Abstract
PTEN is known to be frequently mutated in uterine cancer and also dephosphorylates FAK. Here, we examined the impact of PTEN alterations on the response to treatment with a FAK inhibitor (GSK2256098). In vitro and in vivo therapeutic experiments were carried out using PTEN mutated and PTEN-wild type models of uterine cancer alone and in combination with chemotherapy. Treatment with GSK2256098 resulted in greater inhibition of pFAKY397 in PTEN-mutated (Ishikawa) than in PTEN-wild type (Hec1A) cells. Ishikawa cells were more sensitive to GSK2256098 than the treated Hec1A cells. Ishikawa cells were transfected with a wild-type PTEN construct and pFAKY397 expression was unchanged after treatment with GSK2256098. Decreased cell viability and enhanced sensitivity to chemotherapy (paclitaxel and topotecan) in combination with GSK2256098 was observed in Ishikawa cells as compared to Hec1a cells. In the Ishikawa orthoptopic murine model, treatment with GSK2256098 resulted in lower tumor weights and fewer metastases than mice inoculated with Hec1A cells. Tumors treated with GSK2256098 had lower microvessel density (CD31), less cellular proliferation (Ki67), and higher apoptosis (TUNEL) rates in the Ishikawa model when compared to the Hec1a model. From a large cohort of evaluable patients, increased FAK and pFAKY397 expression levels were significantly related to poor overall survival. Moreover, PTEN levels were inversely related to pFAKY397 expression. These preclinical data demonstrate that PTEN-mutated uterine cancer responds better to treatment FAK inhibition than does PTEN wild-type cancer. Therefore, PTEN could be a biomarker for predicting response to FAK-targeted therapy during clinical development.
Keywords: Focal adhesion kinase, PTEN, Uterine cancer
Introduction
Uterine cancer is the most common gynecological cancer in the United States (1) and the sixth most common cancer in women worldwide (2). In 2014, an estimated 52,630 new cases of uterine cancer were diagnosed in the United States, and 8,590 patients died of their disease. Five-year survival rates in patients with regional and distant metastasis remain poor, at 67% and 17%, respectively (1). Advanced and recurrent uterine cancers are resistant to traditional chemotherapeutic regimens, pointing to an urgent need for novel drugs and treatment strategies (3). Development of targeted biological therapies is essential to control of locally advanced, metastatic, and recurrent uterine cancer.
Focal adhesion kinase (FAK) is a non-receptor protein tyrosine kinase that is overexpressed in many types of human cancer cells, portending a poor prognosis and decreased survival duration. Previous studies demonstrated a role for FAK in tumor angiogenesis, migration, invasion, and metastasis (4-6). Overexpression and increased phosphorylation of FAK is known to confer a more malignant phenotype of uterine cancer (7). Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) is a lipid phosphatase tumor suppressor gene. Its loss of function by multiple mechanisms is considered to be a causal event in the development of uterine cancer (8-12). Somatic PTEN mutations have been identified in 34-55% of endometrial cancers, (11, 13) and the majority of these are frameshift or point mutations. The loss of PTEN can result from mutations, abnormalities in transcription, regulation of microRNAs by PTEN pseudogenes, or other epigenetic changes. PTEN is known to negatively regulate FAK (14, 15). Mutated PTEN and overexpression of FAK can lead to an uncontrolled PI3K-AKT signaling pathway, which can affect downstream signaling and compromise mismatch repair processes. These DNA mismatch repair abnormalities are common features seen in type 1 uterine cancers (16-18). It is not known whether the interaction between FAK and PTEN has an impact on response to FAK-targeted drugs.
Small molecules that target FAKY397 are attractive for uterine and other cancers. GSK2256098 (GlaxoSmithKline, Collegeville, PA, USA) is a small molecule, ATP competitive, reversible inhibitor that targets FAK activity and Y397 phosphorylation. It is high selective for FAK and is much more selective for FAK than Pyk2, the nearest FAK family member (19, 20). Here, we examined the biological effects of GSK2256098 in orthotopic murine models of uterine cancer and the role of PTEN as a predictor of response.
Materials and Methods
Cell lines and culture conditions
PTEN-mutant (Ishikawa) and PTEN-wild type (Hec1A) uterine cancer cell lines were maintained and propagated in Dulbecco’s modified Eagle’s medium/F-12 medium (50/50; Ishikawa) and McCoy’s 5A medium (Hec1A) supplemented with 10% fetal bovine serum and 1% gentamicin (Life Technologies) at 37°C. Cell lines were obtained from the institutional Cell Line Core laboratory within one year of the work described, and per institutional policy (MD Anderson policy ACA#1044) cell line authentication was performed at least once per year. In this case, authentication was performed within six months of the work described. Authentication was performed by the short tandem repeat method using the Promega Power Plex 16HS kit (Promega). Somatic mutations were detected using a Sequenom MALDI TOF MassArray system (Sequenom). Mycoplasma detection was performed using the MycoAlert Kit (Lonza), and all in vitro experiments were conducted with 60-80% confluent cultures. For all animal experiments, cells were harvested using trypsin-EDTA, neutralized with FBS-containing media, washed, and resuspended to the appropriate cell number in Hanks’ balanced salt solution (HBSS; Gibco, Carlsbad, CA).
Cell viability assay
To test the sensitivity of Ishikawa and Hec1A cells to treatment, 2,000 cells per well were plated into a 96-well plate and allowed to adhere overnight. After 12 hours of serum deprivation, they were treated in triplicate with GSK2256098 at increasing concentrations (0.01-10 μmol/L) in medium without serum. After 24 hours of treatment, cell viability was assessed by adding 50 μL of 0.15% 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma) to each well. After two hours of incubation at 37°C, medium/3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide was removed, 200 μL dimethyl sulfoxide (Sigma) was added to each well, and the absorbance at 570 nm was recorded using a Falcon plate reader (Becton Dickinson Labware). The cell viability was determined by calculating the mean absorbance at 570 nm into a percentage from 100% of the untreated cells’ mean absorbance, as previously described (21). For combined GSK2256098-based treatment and chemotherapy, 1 μM GSK2256098 and a range of paclitaxel, cisplatin, or topotecan doses were tested.
Western blot analysis
Cultured cell lysates were prepared using a modified RIPA buffer including both a protease inhibitor and phosphatase inhibitor, and the protein concentrations were determined using a BCA Protein Assay Reagent kit (Pierce Biotechnology), as previously described (22, 23). The lysates were loaded and separated on 8% and 12% sodium dodecyl sulfate-polyacrylamide gels. The proteins were then transferred to nitrocellulose membranes using electrophoresis (Bio-Rad Laboratories) overnight, blocked with 5% milk, and incubated at 4°C with primary antibodies against total FAK, FAK phosphorylated at tyrosine 397 (pFAKY397; BD Biosciences), total AKT, AKT phosphorylated at serine 473 (pAktS473), and PTEN (Cell Signaling Technology) at dilutions of 1:1,000. After washing with Tris-buffered saline and Tween 20 three times for 10 minutes, the membranes were incubated with 1 μg/mL horseradish peroxidase (HRP)-conjugated horse anti-mouse IgG (Amersham) for total FAK and pFAKY397. The incubation was repeated for total AKT, pAKTS473, and PTEN. To confirm equal sample loading the blots were stripped and re-probed with an antibody specific for β-actin (0.1 μg/mL; Sigma).
PTEN re-expression in PTEN-wild type Ishikawa cells
For generation of stably transfected uterine cancer cell lines, a validated full-length wild-type PTEN plasmid (PLNCX-PTEN) and the empty vector pBABE-puro (PLNCX polylinker; negative control) were used. Twenty-four hours after transfection with PLNCX-PTEN or pBABE-puro, Ishikawa cells were selected in neomycin (InvivoGen; 600 mg/mL) for seven days to remove non-infected cells.
Orthotopic model of uterine cancer
Female 8- to 12-week-old athymic nude mice were purchased from the National Cancer Institute at Frederick Cancer Research and Development Center and housed under pathogen-free conditions. Animal care was provided in accordance with the guidelines of the American Association for Accreditation for Laboratory Animal Care and the U.S. Public Health Service Policy on Human Care and Use of Laboratory Animals, and all animal studies were approved and supervised by the MD Anderson Institutional Animal Care and Use Committee. All studies were conducted in accordance with the GSK Policy on the Care, Welfare and Treatment of Laboratory Animals and were reviewed by the Institutional Animal Care and Use Committee either at GSK or by the ethical review process at the institution where the work was performed. Before injection of uterine cancer cells, each mouse was anesthetized with an intraperitoneal injection of 200 μL of ketamine, and a 0.5-cm incision was made in the left lower flank to optimize exposure to the left uterine horn. The distal portion of the horn was then identified and pulled to the incision, and a 50-μL cell suspension was injected into the lumen of the uterine horn. The incision was then closed with staples. The mice were closely monitored during and following the injections.
For therapeutic experiments, 4 × 106 Ishikawa or Hec1A cells were inoculated into the uterine horn. Following tumor cell injection, the mice were randomized (n = 10 mice per group) according to the following groups: 1) 100 μL of a vehicle control (oral, daily); 2) 75 mg/kg GSK2256098 in 100 μL of vehicle (oral, daily); 3) 2.5 mg/kg paclitaxel in 200 μL of PBS (intraperitoneal, weekly); and 4) GSK2256098 and paclitaxel (doses and frequencies given above). Therapy was initiated 10-14 days after tumor injection. The mice were monitored for adverse effects and sacrificed using cervical dislocation four to six weeks after initiation of treatment. At the completion of each experiment, each mouse’s weight, aggregate tumor weight, location, and number of tumor nodules were recorded for each treatment group. Tumor samples were processed for further analysis via preservation in optimal cutting temperature medium (Miles, Inc.) for frozen section analysis as well as fixed in formalin for paraffin-embedded section analysis.
Immunohistochemistry
Immunohistochemical analyses of uterine tumor sections obtained from the mice were performed for CD31 and Ki67. After heating for 30 minutes at 55-60°C using a hot plate, slides were deparaffinized sequentially in xylene; 100%, 95%, and 80% ethanol; and PBS. Antigen retrieval was performed by heating the slides in HEIR (10× Diva [Biocare Medical] diluted 1:10 in distilled water) in a steamer for 45 minutes. CD31 staining was performed using frozen tissue sections. Slides were fixed in cold acetone, acetone with chloroform (1:1), and acetone for five minutes each. Antigen retrieval was not required. Endogenous peroxidase was blocked by adding 3% hydrogen peroxide in methanol for 12 minutes. After washing, nonspecific proteins were blocked using 5% normal horse serum and 1% normal goat serum in PBS for 20 minutes at room temperature. The slides were incubated with either an anti-human CD31 antibody (1:800, catalog #53370; BD Biosciences - Pharmingen) or an anti-Ki67 antibody (1:200, catalog #RB-90-43-P; Thermo Scientific) in 5% normal horse serum plus 1% normal goat serum in PBS (blocking solution) overnight at 4°C. After washing with PBS, the appropriate HRP-conjugated secondary antibody in blocking solution was added for one hour at room temperature. Slides were stained with DAB substrate (Phoenix Biotechnologies) and counterstained with Gill no. 3 hematoxylin (Sigma). To quantify microvessel density in tumor sections, microvessels in five randomly selected 0.159-mm2 fields in each section were counted at a magnification of ×200. A single microvessel was defined as a discrete cluster of at least three cells that stained positively for CD31. Ki67 positivity was similarly quantified in five randomly selected fields at ×200 magnification. The percentage of Ki67-positive cells was calculated by dividing the number of cells with Ki67-positive nuclei by the total number of cells and then multiplying the quotient by 100 (24).
Immunohistochemical analysis using human tissue microarrays
Formalin-fixed, paraffin-embedded sections of human uterine cancer samples obtained from 91 patients were stained for total FAK and pFAKY397(25, 26). The human biological samples were sourced ethically and their research use was in accord with the terms of the informed consents. Formalin-fixed paraffin sections were deparaffinized in graded xylene as previously described. Antigen retrieval was performed for total FAK staining in target retrieval solution (DAKO Cytomation) using a steamer for 40 minutes, followed by cooling for 30 minutes. Antigen retrieval was not performed for pFAKY397 staining to maintain low background. All slides were washed in PBS; endogenous peroxidase activity was blocked by incubation in 3% hydrogen peroxide in methanol then blocked using 5% normal horse serum in PBS. Primary antibodies were applied at a dilution of 1:50 and incubation occurred overnight. Secondary visualization was achieved using the MACH 4 polymer-linked HRP system (Biocare Medical). A mouse probe was applied for 20 minutes, and then the slides were washed in PBS. A rabbit-HRP was applied for 20 minutes followed by washing in PBS. Room temperature DAB was applied and staining was monitored visually under a bright-field microscope for three to five minutes. The slides were then washed three times in distilled water. Counterstaining was performed using Gill no. 3 hematoxylin for 20 seconds followed by washing in PBS. Slides were dried and mounted using Universal mount and then scored by a board-certified pathologist.
Clinical samples were scored for staining with the pFAKY397 or FAK antibody by a board certified gynecologic pathologist blinded to the clinical outcome of the patients (RAF). pFAKY397 and FAK expression was determined semi-quantitatively by assessing the distribution of the positive cells and the staining intensity in the tumor cells. The distribution of positive cells was rated as follows: 0 points, no staining; 1 point, focal or less than 25%; 2 points, 25%–50%, 3 points, 50%–75%; 4 points, 75%–100%. The staining intensity was rated as focal or weak (1 point), moderate (2 points), or heavy (3 points). Points for intensity and distribution were added, and an overall score ranging from 0 to 2 was assigned. An overall score of 0 was assigned for negative expression of pFAKY397 and FAK if 5% or fewer cells were stained, regardless of the intensity. An overall score of 1 (1–4 points) was designated for weak expression of pFAKY397 and FAK, and an overall score of 2 (5–7 points) was designated for pFAKY397 and FAK overexpression (27).
Immunofluorescence staining
Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) staining of fresh frozen tumor sections was performed using a Promega kit. Four percent paraformaldehyde in PBS was added to slides for 20 minutes at room temperature. After washing in PBS, 0.2% Triton X-100 was added for 15 minutes. Equilibration buffer was added for ten minutes before the addition of TUNEL incubation buffer for one hour at 37°C. The slides were then washed in 2X saline-sodium citrate buffer for 15 minutes. Counterstaining of the sections was performed with Hoechst (1:10,000) for ten minutes. Slides were mounted with propyl gallate and glass cover slips. To quantify the apoptotic cells in the sections, the number of cells with TUNEL-positive nuclei was divided by the total number of cells in five randomly selected fields (×200 magnification), and the quotient was multiplied by 100 and reported as the percent apoptotic (TUNEL-positive) cells.
Statistical analysis
Differences in continuous variables were analyzed using the Student t-test or analysis of variance where appropriate. Two-tailed P values less than 0.05 were considered statistically significant. The SPSS software program (IBM Corporation) was used for all statistical analyses.
Results
Effects of GSK2256098 on FAK and Akt phosphorylation in endometrial cancer cells
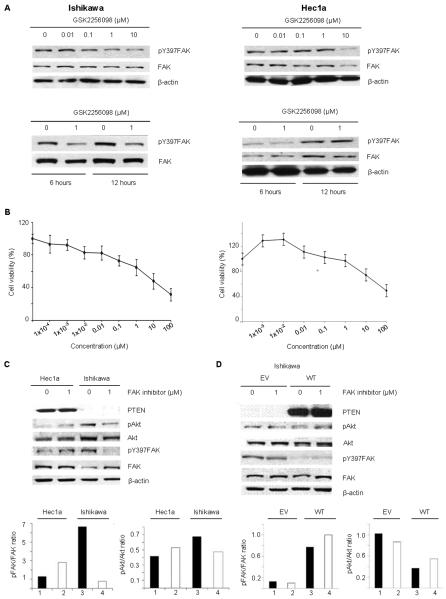
We first tested the effects of treatment with GSK2256098 on inhibition of FAK phosphorylation in PTEN-mutated (Ishikawa) and PTEN-wild type (Hec1a) at increasing concentrations (Fig. 1A). Following treatment with GSK2256098 for 1 hour, there was a substantial decrease in pFAKY397, which was not observed in the Hec1A cells at six or twelve hours. The sensitivity of both cell lines to GSK2256098 was assessed by MTT, and Ishikawa cells had a lower IC50 than Hec1a (Fig. 1B). Western blot and densitometric analysis demonstrated that Ishikawa cells had higher pAKT and pFAKY397 protein expression levels compared to the Hec1A cells (Fig. 1C). To address the role of a PTEN mutation on sensitivity to GSK2256098, we transfected the Ishikawa cells with wild-type PTEN or an empty vector. We did not observe a decrease in pAKT or pFAKY397 expression in the wild-type PTEN-expressing Ishikawa cells after GSK2256098 treatment (Fig. 1D). Cells transfected with the empty vector responded to the treatment in the same manner as non-transfected cells.
Figure 1. The effects of GSK2256098 on inhibition of FAK phosphorylation and endometrial cancer cell viability.
(A) Western blot analysis of Ishikawa and Hec1A cells treated with GSK2256098 at different concentrations, and at 1 μM for 6 and 24 hours. (B) Cell viability of Ishikawa and Hec1a cells after treatment with increasing concentrations of GSK2256098. (C) Western blot and densitometric analysis of Hec1A and Ishikawa cells treated with 1 μM GSK2256098. The Hec1a cells exhibited no decrease in pAkt or pFAKY397 expression 6 hours after treatment with 1 μM GSK2256098. (D) Western blot and densitometric analysis of re-expression of PTEN in Ishikawa cells transfected with PTEN constructs (empty vector [EV] or wild-type PTEN [WT]).
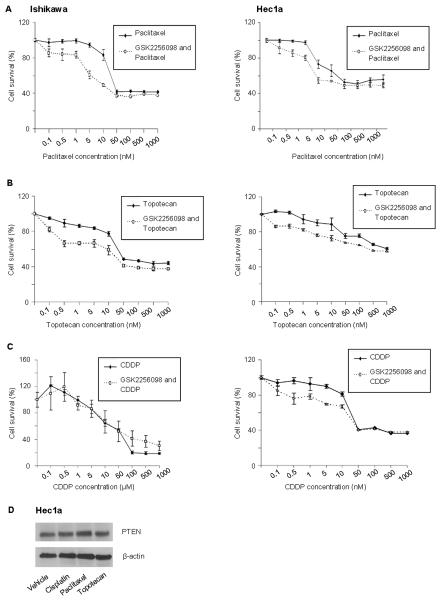
We examined the effects of GSK2256098 in combination with various chemotherapy drugs commonly used in the adjuvant and recurrent setting for uterine cancer treatment. Ishikawa cells were more sensitive to combined treatment with GSK2256098 and paclitaxel (Fig. 2A) and topotecan (Fig. 2B) after 72 hours of treatment than were Hec1A cells. The responses of these two cell lines to treatment with GSK2256098 and cisplatin were similar (Fig. 2C). To determine whether chemotherapy affects PTEN expression, we treated Hec1a cells with paclitaxel, cisplatin, and topotecan and observed no change in PTEN expression (Fig. 2D).
Figure 2. In vitro effects of treatment with GSK2256098 combined with chemotherapy.
Cell viability was assessed for Ishikawa and Hec1a cells after treatment with 1μM of GSK2256098 and paclitaxel (A) for 72 hours, topotecan (B) for 72 hours, and cisplatin (CDDP) (C) for 96 hours at different concentrations. (D) Western blot analysis of expression in Hec1a cells following treatment with paclitaxel, cisplatin or topotecan.
Effects of GSK2256098 in the orthotopic uterine cancer models
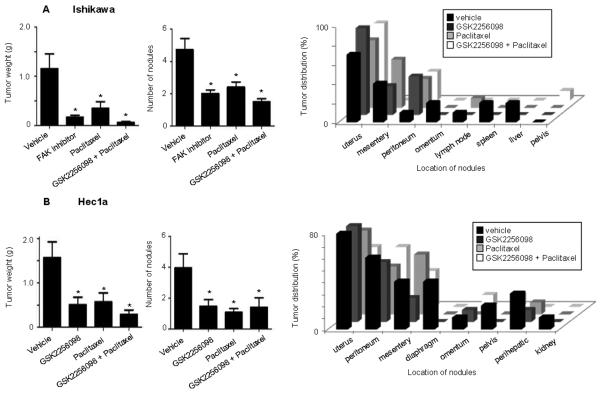
To examine the effect of GSK2256098 in pre-clinical models of uterine cancer, we used orthotopic mouse models of uterine cancer. In this experiment, mice were inoculated with Ishikawa or Hec1a cells. In the Ishikawa model, tumor growth was inhibited to a greater extent in the GSK2256098 monotherapy group (Fig. 3A) as compared to the Hec1A model (Fig. 3B). Paclitaxel combined with GSK2256098 further reduced tumor growth and number of tumor nodules in all models. Extent of distant metastases was also substantially reduced with GSK2256098-based therapy. There was no significant difference between the mean mouse weights in any of the treatment groups in the Ishikawa and Hec1a models (Supplementary Fig. 1A-B).
Figure 3. Effects of treatment with GSK2256098 on uterine tumor growth in vivo.
Mice inoculated with (A) Ishikawa and (B) Hec1A cells received a vehicle (control), GSK2256098, paclitaxel, or a combination of GSK2256098 and paclitaxel beginning ten days after inoculation. Animals were sacrificed four to five weeks after the initiation of therapy. Tumors were harvested and weighed. The mean number of tumor nodules and sites of metastases were recorded. Error bars represent the standard deviation. *P < 0.05 versus control.
Biological effects of GSK2256098 therapy
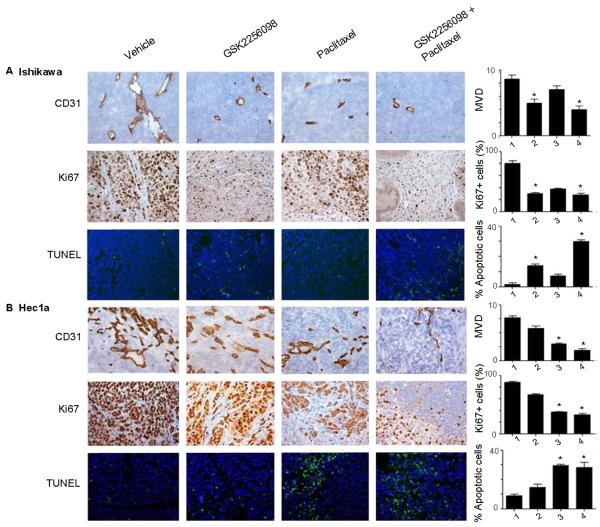
FAK is well-known to play an important role in angiogenesis, proliferation, and apoptosis, so we examined the tumor samples harvested from the in vivo therapy experiments. Evaluating CD31, we observed significantly lower microvessel densities in tumors from mice treated with GSK2256098 and paclitaxel than in tumors from mice in the vehicle control group (P < 0.05; Fig. 4A-B). This was consistent across both models, but Ishikawa tumors had the lowest microvessel density. All tumor models in mice treated with GSK2256098 exhibited less proliferation via Ki67 than control. Ishikawa tumors had the lowest Ki67 expression in response to therapy. Ishikawa tumors had higher apoptotic indices than Hec1A tumors after treatment with GSK2256098. Significant rates of apoptosis were seen in all models that had been treated with combination GSK2256098 and paclitaxel.
Figure 4. Effect of GSK2256098 in vivo on angiogenesis, proliferation, and apoptosis in uterine tumors.
(A) Ishikawa and (B) Hec1A tumors collected at the conclusion of in vivo therapeutic experiments were subjected to CD31, Ki67, and TUNEL staining. Representative sections (final magnification, ×200) are shown for the four treatment groups (vehicle control, GSK2256098, paclitaxel, and a combination of GSK2256098 and paclitaxel). The average number of CD31-positive vessels per field, mean percentage of Ki67-positive cells (proliferative index), and mean percentage of TUNEL fluorescence-positive cells (percentage of apoptotic cells) are shown in the adjoining graphs. Five fields per slide and at least five slides per treatment group were examined and compared using the Student t-test and analysis of variance. *P < 0.05 versus control.
Expression of total FAK and pFAKY397 in human endometrial tumors
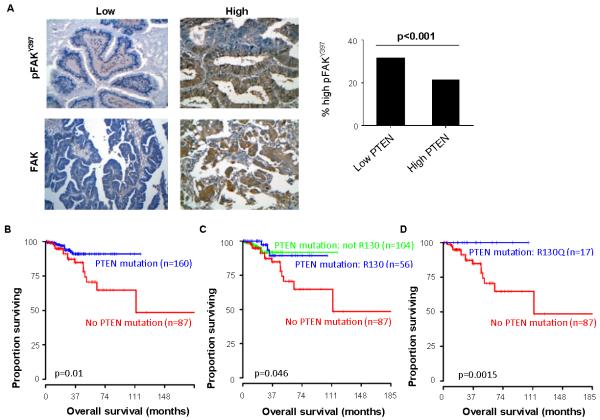
To address whether FAK, pFAKY397, and PTEN expression correlates with survival in human cancers, we examined a cohort of 202 human uterine tumor samples. Representative images of these tumors low or high expression of FAK and pFAKY397 are shown in Fig. 5A. Since PTEN is known to negatively regulate FAK, we sought to determine if there was an association within patient samples. Amongst the samples with high PTEN expression, 43.8% had elevated pFAKY397 (Fig. 5A). Using the Cancer Genome Atlas (TCGA) data, we first evaluated the effect of any PTEN mutation on survival in patients with uterine carcinoma. Patients whose tumors had no PTEN mutations (n=87) had worse overall survival (p=0.01; Fig. 5B). To further evaluate what type of mutation portended a worse overall survival in this cohort, mutations were subclassified into R130 mutations (n=56), a common loss-of-function mutation, and to non-R130 mutations. Patients without a PTEN mutation in their tumor had worse overall survival (p=0.046; Fig. 5C). Another analysis evaluating R130Q (n=17) revealed that this subset of patients had no deaths in the follow-up period (p=0.0015) (Fig. 5D).
Figure 5. Immunohistochemical analysis of total FAK and pFAKY397 expression in human uterine tumor samples.
(A) Representative images of human uterine cancer samples with low or high expression of FAK and pFAKY397. Original magnification, ×250. Percentage of ovarian cancers with high pFAKY397 expression based on tumor PTEN expression. (B) Kaplan-Meier curve of overall survival for patients with uterine carcinoma with and without a PTEN mutation, (C) with a PTEN R130 mutation, PTEN mutation that was not R130, and PTEN mutation, and (D) PTEN R130Q mutation and no PTEN mutation. The log-rank test (two-sided) was used to compare differences between groups.
Discussion
The key findings from our manuscript are that FAK inhibition is most effective in treatment of uterine cancer with a PTEN mutation, suggesting that PTEN is a potential biomarker for predicting a tumor’s response to this treatment. We also demonstrated overexpression of FAK and pFAKY397 in human uterine cancer cells, which is correlated with worse outcomes and poor overall survival.
PTEN is well known to be mutated at high frequencies in human uterine cancer (12, 28). Studies of PTEN expression in a variety of solid malignancies including breast, gastric, esophageal, and uterine cancers and gliobastoma multiforme have concluded that reduced or loss of PTEN has been associated with a poor prognosis and decreased overall survival (29-34). Loss of PTEN function frequently occurs early in type 1 uterine cancer tumorigenesis (12). Other frequently mutated genes in type 1 tumors include FGFR2, ARID1A, CTNNB1, PIK3CA, PIK3R1 and KRAS (35-37). TP53, PIK3CA and PPP2R1A mutations are frequently found in type 2 uterine cancer tumors (38, 39).
PTEN, is a well-characterized tumor suppressor and phosphatase involved in the regulation of the PI3K/AKT signaling pathway (35, 40). PTEN also dephosphorylates FAK, and loss of PTEN function therefore results in a net increase in pFAK expression. PTEN mutations associated with a loss-of-function include R130Q mutations (41). This variant results in decreased phosphatase activity of PTEN and is a common mutation identified within uterine cancer (42). Our findings corroborate that pFAKY397 expression is higher in PTEN-mutant uterine cancer cells than in PTEN wild-type. Treatment with the FAK inhibitor, GSK2256098, prevented phosphorylation of FAK at Y397 in PTEN-mutated uterine cancer cell lines, whereas pFAKY397 expression was not affected in PTEN wild-type uterine cancer cells. After treatment, decreased pFAKY397 expression also correlated with downstream decreases in pAkt expression in a PTEN-mutated cell line. While it is biologically plausible that inhibition of FAK would be most active in PTEN-mutated tumors, translational data are lacking. PTEN is an important candidate biomarker for testing in clinical trials with FAK-targeted drugs.
A number of small molecule FAK inhibitors are under current development as targeted therapies and have been shown in vivo to prevent tumor growth, metastases, vascular permeability, and angiogenesis (43). Previous literature supports increased expression of FAK in uterine cancer to be correlated with higher tumor grade, lymphatic vascular space invasion, and vascular space invasion (10). Our findings related to enhanced anti-tumor activity in combination with traditional adjuvant chemotherapeutics such as paclitaxel and topotecan are also supported by prior studies (44, 45). Additionally, we identified PTEN as a potential predictive biomarker in patients with uterine cancer. Such personalized approaches could allow for rational selection of patients most likely to benefit from anti-FAK therapy.
Conclusion
Our preclinical data demonstrate that GSK2256098 may be therapeutically beneficial to patients with PTEN-mutant uterine cancer, and PTEN represents a potential predictive biomarker.
Supplementary Material
Acknowledgments
We thank Donna Reynolds for assistance with immunohistochemical studies. GlaxoSmithKline provided GSK2256098 for all in vitro and in vivo studies.
Financial Support: Financial support was provided in part by grants from the National Institutes of Health (P50 CA098258, CA 109298, P50 CA083639, U54 CA151668, M. D. Anderson’s Cancer Center Support Grant CA016672), the Ovarian Cancer Research Fund, Inc. (Program Project Development Grant), the U.S. Department of Defense (OC073399, OC093146), the Ann Rife Cox Chair in Gynecology, the Zarrow Foundation, the Marcus Foundation, the Betty Anne Asche Murray Distinguished Professorship, the RGK Foundation, and the Gilder Foundation to A.K. Sood. R.A. Previs, J.M. Hansen, and H.J. Dalton are supported by an NCI-DHHS-NIH T32 Training Grant (T32 CA101642).
Footnotes
Disclosure of Potential Conflicts of Interest: The authors declare that there are no conflicts of interest.
References
- 1.American Cancer Society . Cancer Facts & Figures. 2010. [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Dizon DS. Treatment options for advanced endometrial carcinoma. Gynecol Oncol. 2010;117:373–81. doi: 10.1016/j.ygyno.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–56. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 5.Siesser PM, Hanks SK. The signaling and biological implications of FAK overexpression in cancer. Clin Cancer Res. 2006;12:3233–7. doi: 10.1158/1078-0432.CCR-06-0456. [DOI] [PubMed] [Google Scholar]
- 6.Sood AK, Coffin JE, Schneider GB, Fletcher MS, DeYoung BR, Gruman LM, et al. Biological significance of focal adhesion kinase in ovarian cancer: role in migration and invasion. Am J Pathol. 2004;165:1087–95. doi: 10.1016/S0002-9440(10)63370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canel M, Serrels A, Miller D, Timpson P, Serrels B, Frame MC, et al. Quantitative in vivo imaging of the effects of inhibiting integrin signaling via Src and FAK on cancer cell movement: effects on E-cadherin dynamics. Cancer Res. 2010;70:9413–22. doi: 10.1158/0008-5472.CAN-10-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samarnthai N, Hall K, Yeh IT. Molecular profiling of endometrial malignancies. Obstet Gynecol Int. 2010;2010:162363. doi: 10.1155/2010/162363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oda K, Stokoe D, Taketani Y, McCormick F. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res. 2005;65:10669–73. doi: 10.1158/0008-5472.CAN-05-2620. [DOI] [PubMed] [Google Scholar]
- 10.Gabriel B, Hasenburg A, Waizenegger M, Orlowska-Volk M, Stickeler E, zur Hausen A. Expression of focal adhesion kinase in patients with endometrial cancer: a clinicopathologic study. Int J Gynecol Cancer. 2009;19:1221–5. doi: 10.1111/IGC.0b013e3181b33c61. [DOI] [PubMed] [Google Scholar]
- 11.Kong D, Suzuki A, Zou TT, Sakurada A, Kemp LW, Wakatsuki S, et al. PTEN1 is frequently mutated in primary endometrial carcinomas. Nat Genet. 1997;17:143–4. doi: 10.1038/ng1097-143. [DOI] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Research N. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Risinger JI, Hayes AK, Berchuck A, Barrett JC. PTEN/MMAC1 mutations in endometrial cancers. Cancer Res. 1997;57:4736–8. [PubMed] [Google Scholar]
- 14.Schwock J, Dhani N, Hedley DW. Targeting focal adhesion kinase signaling in tumor growth and metastasis. Expert Opin Ther Targets. 2010;14:77–94. doi: 10.1517/14728220903460340. [DOI] [PubMed] [Google Scholar]
- 15.Chu EC, Tarnawski AS. PTEN regulatory functions in tumor suppression and cell biology. Med Sci Monit. 2004;10:RA235–41. [PubMed] [Google Scholar]
- 16.Shi M, Zhang H, Li M, Xue J, Fu Y, Yan L, et al. Normal endometrial stromal cells regulate survival and apoptosis signaling through PI3K/AKT/Survivin pathway in endometrial adenocarcinoma cells in vitro. Gynecol Oncol. 2011;123:387–92. doi: 10.1016/j.ygyno.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Catasus L, D’Angelo E, Pons C, Espinosa I, Prat J. Expression profiling of 22 genes involved in the PI3K-AKT pathway identifies two subgroups of high-grade endometrial carcinomas with different molecular alterations. Mod Pathol. 2010;23:694–702. doi: 10.1038/modpathol.2010.44. [DOI] [PubMed] [Google Scholar]
- 18.Steinbakk A, Malpica A, Slewa A, Skaland I, Gudlaugsson E, Janssen EA, et al. Biomarkers and microsatellite instability analysis of curettings can predict the behavior of FIGO stage I endometrial endometrioid adenocarcinoma. Mod Pathol. 2011;24:1262–71. doi: 10.1038/modpathol.2011.75. [DOI] [PubMed] [Google Scholar]
- 19.Schultze A, Fiedler W. Clinical importance and potential use of small molecule inhibitors of focal adhesion kinase. Anticancer Agents Med Chem. 2011;11:593–9. doi: 10.2174/187152011796817727. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, He DH, Zajac-Kaye M, Hochwald SN. A small molecule FAK kinase inhibitor, GSK2256098, inhibits growth and survival of pancreatic ductal adenocarcinoma cells. Cell Cycle. 2014;13:3143–9. doi: 10.4161/15384101.2014.949550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Hu W, Bottsford-Miller J, Liu T, Han HD, Zand B, et al. Cross-talk between EphA2 and BRaf/CRaf is a key determinant of response to Dasatinib. Clin Cancer Res. 2014;20:1846–55. doi: 10.1158/1078-0432.CCR-13-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merritt WM, Kamat AA, Hwang JY, Bottsford-Miller J, Lu C, Lin YG, et al. Clinical and biological impact of EphA2 overexpression and angiogenesis in endometrial cancer. Cancer Biol Ther. 2010;10:1306–14. doi: 10.4161/cbt.10.12.13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu W, Liu T, Ivan C, Sun Y, Huang J, Mangala LS, et al. Notch3 Pathway Alterations in Ovarian Cancer. Cancer Res. 2014;74(12):3282–93. doi: 10.1158/0008-5472.CAN-13-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamat AA, Merritt WM, Coffey D, Lin YG, Patel PR, Broaddus R, et al. Clinical and biological significance of vascular endothelial growth factor in endometrial cancer. Clin Cancer Res. 2007;13:7487–95. doi: 10.1158/1078-0432.CCR-07-1017. [DOI] [PubMed] [Google Scholar]
- 25.Wu SY, Yang XB, Gharpure KM, Hatakeyama H, Eqli M, McGuire MH, et al. 2′-OMe-phosphorodithioate-modified siRNAs show increased loading into the RISC complex and enhanced anti-tumour activity. Nat Commun. 2014;5:3459. doi: 10.1038/ncomms4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pecot CV, Rupaimoole R, Yang D, Akbani R, Ivan C, Lu C, et al. Tumour angiogenesis regulation by the miR-200 family. Nat Commun. 2013;4:2427. doi: 10.1038/ncomms3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nick AM, Stone RL, Armaiz-Pena G, Ozpolat B, Tekederli I, Graybill WS, et al. Silencing of p130cas in ovarian carcinoma: a novel mechanism for tumor cell death. J Natl Cancer Inst. 2011;103:1596–612. doi: 10.1093/jnci/djr372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung LW, Hennessy BT, Li J, Yu S, Myers AP, Djordjevic B, et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 2011;1:170–85. doi: 10.1158/2159-8290.CD-11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Depowski PL, Rosenthal SI, Ross JS. Loss of expression of the PTEN gene protein product is associated with poor outcome in breast cancer. Mod Pathol. 2001;14:672–6. doi: 10.1038/modpathol.3880371. [DOI] [PubMed] [Google Scholar]
- 30.Sano T, Lin H, Chen X, Langford LA, Koul D, Bondy ML, et al. Differential expression of MMAC/PTEN in glioblastoma multiforme: relationship to localization and prognosis. Cancer Res. 1999;59:1820–4. [PubMed] [Google Scholar]
- 31.Lee HS, Lee HK, Kim HS, Yang HK, Kim WH. Tumour suppressor gene expression correlates with gastric cancer prognosis. J Pathol. 2003;200:39–46. doi: 10.1002/path.1288. [DOI] [PubMed] [Google Scholar]
- 32.Lee JI, Soria JC, Hassan KA, El-Naggar AK, Tang X, Liu DD, et al. Loss of PTEN expression as a prognostic marker for tongue cancer. Arch Otolaryngol Head Neck Surg. 2001;127:1441–5. doi: 10.1001/archotol.127.12.1441. [DOI] [PubMed] [Google Scholar]
- 33.Terakawa N, Kanamori Y, Yoshida S. Loss of PTEN expression followed by Akt phosphorylation is a poor prognostic factor for patients with endometrial cancer. Endocr Relat Cancer. 2003;10:203–8. doi: 10.1677/erc.0.0100203. [DOI] [PubMed] [Google Scholar]
- 34.Tachibana M, Shibakita M, Ohno S, Kinugasa S, Yoshimura H, Ueda S, et al. Expression and prognostic significance of PTEN product protein in patients with esophageal squamous cell carcinoma. Cancer. 2002;94:1955–60. doi: 10.1002/cncr.0678. [DOI] [PubMed] [Google Scholar]
- 35.McConechy MK, Ding J, Cheang MC, Wiegand KC, Senz J, Tone AA, et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol. 2012;228:20–30. doi: 10.1002/path.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byron SA, Gartside M, Powell MA, Wellens CL, Gao F, Mutch DG, et al. FGFR2 point mutations in 466 endometrioid endometrial tumors: relationship with MSI, KRAS, PIK3CA, CTNNB1 mutations and clinicopathological features. PloS One. 2012;7(2):e30801. doi: 10.1371/journal.pone.0030801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urick ME, Rudd ML, Godwin AK, Sgroi D, Merino M, Bell DW. Cancer Res. 2011;71:4061–7. doi: 10.1158/0008-5472.CAN-11-0549. PIK3R1 (p85alpha) is. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuhn E, Wu RC, Guan B, Wu G, Zhang J, Wang Y, et al. Identification of molecular pathway aberrations in uterine serous carcinoma by genome-wide analyses. J Natl Cancer Inst. 2012;104:1503–13. doi: 10.1093/jnci/djs345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Gallo M, O’Hara AJ, Rudd ML, Urick ME, Hansen NF, O’Neil NJ, et al. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat Genet. 2012;44:1310–5. doi: 10.1038/ng.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leslie NR, Downes CP. PTEN function: how normal cells control it and tumour cells lose it. Biochem J. 2004;382:1–11. doi: 10.1042/BJ20040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han SY, Kato H, Kato S, Suzuki T, Shibata H, Ishii S, et al. Functional evaluation of PTEN missense mutations using in vitro phosphoinositide phosphatase assay. Cancer Res. 2000;60:3147–51. [PubMed] [Google Scholar]
- 42.McConechy MK, Ding J, Senz J, Yang W, Melnyk N, Tone AA, et al. Ovarian and endometrial endometrioid carcinomas have distinct CTNNB1 and PTEN mutation profiles. Mod Pathol. 2014;27:128–34. doi: 10.1038/modpathol.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nature Rev Cancer. 2014;14:598–610. doi: 10.1038/nrc3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halder J, Lin YG, Merritt WM, Spannuth WA, Nick AM, Honda T, et al. Therapeutic efficacy of a novel focal adhesion kinase inhibitor TAE226 in ovarian carcinoma. Cancer Res. 2007;67:10976–83. doi: 10.1158/0008-5472.CAN-07-2667. [DOI] [PubMed] [Google Scholar]
- 45.Kang Y, Hu W, Ivan C, Dalton HJ, Miyake T, Pecot C, et al. Role of focal adhesion kinase in regulating YB-1-mediated paclitaxel resistance in ovarian cancer. J Natl Cancer Inst. 2013;105:1485–95. doi: 10.1093/jnci/djt210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.