Abstract
Several event-related potentials (ERP), including P3, sensory gating (P50), and gamma oscillation, are robustly impaired in patients with schizophrenia (SCZ) and bipolar disorder (BIP). Although these ERPs are known to be heritable, little is known about the specific genetic loci involved and the degree to which they overlap with loci influencing mood and psychotic disorders. In the present study, we conducted GWAS to a) identify common variants associated with ERP endophenotypes, and b) construct polygenic risk scores (PRS) to examine overlap between genetic components of ERPs and mood and psychotic disorders. The sample consisted of 271 patients with SCZ or psychotic BIP diagnosis and 128 controls for whom ERP and genomewide data were available. GWAS were conducted using the full sample. PRS, derived from the Psychiatric Genomics Consortium (PGC) analyses of SCZ, BIP, and major depressive disorder were applied to each ERP phenotype. We identified a region on chromosome 14 that was significantly associated with sensory gating (peak SNP rs10132223, P = 1.27 × 10−9). This locus has not been previously associated with psychotic illness in PGC-GWAS. In the PRS analyses, patients with a higher load of SCZ risk alleles had reduced gamma response whereas patients with a higher load of BIP risk alleles had smaller P3 amplitude. We observed a genomewide significant locus on chromosome 14 for P50. This locus may influence P50 but not psychotic illness. Among patients with psychotic illness, PRS results indicated genetic overlap between SCZ loci and gamma oscillation and between BIP loci and P3 amplitude.
Keywords: schizophrenia, bipolar disorders, event-related potentials, genowide assoication, polygentic risk score, endophenotypes
INTRODUCTION
Event-related potentials (ERPs) reflect synchronized neuronal activities during cognitive processing [Luck, 2005]. Investigators have used various ERPs phenotypes to probe cognitive and information processing deficits in major neuropsychiatric disorders. Several ERP deficits are robustly observed in patients with schizophrenia (SCZ) and bipolar disorder (BIP), including reduced P3 amplitude and delayed latency [Hall et al., 1996; Bramon et al., 2004; Hall et al., 2007; Hall et al., 2009a], impaired sensory gating [Freedman et al., 1996; Hall et al., 2008], and reduced gamma oscillation [Kwon et al., 1999; Spencer et al., 2008b; Roach et al., 2013]. P3 amplitude is related to attention and working memory capacity [Polich and Kok, 1995] whereas its latency reflects stimulus processing speed [McCarthy and Donchin, 1981]. Sensory gating indexes aspects of inhibitory function needed for filtering out repetitive or irrelevant incoming sensory stimuli [Freedman et al., 1994]. Gamma oscillations are believed essential for integrating information within neural circuits and appear to play an important role in perceptual and cognitive processes [Uhlhaas and Singer, 2010]. Deficits in these ERPs aggregate in families of patients with psychotic disorders.[Clementz et al., 1998; Schulze et al., 2008; Perez et al., 2013] Twin studies have demonstrated that these ERPs are heritable [Hall et al., 2006; Hall et al., 2011a] and are genetically correlated with liability to SCZ or psychotic BIP. [Bramon et al., 2005; Hall et al., 2009b] Taken together, these findings suggest that ERP impairments are endophenotypes for psychosis [Turetsky et al., 2007; O’Donnell et al., 2013].
Genome-wide association studies (GWAS) have proven successful in identifying common single nucleotide polymorphisms (SNPs) associated with disease risks for SCZ [Schizophrenia Working Group of the Psychiatric Genomics C, 2014] and BIP [Sklar et al., 2011]. GWAS have also been used for identify SNPs associated with quantitative traits that are putative endophenotypes of SCZ and BIP, including general cognitive ability [Lencz et al., 2014] and gray matter volume [Wang et al., 2013]. However, to our knowledge, no GWAS of sensory gating, gamma, or P3 ERPs in patients with psychotic disorders have been reported to date. On the other hand, studies have used ERP endophenotypes as a tool for characterizing functional or neurobiological effects of risk variants identified by GWAS. For example, Quednow et al. reported that variants of the SCZ risk locus TCF4 are associated with sensory gating deficits [Quednow et al., 2012], and we recently found that the TCF4 SNP rs17512836 risk allele was associated with reduced auditory P3 amplitude and, to a lesser degree, with its latency component [Hall et al., 2013].
GWAS have also demonstrated that a substantial proportion of the heritability of SCZ and BIP is explained by a polygenic component consisting of thousands of common SNPs of small effect [Purcell et al., 2009; Sklar et al., 2011; Schizophrenia Working Group of the Psychiatric Genomics C, 2014]. In addition, cross-disorder GWAS have documented overlapping polygenic risk among SCZ, BIP, and major depressive disorder (MDD), with the strongest effects for BIP and SCZ, indicating pleiotropic effects of some risk variants across conventional diagnostic classifications [Cross-Disorder Group of the Psychiatric Genomics C et al., 2013a; Cross-Disorder Group of the Psychiatric Genomics C et al., 2013b]. The degree to which these risk loci overlap with loci influencing ERPs remains unknown and such knowledge could provide important support for the ERP’s status as putative endophenotypes.
In the current study, our aims were 1) to carry out genome-wide association analyses identifying common variants associated with P3, sensory gating, or gamma oscillation endophenotypes, and 2)to test genetic relationships between the aggregated disease-associated SNP’s effect on each ERP phenotype by constructing polygenic risk scores (PRS) in our case-control sample based on summary statistics from the Psychiatric Genomics Consortium (PGC) of SCZ, BIP, and MDD GWAS. We hypothesized that cumulative polygenic risk for SCZ, BIP, or MDD would predict ERP deficits in our sample.
METHOD
Sample
The sample prior to genetic quality control procedures consisted of 280 patients with psychotic illness and 134 healthy controls (HC). All subjects were assessed using the SCID-IV [First et al., 1997]. Cases were clinically stable patients with a DSM-IV diagnosis of SCZ or schizoaffective disorder (n = 148), BIP type I with psychotic features (n = 129), or psychosis NOS (n = 3). All participants were between 18 and 65 years old with no known neurological disorder, no prior head injury with loss of consciousness, normal hearing confirmed by audiometry, and normal intellectual ability based on the North American Adult Reading Test (NAART) or years of education (high school level or higher). Patients were included if they had no substance abuse (excluding nicotine) in the preceding 6 months or dependence in the preceding 12 months and no history of seizures or ECT treatment in the preceding 12 months. The HC sample was recruited through local advertisements. Additional inclusion criteria for HC were: no current or past history of psychotic disorder, BIP, or a SCZ spectrum disorder, no affective disorder in the preceding 12 months, no substance abuse in the preceding 12 months or previous chronic dependence, and no first-degree relative with a history of psychosis or BIP. All subjects self-reported European ancestry. This study was approved by the McLean Hospital Institutional Review Board. After a complete description of the study, written informed consent was obtained from each subject.
Table I presents demographic and clinical information for the final sample. After genetic quality control procedures (see below), 399 participants were included in the analyses (271 patients and 128 controls). SCZ and BIP patients were older, had fewer years of education, and smoked more cigarettes than control subjects. BIP patients were younger than SCZ patients, included more females, and were receiving a lower mean chlorpromazine (CPZ) equivalent daily dose of antipsychotic medication compared with the SCZ sample (see Table I). The two patient groups did not differ in mean age of illness onset or proportion of current smokers. All but 14 patients (SCZ = 8, BIP = 6) were receiving psychotropic medication. Medicated and non-medicated patients did not differ significantly on any of the ERP measures. The most commonly prescribed non-antipsychotic medications in our sample were: lamotrigine (N = 45), lithium carbonate (N = 58), divalproex sodium (N = 34), bupropion (N = 24), lorazepam (N = 33), and clonazepam (N = 26). The doses of mood stabilizers, antidepressants, anxiolytics, and antipsychotics (in CPZ equivalent daily dose) were not significantly associated with any ERP variable, with the exception of bupropion, for which dose was significantly correlated with P3 latency (R2 = 0.25, P=0.02). SCZ patients who were treated with clozapine antipsychotics (n = 37) did not differ significantly from those who were treated with other antipsychotics in any of the ERP measures.
TABLE I.
Demographic Characteristics of the Sample
| SCZ (n = 141) |
BID (n = 127) |
Controls (n = 128) |
SCZ vs. HC | BID vs. HC | SCZ vs. BID | |
|---|---|---|---|---|---|---|
| Age, yrs | 43.9 (12.5) | 40.0 (13.7) | 33.2 (12.5) | P < 0.001 | P < 0.001 | P = 0.01 |
| Female sex, N (%) | 47 (33) | 67 (53) | 71(55) | P < 0.001 | P = 0.66 | P = 0.001 |
| Education, yrs | 14.3 (2.1) | 15.1 (2.0) | 15.6 (2.1) | P < 0.001 | P = 0.04 | P = 0.004 |
| Smoker, N (%) | 44 (39) | 38 (35) | 9 (9) | P < 0.001 | P < 0.001 | P = 0.48 |
| Age of illness onset | 22.9 (7.8) | 22.4 (8.8) | N/A | N/A | N/A | P = 0.59 |
| CPZ* | 726.5 (910) | 359.2 (338) | N/A | N/A | N/A | P = 0.002 |
Data are presented as mean (SD) unless otherwise indicated. (SCZ schizophrenia, BID bipolar disorder, CPZ chlorpromazine equivalent).
SCZ n=120, BID n=85.
Electrophysiological Recording and Analysis
We applied the same EEG recording and processing procedures as described previously [Hall et al, 2006; Hall et al., 2013]. Briefly, EEG was recorded using the BioSemi Active Two system at a digitization rate of 512 Hz, with a bandpass of DC-104 Hz and a Common Mode Sense (CMS) as the reference (PO2 site). Blinks and eye movements were monitored through electrodes placed on the left temple and above and below the left eye. The EEG data were re-referenced off-line to the averaged mastoid. Subjects were not allowed to smoke for a minimum of 40 min prior to the recordings.
The sensory gating ERP was elicited using the dual-Click paradigm (160 pairs of identical click stimuli, 5-ms duration; 2-ms rise/ fall; 500-ms inter-click interval; 10-s inter-trial interval). Signal processing was performed off-line using NEUROSCAN software (4.3) [Hall et al, 2006; Hall et al, 2011b]. EEG signals were segmented (−100–400ms), filtered (1-Hz high-pass filter), baseline corrected, and artifact rejected if activity exceeding 50 µV between 0 and 75 ms post-stimulus. S1 and S2 waveforms were averaged, digitally filtered (10-Hz high pass), and smoothed. P50 sensory gating ERP are reported at the Cz site and calculated as a ratio (S2/ S1) x 100. A higher ratio reflects more impairment.
P3 amplitude and latency ERPs were elicited by the auditory Oddball paradigm (400 binaural tones; 50-msec duration, 5 ms rise/fall times; 15% 1500 Hz target tones; 85% 1000 Hz standard tones). All participants had >90% accuracy. Signal processing was performed off-line using Brain Vision Analyzer software (Brain Products, Inc, 2000). EEG data were segmented (−100–1000 ms), low-pass filtered (8.5 Hz), baseline corrected, eye-blink corrected using [Gratton et al., 1983], and artifact rejected if activity exceeding >100 µV. P3 amplitude and latency components were automatically detected from the average wave for target tones between 280 and 650 ms at the Pz site [Hall et al., 1999; Salisbury et al., 1999; Hall et al., 2009a].
Auditory steady-state response (ASSR) of gamma oscillation was elicited by the auditory steady state 40-Hz click stimulation paradigm (150 trains of 1-ms white noise clicks, 500-ms duration, 1100-ms stimulus onset asynchrony, 40-Hz stimulation rate) [Spencer et al., 2008a]. Signal processing was performed off-line using Brain Vision Analyzer software (Brain Products, Inc., Germany 2000). Time-frequency analyses were performed using the IDL program (EXELIS Visual Information Solutions, Boulder, Colorado). Single-trial epochs were extracted (−250–800 ms), eye-blink corrected, and artifact rejected if activity exceeding >100 µV. Phase locking and evoked power at Cz were analyzed with the Morlet wavelet transform using the IDL program. Average spectral measures were computed at 20–520 ms and wavelet frequencies between 38–47 Hz, where both evoked phase locking and power were maximal [Spencer et al., 2008a; Spencer et al., 2009]. The evoked phase locking measure at Fz was used for the PRS analyses.
Genotyping and Quality Control Procedure
DNA from blood samples was extracted at the Massachusetts General Hospital Center for Human Genetic Research and geno-typed at the Broad Institute using the Illumina OmniExpress Infinium Platform. Quality control included the following steps: removing individuals with discordant sex information (n = 5), missing genotype rate >5% or heterozygosity rate >3SD (n = 4), shared IBD >0.125 (n = 5), or were non-European ancestry based on principal component analyses (n = 1), resulting in a final sample of N = 399. Exclusion criteria for SNPs were as follows: SNPs on the X or Y chromosome, MAF<0.05, call rate <98%, and P < 1 × 10−6 for deviation from Hardy-Weinberg equilibrium. A total of 664,907 autosomal SNPs passed QC Quality control steps were carried out with PLINK [Purcell et al., 2007].
We performed genotype imputation using a 2-step prephasing and imputation procedure implemented in SHAPHIT and IM-PUTE2 on a total of 1,293 psychosis patients and 381 healthy controls collected at McLean Hospital that included the 399 samples described above [Howie et al., 2011; Delaneau et al., 2012]. We divided the genome into 3 Mb partitions and performed pre-imputation quality control and imputation with the default parameters of the software. The pre-imputation quality control filters include discordant sex information, missing genotype rate per individual, heterozygosity rate, call rate per SNP, deviation from Hardy-Weinberg equilibrium. We used phased haplotypes from the full 1,000 Genomes Project data set as the imputation reference panel. The final imputed dataset comprised 9.7 million autosomal SNPs.
Statistical Analyses
Logistic or linear regression analyses were used to compare the case and control groups on demographic characteristics and each ERP phenotype using STATA (STATA version 12; Stata Corp., College Station, TX). Sex and age were included as covariates.
GWAS analyses
Genome-wide association tests were performed on each ERP phenotype (P3 amplitude, P3 latency, sensory gating, ASSR gamma) in our case-control sample using PLINK. We used linear regression under an additive model to test for association. We adjusted for 10 principal components (PC)in the regression models to correct for potential population stratification. The principal components were obtained using EIGENSOFT [Price et al., 2006].
Polygenic Risk Score Analyses
We used GWAS summary results from the Psychiatric Genomics Consortium (PGC) of SCZ (i.e., SCZ2), BIP, and MDD clumped datasets (http://www.med.unc.edu/pgc/) to derive PRSs for each disease condition. In each disease dataset, we applied five different association P value thresholds (PT < 10−5, PT < 10−4, PT < .001, PT < .05, and PT < .5) and selected SNPs according to these threshods to produce five sets of PRSs for each study participant in our sample. PRS was calculated as a weighted sum of risk allele counts (0,1,2) for each sample in our study. The log of the association odds ratios were used as the weight for PRS [Purcell et al., 2009].
Associations between each of the PRS for SCZ (PRS-SCZ), BIP (PRS-BIP), or MDD (PRS-MDD), and each of the ERP phenotypes (P3 amplitude, P3 latency, sensory gating, ASSR gamma) were tested in our case-control sample using linear regression models in R (R 2.15.3; R Core Team, Vienna, Austria). Effects of diagnosis and diagnosis by PRS interaction were included in the regression model. Age, and the first 10 PC were included as covariates. Partial correlations at each threshold were calculated to estimate the percent of variance explained by the PRSs. P-values ≤0.0125 (α = 0.05 corrected for four phenotypes) were considered statistically significant after multiple testing correction. We also examined the associations of PRSs stratified by cases and control samples separately. All PRS analyses were adjusted for age.
RESULTS
Supplementary Table 1 shows summary statistics for each ERP phenotype by diagnostic group. Compared with HC, patients with SCZ or BIP showed a highly significant impairment of sensory gating (both P < 0.0001), reduced P300 amplitude (both P < 0.0001), evoked gamma responses (both P < 0.0001), and prolonged P3 latency (SCZ: P = 0.001, BIP P < 0.0001). SCZ and BIP patients did not significantly differ on any ERP variable.
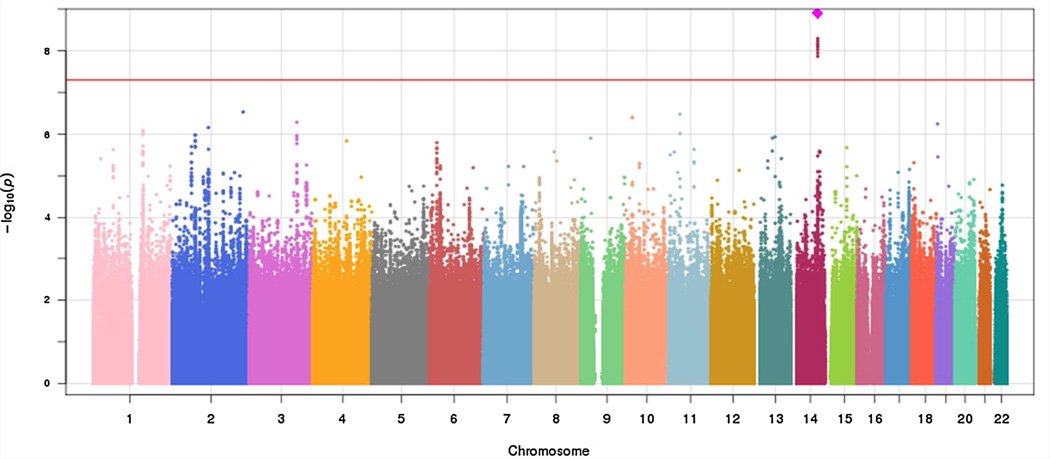
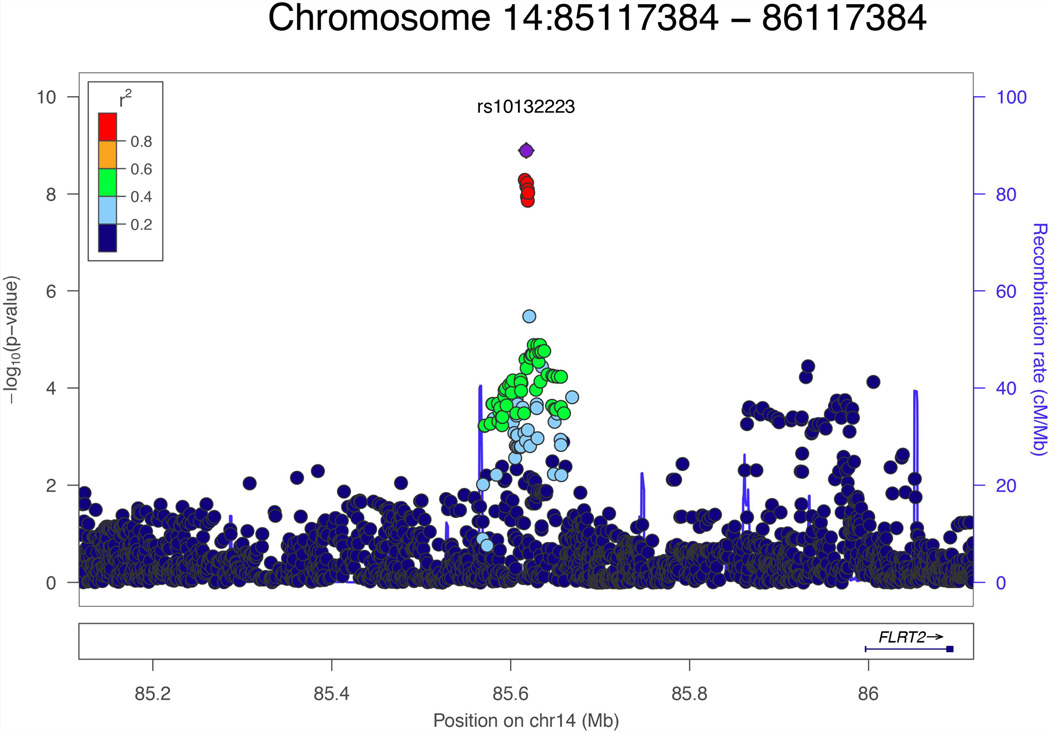
A total of 392 subjects who had P3 ERPs and sensory gating data and a total of 331 subjects who had ASSR gamma data were included in the GWAS analysis. There was no evidence for genomic inflation in each GWAS analysis (P3 amplitude, λ = 0.9916; P3 latency, λ = 1.007; sensory gating, λ = 1.000; sensory gating, λ = 0.9953). As shown in the Manhattan plot (Fig. 1) and Table II, nine SNPs reached the genome-wide significance level of P < 5 × 10−8 for the sensory gating phenotype, with the strongest signal at imputed SNP rs10132223 (P = 1.27 × 10−9). However, rs10149105, a genotyped SNP in strong linkage disequilibrium (LD) with the peak SNP (r2 > 0.8) showed a similar level of association (P = 5.12 × 10−9). Figure 2 shows the regional plot for associated SNPs at the chromosome 14 locus; as shown, all significant SNPs are in strong LD and reflect a single association signal. The nearest gene to this genome-wide significant locus is FLRT2 (located 400 kb from the peak SNP).
FIG. 1.
Manhattan Plot of GWAS results for sensory gating (P50).
TABLE II.
GWAS Results of Significant SNPs Associated With Sensory Gating
| CHR | Location (BP) | SNPs | BETA | SE | P-value | Risk allele |
Ref. allele |
Risk allele frequency |
INFO | Genotyped |
|---|---|---|---|---|---|---|---|---|---|---|
| 14 | 85615940 | rs10149105 | 20.1793 | 3.3717 | 5.12E-09 | T | C | 0.2212 | 1.0503 | Yes |
| 14 | 85617384 | rs10132223 | 21.3939 | 3.4342 | 1.27E-09 | T | G | 0.2072 | 1.056 | No |
| 14 | 85617393 | rs10144282 | −20.0177 | 3.3748 | 6.90E-09 | T | G | 0.7783 | 1.0475 | No |
| 14 | 85617703 | chr14_85617703_I* | 20.0052 | 3.375 | 7.06E-09 | GT | G | 0.2217 | 1.0473 | No |
| 14 | 85618302 | rs10135204 | 19.8278 | 3.3937 | 1.13E-08 | T | C | 0.2202 | 1.0409 | No |
| 14 | 85618459 | rs10147584 | −19.9705 | 3.3506 | 5.87E-09 | T | C | 0.7698 | 1.032 | No |
| 14 | 85619165 | rs9652388 | 19.9258 | 3.3752 | 8.06E-09 | A | G | 0.2216 | 1.0474 | No |
| 14 | 85619232 | rs7157801 | 19.9104 | 3.4278 | 1.36E-08 | C | G | 0.2158 | 1.021 | No |
| 14 | 85619625 | rs7158211 | 19.826 | 3.3742 | 9.39E-09 | T | C | 0.2216 | 1.0482 | No |
INFO, imputation quality score.
Genomic positions are in UCSC hg19/NCBI Build 37.
Chr14_85617703_I is a GT vs. G insertion variant.
FIG. 2.
Regional plot for Chromosome 14 locus associated with Sensory gating.
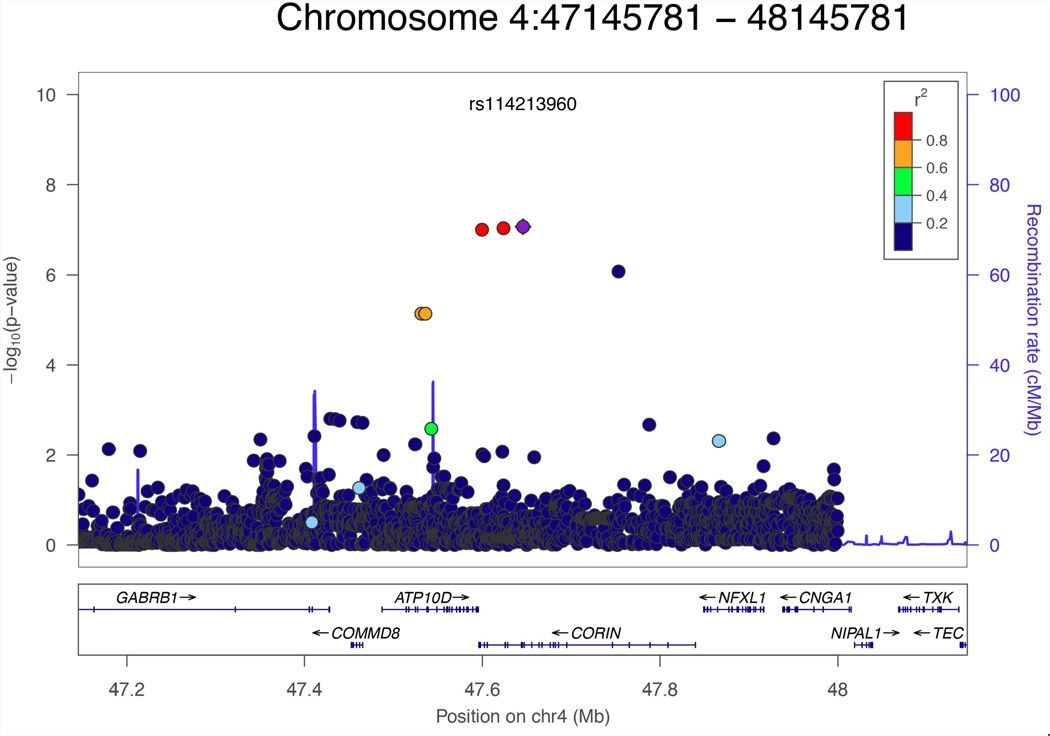
No genomewide significant associations were observed for the other ERPs. However, three SNPs on chromosome 4 approached genomewide significance for ASSR gamma (rs181531738, P = 9.77 E-08; rs146360492, P = 9.05E-08; and rs114213960, P = 8.47E-08, (see Fig. 3). These SNPs were in high LD with each other (r2 > 0.8) and the associated alleles were inversely related to ASSR gamma. These SNPs are located in the intronic region of CORIN and are very close to ATP10D (ATPase, class V, type 10D) and GABRB1 (γ-Aminobutyric acid A receptor, β1)(Fig. 3). Results for SNPs with p < 10−5 for each of the four ERP phenotypes are given in Supplementary Tables 2–5.
FIG. 3.
Regional plot for Chromosome four locus associated with ASSR gamma oscillaton.
Polygenic Score Analysis
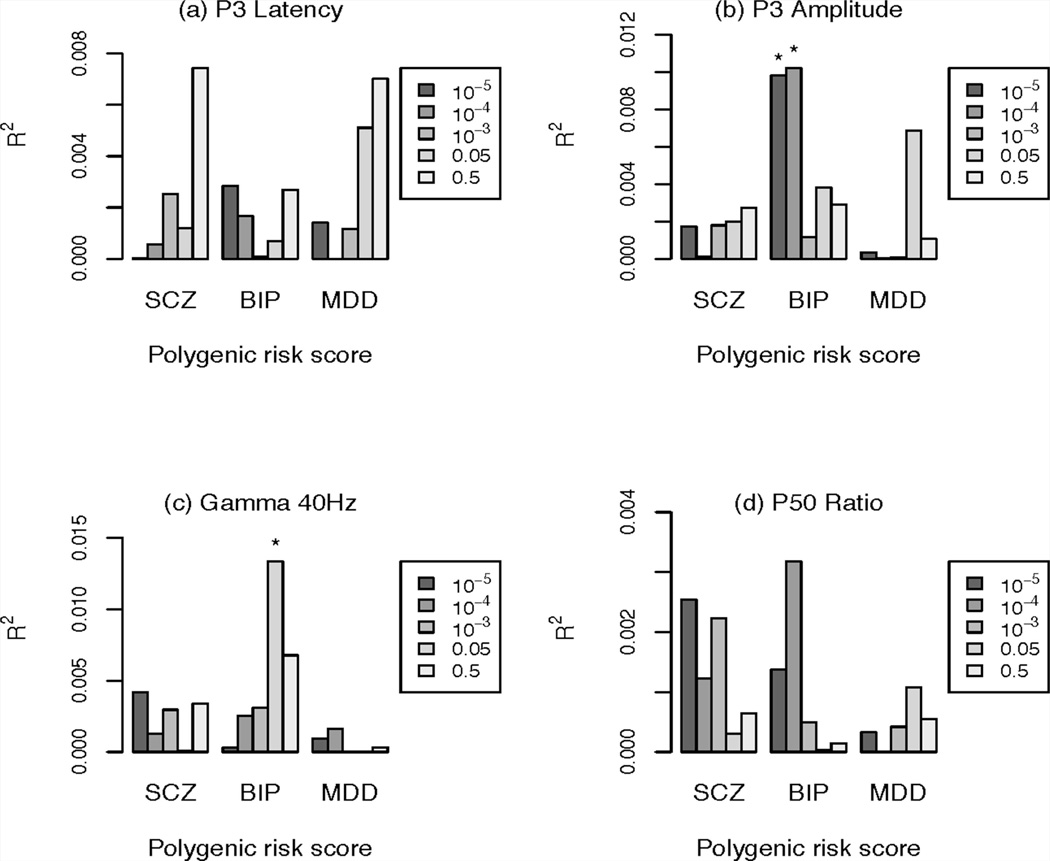
The results of the PRS analyses are shown in Figure 4. The PRS-BIP was nominally associated with reduced P3 amplitude at two thresholds: PT 1e-5 (P = 0.03, R2 = 1%) and PT 1e-4 (P = 0.03, R2 = 1%). Higher genetic risk scores were associated with smaller P3 amplitude. PRS-BIP was also nominally associated with reduced ASSR gamma response at the threshold of PT 0.05 (P = 0.02, R2 = 1.3%). Higher genetic risk scores were associated with smaller ASSR gamma response. These associations did not remain significant after multiple testing correction. Significant interactions between PRS and case-control status were observed in PRS-SCZ with ASSR gamma at PT 1e-4 (P = 0.04) and PT 1e-5 (P = 0.008), as well as in PRS-BIP with ASSR gamma at PT 0.05 (P = 0.047) .
FIG. 4.
The variance explained of different phenotypes by polygenic schizophrenia score (PRS-SCZ, left), bipolar score (PRS-BIP, middle), and major depressive disorder (PRS-MDD, right) for different p-cutoff single nucleotide polymorphism sets (box, right); y axis explained variance by the PRS of this phenotype. SCZ schizophrenia; BIP, bipolar disorder; MDD, major depressive disorder. *P < 0.05. PRS-BIP was nominally associated with reduced P3 amplitude at PT < 10−5 and PT < 10−4. Higher genetic risk scores were associated with smaller P3 amplitude. PRS-BIP was also nominally associated with smaller ASSR gamma response. These associations did not remain significant after multiple testing correction.
Analyses of case and control cohorts separately (Supplementary Figure 1 and 2) revealed that PRS-scz was associated with reduced ASSR gamma in cases at PT 1e-4 (P = 0.04, R2 = 2%), and PT 1e-5 (P = 0.003, R2 = 4%); the latter remained significant after multiple testing correction. PRS-BIP was associated with reduced P3 amplitude in cases at PT 1e-4 (P = 0.02, R2 = 2%) and PT 1e-5 (P = 0.005, R2 = 3%); the latter survived multiple testing correction, though the interaction between PRS-BIP and case-control status was not significant [Supplementary Figure 1]. No significant associations were found in controls.
DISCUSSION
We conducted a GWAS of four heritable ERPs that have been implicated as endophenotypes for psychotic illness: P3 amplitude, P3 latency, P50 sensory gating, and ASSR gamma in a sample of patients with SCZ and psychotic BIP and healthy controls. We observed a single genomewide significant locus associated with sensory gating, including the genotyped SNP rs10149105. Risk alleles were associated with impaired auditory sensory gating (i.e., worse inhibitory control). The observed allele frequencies of these SNPs in control were similar to those reported in the HapMap 3 CEU reference samples. The closest gene, approximately 400 kb upstream, is fibronectin leucine rich transmembrane protein 2 (FLRT2). FLRT2 is expressed in the mammalian developing neocortex [Yamagishi and others 2011] and is important for modulating cortical neuron migration. During development, FLRT2 & FLRT3 ectodomains are shed from neurons and act as repulsive guidance molecules for axons and somata of Unc5-positive neurons [Yamagishi and others 2011]. Deletion of either FLRT2 or Unc5D causes a subset of neurons to migrate prematurely, whereas over-expression of Unc5D has opposite effects. Sensory gating is present at birth and cerebral inhibition develops perinatally, influenced by genetic and in utero factors [Ross and others 2013a; Ross and others 2013b]. A possible link between sensory gating and FLRT2 during development warrants further investigation.
The locus associated with P50 was not associated with SCZ or BIP in the most recent large-scale GWAS of these disorders by the PGC [Schizophrenia Working Group of the Psychiatric Genomics 2014; Sklar and others 2011]. Specifically, the p value for the peak SNP rs10132223 was 0.27 in the PGC-SCZ GWAS and 0.22 in the PGC-BIP GWAS. Thus, this locus appears to influence impairment in sensory gating, but not risk for psychotic illness. The alpha-7 neuronal nicotinic receptor subunit gene (CHRNA7), localized at 15q13–14, has been linked to the P50 sensory gating deficit in SCZ [Freedman and others 1997; Stephens and others 2009]. In our sample, SNPs in the CHRNA7 region were not associated with the P50 sensory gating phenotype (smallest P value = 0.04).
Three SNPs on chromosome 4 approached genomewide significance for the ASSR gamma (see Fig. 3). These SNPs are located in the intronic region of CORIN and are very close to ATP10D (ATPase, class V, type 10D) and GABRB1 (γ-aminobutyric acid A receptor, β 1) (Fig. 3). Although the function of CORIN in the brain is largely unknown, GABRB1 has been linked to beta oscillation in families with alcoholism [Porjesz et al., 2002]. Beta (13–30Hz) and gamma (>30 Hz) oscillations are tightly coordinated in the brain [Uhlhaas and Singer, 2013] and generation of beta and gamma oscillations rely critically on gamma-aminobutyric acid (GABA) ergic N-methyl-D-aspartate (NMDA) networks [Arai and Natsume, 2006; Uhlhaas and Singer, 2013]. Alterations of GABAA receptor gene and expression level are implicated in the pathogenesis of SCZ [Rotaru et al., 2012]. Our preliminary result will need to be confirmed in a larger independent sample, since SNPs associated with gamma oscillation did not survive correction and were relatively rare.
Our second aim was to investigate the overlapping genetic relationships between aggregate effects of SCZ-, BIP- and MDD-SNPs on four ERP phenotypes. Studies of ERPs have characterized P3, sensory gating and ASSR gamma deficits as endophenotypes for SCZ [Bramon et al., 2005; Hall et al., 2007; Hall et al., 2011b] and for psychotic BIP [Muir et al., 1991; Hall et al., 2008; Rass et al., 2010]. Our observations that SCZ and BIP patients did not significantly differ on any ERP variable are consistent with the notion of shared genetic susceptibility between SCZ and BIP [Hall et al., 2012] and that these ERP impairments are endophenotypes for psychosis [Olincy and Martin 2005; Sanchez-Morla et al., 2008; Spencer et al., 2008b; Cross-Disorder Group of the Psychiatric Genomics C et al., 2013a; Cross-Disorder Group of the Psychiatric Genomics et al., 2013b].
We tested the hypothesis that polygenic risk scores for SCZ, BIP, or MDD would associate with ERP phenotypes. In the whole case-control sample analyses, we did not observe a significant correlation. However, analyzing cases and controls separately, we found that a SCZ polygene score was significantly and inversely correlated with ASSR gamma in cases (P = 0.003) at the most stringent PT threshold. [Supplementary Figure 1]. The association explained 4% of the variance in ASSR gamma. A total of 93 SNPs was included in this PRS at P value <10−5 threshold. We also found that BIP-associated SNPs were significantly and inversely associated with P3 amplitude in cases (P = 0.005) at the most stringent threshold [Supplementary Figure 1]. The observed association explained about 3% of the variance in P3 amplitude and only four independent SNPs were included in this threshold cutoff. In fact, the variance explained in P3 amplitude or ASSR gamma dramatically decreased when more SNPs with higher cutoff PT were added [Supplementary Figure 1]. These results suggest that the overlap between common variants so far involved in disorders and ERP phenotypes is restricted to a small subset of SNPs and is seen for ERP variance only among cases. It is possible that the observed SNP effects on P3 amplitude or gamma oscillations are indexing illness severity or some other clinical characteristic. However, the relatively small sample size of ERP subjects may well have limited our ability to detect more extensive overlap. In addition, our sample may not capture the full spectrum of ERP variation because most cases (68% of the sample) were recruited from clinical settings and are thus enriched for more severely ill patients.
Our study has several limitations: first, as noted above, our sample size was modest, raising the possibility of Type II error. For our sample of 399 and alpha level at 0.0125 for the polygenic score analysis, we had 50% power to detect a locus or polygene score accounting for 1.5% of the variance in each phenotype we examined. Nevertheless, our results provide the first genomewide analysis of multiple ERP phenotypes and can inform future, larger studies. Second, we were unable to identify a suitable sample to attempt replication and our results will need further confirmation in independent samples. While cases in our sample were significantly older than controls, age was included as a covariate in the regression models and in the PRS analyses.
In summary, we found genomewide significant association between a locus on chromosome 14 near FLRT2 and the auditory sensory gating phenotype. However, this locus does not appear to be associated with SCZ or BIP based on the largest available GWAS of those disorders. In case-only PRS analyses, we found that a risk score comprising BIP-associated loci (at PT < 10−5) is also associated with reduced P3 amplitude, and a risk score comprising SCZ-associated loci (at PT < 10−5) is associated with reduced ASSR gamma response. Our results provided useful information for future ERP-genetic studies. Larger studies will be needed to clarify the genetic relationships among these phenotypes.
Supplementary Material
Acknowledgments
Grant sponsor: National Institute of Mental Health; Grant number: 1K01MH086714; Grant sponsor: Brain and Behavior Research Foundation; Grant sponsor: National Alliance for Research on Schizophrenia and Depression; Grant number: K24MH094614; Grant sponsor: Ellison Foundation and Anonymous Foundation.
Footnotes
Conflict of interest: Dr. Ongur reported being on a Scientific Advisory Board for Lilly Inc. in 2013. Drs. Hall, Chen, Cohen, Smoller, and Levy reported no biomedical financial interests or potential conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
REFERENCES
- Arai J, Natsume K. The properties of carbachol-induced beta oscillation in rat hippocampal slices. Neurosci Res. 2006;54(2):95–103. doi: 10.1016/j.neures.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Bramon E, McDonald C, Croft RJ, Landau S, Filbey F, Gruzelier JH, Sham PC, Frangou S, Murray RM. Is the P300 wave an endophenotype for schizophrenia? A meta-analysis and a family study. Neuroimage. 2005;27(4):960–968. doi: 10.1016/j.neuroimage.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res. 2004;70(2–3):315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Geyer MA, Braff DL. Poor P50 suppression among schizophrenia patients and their first-degree biological relatives. Am J Psychiatry. 1998;155(12):1691–1694. doi: 10.1176/ajp.155.12.1691. [DOI] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric; Genomics C, Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, Mowry BJ, Thapar A, Goddard ME, Witte JS, Absher D, Agartz I, Akil H, Amin F, Andreassen OA, Anjorin A, Anney R, Anttila V, Arking DE, Asherson P, Azevedo MH, Backlund L, Badner JA, Bailey AJ, Banaschewski T, Barchas JD, Barnes MR, Barrett TB, Bass N, Battaglia A, Bauer M, Bayes M, Bellivier F, Bergen SE, Berrettini W, Betancur C, Bettecken T, Biederman J, Binder EB, Black DW, Blackwood DH, Bloss CS, Boehnke M, Boomsma DI, Breen G, Breuer R, Bruggeman R, Cormican P, Buccola NG, Buitelaar JK, Bunney WE, Buxbaum JD, Byerley WF, Byrne EM, Caesar S, Cahn W, Cantor RM, Casas M, Chakravarti A, Chambert K, Choudhury K, Cichon S, Cloninger CR, Collier DA, Cook EH, Coon H, Cormand B, Corvin A, Coryell WH, Craig DW, Craig IW, Crosbie J, Cuccaro ML, Curtis D, Czamara D, Datta S, Dawson G, Day R, De Geus EJ, Degenhardt F, Djurovic S, Donohoe GJ, Doyle AE, Duan J, Dudbridge F, Duketis E, Ebstein RP, Edenberg HJ, Elia J, Ennis S, Etain B, Fanous A, Farmer AE, Ferrier IN, Flickinger M, Fombonne E, Foroud T, Frank J, Franke B, Fraser C, Freedman R, Freimer NB, Freitag CM, Friedl M, Frisen L, Gallagher L, Gejman PV, Georgieva L, Gershon ES, Geschwind DH, Giegling I, Gill M, Gordon SD, Gordon-Smith K, Green EK, Greenwood TA, Grice DE, Gross M, Grozeva D, Guan W, Gurling H, De Haan L, Haines JL, Hakonarson H, Hallmayer J, Hamilton SP, Hamshere ML, Hansen TF, Hartmann AM, Hautzinger M, Heath AC, Henders AK, Herms S, Hickie IB, Hipolito M, Hoefels S, Holmans PA, Holsboer F, Hoogendijk WJ, Hottenga JJ, Hultman CM, Hus V, Ingason A, Ising M, Jamain S, Jones EG, Jones I, Jones L, Tzeng JY, Kahler AK, Kahn RS, Kandaswamy R, Keller MC, Kennedy JL, Kenny E, Kent L, Kim Y, Kirov GK, Klauck SM, Klei L, Knowles JA, Kohli MA, Koller DL, Konte B, Korszun A, Krabbendam L, Krasucki R, Kuntsi J, Kwan P, Landen M, Langstrom N, Lathrop M, Lawrence J, Lawson WB, Leboyer M, Ledbetter DH, Lee PH, Lencz T, Lesch KP, Levinson DF, Lewis CM, Li J, Lichtenstein P, Lieberman JA, Lin DY, Linszen DH, Liu C, Lohoff FW, Loo SK, Lord C, Lowe JK, Lucae S, MacIntyre DJ, Madden PA, Maestrini E, Magnusson PK, Mahon PB, Maier W, Malhotra AK, Mane SM, Martin CL, Martin NG, Mattheisen M, Matthews K, Mattingsdal M, McCarroll SA, McGhee KA, McGough JJ, McGrath PJ, McGuffin P, McInnis MG, McIntosh A, McKinney R, McLean AW, McMahon FJ, McMahon WM, McQuillin A, Medeiros H, Medland SE, Meier S, Melle I, Meng F, Meyer J, Middeldorp CM, Middleton L, Milanova V, Miranda A, Monaco AP, Montgomery GW, Moran JL, Moreno-De-Luca D, Morken G, Morris DW, Morrow EM, Moskvina V, Muglia P, Muhleisen TW, Muir WJ, Muller-Myhsok B, Murtha M, Myers RM, Myin-Germeys I, Neale MC, Nelson SF, Nievergelt CM, Nikolov I, Nimgaonkar V, Nolen WA, Nothen MM, Nurnberger JI, Nwulia EA, Nyholt DR, O’Dushlaine C, Oades RD, Olincy A, Oliveira G, Olsen L, Ophoff RA, Osby U, Owen MJ, Palotie A, Parr JR, Paterson AD, Pato CN, Pato MT, Penninx BW, Pergadia ML, Pericak-Vance MA, Pickard BS, Pimm J, Piven J, Posthuma D, Potash JB, Poustka F, Propping P, Puri V, Quested DJ, Quinn EM, Ramos-Quiroga JA, Rasmussen HB, Raychaudhuri S, Rehnstrom K, Reif A, Ribases M, Rice JP, Rietschel M, Roeder K, Roeyers H, Rossin L, Rothenberger A, Rouleau G, Ruderfer D, Rujescu D, Sanders AR, Sanders SJ, Santangelo SL, Sergeant JA, Schachar R, Schalling M, Schatzberg AF, Scheftner WA, Schellenberg GD, Scherer SW, Schork NJ, Schulze TG, Schumacher J, Schwarz M, Scolnick E, Scott LJ, Shi J, Shilling PD, Shyn SI, Silverman JM, Slager SL, Smalley SL, Smit JH, Smith EN, Sonuga-Barke EJ, St Clair D, State M, Steffens M, Steinhausen HC, Strauss JS, Strohmaier J, Stroup TS, Sutcliffe JS, Szatmari P, Szelinger S, Thirumalai S, Thompson RC, Todorov AA, Tozzi F, Treutlein J, Uhr M, van den Oord EJ, Van Grootheest G, Van Os J, Vicente AM, Vieland VJ, Vincent JB, Visscher PM, Walsh CA, Wassink TH, Watson SJ, Weissman MM, Werge T, Wienker TF, Wijsman EM, Willemsen G, Williams N, Willsey AJ, Witt SH, Xu W, Young AH, Yu TW, Zammit S, Zandi PP, Zhang P, Zitman FG, Zollner S International Inflammatory Bowel Disease. Genetics C, Devlin B, Kelsoe JR, Sklar P, Daly MJ, O’Donovan MC, Craddock N, Sullivan PF, Smoller JW, Kendler KS, Wray NR. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013a;45(9):984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric. Genomics C, Smoller JW, Crad-dock N, Kendler K, Lee PH, Neale BM, Nurnberger JI, Ripke S, Santangelo S, Sullivan PF. Identification of risk loci with shared effects on five major psychiatric disorders: A genome-wide analysis. Lancet. 2013b;381(9875):1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaneau O, Marchini J, Zagury JF. A linear complexity phasing method for thousands of genomes. Nature methods. 2012;9(2):179–181. doi: 10.1038/nmeth.1785. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders (SCID) New York: New York State Psychiatric Institute, Biometrics Research; 1997. [Google Scholar]
- Freedman R, Adler LE, Bickford P, Byerley W, Coon H, Cullum CM, Griffith JM, Harris JG, Leonard S, Miller C, et al. Schizophrenia and nicotinic receptors. Harv Rev Psychiatry. 1994;2(4):179–192. doi: 10.3109/10673229409017136. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Myles-Worsley M, Nagamoto HT, Miller C, Kisley M, McRae K, Cawthra E, Waldo M. Inhibitory gating of an evoked response to repeated auditory stimuli in schizophrenic and normal subjects. Human recordings, computer simulation, and an animal model. Arch Gen Psychiatry. 1996;53(12):1114–1121. doi: 10.1001/archpsyc.1996.01830120052009. [DOI] [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA. 1997;94(2):587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. Anew method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hall M-H, Rijsdijk FV, Picchioni M, Schulze K, Ettinger U, Toulopoulou T, Murray R, Sham P. Substantial shared genetic influences on schizophrenia and event-related potentials. Am J Psychiatry. 2007;164:804–812. doi: 10.1176/ajp.2007.164.5.804. [DOI] [PubMed] [Google Scholar]
- Hall MH, Levy DL, Salisbury DF, Haddad S, Gallagher PJ, Lohan M, Cohen BM, Ongur D, Smoller JW. Neurohysiologic Effects of GWAS Derived Schizophrenia and Bipolar Risk Variants. Am J Med Genet B Neuropsychiatr Genet. 2013;165B(1):9–18. doi: 10.1002/ajmg.b.32212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Schulze K, Rijsdijk F, Kalidindi S, McDonald C, Bramon E, Murray RM, Sham P. Are auditory P300 and duration MMN heritable and putative endophenotypes of psychotic bipolar disorder? A Maudsley Bipolar Twin and Family Study. Psychol Med. 2009a;39(8):1277–1287. doi: 10.1017/S0033291709005261. [DOI] [PubMed] [Google Scholar]
- Hall MH, Schulze K, Rijsdijk F, Picchioni M, Ettinger U, Bramon E, Freedman R, Murray RM, Sham P. Heritability and Reliability of P300, P50 and Duration Mismatch Negativity. Behavior genetics. 2006;36(6):845–857. doi: 10.1007/s10519-006-9091-6. [DOI] [PubMed] [Google Scholar]
- Hall MH, Schulze K, Sham P, Kalidindi S, McDonald C, Bramon E, Levy DL, Murray RM, Rijsdijk F. Further evidence for shared genetic effects between psychotic bipolar disorder and P50 suppression: A combined twin and family study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(5):619–627. doi: 10.1002/ajmg.b.30653. [DOI] [PubMed] [Google Scholar]
- Hall MH, Smoller JW, Cook NR, Schulze K, Hyoun Lee, Taylor P, Bramon G, Coleman E, Murray MJ, Salisbury RM, Levy DF. Patterns of deficits in brain function in bipolar disorder and schizophrenia: A cluster analytic study. Psychiatry Res. 2012;200(2–3):272–280. doi: 10.1016/j.psychres.2012.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Spencer KM, Schulze K, McDonald C, Kalidindi S, Kravariti E, Kane F, Murray RM, Bramon E, Sham P, Rijsdijk F. The genetic and environmental influences of event-related gamma oscillations on bipolar disorder. Bipolar Disord I. 2011a;13(13):260–271. doi: 10.1111/j.1399-5618.2011.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Taylor G, Salisbury DF, Levy DL. Sensory gating event-related potentials and oscillations in schizophrenia patients and their unaffected relatives. Schizophr Bull. 2011b;37(6):1187–1199. doi: 10.1093/schbul/sbq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Taylor G, Sham P, Schulze K, Rijsdijk F, Picchioni M, Toulopoulou T, Ettinger U, Bramon E, Murray RM, Salisbury DF. The early auditory gamma-band response is heritable and a putative endophenotype of schizophrenia. Schizophr Bull. 2009b;37:778–787. doi: 10.1093/schbul/sbp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie B, Marchini J, Stephens M. Genotype imputation with thousands of genomes. G3. 2011;1(6):457–470. doi: 10.1534/g3.111.001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56(11):1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencz T, Knowles E, Davies G, Guha S, Liewald DC, Starr JM, Djurovic S, Melle I, Sundet K, Christoforou A, Reinvang I, Mukherjee S, DeRosse P, Lundervold A, Steen VM, John M, Espeseth T, Raikkonen K, Widen E, Palotie A, Eriksson JG, Giegling I, Konte B, Ikeda M, Roussos P, Giakoumaki S, Burdick KE, Payton A, Ollier W, Horan M, Donohoe G, Morris D, Corvin A, Gill M, Pendleton N, Iwata N, Darvasi A, Bitsios P, Rujescu D, Lahti J, Hellard SL, Keller MC, Andreassen OA, Deary IJ, Glahn DC, Malhotra AK. Molecular genetic evidence for overlap between general cognitive ability and risk for schizophrenia: A report from the Cognitive Genomics consorTium (COGENT) Mol Psychiatry. 2014;19(2):168–174. doi: 10.1038/mp.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ. Cambridge, Massachusetts: The MIT Press; 2005. An introduction to the event-related potential technique. ISBN 0-262-12277-4. [Google Scholar]
- McCarthy G, Donchin E. A metric for thought: A comparison of P300 latency and reaction time. Science. 1981;211(4477):77–80. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- Muir WJ, St Clair DM, Blackwood DH. Long-latency auditory event-related potentials in schizophrenia and in bipolar and unipolar affective disorder. Psychol Med. 1991;21(4):867–879. doi: 10.1017/s003329170002986x. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, Vohs JL, Krishnan GP, Rass O, Hetrick WP, Morzorati SL. The auditory steady-state response (ASSR): A translational biomarker for schizophrenia. Suppl Clin Neurophysiol. 2013;62:101–112. doi: 10.1016/b978-0-7020-5307-8.00006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olincy A, Martin L. Diminished suppression of the P50 auditory evoked potential in bipolar disorder subjects with a history of psychosis. Am J Psychiatry. 2005;162(1):43–49. doi: 10.1176/appi.ajp.162.1.43. [DOI] [PubMed] [Google Scholar]
- Perez VB, Roach BJ, Woods SW, Srihari VH, McGlashan TH, Ford JM, Mathalon DH. Early auditory gamma-band responses in patients at clinical high risk for schizophrenia. Suppl Clin Neurophysiol. 2013;62:147–162. doi: 10.1016/b978-0-7020-5307-8.00010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Kok A. Cognitive and biological determinants of P300: An integrative review. Biolo Psychol. 1995;41(2):103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, Goate A, Rice JP, O’Connor SJ, Rohrbaugh J, Kuperman S, Bauer LO, Crowe RR, Schuckit MA, Hesselbrock V, Conneally PM, Tischfield JA, Li T-K, Reich T, Begleiter H. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc Natl Acad Sci USA. 2002;99(6):3729–3733. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, deBakker PI, Daly MJ, Sham PC. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quednow BB, Brinkmeyer J, Mobascher A, Nothnagel M, Musso F, Grunder G, Savary N, Petrovsky N, Frommann I, Lennertz L, Spreckelmeyer KN, Wienker TF, Dahmen N, Thuerauf N, Clepce M, Kiefer F, Majic T, Mossner R, Maier W, Gallinat J, Diaz-Lacava A, Toliat MR, Thiele H, Nurnberg P, Wagner M, Winterer G. Schizophrenia risk polymorphisms in the TCF4 gene interact with smoking in the modulation of auditory sensory gating. Proc Natl Acad Sci USA. 2012;109(16):6271–6276. doi: 10.1073/pnas.1118051109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rass O, Krishnan G, Brenner CA, Hetrick WP, Merrill CC, Shekhar A, O’Donnell BF. Auditory steady state response in bipolar disorder: Relation to clinical state, cognitive performance, medication status, and substance disorders. Bipolar Disord. 2010;12(8):793–803. doi: 10.1111/j.1399-5618.2010.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach BJ, Ford JM, Hoffman RE, Mathalon DH. Converging evidence for gamma synchrony deficits in schizophrenia. Suppl Clin Neurophysiol. 2013;62:163–180. doi: 10.1016/b978-0-7020-5307-8.00011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AS, Hunter SK, Groth MA, Ross RG. P50 sensory gating in infants. J Vis Exp. 2013a;82(e50065):1–5. doi: 10.3791/50065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RG, Hunter SK, McCarthy L, Beuler J, Hutchison AK, Wagner BD, Leonard S, Stevens KE, Freedman R. Perinatal choline effects on neonatal pathophysiology related to later schizophrenia risk. Am J Psychiatry. 2013b;170(3):290–298. doi: 10.1176/appi.ajp.2012.12070940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotaru DC, Lewis DA, Gonzalez-Burgos G. The role of glutamatergic inputs onto parvalbumin-positive interneurons: Relevance for schizophrenia. Rev Neurosci. 2012;23(1):97–109. doi: 10.1515/revneuro-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, McCarley RW. P300 topography differs in schizophrenia and manic psychosis. Biol Psychiatry. 1999;45(1):98–106. doi: 10.1016/s0006-3223(98)00208-x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Morla EM, Garcia-Jimenez MA, Barabash A, Martinez-Vizcaino V, Mena J, Cabranes-Diaz JA, Baca-Baldomero E, Santos JL. P50 sensory gating deficit is a common marker of vulnerability to bipolar disorder and schizophrenia. Acta Psychiatr Scandinavica. 2008;117(4):313–318. doi: 10.1111/j.1600-0447.2007.01141.x. [DOI] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric. Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze K, Hall MH, McDonald C, Bramon E, Marshall N, Walshe M, Murray R. Auditory P300 in patients with bipolar disorder and their unaffected relatives. Bipolar Disord. 2008;10(3):377–386. doi: 10.1111/j.1399-5618.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- Sklar P, Ripke S, Scott LJ, Andreassen OA, Cichon S, Craddock N, Edenberg HJ, Nurnberger JI, Jr, Rietschel M, Blackwood D, Corvin A, Flickinger M, Guan W, Mattingsdal M, McQuillin A, Kwan P, Wienker TF, Daly M, Dudbridge F, Holmans PA, Lin D, Burmeister M, Greenwood TA, Hamshere ML, Muglia P, Smith EN, Zandi PP, Nievergelt CM, McKinney R, Shilling PD, Schork NJ, Bloss CS, Foroud T, Koller DL, Gershon ES, Liu C, Badner JA, Scheftner WA, Lawson WB, Nwulia EA, Hipolito M, Coryell W, Rice J, Byerley W, McMahon FJ, Schulze TG, Berrettini W, Lohoff FW, Potash JB, Mahon PB, McInnis MG, Zollner S, Zhang P, Craig DW, Szelinger S, Barrett TB, Breuer R, Meier S, Strohmaier J, Witt SH, Tozzi F, Farmer A, McGuffin P, Strauss J, Xu W, Kennedy JL, Vincent JB, Matthews K, Day R, Ferreira MA, O’Dushlaine C, Perlis R, Raychaudhuri S, Ruderfer D, Hyoun PL, Smoller JW, Li J, Absher D, Thompson RC, Meng FG, Schatzberg AF, Bunney WE, Barchas JD, Jones EG, Watson SJ, Myers RM, Akil H, Boehnke M, Chambert K, Moran J, Scolnick E, Djurovic S, Melle I, Morken G, Gill M, Morris D, Quinn E, Muhleisen TW, Degenhardt FA, Mattheisen M, Schumacher J, Maier W, Steffens M, Propping P, Nothen MM, Anjorin A, Bass N, Gurling H, Kandaswamy R, Lawrence J, McGhee K, McIntosh A, McLean AW, Muir WJ, Pickard BS, Breen G, St Clair D, Caesar S, Gordon-Smith K, Jones L, Fraser C, Green EK, Grozeva D, Jones IR, Kirov G, Moskvina V, Nikolov I, O’Donovan MC, Owen MJ, Collier DA, Elkin A, Williamson R, Young AH, Ferrier IN, Stefansson K, Stefansson H, Thornorgeirsson T, Steinberg S, Gustafsson O, Bergen SE, Nimgaonkar V, Hultman C, Landen M, Lichtenstein P, Sullivan P, Schalling M, Osby U, Backlund L, Frisen L, Langstrom N, Jamain S, Leboyer M, Etain B, Bellivier F, Petursson H, Sigur Sson E, Muller-Mysok B, Lucae S, Schwarz M, Schofield PR, Martin N, Montgomery GW, Lathrop M, Oskarsson H, Bauer M, Wright A, Mitchell PB, Hautzinger M, Reif A, Kelsoe JR, Purcell SM. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43(10):977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009;10:85. doi: 10.1186/1471-2202-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz MA, Shenton ME, McCarley RW. Sensory-evoked gamma oscillations in chronic schizophrenia. Biol Psychiatry. 2008a;63(8):744–747. doi: 10.1016/j.biopsych.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008b;64:369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens SH, Logel J, Barton A, Franks A, Schultz J, Short M, Dickenson J, James B, Fingerlin TE, Wagner B, Hodgkinson C, Graw S, Ross RG, Freedman R, Leonard S. Association of the 5’-upstream regulatory region of the alpha7 nicotinic acetylcholine receptor subunit gene (CHRNA7) with schizophrenia. Schizophr Res. 2009;109(1–3):102–112. doi: 10.1016/j.schres.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: The viability of selected candidate measures. Schizophr Bull. 2007;33(1):69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11(2):100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. High-frequency oscillations and the neurobiology of schizophrenia. Dialogues Clin Neurosci. 2013;15(3):301–313. doi: 10.31887/DCNS.2013.15.3/puhlhaas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Xiang B, Deng W, Wu J, Li M, Ma X, Wang Y, Jiang L, McAlonan G, Chua SE, Sham PC, Hu X, Li T. Genome-wide association analysis with gray matter volume as a quantitative phenotype in first-episode treatment-naive patients with schizophrenia. PloS one. 2013;8(9):e75083. doi: 10.1371/journal.pone.0075083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi S, Hampel F, Hata K, Del Toro D, Schwark M, Kvachnina E, Bastmeyer M, Yamashita T, Tarabykin V, Klein R, Egea J. FLRT2 and FLRT3 act as repulsive guidance cues for Unc5-positive neurons. The EMBO journal. 2011;30(14):2920–2933. doi: 10.1038/emboj.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.