Abstract
Nociceptive plasticity and central sensitization within the spinal cord depend on neurobiological mechanisms implicated in learning and memory in higher neural systems, suggesting that the factors that impact brain-mediated learning and memory could modulate how stimulation affects spinal systems. One such factor is temporal regularity (predictability). The present paper shows that intermittent hindleg shock has opposing effects in spinally transected rats depending upon whether shock is presented in a regular or irregular (variable) manner. Variable intermittent legshock (900 shocks) enhanced mechanical reactivity to von Frey stimuli (hyperreactivity), whereas 900 fixed spaced legshocks produced hyporeactivity. The impact of fixed spaced shock depended upon the duration of exposure; a brief exposure (36 shocks) induced hyperreactivity whereas an extended exposure (900 shocks) produced hyporeactivity. The enhanced reactivity observed after variable shock was most evident 60–180 min after treatment. Fixed and variable intermittent stimulation applied to the sciatic nerve, or the tail, yielded a similar pattern of results. Stimulation had no effect on thermal reactivity. Exposure to fixed spaced shock, but not variable shock, attenuated the enhanced mechanical reactivity (EMR) produced by treatment with hindpaw capsaicin. The effect of fixed spaced stimulation lasted 24 hr. Treatment with fixed spaced shock also attenuated the maintenance of capsaicin-induced EMR. The results show that variable intermittent shock enhances mechanical reactivity, while an extended exposure to fixed spaced shock has the opposite effect on mechanical reactivity and attenuates capsaicin-induced EMR.
Keywords: plasticity, spinal cord injury, pain, learning, capsaicin
INTRODUCTION
Nociceptive stimulation and inflammation can enhance behavioral reactivity to both noxious (hyperalgesia) and non-noxious tactile (allodynia) stimulation. For example, peripheral treatment with the TRPV1 agonist capsaicin enhances pain reports in humans (Simone et al., 1989; LaMotte et al., 1992) and tactile reactivity in animals (Baumann et al., 1991; Sluka et al., 1997; Kim et al., 2007). Research suggests that enhanced pain is due, in part, to the sensitization of nociceptive systems within the spinal cord, a phenomenon known as central sensitization (Woolf and Salter, 2000; Latremoliere and Woolf, 2009). Supporting this, intradermal application of chemical irritants (e.g. capsaicin, mustard oil, formalin, bee venom) induces enhanced mechanical reactivity (EMR) even after communication with the brain has been disrupted (Wall and Woolf, 1984; Sivilotti and Woolf, 1994; You and Arendt-Nielsen, 2005).
Because alterations in spinal nociceptive processing are thought to contribute to the development of prolonged pain symptoms, researchers have sought to identify the physiological mechanisms responsible for the initiation and maintenance of pain states over time. Research has shown that the sensitization of spinal nociceptive systems depends on a form of NMDA receptor (NMDAR) mediated plasticity (Coderre and Melzack, 1992; Willis, 2009; Tan et al., 2011) and that nociceptive sensitization can be induced by a variety of manipulations, including brief exposure to low frequency (< 5 Hz) electrical stimulation (Davies and Lodge, 1987; Woolf and Thompson, 1991; You et al., 2004). Repeated electrical stimulation can increase neural excitability within the dorsal horn (wind-up) and behavioral reactivity to nociceptive stimuli (Mendell, 1966; Herrero et al., 2000).
Recent studies indicate that the sensitization of spinal nociceptive systems may also affect adaptive plasticity within the spinal cord, as indexed by the capacity to learn a simple instrumental task. Spinally transected rats given shock to one hindlimb whenever the leg is extended normally exhibit a progressive increase in flexion duration (the index of learning) that reduces net shock exposure (Grau et al., 1998). Treatments that induce inflammation, EMR, and central sensitization (e.g. peripheral capsaicin or carrageenan) impair this learning (Ferguson et al., 2006; Hook et al., 2008). Interestingly, variable intermittent shock at an intensity that activates C-fibers also impairs instrumental learning and this effect lasts at least 24 hr (Crown et al., 2002; Baumbauer et al., 2008).
We recently discovered that the impact of intermittent shock on learning depends on whether the stimulation occurs in a regular or irregular manner; subjects given 900 intermittent 80-msec shocks in a variable manner (0.2–3.8 s [mean 2 s], rectangular distribution) later fail to learn whereas subjects exposed to an equal number of shocks at a regular (fixed 2 s) exhibit no learning impairment (Baumbauer et al., 2008). Rather, exposure to fixed spaced shock engages an opponent-like process that enables learning and blocks the induction of the learning deficit for up to 24 hr (Baumbauer et al., 2009). The beneficial effect of fixed spaced shock requires extended training, involves a form NMDAR-mediated plasticity and protein synthesis, and depends on the neurotrophin BDNF (Baumbauer et al., 2009).
Elsewhere, we showed that exposure to fixed spaced shock also counters the learning impairment produced by capsaicin administration (Baumbauer and Grau, 2011). However, the more important question is whether fixed spaced shock affects capsaicin-induced EMR. Using a model system, in which spinal mechanisms are surgically isolated from the brain, we show that variable and fixed spaced stimulation affects mechanical reactivity in opposite ways; fixed-spaced shock produces diminished (hypo-) reactivity whereas variable shock induces EMR. The outcome observed depends on the duration of exposure and time of testing. Most importantly, exposure to fixed spaced shock prevented and reversed the EMR produced by capsaicin.
EXPERIMENTAL PROCEDURES
Subjects
Subjects were male Sprague-Dawley rats obtained from Harlan (Houston, TX). Rats were 70–90 days old and weighed 350–400g at the time of spinal cord transection. They were housed in pairs with free access to food and water, and were maintained on a 14–10 hr light-dark cycle. All experiments were carried out in accordance with NIH standards for the care and use of laboratory animals (NIH publications No. 80-23), and were approved by the University Laboratory Animal Care Committee at Texas A&M University. Every effort was made to minimize suffering and limit the number of animals used.
Spinalization surgery
Prior to surgery, the fur over the thoracic portion of the vertebral column was shaved and disinfected with betadine solution. Rats were anesthetized with isoflurane gas. The rat’s head was rendered immobile in a stereotaxic apparatus with a small (5 X 4 X 2.5 cm) gauze pillow under the subject’s chest. An anterior to posterior incision over the second thoracic vertebrae (T2) was made, the tissue just rostral to T2 was cleared using rongeurs, and the cord exposed and cauterized. The remaining gap in the cord was filled with Gelfoam (Pharmacia Corp., Kalamazoo, MI) and the wound was closed with Michel clips (Fisher Scientific, Waltham, MA). Following closure of the wound, the surface of each leg was shaved for electrode placement. Intraperitoneal injections (3 mL) of 0.9% saline solution were administered post-operatively to prevent dehydration. Following surgery, rats were placed in a temperature-controlled environment (25.5 °C) and monitored until awake. All rats were checked every six to eight hours during the 18–24 hr post-surgical period. During this time, hydration was maintained with supplemental injections of saline, and the rats’ bladders and colons were expressed as necessary.
Spinal transections were confirmed by inspecting the cord under a 10× dissection scope, and observing the behavior of the subjects after they recovered to ensure that they exhibited paralysis below the level of the forepaws and did not exhibit any supraspinally-mediated pain responses to legshock.
Stimulation procedures
In all experiments, except Experiment 3, shock was applied using intramuscular shock electrodes constructed from stainless steel wire (0.01 mm2 [36 AWG], magnet wire single beldsol) that were inserted into the tibialis anterior muscle. Shock was administered using a constant current AC shock generator (Model SG-903; BRS/LVE, Laurel MD). This mode of stimulation is regularly used in our laboratory to study learning by instituting a behavioral contingency between the execution of a flexion response and the presentation of shock. From that work, it is known that the effectiveness of stimulation varies with electrode placement. Here, and elsewhere (e.g. Grau et al., 1998), we regularly control for this variability by adjusting the shock intensity so that it elicits a comparable flexion force (0.4 N) across subjects.
Where sciatic stimulation was employed (Experiment 3), the sciatic nerve in one hind leg (counterbalanced across subjects) was accessed by dissecting away the biceps femoris and vastus lateralus muscles to leave the nerve exposed in the popliteal fossa. Bipolar hook electrodes were then placed under the sciatic nerve, with the electrodes 5 mm apart. A test pulse was delivered from the stimulator (model S9; Grass Medical Instruments, Quincy, MA.) to ensure contact between the nerve and electrodes. Once the electrodes were in place, the appropriate stimulation (40 V, 50 Hz, 80 ms bursts, 10 ms pulse width) treatment was administered. Warm mineral oil was applied, as needed, to prevent dehydration of the exposed nerve.
In all experiments, intermittent stimulation was applied using 80 ms shocks spaced an average of 2 s apart. For subjects in the fixed spaced condition, shocks occurred at regular (2-s) intervals. Variable stimulation was created using a rectangular distribution that varied between 0.2 and 3.8 s, with a mean interstimulus interval (ISI) of 2 s. Prior work has shown that variable shock undermines learning (Crown et al., 2002; Baumbauer et al., 2007; Baumbauer et al., 2008; Ferguson et al., 2008; Baumbauer et al., 2009), while fixed shock does not (Baumbauer et al., 2008; Baumbauer et al., 2009).
Tactile reactivity
Reactivity to mechanical stimulation was assessed using von Frey filaments (Stoelting, Wood Dale, IL) by experimenters that were blind to treatment condition. Sensitivity was determined by stimulating the mid-plantar surface of each hindpaw in an ascending order until a flexion response was elicited. Stimuli (range = 4.56 – 6.65) were presented twice to each paw in an ABBA counterbalanced fashion (A = left, B = right), with testing on the same leg separated by a 2 min interval. Because sensory intensity generally increases in a logarithmic fashion (Weber fraction), filament thickness (approximate bending force) was related to behavior using the transformation provided by the manufacturer: Intensity = log10 (10,000 · g). This transformation yields a scale that is approximately linear and amenable to parametric statistical analyses (Tabachnick and Fidell, 2007). Data were converted to change from baseline scores (using difference scores) for purposes of analysis.
Thermal reactivity
Reactivity to radiant heat was assessed in Experiment 4 with an automated tail-flick device. Heat was provided by a 375-W movie light that was focused onto the rat’s tail by means of a condenser lens positioned 8 cm below the light source. The light source illuminated approximately 2 cm of the rat’s tail. Light intensity was controlled by an AC potentiometer (#6681-W, Leviton, Little Neck, NY). The rat’s tail was rested on a 0.5 cm deep groove embedded on an aluminum block positioned 4.7 cm below the condenser lens (for additional details see (Prentice et al., 1996). To avoid tissue damage, test trials were terminated after 8 s of heat exposure. Baseline thermal reactivity was assessed three times at 2 min intervals after tactile reactivity was assessed. As in prior studies (e.g., Crown et al., 2002; Meagher et al., 1990; Prentice et al., 1996) the last two tests were used to compute the baseline score. Behavioral reactivity was assessed at 0, 1, 2, and 3 hr after shock treatment in Experiment 4. At each time point, and after tactile reactivity was assessed, two-tail flick tests were conducted 2 min apart and the average computed.
Capsaicin injections
One percent capsaicin (Sigma-Aldrich, St. Louis, MO) was dissolved in 50 μL of vehicle (Tween 20 [7%] and saline [93%]) and injected subcutaneously into the dorsal surface of the foot. Using the dorsal surface of the paw as the injection site ensured that the resulting edema did not impact any of our assessment procedures (Hook et al., 2008).
Statistics
All behavioral measures were analyzed using an analysis of variance (ANOVA) or an analysis of covariance (ANCOVA). These analyses were supplemented with trend analyses and Bonferroni t-tests. Where appropriate, Tukey’s Honestly Significant Difference (HSD) was used to conduct post hoc analyses. In the case of baseline scores, ANOVAs were routinely used to analyze group differences prior to treatment. Some variability in baseline reactivity was observed, but in no case was there a statistically significant difference between groups.
We controlled for individual variability in baseline reactivity in two ways: 1) By analyzing the test data using an ANCOVA, entering the baseline score as a covariate; and 2) By computing a change from baseline score and analyzing the data using an ANOVA. Both sets of analyses yielded similar patterns of statistical significance. Given this, and the fact that change from baseline scores are often easier to interpret, only those analyses are presented. Significant group differences are indicated in the figures with an ‘*’. In all cases, p < .05 was used to determine statistical significance.
RESULTS
Experiment 1: Fixed and variable spaced stimulation have opposite effects on tactile reactivity
Prior research has shown that exposure to variable and fixed stimulation have divergent effects on spinal learning (Baumbauer et al., 2008, 2009, 2011). Whereas variable stimulation undermines the capacity of the spinal cord to support learning, prolonged exposure to fixed spaced stimulation promotes learning. What is not known is whether these two forms of stimulation have divergent effects on tactile reactivity. This is of interest because the consequences of variable stimulation have been linked to the development of central sensitization and EMR (Ferguson et al., 2006). Our hypothesis was that introducing temporal regularity would alter how intermittent stimulation affects mechanical reactivity, and potentially induce the opposite effect (hyporeactivity).
The experimental design is illustrated at the top of Figure 1. Twenty-four subjects (n = 8 per condition) were randomly assigned to one of three treatment conditions: no shock (Unshk), 900 shocks given at regular (2 s) intervals (fixed shock), or 900 shocks spaced in a variable (0.2–3.8 s, with a mean inter-trial interval of 2 s) manner (variable shock). Tactile reactivity was assessed on each paw prior to legshock and again 30, 60, and 180 min following shock treatment.
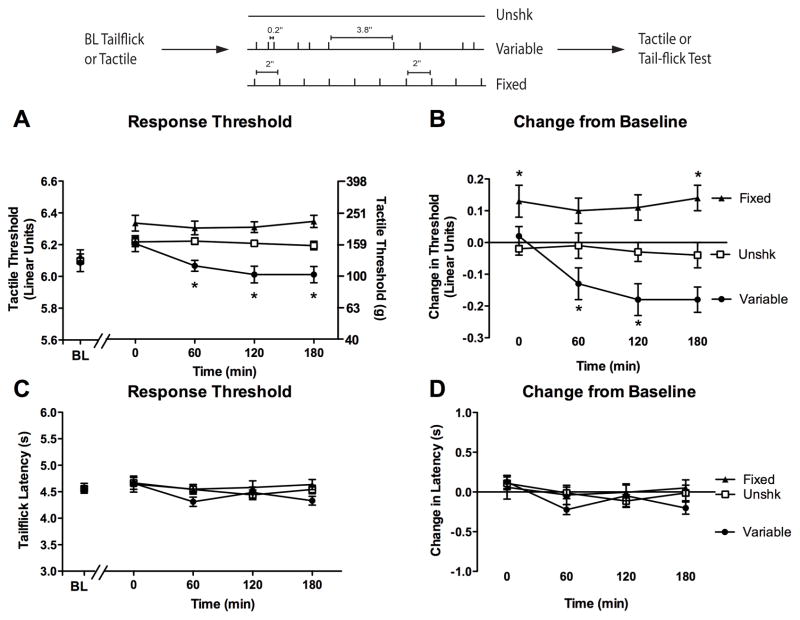
Figure 1.
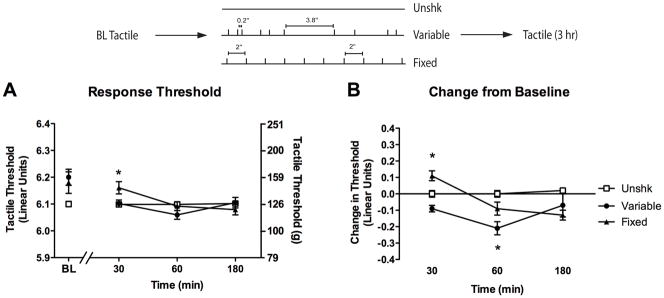
Fixed and variable spaced stimulation have a divergent effect on tactile reactivity. (A) Subjects received a baseline (BL) assessment of reactivity using von Frey monofilaments prior to treatment with 900 variable spaced (filled circles), 900 fixed spaced legshocks (filled triangles), or no shock (Unshk; open squares). Response thresholds were then reassessed at 30, 60, and 180 min after shock treatment. The left y-axis depicts data on a linear scale based on a transformation (log10 [10,000 · g]) of the force required to bend the thinnest filament that produced paw withdrawal. The right y-axis depicts the gram force equivalents. (B) The change from baseline scores. Asterisks indicate statistically significant differences (p < .05), and error bars depict ± SEM. The inset depicts the experimental design.
We first measured baseline reactivity to von Frey stimulation (measured in the units provided by the manufacturer [see Methods]). Baseline thresholds (Figure 1A) ranged from 6.11 ± 0.02 to 6.14 ± 0.04 across groups and did not differ, all Fs < 3.00, p > .05.
After shock treatment (Postshock), subjects were generally less responsive (change from baseline score ± SEM) on the stimulated leg (−0.04 ± 0.03) when compared to the unstimulated leg (−0.12 ± 0.02), F(1, 21) = 5.96, p < .05. However, this effect did not interact with treatment condition or time, all Fs < 2.70, p > .05. Consequently, we collapsed the data across this variable. As anticipated, group differences were most apparent when we controlled for variation in baseline reactivity (Figure 1B). Fixed spaced shock induced a transient hyporeactivity whereas subjects exposed to variable shock were hyperreactive to tactile stimulation. An ANOVA revealed significant main effects of Time and Shock condition, as well as a significant Time X Shock Condition interaction, all Fs > 5.02, p < .01.
Individual one-way ANOVAs were conducted at each time point to explore the impact of shock treatment over time. The ANOVA performed on the 30 min data revealed that subjects in the fixed shock condition exhibited an increase in tactile threshold when compared to all other subjects, F(2, 23) = 14.13, p < .001. Subjects in the variable shock condition demonstrated a nonsignificant decrease in threshold (p = .08). When tactile thresholds were examined 60 min following stimulation, subjects in the variable shock condition exhibited lower thresholds relative to unshocked controls, while rats in the fixed shock condition did not differ from any other condition, F(2, 23) = 7.80, p < .01. No differences in reactivity were detected 180 min following stimulation, F(2, 23) = 2.43, p > .05.
Experiment 2: The impact of fixed spaced stimulation depends on shock number
Experiment 1 showed that an extended exposure to fixed spaced stimulation inhibits tactile reactivity. Others have shown that brief exposure to regularly spaced stimulation (at similar frequencies) can enhance reactivity (Woolf and King, 1987; Sivilotti et al., 1993; Thompson et al., 1993b; Li et al., 1999; Herrero et al., 2000). Together, these observations imply that the effect of fixed stimulation may vary as a function of shock number; administration of 900 shocks may inhibit tactile reactivity, whereas less stimulation enhances responsiveness. The present experiment evaluated this hypothesis by testing mechanical reactivity after different numbers of fixed spaced shocks (36, 180 or 900 shocks).
Forty subjects (n = 10 subjects per condition) were randomly divided into four conditions, and were given 0, 36, 180 or 900 fixed spaced legshocks (see Figure 2). Tactile reactivity was assessed on the plantar surface of each paw prior to legshock and again 30, 60, and 180 min following shock treatment. All subjects were restrained for equivalent periods of time, and stimulation occurred at the end of the restraint period to maintain equal time between shock and successive tactile assessments.
Figure 2.
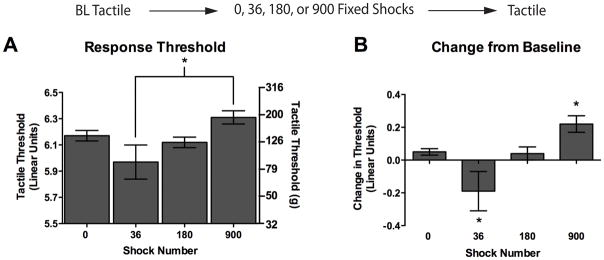
Brief exposure to fixed spaced stimulation causes mechanical hyperreactivty, while an extended exposure causes hyporeactivity. Tactile reactivity was assessed after rats received 0, 36, 180, or 900 fixed legshocks. Assessments were made at 30, 60, and 180 min following legshock. Because time was not a significant factor in our analysis we collapsed across this variable and present mean data. (A) Mean absolute tactile thresholds after shock treatment. The left y-axis depicts linearized data while the right y-axis depicts the gram force equivalents. (B) Mean change from baseline scores. Asterisks indicate statistically significant differences (p < .05), and error bars depict ± SEM. The inset depicts the experimental design.
Baseline mechanical reactivity ranged from 6.07 ± 0.02 to 6.16 ± 0.03 across all conditions and did not differ, all Fs = 1.33, p > .05. After shock treatment, the group differences did not vary across time or leg, all Fs < 1.06, p > .05. Consequently, we collapsed the data across these variables and report the mean post-treatment values. When variation in baseline reactivity was controlled for (Figure 2B), we found that a brief exposure to shock (36 shocks) enhanced reactivity whereas subjects given 900 shocks were less responsive. An ANOVA revealed a significant main effect of shock condition, F(3, 36) = 7.51, p < .01. To further analyze how reactivity varied as a function of shock number, we compared the group means using trend analyses. We found linear and quadratic trends that accounted for 35.19% and 58.74 % of the variance, respectively. Planned comparisons were performed using individual Bonferroni t-tests to control for the number of comparisons made. This analysis showed that, relative to the unshocked controls (0 shocks), rats given 36 shocks were more responsive to tactile stimulation, t(72) = 3.06, p < .05, while rats given 900 shocks exhibited a significant increase in threshold, t(72) = 7.71, p < .01. Rats in the 180 shock condition did not differ from unshocked rats, t(72) = 0.17, p > .05.
Experiment 3: Intermittent stimulation of the sciatic nerve yields similar results
The shift from hyper- to hyporeactivity observed in Experiment 2 behaviorally resembles the well-known phenomena of wind-up and wind-down (Woolf, 1983; Clatworthy and Walters, 1993a; Traub, 1997; Herrero et al., 2000). Collectively, our results suggest that an extended exposure to intermittent shock only produces hyporeactivity if the stimuli occur in a regular manner; if shocks occur in a variable manner EMR is observed (Experiment 1). However, in a prior study (Baumbauer et al., 2008), we reported that variable stimulation of the sciatic nerve induces hyporeactivity, not hyperreactivity. The effects were only measured immediately after stimulation. Perhaps variable stimulation of the sciatic nerve also enhances tactile reactivity, but this effect does not emerge until 60 min after stimulation has ended. The present experiment examines these issues by comparing the impact of variable versus fixed spaced stimulation of the sciatic nerve on mechanical reactivity from 0–180 min after stimulation.
The experimental conditions are illustrated in the upper portion of Figure 3. Eighteen subjects (n = 6 per condition) had their sciatic nerves exposed prior to baseline measurement of mechanical reactivity of both the treated, and untreated, leg. Immediately following baseline assessment, rats received 900 fixed or variable monophasic direct current (DC) shocks (40 V, 50 Hz, 80 ms bursts, 10 ms pulse width) to their exposed sciatic nerves. A separate group of control rats had electrodes placed on their sciatic nerve but no current was applied (Unshk). Using these parameters, we have shown that variable, but not fixed, stimulation undermines learning (Baumbauer et al., 2008). Mechanical reactivity was assessed immediately following stimulation and again every hr over a 3 hr period.
Figure 3.
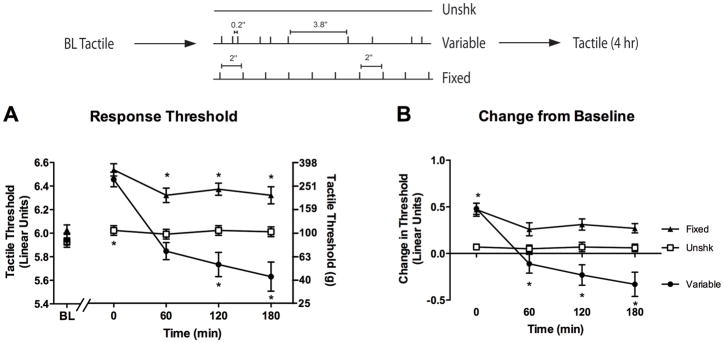
Impact of sciatic nerve stimulation depends upon both temporal regularity and time of testing. Rats were administered 900 variable (filled circles) or 900 fixed (filled triangles) spaced monophasic DC shocks to the sciatic nerve, or no shock (Unshk; open squares), and tactile reactivity was monitored over a period of 3 hr. (A) Mean absolute tactile thresholds prior to (Baseline, BL) and 0, 60, 120, and 180 min after shock treatment. The left y-axis depicts linearized data while the right y-axis depicts the gram force equivalents. (B) Mean change from baseline scores. Asterisks indicate statistically significant differences (p < .05), and error bars depict ± SEM. The inset depicts the experimental design.
Baseline reactivity (Figure 3A) ranged from 5.93 ± 0.03 to 6.03 ± 0.04 and did not differ across conditions, all Fs = 1.46, p > .05. Shock treatment had a similar effect across test legs, F(1, 15) < 1.0, p > .05, and consequently, we collapsed the data across this variable (Figure 3B). When mechanical reactivity was assessed immediately following stimulation, rats in each stimulation condition exhibited hyporeactivity. However, at later time points, rats administered variable sciatic stimulation exhibited enhanced reactivity while rats in the fixed condition did not. An ANOVA conducted on the change from baseline scores revealed significant main effects of Time and Shock condition, as well as a significant Time X Shock condition interaction, all Fs > 7.15, p < .01. Post hoc comparisons confirmed that rats in the variable shock condition exhibited enhanced reactivity relative to rats in the other conditions (p < .05). To further quantify how stimulation affected behavior over time, individual ANOVAs were conducted at each time point. Immediately following sciatic stimulation, both variable and fixed shocked rats were hyporeactive relative to the unshocked condition, F(2, 17) = 13.24, p < .001. Rats given variable stimulation began to exhibit enhanced mechanical reactivity one hr following stimulation, F(2, 17) = 5.87, p < .05 and this effect persisted for the remainder of the 3 hr testing period, all Fs > 10.13, p < .01.
Experiment 4: Intermittent stimulation to the tail has a similar effect on mechanical thresholds and does not affect thermal reactivity on the tail-flick test
Previous work has shown that variable and fixed intermittent shock applied to the tail have differential effects on learning (Baumbauer et al., 2009). While Experiments 1–3 demonstrate that stimulation to one hind limb produces a bilateral effect, it is not known whether stimulation at a remote site affects mechanical reactivity; both here, and in prior studies (Ferguson et al., 2006; Baumbauer et al., 2008; Huie et al., 2012), changes in mechanical reactivity have only been studied after hindleg stimulation. The present experiment explores this issue by testing whether mechanical reactivity to stimuli applied to the hindlimbs is affected by intermittent stimulation applied at a remote dermatome (the tail). In addition, we examine whether stimulation affects tail-withdrawal from noxious radiant heat (tail-flick test).
The experimental design is illustrated in the upper panel of Figure 4. Thirty rats (n = 10 per condition) were tested for baseline tactile reactivity and received 3 baseline tailflick tests at 2 min intervals. Subjects then received 900 fixed or variable tailshocks or no shock (Unshk). Tactile and thermal reactivity were re-assessed immediately after administration of tailshock and again every hour for 3 hours. At each time point, two tail-flick tests were conducted (2 min apart) after tactile reactivity was assessed.
Figure 4.
Fixed and variable tailshock have divergent effects on tactile, but not thermal, reactivity. Following baseline assessments of tactile and thermal responding, rats were administered 900 variable (filled circles) or 900 fixed (filled triangles) tailshocks, or no shock (Unshk). (A) Mean absolute tactile scores prior to treatment (Baseline, BL) and at 0, 60, 120, and 180 min after treatment. The left y-axis depicts linearized data while the right y-axis depicts the gram force equivalents. (B) Mean change from baseline tactile scores. (C) Absolute tail-flick latencies prior to and after shock treatment. (D) Mean change from tail-flick scores. The asterisks indicate statistical significance relative to unshocked (Unshk) controls (p < .05), and error bars depict ± SEM. The inset depicts the experimental design.
Baseline thresholds to mechanical (Figure 4A) and thermal (Figure 4C) stimulation did not differ across groups, all Fs < 1.00, p > .05. As in Experiments 1–3, we controlled for individual variability in behavioral reactivity by computing a change from baseline score (Figures 4B and 4D). Because tailshock had a similar effect on reactivity to tactile stimulation across both hindlegs, F(1, 81) < 1.00, p > .05, we collapsed the data across this variable.
We found that rats exposed to fixed spaced tailshock were hyporeactivity to tactile stimulation (Figure 4B). Exposure to variably spaced tailshock enhanced mechanical reactivity and, as in Experiments 1 and 3, this effect was most evident 60–180 min after stimulation. An ANOVA revealed significant main effects of Time and Shock Condition as well as a significant Time X Shock Condition Interaction, all Fs > 3.19, p < .01. One-way ANOVAs were then performed on the data at each time point to explore the impact of shock treatment over time. Immediately following stimulation (0 min) fixed spaced shocked rats were less responsive relative to the other groups, F(2, 29) = 4.93, p, < .05. At 1 and 2 hr following stimulation, animals in the variable shock condition exhibited lower shock thresholds relative to animals in the unshocked and fixed shock conditions, all Fs > 7.78, p < .01. At 3 hr, rats that received fixed spaced shock were less responsive relative to the other two groups, F(2, 29) = 15.40, p < .001.
Shock treatment did not affect reactivity to thermal stimulation (Figure 4D). An ANOVA confirmed that the groups did not differ, all Fs < 2.45, p > .05.
Experiment 5: Pretreatment with fixed spaced stimulation attenuates capsaicin-induced EMR
Hook et al. (2009) showed that subcutaneous injection of capsaicin into one hind paw undermines instrumental learning when subjects are tested on the contralateral limb. This learning impairment can be blocked by prior treatment with either controllable, or fixed-spaced, shock (Hook et al., 2008; Baumbauer and Grau, 2011). Here we examine whether treatment with fixed-spaced shock affects capsaicin-induced EMR.
Forty-eight subjects (n = 8 per condition) received 900 fixed legshocks, 900 variable legshocks, or nothing (Unshk). Immediately following shock termination, subjects received a subcutaneous injection of 1% capsaicin or physiological saline (50 μL vol) into one hindpaw (see Figure 5). Shock and capsaicin treatment always occurred on the same limb (Treated Leg), and which limb was treated was counterbalanced across subjects. Tactile reactivity was assessed prior to shock treatment (baseline), immediately following shock treatment, and 15–60 min after capsaicin injection.
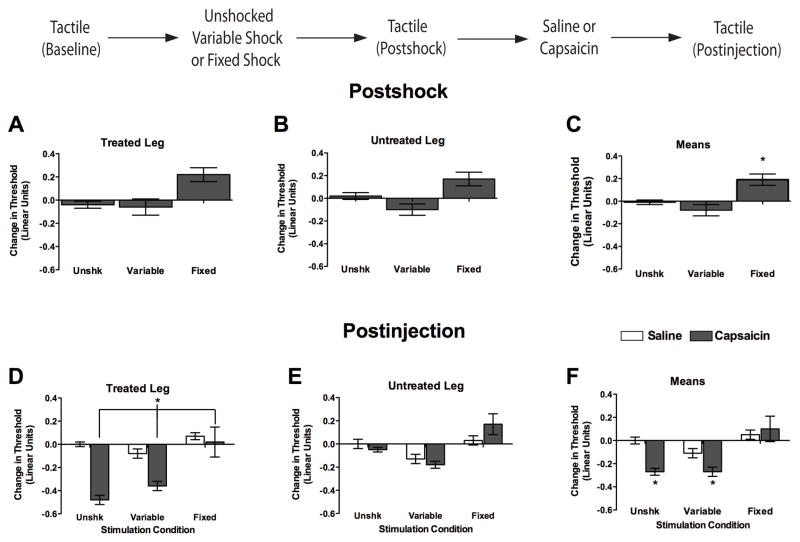
Figure 5.
Pretreatment with fixed, but not variable, spaced stimulation prevents capsaicin-induced EMR. Immediately following assessment of baseline tactile reactivity rats were administered 900 variable legshocks, 900 fixed spaced legshocks, or nothing (Unshk). After the impact of shock treatment on tactile reactivity was assessed (Postshock), subjects received a subcutaneous hindpaw injections of 1% capsaicin (50 μL) or saline to the dorsal surface of one hindpaw (counterbalanced). Injections and stimulation always occurred on the same limb. Tactile reactivity was re-assessed 0–60 min after treatment (Postinjection). (A–C) Tactile reactivity after shock treatment. (D–F) Tactile reactivity after saline (unfilled bars) or capsaicin (filled bars) treatment, averaged over time. In both sets of panels, data are presented for the treated leg (left), untreated leg (center), and mean tactile reactivity (right). Asterisks indicate statistically significant differences (p < .05), and error bars depict ± SEM. The inset depicts the experimental design.
In the previous experiments baseline reactivity was assessed and combined across limbs because shock treatment yielded similar results on the treated (ipsilateral) and untreated (contralateral) legs. In the current experiments, which combined shock and capsaicin treatment, some differences were observed across the treated and untreated legs. Consequently, treated and untreated limbs were maintained as within subjects factors and each limb served as its own baseline. As in Experiments 1–4, a similar pattern of results was observed independent of whether we controlled for baseline reactivity using an ANCOVA on the raw scores or an ANOVA on the change from baseline scores. Because it is generally easier to infer the pattern of statistical significance from graphs of the baseline scores, and because additional panels were required to illustrate the changes observed on both the treated and untreated legs, we only present the change from baseline scores in the subsequent experiments.
Baseline tactile reactivity scores ranged from 6.15 ± 0.04 to 6.23 ± 0.06 and did not differ, all Fs < 2.51, p > .05. After shock treatment, subjects exhibited comparable performance across test legs, all Fs < 1.49, p > .05 (cf. Figure 5A and 5B). As reported above, subjects exposed to fixed spaced shock were less responsive to tactile stimulation. An ANOVA performed on the change from baseline scores yielded a main effect of shock treatment, F(2, 48) = 18.76, p < .001. Post hoc comparisons of the group means (Figure 5C) confirmed that the group given fixed spaced shock was less responsive to tactile stimulation, relative to both the variably shocked and unshocked groups (p < .05)
We next examined tactile reactivity following capsaicin treatment (Figure 5, Postinjection). Because similar results were observed 15–60 min after treatment, all Fs < 1.00, p > .05, the data were collapsed over time. Capsaicin treatment produced a significant reduction in tactile thresholds, and this EMR was most pronounced on the injected paw (Figure 5D). Interestingly, fixed shock prevented the EMR produced by hindpaw capsaicin treatment. An ANOVA conducted on the change from baseline scores revealed significant main effects of Injected paw, Shock condition, and Capsaicin condition, all Fs > 8.05, p < .01. The ANOVA also produced significant Shock X Capsaicin, and Injected paw X Shock condition interactions, all Fs > 4.06, p < .05. The significant Shock X Capsaicin interaction (see Figure 5F) emerged because rats in the unshocked and variable shock conditions given capsaicin were more responsive relative to rats in all other conditions (p < .05), except those given variable shock and saline (p >.05). No other group differences were significant (p > .05).
There are at least two potential interpretations of the above results. One possibility is that exposure to fixed-spaced stimulation engages a process that effectively counters the induction of capsaicin-induced EMR. Alternatively, attenuated responding may be observed in subjects pretreated with fixed spaced shock simply because shock, per se, induces hyporeactivity. By this interpretation, the consequences of fixed spaced shock and capsaicin treatment are additive, and as a result, the shock-induced hyporeactivity subtracts from the capsaicin-induced EMR. To address this possibility, we performed an ANCOVA on the Postinjection scores, treating the Postshock tactile scores as a covariate. As expected, this covariate accounted for a significant proportion of the variance, F(1, 42) = 13.96, p < .001. While the main effect of shock was no longer significant, F(2, 42) < 1.0, p > .05, a significant Shock X Capsaicin interaction remained, F(2, 42) = 5.25, p < .01. Additional analyses performed on the adjusted (least square) means showed that the unshocked and variably shocked capsaicin treated groups were more responsive relative to the capsaicin-treated group that had received fixed spaced shock (p < .05). These analyses imply that fixed spaced stimulation had an effect on capsaicin-induced EMR that was independent of the shock-induced changes in tactile reactivity observed prior to capsaicin treatment.
Experiment 6: Exposure to fixed spaced stimulation has a lasting effect
Experiment 5 showed that fixed spaced shock attenuates the EMR observed after capsaicin treatment. Elsewhere, we have shown that fixed spaced stimulation has a lasting effect on spinal learning that is evident 24–48 hr after stimulation (Baumbauer et al., 2009). Experiment 6 examined whether fixed spaced shock also has a long-term effect on capsaicin-induced EMR.
Eighteen subjects (n = 6 per condition) received 900 fixed legshocks, 900 variable legshocks, or nothing (see Figure 6). Twenty-four hr later, subjects received a subcutaneous injection of 1% capsaicin (50 μL vol) into the hindpaw of the previously treated leg. Tactile reactivity was assessed bilaterally prior to shock treatment (Baseline), immediately following shock treatment (Postshock), 24 hr later prior to capsaicin administration (Preinjection), and again 30, 60, and 180 min after capsaicin injection (Postinjection).
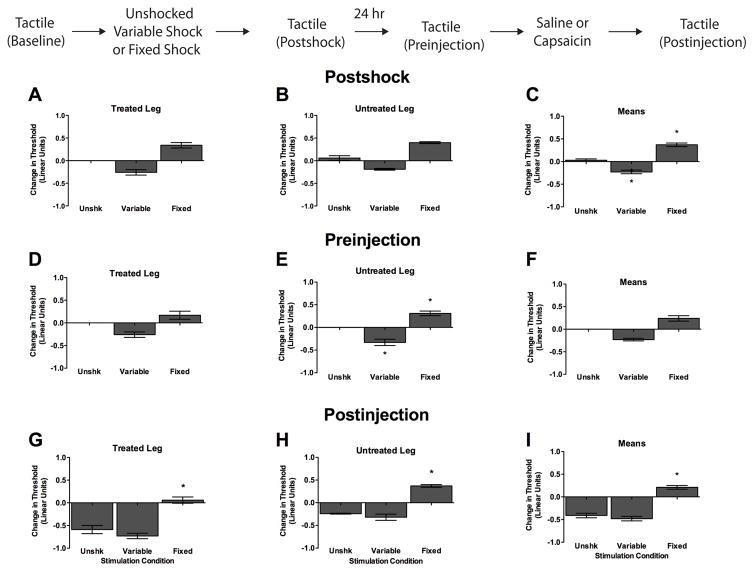
Figure 6.
Treatment with fixed-spaced stimulation prevents capsaicin-induced EMR when administered 24 hr prior to capsaicin. After baseline tactile reactivity was assessed, subjects received 900 fixed spaced legshocks, 900 variable shocks, or nothing (Unshk). Tactile reactivity was then re-assessed (Postshock). The next day, after tactile reactivity was measured (Preinjection), rats received a subcutaneous hindpaw injection of 1% capsaicin (50 μL) in the previously treated leg. Tactile reactivity was then assessed over a period of 3 hr (Postinjection). (A–C) Tactile reactivity after shock treatment. (D–F) Tactile reactivity 24 hr after shock treatment. (G–I) Tactile reactivity after capsaicin treatment, averaged over the 3 hr test period. In each set of panels, data are presented for the Treated Leg (left), Untreated Leg (center), and average across legs (Means, right). Asterisks indicate statistically significant differences (p < .05), and error bars depict ± SEM. The inset depicts the experimental design.
Baseline tactile reactive scores ranged from 6.05 + 0.02 to 6.10 + 0.00 and did not differ across groups, all Fs < 1.86, p > .05. As in previous experiments, exposure to variable shock enhanced reactivity, while exposure to fixed spaced shock inhibited behavioral reactivity relative to the unshocked controls (Figure 6C, Postshock). An ANOVA conducted on the change from baseline scores revealed a significant main effect of Shock condition, F(2, 15) = 56.68, p < .0001. No other statistical effects approached significance, all Fs < 3.60, p > .05. Post hoc analysis of group means confirmed that rats given variable shock exhibited a significant reduction in response thresholds relative to all other conditions. In contrast, rats given fixed spaced stimulation exhibited a significant increase in response thresholds compared to rats in all other conditions (p < .05).
Twenty-four hr following stimulation, we examined tactile reactivity prior to capsaicin treatment (Figure 6, Preinjection). Unshocked rats showed no change in reactivity. Rats previously exposed to variable shock were more responsive to tactile stimulation and this effect was most evident on the shocked leg (Figure 6D). In contrast, fixed spaced shock induced a slight hyporeactivity. An ANOVA performed on the change from baseline scores revealed significant main effects of Stimulated leg and Shock condition, as well as a significant Stimulated leg X Shock condition interaction, F(2, 15) = 28.70, p < .0001. Again, neither the main effect of Leg, nor its interaction with Shock treatment, were statistically significant, both F’s < 2.70, p > .05. Not surprisingly, variation in tactile reactivity 24 hr after shock treatment (Preinjection) was related to the changes observed a day earlier immediately after shock (Postshock). Supporting this, when Preinjection scores were analyzed using an ANCOVA (with Postshock values entered as the covariate), the main effect of shock treatment was no longer significant, F(2, 15) < 1.0, p > .05. However, the Leg x Shock interaction remained statistically significant, F(2, 13) = 4.28, p < .05, which implies that shock treatment had a long-term limb-specific effect that was independent of its acute effect on tactile reactivity. Comparisons of the adjusted (least square) means revealed that this effect emerged because fixed shock induced a long-term hyporeactivity on the contralateral leg (relative to the unshocked and variably shocked rats) that was independent of its immediate (Postshock) effect (p < .05).
Tactile reactivity was then assessed after capsaicin treatment. Because shock treatment had a similar effect on tactile reactivity across both test legs, all Fs < 1.11, p > .05, we collapsed the data across this variable (Figure 6, Postinjection). Capsaicin treatment induced EMR in the unshocked controls, but had no effect on subjects pretreated with fixed spaced shock. An ANOVA revealed significant main effects of Shock condition and Leg, both F’s > 76.37, p < .0001. The Shock X Leg interaction was not significant, F(2, 15) < 1.0, p > .05. Post hoc analysis of group means (Figure 6I) confirmed that unshocked rats, and rats given variable shock prior to capsaicin, exhibited enhanced reactivity relative to rats given fixed stimulation (p < .05).
Again, we examined whether the impact of fixed spaced shock on capsaicin-induced EMR was related to a shock-induced hyporeactivity. We addressed this issue by performing an ANCOVA on the Postinjection scores, using either the Postshock or Preinjection scores as the covariate. Both analyses yielded the same pattern of statistical significance, generating a significant main effect of Shock treatment and Leg, all F’s > 20.28, p < .0001. In both cases, comparisons of the adjusted means (collapsed across leg) showed that fixed spaced shock had a significant effect, relative to both the variably shocked and unshocked groups, that was independent of either its immediate (Postshock) or long-term (Preinjection) effect on tactile reactivity (p < .05).
The sensory response to capsaicin is accompanied by swelling and edema in the affected tissue. To examine whether prior shock treatment affected swelling, we measured the thickness of the injected paw prior to and for 3 hr following capsaicin administration. To control for variation in paw size across subjects, we analyzed the change from pretreatment paw thickness. Swelling was greatest on the injected paw, F(1, 30) = 196.93, p < .001, but was not affected by shock treatment (data not shown), F(2, 15) = 1.47, p > .05.
Experiment 7: Fixed spaced stimulation reverses capsaicin-induced EMR
Fixed spaced shock given immediately before capsaicin treatment attenuated the capsaicin-induced EMR (Experiment 5). Experiment 7 examined whether fixed stimulation given 3 hr after capsaicin treatment can reverse the EMR observed following capsaicin treatment.
We began by testing whether exposure to 900 fixed spaced shocks could attenuate capsaicin-induced EMR. Fixed spaced shock did not have a significant effect, all Fs <1.00, p > .05. Recognizing that the consequences of fixed stimulation strengthen with training (Baumbauer et al., 2008, Baumbauer et al., 2009), we assessed whether increasing stimulus exposure to 1,800 shocks affects capsaicin-induced EMR (Figure 7). Thirty-two rats (n = 8 per condition) were given a subcutaneous injection of 1% capsaicin or physiological saline (50 μL vol) into one hindpaw. Subjects then received 1,800 fixed spaced shocks to the same leg or nothing (Unshk). Mechanical reactivity was assessed: 1) prior to capsaicin treatment (Baseline); 2) 30, 60, and 180 min after capsaicin treatment (Postinjection); and 3) after shock treatment (Postshock). As in Experiments 5 and 6, shock and capsaicin treatment always occurred on the same limb (Treated Leg) and which limb was treated was counterbalanced across subjects.
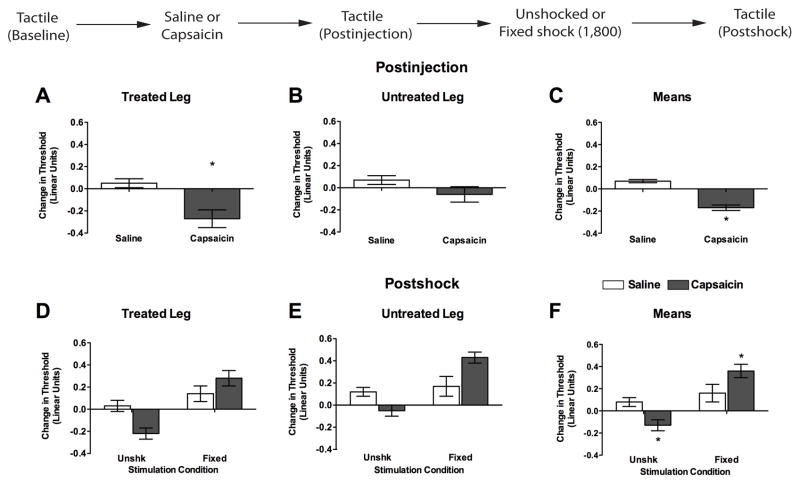
Figure 7.
Exposure to fixed spaced stimulation reverses capsaicin-induced EMR. Rats received baseline assessments of tactile reactivity prior to subcutaneous hindpaw injections of 1% capsaicin or saline (50 μL) into the dorsal surface of one hindpaw. Tactile reactivity was assessed over the next 3 hr (Postinjection). Subjects then received 1,800 fixed spaced shocks, or nothing (Unshk), to the previously injected (Treated) leg. Tactile reactivity was then re-assessed (Postshock). (A–C) Tactile reactivity after capsaicin treatment, averaged over the 3 hr of testing. (D–F) Tactile reactivity after shock treatment in rats that had previously received saline (open bars) or capsaicin (filled bars). In each set of panels, data are presented for the Treated Leg (left), Untreated Leg (center), and average across legs (Means, right). Asterisks indicate statistically significant differences (p < .05), and error bars depict ± SEM. The inset depicts the experimental design.
Baseline tactile reactivity scores ranged from 5.96 + 0.03 to 6.12 + 0.05 and did not differ, all Fs < 1.43, p > .05. Treatment with capsaicin enhanced mechanical reactivity and this effect was most evident on the Treated Leg (Figure 7A). An ANOVA conducted on the change from baseline scores revealed significant main effects of Leg and Capsaicin, as well as significant Injected Leg X Capsaicin interaction, all Fs > 11.57, p < .001. The significant 2-way interaction emerged because subjects given capsaicin exhibited reduced response thresholds, and this effect was more pronounced on the injected paw (p < .05). No other statistical effects approached significance, all Fs < 2.12, p > .05.
Mechanical thresholds were reassessed immediately after shock treatment or equivalent period of restraint (Figure 7, Postshock). Unshocked rats given capsaicin displayed enhanced reactivity while rats administered fixed stimulation were less responsive irrespective of capsaicin treatment. An ANOVA revealed significant main effects of Shock and Leg, both F’s > 15.13, p < .001. In addition, there was significant Shock X Capsaicin treatment interaction, F(1, 20) = 11.29, p < .005. No other terms were significant, all F’s < 1.70, p > .05. Post hoc comparisons of the group means (Figure 7F) showed that the capsaicin unshocked group differed from all other groups (p < .05). In addition, rats given capsaicin followed by fixed spaced shock differed from the unshocked saline controls (p < .05). No other comparisons were significant (p > .05).
DISCUSSION
We have previously shown that variable shock inhibits learning while fixed spaced shock enables learning and counters the consequences of variable stimulation (Baumbauer et al., 2008; Baumbauer et al., 2009). These results suggest that variable and fixed shock have distinct effects on spinal cord plasticity. We have suggested that variable shock may disrupt spinally mediated learning because it induces a central sensitization-like state that diffusely saturates NMDAR-mediated plasticity. Supporting this, treatments that induce central sensitization (e.g., peripheral carrageenan or capsaicin) inhibit learning (Ferguson et al., 2006; Hook et al., 2008). The present paper builds on our previous findings examining learning and explores the generalized impact of stimulation on mechanical reactivity. We showed that variable shock produces an increase in mechanical reactivity that emerged 30–60 min after shock treatment (Experiments 1, 3, and 4) and lasted up to 3 hr (Experiment 3). Variable stimulation enhanced mechanical reactivity independent of whether shock was applied through intramuscular electrodes (Experiment 1), stimulation of the sciatic nerve (Experiment 3), or tail electrodes (Experiment 4). As previously reported (Baumbauer et al., 2008), variable stimulation of the sciatic nerve also produced a transient hyporeactivity (Experiment 3). A transition from hypo- to hyperreactivity may occur because a short-term process masks variable stimulation-induced hyperreactivity or because the underlying processes unfold sequentially over time. Variable stimulation had no effect on thermal reactivity, which suggests that the EMR does not reflect a more general hyperalgesia. A similar pattern has been observed after treatments that induce central sensitization and has been linked to a functional modification of A-beta fibers (Thompson et al., 1996; Linden and Seybold, 1999; Ossipov et al., 2002; Sorkin et al., 2002). A caveat must be acknowledged, however, because we did not test both stimuli at the same location.
An extended exposure to fixed spaced shock induced hyporeactivity rather than mechanical hyperreactivity (Experiments 1–4). As reported by others (Schouenborg and Sjolund, 1983; Schouenborg, 1984; Herrero et al., 2000), the effect of fixed spaced stimulation varied as a function of shock number; a brief (36 shocks) exposure produced enhanced reactivity, whereas an extended exposure (900 shocks) produced hyporeactivity (Experiment 2). An extended exposure to fixed spaced shock produced hyporeactivity independent of whether shock was applied using intramuscular electrodes (Experiments 1–2), sciatic nerve stimulation (Experiment 3), or tailshock (Experiment 4). Fixed spaced shock had no effect on thermal reactivity, which suggests that the mechanical hyporeactivity is not accompanied by a more general hypoalgesia (antinociception). While we acknowledge that this claim is based on a null result, it should be noted that our test of thermal reactivity has proven sensitive to a broad range of environmental stimuli and has been regularly used in our laboratory for over 25 years (e.g., Crown et al., 2002; Grau, 1987; Hook et al., 2009; Meagher et al., 1990; Prentice et al., 1996).
Prior studies showed that exposure to fixed spaced shock can counter the learning deficit induced by capsaicin (Baumbauer and Grau, 2011). We hypothesized that shock treatment had this effect because it countered the induction and maintenance of central sensitization. If this is true, fixed spaced stimulation should also affect capsaicin-induced EMR. The present study explored this issue using a preparation wherein communication to the brain had been surgically disrupted, which assures that the experimental effects reflected interactions at the level of the spinal cord. This procedure has the added advantage of preventing pain/suffering at a conscious (brain-dependent) level during our treatment and test procedures. Under these conditions, prior exposure to fixed spaced, but not variable, shock attenuated the EMR induced by peripheral capsaicin treatment (Experiment 5). Just as fixed spaced shock has a lasting effect on learning (Baumbauer et al., 2009), it too attenuated the response to capsaicin for 24 hr (Experiment 6). Exposure to fixed spaced shock also reduced EMR when given after peripheral capsaicin, suggesting that the shock treatment counters the maintenance of prolonged pain (Experiment 7).
Our results show that the effect of intermittent stimulation depends upon whether shocks are given in a regular or irregular manner. Just as the divergent effects of variable and fixed spaced shock on learning require extended training (Baumbauer et al., 2008; Baumbauer et al., 2009), fixed spaced shock-induced hyporeactivity only emerged after 900 shocks. In contrast, variable shock treatment appears to yield an effect that grows in a monotonic manner to inhibit learning and enhance mechanical reactivity; both here, and in prior studies, increasing the number of shocks presented on a variable schedule from 180 to 900, if anything, enhances the impact of shock treatment (Ferguson et al., 2006; Baumbauer et al., 2008). Finally, just as fixed spaced shock has a lasting protective effect on spinal plasticity, that can counter the learning deficit induced by variable shock or peripheral capsaicin (Baumbauer et al., 2009; Baumbauer and Grau, 2011), fixed spaced shock induces a process that counters behavioral signs of neuropathic pain and this effect is evident 24 hr after treatment.
Locus of action
Both fixed and variable shock treatment had a bilateral effect on tactile reactivity. This is important for a number of reasons. First, it speaks to some alternative interpretations of our results. For example, fixed spaced shock may reduce mechanical reactivity to tactile stimulation because it produces a limb-specific habituation or motor fatigue. The fact that fixed spaced shock impacted performance when subjects were tested on the contralateral leg speaks against both of these accounts and implicates a common (spinal) system. What is more difficult to determine is how fixed spaced stimulation affected capsaicin-induced EMR. Because capsaicin and shock were applied to the same limb, it could be argued that shock treatment had a peripheral effect that reduced the afferent signal produced by capsaicin treatment, and thereby reduced the induction of central sensitization. That explanation, however, would not hold for Experiment 7 where capsaicin-induced central sensitization was induced prior to treatment with fixed spaced shock. Those results, suggest that the key interaction occurs centrally, within the spinal cord.
Within the spinal cord, exposure to fixed spaced stimulation could inhibit capsaicin-induced EMR in a number of ways. One possibility is that both manipulations independently affect motor reactivity, but in opposite ways. From this perspective, the hyporeactivity induced by fixed spaced shock subtracts from the hyperreactivity induced by capsaicin. We addressed this possibility using statistical techniques. At issue is whether the shock-induced alterations in motor reactivity could explain the impact of shock treatment on capsaicin-induced EMR. We found that fixed spaced shock still had a significant effect on EMR after the variance attributable to the shock-induced hyporeactivity was factored out (Experiments 5 and 6). These statistical procedures imply that there are two independent sources of variance, one that is related to the fixed shock induced inhibition of motor reactivity and another that is related to the impact of shock treatment on capsaicin-induced EMR.
Perhaps the strongest evidence against a motoric account comes from studies examining how fixed spaced shock treatment affects a capsaicin-induced inhibition of learning. Prior studies have shown that spinally transected rats given shock to one leg whenever that leg is extended will exhibit a progressive increase in flexion duration, a behavioral phenomenon known as instrumental learning (Grau et al., 1998). Because learning is evident from an increase in shock-induced flexion duration, a motoric explanation would anticipate that factors that enhance reactivity (e.g., capsaicin treatment) would promote learning while manipulations that diminish reactivity (e.g., exposure to fixed spaced shock) would interfere with learning. However, exactly the opposite is observed—pretreatment with capsaicin inhibits learning while prior exposure to fixed spaced shock promotes learning and both effects last at least 24 hr (Baumbauer et al., 2009; Baumbauer and Grau, 2011; Huie et al., 2008). More importantly, exposure to fixed spaced shock before, or after, capsaicin treatment blocks its long-term effect on instrumental learning (Baumbauer and Grau, 2011). The most parsimonious explanation of these findings is that exposure to fixed spaced shock engages a process that opposes the central consequences of capsaicin treatment.
Potential mechanisms for discriminating regular versus irregular stimulation
Our work highlights the importance of temporal regularity and demonstrates that variable and fixed spaced stimulation have divergent effects on spinal cord plasticity. How could spinal systems make this discrimination? One possibility is that fixed and variable stimuli engage distinct fiber pathways. Indeed, similar results have been observed in the visual cortex where regularly spaced low-frequency stimulation results in long-term depression (LTD), while low frequency stimulation administered in a variable manner shifts the balance of plasticity toward potentiation (Perrett et al., 2001). However, from this perspective, it is not obvious why a brief exposure (180 shocks or less) to fixed or variable stimulation have common effects (Baumbauer et al., 2008; Baumbauer et al., 2009). An alternative interpretation is suggested by the fact that the fixed spaced shock effect requires extended training, depends on a form of NMDAR-mediated plasticity, and de novo protein synthesis, factors generally associated with learning and memory (Morris et al., 1986; Schafe and LeDoux, 2000; Izquierdo et al., 2006). As others have noted (Pavlov, 1927; Todd et al., 2010; Wan et al., 2010), a regular inter-stimulus interval provides a temporal cue that can be used to predict the occurrence of stimulation. From this perspective, time can act as a kind of Pavlovian conditioned stimulus (CS) that predicts shock occurrence (the unconditioned stimulus [US]). Previous work has shown that spinal neurons can support Pavlovian conditioning (Durkovic, 1975; Joynes and Grau, 1996). This account assumes that a physiological cue is available that tracks time within the relevant intervals and functions as a Pavlovian CS. Such a cue could be provided by a process that decays at a fixed rate, yielding a kind of hourglass (Boulos and Terman, 1980). Alternatively, a temporal cue could be provided by the central pattern generator (CPG) used to induce rhythmic stepping (Kiehn, 2006; Baumbauer et al., 2008; McCrea and Rybak, 2008; Baumbauer et al., 2009). Indirect evidence for this view comes from data demonstrating that the effective frequency for fixed stimulation lies within the frequency range of coordinated stepping (Roy et al., 1991; de Leon et al., 1994).
Engagement of spinal antinociceptive systems
Here we report that intermittent shock does not impact tailflick behavior, suggesting that the observed mechanical hyporeactivity may not result from a global spinally mediated antinociceptive response. This finding is surprising because the intensity of shock utilized in the current work is likely sufficient to be considered painful in intact animals. Previous work has shown that shock of lower intensity engages supraspinally-mediated indicators of pain responding, such as vocalization (Illich et al., 1995). However, in the intact animal, whether antinociceptive/analgesic mechanisms are engaged is determined by a different set of physiological parameters (Grau, 1987; Meagher et al., 1993). For example, brain-dependent systems support the development of antinociception (via descending modulation) at much lower shock intensities, and, in contrast to the present findings, the application of intermittent stimulation generally produces antinociception (Grau et al., 1981). What is interesting is that stimulation schedule (intermittent vs. continuous shock) determines whether analgesia is opioid or non-opioid dependent; continuous shock engages non-opioid dependent analgesia, while intermittent shock engages opioid-dependent analgesia (Terman et al, 1984).
Summary and conclusion
Researchers have recognized for many years that wind-up and central sensitization may depend on similar mechanisms. However, this simple idea was challenged by the fact that agents that induce central sensitization appear to have a monotonic effect, yielding greater allodynia as the magnitude of inflammation is increased, whereas exposure to fixed spaced nociceptive stimulation generally has a non-monotonic effect, with a brief exposure producing hyperreactivity to mechanical stimulation while an extended exposure yielded hyporeactivity (Clatworthy and Walters, 1993b, a; Traub, 1997; Herrero et al., 2000). While further work is needed to confirm our observations at a physiological level, the results demonstrate behaviorally that a monotonic effect is observed after variable stimulation, a stimulus schedule that more closely resembles the erratic pattern of C-fiber activity following inflammation (Sandkuhler et al., 2000; Ji et al., 2003). Conversely, our results suggest non-monotonic effects have been observed in prior studies because the stimulus pulses were presented in a regular manner. Beyond providing a potential solution to a current enigma, our results suggest that exposure to fixed spaced shock may have a lasting effect that counters inflammation-induced allodynia through interactions that occur within the spinal cord. Further, we have shown that intermittent shock can produce an EMR that lasts for hr when it occurs in a variable manner. In this way, variable stimulation has an effect that appears distinct from prior reports of wind-up associated EMR, which typically decays within minutes.
In some regards, the effect of fixed spaced shock resembles the inhibition of nociceptive processing observed after a continuous stream of shock pulses (transcutaneous electrical nerve stimulation [TENS]) (Ainsworth et al., 2006; DeSantana et al., 2009). TENS, however, appears to affect pain through a distinct process that is tied to its capacity to elicit antinociception (King et al., 2005; Hingne and Sluka, 2008). The mechanisms also appear different in duration; while TENS-induced antinociception generally wanes soon after termination (DeSantana et al., 2009), the effect of fixed spaced stimulation lasts 24 hr (Experiment 6; Baumbauer et al., 2009). Further work is needed to determine the stimulus conditions under which this fixed spaced shock effect emerges, whether it can be induced at non-nociceptive intensity levels (which would enhance its clinical usefulness), and whether it is effective in intact subjects.
Spinal neurons are sensitive to temporal relationships.
Exposure to irregular stimulation enhances tactile reactivity.
Exposure to regular stimulation diminishes tactile reactivity.
Exposure to regular stimulation prevents and reverses capsaicin-induced sesnsitivity.
Acknowledgments
We would like to thank Drs. Michelle Hook, Sandra Garraway and Kevin Hoy for their helpful comments. The present work was supported by NS041548 and HD058412.
List of abbreviations
- ANCOVA
Analysis of Covariance
- ANOVA
Analysis of Variance
- BDNF
brain-derived neurotrophic factor
- BL
baseline
- CS
conditioned stimulus
- CPG
central pattern generator
- EMR
enhanced mechanical reactivity
- ISI
interstimulus interval
- NMDAR
N-methyl D-aspartic acid receptor
- TENS
transcutaneous electrical nerve stimulation
- TRPV1
transient receptor potential cation channel subfamily V member 1
- Tukey HSD
Tukey Honestly Significant Difference
- US
unconditioned stimulus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ainsworth L, Budelier K, Clinesmith M, Fiedler A, Landstrom R, Leeper BJ, Moeller L, Mutch S, O’Dell K, Ross J, Radhakrishnan R, Sluka KA. Transcutaneous electrical nerve stimulation (TENS) reduces chronic hyperalgesia induced by muscle inflammation. Pain. 2006;120:182–187. doi: 10.1016/j.pain.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- Baumbauer KM, Grau JW. Timing in the absence of supraspinal input III: regularly spaced cutaneous stimulation prevents and reverses the spinal learning deficit produced by peripheral inflammation. Behav Neurosci. 2011;125:37–45. doi: 10.1037/a0022009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbauer KM, Hoy KC, Jr, Huie JR, Hughes AJ, Woller SA, Puga DA, Setlow B, Grau JW. Timing in the absence of supraspinal input I: variable, but not fixed, spaced stimulation of the sciatic nerve undermines spinally-mediated instrumental learning. Neuroscience. 2008;155:1030–1047. doi: 10.1016/j.neuroscience.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbauer KM, Huie JR, Hughes AJ, Grau JW. Timing in the absence of supraspinal input II: regularly spaced stimulation induces a lasting alteration in spinal function that depends on the NMDA receptor, BDNF release, and protein synthesis. J Neurosci. 2009;29:14383–14393. doi: 10.1523/JNEUROSCI.3583-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbauer KM, Young EE, Hoy KC, Jr, Joynes RL. Neurokinin receptors modulate the impact of uncontrollable stimulation on adaptive spinal plasticity. Behav Neurosci. 2007;121:1082–1094. doi: 10.1037/0735-7044.121.5.1082. [DOI] [PubMed] [Google Scholar]
- Boulos Z, Terman M. Food availability and daily biological rhythms. Neurosci Biobehav Rev. 1980;4:119–131. doi: 10.1016/0149-7634(80)90010-x. [DOI] [PubMed] [Google Scholar]
- Clatworthy AL, Walters ET. Activity-dependent depression of mechanosensory discharge in Aplysia. J Neurophysiol. 1993a;70:1195–1209. doi: 10.1152/jn.1993.70.3.1195. [DOI] [PubMed] [Google Scholar]
- Clatworthy AL, Walters ET. Rapid amplification and facilitation of mechanosensory discharge in Aplysia by noxious stimulation. J Neurophysiol. 1993b;70:1181–1194. doi: 10.1152/jn.1993.70.3.1181. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J Neurosci. 1992;12:3665–3670. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Crown ED, Ferguson AR, Joynes RL, Grau JW. Instrumental learning within the spinal cord: IV. Induction and retention of the behavioral deficit observed after noncontingent shock. BehavNeurosci. 2002;116:1032–1051. doi: 10.1037//0735-7044.116.6.1032. [DOI] [PubMed] [Google Scholar]
- Davies SN, Lodge D. Evidence for involvement of N-methylaspartate receptors in ‘wind-up’ of class 2 neurones in the dorsal horn of the rat. Brain Res. 1987;424:402–406. doi: 10.1016/0006-8993(87)91487-9. [DOI] [PubMed] [Google Scholar]
- de Leon R, Hodgson JA, Roy RR, Edgerton VR. Extensor- and flexor-like modulation within motor pools of the rat hindlimb during treadmill locomotion and swimming. Brain Res. 1994;654:241–250. doi: 10.1016/0006-8993(94)90485-5. [DOI] [PubMed] [Google Scholar]
- DeSantana JM, Da Silva LF, De Resende MA, Sluka KA. Transcutaneous electrical nerve stimulation at both high and low frequencies activates ventrolateral periaqueductal grey to decrease mechanical hyperalgesia in arthritic rats. Neuroscience. 2009;163:1233–1241. doi: 10.1016/j.neuroscience.2009.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkovic RG. Classical conditioning, sensitization and habituation in the spinal cat. Physiology & Behavior. 1975;14:297–304. doi: 10.1016/0031-9384(75)90037-2. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Bolding KA, Huie JR, Hook MA, Santillano DR, Miranda RC, Grau JW. Group I metabotropic glutamate receptors control metaplasticity of spinal cord learning through a protein kinase C-dependent mechanism. J Neurosci. 2008;28:11939–11949. doi: 10.1523/JNEUROSCI.3098-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AR, Crown ED, Grau JW. Nociceptive plasticity inhibits adaptive learning in the spinal cord. Neuroscience. 2006;141:421–431. doi: 10.1016/j.neuroscience.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Grau JW. Activation of the opioid and nonopioid analgesic systems: evidence for a memory hypothesis and against the coulometric hypothesis. J Exp Psychol Anim Behav Process. 1987;13:215–225. [PubMed] [Google Scholar]
- Grau JW, Barstow DG, Joynes RL. Instrumental learning within the spinal cord: I. Behavioral properties. BehavNeurosci. 1998;112:1366–1386. doi: 10.1037//0735-7044.112.6.1366. [DOI] [PubMed] [Google Scholar]
- Grau JW, Hyson RL, Maier SF, Madden J, Barchas JD. Long-term stress-induced analgesia and activation of the opiate system. Science. 1981;213:1409–1411. doi: 10.1126/science.7268445. [DOI] [PubMed] [Google Scholar]
- Groth R, Aanonsen L. Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or trkB, prevent inflammation-induced hyperalgesia. Pain. 2002;100:171–181. doi: 10.1016/s0304-3959(02)00264-6. [DOI] [PubMed] [Google Scholar]
- Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Hingne PM, Sluka KA. Blockade of NMDA receptors prevents analgesic tolerance to repeated transcutaneous electrical nerve stimulation (TENS) in rats. J Pain. 2008;9:217–225. doi: 10.1016/j.jpain.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook MA, Huie JR, Grau JW. Peripheral inflammation undermines the plasticity of the isolated spinal cord. Behav Neurosci. 2008;122:233–249. doi: 10.1037/0735-7044.122.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook MA, Moreno G, Woller S, Puga D, Hoy K, Jr, Balden R, Grau JW. Intrathecal morphine attenuates recovery of function after a spinal cord injury. J Neurotrauma. 2009;26:741–752. doi: 10.1089/neu.2008.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huie JR, Garraway SM, Baumbauer KM, Hoy KC, Jr, Beas BS, Montgomery KS, Bizon JL, Grau JW. Brain-derived neurotrophic factor promotes adaptive plasticity within the spinal cord and mediates the beneficial effects of controllable stimulation. Neuroscience. 2012;200:74–90. doi: 10.1016/j.neuroscience.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illich PA, King TE, Grau JW. Impact of shock on pain reactivity: I. Whether hypo- or hyperalgesia is observed depends on how pain reactivity is tested. Journal of Exp Psychol Anim Behav Process. 1995;21:331–347. doi: 10.1037//0097-7403.21.4.331. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Bevilaqua LR, Rossato JI, Bonini JS, Medina JH, Cammarota M. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29:496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Jakeman LB, Wei P, Guan Z, Stokes BT. Brain-derived neurotrophic factor stimulates hindlimb stepping and sprouting of cholinergic fibers after spinal cord injury. Exp Neurol. 1998;154:170–184. doi: 10.1006/exnr.1998.6924. [DOI] [PubMed] [Google Scholar]
- Ji R-R, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends in neurosciences. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Grau JW. Mechanisms of Pavlovian conditioning: Role of protection from habituation in spinal conditioning. BehavNeurosci. 1996;110:1375–1387. doi: 10.1037//0735-7044.110.6.1375. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Kim HK, Schattschneider J, Lee I, Chung K, Baron R, Chung JM. Prolonged maintenance of capsaicin-induced hyperalgesia by brief daily vibration stimuli. Pain. 2007;129:93–101. doi: 10.1016/j.pain.2006.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King EW, Audette K, Athman GA, Nguyen HO, Sluka KA, Fairbanks CA. Transcutaneous electrical nerve stimulation activates peripherally located alpha-2A adrenergic receptors. Pain. 2005;115:364–373. doi: 10.1016/j.pain.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Kristensen JD, Svensson B, Gordh T., Jr The NMDA-receptor antagonist CPP abolishes neurogenic ‘wind-up pain’ after intrathecal administration in humans. Pain. 1992;51:249–253. doi: 10.1016/0304-3959(92)90266-E. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Lundberg LE, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol. 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain. 1999;79:75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]
- Linden DR, Seybold VS. Spinal neurokinin3 receptors mediate thermal but not mechanical hyperalgesia via nitric oxide. Pain. 1999;80:309–317. doi: 10.1016/s0304-3959(98)00222-x. [DOI] [PubMed] [Google Scholar]
- Mannion RJ, Costigan M, Decosterd I, Amaya F, Ma QP, Holstege JC, Ji RR, Acheson A, Lindsay RM, Wilkinson GA, Woolf CJ. Neurotrophins: peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A. 1999;96:9385–9390. doi: 10.1073/pnas.96.16.9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev. 2008;57:134–146. doi: 10.1016/j.brainresrev.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher MW, Chen P, Salinas JA, Grau JW. Activation of the opioid and nonopioid hypoalgesic systems at the level of the brainstem and spinal cord: Does a coulometric relation predict the emergence or form of environmentally-induced hypoalgesia? Behav Neurosci. 1993;107:493–505. doi: 10.1037//0735-7044.107.3.493. [DOI] [PubMed] [Google Scholar]
- Meagher MW, Grau JW, King RA. Role of supraspinal systems in environmentally induced antinociception: effect of spinalization and decerebration on brief shock-induced and long shock-induced antinociception. Behav Neurosci. 1990;104:328–338. doi: 10.1037//0735-7044.104.2.328. [DOI] [PubMed] [Google Scholar]
- Mendell LM. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966;16:316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Zhang ET, Carvajal C, Gardell L, Quirion R, Dumont Y, Lai J, Porreca F. Selective mediation of nerve injury-induced tactile hypersensitivity by neuropeptide Y. J Neurosci. 2002;22:9858–9867. doi: 10.1523/JNEUROSCI.22-22-09858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett SP, Dudek SM, Eagleman D, Montague PR, Friedlander MJ. LTD Induction in Adult Visual Cortex: Role of Stimulus Timing and Inhibition. J Neurosci. 2001;21:2308–2319. doi: 10.1523/JNEUROSCI.21-07-02308.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice TW, Joynes RL, Meagher MW, Grau JW. Impact of shock on pain reactivity: III. The magnitude of hypoalgesia observed depends on test location. Behav Neurosci. 1996;110:528–541. doi: 10.1037//0735-7044.110.3.528. [DOI] [PubMed] [Google Scholar]
- Roy RR, Hutchison DL, Pierotti DJ, Hodgson JA, Edgerton VR. EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J Appl Physiol. 1991;70:2522–2529. doi: 10.1152/jappl.1991.70.6.2522. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, Benrath J, Brechtel C, Ruscheweyh R, Heinke B. Synaptic mechanisms of hyperalgesia. Prog Brain Res. 2000;129:81–100. doi: 10.1016/S0079-6123(00)29007-9. [DOI] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. Journal of Neuroscience. 2000;20:96RC. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouenborg J. Functional and topographical properties of field potentials evoked in rat dorsal horn by cutaneous C-fibre stimulation. J Physiol. 1984;356:169–192. doi: 10.1113/jphysiol.1984.sp015459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouenborg J, Sjolund BH. Activity evoked by A- and C-afferent fibers in rat dorsal horn neurons and its relation to a flexion reflex. J Neurophysiol. 1983;50:1108–1121. doi: 10.1152/jn.1983.50.5.1108. [DOI] [PubMed] [Google Scholar]
- Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol. 1994;72:169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- Sivilotti LG, Thompson SW, Woolf CJ. Rate of rise of the cumulative depolarization evoked by repetitive stimulation of small-caliber afferents is a predictor of action potential windup in rat spinal neurons in vitro. J Neurophysiol. 1993;69:1621–1631. doi: 10.1152/jn.1993.69.5.1621. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Rees H, Chen PS, Tsuruoka M, Willis WD. Inhibitors of G-proteins and protein kinases reduce the sensitization to mechanical stimulation and the desensitization to heat of spinothalamic tract neurons induced by intradermal injection of capsaicin in the primate. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 1997;115:15–24. doi: 10.1007/pl00005675. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Yu AL, Junger H, Doom CM. Antibody directed against GD(2) produces mechanical allodynia, but not thermal hyperalgesia when administered systemically or intrathecally despite its dependence on capsaicin sensitive afferents. Brain Res. 2002;930:67–74. doi: 10.1016/s0006-8993(01)03408-4. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5. Pearson/Allyn & Bacon; Boston: 2007. [Google Scholar]
- Tan PH, Yu SW, Lin VC, Liu CC, Chien CF. RNA interference-mediated gene silence of the NR1 subunit of the NMDA receptor by subcutaneous injection of vector-encoding short hairpin RNA reduces formalin-induced nociception in the rat. Pain. 2011;152:573–581. doi: 10.1016/j.pain.2010.11.026. [DOI] [PubMed] [Google Scholar]
- Terman GW, Shavit Y, Lewis JW, Cannon JT, Liebskind JC. Intrinsic Mechanisms of Pain Inhibition: Activation by Stress. Science. 1984;226:1270–1277. doi: 10.1126/science.6505691. [DOI] [PubMed] [Google Scholar]
- Thompson SW, Dray A, Urban L. Injury-induced plasticity of spinal reflex activity: NK1 neurokinin receptor activation and enhanced A- and C-fiber mediated responses in the rat spinal cord in vitro. J Neurosci. 1994;14:3672–3687. doi: 10.1523/JNEUROSCI.14-06-03672.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SW, Dray A, Urban L. Leukemia inhibitory factor induces mechanical allodynia but not thermal hyperalgesia in the juvenile rat. Neuroscience. 1996;71:1091–1094. doi: 10.1016/0306-4522(95)00537-4. [DOI] [PubMed] [Google Scholar]
- Thompson SW, Urban L, Dray A. Contribution of NK1 and NK2 receptor activation to high threshold afferent fibre evoked ventral root responses in the rat spinal cord in vitro. Brain Res. 1993a;625:100–108. doi: 10.1016/0006-8993(93)90142-a. [DOI] [PubMed] [Google Scholar]
- Thompson SW, Woolf CJ, Sivilotti LG. Small-caliber afferent inputs produce a heterosynaptic facilitation of the synaptic responses evoked by primary afferent A-fibers in the neonatal rat spinal cord in vitro. J Neurophysiol. 1993b;69:2116–2128. doi: 10.1152/jn.1993.69.6.2116. [DOI] [PubMed] [Google Scholar]
- Todd TP, Winterbauer NE, Bouton ME. Interstimulus interval as a discriminative stimulus: evidence of the generality of a novel asymmetry in temporal discrimination learning. Behav Processes. 2010;84:412–420. doi: 10.1016/j.beproc.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RJ. Spinal modulation of the induction of central sensitization. Brain Res. 1997;778:34–42. doi: 10.1016/s0006-8993(97)00946-3. [DOI] [PubMed] [Google Scholar]
- Wall PD, Woolf CJ. Muscle but not cutaneous C-afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. JPhysiol. 1984;356:443–458. doi: 10.1113/jphysiol.1984.sp015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M, Djourthe M, Taylor KM, Balsam PD. Relative temporal representations in Pavlovian conditioning. Behav Processes. 2010;83:154–161. doi: 10.1016/j.beproc.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD., Jr The role of TRPV1 receptors in pain evoked by noxious thermal and chemical stimuli. Exp Brain Res. 2009;196:5–11. doi: 10.1007/s00221-009-1760-2. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. C-primary afferent fibre mediated inhibitions in the dorsal horn of the decerebrate-spinal rat. Exp Brain Res. 1983;51:283–290. doi: 10.1007/BF00237204. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, King AE. Physiology and morphology of multireceptive neurons with C-afferent fiber inputs in the deep dorsal horn of the rat lumbar spinal cord. J Neurophysiol. 1987;58:460–479. doi: 10.1152/jn.1987.58.3.460. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Yajima Y, Narita M, Narita M, Matsumoto N, Suzuki T. Involvement of a spinal brain-derived neurotrophic factor/full-length TrkB pathway in the development of nerve injury-induced thermal hyperalgesia in mice. Brain Res. 2002;958:338–346. doi: 10.1016/s0006-8993(02)03666-1. [DOI] [PubMed] [Google Scholar]
- You HJ, Arendt-Nielsen L. Unilateral subcutaneous bee venom but not formalin injection causes contralateral hypersensitized wind-up and after-discharge of the spinal withdrawal reflex in anesthetized spinal rats. Exp Neurol. 2005;195:148–160. doi: 10.1016/j.expneurol.2005.04.009. [DOI] [PubMed] [Google Scholar]
- You HJ, Morch CD, Arendt-Nielsen L. Electrophysiological characterization of facilitated spinal withdrawal reflex to repetitive electrical stimuli and its modulation by central glutamate receptor in spinal anesthetized rats. Brain Res. 2004;1009:110–119. doi: 10.1016/j.brainres.2004.02.053. [DOI] [PubMed] [Google Scholar]