Abstract
Background
Immunosuppression is a mainstay of therapy for both induction and maintenance of remission for inflammatory bowel disease (IBD). Women who are chronically immunosuppressed have been shown to be at higher risk of developing cervical high-grade dysplasia and/or carcinoma. There is contradictory data whether immunosuppressed patients with IBD have the same risk profile for cervical cancer as patients with solid organ transplant or HIV infection.
Objective
To determine if the risk of cervical high-grade dysplasia and/or cancer is higher in patients with IBD on immunosuppressive therapy compared to the rates in the general population.
Methods
The studies were restricted to full text retrospective cohort studies and case controls that had a high (6-9) Newcastle- Ottawa Score.
Results
All pooled analyses were based on a random effects model. Five cohort studies and three case control studies of patients with IBD on any immunosuppression with cervical high-grade dysplasia/cancer (n=995) were included in the meta-analysis. The total IBD population in these studies was 77,116. IBD patients had an increased risk of cervical high-grade dysplasia/cancer compared to healthy controls (OR= 1.34, 95% CI: 1.23-1.46). Heterogeneity was detected (I2 = 34.23, Q= 10.64, df = 7; p = 0.15). The source was found to be the type of study, as well as the OR presented (crude vs. adjusted).
Conclusions
There is sufficient evidence to suggest an increased risk of cervical high-grade dysplasia/cancer in patients with IBD on immunosuppressive medications compared to the general population. Given this increased risk, increased screening intervals are indicated.
Keywords: Inflammatory Bowel disease, cervical cancer, high-grade cervical dysplasia, immunosuppression
Introduction
Immunosuppression is a mainstay of therapy for induction and maintenance of remission for moderate to severe inflammatory bowel disease (IBD), Crohn’s Disease (CD) and Ulcerative Colitis (UC), including immunomodulators as well as anti-TNF agents. 1 Immunosuppression in the context of both solid organ transplant as well as HIV has been associated with a higher rate of opportunistic infections and malignancies, 2-10 including non-melanoma skin cancers and lymphomas. Cancers have also been linked to viral infections in these vulnerable populations. For example, Epstein-Barr virus has been identified as a risk factor for various forms of lymphoma, gastric cancer and nasopharyngeal cancer in both post-transplant and HIV patients. 11
Another carcinogenic virus, Human papilloma virus or HPV, is a sexually transmitted virus and is the causal risk factor for cervical cancer, which is the second most common cancer among women worldwide. 12 HPV plays a necessary role in carcinogenesis and the development of cervical cancer. 12,13 In the United States and Western Europe, women with HIV/AIDS have several-fold higher rates of cervical cancer compared with the general population. 14 The American College of Obstetricians and Gynecologists and the American Society for Colposcopy and Cervical Pathology in their latest 2013 guidelines recommend cervical cancer screening with Pap tests every 3 years or 5 year with HPV testing for women with no risk factors. 15 They recognize that these recommendations do not apply to women who are immunosuppressed. Guidelines for HIV infected patients recommend annual screening without HPV testing. 14 These guidelines are applied to all immunocompromised patients, regardless of the cause of their immunosuppressed state. IBD patients may be on varying levels of immunosuppressive therapies, depending on disease severity, 1 and thus annual Pap tests are recommended for women on immunomodulators (Imuran, 6-Mercaptopurine or Methotrexate), anti-TNFs or a combination of both. However, the risk of cervical cancer among patients with IBD on immunosuppressive medication is not well understood and the evidence in current literature is mixed with respect to whether the risk of cervical high-grade dysplasia and cancer is actually elevated in women with IBD. It is thought that women with IBD who are exposed to HPV while on immunosuppressive medication are likely at an increased risk of cervical dysplasia. Current published studies generally lack patients’ HPV status and have not conclusively demonstrated an increased risk of cervical dysplasia. 16
The intention of this meta-analysis is to review the current literature to assess the risk of high-grade dysplasia and cervical cancer in female patients with IBD on immunosuppressive medications compared to the general population.
Methods
Eligibility criteria
The studies selected are case controls, retrospective chart reviews and matched cohorts on patients with IBD on immunosuppressive medication reporting the rates of cervical high-grade dysplasia/cancer compared to the general population. The studies were restricted to English studies from 1980-2014. The study patients included any patient with a diagnosis of IBD on immunosuppressive medication (including but not restricted to azathioprine, 6-mercaptopurine, methotrexate, infliximab, adalimumab, and prednisone) and high-grade dysplasia (HSIL) on Pap smear or CIN 2/3 or cervical cancer on biopsy. The control patients were general population controls with no history of IBD. Studies that included patients with IBD in the control group were excluded. The exposure of interest is treatment with immunosuppressive medications, and as such, studies that exclusively addressed cancer rates in IBD patients not on immunosuppressive medications were excluded. The outcome was measured by an odds ratio (OR) with associated 95% confidence intervals for patients who developed high-grade cervical dysplasia/cancer on immunosuppressive medications compared to patients in the general population without IBD.
Information Sources
Studies were identified by the electronic databases Embase, Pubmed and Web of Science, searching for English studies, between the years 1980 to 2014. The bibliographies of the articles found were used to find further articles. The last literature search was performed Aug 11, 2014.
Search
The following key words were used in MeSH for Pubmed: Inflammatory Bowel Disease, cervical cancer, uterine cervical neoplasms, Crohn’s Disease, colitis, ulcerative, uterine cervical dysplasia and uterine cervical diseases. See Appendix 1 for an example of the search strategy.
Study selection
The three authors performed the eligibility assessment independently and in an un-blinded manner, and any disagreements between reviewers were resolved by consensus. Studies were screened using the title and abstract. They were restricted to full articles published or accepted for publication in English. The type of studies included in the meta-analysis included: population-based cohort studies, retrospective cohort studies and case controls. The following types of studies were excluded: case reports, abstracts, editorials, prior meta-analyses and reviews. (See Figure 1)
Figure 1.
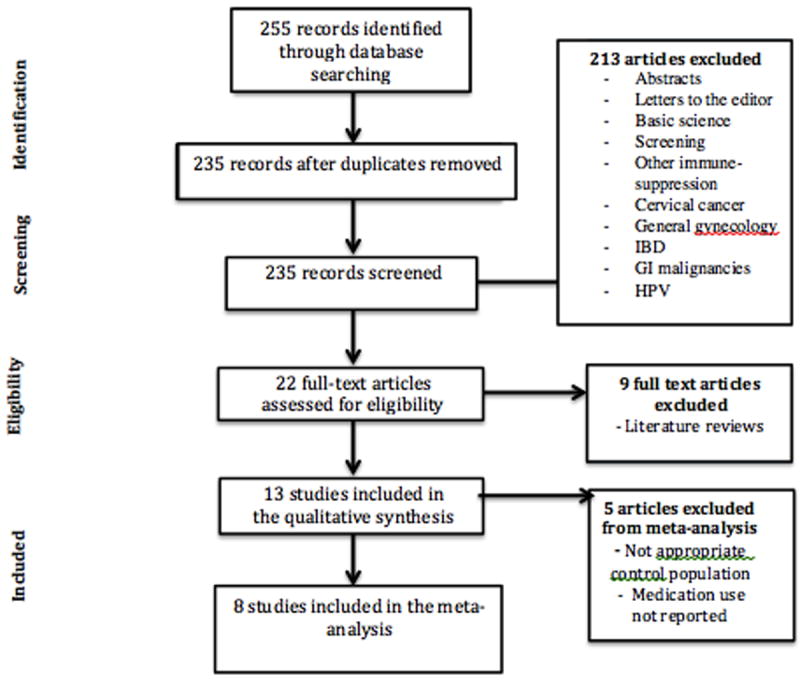
Study Selection
Data Collection Process
We developed a data extraction sheet and refined it after the first round of data collection. All authors extracted the data from the studies included in the meta-analysis. A second reviewer confirmed all the extracted data. Any disagreements were discussed among all three reviewers. Two authors were contacted for additional information from their studies. The additional information supplied by the author for one study allowed for calculation of the OR based on raw data. 17 The authors of the Bernstein et al. were contacted and were not able to supply the necessary information to perform calculations and as such this study was excluded do to the lack of an adequate and comparable metameter. 18 In the cases where the metameter was missing, the OR and 95% confidence intervals were calculated from the raw data using the Comprehensive Meta-Analysis software. In one instance a study reported the incidence rate ratio and given the rarity of cervical cancer this was interpreted as interchangeable with an OR. 19 To ensure that the data in each study was only used in the one study, we cross-referenced author names and institutions between the studies included in the meta-analysis.
Data items
Information extracted from each study included: the characteristics of the patients with IBD as well as the controls (including confirmation of diagnosis, the number of patients with CD versus UC, treatment type, length of immunosuppressive treatment, Pap and/or cervical biopsy results, follow-up time). The location of study, study type, outcome measure, and confidence interval were also extracted. The Newcastle-Ottawa Score was used to rank the study quality. The age of the patients, smoking, status and ethnicity were not available or recorded for all the studies. These factors were controlled for in the case controls.
Risk of Bias in Individual Studies
To explore variability in the study results (heterogeneity) we pre-specified the following hypotheses before conducting the analysis: the studies would differ by class of immunosuppression, duration of use and dosage, as well as IBD type (CD versus UC). Another cause of heterogeneity will likely be due to the pooled data for high-grade cervical dysplasia and cancer. Two reviewers scored the studies independently, with a third reviewer arbitrating in the case of disagreements.
Summary Measures
The primary measure of effect was odds ratios (OR) and 95% confidence intervals. In the majority of articles the OR and confidence intervals were available either adjusted or unadjusted. The adjusted OR was used when available. For certain articles the crude OR was calculated from the raw data. The incidence rate ratio from the article by Marehbian et al. was used as an OR for the meta-analysis. 19 This can be justified, as high-grade dysplasia/cervical cancer is a rare event such that the IRR can be exchangeable with the OR as an effect measure.
Planned Methods of Analysis
The a priori hypothesis for this meta-analysis is that there would be significant heterogeneity amongst the studies. For this reason we chose to analyze the data using the random effects model. We assessed for heterogeneity using a Q statistic and an I2 and proceeded with subgroup analyses. Classic fail-safe and Orwins fail-safe were used to determine publication bias. Finally a fixed effects meta-regression by publication year was performed.
Risk of Bias Across Studies
Publication bias was assessed with a funnel plot model and further analyzed using Duval and Tweedies trim and fill analysis.
Additional Analyses
Further stratification by study type (cohort versus case control) and OR (adjusted versus crude) was performed. In an optimal setting we would have liked to further stratify the studies based on IBD type (CD versus UC), extent of cervical dysplasia (high-grade dysplasia versus cancer) as well as immunosuppressive drug class and duration of therapy however, this was not possible based on available data. One study-removed analysis was also performed.
Results
Study Selection
A total of 8 studies were identified for inclusion in the review. The search of PubMed, EMBASE and Web of Science databases provided a total of 255 citations. After adjusting for duplicates 235 remained. Of these, 213 studies were discarded because after reviewing the abstracts it appeared that these papers clearly did not meet the inclusion criteria. The full text of the remaining 22 citations was examined in more detail. It appeared that 9 studies did not meet the inclusion criteria as described, and therefore 13 studies were included in qualitative synthesis. However, 5 were ultimately not included in the meta-analysis due to inappropriate control group or unclear reporting of medication use in the IBD population. Only one of these five studies used IBD patients not on therapy as their control group. Four unpublished relevant studies were obtained but were not included in the analysis. See flow diagram (Figure 1).
Methods
Eight studies were selected for review. Three studies were case-control studies and five were cohort studies all published in English. The duration of follow up and time on immunosuppressive medication was variable but ranged from 2-36 years depending on the availability of data in each database used.
Participants
The included studies involved 77,116 participants with inflammatory bowel disease. The main inclusion criteria included females (16 years or older) with a confirmed diagnosis of IBD on any immunosuppressive medication for IBD and at least one Pap test within the defined study period (either prior to inclusion into the study or at the time of inclusion) (Table 1). Controls were women without IBD who had a Pap test during the defined study period and were matched by age in 7 of the studies. Confounders such as smoking status and OCP use were often controlled for as well (Table 2).
Table 1.
Summary Table of Studies Evaluating Risk of Cervical High-Grade Dysplasia/Cancer in IBD patients on immunosuppressive medications.
| Source | Country | Study Type | # IBD with dysplasia/cancer | # IBD Total | Inclusion Criteria | Medications Used |
|---|---|---|---|---|---|---|
| Kim, 2014 | US | Cohort | 59 | 25176 | Women age ≥18 with SID, including IBD based on (ICD-9) codes with ≥1 disease specific medication prescription | IM, steroids |
| Bhatia, 2006 | US | Cohort | 4 | 116 | Women age 18-80 with confirmed IBD and at least one Pap smear in the previous five years for inclusion in the study | IM, steroids, biologics |
| Kane, 2008 | US | Case control | 15 | 40 | Female patients with a history of IBD with a confirmed normal Pap smear prior to IBD diagnosis | IM, steroids |
| Lees, 2009 | Scotland | Case control | 25 | 362 | Women with a diagnosis of IBD prior to age 60 | IM, biologics |
| Marehbian, 2009 | US | Cohort | 70 | 22310 | Patients with at least one claim for crohn’s disease by ICD-9 codes | IM, steroids, biologics |
| Hutfless, 2008 | US | Cohort | 10 | 1165 | women age 15–68 with confirmed IBD from January 1996 to 30 June 2006 | IM, steroids, biologics |
| Rungoe, 2014 | Denmark | Cohort | 678 | 27408 | Women with a confirmed diagnosis of either UC or CD after January 1, 1979 residing in Denmark | IM, steroids, biologics |
| Singh 2009 | Canada | Case control | 134 | 539 | Women ≥18 from Manitoba who had an abnormal Pap test result between Jan. 2002 and Dec. 2006. | IM, steroids |
Table 2.
Quality Measures for Each Study
| Studies | Matched Control Group | Controlled for Confounding | Crude vs Adjusted OR | Newcastle-Ottawa Score |
|---|---|---|---|---|
| Kim, 2014 | no- women with HTN | yes | crude OR | 9 |
| Bhatia, 2006 | yes-age matched | no | crude OR | 6 |
| Kane, 2008 | yes-age, race and parity | yes | adjusted | 8 |
| Lees, 2009 | yes-age and geographical location | yes | crude OR | 7 |
| Marehbian, 2009 | yes-age, gender, health plan, available follow up | yes | adjusted | 8 |
| Hutfless, 2008 | yes-age matched | yes | crude OR | 8 |
| Rungoe, 2014 | yes-age matched | yes | crude OR | 9 |
| Singh 2009 | yes-age, number of Paps, health coverage | yes | adjusted | 8 |
Intervention
There is no true intervention in these studies however the exposure of interest is the use of immunosuppressive medications. Studies counted all immunosuppressive medications including steroids, imuran, azathioprine, 6-mercaptopurine, methotrexate, infliximab and adalimumab equally. Monotherapy and combination therapy were also equally grouped for assessment of the primary outcome. However, a few studies performed a subgroup analysis in which they stratified by medication class.
Primary Outcomes
In all studies the primary outcome assessed was risk of cervical cancer or high-grade dysplasia in patients with IBD on immunosuppressive medications compared to a healthy population without IBD. Most studies presented either crude or adjusted ORs. One study presented hazard ratios. Odds ratios were calculated from raw data if not provided. One study presented a risk ratio which was assumed to be equivalent to the OR given the rarity of cervical cancer. Seven of the eight studies evaluated and controlled for additional confounders, however not all presented the adjusted OR (Table 2).
Secondary and additional outcomes
These included risk of cervical cancer in IBD patients by medication class. These analyses varied between studies as some compared these subgroups to a healthy population and others used an IBD population not on immunosuppressive treatment as their comparison group. As the subgroup analyses were not consistent throughout the 8 studies they were not included in the meta-analysis.
Risk of Bias within studies
Variability in studies (heterogeneity) was assessed using the Newcastle-Ottawa score (Table 2). Other potential sources of heterogeneity included whether confounders were controlled for and if a matched control group was used. Final sources of variability between studies included what medications the patients with IBD were taking and the duration of therapy as well as the proportion of CD and UC patients in each study.
Individual Studies
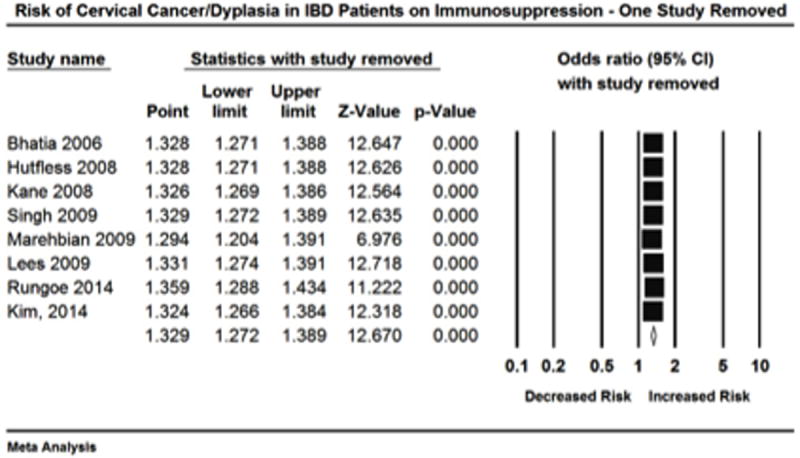
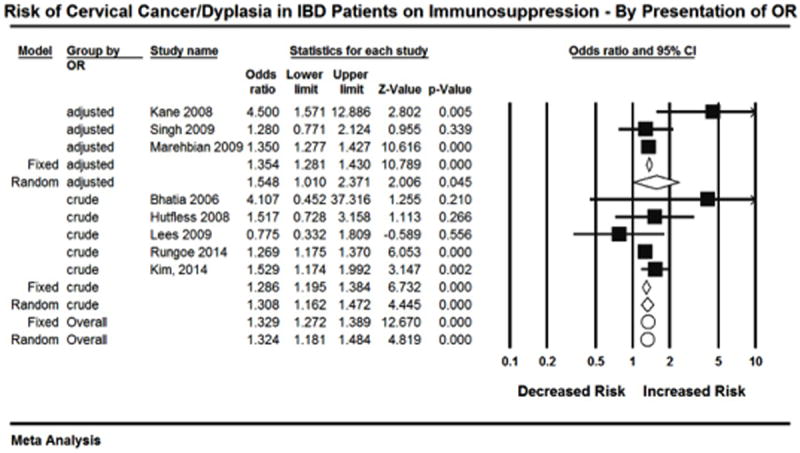
The overall estimate for risk of high-grade dysplasia/cervical cancer in IBD patients on immunosuppressive medication is based on 8 studies. For each study an OR was either presented or obtained from raw data (Figure 2). Given the a priori hypothesis of heterogeneity the random effects model was used, which revealed that patients with IBD on immunosuppressive therapy had an increased risk of high-grade dysplasia/cervical cancer compared to healthy controls (OR= 1.34, 95% CI: 1.23-1.46). Heterogeneity was detected (I2 = 34.23, Q= 10.64, df = 7; p = 0.15), however the non-significant p value for Q suggests that minimal to moderate heterogeneity was present. Retrospective exploration of the heterogeneity did not identify one study in particular that seemed to differ from the others. On one study removed analysis point estimated remained uniform (Figure 3).
Figure 2.
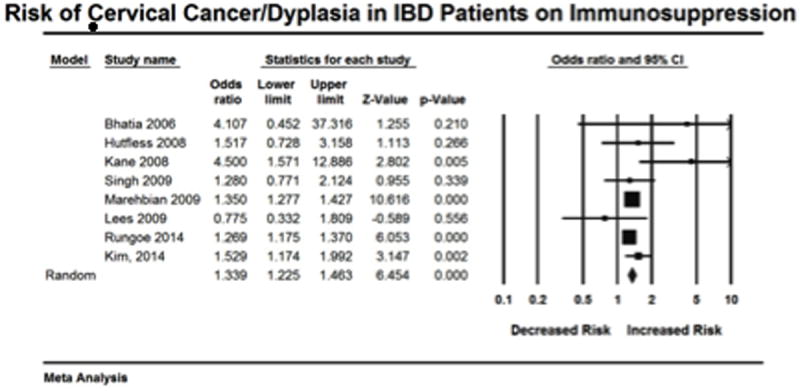
Figure 3.
To explore this heterogeneity further, subgroup analysis was performed. Analysis was first done according to study type. Cohort studies resulted in a significant point estimate (OR=1.328, 95% CI: 1.127-1.448) ’(Figure 4). This subgroup of 5 studies had an I2=0%. However, the point estimate for the three case control studies was not significant (OR=1.374, 95% CI: 0.919-2.053). This subgroup had an I2=70.2%. The total within the groups resulted in a Q value of 10.6 (p=0.10). The total between these two groups resulted in a Q value of 0.03 (p=0.87).
Figure 4.
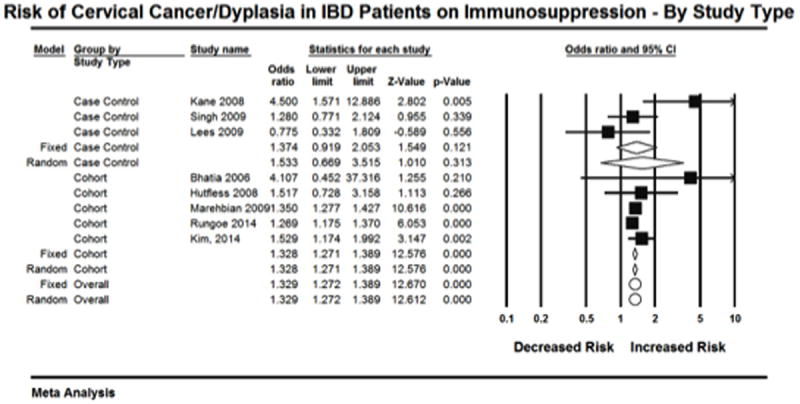
Subgroup analysis was also done comparing studies that presented adjusted versus crude OR. Three studies presented adjusted ORs, and this analysis resulted in a significant point estimate (OR=1.354, 95% CI: 1.281-1.430)(Figure 5). However, this subgroup had an I2=60.5%. The point estimate for the five studies that presented crude OR was also significant (OR=1.285, 95% CI: 1.195-1.384). This subgroup had an I2=8.9%. The total within the groups resulted in a Q value of 9.4 (p=0.15). The total between these two groups resulted in a Q value of 1.18 (p=0.276).
Figure 5.
In order to assess for publication bias a funnel plot was drawn (Figure 6). This did show evidence of asymmetry. Therefore Duval and Tweedies trim and fill analysis was done revealing an addition of 2 studies to the left of the mean (Figure 7).
Figure 6.
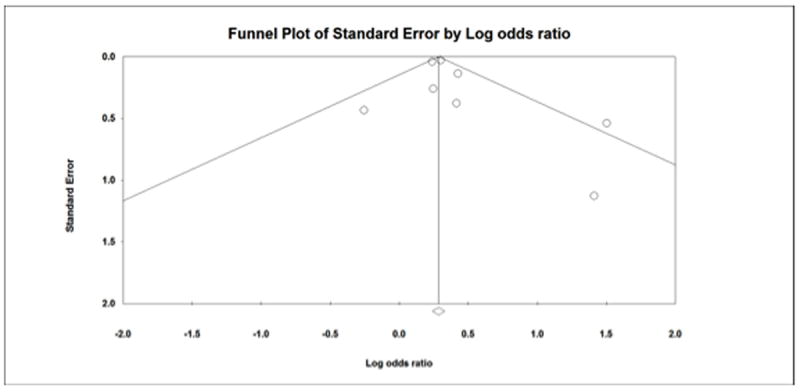
Figure 7.

Classic fail-safe revealed a p=0.00 and 160 studies were needed to bring the p value >0.05. Orwins fail-safe reported needing 8 studies needed to bring the OR less than 1.1, which was designated as the trivial OR.
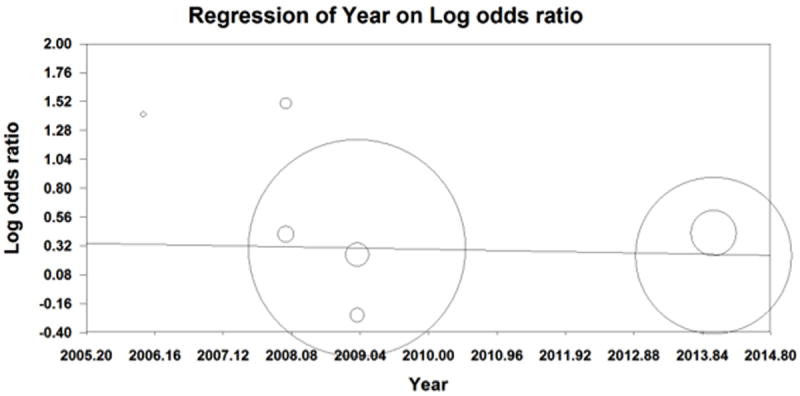
Cumulative analysis was also done by publication year (Figure 8). Older publications appeared to have larger point estimates but also had wider confidence intervals. Therefore, a meta-regression was done by publication year. The results of a fixed effect regression revealed β=-0.01 suggesting for every increase in 1 year the log Odds of getting high-grade dysplasia or cervical cancer decreased by 0.01 (Figure 9). However, this was result was non-significant (p=0.264).
Figure 8.
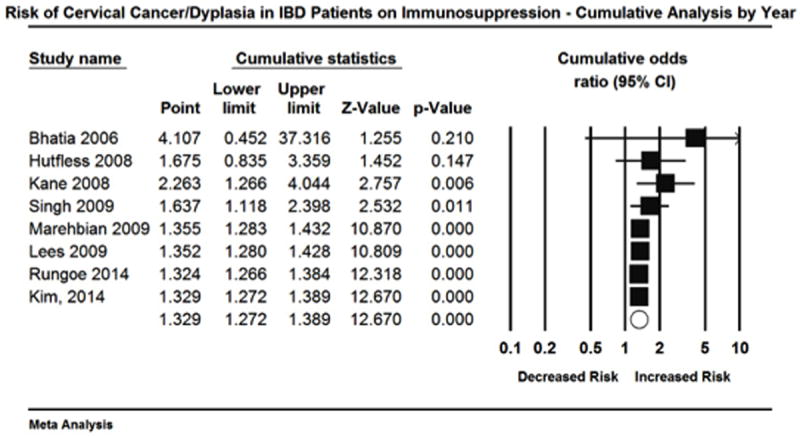
Figure 9.
Discussion
In this meta-analysis, we have demonstrated that there is an increased risk of high-grade cervical dysplasia and cervical cancer among patients with inflammatory bowel disease who are on immunosuppressive medications (OR 1.34, 95% CI:1.23-1.46) compared to the general population. Although there was a range of study designs in the studies reviewed, the overall quality of the studies was good. Six of the eight studies scored 8 or better (out of 9) on the Newcastle-Ottawa scale.
One of the major questions regarding cervical cancer risk in this population is increased risk associated with long-term use of immunosuppression as these therapies are a mainstay of both and induction and maintenance therapy for IBD. Prior studies have demonstrated an increased risk of cervical cancer among immunosuppressed patients in the transplant and HIV populations, 20 however whether this same risk applies to the immunosuppression associated with the treatment of IBD is less clear.
In only a minority of the studies were they able to stratify by medication class. Hutfless, et al. compared IBD patients to age- matched controls and demonstrated 10 cases of cervical cancer among 1165 women with IBD (adjusted OR=1.45; 95% CI: 0.74-2.84). 22 The risk of cervical cancer and dysplasia was further stratified by medication class. For 5-ASAs the OR=1.65, for corticosteroids the OR=2.79 and for immunomodulators the OR= 3.45 (all p> 0.05), with no cervical cancers associated with infliximab exposure. 22 This may indicate a increased risk of cervical cancer and dysplasia among IBD patients in general compared to the baseline population given the increase risk seen with 5-ASAs alone. One study did directly compare IBD patients on and off immunosuppression in a subgroup analysis. Kane et al. found significantly increased proportions of high-risk cervical abnormalities in patients with IBD who had been exposed to immunosuppressive therapy when compared to IBD patients who had not been exposed to immunosuppression. 23 Similarly, Singh, et al. did report an increased risk of cervical abnormalities among patients with combined corticosteroids and immunosuppressants (not defined further) as compared to IBD patients who did not use either corticosteroids and immunosuppressants, OR 1.46 (95% CI: 1.09-1.81). 25
Despite the concerns expressed by gastroenterologists and gynecologists regarding the potential increased risks for cervical abnormalities and ultimately cervical cancer in the IBD population, there remains a relative paucity of studies within this area. 22,29-31 Although the studies that have been performed have provided important information and improved our understanding of this association, they are varied in their study design, control populations, and most importantly their reports of the degree and duration of immunosuppression in the study patients. Furthermore, IBD is not a singular disease entity. The majority of the studies did not clearly separate UC and CD to assess risk separately.
Another limitation of this meta-analysis is the reporting of high-grade cervical dysplasia and cancer as a single entity. If immunosuppression for IBD accelerates the development of high-grade cervical lesions, this would confirm the need for shorter screening intervals, which are currently recommended for all immunosuppressed women. However, even though high-grade lesions can progress to cervical cancer, 6 to 50% will regress spontaneously. 33 Thus high-grade dysplasia and cervical cancer represent separate disease entities along a continuum. It would be preferable to evaluate their risks separately in a subgroup analysis.
This study does have multiple strengths. Using a broad search strategy encompassing multiple databases, we were able to capture all available studies addressing the relationship between cervical cancer and patients with IBD on immunosuppressive therapy. A uniform comparison group was needed to perform this analysis and the majority of the studies did use a healthy population, although an IBD group off immunosuppression would have been preferable.
There is ongoing concern regarding the increased risk of cervical abnormalities in women with IBD on immunosuppressive therapy, and based on this study this concern seems relatively well founded. 29-32 However, due to the limited number of studies and a large variation in data reporting, we were unable to further stratify our meta-analysis by level of immunosuppression, individual drugs, drug combinations used, or duration of immunosuppression. This is a limitation of our meta-analysis, as this likely plays a significant role in the overall risk for development of cervical dysplasia and ultimately cervical cancer. Given continued development of novel therapies for treatment of IBD including biologic agents, this risk will continue to be an area of concern. The ability to stratify our meta-analysis would have allowed for more informative counseling of these women with regards to cervical cancer screening. Based on the increased risk identified in this study we would advise that women with IBD on immunosuppression continue with annual screening per current guidelines.
Acknowledgments
The authors would like to acknowledge Dr. Michael A. Stoto for his help and guidance during the preparation of this manuscript.
Appendix 1
Search strategy
The pubmed criteria for the searches: ((((((“Uterine Cervical Diseases”[Mesh]) AND “Uterine Cervical Dysplasia”[Mesh]) AND “Cervical Intraepithelial Neoplasia”[Mesh]) AND “Uterine Cervical Neoplasms”[Mesh]) AND “Colitis, Ulcerative”[Mesh]) AND “Crohn Disease”[Mesh]) AND “Inflammatory Bowel Diseases”[Mesh], as well as ((“Uterine Cervical Diseases”[Mesh] AND “Uterine Cervical Dysplasia”[Mesh]) AND “Uterine Cervical Neoplasms”[Mesh]) AND “Inflammatory, and Inflammatory bowel disease and cervical dysplasia.
Footnotes
Disclosures: The authors have no financial disclosure
References
- 1.Sandborn WJ, Feagan BG, Lichtenstein GR. Medical management of mild to moderate Crohn’s disease: evidence-based treatment algorithms for induction and maintenance of remission. Aliment Pharmacol Ther. 2007;26:987–1003. doi: 10.1111/j.1365-2036.2007.03455.x. [DOI] [PubMed] [Google Scholar]
- 2.Cutrell J, Bedimo R. Non-AIDS-defining cancers among HIV-infected patients. Curr HIV/AIDS Rep. 2013;10:207–216. doi: 10.1007/s11904-013-0166-8. [DOI] [PubMed] [Google Scholar]
- 3.Dantal J, Hourmant M, Cantarovich D, et al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet. 1998;351:623–628. doi: 10.1016/S0140-6736(97)08496-1. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher MP, Kelly PJ, Jardine M, et al. Long-term cancer risk of immunosuppressive regimens after kidney transplantation. J Am Soc Nephrol. 2010;21:852–858. doi: 10.1681/ASN.2009101043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen P, Hansen S, Moller B, et al. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol. 1999;40:177–186. doi: 10.1016/s0190-9622(99)70185-4. [DOI] [PubMed] [Google Scholar]
- 6.Kalil AC, Florescu MC, Grant W, et al. Risk of serious opportunistic infections after solid organ transplantation: interleukin-2 receptor antagonists versus polyclonal antibodies. A meta-analysis. Expert Rev Anti Infect Ther. 2014;12:881–896. doi: 10.1586/14787210.2014.917046. [DOI] [PubMed] [Google Scholar]
- 7.Miro JM, Aguero F, Duclos-Vallee JC, et al. Infections in Solid Organ Transplant HIV-infected patients. Clin Microbiol Infect. 2014 doi: 10.1111/1469-0691.12754. [DOI] [PubMed] [Google Scholar]
- 8.Rubinstein PG, Aboulafia DM, Zloza A. Malignancies in HIV/AIDS: from epidemiology to therapeutic challenges. AIDS. 2014;28:453–465. doi: 10.1097/QAD.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahapur PR, Bidri RC. Recent trends in the spectrum of opportunistic infections in human immunodeficiency virus infected individuals on antiretroviral therapy in South India. J Nat Sci Biol Med. 2014;5:392–396. doi: 10.4103/0976-9668.136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang CC, Silverberg MJ, Abrams DI. Non-AIDS-Defining Malignancies in the HIV-Infected Population. Curr Infect Dis Rep. 2014;16 doi: 10.1007/s11908-014-0406-0. 406-014-0406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neparidze N, Lacy J. Malignancies associated with epstein-barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12:358–371. [PubMed] [Google Scholar]
- 12.Carter JR, Ding Z, Rose BR. HPV infection and cervical disease: a review. Aust N Z J Obstet Gynaecol. 2011;51:103–108. doi: 10.1111/j.1479-828X.2010.01269.x. [DOI] [PubMed] [Google Scholar]
- 13.Chan JK, Berek JS. Impact of the human papilloma vaccine on cervical cancer. J Clin Oncol. 2007;25:2975–2982. doi: 10.1200/JCO.2007.10.8662. [DOI] [PubMed] [Google Scholar]
- 14.Mofenson LM, Brady MT, Danner SP, et al. Guidelines for the Prevention and Treatment of Opportunistic Infections among HIV-exposed and HIV-infected children: recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR Recomm Rep. 2009;58:1–166. [PMC free article] [PubMed] [Google Scholar]
- 15.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137:516–542. doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 16.Velayos F. Managing risks of neoplasia in inflammatory bowel disease. Curr Gastroenterol Rep. 2012;14:174–180. doi: 10.1007/s11894-012-0247-7. [DOI] [PubMed] [Google Scholar]
- 17.Rungoe C, Simonsen J, Riis L, et al. Inflammatory Bowel Disease and Cervical Neoplasia: A population-based nationwide cohort study. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 18.Bernstein CN, Blanchard JF, Kliewer E, et al. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.Marehbian J, Arrighi HM, Hass S, et al. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104:2524–2533. doi: 10.1038/ajg.2009.322. [DOI] [PubMed] [Google Scholar]
- 20.Busnach G, Piselli P, Arbustini E, et al. Immunosuppression and cancer: A comparison of risks in recipients of organ transplants and in HIV-positive individuals. Transplant Proc. 2006;38:3533–3535. doi: 10.1016/j.transproceed.2006.10.144. [DOI] [PubMed] [Google Scholar]
- 21.Connell WR, Kamm MA, Dickson M, et al. Long-term neoplasia risk after azathioprine treatment in inflammatory bowel disease. Lancet. 1994;343:1249–1252. doi: 10.1016/s0140-6736(94)92150-4. [DOI] [PubMed] [Google Scholar]
- 22.Hutfless S, Fireman B, Kane S, et al. Screening differences and risk of cervical cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2008;28:598–605. doi: 10.1111/j.1365-2036.2008.03766.x. [DOI] [PubMed] [Google Scholar]
- 23.Kane S, Khatibi B, Reddy D. Higher incidence of abnormal Pap smears in women with inflammatory bowel disease. Am J Gastroenterol. 2008;103:631–636. doi: 10.1111/j.1572-0241.2007.01582.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim SC, Glynn RJ, Giovannucci E, et al. Risk of high-grade cervical dysplasia and cervical cancer in women with systemic inflammatory diseases: a population-based cohort study. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh H, Demers AA, Nugent Z, et al. Risk of cervical abnormalities in women with inflammatory bowel disease: a population-based nested case-control study. Gastroenterology. 2009;136:451–458. doi: 10.1053/j.gastro.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Bhatia J, Bratcher J, Korelitz B, et al. Abnormalities of uterine cervix in women with inflammatory bowel disease. World J Gastroenterol. 2006;12:6167–6171. doi: 10.3748/wjg.v12.i38.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lees CW, Critchley J, Chee N, et al. Lack of association between cervical dysplasia and IBD: a large case-control study. Inflamm Bowel Dis. 2009;15:1621–1629. doi: 10.1002/ibd.20959. [DOI] [PubMed] [Google Scholar]
- 28.Jess T, Horvath-Puho E, Fallingborg J, et al. Cancer risk in inflammatory bowel disease according to patient phenotype and treatment: a Danish population-based cohort study. Am J Gastroenterol. 2013;108:1869–1876. doi: 10.1038/ajg.2013.249. [DOI] [PubMed] [Google Scholar]
- 29.Greywoode R, LaFond J, Fine S, et al. Women with inflammatory bowel disease do not receive adequate cervical cancer screening or pregnancy counseling. Inflamm Bowel Dis. 2013;19:E6–7. doi: 10.1002/ibd.22836. [DOI] [PubMed] [Google Scholar]
- 30.Mahadevan U. Cervical neoplasia risk in IBD: truth or hysteria? Inflamm Bowel Dis. 2009;15:1619–1620. doi: 10.1002/ibd.20957. [DOI] [PubMed] [Google Scholar]
- 31.Zabana Y, Barreiro M, Manosa M, et al. Cervical dysplasia in immunosuppressed IBD women. Inflamm Bowel Dis. 2009;15:1774. doi: 10.1002/ibd.20878. [DOI] [PubMed] [Google Scholar]
- 32.Hutfless S, Fireman B, Kane S, et al. Screening differences and risk of cervical cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2008;28:598–605. doi: 10.1111/j.1365-2036.2008.03766.x. [DOI] [PubMed] [Google Scholar]
- 33.Trimble CL, Piantadosi S, Gravitt P, et al. Spontaneous regression of high-grade cervical dysplasia: effects of human papillomavirus type and HLA phenotype. Clin Cancer Res. 2005;11:4717–4723. doi: 10.1158/1078-0432.CCR-04-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]


