doi: 10.1159/000309489. [DOI] [PubMed] [Google Scholar]
- 29.Mendrinos E, Pournaras CJ. Topographic variation of the choroidal watershed zone and its relationship to neovascularization in patients with age-related macular degeneration. Acta Ophthalmol. 2009;87(3):290–296. doi: 10.1111/j.1755-3768.2008.01247.x. [DOI] [PubMed] [Google Scholar]
- 30.Pauleikhoff D, Spital G, Radermacher M, et al. A fluorescein and indocyanine green angiographic study of choriocapillaris in age-related macular disease. Arch Ophthalmol. 1999;117(10):1353–1358. doi: 10.1001/archopht.117.10.1353. [DOI] [PubMed] [Google Scholar]
- 31.Gaudric A, Coscas G, Bird AC. Choroidal ischemia. Am J Ophthalmol. 1982;94(4):489–498. doi: 10.1016/0002-9394(82)90242-2. [DOI] [PubMed] [Google Scholar]
- 32.Zaharia M, Olivier P, Lafond G, et al. Lobular delayed choroidal perfusion as an early angiographic sign of diabetic retinopathy: a preliminary report. Can J Ophthalmol. 1987;22(5):257–261. [PubMed] [Google Scholar]
- 33.CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135(2):194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 35.Grunwald JE, Hariprasad SM, DuPont J. Effect of aging on foveolar choroidal circulation. Arch Ophthalmol. 1998;116(2):150–154. doi: 10.1001/archopht.116.2.150. [DOI] [PubMed] [Google Scholar]
- 36.Papadopoulou DN, Mendrinos E, Mangoris G, et al. Intravitreal Ranibizumab may induce retinal arteriolar vasoconstriction in patients with neovascular age-related macular degeneration. Ophthalmol. 2009;116(9):1755–1761. doi: 10.1016/j.ophtha.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Fontaine O, Olivier S, Cordahi G, et al. The effect of intravitreal injection of bevacizumab on retinal blood flow in patients with neovascular macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(10):7400–7405. doi: 10.1167/iovs.10-6646. [DOI] [PubMed] [Google Scholar]
- 38.Yamazaki T, Koizumi H, Yamagishi T, et al. Subfoveal choroidal thickness after ranimizumab therapy for neovascular age-related macular degeneration: 12- months results. Ophthalmology. 2012;119(8):1621–1627. doi: 10.1016/j.ophtha.2012.02.022. [DOI] [PubMed] [Google Scholar]