Abstract
Despite the availability of several therapies for rheumatoid arthritis (RA) that target the immune system, a large number of RA patients fail to achieve remission. Joint-lining cells, called fibroblast-like synoviocytes (FLS), become activated during RA and mediate joint inflammation and destruction of cartilage and bone. We identify RPTPσ, a transmembrane tyrosine phosphatase, as a therapeutic target for FLS-directed therapy. RPTPσ is reciprocally regulated by interactions with chondroitin sulfate or heparan sulfate containing extracellular proteoglycans in a mechanism called the proteoglycan switch. We show that the proteoglycan switch regulates FLS function. Incubation of FLS with a proteoglycan-binding RPTPσ decoy protein inhibited cell invasiveness and attachment to cartilage by disrupting a constitutive interaction between RPTPσ and the heparan sulfate proteoglycan syndecan-4. RPTPσ mediated the effect of proteoglycans on FLS signaling by regulating the phosphorylation and cytoskeletal localization of ezrin. Furthermore, administration of the RPTPσ decoy protein ameliorated in vivo human FLS invasiveness and arthritis severity in the K/BxN serum transfer model of RA. Our data demonstrate that FLS are regulated by an RPTPσ-dependent proteoglycan switch in vivo, which can be targeted for RA therapy. We envision that therapies targeting the proteoglycan switch or its intracellular pathway in FLS could be effective as a monotherapy or in combination with currently available immune-targeted agents to improve control of disease activity in RA patients.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease that ultimately results in joint destruction, and although there has been much recent progress in therapies for RA, many patients still fail to achieve remission. There are currently no treatments in the clinic that target fibroblast-like synoviocytes (FLS), which are key orchestrators in RA pathogenesis. FLS are specialized synovial lining cells that secrete synovial fluid and extracellular matrix (ECM) and provide structure to the joint. In RA, FLS mediate joint destruction by invading cartilage and promoting inflammation and bone erosion (1).
The behavior of FLS is regulated by several intracellular pathways involving protein tyrosine phosphorylation (2). Although protein tyrosine phosphatases (PTPs) are important regulators of signaling, they remain uncharacterized in FLS. We previously reported that several PTPs are highly expressed in FLS (3). In the attempt to ascertain if any of these PTPs regulate FLS pathogenic behavior in RA, we assessed FLS from arthritic mice and identified a transmembrane PTP belonging to the R2A subclass (4), called RPTPσ (gene Ptprs), as a highly expressed phosphatase in arthritic FLS.
In neurons, engagement of RPTPσ N-terminal extracellular immunoglobulin-like domains 1 and 2 (Ig1&2) by heparan sulfate (HS) or chondroitin sulfate (CS) glycosaminoglycan (GAG) moieties of various proteoglycans (PGs) controls axonal extension (5, 6). The interaction of RPTPσ Ig1&2 with HS-containing PG induces RPTPσ oligomerization and functional inactivation, which promotes axonal extension. On the other hand, CS-containing PG can compete with HS-containing PG for binding to the same RPTPσ Ig1&2 domains, de-clustering RPTPσ and inhibiting axonal extension (6). This mechanism has been termed the “PG switch” (6), and it mediates inhibition of axonal growth through CS-rich repair tissue after spinal cord injuries. Inhibition of RPTPσ through in vivo short hairpin RNA or a cell-permeable peptide able to induce phosphatase oligomerization can induce neuronal regeneration in models of spinal cord contusion (7, 8). It is unclear whether the PG switch depends on RPTPσ phosphatase activity and what intracellular signaling pathways mediate its actions. It is also unknown whether the PG switch exists in tissues outside the central nervous system.
The joint is composed of highly PG-rich tissue, and CS is the predominant GAG in cartilage (9). On the other hand, HS-containing PGs are primarily located on cell surfaces where they mediate interaction between cells and surrounding ECM. In FLS, the HS PG syndecan-4 is required for the attachment of FLS to cartilage (10), an important pathogenic FLS behavior (1). However, the regulation of FLS intracellular signaling by PG has not been addressed.
Here, we found that RPTPσ expression is up-regulated during arthritis progression, and that the PG switch is active in FLS. Manipulation of the PG switch using exogenous CS or recombinant PG-binding decoy RPTPσ protein inhibited FLS invasion, migration, and attachment to cartilage. We identified the intracellular mechanism of action of the PG switch as the dephosphorylation of the RPTPσ substrate ezrin, which disrupts its cytoskeletal localization during migration. Finally, we show that RPTPσ Ig1&2 treatment has therapeutic potential for arthritis in vivo by inhibiting attachment of FLS to cartilage and decreasing disease severity.
RESULTS
RPTPσ is selectively expressed on FLS in arthritic synovium
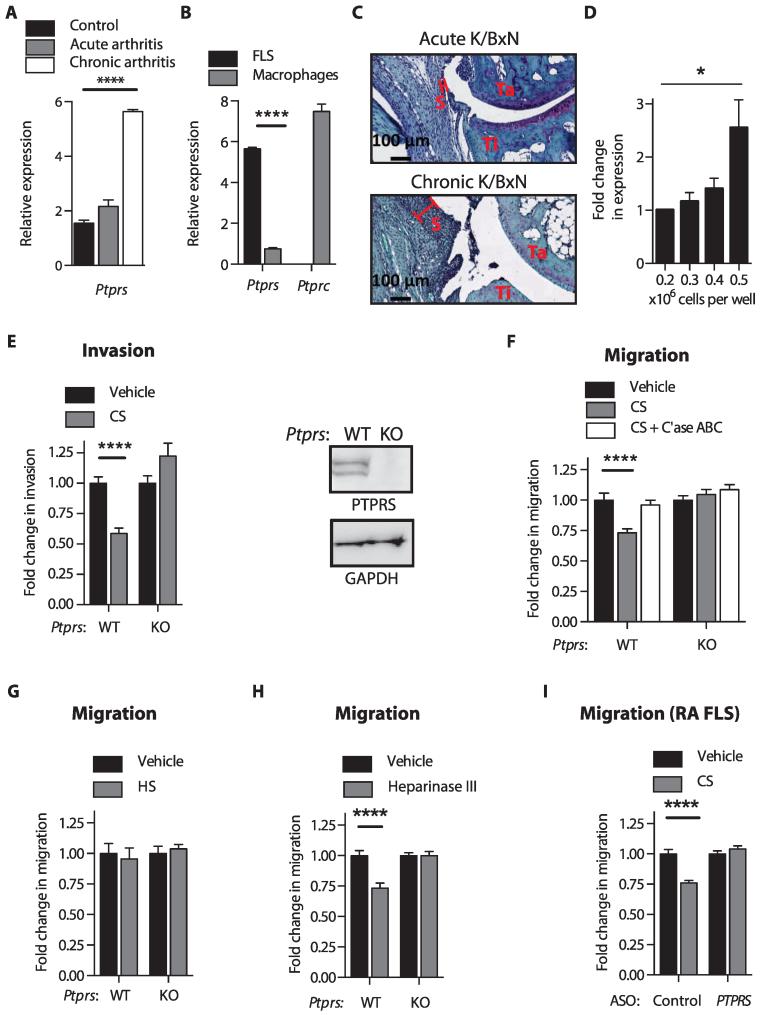
Several PTPs are highly expressed in FLS (3). In the attempt to identify if any of these PTPs regulate the pathologic behavior of FLS in RA, we assessed fluorescence activated cell sorting–isolated FLS from mice subjected to passive transfer of K/BxN serum (11)—an FLS-dependent model of RA (1)—and discovered that a transmembrane PTP called RPTPσ (gene Ptprs) was induced during arthritis progression (Fig. 1A and table S1). In comparison, RPTPσ expression was minimal in synovial macrophages from the same mice (Fig. 1B and table S1), supporting previous reports that RPTPσ is almost absent in immune cells (12). To determine what induces RPTPσ expression during chronic arthritis, we considered the formation of the FLS-rich pannus during chronic arthritis (13). As shown in Fig. 1C, pannus formation is much more pronounced during chronic K/BxN arthritis compared to acute K/BxN arthritis. We therefore hypothesized that pannus formation in chronic arthritis could increase Ptprs expression due to increased cell density of FLS, as was previously described in 3T3 cells (14). Figure 1D and table S1 shows that Ptprs expression increases with increasing cell density of cultured FLS, with an average ~2.5-fold increase in expression in densely crowded cells compared to subconfluent cells. Thus, RPTPσ is a transmembrane PTP highly expressed in FLS and is induced in the arthritic synovial lining.
Fig. 1. RPTPσ-dependent PG switch controls FLS invasiveness and migration.
(A) Ptprs expression in freshly sorted FLS from joints of nonarthritic mice or mice with K/BxN serum–induced arthritis. (B) Ptprs and Ptprc expression in freshly sorted FLS and synovial macrophages from mice with chronic K/BxN arthritis. (A and B) Mean ± SEM of expression relative to Polr2a; ****P < 0.0001, unpaired t test and Welch’s correction. (C) Pathology of safranin O–stained ankles from mice on day 14 of acute, or day 24 of chronic, K/BxN arthritis. Representative of three ankles per group; S, synovial lining; Ta, talus; Ti, tibia. (D) Ptprs expression in mouse FLS cultured at 0.2 × 106 (subconfluent) to 0.5 × 106 (maximum density) cells per well of a six-well plate. Mean ± SEM of expression relative to Polr2a (n = 3; *P = 0.0418, two-tailed unpaired t test). (E) (Left) Invasion of wild-type (WT) or Ptprs KO FLS through Matrigel in response to 5% fetal bovine serum (FBS) in the presence or absence of CS (100 μg/ml). (Right) Western blot of RPTPσ expression in Ptprs WT and KO FLS. (F) WT or Ptprs KO FLS migrated through Transwells in response to 5% FBS in the presence or absence of CS with or without chondroitinase (C’ase) ABC treatment. (G and H) Transwell migration of WT or Ptprs KO FLS in response to 5% FBS (G) in the presence or absence of HS (100 μg/ml) or (H) with or without pretreatment with 10 mU of heparinase III. (I) RA FLS were treated for 7 days with cell-permeable antisense oligonucleotides (ASO) to knock down PTPRS or control ASO and allowed to migrate through Transwells in response to 5% FBS in the presence of CS (100 μg/ml) or vehicle. (E to I) Mean ± SEM fold change of invasion or migration relative to the vehicle-treated cells from the same experiment is shown. Data are from three independent experiments (n = 45 fields; ****P < 0.0001, Mann-Whitney).
The RPTPσ-dependent PG switch is functional in FLS and regulates FLS invasiveness and migration
We reported that RPTPσ is highly expressed in human RA FLS (3). Further investigations in FLS from osteoarthritis (OA) patients showed that RPTPσ is similarly highly expressed in OA FLS (fig. S1 and table S2). Because RPTPσ is induced during arthritis and PGs are one of the main components of the joint ECM, we sought to determine whether the RPTPσ-dependent PG switch exists in FLS and if it regulates FLS pathogenic behavior. Serum-induced FLS invasiveness through Matrigel-coated Transwell chambers (Fig. 1E) and migration through uncoated Transwell chambers (Fig. 1F) were decreased in the presence of exogenous cartilage-derived CS, and these phenomena were dependent on RPTPσ expression. On the other hand, in contrast to exogenous CS, treatment with exogenous HS did not affect FLS migration (Fig. 1G). Because CS competes with HS for binding to RPTPσ Ig1&2 domains, we hypothesized that the effect of CS on FLS behavior is due to disruption of a constitutive interaction between RPTPσ and cell surface HS. Removal of HS, by either heparinase III digestion or knockdown of Ext1, a crucial component of the HS biosynthetic pathway, from FLS was sufficient to induce RPTPσ-dependent inhibition of serum-induced cell migration (Fig. 1H and fig. S2). These results demonstrate that the PG switch exists in mouse FLS, and we then wished to determine whether this occurs in human FLS. Indeed, treatment of primary FLS from RA patients (RA FLS) with CS inhibited migration in an RPTPσ-dependent manner (Fig. 1I and fig. S3).
Decoy RPTPσ Ig1&2 competes for FLS surface HS and regulates FLS invasiveness, migration, and attachment to cartilage
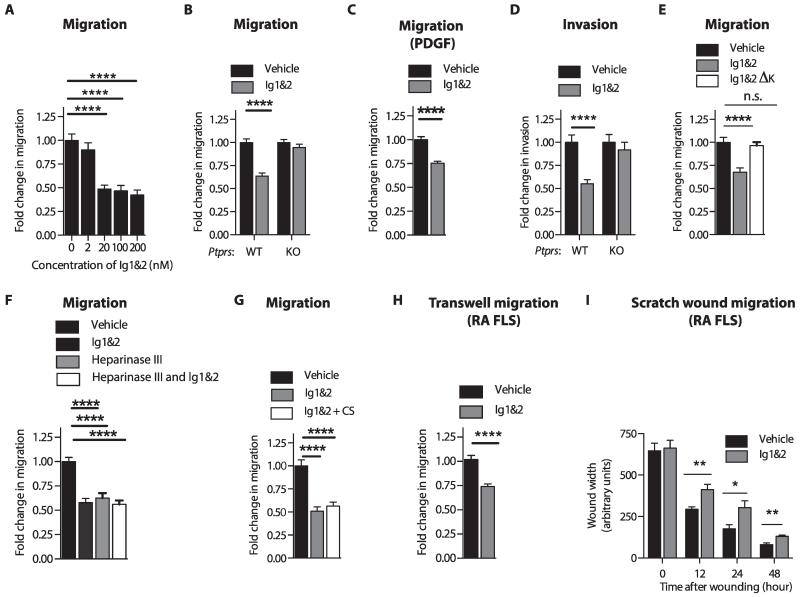
To test if disrupting the interaction between RPTPσ and HS replicates the effect of CS on FLS migration, we incubated FLS with various concentrations of PG-binding RPTPσ Ig1&2 (herein called “Ig1&2”) recombinant fragment during migration (6). Ig1&2 inhibited FLS migration starting at a 20 nM concentration, which is compatible with the KD (dissociation constant) ≈ 10 to 20 nM between the Ig1&2 domain and HS (6). Increasing the concentration of Ig1&2 beyond 20 nM did not further inhibit FLS migration (Fig. 2A). We then used 20 nM Ig1&2 to determine its effect on FLS migration and invasiveness. Treatment of FLS with Ig1&2 inhibited migration of FLS in response to serum and platelet-derived growth factor (PDGF), a prominent growth factor in the synovium of RA patients (Fig. 2, B and C), as well as invasiveness of FLS (Fig. 2D), in an RPTPσ-dependent manner. Furthermore, Ig1&2 ΔK, a non-GAG binding mutant form of Ig1&2, in which lysine residues K67, K68, K70, and K71 have been mutated to alanine residues (6), was not able to affect migration of FLS (Fig. 2E), confirming that the effect of Ig1&2 is through its binding to HS. Ig1&2 treatment did not affect FLS survival (fig. S4 and table S3) or response to cytokine stimulation (fig. S5 and table S4); therefore, we determined that the effect of Ig1&2 is selective for migration and invasiveness of FLS. Treatment with Ig1&2 together with heparinase III or CS did not lead to additional inhibition of FLS migration (Fig. 2, F and G). We conclude that constitutive interaction between RPTPσ and HS-containing PG on the cell surface promotes PDGF-induced FLS invasiveness and migration. This interaction can be abrogated by decoy Ig1&2 or by CS, which effectively competes with endogenous HS for binding to the Ig1&2 domains of RPTPσ. Ig1&2 was also effective in human FLS, because treatment of RA FLS with Ig1&2 also caused inhibition of migration in Transwell assays (Fig. 2H and table S5) as well as in scratch wound assays (Fig. 2I).
Fig. 2. RPTPσ Ig1&2 inhibits FLS migration and invasiveness in an RPTPσ-dependent manner.
(A) FLS migrated through Transwells in response to 5% FBS in the presence of the indicated concentrations of Ig1&2. (B and C) Migration of WT (B and C) or Ptprs KO FLS (B) through Transwells in response to (B) 5% FBS or (C) PDGF-BB (50 ng/ml) in the presence of 20 nM Ig1&2 or vehicle. (D) Invasion of WT or Ptprs KO FLS through Matrigel in response to 5% FBS in the presence of 20 nM Ig1&2 or vehicle. (E) Migration of FLS through Transwells in response to 5% FBS in the presence of vehicle or 20 nM Ig1&2 or non-GAG binding Ig1&2 ΔK mutant. n.s., not significant. (F and G) FLS migrated through Transwells in response to 5% FBS in the presence of (F) vehicle or 20 nM Ig1&2, with or without 10 mU of heparinase III pretreatment, or (G) vehicle, 20 nM Ig1&2, CS (100 μg/ml), or both Ig1&2 and CS. (H) RA FLS migrated through Transwells in response to 5% FBS in the presence of vehicle or 20 nM Ig1&2. (I) RA FLS monolayers were serum-starved before scratch-wounding and stimulated with 10% FBS in the presence of 20 nM Ig1&2 or vehicle. Wound width was measured at three points at the indicated times. (A to H) Mean ± SEM fold change of invasion or migration relative to vehicle-treated cells. Data are from three independent experiments (n = 45 fields; ****P < 0.0001, Mann-Whitney). (I) Mean ± SEM wound width from two independent experiments (n = 6 fields; 12 hours, **P = 0.0087; 24 hours, *P = 0.0411; 48 hours, **P = 0.0043; Mann-Whitney).
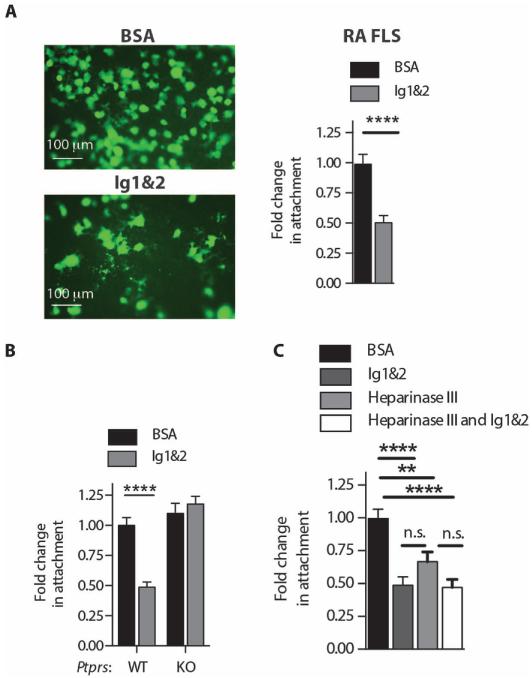
Attachment of RA FLS to cartilage correlates with cartilage damage in RA (1) and is dependent on surface HS expression on FLS as well as damage and subsequent PG exposure on cartilage upon treatment with inflammatory cytokines such as interleukin-1β (IL-1β) (10). Ig1&2 reduced mouse FLS attachment to ECM (fig. S6 and table S6) and significantly diminished the ability of RA FLS (Fig. 3A) and mouse FLS (Fig. 3B and table S7) to attach to IL-1β–pretreated cartilage. This effect of Ig1&2 on FLS cartilage attachment was dependent on RPTPσ expression (Fig. 3B). RPTPσ-deficient FLS displayed normal attachment to cartilage (Fig. 3B), suggesting that RPTPσ does not simply act as an adhesion molecule for cartilage-expressed PG. We confirmed that removal of surface HS from FLS by heparinase III digestion was sufficient to inhibit attachment to cartilage (10), whereas treatment with heparinase III and Ig1&2 did not lead to additive inhibition of cartilage attachment (Fig. 3C and table S7). We conclude that the interaction of RPTPσ with FLS cell surface HS modulates intracellular signaling to promote FLS attachment to cartilage.
Fig. 3. RPTPσ Ig1&2 decreases FLS attachment to cartilage in vitro in an RPTPσ-dependent manner.
(A) RA FLS were allowed to attach to cartilage explants in the presence of 20 nM bovine serum albumin (BSA) or Ig1&2. Representative images (left) and quantification of RA FLS (right) attached to cartilage explants in vitro in the presence of 20 nM Ig1&2 or BSA (n = 20 fields; ****P < 0.0001, Mann-Whitney). (B) Quantification of Ptprs WT or KO FLS attached to cartilage explants in vitro in the presence of 20 nM Ig1&2 or BSA (n = 15 fields; ****P < 0.0001, Mann-Whitney). (C) Quantification of Ptprs WT FLS attached to cartilage explants in vitro in the presence of 20 nM Ig1&2 or BSA with or without pretreatment with heparinase III (10 mU/ml) (n = 15 fields; **P = 0.0033, ****P < 0.0001, Mann-Whitney). (A to C) Mean ± SEM of fold change of number of cells attached to cartilage per 100× field relative to the BSA-treated cells from the same experiment is shown. Data are from three independent experiments.
Regulation of FLS migration by RPTPσ Ig1&2 is dependent on syndecan-4
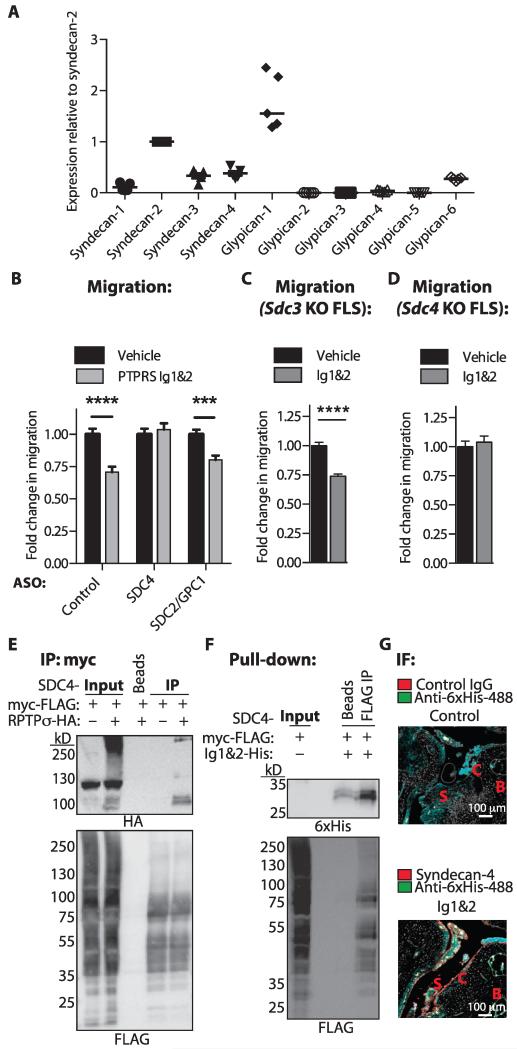
We next sought to determine which HS-containing PG on FLS is responsible for the effect of RPTPσ Ig1&2. We assessed the expression of cell surface HS PG syndecan (SDC) and glypican (GPC) family members in human RA FLS and found SDC2, SDC3, SDC4, and GPC1 to be the most highly expressed (Fig. 4A and table S8). We then designed cell-permeable ASO to knock down expression of these HS PG and identified an ASO able to knock down SDC2 and GPC1 (SDC2/GPC1 ASO) and a second ASO able to knock down SDC4 (SDC4 ASO; fig. S7 and table S9). Ig1&2 was able to inhibit the migration of RA FLS treated with control or SDC2/GPC1 ASO, but not of cells treated with the SDC4 ASO (Fig. 4B). We also assessed the effect of Ig1&2 on the migration of FLS from Sdc3 and Sdc4 knockout (KO) mice. Ig1&2 was able to inhibit migration of Sdc3 KO FLS (Fig. 4C), but not Sdc4 KO FLS (Fig. 4D), suggesting that syndecan-4 is the HS PG that physiologically regulates RPTPσ in FLS. This conclusion is in line with the known role of syndecan-4 as a promoter of FLS cartilage attachment (10).
Fig. 4. Regulation of FLS migration by RPTPσ Ig1&2 is dependent on syndecan-4.
(A) mRNA expression of syndecan and glypican family members in RA FLS. Mean ± SEM of expression normalized to POL2RA house-keeping gene and relative to SDC2; n = 5 RA lines. (B) RA FLS were treated with cell-permeable ASO to knock down SDC2/GPC1 or SDC4 or control ASO and allowed to migrate through Transwells in response to 5% FBS in the presence of 20 nM Ig1&2 or vehicle. Mean ± SEM of the fold change of migration relative to that of the same cells treated with vehicle. Data are representative of three independent experiments (n = 45 fields; ***P = 0.0002, ****P < 0.0001, Mann-Whitney). (C and D) Sdc3 (C) or Sdc4 (D) KO FLS were allowed to migrate through Transwells for 4 hours in response to 5% FBS in the presence of vehicle or 20 nM Ig&2. Mean ± SEM fold change of migration relative to that of the same cells treated with vehicle. Data are representative of two independent experiments in triplicate (n = 30 fields; ****P < 0.0001, Mann-Whitney). (E) Syndecan-4 was coexpressed with empty vector or RPTPσ in HEK293T cells. Representative Western blot of input and immunoprecipitation of syndecan-4 probed with anti-hemagglutinin (HA) and anti-FLAG antibodies (n = 3). (F) Syndecan-4 was expressed in HEK293T cells, immunoprecipitated, and then incubated with Ig1&2. Representative Western blot of input and pull-down of Ig1&2 probed with anti-6×His (Ig1&2) and anti-FLAG antibodies (representative of n = 2 independent experiments). (G) Ankle sections of mice with chronic arthritis were incubated with purified Ig1&2 for 2 hours and then stained for syndecan-4 and 6×His (Ig1&2) and imaged by confocal microscopy. Representative image of three ankles from three individual mice; S, synovial infiltrate; B, bone; C, cartilage.
We next performed immunoprecipitation studies to confirm that RPTPσ indeed interacts with syndecan-4. Figure 4E shows that syndecan-4 coprecipitated with RPTPσ when both proteins were overexpressed in human embryonic kidney (HEK) 293T cells. Further, to determine whether Ig1&2 interacts with syndecan-4, we immunoprecipitated overexpressed syndecan-4 from HEK293T cells and found that it was able to pull down purified recombinant Ig1&2 (Fig. 4F). We also studied the colocalization between Ig1&2 and endogenous syndecan-4 by incubating recombinant Ig1&2 with ankle sections from mice with chronic K/BxN arthritis and performing immunofluorescence microscopy. As shown in Fig. 4G, Ig1&2 colocalizes with syndecan-4 at the interface between pannus and cartilage. These results strongly support a model where RPTPσ, as well as the PG-binding decoy receptor fragment Ig1&2, interacts with syndecan-4.
RPTPσ catalytic activity is necessary for the PG switch
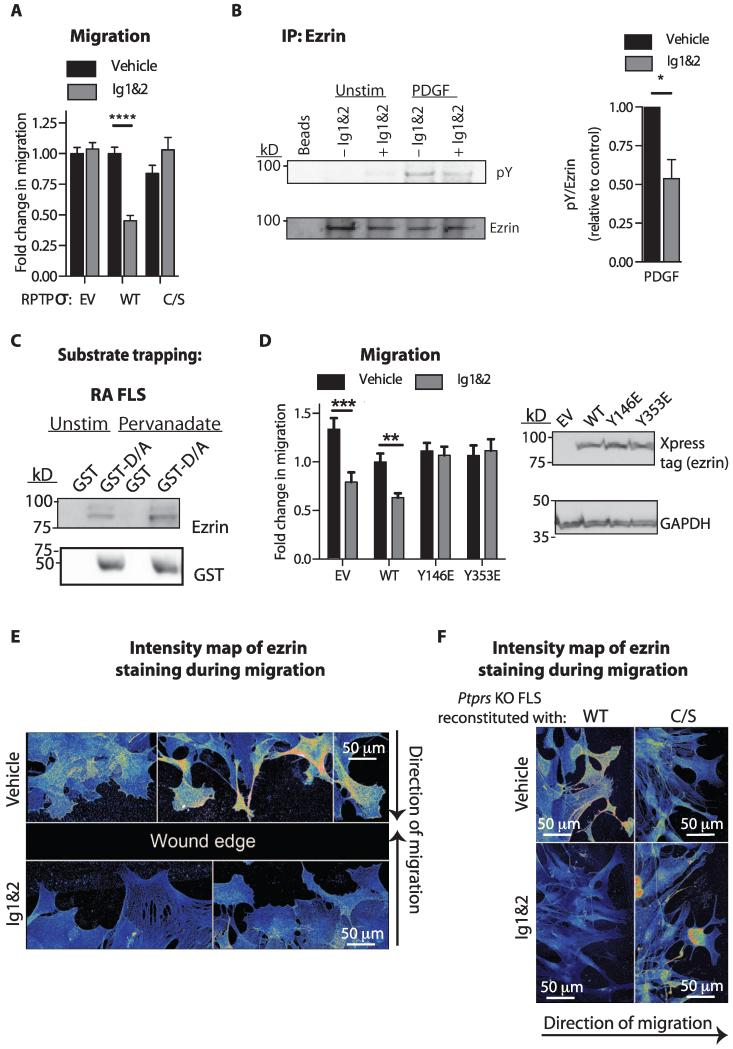
To assess whether the mechanism of action of the PG switch in FLS requires RPTPσ phosphatase activity, we reconstituted Ptprs KO FLS with full-length wild-type RPTPσ or an inactive C1548S/C1839S mutant (C/S) (fig. S8) and assessed the effect of Ig1&2 on FLS migration. Expression of wild-type, but not phosphatase-dead (C/S), RPTPσ restored sensitivity of Ptprs KO FLS to Ig1&2, providing the first direct evidence that RPTPσ catalytic activity is necessary for the PG switch (Fig. 5A).
Fig. 5. Ezrin is regulated by the PG switch through RPTPσ-mediated dephosphorylation.
(A) Transwell migration in response to 5% FBS of Ptprs KO FLS expressing WT or C/S RPTPσ or empty vector (EV) and treated with vehicle or Ig1&2. Mean ± SEM fold change of migration relative to vehicle-treated EV FLS from the same experiment (n = 45 fields; ****P < 0.0001, Mann-Whitney). Data are from three independent experiments. (B) FLS were unstimulated or stimulated with PDGF (50 ng/ml) for 15 min in the presence of 20 nM Ig1&2 or vehicle, and ezrin was immunoprecipitated from cell lysates. Representative Western blot (left) and densitometric analysis (right) of PDGF-induced ezrin phosphorylation from three independent experiments. Mean ± SEM (n = 3; *P = 0.0138, one-tailed unpaired t test). (C) Western blot of lysates from unstimulated or pervanadate-stimulated RA FLS incubated with glutathione S-transferase (GST) or GST-RPTPσ D1516A substrate-trapping mutant. (D) Transwell migration in response to 5% FBS of mouse FLS expressing WT or phosphomimetic Y146E or Y353E ezrin mutants or EV and treated with vehicle or Ig1&2. (Left) Mean ± SEM fold change of migration relative to the vehicle-treated EV sample from the same experiment (n = 30 fields; ****P < 0.0001, Mann-Whitney). (Right) Representative Western blot of lysates from FLS transfected with EV or WT or phosphomimetic mutant ezrin and immunoblotted for Xpress tag or glyceraldehyde phosphate dehydrogenase (GAPDH) (loading control). Data are from three independent experiments. (E and F) Monolayers of (E) WT FLS or (F) Ptprs KO FLS expressing WT or C/S RPTPσ were scratch-wounded and stimulated with PDGF for 8 hours in the presence of vehicle or 20 nM Ig1&2. Pseudocolored map of intensity of ezrin staining in cells migrating toward the wound edge (blue, low; red, high). Representative fields are from three independent experiments.
Ezrin is a substrate of RPTPσ in FLS, and its phosphorylation and cytoskeletal localization is regulated by the PG switch
We next endeavored to identify the substrate dephosphorylated by RPTPσ in response to the PG switch. We prioritized cytoskeleton-associated proteins whose tyrosine phosphorylation promotes cell adhesion and migration, and thus examined the effect of Ig1&2 on phosphorylation of ezrin, focal adhesion kinase (FAK), paxillin, and p130Cas. We discovered that phosphorylation of ezrin was decreased in PDGF-stimulated FLS treated with Ig1&2 (Fig. 5B and table S10), whereas phosphorylation of FAK, paxillin, and p130Cas was unaffected (fig. S9). A recent report also identified ezrin as an RPTPσ substrate in colon epithelial cells (15). To confirm that RPTPσ directly interacts with ezrin in FLS, we performed a substrate-trapping assay using an RPTPσ D1516A mutant catalytic domain (D/A) and found that it precipitated ezrin from RA FLS lysates (Fig. 5C).
We next hypothesized that because ezrin is a substrate of RPTPσ, tyrosine phosphorylation of ezrin was necessary for the effect of Ig1&2 on FLS migration. Mouse FLS were transfected with wild-type ezrin or phosphomimetic ezrin mutants, which contain tyrosine–to–glutamic acid mutations on residues 146 and 353, which are the mouse homologues of the ezrin tyrosines targeted by human RPTPσ in colonic epithelial cells (15). Phosphomimetic mutants represent constitutively phosphorylated tyrosine residues that are insensitive to dephosphorylation. Figure 5D shows that the migration of FLS overexpressing phosphomimetic ezrin mutants with mutations at residues 146 and 353 was unaffected by Ig1&2 treatment. This demonstrates that the ability of Ig1&2 to reduce migration of mouse FLS is dependent on ezrin tyrosine dephosphorylation.
Tyrosine phosphorylation of ezrin regulates cell migration through its association with actin (16). In PDGF-stimulated migrating cells, ezrin colocalized with the actin cytoskeleton (fig. S10) and displayed accumulation at the leading edge (Fig. 5E). In contrast, upon Ig1&2 treatment, ezrin displayed reduced colocalization with the actin cytoskeleton and no enrichment at the leading migration edge (Fig. 5E and fig. S10). This effect was dependent on RPTPσ catalytic activity, because Ptprs KO FLS reconstituted with wild-type, but not phosphatase-dead (C/S), RPTPσ displayed disruption of ezrin localization upon Ig1&2 treatment (Fig. 5F).
We conclude that triggering the PG switch results in RPTPσ-mediated dephosphorylation of ezrin, which disrupts ezrin localization at the cytoskeleton during migration. Our data support previous reports that ezrin is necessary for RA FLS invasiveness (17, 18).
The phosphorylation of β-catenin, which is a known substrate of RPTPσ in other tissues (19) and regulates migration of other cell types (20), was unaffected by Ig1&2 treatment of FLS (fig. S11). Cadherin-11, a critical FLS migration regulator (21, 22), did not mediate the effect of the PG switch, and its functions were unaffected by incubation of FLS with Ig&2 (fig. S11). Treatment with Ig1&2 did not alter PDGF receptor (PDGFR) expression or activation of Akt or mitogen-activated protein kinases (MAPKs) downstream of the PDGFR (fig. S12), suggesting that the PG switch selectively modulates the ezrin pathway in PDGF-stimulated FLS.
Decoy RPTPσ Ig1&2 is active in vivo and decreases arthritis severity
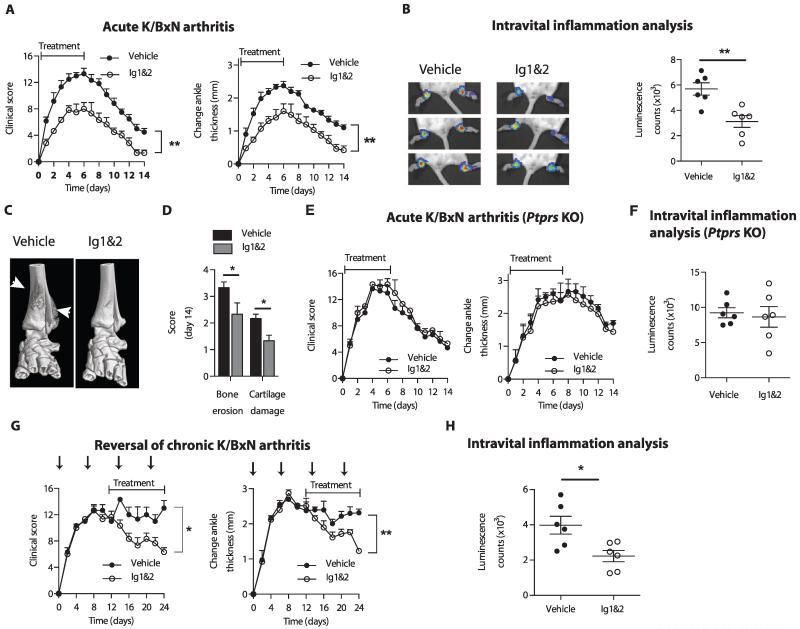
We next examined whether the PG switch regulates FLS behavior in vivo. We assessed the effect of Ig1&2 on arthritis development and severity in mice injected with K/BxN serum. In this model, FLS have a demonstrated pathogenic role (1). Treatment of mice with Ig1&2 in parallel to arthritis induction resulted in decreased arthritis severity compared to control-treated mice as assessed by clinical score and ankle thickness (Fig. 6A and table S11), intravital joint inflammation (Fig. 6B and table S11), microcomputed tomography (microCT) of bone (Fig. 6C), and histological analysis of cartilage damage and bone erosion (Fig. 6D and table S11). As expected, Ig1&2 had no effect on arthritis severity of Ptprs KO mice (Fig. 6, E and F, and table S11), which developed disease similar to wild-type littermates (fig. S13 and table S12). Ig1&2 treatment also reversed severity of established arthritis (Fig. 6, G and H, and table S11). Together, these results show that interference with the PG switch in vivo has an RPTPσ-dependent therapeutic effect on arthritis.
Fig. 6. RPTPσ Ig1&2 administration decreases arthritis severity in an RPTPσ-dependent manner.
(A to D) BALB/c mice were induced with acute K/BxN arthritis and treated with vehicle or 0.5 mg of Ig1&2 intravenously daily (days 0 to 6). (A) Clinical score (left) and change in ankle thickness (right) (n = 6; **P = 0.0022, Mann-Whitney). (B) Ankle luminescence (left) and luminescence counts (right) of mice treated with inflammation probe on day 4 of arthritis (n = 6; **P = 0.0043, Mann-Whitney). (C) Three-dimensional microCT of ankles on day 14. White arrows indicate bone erosion or reactive bone deposition. (D) Histopathological score of bone erosion and cartilage damage of ankle sections from mice in (A) on day 14 (n = 6; bone erosion *P = 0.0299, cartilage damage *P = 0.0056, two-tailed unpaired t test). (E and F) Ptprs KO mice were induced with arthritis as in (A). (E) Clinical score (left) and change in ankle thickness (right); n = 3 per group. (F) Mice from (E) were injected with RediJect Inflammation Probe on day 4 of arthritis induction. Mean ± SEM of luminescence in each ankle joint measured. n = 3 mice per treatment. (G and H) BALB/c mice were induced with chronic K/BxN arthritis (serum administration shown by arrows) and treated daily with vehicle or 0.5 mg of Ig1&2 intravenously (days 12 to 24). (G) Clinical score and change in ankle thickness (n = 3; *P = 0.0135, **P = 0.0058, two-tailed unpaired t test). (H) Mice were injected with RediJect Inflammation Probe on day 20 of arthritis, and luminescence in each ankle joint was measured. Graphs represent mean ± SEM (n = 6; *P = 0.0260, Mann-Whitney).
Decoy RPTPσ Ig1&2 reduces FLS attachment to cartilage in vivo
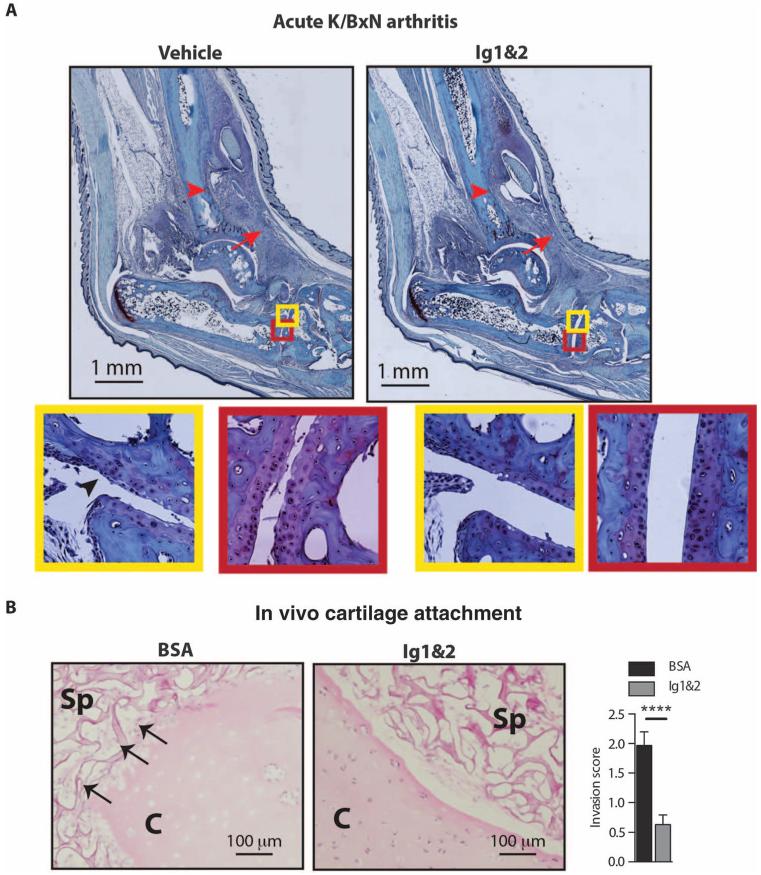
FLS critically mediate cartilage damage in the K/BxN model (1). In line with our observation that Ig1&2 inhibits FLS migration and attachment to cartilage ex vivo (Fig. 3, A and B), the reduced cartilage damage in Ig1&2-treated mice (Figs. 6D and 7A, red insets) correlated with the reduced FLS crawling across the surface of cartilage in arthritic joints (Fig. 7A, yellow insets). To further confirm that Ig1&2 inhibits the ability of FLS to damage cartilage in vivo, we used the severe combined immunodeficiency (SCID) model of FLS cartilage coengraftment (23). Ig1&2 administration markedly decreased RA FLS invasion of cartilage in this model (Fig. 7B).
Fig. 7. RPTPσ Ig1&2 decreases FLS attachment to cartilage in vivo.
(A) BALB/c mice were induced with acute K/BxN arthritis and treated with vehicle or 0.5 mg of Ig1&2 intravenously daily (days 0 to 6). Pathology of safranin O–stained ankle sections from mice on day 14 of arthritis. Arrow indicates inflammation; arrowhead indicates bone erosion. FLS crawling over cartilage (arrow) (yellow insets) and reduced chondrocyte layer (red insets) in (left) vehicle-treated versus (right) Ig1&2-treated mice. Cartilage PG content (red insets) assessed by safranin O staining. (B) SCID mice were implanted subcutaneously with cartilage explants and RA FLS in surgical sponge and treated with BSA or Ig1&2 intravenously for 35 days. (Left) Representative images showing cartilage (C) and sponge (Sp). Arrows indicate FLS attaching to and invading cartilage. (Right) Mean ± SEM invasion scores; n = 3 mice per group (n = 30 fields; ****P < 0.0001, Mann-Whitney).
DISCUSSION
A major unmet medical need in RA is that despite the availability of several disease-modifying agents, a substantial fraction of patients fails to achieve remission. A proposed solution to this problem is the development of therapies that target FLS, a nonimmunological joint-resident cell type that drives RA pathogenesis (1). FLS-associated molecules that could be targeted for adjuvant therapy for RA are highly sought after; however, so far, only one such candidate, the transmembrane adhesion molecule cadherin-11, has been identified (1). We identified another therapeutic target for FLS-directed therapy in the R2A subclass PG-binding RPTPσ tyrosine phosphatase, an enzyme that has been reported to be almost absent in immune cells (12). Here, we show that the expression of RPTPσ is induced in FLS during arthritis, and that the RPTPσ-mediated PG switch selectively regulates FLS pathogenic behaviors. We also developed an approach to pharmacologically manipulate the phosphatase through its extracellular domain, and we show that targeting RPTPσ ameliorates established arthritis in mice.
Two additional members of the R2A subclass of PTPs, RPTPΔ and RPTP-LAR, can also bind PG (6, 24); however, their expression is minimal in RA FLS (3) and undetectable in FLS from arthritic mice (fig. S14 and table S13). Along with the ineffectiveness of the PG switch in FLS lacking expression of RPTPσ from mice and RA patients, we conclude that the PG switch is specifically mediated in FLS by RPTPσ.
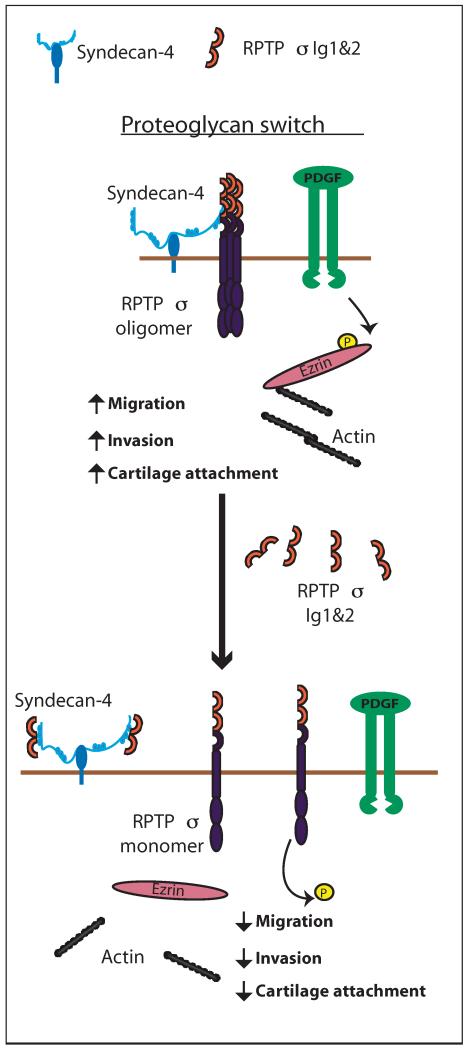
Dissociation of RPTPσ from surface HS-containing PG using CS or with Ig1&2, a recombinant HS-binding decoy fragment of RPTPσ, is sufficient to “flip” the PG switch to the “on” state, leading to functional activation of the phosphatase and inhibition of migration, invasion, and cartilage attachment. Our data provide mechanistic support to previously proposed models where CS releases RPTPσ from a constitutive interaction with HS-containing PG on neuronal surfaces, thus declustering and functionally activating the enzyme (5, 6, 25, 26). However, a notable difference in the physiology of the PG switch between neurons and FLS is that addition of exogenous HS does not enhance FLS migration and invasion. A possible explanation is that in the absence of CS, most RPTPσ is bound to HS on the surface of FLS and kept in a functionally inactive state (Fig. 8).
Fig. 8. Working model for the RPTPσ-dependent PG switch in FLS.
RPTPσ interacts with the HS PG syndecan-4 on the surface of FLS and is maintained in an inactive oligomeric state. Tyrosine phosphorylation of ezrin downstream of the PDGFR promotes its localization to the actin cytoskeleton, enabling cell migration and invasion. Disruption of the RPTPσ-HS interaction by the HS-binding decoy RPTPσ Ig1&2 fragment displaces RPTPσ from HS. This leads to dephosphorylation of ezrin and disassociation of ezrin from the actin cytoskeleton, decreasing FLS migration, invasion, and attachment to cartilage.
In addition to cell migration and invasion, another pathogenic FLS phenotype that is prominently regulated by the PG switch is cartilage attachment. It is known that in FLS, the HS PG syndecan-4 is required for the attachment of FLS to cartilage (10). Our study also points to syndecan-4 as the main HS PG responsible for the physiological regulation of RPTPσ function in FLS. Thus, we speculate that syndecan-4 on the surface of FLS promotes cartilage attachment through oligomerization and inactivation of RPTPσ. The fact that inactivation of RPTPσ per se does not reduce the ability of FLS to attach to cartilage rules out that RPTPσ simply acts as a cartilage adhesion molecule and supports our hypothesis that the PG switch regulates cell adhesiveness through RPTPσ-mediated regulation of intracellular signaling. Further experimentation is necessary to elucidate the downstream molecular mediators of FLS adhesion that are regulated by the PG switch.
The intracellular mechanism behind the PG switch has not yet been addressed. We show that the switch is dependent on the phosphatase activity of RPTPσ and that in FLS, RPTPσ couples ECM composition with intracellular signaling through PG switch–regulated dephosphorylation of ezrin (Fig. 8). Ezrin is a cytoskeleton-associated protein that is involved in invasiveness of RA FLS (18). Tyrosine phosphorylation of ezrin has been shown to regulate the migration and invasion of various cell types through the modulation of its localization and association with actin (16). It remains to be elucidated whether the functional activation of RPTPσ that occurs as a result of flipping the PG switch on the surface of FLS depends on catalytic activation of the phosphatase, increased access of RPTPσ to ezrin after monomerization, or on additional unknown molecular mechanism(s).
We find that flipping the PG switch in vivo with Ig1&2 decreases arthritis severity and cartilage damage in the K/BxN serum transfer model of arthritis. Administration of Ig1&2 was also able to reverse established arthritis in a model of chronic K/BxN serum transfer arthritis. Lack of efficacy of Ig1&2 in Ptprs KO mice provides strong evidence that Ig1&2 exerts its antiarthritic effect by targeting RPTPσ. Evidence of reduced cartilage damage and FLS crawling onto cartilage and decreased invasion of RA FLS into cartilage implants in mice treated with Ig1&2 correlates well with the above-mentioned in vitro evidence and strongly points to an FLS-specific effect of Ig1&2 in vivo.
Arthritis that develops in the passive K/BxN model depends on innate immunity (11) and FLS (1). Although an effect of Ig1&2 on innate immune cells cannot be entirely ruled out, it appears to be unlikely based on the fact that RPTPσ is minimally or not expressed in synovial macrophages (Fig. 1B) and reportedly in other immune cells (12). Also, incubation with Ig1&2 had no effect on macrophage activation (fig. S15 and table S14).
Because of the known RA-protecting action of spinal cord lesions/denervation in humans and in the K/BxN model (27), the established role of RPTPσ as an inhibitor of axonal growth raises the question whether Ig1&2 decreases severity of arthritis in mice by reducing pro-arthritic neural input. Indeed, Ig1&2 is unlikely to penetrate the blood-brain barrier after parenteral injection but could still affect peripheral neuroautonomic or sensory fibers. However, inhibition of sympathetic or parasympathetic input does not affect arthritis severity in the K/BxN model (fig. S16 and table S15) (27). Deficiency of the transient receptor potential vanilloid cation channel (Trpv1 KO), a proinflammatory receptor on sensory nerve fibers (28), does not affect disease severity in this model as well (27), and arthritic Trpv1 KO mice were sensitive to Ig1&2 treatment (fig. S16 and table S15). Because arthritis protection by denervation in the K/BxN model is mediated by alteration in endothelial permeability (27), we also assessed whether administration of Ig1&2 reduces early vascular permeabilization caused by injection of K/BxN serum; however, we did not see any difference between Ig1&2-treated and untreated mice (fig. S17 and table S16). We conclude that it is unlikely that the arthritis protection by Ig1&2 in the K/BxN model is directly or indirectly mediated by actions at the central or peripheral nervous system level or on vascular permeability.
There are interesting parallels between the phenotype of mice treated with Ig1&2 and that of mice that are deficient in, or treated with inhibitors of, cadherin-11, an FLS-selective adhesion molecule and a proposed drug target for therapy of RA (1). Lack or inactivation of cadherin-11 similarly impairs FLS migration and invasiveness ex vivo, and prevents and reverses arthritis in the K/BxN serum transfer model. Cadherin-11 (Cdh11) KO mice display a similar reduction in cartilage damage, which correlates with reduced crawling of FLS onto cartilage (1). We show that the pathways controlled by cadherin-11 and the PG switch in FLS are nonoverlapping. Overall, the finding that the manipulation of two independent pathways controlling FLS migration and adhesion results in a similar reduction in severity of arthritis supports the idea that these are key phenotypes underlying the pathogenic action of FLS in RA. Recent findings that migration and adhesion pathways are hotspots of epigenetic modification in FLS from human RA patients lend further support to this model (29).
The joint is a PG-rich environment, yet little is known about how PGs affect intracellular signaling in joint-lining cells. Our study shows that RPTPσ mediates the action of extracellular PG on FLS functions through the PG switch (5, 6). The role of this mechanism in joint physiology remains to be fully understood. Because FLS do not attach well to intact cartilage [consisting primary of CS PG (9, 30)], and cartilage PG loss is necessary for FLS attachment (10, 23), we speculate that RPTPσ is one of the mediators of physiological inhibition of FLS attachment and invasion of cartilage in healthy joints. Therefore, we hypothesize that treatment of arthritic joints, where CS-mediated inhibition might be reduced following cartilage damage (31, 32), with Ig1&2 restores a physiological safety mechanism that deters FLS from damaging the joint. However, the observation that inactivation of RPTPσ in mice does not lead to joint pathology and does not influence K/BxN serum–induced arthritis suggests that additional mechanisms, perhaps mediated by expression of CS receptors NgRs (33) on FLS, can compensate for the loss of RPTPσ to avoid cartilage damage by FLS in physiological or pathological conditions.
It has been proposed that agents that selectively target FLS in RA without causing significant immune suppression could be combined with current disease-modifying antirheumatic drugs (DMARDs) to improve disease control and remission rates (2). In addition, recent studies of RA synovial pathology suggest that disease might be driven by FLS activation more in some patients than others, suggesting that FLS-targeted interventions could be effective as a monotherapy in the appropriate subset of patients (34).
Cadherin-11 is currently the only validated target for FLS-directed therapy for RA, and anti–cadherin-11 antibodies are in preclinical development as first-in-class agents for combination therapies for RA (2). Similar to anti–cadherin-11 antibodies, Ig1&2 was able to reverse established disease in an FLS-dependent model of RA. Also, like cadherin-11, RPTPσ displays minimal or no expression in immune cells (Fig. 1B and fig. S15) (12), suggesting that drugging RPTPσ is unlikely to result in immune suppression. Therefore, we propose that RPTPσ Ig1&2 or other biologics or small molecules able to selectively disrupt the interaction between RPTPσ and HS on the surface of FLS are attractive candidates for development of FLS-directed therapies to complement existing DMARDs for RA. The absolute conservation between human and mouse RPTPσ Ig1&2 increases confidence in the translational potential of our proposed approach for therapy for RA.
There are a number of limitations of our study. For example, the molecular events that lead to triggering of ezrin dephosphorylation by RPTPσ after activation of the extracellular PG switch remain to be addressed. Because syndecan-4 is known to oligomerize and trigger intracellular signaling (35), it is possible that syndecan-4–mediated signaling also contributes to the signal transduction of the PG switch in FLS. Additional limitations will need to be addressed before translation of our findings into the clinic is attempted. These include the need for preclinical studies characterizing the pharmacology of Ig1&2 and its efficacy in combination with available DMARDs in models of RA. Possible effects of Ig1&2 on immune cells and on other organs and systems will also need to be quantified in appropriate models to assess the therapeutic potential of Ig1&2 and its suitability for combination with other therapeutic agents.
MATERIALS AND METHODS
Study design
The objective of this study was to demonstrate that the RPTPσ-mediated PG switch regulates invasiveness and migration of FLS, and that it can be manipulated in vivo using a decoy RPTPσ Ig1&2 fragment to decrease cartilage destruction and arthritis in a mouse model. This was achieved through ex vivo FLS experiments and in vivo studies of arthritis development in Ig1&2-treated mice. Mice genetically manipulated to lack RPTPσ were used to demonstrate target specificity. For ex vivo experiments, FLS lines were acquired from multiple different human patients or from multiple littermate pairs of mice. For in vivo experiments, mice were randomly assigned to different treatments and blinding was achieved by scoring mice before reading their ear tag. Experimental endpoints for the acute K/BxN model of arthritis were determined to be at least 12 days after serum injection, when disease resolution is achieved; for the chronic K/BxN model, the endpoint was determined to be at least 10 days after disease plateau. Clinical endpoints were determined by Institutional Animal Care and Use Committee–approved protocols (#AP140-NB4-0610) at the La Jolla Institute for Allergy and Immunology (LJI) and were never reached throughout the experiments. The number of replicate measurements and mice is stated in each figure legend. Sample sizes were determined using pilot studies and power calculations on preliminary data to achieve statistical power of 0.90 (α = 0.05).
Statistical analysis
For comparison of treatment groups, Mann-Whitney and unpaired t tests were performed where appropriate as reported in the figure legends. All statistical analyses were performed using GraphPad Prism software (version 6.0). Statistical significance was achieved when P < 0.05.
Supplementary Material
Acknowledgments
We are grateful to the University of California, San Diego (UCSD) Clinical and Translational Research Institute Biorepository for the FLS lines; to E. Girardi (LJI) for advice and assistance with reagents; to I. Shaked (LJI) for the advice and assistance with pharmacological blockage of nerves; to C. Benoist (Harvard University) for KRN mice; to M. Brenner (Harvard University) for cadherin-11 KO FLS; and to J. Bowden (Aarhus University/UCSD) for assistance with statistical analysis.
Funding: This work was supported by LJI institutional funding (N.B.), NIH (AR066053 to N.B.; AR47825, AI070555, and UL1TR000100 to G.S.F.; and AR064202 to J.D.E.). K.M.D. was supported by a Canadian Institutes for Health Research Fellowship. S.M.S. was supported by a postdoctoral fellowship from the Juvenile Diabetes Research Foundation.
Footnotes
www.sciencetranslationalmedicine.org/cgi/content/full/7/288/288ra76/DC1
Materials and Methods
Fig. S1. Expression of PTPRS in OA and RA FLS.
Fig. S2. Reduction of surface HS inhibits migration of FLS in an RPTPσ-dependent manner.
Fig. S3. Knockdown of PTPRS in RA FLS.
Fig. S4. RPTPσ Ig1&2 does not affect FLS survival.
Fig. S5. RPTPσ Ig1&2 treatment does not affect FLS response to cytokine stimulation.
Fig. S6. RPTPσ Ig1&2 decreases cell attachment to fibronectin.
Fig. S7. Knockdown of SDC2/GPC1 and SDC4 in RA FLS.
Fig. S8. Reconstitution of Ptprs KO FLS with RPTPσ mutants.
Fig. S9. RPTPσ Ig1&2 does not affect paxillin, FAK, or p130Cas phosphorylation in FLS.
Fig. S10. RPTPσ Ig1&2 decreases colocalization of ezrin with the actin cytoskeleton during migration.
Fig. S11. RPTPσ Ig1&2 does not alter β-catenin or cadherin-11 phosphorylation and does not affect cadherin-11 functions in FLS.
Fig. S12. RPTPσ Ig1&2 does not affect PDGFR expression or downstream Akt/MAPK signaling in FLS.
Fig. S13. Ptprs deficiency does not affect severity of K/BxN acute serum transfer arthritis.
Fig. S14. Expression of R2A subclass of PTPs in FLS.
Fig. S15. RPTPσ Ig1&2 does not affect macrophage response to stimulation.
Fig. S16. Blockade of peripheral nervous system arms does not affect arthritis in the K/BxN model.
Fig. S17. RPTPσ Ig1&2 does not affect K/BxN serum–induced vascular permeabilization of peripheral joints.
Table S1. Source data for Fig. 1 (A, B, and D).
Table S2. Source data for fig. S1.
Table S3. Source data for fig. S4.
Table S4. Source data for fig. S5.
Table S5. Source data for Fig. 2H.
Table S6. Source data for fig. S6.
Table S7. Source data for Fig. 3 (B and C).
Table S8. Source data for Fig. 4A.
Table S9. Source data for fig. S7 (A and B).
Table S10. Source data for Fig. 5B.
Table S11. Source data for Fig. 6 (A to H).
Table S12. Source data for fig. S13A.
Table S13. Source data for fig. S14.
Table S14. Source data for fig. S15.
Table S15. Source data for fig. S16 (A to C).
Table S16. Source data for fig. S17.
Source Data 1. Full Western blots (Fig. 1E).
Source Data 2. Full Western blots (Fig. 4, E and F).
Source Data 3. Full Western blots (Fig. 5, B to D).
Source Data 4. Flow cytometry data (fig. S2A).
Source Data 5. Flow cytometry gating (fig. S4).
Source Data 6. Flow cytometry gating (fig. S8).
Source Data 7. Full Western blots (fig. S9).
Source Data 8. Full Western blots (fig. S11).
Source Data 9. Full Western blots (fig. S12).
Author contributions: K.M.D., S.M.S., C.S., M.N.D.S., W.B.K., E.C., C.H.C., N.M., R.L.-B., and D.L.B. contributed to acquisition of data. K.M.D., S.M.S., M.C., H.A.A., T.M., J.D.E., M.L.T., G.S.F., A.R.A., and N.B. contributed to study conception and design. K.M.D., S.M.S., C.S., M.N.D.S., W.B.K., B.B., C.F., M.C., R.L.S., T.M., J.D.E., M.L.T., G.S.F., A.R.A., and N.B. contributed to analysis and interpretation of data.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: LJI holds a pending patent “PTPRS and proteoglycans in autoimmune disease,” PCT/US2013/051723, with S.M.S. and N.B. named as inventors.
Supplementary Material can be found in the online version of this article at http://stm.sciencemag.org/content/suppl/2015/05/18/7.288.288ra76.DC1.html
REFERENCES AND NOTES
- 1.Lee DM, Kiener HP, Agarwal SK, Noss EH, Watts GF, Chisaka O, Takeichi M, Brenner MB. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315:1006–1010. doi: 10.1126/science.1137306. [DOI] [PubMed] [Google Scholar]
- 2.Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: Passive responders and imprinted aggressors. Nat. Rev. Rheumatol. 2013;9:24–33. doi: 10.1038/nrrheum.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanford SM, Maestre MF, Campbell AM, Bartok B, Kiosses WB, Boyle DL, Arnett HA, Mustelin T, Firestein GS, Bottini N. Protein tyrosine phosphatase expression profile of rheumatoid arthritis fibroblast-like synoviocytes: A novel role of SH2 domain–containing phosphatase 2 as a modulator of invasion and survival. Arthritis Rheum. 2013;65:1171–1180. doi: 10.1002/art.37872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPσ is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coles CH, Shen Y, Tenney AP, Siebold C, Sutton GC, Lu W, Gallagher JT, Jones EY, Flanagan JG, Aricescu AR. Proteoglycan-specific molecular switch for RPTPσ clustering and neuronal extension. Science. 2011;332:484–488. doi: 10.1126/science.1200840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou H-X, Li X-Y, Li F-Y, Liu C, Liang Z-P, Liu S, Zhang B, Wang T-Y, Chu T-C, Lu L, Ning G-Z, Kong X-H, Feng S-Q. Targeting RPTPσ with lentiviral shRNA promotes neurites outgrowth of cortical neurons and improves functional recovery in a rat spinal cord contusion model. Brain Res. 2014;1586:46–63. doi: 10.1016/j.brainres.2014.08.048. [DOI] [PubMed] [Google Scholar]
- 8.Lang BT, Cregg JM, DePaul MA, Tran AP, Xu K, Dyck SM, Madalena KM, Brown BP, Weng YL, Li S, Karimi-Abdolrezaee S, Busch SA, Shen Y, Silver J. Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature. 2014;518:404–408. doi: 10.1038/nature13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knudson CB, Knudson W. Cartilage proteoglycans. Semin. Cell Dev. Biol. 2001;12:69–78. doi: 10.1006/scdb.2000.0243. [DOI] [PubMed] [Google Scholar]
- 10.Korb-Pap A, Stratis A, Mühlenberg K, Niederreiter B, Hayer S, Echtermeyer F, Stange R, Zwerina J, Pap T, Pavenstädt H, Schett G, Smolen JS, Redlich K. Early structural changes in cartilage and bone are required for the attachment and invasion of inflamed synovial tissue during destructive inflammatory arthritis. Ann. Rheum. Dis. 2012;71:1004–1011. doi: 10.1136/annrheumdis-2011-200386. [DOI] [PubMed] [Google Scholar]
- 11.Kouskoff V, Korganow A-S, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 12.Arimura Y, Yagi J. Comprehensive expression profiles of genes for protein tyrosine phosphatases in immune cells. Sci. Signal. 2010;3:rs1. doi: 10.1126/scisignal.2000966. [DOI] [PubMed] [Google Scholar]
- 13.Müller-Ladner U, Ospelt C, Gay S, Distler O, Pap T. Cells of the synovium in rheumatoid arthritis. Synovial fibroblasts. Arthritis Res. Ther. 2007;9:223. doi: 10.1186/ar2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celler JW, Luo X, Gonez LJ, Böhmer FD. mRNA expression of two transmembrane protein tyrosine phosphatases is modulated by growth factors and growth arrest in 3T3 fibroblasts. Biochem. Biophys. Res. Commun. 1995;209:614–621. doi: 10.1006/bbrc.1995.1544. [DOI] [PubMed] [Google Scholar]
- 15.Murchie R, Guo C-H, Persaud A, Muise A, Rotin D. Protein tyrosine phosphatase σ targets apical junction complex proteins in the intestine and regulates epithelial permeability. Proc. Natl. Acad. Sci. U.S.A. 2014;111:693–698. doi: 10.1073/pnas.1315017111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mak H, Naba A, Varma S, Schick C, Day A, SenGupta SK, Arpin M, Elliott BE. Ezrin phosphorylation on tyrosine 477 regulates invasion and metastasis of breast cancer cells. BMC Cancer. 2012;12:82. doi: 10.1186/1471-2407-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh K, Colmegna I, He X, Weyand CM, Goronzy JJ. Synoviocyte stimulation by the LFA-1–intercellular adhesion molecule-2-Ezrin–Akt pathway in rheumatoid arthritis. J. Immunol. 2008;180:1971–1978. doi: 10.4049/jimmunol.180.3.1971. [DOI] [PubMed] [Google Scholar]
- 18.Xiao Y, Sun M, Zhan Z, Ye Y, Huang M, Zou Y, Liang L, Yang X, Xu H. Increased phosphorylation of ezrin is associated with the migration and invasion of fibroblast-like synoviocytes from patients with rheumatoid arthritis. Rheumatology. 2014;53:1291–1300. doi: 10.1093/rheumatology/keu013. [DOI] [PubMed] [Google Scholar]
- 19.Siu R, Fladd C, Rotin D. N-cadherin is an in vivo substrate for protein tyrosine phosphatase sigma (PTPσ) and participates in PTPσ-mediated inhibition of axon growth. Mol. Cell Biol. 2007;27:208–219. doi: 10.1128/MCB.00707-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller T, Choidas A, Reichmann E, Ullrich A. Phosphorylation and free pool of β-catenin are regulated by tyrosine kinases and tyrosine phosphatases during epithelial cell migration. J. Biol. Chem. 1999;274:10173–10183. doi: 10.1074/jbc.274.15.10173. [DOI] [PubMed] [Google Scholar]
- 21.Kiener HP, Niederreiter B, Lee DM, Jimenez-Boj E, Smolen JS, Brenner MB. Cadherin 11 promotes invasive behavior of fibroblast-like synoviocytes. Arthritis Rheum. 2009;60:1305–1310. doi: 10.1002/art.24453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang SK, Noss EH, Chen M, Gu Z, Townsend K, Grenha R, Leon L, Lee SY, Lee DM, Brenner MB. Cadherin-11 regulates fibroblast inflammation. Proc. Natl. Acad. Sci. U.S.A. 2011;108:8402–8407. doi: 10.1073/pnas.1019437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller-Ladner U, Kriegsmann J, Franklin BN, Matsumoto S, Geiler T, Gay RE, Gay S. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am. J. Pathol. 1996;149:1607–1615. [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher D, Xing B, Dill J, Li H, Hoang HH, Zhao Z, Yang X-L, Bachoo R, Cannon S, Longo FM, Sheng M, Silver J, Li S. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J. Neurosci. 2011;31:14051–14066. doi: 10.1523/JNEUROSCI.1737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aricescu AR, McKinnell IW, Halfter W, Stoker AW. Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase σ. Mol. Cell Biol. 2002;22:1881–1892. doi: 10.1128/MCB.22.6.1881-1892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duan Y, Giger RJ. A new role for RPTPσ in spinal cord injury: Signaling chondroitin sulfate proteoglycan inhibition. Sci. Signal. 2010;3:pe6. doi: 10.1126/scisignal.3110pe6. [DOI] [PubMed] [Google Scholar]
- 27.Stangenberg L, Burzyn D, Binstadt BA, Weissleder R, Mahmood U, Benoist C, Mathis D. Denervation protects limbs from inflammatory arthritis via an impact on the microvasculature. Proc. Natl. Acad. Sci. U.S.A. 2014;111:11419–11424. doi: 10.1073/pnas.1410854111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alawi K, Keeble J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol. Ther. 2010;125:181–195. doi: 10.1016/j.pharmthera.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Nakano K, Whitaker JW, Boyle DL, Wang W, Firestein GS. DNA methylome signature in rheumatoid arthritis. Ann. Rheum. Dis. 2013;72:110–117. doi: 10.1136/annrheumdis-2012-201526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martel-Pelletier J, Boileau C, Pelletier J-P, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract. Res. Clin. Rheumatol. 2008;22:351–384. doi: 10.1016/j.berh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell NS, Shepard N. Changes in proteoglycan and collagen in cartilage in rheumatoid arthritis. J. Bone Joint Surg. Am. 1978;60:342–348. [PubMed] [Google Scholar]
- 32.Murphy G, Lee MH. What are the roles of metalloproteinases in cartilage and bone damage? Ann. Rheum. Dis. 2005;64:iv44–iv47. doi: 10.1136/ard.2005.042465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG, Zheng B, Liepmann CD, Katagiri Y, Benowitz LI, Geller HM, Giger RJ. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat. Neurosci. 2012;15:703–712. doi: 10.1038/nn.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pitzalis C, Kelly S, Humby F. New learnings on the pathophysiology of RA from synovial biopsies. Curr. Opin. Rheumatol. 2013;25:334–344. doi: 10.1097/BOR.0b013e32835fd8eb. [DOI] [PubMed] [Google Scholar]
- 35.Elfenbein A, Simons M. Syndecan-4 signaling at a glance. J. Cell Sci. 2013;126:3799–3804. doi: 10.1242/jcs.124636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson KM, Uetani N, Manitt C, Elchebly M, Tremblay ML, Kennedy TE. Receptor protein tyrosine phosphatase σ inhibits axonal regeneration and the rate of axon extension. Mol. Cell. Neurosci. 2003;23:681–692. doi: 10.1016/s1044-7431(03)00120-9. [DOI] [PubMed] [Google Scholar]
- 37.Elchebly M, Wagner J, Kennedy TE, Lanctôt C, Michaliszyn E, Itié A, Drouin J, Tremblay ML. Neuroendocrine dysplasia in mice lacking protein tyrosine phosphatase σ. Nat. Genet. 1999;21:330–333. doi: 10.1038/6859. [DOI] [PubMed] [Google Scholar]
- 38.Monach PA, Mathis D, Benoist C. The K/BxN arthritis model. Curr. Protoc. Immunol. 2008 doi: 10.1002/0471142735.im1522s81. Chapter 15, Unit 15.22. [DOI] [PubMed] [Google Scholar]
- 39.Uetani N, Bertozzi K, Chagnon MJ, Hendriks W, Tremblay ML, Bouchard M. Maturation of ureter-bladder connection in mice is controlled by LAR family receptor protein tyrosine phosphatases. J. Clin. Invest. 2009;119:924–935. doi: 10.1172/JCI37196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvaro-Gracia JM, Zvaifler NJ, Brown CB, Kaushansky K, Firestein GS. Cytokines in chronic inflammatory arthritis. VI. Analysis of the synovial cells involved in granulocyte-macrophage colony-stimulating factor production and gene expression in rheumatoid arthritis and its regulation by IL-1 and tumor necrosis factor-alpha. J. Immunol. 1991;146:3365–3371. [PubMed] [Google Scholar]
- 41.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA, Jr., Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 42.Hwang H-Y, Olson SK, Brown JR, Esko JD, Horvitz HR. The Caenorhabditis elegans genes sqv-2 and sqv-6, which are required for vulval morphogenesis, encode glycosaminoglycan galactosyltransferase II and xylosyltransferase. J. Biol. Chem. 2003;278:11735–11738. doi: 10.1074/jbc.C200518200. [DOI] [PubMed] [Google Scholar]
- 43.Pap T, van der Laan WH, Aupperle KR, Gay RE, Verheijen JH, Firestein GS, Gay S, Neidhart M. Modulation of fibroblast-mediated cartilage degradation by articular chondrocytes in rheumatoid arthritis. Arthritis Rheum. 2000;43:2531–2536. doi: 10.1002/1529-0131(200011)43:11<2531::AID-ANR21>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 44.Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M, Corr M. Caspase 1–independent activation of interleukin-1β in neutrophil-predominant inflammation. Arthritis Rheum. 2009;60:3642–3650. doi: 10.1002/art.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.