Introduction
Although a dramatic improvement in outcome was initially observed for adults with acute lymphoblastic leukemia (ALL) after incorporation of active chemotherapy agents, a period of stagnation ensued owing to limitations encountered with further dose intensification of standard chemotherapeutics. Recently, significant advances have been achieved with the incorporation of targeted therapy agents such as tyrosine kinase inhibitors for Philadelphia (Ph) positive ALL. Targeting leukemia surface antigens with monoclonal antibodies is another promising strategy. Herein a comprehensive review of available data regarding the use of rituximab for the treatment of Burkitt-type leukemia/lymphoma and CD20 positive precursor B-cell ALL was performed.
Immunophenotypic classification of ALL
The prognostic relevance of immunological subtypes of ALL relates to their association with particular cytogenetic and molecular aberrancies. For example, pro-B cell ALL (universally CD10- CD20-) is associated with the adverse karyotype of t(4:11) which involves the mixed lineage leukemia (MLL) proto-oncogene [1]. The subclassification of ALL by flow cytometric immunophenotyping not only characterizes the disease, but also delineates potential therapeutic interventions by detecting surface antigens such as CD19, CD20, CD22, CD33 or CD52, which can be targeted by specific monoclonal antibodies (MoAbs) [2]. The CD20 molecule in particular is a B-lineage specific antigen expressed on both normal and malignant cells during nearly all stages of B-cell differentiation (except for hematopoeitic stem cells and plasma cells). Heterogeneity in the expression of CD20 among various B-cell malignancies has been well described [3]. For example, CD20 expression (defined as ≥ 20% of leukemia cells positive) ranges from 40% to 50% in precursor B-cell ALL compared with 80% to 90% in mature B-cell or Burkitt-type leukemia/lymphoma.
Prognostic significance of CD20 expression in precursor B-cell ALL
The CD20 molecule is a 33 to 37 kDa nonglycosylated hydrophobic transmembrane phosphoprotein that forms tetramers. The gene for CD20 has been mapped to chromosome 11 at position q12-13, centromeric to the Bcl-1 locus, which is involved in the translocation t(11;14)(q13;q32) [4]. The CD20 antigen is not internalized or secreted upon antibody binding, although circulating CD20 (bound to other proteins, cell membrane fragments, or large membrane complexes presumably after cells have undergone apoptosis) has been detected in the plasma of patients with B-cell malignancies [5]. CD20 functions as a calcium channel which ultimately influences cell-cycle progression and differentiation via downstream signaling pathways. The modulations of surface CD20 and the resulting alterations in intracellular Ca++ metabolism affect apoptosis pathways and levels of the proapoptotic proteins sarco/endoplasmic reticulum Ca(2+) (SERCA3) and Bax/Bak [6]. The constitutive activation of survival pathways involving nuclear factor-κB (NF-κB) and extracellular receptor kinase (ERK1/2) induced by CD20 results in the overexpression of the anti-apoptotic protein Bcl-2 and associated Bcl-2 genes [7]. The hypothesis generated from these observations is that CD20 expression confers increased drug resistance via these mechanisms, resulting in the persistence of leukemia subclones which eventually resurge and lead to recurrence of the disease.
The prognostic relevance of CD20 expression in de novo childhood precursor B-cell ALL has been investigated with conflicting results. CD20 expression was evaluated both by the traditional arbitrary cut point of 20% and by mean fluorescence intensity (MFI) in 1231 children treated with risk-adapted Pediatric Oncology Group (POG) protocols [8]. Absolute CD20 expression and increasing MFI of CD20 were both independently associated with a significantly inferior event-free survival (EFS) irrespective of other known prognostic factors such as age and karyotypic aberrancies. Jeha et al [9] retrospectively studied the influence of CD20 expression on outcome in 359 children with de novo precursor B-cell ALL treated on sequential St. Jude Total Therapy protocols. The overall incidence of CD20 expression was 48%, but decreased in frequency at the extremes of the age spectrum (younger than 1 year and older than 10 years). In contrast to the POG experience, CD20 expression was associated with a slightly more favorable prognosis (5-year EFS rate of 84% ± 2.9% versus 78% ± 3.1%, p=0.08; 5-year overall survival [OS] rate of 88% ± 2.5% versus 83% ± 2.8%, p=0.13). It was postulated that the disparate results could be accounted for by differences in the intensity of chemotherapy and/or the application of risk-adapted strategies between the POG and St. Jude chemotherapy regimens.
The significance of CD20 expression was then evaluated in 253 adolescents and adults with de novo precursor B-cell ALL treated in the pre-rituximab era with one of two sequential chemotherapy regimens of increasing intensity (VAD/CVAD [vincristine, doxorubicin, and dexamethasone/cyclophosphamide and VAD] or hyper-CVAD [fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high dose methotrexate and cytarabine]) [10]. Forty-seven percent of the cases were CD20 positive as defined by the traditional cut point of 20%. There were no significant associations between CD20 expression and the standard prognostic factors. Complete remission (CR) rates were similar within the regimens regardless of CD20 status (positive versus negative). However, CD20 expression was associated with a higher relapse rate (71% versus 53% for VAD/CVAD, p=.08; 61% versus 37% for hyper-CVAD, p=.005), lower 3-year CR duration (CRD) rates (22% versus 58% for hyper-CVAD, p=<.001), and lower 3-year overall OS rates (27% versus 60% for hyper-CVAD, p=.003). The negative influence of CD20 expression on outcome appeared to be most pronounced in the younger subsets (e.g., 3-year OS rates for hyper-CVAD if age 30 years or younger 35% versus 85%, p=.009; if age 31 – 59 years 28% versus 54%, p=.04). The elderly subgroup (age 60 years or older) did poorly irrespective of CD20 status; 3-year OS rates were 20% for hyper-CVAD.
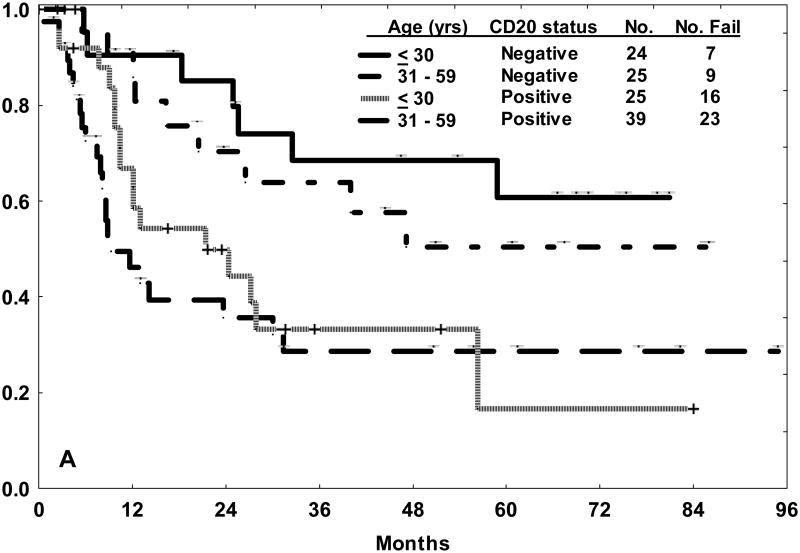
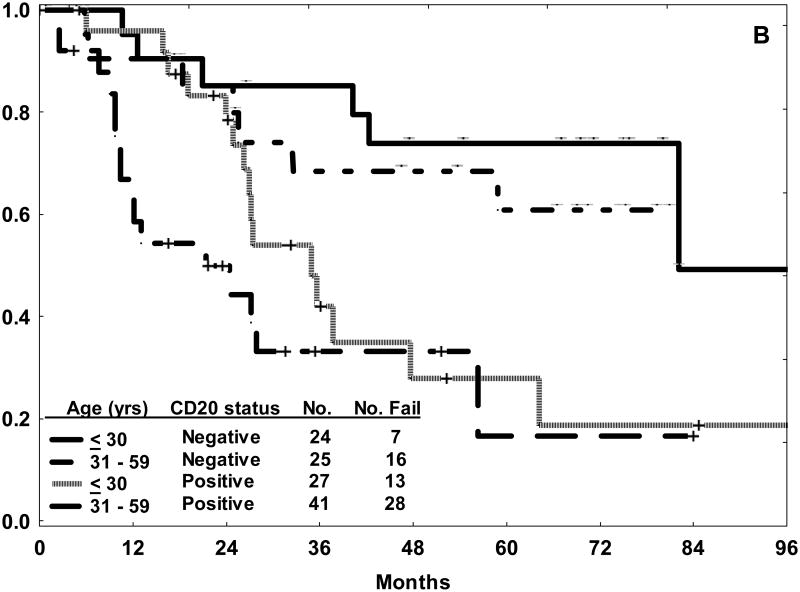
In this study, multivariate analysis for EFS identified older age, leukocyte count ≥ 30 × 109/L, presence of the Philadelphia chromosome, high systemic risk classification, and CD20 expression as independent adverse factors. Alternative cut points of CD20 expression such as 10% or 30% were also examined for prognostic relevance. The rate of CD20 expression increased from 47% to 67% with use of the 10% demarcation for CD20 positivity. The negative influence of CD20 expression on outcome (with to respect to CRD and OS) within each of the age subsets was nearly identical to that observed with the traditional cut point of 20% (Figure 1). There was no correlation between increasing levels of CD20 expression and outcome, rather the influence of CD20 expression appeared to be an “all or nothing phenomenon” [10].
Figure 1.
Outcome for precursor B-cell ALL treated with hyper-CVAD in the pre-rituximab era by age category and CD20 status (positive or negative) using the 10% demarcation of expression. The 3-year rates for CRD (A) and OS (B) were nearly identical to those observed with the traditional cut point of 20% for CD20 expression.10
Another retrospective analysis of CD20 expression in 143 adolescents and adults with de novo precursor B-cell ALL treated with the pediatric-inspired Group for Research in Adult Acute Lymphoblastic Leukemia (GRAALL)-2003 regimen identified a higher cumulative incidence of relapse (39% [95% CI, 25 – 55] versus 20% [95% CI, 13 – 31], p=0.02) in the CD20 positive subset, although this did not translate into a difference in disease-free survival (DFS) [11,12]. It also was noted that the negative prognostic influence of elevated leukocyte count or corticosteroid resistance on outcome was observed only in the CD20 positive subset, suggesting intrinsic resistance of the CD20 positive lymphoblasts to the dose intensification of alkylating agents applied in these scenarios.
Upregulation of CD20 expression in precursor B-cell ALL
The modulation of surface antigens on viable lymphoblasts during the induction phase of chemotherapy has been noted by several investigators. Serial analyses of surface leukemia antigen expression were performed on bone marrow (BM) samples collected from children with de novo precursor B-cell ALL during the induction phase of chemotherapy administered per the Italian Association of Pediatric Hematology and Oncology/Berlin-Frankfurt-Munster (AIEOP-BFM) ALL 2000 protocol. The quantitative surface expressions of CD10 and CD34 were down modulated, whereas the expressions of CD19, CD20, CD45RA and CD11a were upregulated [13]. These findings were attributed to glucocorticoid effects since the modulations were detected as early as a few days after initiation of the prednisone phase of therapy [14].
The modulation of CD20 on lymphoblasts in BM and peripheral blood (PB) samples was evaluated further during the induction phase of therapy (AIEOP-BFM ALL 2000 protocol) for 159 children with precursor B-cell ALL.[15] Expression of CD20 (using the traditional cut point of 20%) at diagnosis was noted in 46% and 51% of BM and PB samples, respectively. There was a good correlation in expression levels between paired BM and PB samples, although significant variance (mean 13% ± 17%) was observed. There was no significant correlation between CD20 expression at diagnosis and age, BFM risk group, specific immunophenotype, or relapse. Subsequent specimens including PB samples collected on day 8 and BM samples collected on day 15 of the induction chemotherapy showed significantly increased levels of CD20 by expression and MFI. Notably, individual patient samples exhibited a significant increase in the proportion of CD20 positive lymphoblasts even if CD20 expression had been negative (by definition) at diagnosis, suggesting that therapy with anti-CD20 MoAbs may be applicable even in the CD20 negative subset. The one exception was the favorable category of TEL-AML1-rearranged cases in which the low baseline CD20 expression did not change significantly during induction chemotherapy. In contrast, samples from the high BFM risk group exhibited very high levels of CD20 expression (80% or more) at essentially all collection time points.
In this study, the upregulation of CD20 to a MFI of at least 50 (equivalent to CD20 expression of 80%) translated to a more efficient cytolysis of the lymphoblasts after in vitro exposure to rituximab [15]. In the cases positive for minimal residual disease (MRD, defined as ≥ 0.1% lymphoblasts) at end-induction (day 33), CD20 expression at least 90% (or at least 80%) was observed in 52% (67%), as opposed to 5% (8%) at the time of diagnosis and 20% (25%) on day 15 of therapy. These findings certainly suggest that MoAb therapy directed against CD20 should be further investigated as a therapeutic intervention to eradicate residual disease.
Upregulation of surface CD20 expression via pharmacological maneuvers may improve the efficacy of MoAb therapy. Venugopal and colleaguesdemonstrated an increase in surface CD20 expression on chronic lymphocytic leukemia (CLL) cells in vitro after exposure to the cytokines interleukin-4, granulocyte-macrophage colony-stimulating factor (GM-CSF), or tumor necrosis factor-α [16]. However, neither upregulation of CD20 nor an increase in the proportion of cells expressing CD20 were observed in patients with CLL who underwent PB sampling at various time points within a 24 hour span after a single dose of GM-CSF [17]. Despite these findings, GM-CSF has still been utilized as an adjunct to therapy with rituximab for CLL and indolent B-cell lymphomas owing to its enhancement of antibody-dependent cellular cytotoxicity (ADCC) and augmentation of innate immunity against malignant cells [18,19]. Whether cytokines truly modulate CD20 expression remains to be elucidated. In vitro exposure of B-lineage non-Hodgkin lymphoma (NHL) cells to hypomethylating agents or bryostatin-1 (a modulator of the protein kinase C [PKC] pathway) has been shown to increase surface CD20 expression via processes detailed further in the section on mechanisms of resistance to rituximab [20,21].
Principles of monoclonal antibody therapy with rituximab
The activity of the chimeric MoAb rituximab (Rituxan®, Genentech, South San Francisco, CA, USA) is generally mediated by modulation of the CD20 receptor via induction of ADCC, complement-dependent cytotoxicity (CDCC), and/or direct apoptosis [22]. Rituximab is an IgG1 immunoglobulin that contains murine variable region sequences and human constant κ and Fc region sequences. Rituximab recognizes a discontinuous epitope composed of the amino acids 170ANPS173 (alanine, asparagine, proline, serine) within the small extracellular domain of CD20 [23]. Rituximab received approval by the Food and Drug Administration (FDA) in 1997 for the treatment of relapsed or refractory CD20 positive low-grade or follicular B-cell NHL based on its single agent activity in the pivotal trials [24-26]. It was the first MoAb approved for the therapeutic treatment of malignancy.
Therapy with MoAbs directed against lymphoma or leukemia surface antigens is an attractive targeted treatment approach since it is subtype specific. When compared with chemotherapeutics, MoAbs generally effect cytolysis by different or complementary mechanisms, and therefore may have efficacy against malignant clones which are resistant to cytostatic drugs. The incorporation of MoAb into therapeutic regimens may therefore be particularly beneficial in instances where further intensification of chemotherapy is impossible, particularly when there is minimal overlapping toxicity. The clinical benefits of combining rituximab with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy for diffuse large B-cell lymphoma (DLBCL) were subsequently confirmed in randomized clinical trials; improvements in EFS and OS were evident not only in younger patients with good risk features, but also in the elderly subsets [27-29].
Rituximab-based chemoimmunotherapy for adult Burkitt-type leukemia/lymphoma
Burkitt-type lymphoma (BL) and mature B-cell ALL are composed of high grade rapidly proliferating small noncleaved B lymphoid cells with a mature B-lineage immunophenotype characterized by expression of monotypic surface IgM, CD19, CD20, CD22, CD10, Bcl-6, and CD79a (negative for CD5, CD23, Bcl-2, and nuclear terminal deoxyribonucleotide transferase [TdT]). For mature B-cell ALL, the typical L3 morphology as per the French-American-British (FAB) classification and the presence of one of the characteristic translocations involving the proto-oncogene c-myc on band 8q24 [t8;14(q24;q32); t(2;8)(p12;q24); t(8;22)(q24;11)] have been hallmarks of the disease; an additional t(14;18)(q32;q21) may confer a worse prognosis owing to over expression of the anti-apoptotic protein bcl-2 [30,31]. Prognosis has improved significantly with use of short-term dose-intensive multiagent chemotherapy regimens, except for the elderly subgroup, which tend to be underrepresented in clinical trials (Table 1). The poor prognosis of older age (60 years or older) was initially reported upon reviewing the outcome of mature B-cell ALL after chemotherapy per the hyper-CVAD regimen [32]. A subsequent reanalysis of several multinational clinical trials of various chemotherapy regimens used for BL identified that 2-year OS rates were lower in most series for the subgroups aged 40 years or older [33].
Table 1. Regimens for adult Burkitt-type leukemia/lymphoma.
| Study | Therapy | No. | Age (yrs) | %CR | % Continuous CR (X yrs) | % OS (X yrs) | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Median | % ≥ 60 [% ≥ 55] | ||||||
| No rituximab | |||||||
|
| |||||||
| Hoelzer [89] | B-NHL83 | 24 | 33 | 0 | 63 | 50 (8) | 49 (8) |
| B-NHL86 | 35 | 36 | 10 | 74 | 71 (4) | 51 (4) | |
| Hoelzer [40] | B-NHL 90 | 45 | — | [100] | 71 | — | 39 (6) |
| Magrath [90] | 89-C-41 (CODOX-M/IVAC) | 20 | 25 | 0 | 89 | 89 (2) | 74 (4) |
| Hyper-CVAD | 26 | 58 | 46 | 81 | 61 (3) | 49 (3) | |
| Thomas [32] | Age < 60 yrs | 14 | 38 | — | 93 | 83 (3) | 77 (3) |
| Age ≥ 60 yrs | 12 | — | — | — | — | 17 (3) | |
| CALGB 9521 | |||||||
| Rizzieri [91] | Cohort 1 | 52 | 44 | 19 | 79 | 66 (3) | 54 (3) |
| Cohort 2 | 40 | 50 | 23 | 68 | 67 (3) | 50 (3) | |
| Di Nicola [92] | CMVVP-16/Ara-C/CDDP | 22 | 36 | — | 77 | 68 (2) | 77 (2) |
| Lacasce [93] | Modified CODOX-M/IVAC | 14 | 47 | — | 86 | 64 (2) | 71 (2) |
| Divine [94] | LMB95 | 72 | 33 | — | 72 | — | 70 (2) |
| Hyper-CVAD | 48 | 48 | 33 | 85 | 60 (3) | 53 (3) | |
| Thomas [38] | Age < 60 yrs | 25 | — | — | — | 73 (3) | 70 (3) |
| Age ≥ 60 yrs | 23 | — | — | — | 44 (3) | 19 (3) | |
| Mead [95] | Modified CODOX-M/IVAC | 53 | 37 | 9 | — | — | 67 (2) |
|
| |||||||
| With rituximab | |||||||
|
| |||||||
| Hyper-CVAD + rituximab | 31 | 46 | 29 | 86 | 88 (3) | 89 (3) | |
| Thomas [38] | Age < 60 yrs | 22 | — | — | — | 88 (3) | 90 (3) |
| Age ≥ 60 yrs | 9 | — | — | — | 100 (3) | 89 (3) | |
| Oriol [46] | PETHEMA* | 17 | 36 | 0 | 88 | 93 (2) | 82 (2) |
| GMALL B-ALL/NHL 2002 | |||||||
| BL | |||||||
| Hoelzer [42] | Age < 55 yrs | 146 | 36 | [18] | 90 | — | 91 (3) |
| Age ≥ 55 yrs | — | 84 (3) | |||||
| B-ALL | |||||||
| Age < 55 yrs | 84 | 46 | [41] | 83 | — | 79 (3) | |
| Age ≥ 55 yrs | — | 39 (3) | |||||
— Not available or not reported;
Adopted from GMALL B-ALL/NHL 2002
CR, complete remission; OS, overall survival; NHL, non-Hodgkin lymphoma; CODOX-M/IVAC, cyclophosphamide, vincristine, doxorubicin, high dose methotrexate alternating with ifosfamide, etoposide, high dose cytarabine; hyper-CVAD, fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone alternating with high dose methotrexate and cytarabine; CALGB, Cancer and Leukemia Group B; CMVVP-16/Ara-C/CDDP, cyclophosphamide, doxorubicin, high dose methotrexate, vincristine, etoposide, cytarabine, cisplatin; LMB, Lymphoma Malignancy B; PETHEMA, Programa para el Estudio de la Terapéutica en Hemopatías Malignas; GMALL, German Multicenter Study Group for Adult ALL; BL, Burkitt lymphoma; B-ALL, mature B-cell ALL
Significant activity of therapy with single agent rituximab was initially observed in children with relapsed or refractory BL or mature B-cell ALL [34]. Indeed, caspase-independent apoptosis has been reported in BL-derived cell lines after in vitro exposure to rituximab [35]. Rituximab has also been shown to sensitize B-lineage NHL cell lines to chemotherapeutic agents in vitro via selective down regulation of the anti-apoptotic proteins Bcl-2 and Bcl-XL [36,37]. Bcl-XL protects cells from cytotoxicity, thus conferring a multidrug-resistant phenotype; down regulation of Bcl-XL induced by rituximab could modulate this effect. Rituximab has therefore been incorporated into established regimens for Burkitt-type leukemia/lymphoma in order to exploit the universally high CD20 expression and potential synergy with chemotherapeutic agents, particularly given the successes observed with chemoimmunotherapy approaches for DLBCL and other lymphoproliferative disorders. Given the relative rarity of these disease entities, these nonrandomized clinical trials have relied on historical comparisons for determination of clinical benefit.
As alluded to earlier, outcome for the elderly subset of de novo BL and mature B-cell ALL treated with the hyper-CVAD was particularly poor. The 3-year OS rates ranged from 17% – 19% compared with 70% – 77% for their younger counterparts, in part attributable to a higher induction mortality (10%) incurred from infections and to a higher incidence of disease recurrence (50%) [32,38]. Further intensification of the chemotherapy was not deemed feasible. Standard dose rituximab (375 mg/m2) was therefore incorporated into the first 4 of 8 cycles of intensive chemotherapy (8 treatments given days 1 and 11 of the hyper-CVAD cycles, days 1 and 8 of the high dose methotrexate/cytarabine cycles) [38]. No other modifications were made to the hyper-CVAD regimen except for use of a “protective environment” for the elderly patients during induction chemotherapy until neutrophil recovery > 500. An analysis of 31 patients (29% age 60 years or older) treated with hyper-CVAD and rituximab demonstrated clinical benefit from the addition of rituximab, particularly for the elderly subset [38]. Although overall the CR rates were similar (86% versus 85%), the CRD, EFS and OS rates for the entire group treated with the combination were 91%, 89% and 89% compared with 66%, 52% and 53% (p<.05 for all comparisons), respectively, for the historical cohort treated with hyper-CVAD alone (Table 1). The improvement in outcome was most apparent for the elderly subgroup, with respective rates of 100%, 89% and 89% compared with 44%, 19% and 19% for hyper-CVAD without rituximab, owing to amelioration of induction deaths and absence of disease recurrence. Multivariate analysis for DFS identified younger age and treatment with rituximab as independent factors predictive for favorable outcome.
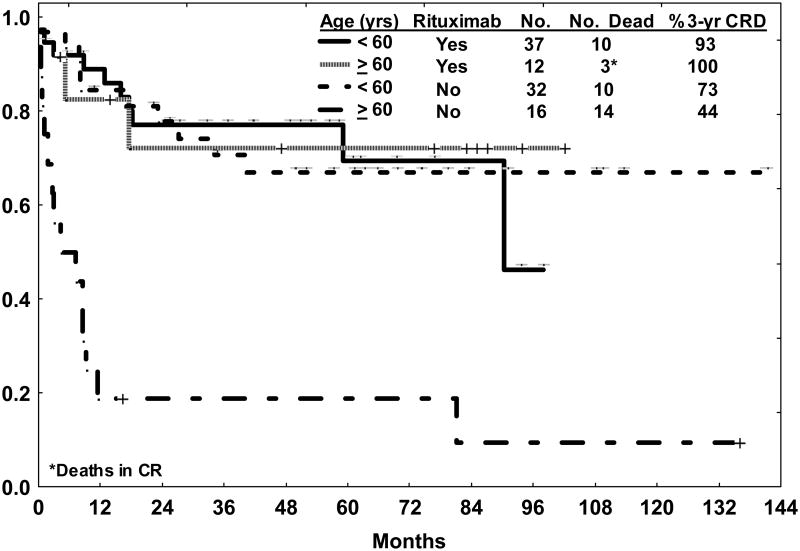
A recent update of outcome with the hyper-CVAD and rituximab regimen inclusive of 49 patients with de novo BL or mature B-cell ALL continued to show apparent clinical benefit from the addition of rituximab, predominantly for the elderly subset (Figure 2) [39]. Of note, with long-term follow-up (median 46 months), three of 37 (8%) younger patients treated with chemoimmunotherapy subsequently developed secondary blood dyscrasias (acute myelogenous leukemia [AML] at 7 years, myelodysplastic syndrome at 3½ years, AML with t(8;21) at 3 years) without recurrence of the Burkitt disease, suggesting that monitoring for late toxicities beyond 2 years after chemoimmunotherapy is warranted.
Figure 2.
Overall survival for de novo Burkitt leukemia/lymphoma by age and therapy (hyper-CVAD with or without rituximab). Late events in the younger group treated with hyper-CVAD and rituximab were related to secondary blood dyscrasias (refer to text).
Elderly patients older than 55 years with BL and mature B-cell ALL also had a poor outcome with the former protocol B-NHL90 without rituximab. The CR rate in 45 patients was 71% with an OS rate of 39% [40]. In the ensuing chemoimmunotherapy regimen developed for BL, mature B-cell ALL, and DLBCL by the German Multicenter Study Group for Adult ALL (GMALL-B-ALL/NHL 2002), eight standard doses of rituximab were applied (1 dose prior to each of the six chemotherapy cycles and 2 doses for consolidation) [41]. Other modifications to the former protocol B-NHL90 included the addition of cycles containing high dose cytarabine (2 g/m2) besides other drugs (cycle C). Patients younger than 55 years of age received 6 cycles (ABCABC) of therapy with 1.5 g/m2 of intravenous methotrexate, whereas older patients underwent dose reduction of the methotrexate to 0.5 g/m2 without cycle C (ABABAB). Outcome for the BL (n=146, median age 36 years, 18% > 55 years) and mature B-cell ALL (n=84, median age 46, 41% > 55 years) subgroups was recently reported [42]. The CR rate was 90% and 83% with induction death rates of 3% and 11%, respectively. The 3-year OS rates were influenced by age (younger versus older) within the subtypes: rates were 91% versus 84% for BL and 84% versus 39% for mature B-cell ALL, respectively. The worse outcome noted in the older patients with mature B-cell ALL was attributed to the high incidence of CNS relapses, likely related to the predetermined dose reductions of systemic methotrexate and omission of the high dose cytarabine chemotherapy cycles.
Standard dose rituximab has been incorporated into other previously established regimens for BL such as dose-modified CODOX-M/IVAC (cyclophosphamide, vincristine, doxorubicin, and high dose methotrexate alternating with ifosfamide, etoposide, and high dose cytarabine) and dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) with encouraging preliminary results [43,44]. Several studies have recently shown that the use of rituximab and intensive chemotherapy for HIV-related BL is feasible and can result in outcomes similar to the HIV negative population, particularly in the setting of effective HAART (highly active anti-retroviral therapy) [43,45,46].
Rituximab-based chemoimmunotherapy for adult Ph negative precursor B-cell ALL
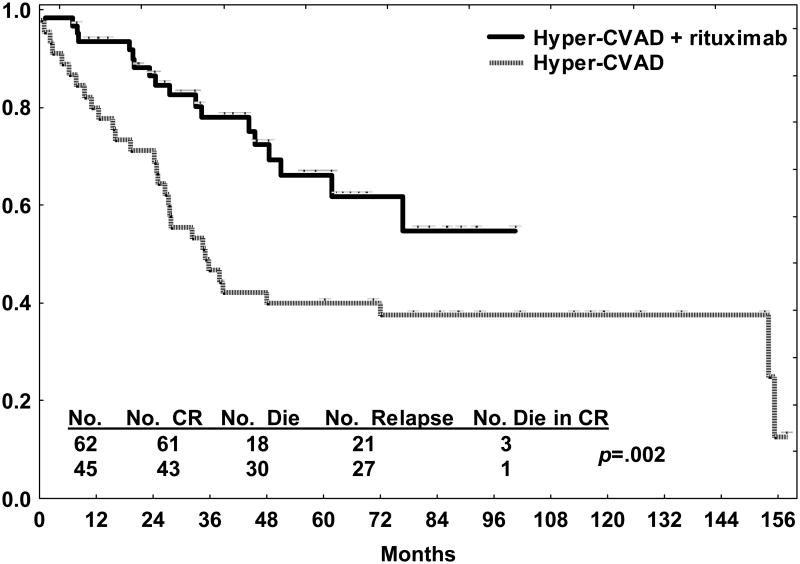
Rituximab has also been incorporated into a modified hyper-CVAD regimen for adolescents and adults with de novo CD20 positive precursor B-cell ALL in a similar fashion to the regimen designed for BL or mature B-cell ALL. Comparative details of the modified hyper-CVAD chemoimmunotherapy regimens implemented since 1999 are provided in Table 2 [39,47]. Since May 2000, 220 adolescents and adults with de novo ALL or lymphoblastic lymphoma were treated with the modified hyper-CVAD regimens, incorporating rituximab for the CD20 positive precursor B-cell subset (subsequent to October 2006 adolescents and young adults age 30 years or less have been treated with an augmented BFM regimen without rituximab) [48]. CD20 expression was noted in 53% of the 143 cases of precursor B-cell ALL. Overall CR rate was 94%, and was similar regardless of CD20 expression. In the CD20 positive subset, rituximab improved outcome compared with the historical experience with hyper-CVAD, with 3-year CRD rates (68% versus 28%, p< .001) and OS rates (68% versus 35%, p=.01) approaching those of the CD20 negative counterparts (Figure 3). In contrast to the experience with Burkitt-type leukemia/lymphoma, rituximab was not beneficial for the elderly group (age 60 years or older), with 3-year OS rates 48% versus 35%, p NS.
Table 2. Hyper-CVAD and Modified Hyper-CVAD Chemoimmunotherapy Regimens.
| Modified hyper-CVAD (± rituximab) | Hyper-CVAD (1992-1999) | |||
|---|---|---|---|---|
|
|
||||
| Without Intensification (2001 – present) | With Intensification (2000 – 2001) | |||
| Induction | Hyper-CVAD (laminar air flow rooms if ≥ 60 yrs) | Y (Y) | Y (Y) | Y (N) |
| Consolidation | ||||
| Cycle 2 (intensification) | Liposomal daunorubicin 150 mg/m2 IV days 1–2 | N | Y | N |
| Cytarabine 1.5 g/m2 CI IV days 1–2 | ||||
| Prednisone 200 mg days 1–5 | ||||
| Cycles 2, 4, 6, 8 or Cycles 3, 5, 7, 9 | Methotrexate 200 mg/m2, then 800 mg/m2 IV day 1 | Y | Y | Y |
| Cytarabine 3 g/m2 IV q12h × 3 days 2–3 | ||||
| Solu-Medrol 50 mg IV q12h × 6 days 1–3 | ||||
| Cycles 1, 3, 5, 7 or Cycles 1, 4, 6, 8 | Cyclophosphamide 300 mg/m2 IV q12h × 6 days 1–3 | Y | Y | Y |
| Dexamethasone 40 mg days 1–4, 11–14 | ||||
| Doxorubicin 50 mg/m2 CI IV day 4 | ||||
| Vincristine 2 mg IV days 1, 11 | ||||
| IF CD20 ≥ 20%: 8 doses rituximab 375 mg/m2 | ||||
| Cycles 1–4 | ||||
| Rituximab days 1, 11 (hyper-CVAD) | Y | Y | N | |
| Rituximab days 1, 8 (LDNR- or MTX-cytarabine) | ||||
| CNS prophylaxis (No. intrathecals) | Risk adapted (LDH > 1400, S + G2M ≥ 14%) | |||
| High | 8 | 16 | ||
| Indeterminate | 8 | 8 | ||
| Low | 6 | 4 | ||
| Maintenance | ||||
| POMP (6-mercaptopurine, VCR, methotrexate, prednisone) | Months 1–5, 8–17, 20–30 | Months 1–6, 8–10, 12–24 | ||
| Intensification | Hyper-CVAD | Months 6, 18 | N | |
| Rituximab 375 mg/m2 days 1, 11 if CD20 ≥ 20% | ||||
| Intensification | Methotrexate 100 mg/m2 IV day 1 weekly × 4 | Months 7, 19 | Months 7, 11 | |
| L-asparaginase 20,000 units IV day 2 weekly × 4 | ||||
Figure 3.
Overall survival by therapy (modified hyper-CVAD with rituximab compared with hyper-CVAD alone) in the younger subsets (age less than 60 years) with de novo CD20 positive precursor B-cell ALL. No difference in survival was observed with rituximab for the elderly subset (not shown).
In the elderly patients (> 55 years) with CD20 positive precursor B-cell ALL treated according to the GMALL 2002 protocol, standard dose rituximab was administered prior each cycle of dose-reduced chemotherapy and as consolidation for a total of 8 applications. In an interim analysis of 26 patients, the CR rate was 63% with a 1-year OS rate of 54% [41]. The younger patients with CD20 positive precursor B-cell ALL were treated per the GMALL 07/2003 protocol according to risk group. For the high risk (HR) patients (leukocyte count > 30 × 109/L or late achievement of CR), 3 standard doses of rituximab were administered immediately prior to each phase of induction (phase I, II), and the first consolidation chemotherapy cycle followed by allogeneic stem cell transplantation (SCT). The standard risk (SR) patients received 8 applications of standard dose rituximab before (day -1) the induction (phase I, II), reinduction, and six consolidation chemotherapy cycles. Preliminary results were reported for 185 patients with CD20 positive precursor B-cell ALL (133 SR, 52 HR); 117 (63%) received rituximab and were compared to the 70 patients who were treated with the same chemotherapy regimen prior to the incorporation of rituximab [49]. For SR patients, no difference in CR rate (94% versus 93%) was observed. However, the molecular CR rate (less than 10−4) was superior with the rituximab therapy, 60% versus 19% at day 21 and 89% versus 57% at week 16. Improvement in the 3-year rates for CRD (64% versus 48%, p=0.009) and for OS (75% versus 54%) were observed with the addition of rituximab. For HR pts, the 3-year rates for OS were 54% versus 32%. Allogeneic SCT was performed in 66% of the HR patients in first CR; OS was superior for the patients treated with rituximab (75% versus 40%) and was attributed to a reduction in the relapse rate. There was no difference in the incidence of deaths in first CR with the addition of rituximab (4% versus 3%).
The results of these two phase II nonrandomized clinical trials suggest that the addition of rituximab to established chemotherapy regimens for adolescents and adults with CD20 positive precursor B-cell ALL can improve outcome. Prospective clinical trials to further delineate the potential benefits of rituximab in a randomized fashion are either underway (randomization to rituximab versus no rituximab for CD20 positive precursor B-cell ALL) or planned (randomization of rituximab versus no rituximab for precursor B-cell ALL irrespective of CD20 expression). Based on preponderance of the data, the role of rituximab in the firstline treatment of adolescents and young adults with precursor B-cell ALL now treated with pediatric-based regimens should also be explored as a potential therapeutic intervention to eradicate MRD.
Rituximab therapy in pediatric Burkitt-type leukemia/lymphoma and precursor B-cell ALL
Until recently, there was a paucity of data regarding the use of rituximab for the treatment of BL or mature B-cell ALL in children and adolescents, despite extensive experience in the setting of posttransplant lymphoproliferative disease. Attias and Weitzman [50] reviewed the available literature up to the year 2007 and determined there was sufficient evidentiary support for the efficacy of rituximab as a single agent or in combination with chemotherapy, even in heavily pretreated patients. Subsequently, a clinical trial of rituximab combined with ifosfamide, carboplatin, and etoposide (RICE) for relapsed/refractory NHL or mature B-cell ALL was conducted by the Children's Oncology Group (COG ANHL0121) [51]. The overall response rate was 64% in 14 patients with BL or mature B-cell ALL; there was no significant difference in toxicity profile compared with the historical experience with ICE. It was further postulated that the incorporation of rituximab into effective frontline chemotherapy could offset the negative prognostic influence of dose reductions (implemented for toxicities such stomatitis, typhilitis and infections) on EFS for children with de novo BL or mature B-ALL [52]. EFS was also inferior if features such as poor COP response, combined BM/central nervous system (CNS) disease, high tumor burden, and/or complex karyotype were present. Rituximab was therefore incorporated into French-American-British/Lymphoma Malignancy B (FAB/LMB) chemotherapy (4 doses in subpilot, 6 doses in pilot) for 48 children and adolescents with de novo intermediate risk advanced stage NHL (COG ANHL01P1), including 28 (59%) with BL. A preliminary report indicated an improvement in the 1-year EFS (96% versus 86%) compared with the historical experience with FAB/LMB96 without rituximab [53]. Randomized clinical trials are planned to confirm these findings.
Rituximab-based chemoimmunotherapy with allogeneic stem cell transplant for ALL
Investigators have reported an association between prior therapy with rituximab and a reduced incidence of acute and chronic graft-versus-host disease (aGVHD, cGVHD) after allogeneic SCT. Rituximab also has been efficacious as therapy for steroid-refractory GVHD, likely via depletion of donor and host antigen-presenting B-cells which prime the T-cells responsible for GVHD. In support of this hypothesis is the observation that infusion of a higher number of CD20 positive B-cells from peripheral blood graft products correlated with a higher incidence of aGVHD [54].
There is limited data regarding the use of rituximab for CD20 positive precursor B-cell ALL in the setting of allogeneic SCT. Kebriaei and colleagues [55] incorporated four weekly infusions of standard dose rituximab (starting day -7) into the standard preparative regimen of cyclophopshamide (Cy) and total body irradiation (TBI) for adolescents and adults with CD20 positive ALL. There was no delay in engraftment with the addition of rituximab. The cumulative incidence of aGVHD after matched sibling or matched unrelated donor SCT using the rituximab-based regimen was lower than that observed with the cohort of CD20 negative ALL patients treated over the same time period with the Cy/TBI regimen without rituximab (17% versus 39%, p=0.07). Whether the addition of rituximab truly influenced outcomes such as survival and relapse-free survival could not be ascertained since there were disparities with respect to the proportion of cases with B-lineage ALL (20% with T-lineage in the comparative group) and disease status at transplant (e.g., in the rituximab group 74% were beyond first CR or had active disease whereas in the comparative group 54% were in first CR). Notably, the reduction in incidence of aGVHD with the use of rituximab did not appear to result in a significantly increased risk of relapse, as the direct anti-leukemic effects of rituximab likely offset any corresponding attenuation of graft-versus-leukemia (GVL) effect.
Rituximab intrathecally for central nervous system disease in ALL
Following systemic administration of rituximab, only 0.1% to 1.7% of the serum level was detectable in the cerebrospinal fluid (CSF), even in cases where the blood-brain barrier was likely disrupted [56,57]. The CD20 antigen is not expressed by normal neurons or glial cells. Rituximab has been therefore been used intrathecally for the management of leptomeningeal infiltration in various lymphoproliferative disorders, including NHL [58]. There is limited data regarding the efficacy of this approach in CD20 positive ALL [59].
A small cohort of children and adolescents with CNS disease failing to respond triple intrathecal (IT) chemotherapy (methotrexate, cytarabine, hydrocortisone) underwent four weekly IT instillations of rituximab at a flat dose of 10 mg (in 6 mL of normal saline), followed by resumption of the triple IT therapy. All seven patients cleared the CSF of malignant cells by the fourth dose of rituximab without significant toxicity. Although systemic relapses were noted in a few of the patients, the CNS was not a site of disease recurrence in any of the cases. The incorporation of rituximab concurrently with standard IT chemotherapy for de novo BL, mature B-cell ALL or CD20 positive precursor B-cell presenting with CNS involvement warrants further study in clinical trials.
Effects of rituximab on detection of minimal residual disease in precursor B-cell ALL
The use of multiparameter flow cytometry (MFC) for the detection and quantitation of MRD after induction and consolidation chemotherapy has been well established in childhood ALL [60]. The technique relies on aberrant leukemia-associated antigen expression patterns which allow discrimination from normal marrow precursor cells. The modulation of various surface leukemia antigens during induction chemotherapy (data from the AIEOP-BFM ALL 2000 protocol discussed earlier) did not compromise the ability to detect residual lymphoblasts [13]. In childhood ALL, the detection of MRD by MFC at levels 0.01% or greater at specific time points during chemotherapy has been associated with a higher propensity for disease recurrence. Risk-adapted therapeutic strategies have thus been implemented in these scenarios. Accurate assessments of MRD by MFC are therefore paramount.
Hematogones are normally occurring immature B-lineage cells which resemble malignant lymphoblasts morphologically and immunophenotypically. Hematogones may be particularly prominent in the regeneration phase following chemotherapy. The phenotypic stability and characteristic expression of antigens of these benign B-cell precursors has been well-established [61]. In contrast, neoplastic lymphoblasts have aberrant antigen expression, maturational arrest, and immunophenotypic asynchrony (co-expression of early and late antigens). Despite these disparities, type 1 hematogones tend to have a similar phenotype to B-lymphoblasts (CD34+ TdT+). The use of MFC to detect MRD after induction and consolidation chemotherapy per the modified hyper-CVAD regimen (with or without rituximab) for de novo CD20 positive precursor B-cell ALL (discussed earlier) was reviewed [39]. Rituximab appeared to induce a B-cell maturation arrest manifested by the detection of immature CD34+ TdT+ CD20- B-cells in the marrow, potentially leading to a false positive diagnosis of MRD [62]. The use of additional markers such as CD38 (uniformly bright), CD58 (moderately positive) and/or CD9 (most cells positive) established a pattern of expression consistent with hematogones rather than residual ALL. The CD81 surface marker appears to be exceptionally discriminatory with respect to distinguishing residual precursor B-cell ALL (CD81 underexpressed) from hematogones (CD81 universally bright) [63]. The effect of rituximab on the immunophenotype of marrow B-cell precursors could persist for as long as six months after completion of the immunotherapy. These observations should be taken into consideration when performing MRD assays by MFC after rituximab-based chemoimmunotherapy.
Toxicity of rituximab
Rituximab is generally well-tolerated with the most common toxicity manifested by mild to moderate first infusion reactions, although life-threatening anaphylaxis has been reported. In highly proliferative disease states such as Burkitt leukemia/lymphoma, the rapid cytolysis induced by rituximab may result in tumor lysis syndrome (TLS); the use of rasburicase for prophylaxis of TLS is considered mandatory. Other potentially serious events associated with rituximab therapy have included reactivation of hepatitis B in chronic carriers leading to fulminant hepatic failure, severe mucocutaneous reactions, and progressive multifocal leukoencephalopathy (PML) [64]. Prophylaxis of hepatitis B carriers with the antiviral agent lamivudine has been effective in preventing reactivation when commenced one week prior to initiation of rituximab and continued for at least one year after completion of therapy [65]. As a rare demyelinating infection of the CNS caused by the JC polyomavirus, PML is generally lethal with a case-fatality ratio of 90%. A recent report summarized at least 50 cases, over a 10 year period following approval of by the FDA, for which the development of PML was associated with rituximab (generally in the setting of prior or concurrent immunosuppressive therapy) [66]. Other rarely reported hematological or immunological events occurring subsequent to the completion of rituximab therapy include late onset neutropenia and/or transient hypogammaglobulinemia, neither usually associated with serious infections. Rituximab has rarely been linked to development of corticosteroid-responsive interstitial lung disease; it is an elusive diagnosis requiring exclusion of an underlying infectious etiology.
Mechanisms of resistance to rituximab
Several potential mechanisms of resistance to rituximab have been characterized [67]. Development of rituximab-resistant B-lineage NHL cell lines (RRCL) has allowed the study of secondary or acquired resistance. The loss of protein expression, membrane exposure or structural changes of CD20 can lead to primary resistance. Reduced expression of surface CD20 has been associated with hypermethylation of the transcription factor PU.1-encoding gene which reputedly regulates CD20 expression [21]. In vitro exposure to the hypomethylating agent 5-azacytidine restored CD20 mRNA expression of primary B-NHL cells [20]. Alterations of CD20 surface expression have been associated with increased histone acetylation, suggesting a possible role for histone deacetylase (HDAC) inhibitors [6]. Conformational changes of the CD20 protein affecting its affinity for rituximab have also been described [6]. The binding of rituximab to CD20 results in translocation and clustering of CD20 molecules into cholesterine-enriched lipid rafts. As inhibitors of cholesterol synthesis, statins have been shown to decrease rituximab-induced ADCC and CDCC [68]. The Fc region of rituximab is recognized by the complement component C1q, which activates the classical complement pathway and results in cytolysis via formation of the membrane attack complex (MAC). Specific Fc region or complement component polymorphisms, such as FgammaRIIIb (Val/Phe158), have been associated with reduced efficacy and increased propensity for disease recurrence after rituximab-based therapy [69].
Within signaling platforms of the lipid rafts, CD20 is indirectly associated with the Src tyrosine kinases Lyn, Fyn and Lck [70]. This process results in binding of downstream targets, triggering several intracellular signaling cascades, including NF-κB, phosphoinositide-3-kinase (PI3K)/Akt, p38 mitogen-activated kinases (MAPK), and ERK1/2 [7,37,71,72]. Rituximab resistance has been associated with upregulation of these pro-proliferative signaling pathways. Resistance mechanisms also include upregulation of the anti-apoptotic Bcl-2 protein family members Bcl-2, Bcl-XL and Mcl-1 as well as down-regulation of proapoptotic Bak and Bax proteins [73,74].
Pharmacological targeting of NF-κB with bortezomib, histone deacetylase with vorinostat (SAHA), and Bcl-2 with the bcl-2 antisense oligonucleotide oblimersen may therefore restore sensitivity to rituximab. Interestingly, these agents have single agent activity in a variety of lymphoproliferative disorders, and have been incorporated into chemoimmunotherapy regimens. Examples include bortezomib and hyper-CVAD with rituximab in de novo mantle cell lymphoma and oblimersen with FCR (fludarabine, cyclophosphamide, and rituximab) in relapsed or refractory CLL [75,76]. Alterations of the PI3K/Akt pathway induced by inhibitors of the mammalian target of rapamycin (mTOR) (e.g., temsirolimus and everolimus [RAD001]) appear to reverse resistance of lymphoblasts to anthracyclines and vinca alkaloids in vitro; combination therapy trials incorporating everolimus into hyper-CVAD and rituximab are planned [77].
The down-regulation of surface CD20 expression, increase in inhibitory complement-regulatory proteins CD55 & CD59, and upregulation of surface CD52 expression also appear to effect resistance to rituximab [78]. The CD52 antigen is a member of the glycosylphosphatidylinositol (GPI) anchored membrane glycoproteins which appears to function in normal T-cell activation, release of cytokines, and signal transduction [79]. Targeting the CD52 antigen in vitro with alemtuzumab in RRCL induced significant cytolysis via CDCC comparable to what has been observed with rituximab in sensitive cell lines [78]. Blocking the CD52 antigen using anti-CD52 F(ab')2 fractions partially restored rituximab-associated CDCC, suggesting that CD52 may serve as an inhibitory protein [78]. An in vivo animal model of primary human ALL that co-expressed CD20 and CD52 was developed to assess response to rituximab, alemtuzumab and the combination [80]. Whereas significant levels of residual ALL were identified in nearly all cases after administration of either MoAb singly, 90% achieved CR with the combination of rituximab and alemtuzumab. Faderl and colleagues [81] studied a regimen of 4 weekly standard doses of rituximab co-administered with alemtuzumab (7 full doses after 2 challenge doses) in relapsed or refractory CLL and other lymphoproliferative disorders, establishing the activity and tolerability of the regimen. The combination of rituximab with alemtuzumab could therefore be of further interest in the treatment of CD20 positive precursor B-cell ALL, based on the preclinical and clinical data.
Notably, all of these mechanisms detailed above not only play a role in the primary or acquired resistance of lymphoblasts to rituximab, but also in the sensitivity of the leukemia clones to chemotherapeutics. The countermeasures described which alter these pathways of leukemogenesis and resistance need to be studied further in the context of multi-targeted chemoimmunotherapy.
Novel anti-CD20 monoclonal antibodies
Ofatumumab (HuMax-CD20; GlaxoSmithKline, Collegeville, PA, USA and Genmab, Copenhagen, Denmark) is a second generation fully human anti-CD20 IgG1 monoclonal antibody. This MoAb is directed at a unique small loop epitope of CD20 (different binding site than rituximab) with a longer release time from the target site and a superior CDCC effect compared with rituximab [82]. In vitro, ofatumumab exhibits its activity at a lower number of CD20 molecules per cell than rituximab, suggesting it would be more efficacious in diseases with inherently low CD20 expression, such as CLL [83]. It has indeed demonstrated promising clinical activity with minimal infusional related toxicity in double refractory (fludarabine and alemtuzumab) or bulky fludarabine refractory CLL [84]. The use of ofatumumab in lieu of rituximab in frontline chemotherapy regimens for Burkitt-type leukemia/lymphoma and CD20 positive precursor B-cell ALL warrants investigation. Other candidate fully human anti-CD20 MoAbs undergoing development include veltuzumab (Immunomedics, Inc., Morris Plains, NJ) in intravenous and subcutaneous formulations and ocrelizumab (Biogen Idec, Inc., Genentech, Inc., Roche Holding AG and Chugai Pharmaceuticals Co. Ltd) [85,86].
Future directions
The incorporation of rituximab into frontline chemotherapy regimens for Burkitt-type leukemia/lymphoma appears to improve outcome. Preliminary data regarding the use of rituximab for CD20 positive precursor B-cell ALL suggest its use may also be beneficial, particularly for the younger subsets. Additional prospective studies are needed in order to confirm these findings and to determine the optimal use of rituximab in the treatment of CD20 positive (and possibly the CD20 negative subset based on upregulation of CD20 surface antigen expression) precursor B-cell ALL. To date, the development of these chemoimmunotherapy regimens has been entirely empiric. Future clinical trials should be guided by (1) levels of CD20 expression, (2) degree of CD20 modulation during induction chemotherapy, (3) presence or absence of circulating CD20 antigen, and (4) levels of rituximab. Unanswered questions include the potential benefits of early CD20 “saturation” (e.g., more frequent or higher dosing of rituximab) versus administration solely to exploit chemosensitization (e.g., standard dose concurrently with each cycle of chemotherapy). The incorporation of novel therapeutics which counter mechanisms of resistance to rituximab (e.g. anti-Bcl-2 agents, mTOR inhibitors) may also improve the efficacy of chemoimmunotherapy by directly targeting the lymphoma or leukemia clone. The use of rituximab in combination with other MoAbs such as epratuzumab or alemtuzumab for which safety and efficacy data exist, may also be beneficial for reasons detailed previously [81,87,88]. Although the preliminary data regarding the clinical activity of the novel anti-CD20 MoAbs appears promising, the improved efficacy alone is unlikely to ameliorate the need for multi-targeted approaches required for a curative strategy in the treatment of adult ALL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liedtke M, Cleary ML. Therapeutic targeting of MLL. Blood. 2009;113:6061–6068. doi: 10.1182/blood-2008-12-197061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig FE, Foon KA. Flow cytometric immunophenotyping for hematologic neoplasms. Blood. 2008;111:3941–3967. doi: 10.1182/blood-2007-11-120535. [DOI] [PubMed] [Google Scholar]
- 3.Ginaldi L, De Martinis M, Matutes E, Farahat N, Morilla R, Catovsky D. Levels of expression of CD19 and CD20 in chronic B cell leukaemias. J Clin Pathol. 1998;51:364–369. doi: 10.1136/jcp.51.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riley JK, Sliwkowski MX. CD20: a gene in search of a function. Semin Oncol. 2000;27:17–24. [PubMed] [Google Scholar]
- 5.Manshouri T, Do KA, Wang X, et al. Circulating CD20 is detectable in the plasma of patients with chronic lymphocytic leukemia and is of prognostic significance. Blood. 2003;101:2507–2513. doi: 10.1182/blood-2002-06-1639. [DOI] [PubMed] [Google Scholar]
- 6.Czuczman MS, Olejniczak S, Gowda A, et al. Acquirement of rituximab resistance in lymphoma cell lines is associated with both global CD20 gene and protein down-regulation regulated at the pretranscriptional and posttranscriptional levels. Clin Cancer Res. 2008;14:1561–1570. doi: 10.1158/1078-0432.CCR-07-1254. [DOI] [PubMed] [Google Scholar]
- 7.Jazirehi AR, Vega MI, Bonavida B. Development of rituximab-resistant lymphoma clones with altered cell signaling and cross-resistance to chemotherapy. Cancer Res. 2007;67:1270–1281. doi: 10.1158/0008-5472.CAN-06-2184. [DOI] [PubMed] [Google Scholar]
- 8.Borowitz MJ, Shuster J, Carroll AJ, et al. Prognostic significance of fluorescence intensity of surface marker expression in childhood B-precursor acute lymphoblastic leukemia. A Pediatric Oncology Group Study. Blood. 1997;89:3960–3966. [PubMed] [Google Scholar]
- 9.Jeha S, Behm F, Pei D, et al. Prognostic significance of CD20 expression in childhood B-cell precursor acute lymphoblastic leukemia. Blood. 2006;108:3302–3304. doi: 10.1182/blood-2006-04-016709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas DA, O'Brien S, Jorgensen JL, et al. Prognostic significance of CD20 expression in adults with de novo precursor B-lineage acute lymphoblastic leukemia. Blood. 2009;113:6330–6337. doi: 10.1182/blood-2008-04-151860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maury S, Huguet F, Pigneux A, et al. Prognostic significance of CD20 expression in adult B-cell precursor acute lymphoblastic leukemia. Blood. 2007;110:832a. abstract 2829. [Google Scholar]
- 12.Huguet F, Leguay T, Raffoux E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27:911–918. doi: 10.1200/JCO.2008.18.6916. [DOI] [PubMed] [Google Scholar]
- 13.Gaipa G, Basso G, Maglia O, et al. Drug-induced immunophenotypic modulation in childhood ALL: implications for minimal residual disease detection. Leukemia. 2005;19:49–56. doi: 10.1038/sj.leu.2403559. [DOI] [PubMed] [Google Scholar]
- 14.Gaipa G, Basso G, Aliprandi S, et al. Prednisone induces immunophenotypic modulation of CD10 and CD34 in nonapoptotic B-cell precursor acute lymphoblastic leukemia cells. Cytometry B Clin Cytom. 2008;74:150–155. doi: 10.1002/cyto.b.20408. [DOI] [PubMed] [Google Scholar]
- 15.Dworzak MN, Schumich A, Printz D, et al. CD20 up-regulation in pediatric B-cell precursor acute lymphoblastic leukemia during induction treatment: setting the stage for anti-CD20 directed immunotherapy. Blood. 2008;112:3982–3988. doi: 10.1182/blood-2008-06-164129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venugopal P, Sivaraman S, Huang XK, Nayini J, Gregory SA, Preisler HD. Effects of cytokines on CD20 antigen expression on tumor cells from patients with chronic lymphocytic leukemia. Leuk Res. 2000;24:411–415. doi: 10.1016/s0145-2126(99)00206-4. [DOI] [PubMed] [Google Scholar]
- 17.Yagci M, Akar I, Sucak GT, Haznedar R. GM-CSF does not increase CD20 antigen expression on chronic lymphocytic leukemia lymphocytes. Leuk Res. 2005;29:735–738. doi: 10.1016/j.leukres.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Schuster SJ, Venugopal P, Kern JC, McLaughlin P. GM-CSF plus rituximab immunotherapy: translation of biologic mechanisms into therapy for indolent B-cell lymphomas. Leuk Lymphoma. 2008;49:1681–1692. doi: 10.1080/10428190802216731. [DOI] [PubMed] [Google Scholar]
- 19.Ferrajoli A. Incorporating the use of GM-CSF in the treatment of chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50:514–516. doi: 10.1080/10428190902763541. [DOI] [PubMed] [Google Scholar]
- 20.Hiraga J, Tomita A, Sugimoto T, et al. Down-regulation of CD20 expression in B-cell lymphoma cells after treatment with rituximab-containing combination chemotherapies: its prevalence and clinical significance. Blood. 2009;113:4885–4893. doi: 10.1182/blood-2008-08-175208. [DOI] [PubMed] [Google Scholar]
- 21.Wojciechowski W, Li H, Marshall S, Dell'Agnola C, Espinoza-Delgado I. Enhanced expression of CD20 in human tumor B cells is controlled through ERK-dependent mechanisms. J Immunol. 2005;174:7859–7868. doi: 10.4049/jimmunol.174.12.7859. [DOI] [PubMed] [Google Scholar]
- 22.Maloney DG. Mechanism of action of rituximab. Anticancer Drugs. 2001;12(Suppl 2):S1–4. [PubMed] [Google Scholar]
- 23.Binder M, Otto F, Mertelsmann R, Veelken H, Trepel M. The epitope recognized by rituximab. Blood. 2006;108:1975–1978. doi: 10.1182/blood-2006-04-014639. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 25.Leget GA, Czuczman MS. Use of rituximab, the new FDA-approved antibody. Curr Opin Oncol. 1998;10:548–551. doi: 10.1097/00001622-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 26.McLaughlin P, Hagemeister FB, Grillo-Lopez AJ. Rituximab in indolent lymphoma: the single-agent pivotal trial. Semin Oncol. 1999;26:79–87. [PubMed] [Google Scholar]
- 27.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 28.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 29.Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 30.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33:451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 31.D'Achille P, Seymour JF, Campbell LJ. Translocation (14;18)(q32;q21) in acute lymphoblastic leukemia: a study of 12 cases and review of the literature. Cancer Genet Cytogenet. 2006;171:52–56. doi: 10.1016/j.cancergencyto.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Thomas DA, Cortes J, O'Brien S, et al. Hyper-CVAD program in Burkitt's-type adult acute lymphoblastic leukemia. J Clin Oncol. 1999;17:2461–2470. doi: 10.1200/JCO.1999.17.8.2461. [DOI] [PubMed] [Google Scholar]
- 33.Kelly JL, Toothaker SR, Ciminello L, et al. Outcomes of patients with Burkitt lymphoma older than age 40 treated with intensive chemotherapeutic regimens. Clin Lymphoma Myeloma. 2009 doi: 10.3816/CLM.2009.n.060. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Vries MJ, Veerman AJ, Zwaan CM. Rituximab in three children with relapsed/refractory B-cell acute lymphoblastic leukaemia/Burkitt non-Hodgkin's lymphoma. Br J Haematol. 2004;125:414–415. doi: 10.1111/j.1365-2141.2004.04925.x. [DOI] [PubMed] [Google Scholar]
- 35.Daniels I, Abulayha AM, Thomson BJ, Haynes AP. Caspase-independent killing of Burkitt lymphoma cell lines by rituximab. Apoptosis. 2006;11:1013–1023. doi: 10.1007/s10495-006-6314-5. [DOI] [PubMed] [Google Scholar]
- 36.Jazirehi AR, Gan XH, De Vos S, Emmanouilides C, Bonavida B. Rituximab (anti-CD20) selectively modifies Bcl-xL and apoptosis protease activating factor-1 (Apaf-1) expression and sensitizes human non-Hodgkin's lymphoma B cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther. 2003;2:1183–1193. [PubMed] [Google Scholar]
- 37.Jazirehi AR, Vega MI, Chatterjee D, Goodglick L, Bonavida B. Inhibition of the RafMEK1/2-ERK1/2 signaling pathway, Bcl-xL down-regulation, and chemosensitization of non-Hodgkin's lymphoma B cells by Rituximab. Cancer Res. 2004;64:7117–7126. doi: 10.1158/0008-5472.CAN-03-3500. [DOI] [PubMed] [Google Scholar]
- 38.Thomas DA, Faderl S, O'Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106:1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 39.Thomas DA, Kantarjian H, Faderl S, et al. Outcome after frontline therapy with the modified hyper-CVAD regimen with or without rituximab for de novo acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma (LL) Blood. 2008;112 abstract 1931. [Google Scholar]
- 40.Hoelzer D, Arnold R, Diedrich H. Successful treatment of Burkitt's NHL and other high-grade NHL according to protocol for mature B-ALL. Blood. 2002;100:159a. abstract. [Google Scholar]
- 41.Gokbuget N, Hoelzer D. Rituximab in the treatment of adult ALL. Ann Hematol. 2006;85:117–119. [Google Scholar]
- 42.Hoelzer D. Recent results in the treatment of Burkitt lymphomas. Ann Oncol. 2008;19(suppl 4):iv83. [Google Scholar]
- 43.Abramson JS, Barnes JA, Toomey CE, et al. Rituximab added to CODOX-M/IVAC is highly effective in HIV-negative and HIV-positive Burkitt lymphoma. Blood. 2008;112 abstract 3595. [Google Scholar]
- 44.Dunleavy K, Little RF, Pittaluga S, et al. A prospective study of dose-adjusted (DA) EPOCH with rituximab in adults with newly diagnosed Burkitt lymphoma: A regimen with high efficacy and low toxicity. Ann Oncol. 2008;19(suppl 4):iv83–iv84. abstract 009. [Google Scholar]
- 45.Cortes J, Thomas D, Rios A, et al. Hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone and highly active antiretroviral therapy for patients with acquired immunodeficiency syndrome-related Burkitt lymphoma/leukemia. Cancer. 2002;94:1492–1499. doi: 10.1002/cncr.10365. [DOI] [PubMed] [Google Scholar]
- 46.Oriol A, Ribera JM, Bergua J, et al. High-dose chemotherapy and immunotherapy in adult Burkitt lymphoma: comparison of results in human immunodeficiency virus-infected and noninfected patients. Cancer. 2008;113:117–125. doi: 10.1002/cncr.23522. [DOI] [PubMed] [Google Scholar]
- 47.Thomas DA, O'Brien S, Cortes J, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood. 2004;104:1624–1630. doi: 10.1182/blood-2003-12-4428. [DOI] [PubMed] [Google Scholar]
- 48.Rytting M, Thomas D, Franklin A, et al. Young adults with acute lymphoblastic leukemia (ALL) treated with adapted augmented Berlin-Frankfurt-Munster (ABFM) therapy. Blood. 2008;112 abstract 3957. [Google Scholar]
- 49.Hoelzer D, Huettmann A, Kaul F, et al. Immunochemotherapy with rituximab in adult CD20 B-precusor ALL improves molecular CR rate and outcome in standard risk (SR) as well as in high risk (HR) patients with SCT. Haematologica. 2009;94(suppl 2) abstract 481. [Google Scholar]
- 50.Attias D, Weitzman S. The efficacy of rituximab in high-grade pediatric B-cell lymphoma/leukemia: a review of available evidence. Curr Opin Pediatr. 2008;20:17–22. doi: 10.1097/MOP.0b013e3282f424b0. [DOI] [PubMed] [Google Scholar]
- 51.Griffin TC, Weitzman S, Weinstein H, et al. A study of rituximab and ifosfamide, carboplatin, and etoposide chemotherapy in children with recurrent/refractory B-cell (CD20+) non-Hodgkin lymphoma and mature B-cell acute lymphoblastic leukemia: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2009;52:177–181. doi: 10.1002/pbc.21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cairo MS, Gerrard M, Sposto R, et al. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007;109:2736–2743. doi: 10.1182/blood-2006-07-036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cairo MS, Lynch J, Harrison L, et al. Safety, efficacy, and rituximab levels following chemoimmunotherapy (rituximab + FAB chemotherapy) in children and adolescents with mature B-cell non-Hodgkin lymhoma (B-NHL): A Children's Oncology Group Report. Blood. 2008;112 [Google Scholar]
- 54.Iori AP, Torelli GF, De Propris MS, et al. B-cell concentration in the apheretic product predicts acute graft-versus-host disease and treatment-related mortality of allogeneic peripheral blood stem cell transplantation. Transplantation. 2008;85:386–390. doi: 10.1097/TP.0b013e3181622e36. [DOI] [PubMed] [Google Scholar]
- 55.Kebriaei P, Saliba RM, Ma C, et al. Allogeneic hematopoietic stem cell transplantation after rituximab-containing myeloablative preparative regimen for acute lymphoblastic leukemia. Bone Marrow Transplant. 2006;38:203–209. doi: 10.1038/sj.bmt.1705425. [DOI] [PubMed] [Google Scholar]
- 56.Rubenstein JL, Combs D, Rosenberg J, et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101:466–468. doi: 10.1182/blood-2002-06-1636. [DOI] [PubMed] [Google Scholar]
- 57.Neuwelt EA, Specht HD, Hill SA. Permeability of human brain tumor to 99mTc-glucoheptonate and 99mTc-albumin. Implications for monoclonal antibody therapy. J Neurosurg. 1986;65:194–198. doi: 10.3171/jns.1986.65.2.0194. [DOI] [PubMed] [Google Scholar]
- 58.Schulz H, Pels H, Schmidt-Wolf I, Zeelen U, Germing U, Engert A. Intraventricular treatment of relapsed central nervous system lymphoma with the anti-CD20 antibody rituximab. Haematologica. 2004;89:753–754. [PubMed] [Google Scholar]
- 59.Jaime-Perez JC, Rodriguez-Romo LN, Gonzalez-Llano O, Chapa-Rodriguez A, Gomez-Almaguer D. Effectiveness of intrathecal rituximab in patients with acute lymphoblastic leukaemia relapsed to the CNS and resistant to conventional therapy. Br J Haematol. 2009;144:794–795. doi: 10.1111/j.1365-2141.2008.07497.x. [DOI] [PubMed] [Google Scholar]
- 60.Campana D. Minimal residual disease in acute lymphoblastic leukemia. Semin Hematol. 2009;46:100–106. doi: 10.1053/j.seminhematol.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalff A, Juneja S. B-acute leukemic lymphoblasts versus hematogones: the wolf in sheep's clothing. Leuk Lymphoma. 2009;50:523–524. doi: 10.1080/10428190902725839. [DOI] [PubMed] [Google Scholar]
- 62.Siami K, Awagu S, Cooper DG, et al. Effects of rituximab on the immunophenotype of benign B-cell precursors: Implications for flow cytometric minimal residual disease detection in precursor B-acute lymphoblastic leukemia. Lab Invest. 2006;86(Suppl 1):246A–247A. [Google Scholar]
- 63.Muzzafar T, Medeiros J, Wang S, Brahamandam A, Thomas DA, Jorgensen JL. Aberrant underexpression of CD81 in precursor B-cell acute lymphoblastic leukemia: Utility in detection of minimal residual disease by flow cytometry. Amer J Clin Path. 2009 doi: 10.1309/AJCP02RPVOKTNWEC. in press. [DOI] [PubMed] [Google Scholar]
- 64.Ram R, Ben-Bassat I, Shpilberg O, Polliack A, Raanani P. The late adverse events of rituximab therapy - rare but there! Leuk Lymphoma. 2009:1–13. doi: 10.1080/10428190902934944. [DOI] [PubMed] [Google Scholar]
- 65.Lalazar G, Rund D, Shouval D. Screening, prevention and treatment of viral hepatitis B reactivation in patients with haematological malignancies. Br J Haematol. 2007;136:699–712. doi: 10.1111/j.1365-2141.2006.06465.x. [DOI] [PubMed] [Google Scholar]
- 66.Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113:4834–4840. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stolz C, Schuler M. Molecular mechanisms of resistance to rituximab and pharmacological strategies for its circumvention. Leuk Lymphoma. 2009;50:873–885. doi: 10.1080/10428190902878471. [DOI] [PubMed] [Google Scholar]
- 68.Winiarska M, Bil J, Wilczek E, et al. Statins impair antitumor effects of rituximab by inducing conformational changes of CD20. PLoS Med. 2008;5:e64. doi: 10.1371/journal.pmed.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hatjiharissi E, Xu L, Santos DD, et al. Increased natural killer cell expression of CD16, augmented binding and ADCC activity to rituximab among individuals expressing the Fc{gamma}RIIIa-158 V/V and V/F polymorphism. Blood. 2007;110:2561–2564. doi: 10.1182/blood-2007-01-070656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deans JP, Kalt L, Ledbetter JA, Schieven GL, Bolen JB, Johnson P. Association of 75/80-kDa phosphoproteins and the tyrosine kinases Lyn, Fyn, and Lck with the B cell molecule CD20. Evidence against involvement of the cytoplasmic regions of CD20. J Biol Chem. 1995;270:22632–22638. doi: 10.1074/jbc.270.38.22632. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki E, Umezawa K, Bonavida B. Rituximab inhibits the constitutively activated PI3K-Akt pathway in B-NHL cell lines: involvement in chemosensitization to drug-induced apoptosis. Oncogene. 2007;26:6184–6193. doi: 10.1038/sj.onc.1210448. [DOI] [PubMed] [Google Scholar]
- 72.Vega MI, Huerta-Yepaz S, Garban H, Jazirehi A, Emmanouilides C, Bonavida B. Rituximab inhibits p38 MAPK activity in 2F7 B NHL and decreases IL-10 transcription: pivotal role of p38 MAPK in drug resistance. Oncogene. 2004;23:3530–3540. doi: 10.1038/sj.onc.1207336. [DOI] [PubMed] [Google Scholar]
- 73.Stolz C, Hess G, Hahnel PS, et al. Targeting Bcl-2 family proteins modulates the sensitivity of B-cell lymphoma to rituximab-induced apoptosis. Blood. 2008;112:3312–3321. doi: 10.1182/blood-2007-11-124487. [DOI] [PubMed] [Google Scholar]
- 74.Olejniczak SH, Hernandez-Ilizaliturri FJ, Clements JL, Czuczman MS. Acquired resistance to rituximab is associated with chemotherapy resistance resulting from decreased Bax and Bak expression. Clin Cancer Res. 2008;14:1550–1560. doi: 10.1158/1078-0432.CCR-07-1255. [DOI] [PubMed] [Google Scholar]
- 75.Romaguera J, Fayad L, McLaughlin P, et al. Phase 1 trial of bortezomib in combination with rituximab-hyper-CVAD/methotrexate and cytarabine for untreated mantle cell lymphoma. Blood. 2008;112 doi: 10.1111/j.1365-2141.2010.08315.x. abstract 3051. [DOI] [PubMed] [Google Scholar]
- 76.O'Brien S, Moore JO, Boyd TE, et al. Randomized phase III trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2007;25:1114–1120. doi: 10.1200/JCO.2006.07.1191. [DOI] [PubMed] [Google Scholar]
- 77.Chanan-Khan A. Bcl-2 antisense therapy in hematologic malignancies. Curr Opin Oncol. 2004;16:581–585. doi: 10.1097/01.cco.0000142074.67968.eb. [DOI] [PubMed] [Google Scholar]
- 78.Cruz RI, Hernandez-Ilizaliturri FJ, Olejniczak S, et al. CD52 over-expression affects rituximab-associated complement-mediated cytotoxicity but not antibody-dependent cellular cytotoxicity: preclinical evidence that targeting CD52 with alemtuzumab may reverse acquired resistance to rituximab in non-Hodgkin lymphoma. Leuk Lymphoma. 2007;48:2424–2436. doi: 10.1080/10428190701647879. [DOI] [PubMed] [Google Scholar]
- 79.Ginaldi L, De Martinis M, Matutes E, et al. Levels of expression of CD52 in normal and leukemic B and T cells: correlation with in vivo therapeutic responses to Campath-1H. Leuk Res. 1998;22:185–191. doi: 10.1016/s0145-2126(97)00158-6. [DOI] [PubMed] [Google Scholar]
- 80.Nijmeijer B, van Schie MLJ, Willemze R, Falkenburg JHF. Rituximab and alemtuzumab in combination, but not alone, induce complete remissions in a preclinical animal model of primary human ALL: Rationale for combination treatment. Blood. 2008;112 abstract 2833. [Google Scholar]
- 81.Faderl S, Thomas DA, O'Brien S, et al. Experience with alemtuzumab plus rituximab in patients with relapsed and refractory lymphoid malignancies. Blood. 2003;101:3413–3415. doi: 10.1182/blood-2002-07-1952. [DOI] [PubMed] [Google Scholar]
- 82.Castillo J, Milani C, Mendez-Allwood D. Ofatumumab, a second-generation anti-CD20 monoclonal antibody, for the treatment of lymphoproliferative and autoimmune disorders. Expert Opin Investig Drugs. 2009;18:491–500. doi: 10.1517/13543780902832679. [DOI] [PubMed] [Google Scholar]
- 83.Teeling JL, Mackus WJ, Wiegman LJ, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362–371. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 84.Osterborg A, Kipps TJ, Mayer J, et al. Ofatumumab (HuMax-CD20), a novel CD20 monoclonal antibody, is an active treatment for patients with CLL refractory to both fludarabine and alemtuzumab or bulky fludarabine-refractory disease: Results from the planned interim analysis of an international pivotal trial. Blood. 2008;112 abstract 328. [Google Scholar]
- 85.Morschhauser F, Leonard JP, Fayad L, et al. Humanized Anti-CD20 Antibody, Veltuzumab, in Refractory/Recurrent Non-Hodgkin's Lymphoma: Phase I/II Results. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.19.9117. [DOI] [PubMed] [Google Scholar]
- 86.Hutas G. Ocrelizumab, a humanized monoclonal antibody against CD20 for inflammatory disorders and B-cell malignancies. Curr Opin Investig Drugs. 2008;9:1206–1215. [PubMed] [Google Scholar]
- 87.Leonard JP, Coleman M, Ketas J, et al. Combination antibody therapy with epratuzumab and rituximab in relapsed or refractory non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:5044–5051. doi: 10.1200/JCO.2005.13.821. [DOI] [PubMed] [Google Scholar]
- 88.Leonard JP, Schuster SJ, Emmanouilides C, et al. Durable complete responses from therapy with combined epratuzumab and rituximab: final results from an international multicenter, phase 2 study in recurrent, indolent, non-Hodgkin lymphoma. Cancer. 2008;113:2714–2723. doi: 10.1002/cncr.23890. [DOI] [PubMed] [Google Scholar]
- 89.Hoelzer D, Ludwig WD, Thiel E, et al. Improved outcome in adult B-cell acute lymphoblastic leukemia. Blood. 1996;87:495–508. [PubMed] [Google Scholar]
- 90.Magrath IT, Janus C, Edwards BK, et al. An effective therapy for both undifferentiated (including Burkitt's) lymphomas and lymphoblastic lymphomas in children and young adults. Blood. 1984;63:1102–1111. [PubMed] [Google Scholar]
- 91.Rizzieri DA, Johnson JL, Niedzwiecki D, et al. Intensive chemotherapy with and without cranial radiation for Burkitt leukemia and lymphoma: final results of Cancer and Leukemia Group B Study 9251. Cancer. 2004;100:1438–1448. doi: 10.1002/cncr.20143. [DOI] [PubMed] [Google Scholar]
- 92.Di Nicola M, Carlo-Stella C, Mariotti J, et al. High response rate and manageable toxicity with an intensive, short-term chemotherapy programme for Burkitt's lymphoma in adults. Br J Haematol. 2004;126:815–820. doi: 10.1111/j.1365-2141.2004.05141.x. [DOI] [PubMed] [Google Scholar]
- 93.Lacasce A, Howard O, Lib S, et al. Modified magrath regimens for adults with Burkitt and Burkitt-like lymphomas: preserved efficacy with decreased toxicity. Leuk Lymphoma. 2004;45:761–767. doi: 10.1080/1042819031000141301. [DOI] [PubMed] [Google Scholar]
- 94.Divine M, Casassus P, Koscielny S, et al. Burkitt lymphoma in adults: a prospective study of 72 patients treated with an adapted pediatric LMB protocol. Ann Oncol. 2005;16:1928–1935. doi: 10.1093/annonc/mdi403. [DOI] [PubMed] [Google Scholar]
- 95.Mead GM, Barrans SL, Qian W, et al. A prospective clinicopathologic study of dose-modified CODOX-M/IVAC in patients with sporadic Burkitt lymphoma defined using cytogenetic and immunophenotypic criteria (MRC/NCRI LY10 trial) Blood. 2008;112:2248–2260. doi: 10.1182/blood-2008-03-145128. [DOI] [PMC free article] [PubMed] [Google Scholar]