Abstract
All extant species of flatfish (order Pleuronectiformes) are thought to descend from a common ancestor, and therefore to represent a monophyletic group. This hypothesis is based largely on the dramatic bilateral asymmetry and associated ocular migration characteristics of all flatfish. Yet, molecular-based phylogenetic studies have been inconclusive on this premise. Support for flatfish monophyly has varied with differences in taxonomic and gene region sampling schemes. Notably, the genus Psettodes has been found to be more related to non-flatfishes than to other flatfishes in many recent studies. The polyphyletic nature of the Pleuronectiformes is often inferred to be the result of weak historical signal and/or artifact of phylogenetic inference due to a bias in the data. In this study, we address the question of pleuronectiform monophyly with a broad set of markers (from six phylogenetically informative nuclear loci) and inference methods designed to limit the influence of phylogenetic artifacts. Concomitant with a character-rich analytical strategy, an extensive taxonomic sampling of flatfish and potential close relatives is used to increase power and resolution. Results of our analyses are most consistent with a non-monophyletic Pleuronectiformes with Psettodes always being excluded. A fossil-calibrated Bayesian relaxed clock analysis estimates the age of Pleuronectoidei to be 73 Ma, and the time to most recent common ancestor of Pleuronectoidei, Psettodes, and other relative taxa to be 77 Ma. The ages are much older than the records of any fossil pleuronectiform currently recognized. We discuss our findings in the context of the available morphological evidence and discuss the compatibility of our molecular hypothesis with morphological data regarding extinct and extant flatfish forms.
Keywords: Acanthomorpha, Pleuronectiformes, Phylogeny, Model of Evolution, Non-stationary Evolution, Base Composition Heterogeneity
Graphical Abstract
1. Introduction
1.1 Current State of Flatfish Systematics
Flatfish (Percomorpha: Pleuronectiformes) have received attention in evolutionary biology from Darwin’s time (Darwin, 1872) because of their pronounced cranial asymmetry, which requires the ontogenetic migration of an eye from one side of the head to the other (Frazzetta, 2012). The lack of extant species with incipient or partial cranial asymmetry opens questions about evolutionary tempo and mode of the morphological change (sudden vs. gradual evolutionary change) and room for speculation on the evolutionary scenarios that would promote the evolution of asymmetry (Janvier, 2008). For example, Lamarck proposed a scenario of adaptive evolution in which flatfish ancestors lived in exceedingly shallow water and lied flatly on the sea bed (Lamarck, 1809). Flatfish and the absence of intermediate forms were discussed as early challenges to theories of evolutionary change through the accumulation of a series of small steps (Darwin, 1872; Mivart, 1871). The recent discovery of fossils showing an intermediate degree of asymmetry casts those early debates in a new light by showing how the current marked asymmetry could have arisen (Friedman, 2008, 2012). However, pleuronectiform monophyly remains a topic of ongoing debate (e.g., Amaoka, 1969; Chabanaud, 1949; Chapleau, 1993; Dettai and Lecointre, 2005).
Support for pleuronectiform monophyly is based largely on the dorsoventrally compressed morphology that the group’s common name highlights. Three synapomorphies have been identified in support of flatfish monophyly: (1) cranial asymmetry as a result of the migration of the eyes, (2) the dorsal fin positioned dorsal to the skull, and (3) the presence of the recessus orbitalis (Chapleau, 1993). The recessus orbitalis is a muscular sac in the orbit that can be filled with fluid enabling the eyes to protrude above the head while a flatfish is lying on the substrate (Cole and Johnstone, 1902; Holt, 1894). Flatfish begin life as bilaterally symmetric larvae, but develop asymmetry through development as one eye migrates dorsally across the head and cranium to the opposite side (Brewster, 1987). Pleuronectiformes is a species-rich group with approximately 700 recognized, extant species, 134 genera, and 14 families. It is considered to be derived from a perciform (perch-like) lineage (Chapleau, 1993; Chen et al., 2003; Munroe, 2005; Nelson, 2006). The core of flatfish species diversity occurs in the tropics but about one fourth of the species are found in temperate waters (Hensley, 1997; Munroe, 2005).
According to the otolith fossil record, early pleuronectiforms could have been present in the Late Paleocene-Early Eocene, 57 to 53 Ma (Munroe, 2005; Schwarzhans, 1999). The oldest crown flatfish fossil skeleton known is a representative of unknown affinity to extant forms of bothids from the Lutetian, Eocene (around 45 million years ago; Chanet, 1997, 1999; Norman, 1934). Shortly after this period, several different pleuronectiform lineages suddenly appear in the fossil record along with other diverse acanthomorph fishes (Chanet, 1997; Munroe, 2005; Patterson, 1993; Schwarzhans, 1999). Among fossil flatfishes, Soleidae is the best represented family (Chanet, 1999). Extant intermediary forms between symmetrical and asymmetrical fish do not exist, though they are present in the fossil record at approximately 40 to 50 million years ago (Friedman, 2008, 2012).
Phylogenetic studies appear to be converging on a consensus but not yet fully defined placement of flatfish among one of the major acanthomorph clades: clade L or the Carangimorpha sensu Li et al. (2009). Evidence for clade L was first reported by Chen et al. (2003) from multiple gene sequence data. Currently, this clade comprises disparate perciform taxa encompassing carangids (jacks), echeneids (remoras), coryphaenids (dolphinfishes), rachycentrids (cobia), sphyraenids (barracudas), menids (moonfish), polynemids (threadfins), xiphiids (swordfish), istiophorids (billfishes), toxotids (archerfishes), centropomids (snooks), latids (Nile perches and allies) (Betancur-R. et al., 2013; Chen et al., 2003, 2007; Li et al., 2009; Little et al., 2010; Near et al., 2012; Smith and Craig, 2007; Smith and Wheeler, 2006; Wainwright et al., 2012). Lactarius (false trevally) has been recognized as part of the Carangimorpha in this study. Yet, questions regarding when flatfishes evolved, and how these diverse lineages are related to each other and to other percomorphs in the clade L remain unresolved (Azevedo et al., 2008; Berendzen and Dimmick, 2002; Chapleau, 1993; Chen et al., 2003; Dettai and Lecointre, 2005; Li et al., 2009; Little et al., 2010; Roje, 2010; Shi et al., 2011; Smith and Wheeler, 2006). Moreover, molecular studies have not consistently shown flatfishes to be a monophyletic group with Psettodidae and a few taxa exhibiting base composition bias often excluded (Chen et al., 2003; Dettai and Lecointre, 2005; Li et al., 2009; Near et al., 2012; Smith and Wheeler, 2006).
1.2 Psettodes (spiny turbot) and pleuronectiform polyphyly
Psettodidae contains a single genus (Psettodes) with three recognized species (Nelson, 2006). The condition of the three pleuronectiform synapomorphies differs between Psettodes and other pleuronectiforms. Generally in pleuronectiforms the eyes are on the same side of the head, but in the case of Psettodes one eye is at the dorsal midline (Friedman, 2008). This condition affects the insertion of the dorsal fin in Psettodes, which unlike that in other flatfish, is posterior to the eye (Nelson, 2006). The recessus orbitalis is assumed by Chapleau (1993) to be present among all flatfishes including Psettodes, but it may in fact not be found in Psettodes (Chabanaud, 1937). Chabanaud (1937) notes that the eyes of Psettodes cannot be extended and do not have any skin folds around the eyes unlike pleuronectoids, which can extend the eyes and have skin folds around the eyes. In addition, Psettodes has distinct characteristics that are not typical of other flatfish. Populations of species of Psettodes may include both left- and right-sided fish, a characteristic termed antisymmetry. In contrast, populations of other pleuronectiform species have a tendency to be uniformly left or right sided (Palmer, 1996). Psettodes retains many characters considered to be ancestral in Pleuronectiformes. Chapleau (1993) lists the following: palatine teeth (character 4), toothed plates on basihyal (character 5), a basisphenoid (character 6), spines in median fins (character 7), absent or not well developed sciatic portion of urohyal, (character 8), presence of uroneural 1 (character 9), elongated shape of second infrapharyngobranchial (character 10), a large maxilla (character 11), and a parhypural the articulating with vertebral column (character 35). Other characters that may be considered primitive in Psettodes are the presence of a macula neglecta in the inner ear (Platt, 1983) and vertical barring (Hewer, 1931). Psettodes bodies are almost rounded and do not have the associated bilateral asymmetry in musculature typical of other flatfishes (Munroe, 2005) and often swim in an upright orientation (Hensley, 1999). The distinct morphology of Psettodes earned it an early characterization as “simply an asymmetric ercoid” (Regan, 1910). The theory that Psettodes arose from a different lineage is not new, and several authors outline the similarities of Psettodes to percoids (Amaoka, 1969; Hubbs, 1945; Kyle, 1921; Norman, 1934; Regan, 1910, 1929). The scarcity of shared derived characters among percoid families severely limits the phylogenetic utility of these observations (Chapleau, 1993; Gosline, 1971; Johnson, 1984). Psettodes is now considered to be most closely related to other flatfishes and to be the most basal lineage of the Pleuronectiformes (Chapleau, 1993; Friedman, 2012; Munroe, 2005). In the current system of fish classification, the suborder Psettodoidei (including a sole family Psettodidae) is the sister lineage of all other living flatfish species, which are grouped in the suborder Pleuronectoidei (Nelson, 2006).
From a molecular-based perspective, the inferred phylogenetic placement of Psettodes and other flatfish taxa has varied between studies depending on genes surveyed or inference method employed (Dettai and Lecointre, 2005). The salient pattern is that psettodids are not grouped with other pleuronectiform taxa (Dettai and Lecointre, 2005; Li et al., 2009; Smith and Wheeler, 2006). It remains to be determined if methodological artifacts (e.g., base composition or long-branch attraction) are responsible for the non-monophyly of flatfish. For this study we attempt to resolve that question by increasing the number of independent data sources (more genes) and by recognizing and addressing sources of phylogenetic artifacts.
1.3 Data Sources
To improve phylogenetic resolution we examined six independent sources of characters in the form of single copy protein-coding nuclear genes. Increasing the number of independent data points and sites is a well established strategy for improving the accuracy of phylogenetic inference (e.g., Cao et al., 1994; Chen and Mayden, 2010; Mitchell et al., 2000; Russo et al., 1996; Wolf et al., 2004; Wortley et al., 2005). Studies of acanthomorph phylogeny including a sampling of Pleuronectiformes from divergent lineages and based on evidence from more than one locus have used three data sources (Chen et al., 2003; Smith and Wheeler, 2006) or four data sources (Dettai and Lecointre, 2005; Li et al., 2009). It is important to consider that increasing the number of traits will not circumvent problems due to substitution saturation, difficulties in alignment, and/or lack of information due to strong sequence conservation (Smith and Wheeler, 2006) as noted by Chen et al. (2003; 2008) and Li et al. (2009). The genes sequenced here were selected in part because they can be aligned with little or no ambiguity and have been reported to be phylogenetically informative (Chen, 2001; Chen et al., 2003, 2008; Dettai and Lecointre, 2005; López et al., 2004).
1.4 Phylogenetic Artifacts
The most commonly used implementations of nucleotide substitution models for phylogenetics assume that nucleotide frequencies remain relatively stable across all the lineages being examined. However, there is ample evidence of base composition shifts at different levels of phylogenetic divergence (Akashi et al., 1998; Eyre-Walker, 1999; Galtier and Gouy, 1995; Mooers and Holmes, 2000). Relying on an incorrect substitution model can mislead phylogenetic inference by affecting branch length estimation (Posada, 2001) or estimating an incorrect topology (Bruno and Halpern, 1999; Penny et al., 1994) and support for resulting topologies can also be biased (Buckley and Cunningham, 2002). Convergent base composition can result in organisms being improperly associated in phylogenies as a result of similarity in overall frequencies of nucleotides (Delsuc et al., 2005; Foster and Hickey, 1999; Phillips et al., 2004; Steel et al., 1993). In cases where a molecular hypothesis opposes a well established morphological hypothesis, it is often thought that base composition bias is at fault (e.g., Li and Ortí, 2007; Sheffield et al., 2009). However, identifying when the degree of deviation from base composition stationarity will mislead phylogenetic inference is problematic (Jermiin et al., 2004). In addition to base composition non-stationarity, long branch attraction can also contribute to artificial support for groupings not corresponding to true clades (Bergsten, 2005; Felsenstein, 1978).
We approach the question of pleuronectiform monophyly with the intent of identifying and eliminating possible biases in the data. We exhaustively evaluate our sequence data by taxon, gene, and codon position for evidence of compositional bias or saturation and remove or recode affected characters. A broad sampling of pleuronectiforms and possible relatives is used to reduce occurrences of long-branch attraction in the dataset and increase accuracy (Hillis, 1998; Hillis et al., 2003). Because simply eliminating data partitions or taxa, and using alternative sequence codings to reduce compositional bias comes at the cost of potential phylogenetic signal, we also employ several phylogenetic methods that have been designed to account for non-stationarity of base composition evolution (e.g., p4; Foster, 2004). We review existing morphological evidence to assess the compatibility between molecular and morphological sources concerning the question of pleuronectiform monophyly.
2. Materials and methods
2.1 Taxon and Character Sampling
We assembled a taxonomic sample representing all major divisions within the Pleuronectiformes including Psettodes (25 taxa). In addition, we sampled heavily (15 taxa) within acanthomorph clade L, the Carangimorpha (Chen et al., 2003; Li et al., 2009) to capture potential sister taxa of pleuronectiforms (see introduction). Finally, a broad sampling of 48 additional percomorph taxa representing main lineages recently identified in molecular analyses (Chen et al., 2003, 2007; Li et al., 2009; Miya et al., 2003; Smith and Craig, 2007; Wainwright et al., 2012) were included to evaluate the support for acanthomorph clade L. Two beryciforms were used as outgroups to root the percomorph tree. Tissue samples were obtained from collections performed by W.-J. Chen or the University of Kansas tissue collections (Table A). In addition to newly reported sequences, publicly available sequences from GenBank were included this study (Table A).
2.2 DNA data
Total genomic DNA was isolated from samples using Qiagen DNEasy spin-column or QIAmp kits following manufacturer’s directions. Fragments of six nuclear protein-coding genes were amplified for this study. The nuclear protein-coding genes used in the study are Recombination Activating Gene 1 (RAG1), Rhodopsin (RH), Early Growth Response Protein genes 1, 2B, and 3 (E1, E2B, E3), and Mixed-lineage Leukemia (MLL). Primer sequences and sources are given in Table B. The temperature cycling profile used for amplification of RAG1 had an initial denaturation step of 95° C for 4 min, followed by 35 cycles of 95° C for 40 sec, 53 ° C for 40 sec, and 72 ° C for 90 sec, and a final extension of 72° C for 7 min. For the other five genes a similar profile was used, but the annealing temperature was raised to 55° C and the extension time was reduced to 60 sec. Either Takara ExTaq or Promega GoTaq Flexi were used. For amplifications using Promega GoTaq Flexi, PCR reagent concentrations were 1X Promega GoTaq Flexi reaction buffer, 0.25 mM dNTP’s, 2.0 mM MgCl2, 0.4 μM forward primer, 0.4 μM reverse primer, 0.025 U/μL GoTaq Flexi Taq polymerase, and 1 μL template DNA (variable concentrations). Reagent concentrations for reactions using Takara ExTaq were 1X Takara ExTaq reaction buffer, 0.8 mM dNTP’s, 2.0 mM MgCl2, 0.2 μM forward primer, 0.2 μM reverse primer, 0.5 U/μL Takara ExTaq polymerase. Diluted DNA extractions of varying concentrations were added at a ratio of 2.5 μL for a 25 μL reaction. Unpurified PCR products were sent to multiple commercial institutions for purification and Sanger sequencing. Raw sequence output was examined, edited and assembled using the features implemented in CodonCode Aligner Version 3.7.1.1 (by CodonCode Corp., Dedham, MA, USA).
Assembled DNA sequences were managed using Se-Al v2.0a11 (available at http://tree.bio.ed.ac.uk/software/seal/) and Mesquite 2.75 (Maddison and Maddison, 2011). Compiled sequences were initially aligned with MUSCLE (Edgar, 2004a, 2004b) using the online server at http://www.ebi.ac.uk/Tools/muscle/index.html. Alignments were then adjusted manually to ensure that the placement of inferred insertions/deletions (indels) followed the expected codon structure. Regions containing large indels (e.g., tandem repeats in EGR genes) showing high dissimilarity in sequence length, which may produce invalid assertions of homology were discarded from the phylogenetic analyses. We trimmed the 5′- and 3′-ends of some sequences to reduce the number of sites with missing data.
2.3 Stationary Phylogenetic Analyses
For the initial phylogenetic analyses, we had two expectations for variability in the data since the most constrained codon position is the second and the least is the third position (Alff-Steinberger, 1969; Haig and Hurst, 1991; Kimura, 1980; Woese, 1965). Consequently, we expected stronger and more numerous deviations from base composition homogeneity at the third codon position than at other positions. Secondly, at the time scales we are investigating, third codon positions could be mutationally saturated and recoding to purines and pyrimidines (RY) would be useful for reducing both saturation and base composition bias (e.g., Chen et al., 2008; Delsuc et al., 2003; Phillips and Penny, 2003; Phillips et al., 2004).
To determine if certain taxon/marker combinations showed significant deviation in base composition, we created alignments of variable sites for each codon position and tested each alignment using the Chi-squared test for base composition homogeneity implemented in TreePuzzle version 5.2 (Schmidt et al., 2002). Systematic biases across markers were evaluated based on repeated failures to pass the test of homogeneity and helped establish whether genome wide biases are present in the taxa in this study. Based on results of tests for stationarity, we generated the following three alternative codings of the data set for phylogenetic inference: 1) all codon positions retained for all genes (1N2N3N); 2) all codon positions retained, third codon positions recoded as purines and pyrimidines (1N2N3RY); and 3) third codon positions discarded (1N2N). We also generated alignments following these three data schemes with no missing data to assess the influence of missing data on inferred relationships.
Phylogenetic analysis of the 1N2N3N, 1N2N3RY, and 1N2N datasets for all taxa and those with no missing data was conducted in RAxML 7.2.8 under a partitioned maximum likelihood (ML) approach using the general time reversible model of nucleotide evolution (GTR) (Stamatakis, 2006) with a four category gamma distribution (Γ), invariant sites (I) and automatic stopping of bootstrap replicates. Data were partitioned by gene and codon position. For the alignments containing all taxa, we evaluated the stability of the resulting topology using RogueNaRok (Aberer et al., 2013). Rogue taxa, those that fail to find a consistent placement among pseudoreplicate analyses (Aberer et al., 2013) were removed and the edited alignment reanalyzed.
2.4 Alternative Phylogenetic Analyses
We also conducted analyses implementing models designed to alleviate issues of compositional heterogeneity. We used the three data coding schemes (1N2N3N, 1N2N3RY, and 1N2N) partitioned by gene in these analyses and used the programs Phylobayes 3.3.b (Lartillot et al., 2009) and p4 (Foster, 2004). Phylobayes implements a CAT-GTR model (Lartillot and Philippe, 2004) that allows for more variation in nucleotide evolution than the more widely used substitution models. In Phylobayes we ran an analysis for each of the three data schemes with two chains for at least 500 cycles. After 500 cycles, a sampling every 100 cycles was done to check convergence of the two chains. The program was allowed to run until all discrepancies between the chains were less than 0.3 and all effective sample sizes (ESS) were greater than 50 as recommended by the software developers.
We conducted two different analyses in p4 differing on the treatment of rate matrices and base composition vectors. In both cases the estimate of the α shape parameter for the Γ distribution was linked across partitions with unlinked relative rates for each partition. We used the GTR+I+Γ model of nucleotide evolution in p4 for each data partition. When more than one base composition vector or rate matrix was specified, the additional vector or matrix was constrained to represent at least two taxa. The placement of additional base composition vectors and rate matrices was at first placed randomly, then allowed to vary within the MCMC tree search. The first strategy was to retain a single rate matrix and proportion of invariant sites per partition to reduce parameterization. Each partition was then permitted to have multiple base composition vectors. In our second strategy, we allowed multiple rate matrices and base composition vectors in each partition. We began with a basic Markov chain Monte Carlo (MCMC) Bayesian tree search with p4 using four chains, sampled every 1,000 steps, and a total run length of 1,000,000 steps. We subsequently modified MCMC parameters to reach adequate sampling and mixing. In all p4 analyses, we discarded 10% of samples as burnin.
2.5 Divergence Time Estimation
We estimated divergence times using the simultaneous Bayesian phylogenetic inference and divergence time approach (Drummond et al., 2006) with a Bayesian relaxed clock model with uncorrelated lognormal rate heterogeneity as implemented in BEAST version 1.7.2 (Drummond et al., 2012). Given the highly congruent phylogenetic trees produced by the analysis described above, we only employed the 1N2N3RY data coding scheme in the divergence time analysis. We generated a starting tree for this analysis by partitioning the data by gene and constraining ingroups, outgroups, and the time to most recent common ancestor (TMRCA) of the ingroup. We calibrated the root of the tree at 150 million years ago (Ma) using the first appearance of euteleost and ostariophysan fish in the fossil record at a minimum of 149.85 Ma (Benton et al., 2009). The root age was chosen so that subsequent constraints forced on the starting tree would be compatible. An uncorrelated relaxed clock was used to generate the input tree with a Markov chain Monte Carlo (MCMC) chain of 100 million generations sampled every 5,000 generations. All partitions were modeled under a GTR+I+Γ model of nucleotide evolution. We applied a 10% burnin and examined the MCMC run output with Tracer v 1.5 to determine whether the analyses resulted in sufficiently sampled parameters (Drummond et al., 2012). The resulting topology was incorporated as the starting tree into the following divergence time analysis.
The alignment was partitioned by gene and each partition was modeled under a GTR+I+Γ model of evolution. We included settings in BEAST to use ambiguities across all partitions and to unlink the uncorrelated relaxed clock for each data partition. Based on the results from the ML tree search in this study, we assigned lognormal fossil constraint distributions at well-supported nodes (Table D). We did not use any fossil pleuronectiform fossils as calibration points to minimize the effect of prior assumptions on pleuronectiform relationships and age of lineages.
Two independent runs of 100 million generations sampled every 10,000 generations were generated. After verifying adequate sampling and convergence with Tracer v 1.5, we applied a 10% burnin and combined the tree files with LogCombiner. The final maximum clade credibility tree with mean heights was generated with TreeAnnotator.
3. Results
3.1 Taxon and Character Sampling
Sequence data from a total of 90 taxa are examined in this analysis. This taxonomic sample includes 25 pleuronectiforms (Table A). No taxon has more than two missing genes in our data matrix. Sixty-seven of the 90 taxa did not have any missing sequence data.
3.2 Alignment
After end-trimming and concatenation, our final alignment spans 5,664 nucleotide sites. The aligned sequence matrix of combined genes (90 taxa) includes about 7.6% missing nucleotides and gapped sites; a text file with the concatenated alignment is available from the Dryad repository (doi:xxxx/dryad.xxx). The 1N2N3N alignment includes 3,034 variable sites, of which 2,525 are parsimony informative. When recoded as 1N2N3RY the alignment contains 2,396 variable sites and 1,821 parsimony informative sites. Excluding first codon positions (1N2N) produces an alignment of 3,776 characters. Of these, 1,239 are variable and 838 are parsimony informative.
3.3 Base composition changes
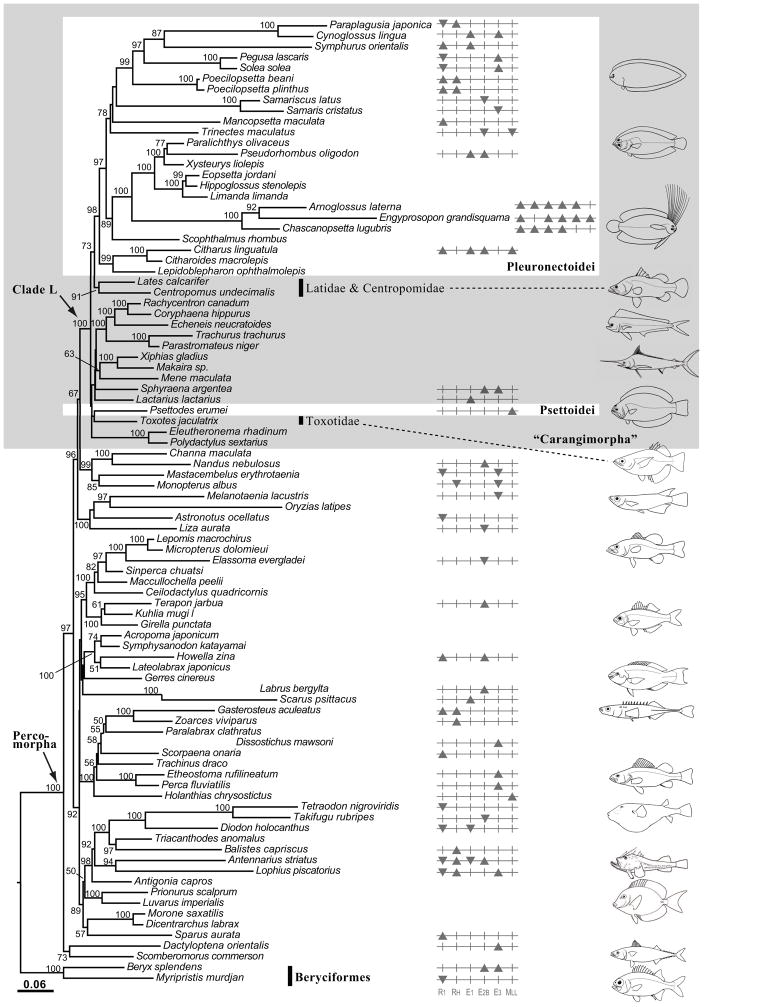
Forty-seven taxa contain compositional biases in one or more genes at variable sites (Table C and Fig. 1). Psettodes erumei exhibits compositional bias towards higher GC content only in the MLL gene, but not a broader genome wide base composition bias. In contrast, systematic base composition bias in other pleuronectiform taxa is evident (e.g., Bothidae). In other percomorph taxa, GC and AT bias are only evident in lophiiforms. We detect no evidence of unusual base composition biases in the non-pleuronectiform cargangimorphs included in our sample.
Fig. 1.
A maximum-likelihood (ML) tree generated under a GTR+I+Γ model of sequence evolution in RAxML, depicting phylogenetic positions of the flatfishes (Pleuronectiformes) (taxa within the white rectangle boxes) in relation to other percomorph taxa. All taxa are included in the analysis with data partitioned by gene and codon position and third codons recoded as purines or pyrimidines (1N2N3RY). Values at nodes represent bootstrap values. Those values below 50% are not shown. Taxa with significant higher GC content and lower GC content with respect to gene partitions, as detected by chi-square tests, are indicated as black up-pointing and down-pointing triangles, respectively, after the taxon names.
3.4 Stationary Phylogenetic Analyses
Results of ML phylogenetic analyses using the different combination of data and taxa described in the methods, consistently find a non-monophyletic pleuronectiformes with Psettodes always excluded (Fig. 1, Table 1). The monophyly of the suborder Pleuronectoidei (Pleuronectiformes minus Psettodes) is supported. Important relationships and bootstrap support are summarized in Table 1. The sister group relationship of Pleuronectoidei and Centropomidae (sensu Greenwood (1976), Lates + Centropomus) is consistently inferred across all ML analyses. The placement of Psettodes varied with taxon sampling and data scheme. Evaluation of ML results from the alignments containing all taxa with RogueNaRok identified rogue taxa in 1N2N3N and 1N2N data schemes. Importantly, this analysis does not identify Psettodes as a possible rogue taxon. ML searches with rogue taxa removed from 1N2N3N and 1N2N searches again resolve a non-monophyletic Pleuronectiformes (Table 1). Finally, all of the analyses strongly support the monophyletic “Carangimorpha” (Clade L, ML bootstrap value = 100%; Posterior probability = 1). Carangimorpha in this study includes recognized taxa from previous molecular studies plus a perciform family, Lactariidae. Lactariidae contains only one species, Lactarius lactarius, widely distributed in Indo-West Pacific (Nelson, 2006).
Table 1.
Summary of key relationships and support values for phylogenetic analyses of different data coding schemes and taxon composition. Centopomidae includes Lates and Centropomus (Greenwood, 1976; Li et al., 2011). For each analysis the basic characteristics and outcomes are reported.
| Data Scheme | Numer of Partitions | Pleuronectiformes Monophyletic? | Bootstrap Support or Posterior Probability For Pleuronectoidei | Sister Group of Pleuronectoidei | Bootstrap Support or Posterior Probability for Sister of Pleuronectoidei | Sister of Psettodes | Bootstrap Support or Posterior Probability for Sister of Psettodes |
|---|---|---|---|---|---|---|---|
|
ML Analysis
| |||||||
| 1N2N3N | 18 | No | 100 | Centropomidae | 32 | Toxotes jaculatrix | 27 |
| 1N2N3RY | 18 | No | 98 | Centropomidae | 73 | Toxotes jaculatrix | 39 |
| 1N2N | 18 | No | 70 | Centropomidae | 67 | Other Carangimorpha, not Pleuronectifomres + Centropomidae excluding Polydactylus sextarius and Eleutheronema rhadinum | 21 |
| 1N2N3N No Missing Data | 18 | No | 100 | Centropomidae | 34 | Eleutheronema rhadinum | 47 |
| 1N2N3RY No Missing Data | 18 | No | 99 | Centropomidae | 81 | Pleuronectiformes+Other Carangimorpha excluding Eleutheronema rhadinum | 34 |
| 1N2N No Missing Data | 18 | No | 92 | Centropomidae | 87 | Other Carangimorpha excluding Toxotes jaculatrix and Eleutheronema rhadinum | 16 |
| 1N2N3N Rogue Taxa Removed | 18 | No | 100 | Centropomidae | 29 | Polydactylus sextarius + Eleutheronema rhadinum | 36 |
| 1N2N Rogue Taxa Removed | 18 | No | 82 | Centropomidae | 75 | Other Carangimorpha, not Pleuronectifomres + Centropomidae, excluding Polydactylus sextarius and Eleutheronema rhadinum | 42 |
|
GTR-CAT Model
| |||||||
| 1N2N3RY | 6 | No | 0.87 | Centropomidae | 0.5 | Part of four branch polytomy at base of Carangimorpha | 0.99 |
| 1N2N | 6 | No | 0.94 | Polytomy including Centropomidae | - | Part of five branch polytomy at base of Carangimorpha | - |
|
p4 Multiple Composition Vectors
| |||||||
| 1N2N3N | 6 | No | 59 | Centropomidae | 54 | Eleutheronema rhadinum + Polydactylus sextarius | 100 |
| 1N2N3RY | 6 | No | 100 | Centropomidae | 98 | Other Carangimorpha excluding Toxotes jaculatrix, Eleutheronema rhadinum and Polydactylus sextarius | 99 |
| 1N2N | 6 | No | 90 | Centropomidae | 90 | Pleuronectiformes+Other Carangimorpha excluding Eleutheronema rhadinum and Polydactylus sextarius | 66 |
|
p4 Multiple Composition Vectors and Rate Matrices
| |||||||
| 1N2N3N | 6 | No | 61 | Centropomidae | 56 | Eleutheronema rhadinum + Polydactylus sextarius | 100 |
| 1N2N3RY | 6 | No | 100 | Centropomidae | 97 | Other Carangimorpha excluding Toxotes jaculatrix, Eleutheronema rhadinum and Polydactylus sextarius | 99 |
| 1N2N | 6 | No | 98 | Centropomidae | 100 | Other Carangimorpha excluding Toxotes jaculatrix, Eleutheronema rhadinum and Polydactylus sextarius | 65 |
|
BEAST
| |||||||
| 1N2N3RY | 6 | No | 1 | Centropomidae | 0.98 | Toxotes jaculatrix | 0.52 |
3.5 Alternative Phylogenetic Analyses
Use of the CAT-GTR model in Phylobayes does not result in convergence with the 1N2N3N data scheme. In the case of 1N2N3N data scheme, long run time permits high ESS for each parameter but variation between chains remains greater than 0.3. With the other two data schemes, convergence was reached and the topologies generated by the 1N2N3RY and 1N2N coding schemes are summarized in Table 1.
Analysis of the data with p4 varied in base composition vectors and rate matrices assigned to each data scheme. In analyses of the 1N2N3N dataset we assigned six base composition vectors with a run length of 5 million generations; and four base composition vectors and four rate matrices with a run length of 3 million generations in a second analysis. In analyses of the 1N2N3RY matrix, five base vectors were modeled on the tree in addition to a single rate matrix; and three base vectors and three rate matrices in a second analysis. Both of these 1N2N3RY analyses ran for 3 million generations. In analyses of the 1N2N matrix, we allowed four base vectors and one rate matrix; and three base vectors and three rate matrices in a second analysis. Both of these runs had lengths of 3 million generations. In all six p4 analyes, Pleuronectiformes is polyphyletic. Pleuronectoidei remains monophyletic whereas Psettodes is more closely related to non-pleuronectiform taxa (Table 1). The placement of Psettodes is inconsistent between analyses.
3.6 Divergence Time Estimation
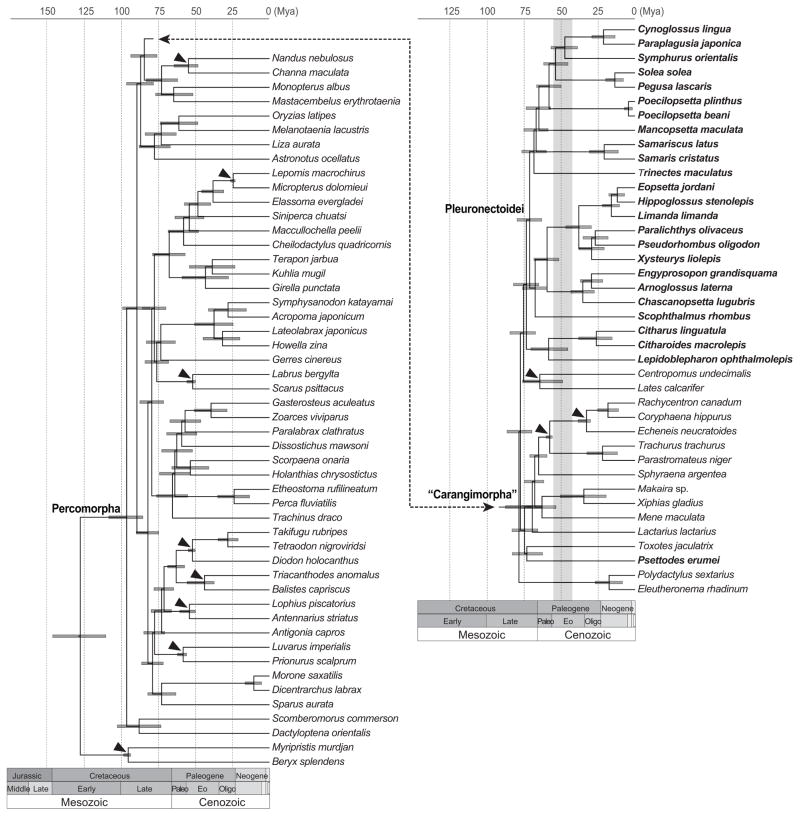
Simultaneous Bayesian tree inference and divergence time estimation results in a paraphyletic Pleuronectiformes, but monophyletic Pleuronectoidei and Carangimorpha, which includes Psettodes (Fig. 2 and Table 1). We find the estimated divergence time for the split between Pleuronectoidei and Centropomidae to have a mean age of 75.3 Ma (95% highest posterior density (HPD) 67.3 – 84.5), and the time to MRCA of the Pleuronectoidei to be 73.4 Ma (95% HPD 65.1 – 82.1). The origin of the carangimorphs dates back to 78.4 Ma (95% HPD 65.2 – 130.0). The time to the divergence of Psettodes from other fishes in our sample is estimated to have a mean of 77.4 Ma (95% HPD 69.7 – 86.5 Ma).
Fig. 2.
Timetree based on a Bayesian relaxed clock calibrated by fossils and distributions described in Supplementary Material Table D. The timescale is in millions of years ago (Ma). Horizontal bars at nodes represent 95% highest posterior densities and black triangles indicate fossil calibrated nodes. Pleuronectiform taxa are highlighted in bold. Vertical bar in light gray indicates the period when flatfish intermediates were present according to fossil records.
4. Discussion
4.1 Non-monophyletic Pleuronectiformes and the sister of the Pleuronectoidei
Combined, the results from all our analyses indicate that the six-gene (~ 5.5 k bp) dataset is incongruent with a monophyletic Pleuronectiformes. The genus Psettodes is consistently excluded from the Pleuronectiformes across analyses. We find the Pleuronectoidei to be monophyletic, in agreement with previous molecular and morphological studies (Azevedo et al., 2008; Berendzen and Dimmick, 2002; Chapleau, 1993). We identify the sister-taxa of the Pleuronectoidei to be the Centropomidae (including Latidae; see below). We addressed several potential biases that may have misled phylogenetic inference. Our taxonomic sampling is broad and specifically targets clade L percomorphs as potential sister lineages to pleuronectiform clades. We include multiple independent loci with a substantial degree of variability that could be aligned with high confidence. Further, we evaluated the loci for base composition homogeneity and implemented alternative treatments of third codon positions (RY recoding and deletion). We evaluated the stability of phylogenetic inference by using only taxa with no missing data and by excluding potentially problematic taxa as identified by the approach implemented in RogueNaRok. We also used several alternative molecular evolution models. No treatment of the data yielded support of a monophyletic Pleuronectiformes.
Pleuronectioid sister-taxa, Centropomidae sensu Greenwood (1976) includes two currently recognized perciform families Latidae and Centropomidae (Nelson, 2006). The evolutionary affinity for these two families was confirmed by recent molecular studies (Chen et al., 2007; Li et al., 2011; Near et al., 2012) and this study. Two morphological features used to unite these two groups in single assemblage are: (1) expanded neural arch and spine on the 2nd vertebrae often embracing the spine of the first vertebra; (2) and, pored lateral-line scales extending to the posterior edge of the caudal fin. Although determination of these features (e.g., morphology of the second neural spine) remain highly subjective and the posterior extension of the lateral line may be present in other percomorphs (e.g., Sciaenidae; Mooi and Gill, 1995; Otero, 2004), extant and extinct flatfishes share a posterior extensive lateral line with centropomids (Fukuda et al., 2010; Yamanaka et al., 2010).
Psettodes is placed within the Carangimorpha, however there is no consistent support for any particular sister lineage for this genus. It is already recognized that Psettodes is a divergent flatfish lineage that has been interpreted as basally divergent among the flatfishes. It is recognized as a separate suborder in morphological studies (Chapleau, 1993; Friedman, 2012). Regardless its morphological distinctiveness, Psettodes is thought to possess the synapomorphies proposed for the Pleuronectiformes by Chapleau (1993). However, the presence of the recessus orbitalis has not been systematically evaluated among flatfish groups (Hensley, 1997), and may not be present in Psettodes. Chabanaud (1937) while noting that Psettodes cannot protrude its eyes and lacks skin folds around the eyes which would suggest it can, did not examine Psettodes for the presence of the recessus orbitalis. Determining the condition of this character in Psettodes will help establish the extent to which the morphological and molecular lines of evidence conflict. The traits that Psettodes shares with percoids (Amaoka, 1969; Hubbs, 1945; Norman, 1934; Regan, 1910, 1929) do not provide synapomorphies to identify a potential sister group for Psettodes. In light of the molecular evidence, does a review of the existing literature reveal potentially informative traits linking Psettodes to non-flatfish groups? Work predating the development of cladistics placed Psettodes among serranids (Norman, 1934). A cladistic analysis identified four synapomorphies supporting serranid monophyly (Johnson, 1983). Psettodes shares two of these four characters with serranids (no third preural cartilage and no procurrent spur). Both traits are reductive and may represent independent losses (Chapleau, 1993; Johnson, 1983). Finally, aspects of the head musculature of Psettodes have been used to suggest affinity to carangids (Kyle, 1921), and we find Psettodes to be a close relative of carangids with this molecular dataset. A broad analysis of morphological variation among pleuronectiforms and acanthomorphs may add clarity to nature of the apparent conflict between morphology and molecular-based hypotheses of pleuronectiform relationships.
4.2 How does accepting a polyphyletic pleuronectiforms affect the interpretation of extinct intermediate flatfish lineages?
†Amphistium is found in deposits from the Ypresian and Lutetian (40.4–55.8 Ma) while †Heteronectes is documented from the Ypresian (48.6–55.8 Ma; Walker and Geismann, 2009; Friedman, 2012). Both are much younger than the estimated ages of the origin of Pleuronectoidei and Carangimorpha (Fig. 2). We estimate a mean age of 73.4 Ma with a 95% HPD range of 65.1–82.1 Ma for the time to MRCA of extant Pleuronectoidei. The age of crown pleuronectiforms predating known flatfish intermediates is consistent with the fact that †Amphistium and †Heteronectes occurred in strata that also contain fossils showing complete cranial asymmetry (Chanet, 1997, 1999; Friedman, 2008). Although preservation of these fossils makes it difficult to fully evaluate all relevant characters, †Amphistium and †Heteronectes are characterized by cranial asymmetry. Cranial asymmetry in †Amphistium and †Heteronectes is not a complete compared to extant flatfishes (Friedman, 2008). With regards to pleuronectiform synapomorphies, †Amphistium has derived pleuronectiform features unrelated to asymmetry that cannot be evaluated in †Heteronectes: 1) a dorsal fin that is anteriorly extensive, 2) anteriorly curved neural spines of the abdominal region, and 3) a procumbent first pterygiophore of the dorsal fin (Friedman, 2008).
†Amphistium and †Heteronectes both have traits in common with Psettodes that are considered primitive for pleuronectiforms (Friedman, 2012). †Amphistium and Psettodes differ with regards to a ventrally directed sciatic process (character 8 of Chapleau (1993); Friedman, 2008, 2012); however share character states considered primitive for flatfish otherwise. †Heteronectes can be evaluated for five of seven osteological characters that are considered informative for flatfish relationships, four of which are shared with Psettodes in a primitive state (Friedman, 2012). The fifth character, cranial asymmetry, is incomplete and considered by Friedman (2012) to be sufficient to place †Heteronectes as a flatfish. Otherwise †Amphistium and †Heteronectes show general percomorph character states including presence of dorsal and anal fine spines, and in the case of †Heteronectes a procurrent spur, found only in Psettodes amongst extant flatfishes. These characteristics have been offered as evidence to place the two fossil taxa as stem lineages of a monophyletic Pleuronectiformes with †Amphistium higher up along the stem (Friedman, 2008, 2012). If the pleuronectiforms as currently defined include representatives of two divergent lineages, then it will be important to re-evaluate the affinities of †Amphistium or †Heteronectes to adequately characterize the evolution of bilateral asymmetry in fishes. In light of the phylogenetic hypothesis supported by molecular evidence in this study, it will be especially valuable to review morphological variation in Psettodes and the two fossil genera to test the stem placement of the fossil taxa. It is possible that either †Amphistium or †Heteronectes are not stem members of a monophyletic Pleuronectiformes [sensu Chapleau (1993)] or the Pleuronectoidei, and might be a stem lineage of Psettodoidei or related to other lineages of percomorphs (Friedman, 2012).
Our results support parallel evolution of the flatfish body form with pronounced cranial asymmetry in two fish lineages with extant representatives. A growing number of molecular-based phylogenetic studies offer evidence rejecting monophyly of Pleuronectiformes as the result of alternative placements for the genus Psettodes (e.g., Near et al., 2012). The evidence is found in different taxonomic and gene fragment samples. The potential biases in base composition across taxa may mislead our conclusion about monophyly or non-monophyly of the Pleuronectiformes, and possibly affect our inference of intra-pleuronectiform phylogeny when fewer gene markers and/or inappropriate phylogenetic reconstruction methods used. For instance, a monophyletic Cynoglossidae (GC biased) was only found with 1N2N3N coding by p4 in our study. However given consistent results across a broad range of treatments of the sequence data, we find it unlikely that our non-monophyletic Pleuronectiformes is the product of artifacts of phylogenetic reconstruction.
The results of our study support at least two independent origins of a flatfish body form with pronounced cranial asymmetry. If further phylogenetic analyses corroborate this finding, the evolution of cranial asymmetry should prove a rich research topic for understanding parallel evolution of complex traits. If parallel evolution of body asymmetry is confirmed by further research, it would suggest that major morphological adaptations can take place in the context of relatively modest degrees of divergence at the coding sequence level and point to important roles for regulatory changes in the evolution of complex morphological adaptations.
Supplementary Material
Taxa included in this study, the corresponding accession to the tissue (if any), and corresponding GenBank accession.
Primers used in this study, gene targeted, and source of the primers.
Taxa that failed at least one chi-square test of base composition heterogeneity at one gene.
Prior characteristics of calibration points used in divergence time estimation.
Highlights.
This study adds to the growing case for rejecting the monophyly the flatfish group (fish order Pleuronectiformes).
Accounting for artifacts in phylogenetic inference and violations of substitution models rejects pleuronectiform monophyly.
We find the Pleuronectodei to be monophyletic.
The sister group of Pleuronectodei is Centropomidae sensu Greenwood (1976).
Acknowledgments
Sebastien Lavoué provided assistance with the Bayesian divergence time analysis. We want to thank Jhen-Nien Chen, Pei-Chun Lo, and Hsin Lee for their efforts in sequencing. Bruno Chanet and Jordan S. Metzgar provided helpful comments on earlier versions of the manuscript. Our gratitude goes to Bruno Chanet, Sébastien Lavoué, Samuel Iglésia, Guy Duhamel, Richard L. Mayden, Kwang-Tsao Shao, Mao-Ying Lee, Labbish L. Chao, University of Kansas Natural History Museum and the Scripps Institution of Oceanography Marine Vertebrates Collection for sharing tissue samples (via H.-J. Walker). Computational support was provided by UA Life Science Informatics, Grant Number P20RR016466 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). This work was supported by a joint award from the Taiwanese National Science Council/U.S. National Science Foundation (OISE 1210243 to M.A.C.), the Taiwanese National Science Council (NSC 99-2611-M-002-001-MY2 and NSC 101-2611-M-002 -016 -MY3 to W.J.C.), and the U.S. National Science Foundation (DEB 0963767 to J.A.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Matthew A. Campbell, Email: macampbell2@alaska.edu.
Wei-Jen Chen, Email: wjchen.actinops@gmail.com.
J. Andrés López, Email: andresl.fish@gmail.com.
References
- Aberer AJ, Krompass D, Stamatakis A. Pruning rogue taxa improves phylogenetic accuracy: An efficient algorithm and webservice. Syst Biol. 2013;62:162–166. doi: 10.1093/sysbio/sys078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akashi H, Kliman R, Eyre-Walker A. Mutation pressure, natural selection, and the evolution of base composition in Drosophila. Genetica. 1998;102–103:49–60. [PubMed] [Google Scholar]
- Alff-Steinberger C. The genetic code and error transmission. P Natl Acad Sci USA. 1969;64:584–591. doi: 10.1073/pnas.64.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaoka K. Studies on the sinistral flounder found in the waters around Japan. Taxonomy, anatomy, and phylogeny. J Shimonoseki Univ Fish. 1969;18:65–340. [Google Scholar]
- Azevedo MFC, Oliveira C, Pardo BG, Martínez P, Foresti F. Phylogenetic analysis of the order Pleuronectiformes (Teleostei) based on sequences of 12S and 16S mitochondrial genes. Genet Mol Biol. 2008;31:284–292. [Google Scholar]
- Benton MJ, Donoghue PCJ, Asher RJ. Calibrating and constraining molecular clocks. In: Hedges SB, Kumar S, editors. The Timetree of Life. Oxford University Press; Oxford: 2009. pp. 35–86. [Google Scholar]
- Berendzen PB, Dimmick WW. Phylogenetic relationships of Pleuronectiformes based on molecular evidence. Copeia. 2002;2002:642–652. [Google Scholar]
- Bergsten J. A review of long-branch attraction. Cladistics. 2005;21:163–193. doi: 10.1111/j.1096-0031.2005.00059.x. [DOI] [PubMed] [Google Scholar]
- Betancur RR, Broughton RE, Wiley EO, Carpenter K, López JA, Li C, Holcroft NI, Arcila D, Sanciangco M, Cureton JC, II, Zhang F, Buser T, Campbell MA, Ballesteros JA, Roa-Varon A, Willis S, Borden WC, Rowley T, Reneau PC, Hough DJ, Lu G, Grande T, Arratia G, Ortí G. PLoS Currents. 1. 2013. The Tree of Life and a new classification of bony fishes. 2013 Apr 18 [last modified: 2013 Apr 23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster B. Eye migration and cranial development during flatfish metamorphosis: a reappraisal (Teleostei: Pleuronectiformes) J Fish Biol. 1987;31:805–833. [Google Scholar]
- Bruno WJ, Halpern AL. Topological bias and inconsistency of maximum likelihood using wrong models. Mol Biol Evol. 1999;16:564–566. doi: 10.1093/oxfordjournals.molbev.a026137. [DOI] [PubMed] [Google Scholar]
- Buckley TR, Cunningham CW. The effects of nucleotide substitution model assumptions on estimates of nonparametric bootstrap support. Mol Biol Evol. 2002;19:394–405. doi: 10.1093/oxfordjournals.molbev.a004094. [DOI] [PubMed] [Google Scholar]
- Cao Y, Adachi J, Janke A, Pääbo S, Hasegawa M. Phylogenetic relationships among eutherian orders estimated from inferred sequences of mitochondrial proteins: Instability of a tree based on a single gene. J Mol Evol. 1994;39:519–527. doi: 10.1007/BF00173421. [DOI] [PubMed] [Google Scholar]
- Chabanaud P. Les téléostéens dyssymétriques du Mokkatam Inférieur de Tourah. Mém Inst Egypte. 1937;31:1–122. [Google Scholar]
- Chabanaud P. Le problème de la phylogénèse des Heterosomata. Bull Inst Oceanogr. 1949;950:1–24. [Google Scholar]
- Chanet B. A cladistic reappraisal of the fossil flatfishes record consequences on the phylogeny of the Pleuronectiformes (Osteichthyes: Teleostei) Ann Sci Nat Zooll. 1997;18:105–117. [Google Scholar]
- Chanet B. Supposed and true flatfishes [Teleostei: Pleuronectiformes] from the Eocene of Monte Bolca, Italy. Stud Ric Giacim Terz Bolca. 1999;8:220–243. [Google Scholar]
- Chapleau F. Pleuronectiform relationships: A cladistic reassessment. B Mar Sci. 1993;52:516–540. [Google Scholar]
- Chen W-J. PhD thesis. University of Paris VI; 2001. La répétitivité des clades omme critère de la fiabilité: application à la phylogénie des Acanthomorpha (Teleostei) et des Notothenioidei (acanthomorphes antarctiques) [Google Scholar]
- Chen WJ, Bonillo C, Lecointre G. Repeatability of clades as a criterion of reliability: a case study for molecular phylogeny of Acanthomorpha (Teleostei) with larger number of taxa. Mol Phylogenet Evol. 2003;26:262–288. doi: 10.1016/s1055-7903(02)00371-8. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Mayden RL. A phylogenomic perspective on the new era of Ichthyology. BioScience. 2010;60:421–432. [Google Scholar]
- Chen WJ, Miya M, Saitoh K, Mayden RL. Phylogenetic utility of two existing and four novel nuclear gene loci in reconstructing Tree of Life of ray-finned fishes: The order Cypriniformes (Ostariophysi) as a case study. Gene. 2008;423:125–134. doi: 10.1016/j.gene.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Ruiz-Carus R, Ortí G. Relationships among four genera of mojarras (Teleostei: Perciformes: Gerreidae) from the western Atlantic and their tentative placement among percomorph fishes. J Fish Biol. 2007;70:202–218. [Google Scholar]
- Cole FJ, Johnstone J. Pleuronectes (The plaice.) Mem Liverpool Mar Biol Comm. 1902;8:1–252. [Google Scholar]
- Darwin C. The Origin of Species by Means of Natural Selection. John Murray; London: 1872. [Google Scholar]
- Delsuc F, Brinkmann H, Philippe H. Phylogenomics and the reconstruction of the tree of life. Nat Rev Genet. 2005;6:361–375. doi: 10.1038/nrg1603. [DOI] [PubMed] [Google Scholar]
- Delsuc F, Phillips MJ, Penny D. Comment on “Hexapod origins: Monophyletic or paraphyletic? Science. 2003;301:1482. doi: 10.1126/science.1086558. [DOI] [PubMed] [Google Scholar]
- Dettai A, Lecointre G. Further support for the clades obtained by multiple molecular phylogenies in the acanthomorph bush. CR Biol. 2005;328:674–689. doi: 10.1016/j.crvi.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Drummond AJ, Ho SYW, Phillips MJ, Rambaut A. Relaxed phylogenetics and dating with confidence. PLoS Bio. 2006;4:e88. doi: 10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012;29:1969–73. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004a;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004b;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre-Walker A. Evidence of selection on silent site base composition in mammals: Potential implications for the evolution of isochores and junk DNA. Genetics. 1999;152:675–683. doi: 10.1093/genetics/152.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Cases in which parsimony or compatibility methods will be positively misleading. Syst Zool. 1978;27:401–410. [Google Scholar]
- Foster PG. Modeling compositional heterogeneity. Syst Biol. 2004;53:485–495. doi: 10.1080/10635150490445779. [DOI] [PubMed] [Google Scholar]
- Foster PG, Hickey DA. Compositional bias may affect both DNA-based and protein-based phylogenetic reconstructions. J Mol Evol. 1999;48:284–290. doi: 10.1007/pl00006471. [DOI] [PubMed] [Google Scholar]
- Frazzetta T. Flatfishes, turtles, and bolyerine snakes: Evolution by small steps or large, or both? Evol Biol. 2012;39:30–60. [Google Scholar]
- Friedman M. The evolutionary origin of flatfish asymmetry. Nature. 2008;454:209–212. doi: 10.1038/nature07108. [DOI] [PubMed] [Google Scholar]
- Friedman M. Osteology of †Heteronectes chaneti (Acanthomorpha, Pleuronectiformes), an Eocene stem flatfish, with a discussion of flatfish sister-group relationships. J Vertebr Paleontol. 2012;32:735–756. [Google Scholar]
- Fukuda E, Nakae M, Asaoka R, Sasaki K. Branching patterns of trunk lateral line nerves in Pleuronectiformes: uniformity and diversity. Ichthyol Res. 2010;57:148–160. [Google Scholar]
- Galtier N, Gouy M. Inferring phylogenies from DNA sequences of unequal base compositions. P Natl Acad Sci USA. 1995;92:11317–11321. doi: 10.1073/pnas.92.24.11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosline WA. Functional Morphology and Classification of Teleostean Fishes. University of Hawaii Press; Honolulu: 1971. [Google Scholar]
- Greenwood PH. A review of the family Centropomidae (Pisces, Perciformes) Bull Br Mus Nat Hist (Zool) 1976;29:1–81. [Google Scholar]
- Haig D, Hurst L. A quantitative measure of error minimization in the genetic code. J Mol Evol. 1991;33:412–417. doi: 10.1007/BF02103132. [DOI] [PubMed] [Google Scholar]
- Hensley DA. An overview of the systematics and biogeography of the flatfishes. J Sea Res. 1997;37:187–194. [Google Scholar]
- Hensley DA. Psettoididae. Spiny turbots. In: Carpenter KE, Niem V, editors. FAO Identification guide for fishery purposes. The Wesern Central Pacific. Food and Agriculture Organization of the United Nations; Rome: 1999. pp. 3792–3793. [Google Scholar]
- Hewer HR. Studies in colour-changes in fish —Part V The colour-patterns in certain flat-fish and their relation to the environment. Zool J Linn Soc-Lond. 1931;37:493–513. [Google Scholar]
- Hillis DM. Taxonomic sampling, phylogenetic accuracy, and investigator bias. Syst Biol. 1998;47:3–8. doi: 10.1080/106351598260987. [DOI] [PubMed] [Google Scholar]
- Hillis DM, Pollock DD, McGuire JA, Zwickl DJ. Is sparse taxon sampling a problem for phylogenetic inference? Syst Biol. 2003;52:124–126. doi: 10.1080/10635150390132911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt EWL. Studies in teleostean morphology from the Marine Laboratory at Cleethorpes. Proc Zool Soc London. 1894;1894:413–446. [Google Scholar]
- Hubbs CL. Phylogenetic position of the Citharidae, a family of flatfishes. Misc Pub, Museum Zool Univ Mich. 1945;63:1–38. [Google Scholar]
- Janvier P. Palaeontology: Squint of the fossil flatfish. Nature. 2008;454:169–170. doi: 10.1038/454169a. [DOI] [PubMed] [Google Scholar]
- Jermiin LS, Ho SYW, Ababneh F, Robinson J, Larkum AWD. The biasing effect of compositional heterogeneity on phylogenetic estimates may be underestimated. Syst Biol. 2004;53:638–643. doi: 10.1080/10635150490468648. [DOI] [PubMed] [Google Scholar]
- Johnson GD. Niphon spinosus: A primitive epinepheline serranid, with comments on the monophyly and intrarelationships of the Serranidae. Copeia. 1983;1983:777–787. [Google Scholar]
- Johnson GD. Percoidei: development and relationships. In: Moser HG, Richards WJ, Cohen DM, Fahay MP, Kendall AW Jr, Richardson SL, editors. Ontogeny and Systematics of Fishes. American Society of Ichthyology and Herpetology; Lawrence, Kansas: 1984. pp. 464–478. [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kyle HM. The asymmetry, metamorphosis and origin of flat-fishes. Philos T Roy Soc B. 1921;211:75–129. [Google Scholar]
- Lamarck JB. Philosophie Zoologique. Chez Detu; Paris: 1809. [Google Scholar]
- Lartillot N, Lepage T, Blanquart S. PhyloBayes 3: a Bayesian software package for phylogenetic reconstruction and molecular dating. Bioinformatics. 2009;25:2286–2288. doi: 10.1093/bioinformatics/btp368. [DOI] [PubMed] [Google Scholar]
- Lartillot N, Philippe H. A Bayesian mixture model for across-site heterogeneities in the amino-acid replacement process. Mol Biol Evol. 2004;21:1095–1109. doi: 10.1093/molbev/msh112. [DOI] [PubMed] [Google Scholar]
- Li B, Dettaï A, Cruaud C, Couloux A, Desoutter-Meniger M, Lecointre G. RNF213, a new nuclear marker for acanthomorph phylogeny. Mol Phylogenet Evol. 2009;50:345–363. doi: 10.1016/j.ympev.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Li C, Ortí G. Molecular phylogeny of Clupeiformes (Actinopterygii) inferred from nuclear and mitochondrial DNA sequences. Mol Phylogenet Evol. 2007;44:386–398. doi: 10.1016/j.ympev.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Li C, Ricardo BR, Smith LW, Ortí G. Monophyly and interrelationships of Snook and Barramundi (Centropomidae sensu Greenwood) and five new markers for fish phylogenetics. Mol Phylogenet Evol. 2011;60:463–471. doi: 10.1016/j.ympev.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Little AG, Lougheed SC, Moyes CD. Evolutionary affinity of billfishes (Xiphiidae and Istiophoridae) and flatfishes (Plueronectiformes): Independent and trans-subordinal origins of endothermy in teleost fishes. Mol Phylogenet Evol. 2010;56:897–904. doi: 10.1016/j.ympev.2010.04.022. [DOI] [PubMed] [Google Scholar]
- López JA, Chen WJ, Ortí G. Esociform phylogeny. Copeia. 2004;2004:449–464. [Google Scholar]
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. Version 2.75. 2011 http://mesquiteproject.org.
- Mitchell A, Mitter C, Regier JC. More taxa or more characters revisited: combining data from nuclear protein-encoding genes for phylogenetic analyses of Noctuoidea (Insecta: Lepidoptera) Syst Biol. 2000;49:202–224. [PubMed] [Google Scholar]
- Mivart GJ. On the genesis of species. MacMillan; London: 1871. [Google Scholar]
- Miya M, Takeshima H, Endo H, Ishiguro NB, Inoue JG, Mukai T, Satoh TP, Yamaguchi M, Kawaguchi A, Mabuchi K, Shirai SM, Nishida M. Major patterns of higher teleostean phylogenies: a new perspective based on 100 complete mitochondrial DNA sequences. Mol Phylogenet Evol. 2003;26:121–138. doi: 10.1016/s1055-7903(02)00332-9. [DOI] [PubMed] [Google Scholar]
- Mooers AO, Holmes EC. The evolution of base composition and phylogenetic inference. Trends Ecol Evol. 2000;15:365–369. doi: 10.1016/s0169-5347(00)01934-0. [DOI] [PubMed] [Google Scholar]
- Mooi RD, Gill AC. Association of epaxial musculature with dorsal-fin pterygiophores in acanthomorph fishes, and its phylogenetic significance. Bull Br Mus Nat Hist (Zool) 1995;61:121–137. [Google Scholar]
- Munroe TA. Systematic diversity of the Pleuronectiformes. In: Gibson RN, editor. Flatfishes: biology and exploitation. Blackwell; Oxford: 2005. pp. 10–41. [Google Scholar]
- Near TJ, Eytan RI, Dornburg A, Kuhn KL, Moore JA, Davis MP, Wainwright PC, Friedman M, Smith WL. Resolution of ray-finned fish phylogeny and timing of diversification. P Natl Acad Sci USA. 2012;109:13698–13703. doi: 10.1073/pnas.1206625109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JS. Fishes of the World. John Wiley & Sons, Inc; Hoboken, New Jersey: 2006. [Google Scholar]
- Norman JR. Psettodidae, Bothidae, Pleuronectidae. Vol. 2. Printed by order of the Trustees of the British Museum; London: 1934. A systematic monograph of the flatfishes (Hetersomata) [Google Scholar]
- Otero O. Anatomy, systematics and phylogeny of both Recent and fossil latid fishes (Teleostei, Perciformes, Latidae) Zool J Linn Soc-Lond. 2004;141:81–133. [Google Scholar]
- Palmer AR. From symmetry to asymmetry: Phylogenetic patterns of asymmetry variation in animals and their evolutionarysignificance. P Natl Acad Sci USA. 1996;93:14279–14286. doi: 10.1073/pnas.93.25.14279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson C. An overview of the early fossil record of acanthomorphs. Bull Mar Sci. 1993;52:29–59. [Google Scholar]
- Penny D, Steel MA, Lockhart PJ, Hendy MD. The role of models in reconstructing evolutionary trees. In: Scotland RW, Siebert DJ, Williams DM, editors. Models in Phylogeny Reconstruction. Oxford University Press; Oxford: 1994. pp. 211–230. [Google Scholar]
- Phillips MJ, Delsuc F, Penny D. Genome-scale phylogeny and the detection of systematic biases. Mol Biol Evol. 2004;21:1455–1458. doi: 10.1093/molbev/msh137. [DOI] [PubMed] [Google Scholar]
- Phillips MJ, Penny D. The root of the mammalian tree inferred from whole mitochondrial genomes. Mol Phylogenet Evol. 2003;28:171–185. doi: 10.1016/s1055-7903(03)00057-5. [DOI] [PubMed] [Google Scholar]
- Platt C. Retention of generalized hair cell patterns in the inner ear of the primitive flatfish Psettodes. Anat Rec. 1983;207:503–508. doi: 10.1002/ar.1092070311. [DOI] [PubMed] [Google Scholar]
- Posada D. The effect of branch length variation on the selection of models in molecular evoltuion. J Mol Evol. 2001;52:434–444. doi: 10.1007/s002390010173. [DOI] [PubMed] [Google Scholar]
- Regan CT. The origin and evolution of the teleostean fishes of the order Heterosomata. Ann Mag Nat Hist. 1910;8:484–496. [Google Scholar]
- Regan CT. Fishes. Heterosomata. Encyclopedia Britannica; London: 1929. pp. 324–325. [Google Scholar]
- Roje DM. Incorporating molecular phylogenetics with larval morphology while mitigating the effects of substitution saturation on phylogeny estimation: A new hypothesis of relationships for the flatfish family Pleuronectidae (Percomorpha: Pleuronectiformes) Mol Phylogenet Evol. 2010;56:586–600. doi: 10.1016/j.ympev.2010.04.036. [DOI] [PubMed] [Google Scholar]
- Russo CA, Takezaki N, Nei M. Efficiencies of different genes and different tree-building methods in recovering a known vertebrate phylogeny. Mol Biol Evol. 1996;13:525–536. doi: 10.1093/oxfordjournals.molbev.a025613. [DOI] [PubMed] [Google Scholar]
- Schmidt HA, Strimmer K, Vingron M, von Haeseler A. TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics. 2002;18:502–504. doi: 10.1093/bioinformatics/18.3.502. [DOI] [PubMed] [Google Scholar]
- Schwarzhans W. Piscium Catalogus: Otolithi Piscium. Munchen: Verlag Dr. Friedrich Pfeil; 1999. [Google Scholar]
- Sheffield NC, Song H, Cameron SL, Whiting MF. Nonstationary evolution and compositional heterogeneity in beetle mitochondrial phylogenomics. Syst Biol. 2009;58:381–394. doi: 10.1093/sysbio/syp037. [DOI] [PubMed] [Google Scholar]
- Shi W, Kong XY, Wang ZM, Jiang JX. Utility of tRNA genes from the complete mitochondrial genome of Psetta maxima for implying a possible sister-group relationship to the Pleuronectiformes. Zool Stud. 2011;50:665–681. [Google Scholar]
- Smith WL, Craig MT. Casting the Percomorph net widely; The importance of broad taxonomic sampling in the search for the placement of serranid and percid fishes. Copeia. 2007;2007:35–55. [Google Scholar]
- Smith WL, Wheeler WC. Venom evolution widespread in fishes: A phylogenetic road map for the bioprospecting of piscine venoms. J Hered. 2006;97:206–217. doi: 10.1093/jhered/esj034. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Steel MA, Lockhart PJ, Penny D. Confidence in evolutionary trees from biological sequence data. Nature. 1993;364:440–442. doi: 10.1038/364440a0. [DOI] [PubMed] [Google Scholar]
- Wainwright PC, Smith WL, Price SA, Tang KL, Sparks JS, Ferry LA, Kuhn KL, Eytan RI, Near TJ. The Evolution of pharyngognathy: A phylogenetic and functional appraisal of the pharyngeal jaw key innovation in labroid fishes and beyond. Syst Biol. 2012;61:1001–1027. doi: 10.1093/sysbio/sys060. [DOI] [PubMed] [Google Scholar]
- Walker JD, Geismann JW. 2009 GSA Geologic Time Scale. GSA Today. 2009 Apr-May;:60–61. [Google Scholar]
- Woese CR. On the evolution of the genetic code. P Natl Acad Sci USA. 1965;54:1546–1552. doi: 10.1073/pnas.54.6.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf YI, Rogozin IB, Koonin EV. Coelomata and not Ecdysozoa: Evidence from genome-wide phylogenetic analysis. Genome Res. 2004;14:29–36. doi: 10.1101/gr.1347404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortley AH, Rudall PJ, Harris DJ, Scotland RW. How much data are needed to resolve a difficult phylogeny? Case study in Lamiales. Syst Biol. 2005;54:697–709. doi: 10.1080/10635150500221028. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Nakae M, Fukuda E, Sasaki K. Monophyletic origin of the dorsally arched lateral line in Teleostei: evidence from nerve innervation patterns. Ichthyol Res. 2010;57:49–61. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Taxa included in this study, the corresponding accession to the tissue (if any), and corresponding GenBank accession.
Primers used in this study, gene targeted, and source of the primers.
Taxa that failed at least one chi-square test of base composition heterogeneity at one gene.
Prior characteristics of calibration points used in divergence time estimation.