Abstract
Background
In previous studies of frontline therapy for adult acute lymphoblastic leukemia (ALL), early treatment with higher doses of anthracyclines has been reported to improve outcome. The current study was conducted to evaluate whether addition of anthracycline-based consolidation chemotherapy (Course 2) with liposomal daunorubicin (150 mg/m2 intravenously [IV] on Days 1 and 2) and cytarabine (1.5 g/m2 IV on Days 1 and 2) to the standard hyper-CVAD regimen (fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high dose methotrexate and cytarabine) would improve outcome.
Methods
Sixty-eight consecutive adults with de novo ALL or lymphoblastic lymphoma were treated with this modified hyper-CVAD regimen inclusive of rituximab for CD20 expression ≥20%.
Results
Sixty-three (93%) patients achieved complete response (CR). With a median follow-up of 90 months, the 5-year CR duration (CRD) and overall survival (OS) rates were 46% and 44%, respectively. Compared with 208 patients treated with standard hyper-CVAD (rates of 45% and 47%, respectively; P = not significant), outcome with the modified hyper-CVAD regimen was not improved overall. Outcome was improved by the addition of rituximab for the CD20-positive subset (rates of CRD and OS of 50% and 53%, respectively), whereas anthracycline intensification worsened outcome for the CD20-negative subset (rates of CRD and OS of 41% and 35%, respectively; P = .01) compared with standard hyper-CVAD. A high mortality rate related to infections in CR was noted among patients aged 60 years or older.
Conclusions
In the context of the hyper-CVAD regimen, early anthracycline intensification did not improve outcome for adults with de novo ALL or lymphoblastic lymphoma.
Keywords: acute lymphoblastic leukemia, hyper-CVAD regimen, anthracycline intensification, liposomal preparation of daunorubicin
The outcome of childhood acute lymphoblastic leukemia (ALL) has improved significantly over the past 50 years. Progress has occurred predominantly as a result of judicious application of combinations of anti-ALL drugs in optimized sequences of induction, consolidations, maintenance, and central nervous system (CNS) prophylaxis.1-3 The complete response (CR) rates with modern frontline intensive regimens in pediatric ALL are currently 95% or better, and the cure rates exceed 80%. Using similar therapeutic strategies in adult ALL has improved the CR rates to 90%, but overall the cure rates remain in the range of 30% to 50%.4-7 In younger adults with de novo ALL, the application of pediatric regimens that intensify the relatively nonmyelosuppressive components, such as corticosteroids, vincristine, and asparaginase, has shown promising results.8-11 Alternative approaches to intensification include the incorporation of targeted agents for specific subtypes of adult ALL. These approaches have significantly improved the prognosis of subsets previously associated with particularly poor outcomes with chemotherapy alone, exemplified by the addition of rituximab for Burkitt type and mature B-cell ALL and by the use of selective Bcr-Abl tyrosine kinase inhibitors for Philadelphia chromosome-positive ALL.12-14
Despite the success of these interventions, further progress is needed, especially for adults with other ALL subtypes. The use of autologous intensification in first CR has proven to be inferior to continuation of chemotherapy.15 Several alternative therapeutic strategies have been proposed, including allogeneic stem cell transplantation (SCT) in first remission (for both standard and high-risk patients15,16), incorporation of novel agents into frontline therapy such as monoclonal antibodies (eg, rituximab17) directed against surface antigens on lymphoblasts, and liposomal preparations of standard chemotherapeutics (eg, sphingosomal vincristine18,19).
Todeschini et al20 studied dose-intensive anthracycline regimens and the impact of this therapeutic strategy on outcome for adult ALL. They first showed that the cumulative dose and the dose intensity of daunorubicin delivered during induction therapy was a significant prognostic factor for survival.20 They subsequently developed a frontline chemotherapy regimen that incorporated higher doses of daunorubicin during induction. The median cumulative dose of daunorubicin delivered was 275 mg/m2, and the dose intensity was 38 mg/m2 weekly.21 The investigators reported a higher CR rate with this regimen compared with their previous experience, and a favorable 6-year estimated survival rate of 55%. Other investigators have also reported improved outcomes with higher doses of anthracyclines for adult ALL.22-24
Although no significant toxicities were observed in the study conducted by Todeschini and others,21 a higher cumulative dose of anthracyclines may be associated with significant and delayed cardiotoxicity, which would be of importance in a potentially curable population. Encapsulated formulations of chemotherapeutic agents have been shown to improve efficacy and to reduce toxicity. The liposomal preparation of daunorubicin appears to achieve greater neoplastic tissue concentrations, better tumor cell delivery, and immunity to modulation by the multidrug resistance (MDR) drug transport system.25-27 It has been administered in cumulative doses >1000 mg/m2 without cardiotoxicity.25 Our previous studies with liposomal daunorubicin and cytarabine in acute leukemias had demonstrated efficacy and safety.28-30 Phase 1 studies in acute myelogenous leukemia (AML) determined that liposomal daunorubicin could be given safely at a dose of 100 to 125 mg/m2 daily for 3 days (total dose 300-375 mg/m2 per course). Among 42 patients treated, 79% of whom had previously received anthracycline-based chemotherapy, the incidence of cardiotoxicity was only 2%.29
The hyper-CVAD regimen (fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high dose methotrexate and cytarabine), an intensive program modeled after a pediatric regimen designed for childhood Burkitt lymphoma/leukemia, has proven to be an effective frontline therapy for adults with de novo ALL and lymphoblastic lymphoma.4,5,31,32 On the basis of the encouraging data with early anthracycline intensification, we modified the standard hyper-CVAD regimen by incorporating a postinduction course of liposomal daunorubicin-based chemotherapy to confirm the results reported by other investigators, and to test the hypothesis that this intervention might improve outcome by overcoming leukemia resistance with elimination of residual malignant clones. On the basis of our previous findings regarding the poor prognostic impact of CD20 expression on outcome in B-lymphoblastic leukemia and the encouraging results with hyper-CVAD with rituximab in Burkitt type ALL, we also incorporated the anti-CD20 monoclonal antibody rituximab.12,33 Herein we report the long-term outcome after treatment with this modified hyper-CVAD regimen, expanding on the previously reported experience in a small cohort of patients with lymphoblastic lymphoma.32
Materials and Methods
Study Group
Adults with previously untreated ALL or lymphoblastic lymphoma referred to our institution from May 2000 until November 2001 were treated with the modified hyper-CVAD regimen (Table 1). The protocol was approved by the institutional review board at The University of Texas M. D. Anderson Cancer Center. Informed consent for participation was obtained in accordance with institutional guidelines and the Declaration of Helsinki.
Table 1. Hyper-CVAD and Modified Hyper-CVAD Chemoimmunotherapy Regimens.
| Regimen | Modified Hyper-CVAD [2000-2001] | Hyper-CVAD [1992-1999] |
|---|---|---|
| Induction | ||
| Hyper-CVAD [laminar air flow rooms if age ≥60 years] | Y [Y] | Y [N] |
| Rituximab, 375 mg/m2 IV on Days 1 and 11 if CD20 ≥20% | Y | N |
| Consolidation | ||
| Cycle 2 | ||
| Liposomal daunorubicin, 150 mg/m2 IV daily over 12 hours on Days 1-2; cytarabine, 1.5 g/m2 CI IV daily on Days 1-2; prednisone, 200 mg PO daily on Days 1-5 | Y | N |
| Cycles 2, 4, 6, 8 or Cycles 3, 5, 7, 9 | ||
| MTX, 200 mg/m2 IV over 2 hours, then 800 mg/m2 IV over 22 hours on Day 1; cytarabine, 3 g/m2 [1 g/m2 if age ≥60 years] IV over 2 hours every 12 hours 4× on Days 2-3; Solu-Medrol, 50 mg IV every 12 hours 6× on Days 1-3; citrovorum factor, 50 mg IV 12 hours after end of MTX then 15 mg IV every 6 hours 8× or until MTX level <0.1 lmol/L; acetazolamide PO if urine pH <7 | Y | Y |
| Cycles 1, 3, 5, 7 or Cycles 1, 4, 6, 8 | ||
| Cyclophosphamide, 300 mg/m2 IV over 2 hours every 12 hours 6× on Days 1-3; mesna, 600 mg/m2 CI IV daily on Days 1-3 [1 hour before cyclophosphamide until 12 hours after final dose of cyclophosphamide]; dexamethasone, 40 mg IV or PO daily on Days 1-4 and 11-14; doxorubicin, 50 mg/m2 CI IV over 2-24 hours | Y | Y |
| on Day 4 [48 hours if EF <50%]; vincristine, 2 mg IV on Days 1 and 11 | ||
| Cycles 1-4 | ||
| If CD20 ≥20%: 8 doses of rituximab, 375 mg/m2 IV; Days 1 and 11 [hyper-CVAD]; Days 1 and 8 [LDNR- or MTX-cytarabine] | Y | N |
| Central nervous system prophylaxis | ||
| MTX 12 mg [6 mg if Ommaya] on Day 2; cytarabine 100 mg on Day 7 or 8 | Y | Y |
| Risk adapted [LDH ≥1400 U/L, S+G2M ≥14%] number of intrathecal treatments | ||
| High [1 elevated] | 8 | 16 |
| Indeterminate [1 unknown] | 8 | 8 |
| Low | 6 | 4 |
| Maintenance | ||
| POMP | ||
| 6-Mercaptopurine 50 mg PO TID; vincristine 2 mg IV on Day 1 every 28 days; MTX 20 mg/m2 PO or IV weekly; prednisone 200 mg PO daily on Days 1-5 | Months 1-5, 8-17, 20-30 | Months 1-6, 8-10, 12-24 |
| Intensifications | ||
| Hyper-CVAD; rituximab 375 mg/m2 IV on Days 1, 11 if CD20 ≥20% | Months 6, 18 | N |
| MTX 100 mg/m2 IV on Day 1 weekly 4×; L-asparaginase 20,000 U IV on Day 2 weekly 4× | Months 7, 19 | Months 7, 11 |
| Supportive care | ||
| IV/PO alkalinization in all courses; rasburicase/allopurinol for induction | ||
| G-CSF 10 μg/kg subcutaneously daily until ANC >109/L starting 24 hours after completion [eg, Day 4 or 5] | ||
| Duration of doxorubicin infusions increased from 2 hours to 24 hours [48 hours if EF <50%] for modified hyper-CVAD regimen for cardioprotection | ||
| Leucovorin [citrovorum factor] rescue: 50-100 mg IV every 4-6 hours if MTX levels elevated at the end of infusion [0 hour, confirmed on repeat sample] to >20 μmol/L, >1 μmol/L at 24 hours, or >0.1 μmol/L at 48 hours |
Hyper-CVAD indicates fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone; Y, yes; N, no; IV, intravenous; CI, continuous infusion; PO, orally; MTX, methotrexate; EF, ejection fraction; LDNR, liposomal daunorubicin; LDH, lactate dehydrogenase; POMP, 6-mercaptopurine, MTX, vincristine, prednisone; TID, 3× daily; G-CSF, granulocyte colony-stimulating factor; ANC, absolute neutrophil count; S+G2M, proliferative index.
Eligibility criteria included age at least 15 years in the absence of other active malignancy with expected consequent death within 12 months or known positivity for human immunodeficiency virus-1. No exclusions were made because of performance status; cardiac, hepatic, or renal function; or concomitant active infection. Mature B-cell ALL, Burkitt leukemia/lymphoma, or Philadelphia-positive ALL subtypes were treated on separate protocols, and were also excluded from the comparative historical control group treated with standard hyper-CVAD.12,13 Entry criteria were similar during the 2 study periods.
The diagnosis of ALL was established according to World Health Organization criteria.34 Pretreatment evaluations included history/physical examination; complete blood/platelet/differential counts; serum chemistry, including hepatic and renal function studies; bone marrow aspiration/biopsy for morphologic analysis and immunohistochemical staining; and karyotyping. Flow cytometry was performed to establish lineage, CD20 expression, and DNA cell cycle as previously described.33
Therapy
A detailed comparison of the modified and standard hyper-CVAD regimens is provided in Table 1. The modified hyper-CVAD regimen32 is similar to standard hyper-CVAD except for: 1) addition of consolidation with Course 2 of liposomal daunorubicin-cytarabine (9 vs 8 total cycles of induction-consolidation); 2) addition of 8 standard doses of rituximab in the first 4 courses for CD20-positive ALL; 3) alteration in number of intrathecal (IT) treatments based on CNS risk (reduced from 16 to 8 for high risk, increased from 4 to 6 for low risk); 4) administration of induction course in a laminar airflow room or Protective Environment (PE) if age ≥60 years; 5) extension of the 6-mercaptopurine, methotrexate, vincristine, prednisone (POMP) maintenance phase by 6 months; and 6) addition of early and late intensifications with hyper-CVAD (±rituximab) followed by methotrexate and L-asparaginase during the maintenance phase. Allogeneic SCT in first CR was considered for the poor risk karyotype t(4;11)(q21;q23) or failure to achieve marrow CR after 1 course.
The major modifications to the induction-consolidation phase of the standard hyper-CVAD regimen included the addition of Course 2 with liposomal daunorubicin 150 mg/m2 intravenously (IV) over 12 hours daily on Days 1 and 2 beginning 6 hours after completion of rituximab (if administered); cytarabine 1.5 g/m2 continuous infusion IV on Days 1 and 2; and prednisone 200 mg orally daily on Days 1 to 5. If bone marrow lymphoblasts were CD20-positive (≥20%), rituximab 375 mg/m2 IV was given for 8 total doses over the first 4 courses (Table 1). The intensive cycles were administered every 21 days or earlier (at least 14 days apart) when the white blood cell count was ≥3 × 109/L (at least 24 hours from last dose of granulocyte colony-stimulating factor) and untransfused platelet count was ≥50 to 60 × 109/L. Antibiotic prophylaxis during the dose-intensive (induction-consolidation) and maintenance phases was given as previously described.32
Therapy for active CNS leukemia at presentation included alternating IT treatments twice weekly during the induction course until the cerebrospinal fluid cell count normalized and cytological examination was negative for malignant cells on 2 sequential assessments, then weekly for 4 weeks, then according to the prophylactic schedule for all remaining cycles of intensive chemotherapy. Therapeutic radiation therapy was given if indicated (eg, 24-30 Gray in 10-12 fractions to the base of the skull for cranial nerve palsies). No prophylactic cranial irradiation was administered.
Dose reductions were applied as follows: 1) cytarabine 1 g/m2 for age ≥60 years, creatinine ≥1.5 mg/dL, or methotrexate level at 0 hour (repeated) ≥20 μmol/L; 2) vincristine 1 mg for total bilirubin >2 mg/dL or grade 2 persistent peripheral neuropathy; 3) vincristine eliminated for total bilirubin >3 mg/dL, grade 3 to 4 peripheral neuropathy, or grade 3 to 4 ileus; 4) doxorubicin decreased by 50% for total bilirubin 2 to 3 mg/dL, by 75% if 3 to 5 mg/dL, and eliminated if >5 mg/dL; and 5) methotrexate decreased by 50% for calculated creatinine clearance 10 to 50 mL/min (eliminated if <10 mL/min) or if pleural effusions/ascites (with thoracentesis/paracentesis as feasible), or decreased by 25% to 50% for delayed excretion, nephrotoxicity, or grade 3 or greater mucositis with prior courses.
Response and Toxicity Criteria and Statistical Methods
Response criteria were standard.5 Survival was measured from start of therapy until death from any cause. CR duration (CRD) was measured from achievement of CR until evidence of leukemia recurrence (≥10% lymphoblasts in bone marrow or extramedullary disease recurrence). Overall survival (OS) and CRD distributions were estimated using the Kaplan-Meier method, and compared using the log-rank test.35 Differences in response rates were analyzed using a chi-square test.
Results
Study Group
Characteristics of the 68 patients treated with the modified hyper-CVAD regimen are summarized in Table 2. Their median age was 40 years (range, 18-83 years); 27 (40%) were 50 years or older, and 16 (24%) were 60 years or older. Leukocyte count ≥30 × 109/L was noted in 16 (24%).
Table 2. Characteristics of 68 Patients Treated With the Modified Hyper-CVAD Regimen.
| Characteristic | Category | No. | % |
|---|---|---|---|
| Age, y | <40 | 34 | 50 |
| 40-59 | 18 | 27 | |
| ≥60 | 16 | 24 | |
| Zubrod performance score | >2 | 1 | 2 |
| Splenomegaly | Yes | 7 | 10 |
| Hepatomegaly | Yes | 5 | 7 |
| Lymphadenopathy | Yes | 22 | 32 |
| CNS disease at diagnosis | Yes | 4 | 6 |
| Mediastinal mass | Yes | 12 | 18 |
| WBC count, ×109/L | ≥30 | 16 | 24 |
| Platelet count, ×109/L | ≤50 | 31 | 46 |
| Hemoglobin level, g/dL | <10 | 44 | 65 |
| LDH level, U/L | >600 | 55 | 81 |
| Creatinine level, mg/dL | ≥1.3 | 7 | 10 |
| Total bilirubin level, mg/dL | ≥1.3 | 5 | 7 |
| Karyotype, n=67 | Diploid | 35 | 52 |
| Insufficient metaphases | 5 | 7 | |
| Hypodiploid | 4 | 6 | |
| t(4;11) | 3 | 4 | |
| Hyperdiploid | 3 | 4 | |
| 6q−; 14q+ | 1 | 2 | |
| Other | 16 | 24 | |
| Diagnosis | ALL | 57 | 84 |
| Lymphoblastic lymphoma | 11 | 16 | |
| FAB classification, n=49 | L1 | 17 | 35 |
| L2 | 32 | 65 | |
| Immunophenotype, n=62 | B lineage | 49 | 79 |
| T lineage | 13 | 21 | |
| Myeloid markers, n=57 | Positive | 25 | 44 |
| Negative | 32 | 56 | |
| CD20 expression ≥20%, n=59 | Positivea | 25 | 42 |
| Negative | 34 | 58 | |
| Systemic risk classificationb | Low | 11 | 16 |
| High | 57 | 83 | |
| CNS risk classification, n=64c | Low | 12 | 19 |
| Indeterminate | 24 | 24 | |
| High | 28 | 44 |
Hyper-CVAD indicates fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone; CNS, central nervous system; WBC, white blood cell count; LDH, lactate dehydrogenase; ALL, acute lymphoblastic leukemia; FAB, French-American-British.
Incidence of CD20 expression by age: 20% if <40 years, 50% if 40-59 years, 60% if 60 years or older.
Systemic risk classification40: high risk, WBC >5 × 109/L, >1 course to complete response, Philadelphia chromosome or CNS disease; low risk, none of these features.
CNS risk classification (does not include CNS disease): low, proliferative index (S+G2M) <14% and LDH ≤1400 U/L; high, S+G2M ≥14% or LDH ≥1400 U/L; indeterminate, value unknown.
Outcome
Sixty-three (93%) patients achieved CR within a median time of 23 days. Other responses included 1 (1%) CR with incomplete platelet recovery, 3 (4%) partial responses, and 1 (1%) induction death, the latter related to systemic bacterial-fungal infections. Response by pre-treatment characteristics is shown in Table 3. The CR rate for patients aged <30 years was 95% versus 100% among patients aged 60 years or older. Given the small cohort size and high CR rates, no significant prognostic factors for response were identified (Table 3).
Table 3. Response, Remission Duration, and Survival After Modified Hyper-CVAD by Pretreatment Characteristics.
| Characteristic | No. CR/Total | % CR | P | % 5-Year CRD | P | % 5-Year Survival | P |
|---|---|---|---|---|---|---|---|
| Age, y | .42 | .82 | .01 | ||||
| <40 | 31/34 | 91 | 55 | 59 | |||
| 40-59 | 16/18 | 89 | 41 | 44 | |||
| ≥60 | 16/16 | 100 | 34 | 13 | |||
| Zubrod performance score | .92 | .16 | .06 | ||||
| 0-1 | 46/50 | 92 | 53 | 50 | |||
| 2 | 16/17 | 94 | 25 | 24 | |||
| 3 | 1/1 | 100 | 100 | 100 | |||
| Hepatomegaly | .52 | .53 | .37 | ||||
| No | 58/63 | 92 | 46 | 42 | |||
| Yes | 5/5 | 100 | 60 | 60 | |||
| WBC, ×109/L | .13 | .12 | .62 | ||||
| ≤5 | 30/31 | 97 | 53 | 47 | |||
| 5.1-49.9 | 22/26 | 85 | 42 | 42 | |||
| ≥50 | 11/11 | 100 | 42 | 36 | |||
| Platelet count, ×109/L | .59 | .06 | .08 | ||||
| <20 | 8/8 | 100 | 15 | 25 | |||
| 20-80 | 29/31 | 94 | 36 | 37 | |||
| >80 | 26/29 | 90 | 69 | 55 | |||
| Albumin level, gm/dL | .42 | .23 | .44 | ||||
| <3 | 15/17 | 88 | 27 | 41 | |||
| ≥3 | 48/51 | 94 | 55 | 45 | |||
| Bilirubin level, mg/dL | .52 | .37 | .52 | ||||
| <1.3 | 58/63 | 92 | 45 | 42 | |||
| >1.3 | 5/5 | 100 | 75 | 60 | |||
| FAB classification | .17 | .60 | .72 | ||||
| L1 | 16/17 | 94 | 29 | 29 | |||
| L2 | 32/32 | 100 | 48 | 46 | |||
| ND | 15/19 | 79 | 62 | 53 | |||
| Immunophenotype | .65 | .35 | .21 | ||||
| T lineage | 12/13 | 92 | 43 | 31 | |||
| B lineage | 47/49 | 96 | 48 | 48 | |||
| Precursor | 12/12 | 100 | 35 | 33 | |||
| CALLA | 35/37 | 95 | 53 | 51 | |||
| Karyotype | .69 | .54 | .34 | ||||
| Diploid | 32/35 | 91 | 50 | 49 | |||
| Other | 31/33 | 94 | 47 | 38 | |||
| Myeloid markers | .10 | .71 | .89 | ||||
| Positive | 23/25 | 92 | 41 | 44 | |||
| Negative | 32/32 | 100 | 50 | 43 | |||
| CD20 expression ≥20% | .23 | .10 | .04 | ||||
| Positive | 25/25 | 100 | 51 | 51 | |||
| Negative | 32/34 | 94 | 39 | 35 | |||
| Systemic risk classification | .82 | .62 | .26 | ||||
| Low | 10/11 | 91 | 47 | 63 | |||
| High | 53/57 | 93 | 47 | 40 |
Hyper-CVAD indicates fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone; CR, complete response; CRD, CR duration; WBC, white blood cell count; FAB, French-American-British; ND, not done; CALLA, common acute lymphoblastic leukemia antigen.
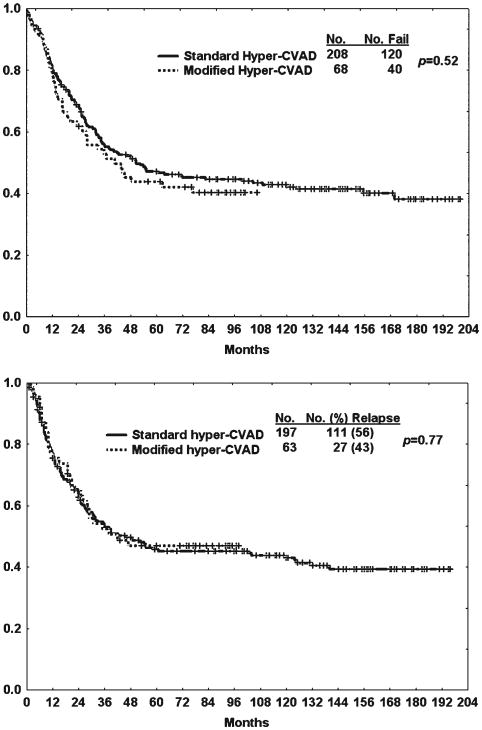
The median follow-up time was 90 months (range, 24-107 months). No patients underwent autologous or allogeneic SCT in first CR. The median OS was 41 months; 5-year OS and CRD rates were 44% and 46%, respectively (Fig. 1). Older age was associated with a significantly worse survival (Table 3). Seven of the 9 deaths in CR were in patients aged 60 years or older (median, 72 years; range, 61-80 years); 6 were related to infections, with 1 from bleeding complications of thrombocytopenia. The other 2 deaths in CR were in younger patients (ages 35 and 37 years), 1 from multiple infections (cytomegalovirus, Pneumocystis jirovecii) on Day 42 of Course 6 of high-dose methotrexate and cytarabine, and the other from complications of hepatitis B reactivation during POMP maintenance therapy. Mortality rates in CR were 44% for older versus 4% for younger subsets, P < .001. Three (all in the older group) of the 9 infection-related deaths occurred after Course 2 of liposomal daunorubicin-cytarabine.
Figure 1.
Comparison of modified fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) versus standard hyper-CVAD with regard to (Top) survival and (Bottom) complete response duration.
CNS Disease
Four patients presented with CNS disease; 3 achieved both systemic and CNS remission, and 2 developed disease recurrence with systemic disease only.
Among 64 patients without initial CNS involvement, 5 (8%) had CNS recurrence: 4 isolated (before systemic disease recurrence) and 1 concomitant with bone marrow recurrence.
Side Effects
Side effects of the modified hyper-CVAD regimen were similar to standard hyper-CVAD, as detailed in previous reports.5,32 During Course 2 of liposomal daunorubicincytarabine, the only severe (grade 3-4) nonhematological toxicity was gastrointestinal (n = 1). The incidence of grade 1 to 2 mucositis was 8%. No significant cardiotoxicity was observed. Thirty-two (48%) patients developed febrile episodes: documented bacterial infections in 3, fungal infections in 2, mixed or multiple infections in 6, and fever of unknown origin in 21. Three older patients died subsequent to Course 2 from myelosuppression-related complications. For the liposomal daunorubicincytarabine courses, the median time to recovery of granulocytes to >109/L was 17 days and to platelet counts >100 × 109/L was 20 days, not significantly different from the hyper-CVAD or methotrexate-cytarabine courses. Four of the 25 patients with CD20-positive ALL treated with modified hyper-CVAD with rituximab subsequently developed secondary myelodysplastic syndrome (n = 3) or AML (n = 1) and were treated accordingly, eventually dying from refractory disease or complications of therapy.
Outcome With First Salvage Therapy
Twenty-seven (43%) of 63 patients who achieved CR with the modified hyper-CVAD regimen subsequently developed disease recurrence within a median of 14 months (range, 2-48 months). There were no significant pretreatment predictors for duration of CR (Table 3). Of the 12 patients with first CRD <1 year, the median age was 40 years (range, 21-72 years). Three (27%) of the 11 treated patients achieved CR with asparaginase-based salvage chemotherapy. Of the 15 patients with first CRD >1 year, the median age was 35 years (range, 19-76 years). Eight (53%) patients achieved CR with hyper-CVAD-based salvage chemotherapy (inclusive of rituximab and/or L-asparaginase). Two of the 12 patients who achieved second CR remained long-term survivors after allogeneic SCT.
Comparison of Modified With Standard Hyper-CVAD
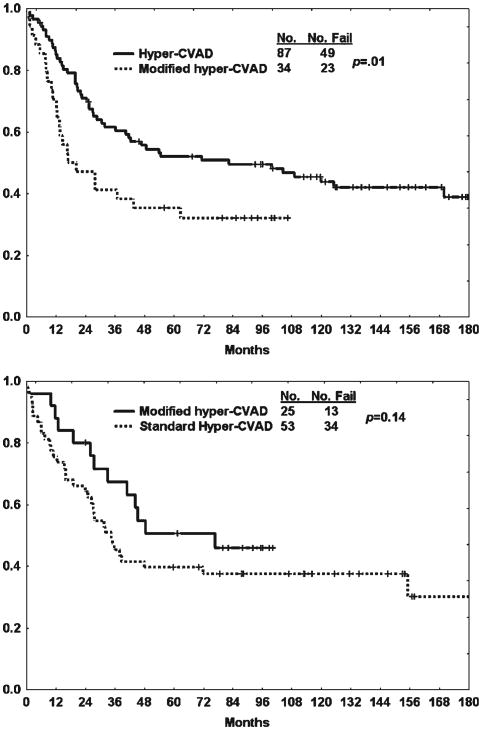
The results with the modified hyper-CVAD regimen were compared with those of 208 patients treated with standard hyper-CVAD. The characteristics of the patients in each treatment cohort were similar (data not shown). There was no difference in response outcomes between the 2 regimens (Table 4). This was expected because the induction therapy is similar except for the incorporation of rituximab for the CD20-positive subset in the modified hyper-CVAD regimen. Similarly, as shown in Figure 1, the rates of OS and CRD were not improved with the addition of liposomal daunorubicin-cytarabine. In fact, when comparing 5-year CRD and OS rates by therapy within the CD20-negative subset, the outcome was worse with the modified hyper-CVAD regimen (41% vs 52% [P = .07] and 35% vs 53% [P = .01], respectively) (Fig. 2, Top). In contrast, the use of rituximab in the modified hyper-CVAD regimen for the CD20-positive ALL subset produced better 5-year CRD and OS rates when compared with standard hyper-CVAD (50% vs 40% [P = .06] and 53% vs 40% [P = .14], respectively) (Fig. 2, Bottom).
Table 4. Response Outcomes With Modified Hyper-CVAD Versus Standard Hyper-CVAD.
| Response | Modified Hyper-CVAD, n = 68, % | Hyper-CVAD, n = 208, % | P |
|---|---|---|---|
| CR | |||
| Overall | 93 | 95 | NS |
| After course 1 | 78 | 77 | |
| CR with incomplete platelet recovery | 1 | 0 | |
| Partial response | 4 | 0 | NS |
| Resistant disease | 0 | 2 | NS |
| Induction mortality | 1 | 3 | NS |
Hyper-CVAD indicates fractionated cyclophosphamide, vincristine, doxoru-bicin, and dexamethasone; CR, complete response; NS, not significant.
Figure 2.
Overall survival by therapy (modified fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone [hyper-CVAD] vs standard hyper-CVAD) is shown within the (Top) CD20-negative and (Bottom) CD20-positive subsets.
Discussion
The aim of the study was to evaluate whether the addition of higher doses of anthracyclines early during the course of frontline therapy might improve the prognosis of de novo non–Burkitt-type Philadelphia-negative adult ALL or lymphoblastic lymphoma. This was based on previous observations reporting encouraging results with this therapeutic strategy.20,21 We elected to use the liposomal preparation of daunorubicin owing to its purported efficacy against MDR and cardioprotective properties.25-27 With this and other modifications to the standard hyper-CVAD regimen, which included additional cycles of hyper-CVAD as early/late intensifications during maintenance therapy, the planned cumulative dose of doxorubicin was 300 mg/m2 and of liposomal daunorubicin was 300 mg/m2. Despite the significant anthracycline exposure, the incidence of severe cardiotoxicity was not increased. Unfortunately, in the context of the hyper-CVAD regimen, the anthracycline intensification early in the consolidation phase (liposomal daunorubicin-cytarabine during Course 2) did not improve the outcome. Overall, the modified hyper-CVAD regimen produced similar 5-year OS and CRD rates compared with standard hyper-CVAD (Fig. 1).
In addition to the early anthracycline intensification, we incorporated standard dose rituximab into the modified hyper-CVAD regimen for CD20-positive ALL subsets in a dosing schema modeled after our experience with Burkitt leukemia/lymphoma.12 The use of rituximab for the CD20-positive B-lymphoblastic subset appeared to partially offset the negative influence of the anthracycline intensification on CRD and OS, which was significantly more evident in the CD20-negative subset (5-year OS rates for modified hyper-CVAD vs standard hyper-CVAD within the CD20-positive subset were 53% vs 40% [P = .14] compared with 35% vs 53% in the CD20-negative subset [P = .01]) (Fig. 2). Preliminary data from an ensuing frontline program treating a larger cohort of CD20-negative patients with de novo ALL or lymphoblastic lymphoma with the same modified hyper-CVAD regimen except for omission of the early anthracycline consolidation cycle have shown that the 3-year CRD and OS rates observed in this study have improved from 50% to 84% and 44% to 63%, respectively.36
In adolescents and young adults, several retrospective studies have shown superior outcomes with pediatric regimens, which generally intensify the nonmyelosuppressive components of therapy (eg, corticosteroids, vincristine, asparaginase) by several-fold (vs conventional adult ALL regimens), with anthracycline intensification reserved for only very high-risk pediatric cases.9-11 The difficulties encountered in the prospective application of pediatric regimens to adults relates to ascertaining the optimal age cutoff that should be used to designate the younger adult most likely to benefit from this therapeutic approach. Huguet and colleagues8 used a pediatric-inspired treatment regimen in adults with de novo ALL up to the age of 60 years. The results of their GRAALL-2003 study showed poorer tolerance of the regimen in adults older than 45 years of age, with a higher incidence of induction deaths (23% vs 5%; P < .001) and deaths in first CR (22% vs 5%; P < .001). Reduced dose intensity of the asparaginase and delays in initiation of consolidation were also noted in this older subgroup, although this did not translate into a higher cumulative incidence of disease recurrence (20% vs 25%; p = not significant).
In our study, 9 (14%) of 63 patients died in first CR after treatment with the modified hyper-CVAD regimen (6 without and 3 with rituximab), mostly related to infectious complications of myelosuppression. These included 7 (44%) of the 16 patients who were 60 years of age or older. The high mortality rate in CR had also been observed in the larger experience with the hyper-CVAD regimens, which has prompted truncation of the dose-intensive phase of chemotherapy and earlier transition to maintenance phase among older patients once a life-threatening complication has occurred.37 Three of these deaths occurred after the liposomal daunorubicin-cytarabine course, again suggesting that the increased anthracycline dose intensity was not beneficial overall, and may have been particularly detrimental among older patients.
In summary, our results with the modified hyper-CVAD regimen incorporating early dose-intensive anthracycline-based consolidation chemotherapy did not show improved outcome compared with the prior experience with standard hyper-CVAD. The augmented hyper-CVAD regimen, which intensifies the dexamethasone and vincristine components and incorporates L-asparaginase38 or pegylated asparaginase,39 has shown encouraging results in the salvage setting, with poorer tolerance in the older adults, as expected. Alternative strategies to chemo-therapy intensification, including incorporation of targeted agents (eg, other monoclonal antibodies directed at other surface antigens on lymphoblasts), use of other novel chemotherapeutic agents with minimal toxicity (eg, sphingosomal vincristine18,19), and integration of modulators of resistance mechanisms (eg, bcl-2 inhibitors, mammalian target of rapamycin inhibitors), will be needed to improve the prognosis of older adults with ALL.
Footnotes
Conflict of Interest Disclosures: The authors made no disclosures.
References
- 1.Pui CH, Sandlund JT, Pei D, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children's Research Hospital. Blood. 2004;104:2690–2696. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 2.Moricke A, Reiter A, Zimmermann M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood. 2008;111:4477–4489. doi: 10.1182/blood-2007-09-112920. [DOI] [PubMed] [Google Scholar]
- 3.Pui CH, Cheng C, Leung W, et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med. 2003;349:640–649. doi: 10.1056/NEJMoa035091. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian HM, O'Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547–561. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian H, Thomas D, O'Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–2801. doi: 10.1002/cncr.20668. [DOI] [PubMed] [Google Scholar]
- 6.Thiebaut A, Vernant JP, Degos L, et al. Adult acute lymphocytic leukemia study testing chemotherapy and autologous and allogeneic transplantation. A follow-up report of the French protocol LALA 87. Hematol Oncol Clin North Am. 2000;14:1353–1366, x. doi: 10.1016/s0889-8588(05)70190-8. [DOI] [PubMed] [Google Scholar]
- 7.Durrant IJ, Richards SM, Prentice HG, Goldstone AH. The Medical Research Council trials in adult acute lymphocytic leukemia. Hematol Oncol Clin North Am. 2000;14:1327–1352. doi: 10.1016/s0889-8588(05)70189-1. [DOI] [PubMed] [Google Scholar]
- 8.Huguet F, Leguay T, Raffoux E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27:911–918. doi: 10.1200/JCO.2008.18.6916. [DOI] [PubMed] [Google Scholar]
- 9.Stock W, La M, Sanford B, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children's Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112:1646–1654. doi: 10.1182/blood-2008-01-130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boissel N, Auclerc MF, Lheritier V, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol. 2003;21:774–780. doi: 10.1200/JCO.2003.02.053. [DOI] [PubMed] [Google Scholar]
- 11.de Bont JM, Holt B, Dekker AW, van der Does-van den Berg A, Sonneveld P, Pieters R. Significant difference in outcome for adolescents with acute lymphoblastic leukemia treated on pediatric vs adult protocols in the Netherlands. Leukemia. 2004;18:2032–2035. doi: 10.1038/sj.leu.2403538. [DOI] [PubMed] [Google Scholar]
- 12.Thomas DA, Faderl S, O'Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106:1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 13.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103:4396–4407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 14.Ravandi F, Thomas D, Kantarjian H, et al. Phase II study of combination of hyper-CVAD with dasatinib in frontline therapy of patients with Philadelphia chromosome (Ph) positive acute lymphoblastic leukemia (ALL) Blood. 2008;112:1005. abstract. Abstract 2921. [Google Scholar]
- 15.Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008;111:1827–1833. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 16.Cornelissen JJ, van der Holt B, Verhoef GE, et al. Myeloablative allogeneic versus autologous stem cell transplantation in adult patients with acute lymphoblastic leukemia in first remission: a prospective sibling donor versus no-donor comparison. Blood. 2009;113:1375–1382. doi: 10.1182/blood-2008-07-168625. [DOI] [PubMed] [Google Scholar]
- 17.Thomas DA, O'Brien S, Kantarjian HM. Monoclonal antibody therapy with rituximab for acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2009;23:949–971, v. doi: 10.1016/j.hoc.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas DA, Sarris AH, Cortes J, et al. Phase II study of sphingosomal vincristine in patients with recurrent or refractory adult acute lymphocytic leukemia. Cancer. 2006;106:120–127. doi: 10.1002/cncr.21595. [DOI] [PubMed] [Google Scholar]
- 19.Thomas DA, Kantarjian HM, Stock W, et al. Phase 1 multicenter study of vincristine sulfate liposomes injection and dexamethasone in adults with relapsed or refractory acute lymphoblastic leukemia. Cancer. 2009;115:5490–5498. doi: 10.1002/cncr.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todeschini G, Meneghini V, Pizzolo G, et al. Relationship between Daunorubicin dosage delivered during induction therapy and outcome in adult acute lymphoblastic leukemia. Leukemia. 1994;8:376–381. [PubMed] [Google Scholar]
- 21.Todeschini G, Tecchio C, Meneghini V, et al. Estimated 6-year event-free survival of 55% in 60 consecutive adult acute lymphoblastic leukemia patients treated with an intensive phase II protocol based on high induction dose of daunorubicin. Leukemia. 1998;12:144–149. doi: 10.1038/sj.leu.2400912. [DOI] [PubMed] [Google Scholar]
- 22.Bassan R, Rohatiner AZ, Rambaldi A, et al. Clinical sensitivity to anthracyclines in PH/BCR+ acute lymphoblastic leukemia. Adv Exp Med Biol. 1999;457:489–499. doi: 10.1007/978-1-4615-4811-9_53. [DOI] [PubMed] [Google Scholar]
- 23.Bassan R, Rohatiner AZ, Lerede T, et al. Role of early anthracycline dose-intensity according to expression of Philadelphia chromosome/BCR-ABL rearrangements in B-precursor adult acute lymphoblastic leukemia. Hematol J. 2000;1:226–234. doi: 10.1038/sj.thj.6200032. [DOI] [PubMed] [Google Scholar]
- 24.Bassan R, Pogliani E, Casula P, et al. Risk-oriented postre-mission strategies in adult acute lymphoblastic leukemia: prospective confirmation of anthracycline activity in standard-risk class and role of hematopoietic stem cell transplants in high-risk groups. Hematol J. 2001;2:117–126. doi: 10.1038/sj/thj/6200091. [DOI] [PubMed] [Google Scholar]
- 25.Gill PS, Espina BM, Muggia F, et al. Phase I/II clinical and pharmacokinetic evaluation of liposomal daunorubicin. J Clin Oncol. 1995;13:996–1003. doi: 10.1200/JCO.1995.13.4.996. [DOI] [PubMed] [Google Scholar]
- 26.Michieli M, Damiani D, Ermacora A, et al. Liposome-encapsulated daunorubicin for PGP-related multidrug resistance. Br J Haematol. 1999;106:92–99. doi: 10.1046/j.1365-2141.1999.01505.x. [DOI] [PubMed] [Google Scholar]
- 27.Michieli M, Damiani D, Ermacora A, Masolini P, Michelutti A, Baccarani M. Liposome encapsulated daunorubicin doubles anthracycline toxicity in cell lines showing a non-PGP related multidrug resistance. Haematologica. 1999;84:1151–1152. [PubMed] [Google Scholar]
- 28.Cortes J, O'Brien S, Estey E, Giles F, Keating M, Kantarjian H. Phase I study of liposomal daunorubicin in patients with acute leukemia. Invest New Drugs. 1999;17:81–87. doi: 10.1023/a:1006216001681. [DOI] [PubMed] [Google Scholar]
- 29.Cortes J, Estey E, O'Brien S, et al. High-dose liposomal daunorubicin and high-dose cytarabine combination in patients with refractory or relapsed acute myelogenous leukemia. Cancer. 2001;92:7–14. doi: 10.1002/1097-0142(20010701)92:1<7::aid-cncr1285>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 30.Cortes J, Kantarjian H, Albitar M, et al. A randomized trial of liposomal daunorubicin and cytarabine versus liposomal daunorubicin and topotecan with or without thalidomide as initial therapy for patients with poor prognosis acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2003;97:1234–1241. doi: 10.1002/cncr.11180. [DOI] [PubMed] [Google Scholar]
- 31.Murphy SB, Bowman WP, Abromowitch M, et al. Results of treatment of advanced-stage Burkitt's lymphoma and B cell (SIg+) acute lymphoblastic leukemia with high-dose fractionated cyclophosphamide and coordinated high-dose methotrexate and cytarabine. J Clin Oncol. 1986;4:1732–1739. doi: 10.1200/JCO.1986.4.12.1732. [DOI] [PubMed] [Google Scholar]
- 32.Thomas DA, O'Brien S, Cortes J, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood. 2004;104:1624–1630. doi: 10.1182/blood-2003-12-4428. [DOI] [PubMed] [Google Scholar]
- 33.Thomas DA, O'Brien S, Jorgensen JL, et al. Prognostic significance of CD20 expression in adults with de novo precursor B-lineage acute lymphoblastic leukemia. Blood. 2009;113:6330–6337. doi: 10.1182/blood-2008-04-151860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunning RD, Borowitz M, Matutes E, et al. Pathology and genetics of tumours of haematopoeitic and lymphoid tissues. In: Jaffe ES, Harris NL, Stein H, editors. World Health Classification of Tumours. Lyon, France: IARC Press; 2001. pp. 111–114. [Google Scholar]
- 35.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1965;53:457–481. [Google Scholar]
- 36.Thomas DA, Kantarjian HM, Faderl S, et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome for patients with de novo Philadelphia negative precursor B-cell acute lymphoblastic leukemia (ALL) Blood. 2009;114:344–345. abstract. Abstract 836. [Google Scholar]
- 37.O'Brien S, Thomas DA, Ravandi F, Faderl S, Pierce S, Kantarjian H. Results of the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimen in elderly patients with acute lymphocytic leukemia. Cancer. 2008;113:2097–2101. doi: 10.1002/cncr.23819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faderl S, Thomas DA, Ravandi F, et al. Intensification of hyper-CVAD with L-asparaginase, vincristine, and dexamethasone (“augmeneted hyper-CVAD”) has activity in adult patients with relapsed/refractory acute lymphoblastic leukemia (ALL) Blood. 2007;110:149b. abstract. Abstract 4324. [Google Scholar]
- 39.Ayoubi M, Thomas DA, Kantarjian H, et al. Augmented hyper-CVAD in adult ALL salvage therapy: the MDACC experience of hyper-CVAD using dose-intense vincristine, dexamethasone and pegaspargase. Blood. 2009;114:802. abstract. Abstract 2031. [Google Scholar]
- 40.Kantarjian HM, Walters RS, Keating MJ, et al. Results of the vincristine, doxorubicin, and dexamethasone regimen in adults with standard- and high-risk acute lymphocytic leukemia. J Clin Oncol. 1990;8:994–1004. doi: 10.1200/JCO.1990.8.6.994. [DOI] [PubMed] [Google Scholar]