Abstract
Gastric acid is of paramount importance for digestion and protection from pathogens but, at the same time, is a threat to the integrity of the mucosa in the upper gastrointestinal tract and may give rise to pain if inflammation or ulceration ensues. Luminal acidity in the colon is determined by lactate production and microbial transformation of carbohydrates to short chain fatty acids as well as formation of ammonia. The pH in the oesophagus, stomach and intestine is surveyed by a network of acid sensors among which acid-sensing ion channels (ASICs) and acid-sensitive members of transient receptor potential ion channels take a special place. In the gut, ASICs (ASIC1, ASIC2, ASIC3) are primarily expressed by the peripheral axons of vagal and spinal afferent neurons and are responsible for distinct proton-gated currents in these neurons. ASICs survey moderate decreases in extracellular pH and through these properties contribute to a protective blood flow increase in the face of mucosal acid challenge. Importantly, experimental studies provide increasing evidence that ASICs contribute to gastric acid hypersensitivity and pain under conditions of gastritis and peptic ulceration but also participate in colonic hypersensitivity to mechanical stimuli (distension) under conditions of irritation that are not necessarily associated with overt inflammation. These functional implications and their upregulation by inflammatory and non-inflammatory pathologies make ASICs potential targets to manage visceral hypersensitivity and pain associated with functional gastrointestinal disorders.
Keywords: Acid-induced pain, acid-related gastrointestinal diseases, acid-sensing ion channels, acid surveillance, gastrointestinal tract, inflammation, primary afferent neurons, proton-gated currents, visceral hypersensitivity
1. Luminal acidity and tissue acidosis in the gastrointestinal tract
Acid is of paramount relevance to digestion. Being one of the prime secretory products of the stomach, hydrochloric acid (HCl) not only promotes digestion (Holzer, 2011a) but also protects the gastrointestinal tract from potentially infectious microorganisms that may be ingested with food (Canani and Terrin, 2010). The parietal cells in the gastric mucosal glands are the most productive source of acid in the body. Equipped with the so-called proton pump, these cells can secrete HCl to build up a luminal H+ concentration that – with an average diurnal pH of 1.5 – is 6 orders of magnitude higher than in the gastric lamina propria (Holzer, 2007a, 2011). The physiological functions of gastric acid comprise the conversion of pepsinogen to pepsin, solubilisation of food components, digestion and absorption of several nutrients, and elimination of ingested pathogens (Pohl et al., 2008), At the same time, the high luminal concentration of HCl endangers the integrity of the mucosa in the stomach and adjacent regions of the gastrointestinal tract. The injurious threat that HCl exerts on the mucosa is kept in check by a network of mucosal defence mechanisms and by the lower oesophageal and pyloric sphincters which control the backflux and propulsion of the acidified gastric juice (Holzer, 2007b). In order to regulate these defence systems according to need, cells that are able to sense acid are required, among which epithelial cells and acid-sensitive neurons play a particular role. Following activation by a drop of extracellular pH, these cells evoke both local and remote homeostatic reactions (Figure 1). When the surveillance and/or defence systems are defective, acid-related diseases including gastro-oesophageal reflux disease, dyspepsia, gastritis and gastroduodenal ulcer disease may ensue.
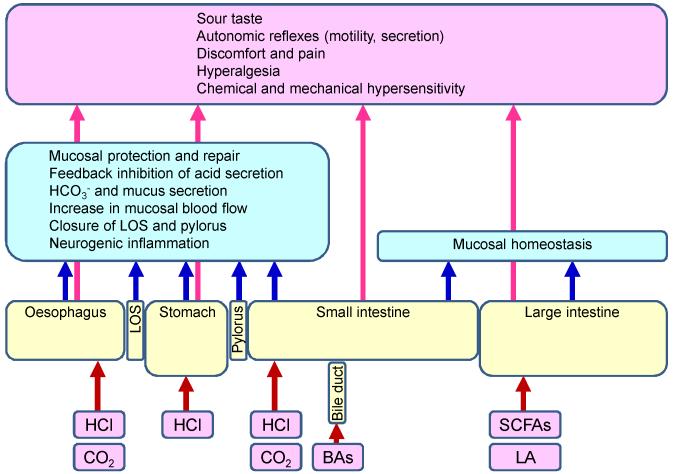
Figure 1.
Acid surveillance in various regions of the gastrointestinal tract. The graph shows various intrinsic sources of acid along the gastrointestinal tract. Acidity is monitored by epithelial cells and other cells of the gastrointestinal tract, notably intrinsic and extrinsic sensory neurons. When activated by acidification, the acid-sensing cells initiate local homeostatic reactions within the gastrointestinal tract (blue boxes) as well as remote warning reactions (pink box) in which primary afferent, cerebral and autonomic neurons are involved. BAs, bile acids; LA, lactic acid; LOS, lower oesophageal sphincter; SCFAs (short chain fatty acids: acetic, butyric and propionic acid).
Deviations from the physiological value of extracellular pH (7.4) occur not only in the lumen of the upper gastrointestinal tract but can be observed throughout the gut including the small and large intestine. The luminal pH profile along the digestive system of healthy subjects exhibits a distinct shape (Fallingborg, 1999; Nugent et al., 2001), with peaks of acidity occurring in the stomach and in the proximal large bowel. In the foregut it is primarily HCl and bicarbonate (HCO3−) secretion that determines the luminal pH, whereas in the colon it is mainly mucosal HCO3− and lactate production as well as microbial transformation of carbohydrates to short chain fatty acids (acetic, butyric and propionic acid) and formation of ammonia that are responsible for luminal acidity. The role which the microbiota plays in the physiological and pathophysiological regulation of acidity along the gut has not yet been fully disclosed, although it is evident that many species of the microbiota are able to generate metabolites that have a bearing on luminal acidity. Conversely, variations in luminal pH can have an impact on microbiota diversity and activity (Tana et al. 2010). Thus, acid-related diseases as well as their treatment with proton pump inhibitors have a significant effect on microbiota diversity in the oesophagus and stomach (Amir et al., 2014). The pH profile along the alimentary canal can be changed by surgical interventions, by inflammatory bowel disease (Nugent et al., 2001; Holzer, 2009) and, very likely, by alterations in the composition of the luminal microbiota.
As in other tissues, acidosis in the gastrointestinal wall can be a pathological condition that may result from excess intake of acid, excess gastric acid secretion, defective acid containment, metabolic acidosis and acidosis due to microbial activity, inflammation, ischaemia (hypoxia), malignant tumour growth and gastrointestinal motor stasis (Holzer, 2011a). Luminal acid threats as well as harmful tissue acidosis are monitored by multiple acid-sensing epithelial cells and neurons and counteracted by cellular mechanisms of acid-base regulation and systemic responses designed to maintain homeostasis. Through these measures, structural injury and functional impairment of the tissue are prevented (Holzer, 2011a).
2. Acid-evoked neurogenic inflammation and pain in the gastrointestinal tract
Krishtal and Pidoplichko (1981) were the first to observe that sensory neurons are able to react to protons in their vicinity. Since then, a plethora of studies has confirmed this discovery and shown that acid is a potent stimulator of primary afferent neurons. As reviewed by Kress and Waldmann (2006), two principal types of proton-gated inward currents in dorsal root ganglion (DRG) neurons can be distinguished. The first type is a fast and rapidly inactivating inward current carried by Na+, which is highly sensitivity to H+ as the threshold activation occurs at a pH of 7. While this type of proton-gated current is seen in most DRG neurons, the second type is observed only in DRG neurons that are excited by capsaicin (Bevan and Geppetti, 1994). This second type of slow and non-desensitizing current is less sensitive to protons, activated only at pH levels below 6.2 (Petersen and LaMotte, 1993) and carried by Na+, K+ and Ca2+ (Zeilhofer et al., 1997). Although several acid-gated ion channels are likely to contribute, the second type of proton-activated conductance in DRG neurons shares many similarities with the acid-evoked current through the transient receptor potential channel of vanilloid type 1 (TRPV1), while the fast and rapidly inactivating proton-gated current resembles currents carried by acid-sensing ion channels (ASICs) (Waldmann et al., 1997; Kress and Waldmann, 2006).
Acid-evoked stimulation of sensory neurons in the gastrointestinal tract and other organs elicits two distinct systemic responses: local release of neuropeptides from the peripheral axons in the tissue as well as autonomic reflexes, sensation and pain (Holzer, 1988; Holzer and Maggi, 1998). By releasing peptide transmitters in the periphery, sensory nerve fibres have the capacity to regulate vascular diameter and permeability and other tissue processes, these effects being embodied in the term neurogenic inflammation. This efferent-like mode of operation of sensory neurons may take place independently of nociception, and it has been hypothesized that some DRG neurons are specialized in controlling peripheral effector mechanisms only, while other DRG neurons may be specialized in the afferent signalling of sensation and pain (Holzer and Maggi, 1998). The neuropeptides mediating the efferent-like mode of operation comprise, among others, calcitonin gene-related peptide (CGRP) and the tachykinins substance P and neurokinin A. Acid-evoked release of CGRP, one of the most potent vasodilator peptides, has been demonstrated in a variety of tissues including the gastric mucosa (Geppetti et al., 1991; Manela et al., 1995; Auer et al., 2010).
There is also ample evidence that acid is a potent stimulus to elicit pain in the upper gastrointestinal tract and other visceral organs and that acidosis contributes to the pain associated with ischaemia and inflammation (Steen et al., 1992; Kress and Waldmann, 2006; Wemmie et al., 2006; Holzer, 2009). Gastric acid-related diseases manifest themselves in mucosal injury and ulceration and are typically diagnosed when patients complain about pain in the upper abdomen and/or thorax. Although gastric ulcer pain may not entirely be explained by the effect of acid (Huang and Hunt, 1996), reflux of acid into the oesophagus or exposure of the duodenum to excess acid is known to make a major contribution to the pain associated with gastro-oesophageal reflux and duodenal ulcer disease (Texter, 1987; Kang and Yap, 1991; Huang and Hunt, 1996; Holzer, 2011a). Comparatively little is still known as to whether acid and acid-related hyperalgesia participate in functional abdominal pain and pain associated with functional gastrointestinal disorders such as heartburn in non-erosive gastro-oesophageal reflux disease (Bulsiewicz et al., 2013), non-cardiac chest pain, functional dyspepsia and irritable bowel syndrome (IBS) (Tana et al., 2010).
3. Acid sensors in the gastrointestinal tract
Acid-sensing cells are present throughout the alimentary canal, which attests to the important physiological role of acid surveillance in the digestive system (Figure 1). Although this review focuses on ASICs, the role of these proton-gated channels in gastrointestinal acid homeostasis can only be appreciated if seen in perspective with the function of the other proton-responsive sensors involved.
As alluded to above, ASICs and TRPV1 play a prominent role in the two major proton-gated currents recorded from mammalian DRG neurons. TRPV1 is a polymodal nocisensor that is receptive not only to acid but also to noxious heat, capsaicin and endovanilloids (Holzer 2008). Being a non-selective cation channel with high permeability for Ca2+, it allows Ca2+ to enter the cell in an acidic environment (Hellwig et al., 2004; Vulcu et al., 2004). TRPV1 opens only if the extracellular pH is lowered towards 6, although mild acidosis in the range of pH 7-6 can sensitize TRPV1 to other stimuli such as capsaicin and heat (Tominaga et al., 1998; Holzer, 2008, 2011b). TRPV1 is not the only TRP channel that responds to acidification. Thus, TRPV4 which is also expressed by DRG neurons is gated by a drop of pH below 6 (Suzuki et al. 2003), and TRPC4 and TRPC5 are able to respond to small decreases of pH from 7.4 to 7.0 (Semtner et al., 2007). Furthermore, a member of the TRPP subfamily, PKD2L1, plays a major role in the sour taste (Huang et al., 2006; Ishimaru et al., 2006).
Two-pore (or tandem-pore) domain K+ (K2P) channels represent one of the subfamilies of the large superfamily of K+ channels. Being primarily background channels, K2P channels subserve a key function in setting the resting membrane potential and thereby the excitability of neurons. Many of the K2P channels such as TASK-1, TASK-2, TASK-3, TRESK, TREK-1, TREK-2 and TRAAK are expressed by sensory neurons and react to modifications of intra- and/or extracellular pH (Goldstein et al., 2005; Duprat et al., 2007; Holzer, 2009). The activity of other K+ channels such as distinct members of the inward rectifier K+ channel (Kir) family (Kir1.1, Kir4.1, Kir5.1 and Kir6.1) and of the voltage-activated K+ channel (Kv) family (Kv1.3, Kv1.4 and Kv11.1) is also modified by extracellular acidification, as is the case with nifedipine-sensitive L-type Ca2+ channels and distinct voltage-gated Na+ channels (Holzer, 2009). Adenosine triphosphate (ATP)-gated P2X purinoceptor channels can likewise be considered as indirect acid sensors because many P2X subunits, such as P2X1, P2X2, P2X3, P2X4, P2X5 and P2X7, are modulated by alterations in extracellular pH (Holzer, 2003). Acidification can either reduce the potency of ATP to gate certain multimeric P2X or sensitize other multimeric P2X receptors to the excitatory effect of ATP (North, 2002; Burnstock, 2007).
Although most acid sensors are ionotropic receptors, G-protein-coupled receptors such as OGR1 have also turned out to respond to extracellular acidosis (Ludwig et al., 2003; Tomura et al., 2005). The sensitivity of OGR1 to extracellular pH changes is in fact extremely high, given that half-maximum activation occurs at pH 7.2-7.5 (Ludwig et al., 2003; Tomura et al., 2005).
4. Expression of acid-sensing ion channels in the gastrointestinal tract
ASICs are members of the voltage-insensitive, amiloride-sensitive epithelial Na+ channel/degenerin family of cation channels (Kellenberger and Schild, 2002; Kress and Waldmann, 2006; Lingueglia, 2007). In these aspects, ASICs are related to other acid-responsive Na+ channels including P2X purinoceptor channels (Kress and Waldmann 2006), the epithelial Na+ channel δ subunit (Waldmann et al., 1995; Yamamura et al., 2008) and BASIC, a bile acid-sensitive ion channel highly expressed in bile ducts (Sakai et al., 1999; Wiemuth et al., 2012). The proton-sensitive members of ASICs expressed in mammals are encoded by 3 different genes (ACCN1, ACCN2 and ACCN3) which are alternatively spliced to produce 5 subunits: ASIC1a, ASIC1b, ASIC2a, ASIC2b and ASIC3. The multimeric structure and molecular properties of ASICs have been described elsewhere (Kress and Waldmann, 2006; Deval et al., 2010; Wemmie et al., 2013) and are being discussed in other chapters of this special issue of Neuropharmacology. ASIC1a, ASIC1b, ASIC2a and ASIC3 are directly gated by protons, whereas ASIC2b responds to acidification only when expressed in heteromultimers with other ASIC subunits, particularly ASIC3. ASICs are sensors of extracellular pH, the threshold for activation of ASIC3 being around a pH of 7.2 (Kress and Waldmann, 2006; Wemmie et al., 2006). The properties of proton-gated currents in ASIC homo- and heteromultimers exhibit diverse dynamics ranging from fast, rapidly inactivating to prolonged and sustained responses (Kellenberger and Schild, 2002; Kress and Waldmann, 2006; Wemmie et al., 2006).
The ASIC subunits present in the gastrointestinal tract occur primarily on the peripheral fibres of extrinsic primary afferent neurons originating from the DRGs and nodose ganglia. There is only sparse information as to whether ASICs are also expressed by other cells such as oesophageal and gastric epithelial cells (Akiba et al., 2008; Yuan et al., 2012). Comparative functional and neurochemical studies indicate that ASIC-mediated currents in native sensory neurons are carried by ASIC1a and ASIC3 homomultimers (Escoubas et al., 2000; Sutherland et al., 2001), ASIC1b-containing channels (Diochot et al., 2012) as well as ASIC2a/ASIC3 and ASIC2b/ASIC3 heteromultimers (Waldmann et al., 1999; Xie et al., 2002; Diochot et al., 2004; Yagi et al., 2006). This diversity in function is paralleled by a regional diversity in the expression of ASIC1, ASIC2 and ASIC3 subunits by glossopharyngeal, vagal and spinal afferent neurons innervating the gut and other visceral organs (Waldmann et al., 1999; Alvarez de la Rosa et al., 2002; Benson et al., 2002; Schicho et al., 2004; Page et al., 2005; Fukuda et al., 2006; Kress and Waldmann, 2006; Wemmie et al., 2006; Holzer, 2007a; Hughes et al., 2007; Lingueglia, 2007). Unlike the TRPV1-mediated proton-gated currents which are largely confined to DRG neurons with unmyelinated fibres, ASIC-mediated currents can be recorded from DRG neurons of small, medium and large diameter with both unmyelinated and myelinated axons (Leffler et al., 2006).
ASIC1a is expressed by sensory neurons as well as neurons in the central nervous system (CNS), whereas ASIC1b is largely confined to primary afferent neurons (Chen et al., 1998; Alvarez de la Rosa et al., 2002). The protein levels of ASIC2a in sensory neurons are relatively low, while appreciable expression of ASIC2b can be found in both sensory and CNS neurons, and ASIC3 has been almost exclusively localized to primary afferent neurons (Lingueglia et al., 1997; Waldmann et al., 1999; Price et al., 2001; Alvarez de la Rosa et al., 2002; Chen et al., 2002; Xie et al., 2002). Retrograde tracing studies indicate that 75 % of the nodose ganglion neurons and 82 % of the DRG neurons projecting to the rat stomach express ASIC3-like immunoreactivity (Schicho et al., 2004). In mouse thoracolumbar DRGs, ASIC3 is expressed in 73 %, ASIC2 in 47 % and ASIC1 in 30 % of the somata projecting to the mouse colon (Hughes et al., 2007).
5. Physiological and pathophysiological implications of acid-sensing ion channels in the gastrointestinal tract
5.1. Sour taste
The sight, smell and taste of food are important stimulants to start the process of digestion. Taste reception takes place at the apical tip of taste receptor cells that form taste buds. After transduction, the taste receptor cells transmit the taste modalities (bitter, sweet, umami, salty, and sour) to afferent neurons. The sour taste modality serves to warn against ingestion of acidic (e.g., spoiled or unripe) food sources (Huang et al., 2006). Several receptor mechanisms have been proposed to mediate the sour taste (Chandrashekar et al., 2006; Holzer, 2009), including ASIC2a and ASIC2b which are expressed in taste buds (Ugawa et al., 2003; Shimada et al., 2006). Although ASIC2 knockout mice respond normally to sour taste stimuli (Richter et al., 2004), genetic analysis of two patients with sour ageusia (Huque et al., 2009) suggests that ASIC subunits (notably ASIC1b,2a,2b,3) are involved in human sour taste (Figure 2). Although species differences may exist (Huque et al., 2009), the TRP channel PKD2L1 (TRPP2) has been recognized to be another important sour taste receptor (Huang et al., 2006; Ishimaru et al., 2006).
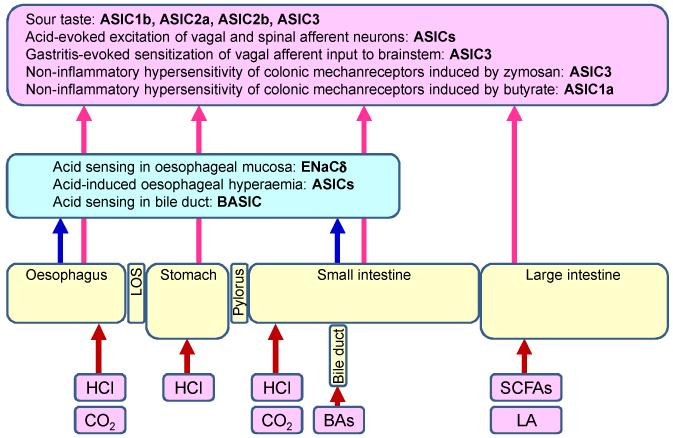
Figure 2.
Pathophysiological implications of ASICs in gastrointestinal acid surveillance, homeostasis, sensation and pain. The graph depicts homeostatic roles of ASICs within the gastrointestinal tract (blue box) and their roles in sensation and pain mediated by primary afferent neurons (pink box). BAs, bile acids; BASIC, bile acid-sensitive ion channel; ENaCδ, epithelial Na+ channel δ subunit, LA, lactic acid; LOS, lower oesophageal sphincter; SCFAs (short chain fatty acids: acetic, butyric and propionic acid).
5.2. Mucosal homeostasis of the upper gastrointestinal tract
The upper gastrointestinal tract (stomach, oesophagus and duodenum) is exposed to high concentrations of HCl secreted by the gastric parietal cells. In order to avoid tissue damage, the secretion of gastric acid must be tightly controlled according to need, and acid-sensitive protective mechanisms must be present in all regions of the gut that may potentially be exposed to excess luminal acid. As is shown below, the role of ASICs in mucosal homeostasis in the upper gut has not yet been fully elucidated and remains an important area of study.
Gastric acid secretion is subject to strong feedback inhibition, given that a decrease of luminal pH below 3 has a concentration-dependent inhibitory effect on HCl and gastrin secretion, and at pH 1 acid output comes to a complete halt (Shulkes et al., 2006). This feedback inhibition is primarily mediated by somatostatin which via paracrine and endocrine pathways inhibits parietal cell function both directly and indirectly through a reduction of gastrin secretion. The output of somatostatin from gastric D cells is stimulated by CGRP released from acid-sensitive primary afferent neurons (Manela et al., 1995; Holzer, 1998). However, the acid sensors of D cells and other endocrine cells in the gastrointestinal mucosa await to be identified. Excess acid also causes release of 5-hydroxytryptamine from enterochromaffin cells in the rat gastric mucosa (Wachter et al., 1998), but whether enterochromaffin cells themselves are able to monitor intraluminal pH is not known.
When the oesophageal, gastric or duodenal mucosa is exposed to excess acid, there is a rapid activation of protective mechanisms leading to a prompt increase in mucus gel thickness, HCO3− secretion and mucosal blood flow (Holzer, 1998; Montrose et al., 2006; Akiba and Kaunitz, 2009). These reactions are initiated in part by acid sensing mechanisms in epithelial cells and in part by acid-sensitive afferent neurons. In the oesophageal and duodenal mucosa, the transduction of the mucosal acid signal involves diffusion of CO2 into the epithelial cells, where it is sensed via carbonic anhydrases and ion transporters. Hydration of CO2 to H+ and HCO3− leads to intracellular acidification and exit of H+ via the basolateral Na+/H+ exchanger of type 1 (Montrose et al., 2006; Akiba and Kaunitz, 2009). As a result, interstitial pH is lowered, which stimulates acid-sensitive afferent nerve terminals to release CGRP (Akiba and Kaunitz, 2009). This vasodilator peptide, in turn, increases blood flow in part via a nitric oxide-dependent mechanism (Holzer, 1998). ASICs and TRPV1 are involved in the oesophageal hyperaemia evoked by luminal acid exposure (Figure 2), since the response is attenuated by the ASIC blocker amiloride and the TRPV1 blocker capsazepine (Akiba et al. 2008). The protective effects elicited by exposure to acid show distinct regional differences. While acid-evoked increases in blood flow are seen in the oesophageal, gastric and duodenal mucosa (Akiba and Kaunitz, 2009), the effect of acid to enhance HCO3− secretion is particularly prominent in the duodenum (Holm et al., 1998). As TRPV1 does not seem to be involved in the acid-evoked duodenal bicarbonate secretion (Kagawa et al., 2003; Aihara et al., 2005), the acid sensors responsible for this protective measure remain unidentified.
The mechanisms that keep the injurious potential of gastric acid in check involve not only adaptations of mucosal function and blood flow but also distinct motor effects. Thus, the lower oesophageal and pyloric sphincters control the reflux of H+ from the stomach into the oesophagus and its passage from the stomach to the duodenum (Holzer, 2007a, 2007b). In their activity, both sphincters are under the control of neural reflexes involving acid-sensitive neurons which adjust sphincter tone to the levels of acid present in the oesophagus, stomach and duodenum (Forster et al., 1990; Lu and Owyang, 1999; Holzer et al., 2003; Holzer, 2007a). The molecular acid sensors involved in the control of oesophago-gastro-duodenal motor activity have not yet been characterized (Holzer, 2009). Besides extrinsic sensory neurons, intrinsic primary afferent neurons of the enteric nervous system are most likely involved, given that they are able to respond to acid (Bertrand et al., 1997; Schicho et al., 2003).
5.3. Acid-induced pain in the oesophagus, stomach and duodenum
The pain associated with gastro-oesophageal reflux and peptic ulcer disease is to a considerable extent related to the presence of gastric acid (Texter, 1987; Kang and Yap, 1991; Huang and Hunt, 1996; Holzer, 2011a, 2011b). In keeping with this contention, drugs blocking gastric acid secretion not only promote healing of the mucosal lesions but also ameliorate the painful symptoms of the disease. Whole-cell voltage-clamp recordings from DRG and nodose ganglion neurons innervating the rat stomach show that the proton-gated currents can to a variable degree be attributed to the gating of ASICs (Figure 2) and TRPV1 (Sugiura et al., 2005). The pH sensitivity and kinetics of these currents are distinctly altered after experimental induction of gastric ulcers (Sugiura et al., 2005).
Challenge of the rat or mouse gastric lumen with supraphysiological HCl concentrations (> 0.15 M) elicits a visceromotor response indicative of pain (Lamb et al., 2003) and causes many neurons in the nucleus of the solitary tract in the brainstem to express c-Fos, a marker of neuronal excitation (Schuligoi et al., 1998; Danzer et al., 2004; Wultsch et al., 2008). The gastric HCl-evoked visceromotor reaction and medullary c-Fos response are absent after vagotomy, but not transection of the sympathetic nerve supply to the stomach (Schuligoi et al., 1998; Lamb et al., 2003). These observations imply that gastric acid-evoked nociception in rodents depends critically on the integrity of the vagal afferent innervation of the upper gastrointestinal tract. Apart from eliciting pain, acid causes sensitization of mechanosensitive afferent pathways from the oesophagus and stomach (Coffin et al., 2001; Medda et al., 2005). Experimentally induced gastritis and gastric ulceration augment the gastric acid-evoked visceromotor reaction and medullary c-Fos response (Lamb et al., 2003; Holzer et al., 2007; Wultsch et al., 2008).
The nociceptive responses of vagal afferent neurons to luminal acidification are likely to depend on multiple acid-sensing mechanisms (Schuligoi et al., 1998; Lamb et al., 2003), given that the acid-evoked excitation of gastric and oesophageal vagal afferent nerve fibres is attenuated after knockout of both ASIC3 (Figure 2) and TRPV1 (Bielefeldt and Davis, 2008). In addition, epithelial Na+ channels containing the δ subunit seem to function as acid sensors in the human oesophagus (Yamamura et al., 2008). When judged by the brainstem c-Fos response to excess gastric acid, it appears that afferent signalling from the normal stomach is preserved in ASIC3 knockout mice. However, the effect of gastritis to enhance the gastric acid-evoked expression of c-Fos in the brainstem is abolished after disruption of the ASIC3 gene (Wultsch et al., 2008). ASIC3 thus seems to play a major role in the inflammatory hyperresponsiveness to gastric acid (Figure 2) as it may occur in gastritis and peptic ulcer disease. Conversely, ASIC2 gene knockout does not alter inflammatory hyperresponsiveness but enhances the medullary c-Fos response to gastric acid challenge of the normal stomach (Wultsch et al., 2008). Although this finding suggests that ASIC2 may normally dampen acid-induced afferent input, it must not be forgotten that compensatory changes in germline knockout mice may obscure the functional implication of the disrupted gene.
5.4. Pain in the lower gastrointestinal tract
Little is known as to whether acid-induced pain can arise in the lower small intestine and colon and whether acid-evoked pain contributes in any way to functional abdominal pain and the discomfort associated with IBS. High levels of acetic and propionic acid produced by an altered microbiota composition have been suggested to contribute to the pain and negative emotions frequently seen in patients with IBS (Tana et al., 2010). In keeping with this contention, acid sensors such as ASICs and TRP channels are expressed by primary afferent neurons innervating the small and large intestine (Hughes et al., 2007; Sugiura et al., 2007; Matricon et al., 2013). In addition, there is increasing evidence that, besides TRP channels, ASIC channels play a role in various models of mechanical hypersensitivity to colorectal distension (Figure 2). This implication is related to the property of molecular acid sensors to be affected by multiple sensory modalities including mechanical stimuli, which also applies to ASICs (Price et al., 2001; Page et al., 2005).
Specifically, both ASIC3 and TRPV1 participate in the effect of zymosan to sensitize colonic mechanoreceptors of the mouse and to cause chronic behavioural hypersensitivity to colorectal distension in the absence of inflammation (Jones et al., 2007). Based on these observations, Jones et al. (2007) have proposed that ASIC3 and TRPV1 contribute to functional (non-inflammatory) visceral hypersensitivity (Figure 2) as is typically seen in IBS. This contention has been confirmed in a rat model of non-inflammatory hyperresponsiveness to colorectal distension, induced by intracolonic administration of butyrate (Matricon et al., 2011, 2013). ASICs play a major role in this model, given that intrathecal administration of the ASIC1a antagonist psalmotoxin-1 completely prevents the development of colonic hypersensitivity (Matricon et al., 2011). The hyperresponsiveness to colorectal distension is associated with an upregulation of ASIC1a in colonic DRG neurons and in the spinal cord (Matricon et al., 2011, 2013). Both the butyrate-induced upregulation of ASIC1a and the butyrate-induced colonic hypersensitivity are prevented by immunoneutralization of nerve growth factor (Matricon et al., 2013). It would therefore seem that this neurotrophic factor operates as an essential interface between the peripheral initiator signal and the spinal mechanism of ASIC-mediated hypersensitivity (Matricon et al., 2013). Collectively, these findings attest ASICs an important part in the type of hypersensitivity and hyperalgesia that many patients with IBS seem to suffer from (Figure 2).
5.5. Upregulation of acid sensors in gastrointestinal pathology
As reviewed above, there is ample evidence that ASICs, in particular ASIC1 and ASIC3, as well as other acid sensors such as TRPV channels, play a role in acid sensing within the gastrointestinal tract and contribute to chemical and mechanical hypersensitivity associated with both inflammatory and ulcerative as well as non-inflammatory conditions. This inference is consistent with the finding that the expression of ASIC3, but not ASIC1 and ASIC2, is enhanced in the colonic mucosa of patients with inflamed Crohn’s disease (Yiangou et al., 2001). Likewise, the immunocytochemical density of ASIC2 and ASIC3, but not ASIC1a, is increased in the gastric mucosa of patients with Henoch-Schönlein purpura, a vasculitis of immunological aetiology (Yuan et al., 2012). These clinical observations are matched by experimental findings that ASIC1a in rat DRG neurons and both ASIC1 and ASIC2 in the rat spinal cord are upregulated along with butyrate-induced hypersensitivity to colorectal distension (Matricon et al., 2011, 2013). The functional implications of ASICs in IBD and their potential role as a target for treatment await to be explored.
6. Conclusions
Acid sensors have proved to be highly relevant to the maintenance of pH homeostasis at the cellular and systemic level. Acid sensing is particularly important in the upper gastrointestinal tract which, with the gastric parietal cells, holds the richest source of acid in the body. Gastric acid secretion is tightly regulated by inhibitory feedback mechanisms, while acid-induced injury of the mucosa gives rise to a systemic warning signal by causing discomfort and pain. Both homeostatic mechanisms require a close surveillance of the acidic environment in the upper gut, which is achieved by multiple acid-sensing systems. The redundancy of molecular acid sensors signifies that quick detection of excess acid is physiologically so important that multiple mechanisms of acid sensing have evolved. Apart from TRP channels, ASICs have emerged as major acid sensors which play a particular role in the acid hypersensitivity under conditions of inflammation and ulceration in the upper gastrointestinal tract. Importantly, their implication in visceral hypersensitivity extends to the lower gut in which they expand their sensory repertoire and play a role in the hyperresponsiveness to mechanical stimuli (distension) under conditions that are not necessarily associated with overt inflammation.
These functional implications and their upregulation by inflammatory and non-inflammatory pathologies make ASICs potential targets to manage visceral hypersensitivity and pain associated with functional gastrointestinal disorders. Experimental blockade of particular ASICs supports such a therapeutic approach. It is intriguing that nature itself provides a lead to development, given that psalmotoxin-1, isolated from the venom of the South American tarantula Psalmopoeus cambridgei, is an ASIC1a blocker (Escoubas et al., 2000), APETx2, isolated from the venom of the sea anemone Anthopleura elegantissima, is an ASIC3 blocker (Diochot et al., 2004), and mambalgins, isolated from the venom of the black mamba, are multi-ASIC blockers (Diochot et al. 2012). However, the toxic nature of these ASIC blockers also issues a warning on the usefulness of selective ASIC blockers: any interference with molecular probes that are physiologically important poses a threat to homeostasis, unless selective inhibition of “excess” acid detectors can be achieved while their physiological function is preserved. The challenge, therefore, is to elucidate differences in the number, location and molecular properties of physiologically relevant and abnormally active ASICs and to pharmacologically differentiate between their physiological and pathological implications (Holzer, 2011a).
ACKNOWLEDGEMENTS
Work performed in the author’s laboratory was supported by the Austrian Science Fund (FWF grants P23017-B18 and P25912-B23).
Footnotes
CONFLICT OF INTEREST
There is no conflict of interest.
References
- Aihara E, Hayashi M, Sasaki Y, Kobata A, Takeuchi K. Mechanisms underlying capsaicin-stimulated secretion in the stomach: comparison with mucosal acidification. J. Pharmacol. Exp. Ther. 2005;315:423–432. doi: 10.1124/jpet.105.087619. [DOI] [PubMed] [Google Scholar]
- Akiba Y, Kaunitz JD. Luminal chemosensing and upper gastrointestinal mucosal defenses. Am. J. Clin. Nutr. 2009;90:826S–831S. doi: 10.3945/ajcn.2009.27462U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiba Y, Mizumori M, Kuo M, Ham M, Guth PH, Engel E, Kaunitz JD. CO2 chemosensing in rat oesophagus. Gut. 2008;57:1654–1664. doi: 10.1136/gut.2007.144378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez de la Rosa D, Zhang P, Shao D, White F, Canessa CM. Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system. Proc. Natl. Acad. Sci. U.S.A. 2002;99:2326–2331. doi: 10.1073/pnas.042688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir I, Konikoff FM, Oppenheim M, Gophna U, Half EE. Gastric microbiota is altered in oesophagitis and Barrett’s oesophagus and further modified by proton pump inhibitors. Environ. Microbiol. 2014 doi: 10.1111/1462-2920.12285. in press. [DOI] [PubMed] [Google Scholar]
- Auer J, Reeh PW, Fischer MJ. Acid-induced CGRP release from the stomach does not depend on TRPV1 or ASIC3. Neurogastroenterol. Motil. 2010;22:680–687. doi: 10.1111/j.1365-2982.2009.01459.x. [DOI] [PubMed] [Google Scholar]
- Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc. Natl. Acad. Sci. U.S.A. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand PP, Kunze WA, Bornstein JC, Furness JB, Smith ML. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am. J. Physiol. 1997;273:G422–G435. doi: 10.1152/ajpgi.1997.273.2.G422. [DOI] [PubMed] [Google Scholar]
- Bevan S, Geppetti P. Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends Neurosci. 1994;17:509–512. doi: 10.1016/0166-2236(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Davis BM. Differential effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation in mice. Am. J. Physiol. 2008;294:G130–G138. doi: 10.1152/ajpgi.00388.2007. [DOI] [PubMed] [Google Scholar]
- Bulsiewicz WJ, Shaheen NJ, Hansen MB, Pruitt A, Orlando RC. Effect of amiloride on experimental acid-induced heartburn in non-erosive reflux disease. Dig. Dis. Sci. 2013;58:1955–1959. doi: 10.1007/s10620-013-2586-0. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Canani RB, Terrin G. Gastric acidity inhibitors and the risk of intestinal infections. Curr. Opin. Gastroenterol. 2010;26:31–35. doi: 10.1097/MOG.0b013e328333d781. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- Chen CC, England S, Akopian AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proc. Natl. Acad. Sci. U.S.A. 1998;95:10240–10245. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ, Zimmer A. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8992–8997. doi: 10.1073/pnas.122245999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin B, Chollet R, Flourie B, Lemann M, Franchisseur C, Rambaud JC, Jian R. Intraluminal modulation of gastric sensitivity to distension: effects of hydrochloric acid and meal. Am. J. Physiol. 2001;280:G904–G909. doi: 10.1152/ajpgi.2001.280.5.G904. [DOI] [PubMed] [Google Scholar]
- Danzer M, Jocic M, Samberger C, Painsipp E, Bock E, Pabst MA, Crailsheim K, Schicho R, Lippe IT, Holzer P. Stomach-brain communication by vagal afferents in response to luminal acid backdiffusion, gastrin, and gastric acid secretion. Am. J. Physiol. 2004;286:G403–G411. doi: 10.1152/ajpgi.00308.2003. [DOI] [PubMed] [Google Scholar]
- Deval E, Gasull X, Noël J, Salinas M, Baron A, Diochot S, Lingueglia E. Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol. Ther. 2010;128:549–558. doi: 10.1016/j.pharmthera.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Diochot S, Baron A, Rash LD, Deval E, Escoubas P, Scarzello S, Salinas M, Lazdunski M. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 2004;23:1516–1525. doi: 10.1038/sj.emboj.7600177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diochot S, Baron A, Salinas M, Douguet D, Scarzello S, Dabert-Gay AS, Debayle D, Friend V, Alloui A, Lazdunski M, Lingueglia E. Black mamba venom peptides target acid-sensing ion channels to abolish pain. Nature. 2012;490:552–555. doi: 10.1038/nature11494. [DOI] [PubMed] [Google Scholar]
- Duprat F, Lauritzen I, Patel A, Honoré E. The TASK background K2P channels: chemo- and nutrient sensors. Trends Neurosci. 2007;30:573–580. doi: 10.1016/j.tins.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Escoubas P, De Weille JR, Lecoq A, Diochot S, Waldmann R, Champigny G, Moinier D, Menez A, Lazdunski M. Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J. Biol. Chem. 2000;275:25116–25121. doi: 10.1074/jbc.M003643200. [DOI] [PubMed] [Google Scholar]
- Fallingborg J. Intraluminal pH of the human gastrointestinal tract. Dan. Med. Bull. 1999;46:183–196. [PubMed] [Google Scholar]
- Forster ER, Green T, Elliot M, Bremner A, Dockray GJ. Gastric emptying in rats: role of afferent neurons and cholecystokinin. Am. J. Physiol. 1990;258:G552–G556. doi: 10.1152/ajpgi.1990.258.4.G552. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Ichikawa H, Terayama R, Yamaai T, Kuboki T, Sugimoto T. ASIC3-immunoreactive neurons in the rat vagal and glossopharyngeal sensory ganglia. Brain Res. 2006;1081:150–155. doi: 10.1016/j.brainres.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Geppetti P, Tramontana M, Evangelista S, Renzi D, Maggi CA, Fusco BM, Del Bianco E. Differential effect on neuropeptide release of different concentrations of hydrogen ions on afferent and intrinsic neurons of the rat stomach. Gastroenterology. 1991;101:1505–1511. doi: 10.1016/0016-5085(91)90385-x. [DOI] [PubMed] [Google Scholar]
- Goldstein SA, Bayliss DA, Kim D, Lesage F, Plant LD, Rajan S. International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol. Rev. 2005;57:527–540. doi: 10.1124/pr.57.4.12. [DOI] [PubMed] [Google Scholar]
- Hellwig N, Plant TD, Janson W, Schäfer M, Schultz G, Schaefer M. TRPV1 acts as proton channel to induce acidification in nociceptive neurons. J. Biol. Chem. 2004;279:34553–34561. doi: 10.1074/jbc.M402966200. [DOI] [PubMed] [Google Scholar]
- Holm M, Johansson B, Pettersson A, Fändriks L. Carbon dioxide mediates duodenal mucosal alkaline secretion in response to luminal acidity in the anesthetized rat. Gastroenterology. 1998;115:680–685. doi: 10.1016/s0016-5085(98)70147-7. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Holzer P. Neural emergency system in the stomach. Gastroenterology. 1998;114:823–839. doi: 10.1016/s0016-5085(98)70597-9. [DOI] [PubMed] [Google Scholar]
- Holzer P. Acid-sensitive ion channels in gastrointestinal function. Curr. Opin. Pharmacol. 2003;3:618–625. doi: 10.1016/j.coph.2003.06.008. [DOI] [PubMed] [Google Scholar]
- Holzer P. Taste receptors in the gastrointestinal tract. V. Acid sensing in the gastrointestinal tract. Am. J. Physiol. 2007a;292:G699–G705. doi: 10.1152/ajpgi.00517.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Role of visceral afferent neurons in mucosal inflammation and defense. Curr. Opin. Pharmacol. 2007b;7:563–569. doi: 10.1016/j.coph.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. The pharmacological challenge to tame the transient receptor potential vanilloid-1 (TRPV1) nocisensor. Br. J. Pharmacol. 2008;155:1145–1162. doi: 10.1038/bjp.2008.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Acid-sensitive ion channels and receptors. Handb. Exp. Pharmacol. 2009;194:283–332. doi: 10.1007/978-3-540-79090-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Acid sensing by visceral afferent neurones. Acta Physiol. (Oxf.) 2011a;201:63–75. doi: 10.1111/j.1748-1716.2010.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol. Ther. 2011b;131:142–170. doi: 10.1016/j.pharmthera.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P, Maggi CA. Dissociation of dorsal root ganglion neurons into afferent and efferent-like neurons. Neuroscience. 1998;86:389–398. doi: 10.1016/s0306-4522(98)00047-5. [DOI] [PubMed] [Google Scholar]
- Holzer P, Painsipp E, Jocic M, Heinemann A. Acid challenge delays gastric pressure adaptation, blocks gastric emptying and stimulates gastric fluid secretion in the rat. Neurogastroenterol. Motil. 2003;15:45–55. doi: 10.1046/j.1365-2982.2003.00382.x. [DOI] [PubMed] [Google Scholar]
- Holzer P, Wultsch T, Edelsbrunner M, Mitrovic M, Shahbazian A, Painsipp E, Bock E, Pabst MA. Increase in gastric acid-induced afferent input to the brainstem in mice with gastritis. Neuroscience. 2007;145:1108–1119. doi: 10.1016/j.neuroscience.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Tränkner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JQ, Hunt RH. pH, healing rate and symptom relief in acid-related diseases. Yale J. Biol. Med. 1996;69:159–174. [PMC free article] [PubMed] [Google Scholar]
- Hughes PA, Brierley SM, Young RL, Blackshaw LA. Localization and comparative analysis of acid-sensing ion channel (ASIC1, 2, and 3) mRNA expression in mouse colonic sensory neurons within thoracolumbar dorsal root ganglia. J. Comp. Neurol. 2007;500:863–875. doi: 10.1002/cne.21204. [DOI] [PubMed] [Google Scholar]
- Huque T, Cowart BJ, Dankulich-Nagrudny L, Pribitkin EA, Bayley DL, Spielman AI, Feldman RS, Mackler SA, Brand JG. Sour ageusia in two individuals implicates ion channels of the ASIC and PKD families in human sour taste perception at the anterior tongue. PLoS One. 2009;4:e7347. doi: 10.1371/journal.pone.0007347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl. Acad. Sci. U.S.A. 2006;103:12569–12574. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RC, Otsuka E, Wagstrom E, Jensen CS, Price MP, Gebhart GF. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology. 2007;133:184–194. doi: 10.1053/j.gastro.2007.04.042. [DOI] [PubMed] [Google Scholar]
- Kagawa S, Aoi M, Kubo Y, Kotani T, Takeuchi K. Stimulation by capsaicin of duodenal HCO3− secretion via afferent neurons and vanilloid receptors in rats: comparison with acid-induced HCO3− response. Dig. Dis. Sci. 2003;48:1850–1856. doi: 10.1023/a:1025480003388. [DOI] [PubMed] [Google Scholar]
- Kang JY, Yap I. Acid and gastric ulcer pain. J. Clin. Gastroenterol. 1991;13:514–516. doi: 10.1097/00004836-199110000-00007. [DOI] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol. Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Kress M, Waldmann R. Acid sensing ionic channels. Curr. Top. Membranes. 2006;57:241–276. [Google Scholar]
- Krishtal OA, Pidoplichko VI. A receptor for protons in the membrane of sensory neurons may participate in nociception. Neuroscience. 1981;6:2599–2601. doi: 10.1016/0306-4522(81)90105-6. [DOI] [PubMed] [Google Scholar]
- Lamb K, Kang YM, Gebhart GF, Bielefeldt K. Gastric inflammation triggers hypersensitivity to acid in awake rats. Gastroenterology. 2003;125:1410–1418. doi: 10.1016/j.gastro.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Leffler A, Mönter B, Koltzenburg M. The role of the capsaicin receptor TRPV1 and acid-sensing ion channels (ASICS) in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice. Neuroscience. 2006;139:699–709. doi: 10.1016/j.neuroscience.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Lingueglia E. Acid-sensing ion channels in sensory perception. J. Biol. Chem. 2007;282:17325–17329. doi: 10.1074/jbc.R700011200. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J. Biol. Chem. 1997;272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- Lu YX, Owyang C. Duodenal acid-induced gastric relaxation is mediated by multiple pathways. Am. J. Physiol. 1999;276:G1501–G1506. doi: 10.1152/ajpgi.1999.276.6.G1501. [DOI] [PubMed] [Google Scholar]
- Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. Proton-sensing G-protein-coupled receptors. Nature. 2003;425:93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- Manela FD, Ren J, Gao J, McGuigan JE, Harty RF. Calcitonin gene-related peptide modulates acid-mediated regulation of somatostatin and gastrin release from rat antrum. Gastroenterology. 1995;109:701–706. doi: 10.1016/0016-5085(95)90376-3. [DOI] [PubMed] [Google Scholar]
- Matricon J, Gelot A, Etienne M, Lazdunski M, Muller E, Ardid D. Spinal cord plasticity and acid-sensing ion channels involvement in a rodent model of irritable bowel syndrome. Eur. J. Pain. 2011;15:335–343. doi: 10.1016/j.ejpain.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Matricon J, Muller E, Accarie A, Meleine M, Etienne M, Voilley N, Busserolles J, Eschalier A, Lazdunski M, Bourdu S, Gelot A, Ardid D. Peripheral contribution of NGF and ASIC1a to colonic hypersensitivity in a rat model of irritable bowel syndrome. Neurogastroenterol. Motil. 2013;25:e740–e754. doi: 10.1111/nmo.12199. [DOI] [PubMed] [Google Scholar]
- Medda BK, Sengupta JN, Lang IM, Shaker R. Response properties of the brainstem neurons of the cat following intra-esophageal acid-pepsin infusion. Neuroscience. 2005;135:1285–1294. doi: 10.1016/j.neuroscience.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Montrose MH, Akiba Y, Takeuchi K, Kaunitz JD. Gastroduodenal mucosal defense. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Fourth Edition Academic Press; San Diego: 2006. pp. 1259–1291. [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Nugent SG, Kumar D, Rampton DS, Evans DF. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut. 2001;48:571–577. doi: 10.1136/gut.48.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, Blackshaw LA. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54:1408–1415. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M, LaMotte RH. Effect of protons on the inward current evoked by capsaicin in isolated dorsal root ganglion cells. Pain. 1993;54:37–42. doi: 10.1016/0304-3959(93)90097-9. [DOI] [PubMed] [Google Scholar]
- Pohl D, Fox M, Fried M, Göke B, Prinz C, Mönnikes H, Rogler G, Dauer M, Keller J, Lippl F, Schiefke I, Seidler U, Allescher HD, Kandahar Study Group Do we need gastric acid? Digestion. 2008;77:184–197. doi: 10.1159/000142726. [DOI] [PubMed] [Google Scholar]
- Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- Richter TA, Dvoryanchikov GA, Chaudhari N, Roper SD. Acid-sensitive two-pore domain potassium (K2P) channels in mouse taste buds. J. Neurophysiol. 2004;92:1928–1936. doi: 10.1152/jn.00273.2004. [DOI] [PubMed] [Google Scholar]
- Sakai H, Lingueglia E, Champigny G, Mattei MG, Lazdunski M. Cloning and functional expression of a novel degenerin-like Na+ channel gene in mammals. J. Physiol. 1999;519:323–333. doi: 10.1111/j.1469-7793.1999.0323m.x. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schicho R, Schemann M, Pabst MA, Holzer P, Lippe IT. Capsaicin-sensitive extrinsic afferents are involved in acid-induced activation of distinct myenteric neurons in the rat stomach. Neurogastroenterol. Motil. 2003;15:33–44. doi: 10.1046/j.1365-2982.2003.00384.x. [DOI] [PubMed] [Google Scholar]
- Schicho R, Florian W, Liebmann I, Holzer P, Lippe IT. Increased expression of TRPV1 receptor in dorsal root ganglia by acid insult of the rat gastric mucosa. Eur. J. Neurosci. 2004;19:1811–1818. doi: 10.1111/j.1460-9568.2004.03290.x. [DOI] [PubMed] [Google Scholar]
- Schuligoi R, Jocic M, Heinemann A, Schöninkle E, Pabst MA, Holzer P. Gastric acid-evoked c-fos messenger RNA expression in rat brainstem is signaled by capsaicin-resistant vagal afferents. Gastroenterology. 1998;115:649–660. doi: 10.1016/s0016-5085(98)70144-1. [DOI] [PubMed] [Google Scholar]
- Semtner M, Schaefer M, Pinkenburg O, Plant TD. Potentiation of TRPC5 by protons. J. Biol. Chem. 2007;282:33868–33878. doi: 10.1074/jbc.M702577200. [DOI] [PubMed] [Google Scholar]
- Shimada S, Ueda T, Ishida Y, Yamamoto T, Ugawa S. Acid-sensing ion channels in taste buds. Arch. Histol. Cytol. 2006;69:227–231. doi: 10.1679/aohc.69.227. [DOI] [PubMed] [Google Scholar]
- Shulkes A, Baldwin GS, Giraud AS. Regulation of gastric acid secretion. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Fourth Edition Academic Press; San Diego: 2006. pp. 1223–1258. [Google Scholar]
- Steen KH, Reeh PW, Anton F, Handwerker HO. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J. Neurosci. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Dang K, Lamb K, Bielefeldt K, Gebhart GF. Acid-sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J. Neurosci. 2005;25:2617–2627. doi: 10.1523/JNEUROSCI.2894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Bielefeldt K, Gebhart GF. Mouse colon sensory neurons detect extracellular acidosis via TRPV1. Am. J. Physiol. 2007;292:C1768–C1774. doi: 10.1152/ajpcell.00440.2006. [DOI] [PubMed] [Google Scholar]
- Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc. Natl. Acad. Sci. U.S.A. 2001;98:711–716. doi: 10.1073/pnas.011404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol. Motil. 2010;22:512–519. e114–e115. doi: 10.1111/j.1365-2982.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- Texter EC. Ulcer pain mechanisms. The clinical features of active peptic ulcer disease and implications for therapy. Scand. J. Gastroenterol. Suppl. 1987;134:1–20. doi: 10.3109/00365528709090135. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina M, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 21:531–543. doi: 10.1016/s0896-6273(00)80564-4. 198. [DOI] [PubMed] [Google Scholar]
- Tomura H, Mogi C, Sato K, Okajima F. Proton-sensing and lysolipid-sensitive G-protein-coupled receptors: a novel type of multi-functional receptors. Cell. Signal. 2005;17:1466–1476. doi: 10.1016/j.cellsig.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Ugawa S, Yamamoto T, Ueda T, Ishida Y, Inagaki A, Nishigaki M, Shimada S. Amiloride-insensitive currents of the acid-sensing ion channel-2a (ASIC2a)/ASIC2b heteromeric sour-taste receptor channel. J. Neurosci. 2003;23:3616–3622. doi: 10.1523/JNEUROSCI.23-09-03616.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulcu SD, Liewald JF, Gillen C, Rupp J, Nawrath H. Proton conductance of human transient receptor potential-vanilloid type-1 expressed in oocytes of Xenopus laevis and in Chinese hamster ovary cells. Neuroscience. 2004;125:861–866. doi: 10.1016/j.neuroscience.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Wachter CH, Heinemann A, Donnerer J, Pabst MA, Holzer P. Mediation by 5-hydroxytryptamine of the femoral vasoconstriction induced by acid challenge of the rat gastric mucosa. J. Physiol. 1998;509:541–550. doi: 10.1111/j.1469-7793.1998.541bn.x. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Voilley N, Lazdunski M. Molecular cloning and functional expression of a novel amiloride-sensitive Na+ channel. J. Biol. Chem. 1995;270:27411–27414. doi: 10.1074/jbc.270.46.27411. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Lingueglia E, De Weille JR, Heurteaux C, Lazdunski M. H+-gated cation channels. Ann. New York Acad. Sci. 1999;868:67–76. doi: 10.1111/j.1749-6632.1999.tb11274.x. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Taugher RJ, Kreple CJ. Acid-sensing ion channels in pain and disease. Nat. Rev. Neurosci. 2013;14:461–471. doi: 10.1038/nrn3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemuth D, Sahin H, Falkenburger BH, Lefèvre CM, Wasmuth HE, Gründer S. BASIC – a bile acid-sensitive ion channel highly expressed in bile ducts. FASEB J. 2012;26:4122–4130. doi: 10.1096/fj.12-207043. [DOI] [PubMed] [Google Scholar]
- Wultsch T, Painsipp E, Shahbazian A, Mitrovic M, Edelsbrunner M, Waldmann R, Lazdunski M, Holzer P. Deletion of the acid-sensing ion channel ASIC3 prevents gastritis-induced acid hyperresponsiveness of the stomach-brainstem axis. Pain. 2008;134:245–253. doi: 10.1016/j.pain.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Price MP, Berger AL, Welsh MJ. DRASIC contributes to pH-gated currents in large dorsal root ganglion sensory neurons by forming heteromultimeric channels. J. Neurophysiol. 2002;87:2835–2843. doi: 10.1152/jn.2002.87.6.2835. [DOI] [PubMed] [Google Scholar]
- Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ. Res. 2006;99:501–509. doi: 10.1161/01.RES.0000238388.79295.4c. [DOI] [PubMed] [Google Scholar]
- Yamamura H, Ugawa S, Ueda T, Nagao M, Joh T, Simada S. Epithelial Na+ channel δ subunit is an acid sensor in the human oesophagus. Eur. J. Pharmacol. 2008;600:32–36. doi: 10.1016/j.ejphar.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Smith JA, Sangameswaran L, Eglen R, Birch R, Knowles C, Williams N, Anand P. Increased acid-sensing ion channel ASIC-3 in inflamed human intestine. Eur. J. Gastroenterol. Hepatol. 2001;13:891–896. doi: 10.1097/00042737-200108000-00003. [DOI] [PubMed] [Google Scholar]
- Yuan LP, Bo Y, Ming G, Zhou QL. Expression of acid-sensing ion channels of gastric mucosa from patients with Henoch-Schönlein purpura. J. Pediatr. Gastroenterol. Nutr. 2012;54:561–563. doi: 10.1097/MPG.0b013e318244255f. [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU, Kress M, Swandulla D. Fractional Ca2+ currents through capsaicin- and proton-activated ion channels in rat dorsal root ganglion neurones. J. Physiol. 1997;503:67–78. doi: 10.1111/j.1469-7793.1997.067bi.x. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]