Abstract
The acid sensing ion channels (ASICs) are proton-gated cation channels expressed throughout the nervous system. ASICs are activated during acidic pH fluctuations, and recent work suggests that they are involved in excitatory synaptic transmission. ASICs can also induce neuronal degeneration and death during pathological extracellular acidosis caused by ischemia, autoimmune inflammation, and traumatic injury. Many endogenous neuromodulators target ASICs to affect their biophysical characteristics and contributions to neuronal activity. One of the most unconventional types of modulation occurs with the interaction of ASICs and neuropeptides. Collectively, FMRFamide-related peptides and dynorphins potentiate ASIC activity by decreasing the proton-sensitivity of steady state desensitization independent of G protein-coupled receptor activation. By decreasing the proton-sensitivity of steady state desensitization, the FMRFamide-related peptides and dynorphins permit ASICs to remain active at more acidic basal pH. Unlike the dynorphins, some FMRFamide-related peptides also potentiate ASIC activity by slowing inactivation and increasing the sustained current. Through mechanistic studies, the modulation of ASICs by FMRFamide-related peptides and dynorphins appears to be through distinct interactions with the extracellular domain of ASICs. Dynorphins are expressed throughout the nervous system and can increase neuronal death during prolonged extracellular acidosis, suggesting that the interaction between dynorphins and ASICs may have important consequences for the prevention of neurological injury. The overlap in expression of FMRFamide-related peptides with ASICs in the dorsal horn of the spinal cord suggests that their interaction may have important consequences for the treatment of pain during injury and inflammation.
1.1 Introduction
The acid sensing ion channels (ASICs) are proton-gated cation channels and members of the degenerin/epithelial sodium channel (DEG/ENaC) super family (1). There are four ASIC genes (ACCN1-4) which encode six known subunit isoforms including ASIC1a and ASIC1b, ASIC2a and ASIC2b, ASIC3, and ASIC4 (2-11). Three subunits combine to form functional homomeric (i.e. ASIC1a) or heteromeric channels (i.e. ASIC1a/ASIC2b), each with characteristic biophysical properties and tissue distributions (12-15). ASICs are enriched in the dorsal root ganglia (DRG), olfactory bulbs, hippocampus, amygdala, cerebellum, and cerebral cortex (16). Broadly speaking, ASIC1b and ASIC3 are found in sensory neurons while ASIC1a, ASIC2a, ASIC2b, and ASIC4 are found in both sensory and central neurons. In central neurons, ASICs are localized to the cell body, dendrites, and dendritic spines (17). ASICs are activated by reductions in extracellular pH and depolarize the membrane. Recent work shows that ASICs are activated during synaptic transmission (18,19). Specifically, acidic pH fluctuations in the synapse are due, at least in part, to proton release from synaptic vesicles within active regions of the brain (19-21). Furthermore, acidic pH fluctuations are a major form of neuromodulation in the retina (22). Thus, protons and ASICs represent a neurotransmitter system that functions in concert with more traditional neurotransmitters, such as glutamate, to mediate neuronal signaling.
Mice with disruptions in individual ASIC genes (ACCN1-3) are healthy, reproduce, and display no obvious signs of dysfunction (17,23,24). Moreover, simultaneous disruption of ASIC1, ASIC2, and ASIC3 results in viable animals (25). ASIC knockout animals, however, do display particular abnormalities in behavioral and sensory transduction. In particular, disruption of ASIC1a, which eliminates proton-gated currents activated by a pH above 5 in central neurons, results in deficiencies in behaviors linked to fear, anxiety, panic, and depression (26-32).Interestingly, disruption of ASIC2 has similar effects suggesting that both ASIC1a and ASIC2 are essential for proper function in the brain (33). Similarly, the localization of ASICs to cutaneous nerve terminals and the involvement of ASICs in sensory transduction suggests that acidic pH fluctuations are also critical for normal sensory inputs (23,24).
No mutations in ACCN1-3 have yet been shown to be the cause of a human disease and no therapeutics have yet been proven to improve human heath by targeting ASICs. However, ASICs are involved in a number of pathophysiological conditions and thus represent novel therapeutic targets in the treatment of neurological injury. ASIC1a activation attenuates seizure duration and ASIC3 may contribute to migraine (34,35). ASIC1a also mediates neurodegeneration and death under pathological conditions that induce long lasting cerebral acidosis. In this way, ASIC1a contributes to neuronal injury in cerebral ischemia (36-39), autoimmune inflammation (40-42), and traumatic injury (43). ASIC1 and ASIC3 also contribute to hyperalgesia (44-48). Through the genetic deletion or use of inhibitors of ASICs, such as amiloride, venom peptides (i.e. psalmotoxin 1 (PcTx1) or mambalgins), the severity and overall presentation of these nervous system diseases in animal models can be alleviated (48,49).
ASICs generally produce transient currents that inactivate in the continued presence of acidic extracellular pH. The exact concentration of protons required to induce activation varies between subunits and is affected by endogenous neuromodulators and toxins. Under certain conditions, the inactivation of ASICs can be incomplete and allow them to produce sustained currents in the continued presence of acidic extracellular pH (50,51). When ASICs are exposed to mildly acidic extracellular pH, which itself is not sufficient for robust activation, they become desensitized at steady state (52). ASICs that have undergone steady state desensitization are refractory to further decreases in extracellular pH. Steady state desensitization allows ASICs to selectively respond to rapid and localized pH fluctuations. In addition, there is an intimate connection between extracellular calcium and ASIC gating. Higher concentrations of calcium attenuate activation and steady state desensitization as well as accelerate the recovery from inactivation. There is thought to be a calcium binding site, as yet unidentified, within the extracellular domain of ASICs that interferes with the protonation of residues linked to activation and steady state desensitization (53,54). However, exactly where these residues reside within ASICs is the subject of debate. ASICs have several structural domains defined by their resemblance to the anatomy of a closed fist(12). The large extracellular domain of the channel is partially responsible for the gating characteristics of ASICs including the proton sensitivity of activation, steady state desensitization, and inactivation. The knuckle and finger domains project from the apical part of the channel and contain residues that are glycosylated (12,55-58). The palm domain is composed of beta sheets and lines the central region of the channel. The thumb domain is exposed to the outside of the channel and is critical for the proton sensitivity of activation (12,59). The wrist domain connects the extracellular to the transmembrane domains and is involved in transducing extracellular signals into changes in gating characteristics (60-63). The channel also contains a “beta-ball” domain which is critical for the conformational changes that accompany gating and is central to the knuckle, palm, and thumb domains (64). One of the most highlighted regions in the extracellular domain is the acidic pocket, an area composed of the thumb, palm, and beta-ball domains of adjacent subunits. This region is integral for activation and steady state desensitization (12,59,60,63,65).
1.2 ASICs and Neuropeptides
The DEG/ENaC superfamily includes FaNaC, a molluscan ion channel that is gated by the neuropeptide FMRFamide (Phe-Met-Arg-Phe-amide) and modulated by acidic pH (9). FaNaC-like receptors have been identified in mollusks and hydra (66,67) but not in mammals. While FMRFamide is not produced in mammals, injection of FMRFamide into the brain can alter behavior and blood pressure in rodents (68,69). Mammals do express FMRFamide-related peptides, including neuropeptide FF (NPFF), neuropeptide AF (NPAF), RFamide related peptide 1 and 3, (RFRP-1, RFRP-3), 26-RFamide, prolactin releasing peptide (PrP), and Kiss1 (68,70-73). The pharmacology of the FMRFamide-related peptides in mammals is thought to arise from the activation of five distinct G protein-coupled receptors (74). However, the FMRFamide-related peptides also have effects that are independent of G protein-coupled receptor activation (75-80). ASICs, like FaNaC, are affected by the FMRFamide-related peptides, but while FaNaC is activated by neuropeptides and modulated by acidic pH, ASICs are activated by acidic pH and modulated by neuropeptides (81,82).
Several aspects of ASIC current are affected by FMRFamide and the FMRFamide-related peptides (Figure 1). Most notably, FMRFamide slows inactivation and induces a pH-dependent sustained current in ASIC1 and ASIC3 (81). Moreover, the sustained current displays reduced ion selectivity compared to the transient peak current. The effect of FMRFamide is observed in Xenopus oocytes expressing different ASIC subunits, cultured cells transfected with different ASIC subunits, and native proton-gated currents from isolated DRG or central neurons (81,83). FMRFamide affects both homomeric ASIC1 and ASIC3 as well as heteromericASIC1b/ASIC3, ASIC1a/2a, ASIC1a/2b, and ASIC3/2a (81,84-87). With ASIC3/2a, FMRFamide slows inactivation and enhances the amplitude of the transient current (84). Homomeric ASIC2a or heteromeric ASIC2a/2b are unaffected by FMRFamide.
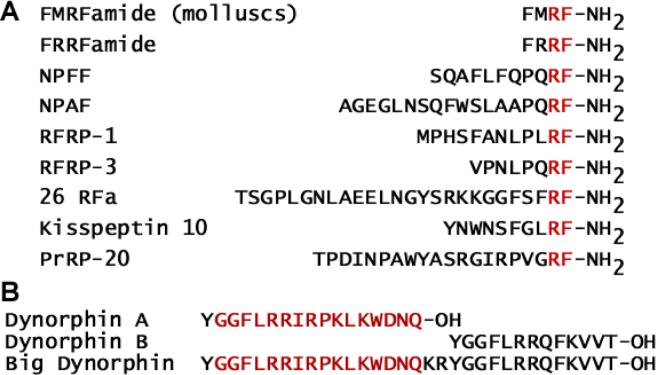
Figure 1.
Amino acid sequenced of the FMRFamide-related peptided and dynorphins, (A) The sequence of FMRFamide and human FMRFamide-related neuropeptided. The “RFamide” moiety is shown in red. (B) Amino acid sequences of the dunorphins. The smallest dynorphin peptide that modulates ASIC is highlighted in red.
In DRG neurons, an extensive complement of ASIC1, ASIC2, and ASIC3 are expressed (14). The increase in the sustained current by FMRFamide is dependent on ASIC3 in DRG neurons, as disruption of ASIC3 eliminates the effect of FMRFamide (81,88,89). Furthermore, while loss of ASIC2 is without effect, loss of ASIC1 from mouse DRG neurons potentiates the effects of FMRFamide (87). These results indicate that ASIC3 preferentially mediates the increase in the sustained current observed with FMRFamide in DRG neurons. In central neurons that do not express ASIC3, FMRFamide slows inactivation and some sustained current is observed, however, the effect is less pronounced than that observed in DRG neurons (83). FMRFamide also decreases the proton sensitivity of steady state desensitization of ASIC1b/ASIC3 (85) and ASIC1a (86). Specifically, FMRFamide reduces the proton-sensitivity of steady state desensitization such that more acidic pH is required to induce desensitization (Figure 2). Endogenous FMRFamide-related peptides also modulate ASIC inactivation and induce sustained current. In particular, NPFF and RFRP-1 increase the sustained proton-gated currents in mouse DRG neurons. Similarly, the mammalian neuropeptides NPFF and QRFP decrease the proton sensitivity of steady state desensitization (87). Ultimately, the specific effect of a given FMRFamide-related peptide is dependent upon its primary sequence in combination with the ASIC subunit and species identity. Furthermore, while the concentration of FMRFamide-related peptide needed to modulate ASICs is high, ASICs are localized to synapses and respond to acidic pH fluctuations within the synaptic cleft. The synaptic cleft is an area that sees a localized and highly concentrated accumulation of neuropeptide after neurotransmitter release and is an ideal location for the modulation of ASICs by the FMRFamide-related peptides.
Figure 2. FRRFamide, FMRFamide, and Dynorphin A Modulation of Human ASIC1a.
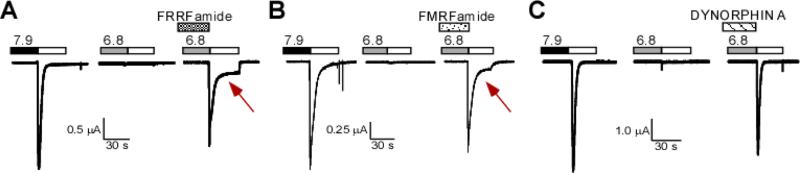
Representative traces of human ASICa expressed in Xenopus oocytes showing the response to FRRFamide (A), FMRFamide (B), or Dynorphin A(1-17) (C), Note that all three peptided inhibit steady-sate desenitization of ASIC and allow activation after incubation with Ph 6.8. Only FMRF Famide and FRRFamide slow inactivation and induce a sustained current (red arrows). Dynorphin A, FRRF amide, and FMRFamide were applied fro two minutes at Ph 7.9 (50100μ) and during the Ph 6.8 condition solution indicated in grey boxes above the trace. The pH 5.0 test solution was applied for 30s as indicated in white boxes the trace.
FMRFamide action generally requires the peptide to be applied before the extracellular solution is acidified. For example, if FMRFamide is present at pH 7.4 prior to a pH reduction sufficient for activation, there is an increase in the sustained current of ASIC1a and ASIC1b/ASIC3 (Figure 2B). If FMRFamide is co-applied during the pH reduction, little change in the current kinetics occurs. Accordingly, the effect of FMRFamide remains regardless of whether or not it is continuously applied during the pH reduction (81,85). These results suggest that FMRFamide initially binds to ASICs in the closed or inactivated state, presumably through a C-terminal arginine, as neutralization of this positive charge to valine attenuates the increase in the sustained proton-gated current in rat DRG neurons (90). The effects of the FMRFamide-related peptides are also pH dependent, being most pronounced at pH 5, and eliminated by increasing extracellular calcium (85,90). These results suggest that the FMRFamide-related peptides have their greatest effects when ASICs are in a protonated state and calcium is not bound. Although exactly how the FMRFamide-related peptides modulate the gating of ASICs is not well understood, recent work suggests that the palm domain is essential for the effects of FMRFamide. In the inactivated state of ASIC1a, L415 in the region that links beta sheets 11 and 12 within the palm domain (β11-β12linker) hydrophobically interacts with I307 in α7 of the thumb domain, V367 in β10 of the palm domain, and L280 in β9 of the palm domain. The L415C and L280C mutations result in an increase in the sustained current of ASIC1a when treated with MTSET. The L280C mutation also potentiates the increase in the sustained and transient currents by the synthetic FMRFamide-related peptide, FRRFa, in ASIC1a. These results suggest that the FMRFamide-related peptides affect the lower palm domain by interfering with the conformational changes necessary for inactivation (91). Despite this, not all of the FMRFamide-related peptides that interact with ASICs slow inactivation; some decrease the proton sensitivity of steady state desensitization. Thus, there are likely additional interactions between the FMRFamide-related peptides and ASICs that are as yet unknown.
In addition to FMRFamide-related peptides, the dynorphin peptides modulate ASICs. Dynorphins are highly basic peptides expressed abundantly throughout the nervous system (Figure 1). Specifically, the dynorphins are expressed in the amygdala and contribute to fear learning (92). Both big dynorphin and dynorphin A decrease the proton-sensitivity of steady state desensitization of ASIC1a and ASIC1b independent of opioid receptor activation (Figure 2C). Big dynorphin also decreases the proton sensitivity of steady state desensitization of proton-gated currents in mouse cortical neurons. Big dynorphin is nearly 1,000 times more potent than dynorphin A in its modulation of ASICs, while dynorphin B is without effect. Thus, like the FMRFamide-related peptides, their primary structures determine not only their potency but their specific modulatory effects. Like the FMRFamide-related peptides, dynorphins are most effective when applied before the extracellular solution is acidified. However, unlike the FMRFamide-related peptides, big dynorphin does not slow inactivation and induce sustained currents. The dynorphins appear to only affect homomeric ASIC1 and heteromeric ASIC1a/2b channels, and the presence of ASIC3 subunits attenuates the effects of dynorphin (93). PcTx1 interacts with the acidic pocket of the extracellular domain and the surrounding area to increase the proton-sensitivity of steady state desensitization of ASIC1a (49,94) and ASIC1a/2b (95). Interestingly, PcTx1 competes with big dynorphin in the modulation of steady state desensitization of ASIC1a, suggesting that they may share a binding site. Furthermore, PcTx1 can also affect pain as intrathecal injections of PcTx1 reduce mechanical and thermal hyperalgesia in mice (44).
The interactions between neuropeptides and ASICs could have a variety of physiological and pathological consequences. The FMRF-amide related peptides are abundant within the spinal cord and both ASICs and the FMRFamide-related peptides localize to the dorsal horn of the spinal cord. In addition, both the FMRFamide-related peptides and ASICs affect pain processing. As FMRFamide decreases the proton-sensitivity of steady state desensitization and slows inactivation, resulting in a sustained proton-gated current in DRG neurons, extracellular acidification and concomitant accumulation of FMRFamide-related peptides during injury and inflammation may contribute to the role of ASICs in hyperalgesia. Taken together, these studies suggest that (1) the FMRFamide-related peptides preferentially interact with the extracellular domain of the inactivated or closed channel and potentiate ASIC activity by decreasing the proton-sensitivity of steady state desensitization and slowing inactivation and (2) the dynorphin peptides preferentially interact near the acidic pocket in the extracellular domain of the channel and potentiate ASIC activity by decreasing the proton-sensitivity of steady state desensitization.
The importance of the interaction between FMRFamide-related peptides and ASICs in the brain is less clear. The FMRFamide-related peptides have predominantly been studied in the hypothalamus, a location where the function of ASICs remains unknown. Thus, although FMRFamide slows inactivation of ASICs in Purkinje neurons from the cerebellum, it is not clear how this effect may contribute to neuronal activity in the brain. On the other hand, dynorphins and ASICs are both localized to the amygdala and involved in fear and anxiety. The dynorphin peptides big dynorphin and dynorphin A decrease the proton-sensitivity of steady state desensitization of proton-gated currents in cortical neurons. This would allow ASICs to continue to respond to reductions in pH even after the basal pH has become modestly acidic. Moreover, big dynorphin enhances neuronal death following prolonged extracellular acidosis (93). Interestingly, mutations in the dynorphin A peptide cause neurodegeneration in spinocerebellar ataxia 23 (SCA23) through non-opioid receptor dependent mechanisms (96). Dynorphins can modulate NMDA receptors as well as ASICs suggesting dynorphins may interact with additional channels (97). Yet, our results suggested that ASICs could play a role (98), and recent work indicates that ASIC1a contributes to neuronal damage in spinocerebellar ataxia and suggest inhibition of ASIC1a activity could provide a novel strategy to attenuate neurological injury in this genetic disease (99). Thus, understanding the interaction between ASICs and neuropeptides may have profound implications for the prevention of ASIC-mediated injuries in conditions of constitutive cerebral acidosis. New insights to promote the development of novel therapeutics that can limit ASIC activity though modulation of steady state desensitization and inactivation are an important first step in this process. In short, preventing the interaction of the FMRFamide-related peptides with ASICs may prove useful in the treatment of hyperalgesia while preventing the interaction of the dynorphin peptides with ASICs may reduce the severity of neuronal injury by preventing neuronal death upon prolonged extracellular acidosis.
The acid sensing ion channels (ASICs) are important for normal behaviors.
ASICs also induce neuronal degeneration in pathological conditions.
ASICs are modulated by neuropeptides.
Dynorphins and RFamide-related peptides can enhance ASIC activity.
These effects are independent of GPCRs.
Neuropeptides may affect ASICs role in pain, behavior, and neuronal injury.
Acknowledgements
This work was possible through a NIH R01 NINDS #NS062967. J. S. Vick was supported from a P30 NINDS Core Grant #NS045758. The authors acknowledge Thomas Sherwood for technical assistance and Kirk Mykytyn for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 2.Akopian AN, Chen CC, Ding Y, Cesare P, Wood JN. A new member of the acid-sensing ion channel family. Neuroreport. 2000;11:2217–2222. doi: 10.1097/00001756-200007140-00031. [DOI] [PubMed] [Google Scholar]
- 3.Babinski K, Le KT, Seguela P. Molecular cloning and regional distribution of a human proton receptor subunit with biphasic functional properties. J Neurochem. 1999;72:51–57. doi: 10.1046/j.1471-4159.1999.0720051.x. [DOI] [PubMed] [Google Scholar]
- 4.Bassler EL, Ngo-Anh TJ, Geisler HS, Ruppersberg JP, Grunder S. Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J Biol Chem. 2001;276:33782–33787. doi: 10.1074/jbc.M104030200. [DOI] [PubMed] [Google Scholar]
- 5.Chen CC, England S, Akopian AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci U S A. 1998;95:10240–10245. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Weille JR, Bassilana F, Lazdunski M, Waldmann R. Identification, functional expression and chromosomal localisation of a sustained human proton-gated cation channel. FEBS Lett. 1998;433:257–260. doi: 10.1016/s0014-5793(98)00916-8. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Anoveros J, Derfler B, Neville-Golden J, Hyman BT, Corey DP. BNaC1 and BNaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proc Natl Acad Sci U S A. 1997;94:1459–1464. doi: 10.1073/pnas.94.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grunder S, Geissler HS, Bassler EL, Ruppersberg JP. A new member of acid-sensing ion channels from pituitary gland. Neuroreport. 2000;11:1607–1611. doi: 10.1097/00001756-200006050-00003. [DOI] [PubMed] [Google Scholar]
- 9.Lingueglia E, Champigny G, Lazdunski M, Barbry P. Cloning of the amiloride-sensitive FMRFamide peptide-gated sodium channel. Nature. 1995;378:730–733. doi: 10.1038/378730a0. [DOI] [PubMed] [Google Scholar]
- 10.Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. J Biol Chem. 1996;271:7879–7882. doi: 10.1074/jbc.271.14.7879. [DOI] [PubMed] [Google Scholar]
- 11.Waldmann R, Champigny G, Voilley N, Lauritzen I, Lazdunski M. The mammalian degenerin MDEG, an amiloride-sensitive cation channel activated by mutations causing neurodegeneration in Caenorhabditis elegans. J Biol Chem. 1996;271:10433–10436. doi: 10.1074/jbc.271.18.10433. [DOI] [PubMed] [Google Scholar]
- 12.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 13.Baron A, Waldmann R, Lazdunski M. ASIC-like, proton-activated currents in rat hippocampal neurons. J Physiol. 2002;539:485–494. doi: 10.1113/jphysiol.2001.014837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci U S A. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hesselager M, Timmermann DB, Ahring PK. pH Dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem. 2004;279:11006–11015. doi: 10.1074/jbc.M313507200. [DOI] [PubMed] [Google Scholar]
- 16.Waldmann R, Lazdunski M. H(+)-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- 17.Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr., Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- 18.Kreple CJ, Lu Y, Taugher RJ, Schwager-Gutman AL, Du J, Stump M, Wang Y, Ghobbeh A, Fan R, Cosme CV, Sowers LP, Welsh MJ, Radley JJ, LaLumiere RT, Wemmie JA. Acid-sensing ion channels contribute to synaptic transmission and inhibit cocaine-evoked plasticity. Nat Neurosci. 2014 doi: 10.1038/nn.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du J, Reznikov LR, Price MP, Zha X.-m., Lu Y, Moninger TO, Wemmie JA, Welsh MJ. Protons are a neurotransmitter that regulates synaptic plasticity in the lateral amygdala. Proceedings of the National Academy of Sciences. 2014:201407018. doi: 10.1073/pnas.1407018111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeVries SH. Exocytosed protons feedback to suppress the Ca2+ current in mammalian cone photoreceptors. Neuron. 2001;32:1107–1117. doi: 10.1016/s0896-6273(01)00535-9. [DOI] [PubMed] [Google Scholar]
- 21.Magnotta VA, Heo H-Y, Dlouhy BJ, Dahdaleh NS, Follmer RL, Thedens DR, Welsh MJ, Wemmie JA. Detecting activity-evoked pH changes in human brain. Proceedings of the National Academy of Sciences. 2012;109:8270–8273. doi: 10.1073/pnas.1205902109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang TM, Holzhausen LC, Kramer RH. Imaging an optogenetic pH sensor reveals that protons mediate lateral inhibition in the retina. Nat Neurosci. 2014;17:262–268. doi: 10.1038/nn.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407:1007–1011. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- 24.Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 25.Kang S, Jang JH, Price MP, Gautam M, Benson CJ, Gong H, Welsh MJ, Brennan TJ. Simultaneous disruption of mouse ASIC1a, ASIC2 and ASIC3 genes enhances cutaneous mechanosensitivity. PloS one. 2012;7:e35225. doi: 10.1371/journal.pone.0035225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, 3rd, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coryell MW, Wunsch AM, Haenfler JM, Allen JE, Schnizler M, Ziemann AE, Cook MN, Dunning JP, Price MP, Rainier JD, Liu Z, Light AR, Langbehn DR, Wemmie JA. Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J Neurosci. 2009;29:5381–5388. doi: 10.1523/JNEUROSCI.0360-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coryell MW, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM, Price M, Schnizler MK, Wemmie JA. Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biol Psychiatry. 2007;62:1140–1148. doi: 10.1016/j.biopsych.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Wemmie JA, Coryell MW, Askwith CC, Lamani E, Leonard AS, Sigmund CD, Welsh MJ. Overexpression of acid-sensing ion channel 1a in transgenic mice increases acquired fear-related behavior. Proc Natl Acad Sci U S A. 2004;101:3621–3626. doi: 10.1073/pnas.0308753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr., Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23:5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dwyer JM, Rizzo SJ, Neal SJ, Lin Q, Jow F, Arias RL, Rosenzweig-Lipson S, Dunlop J, Beyer CE. Acid sensing ion channel (ASIC) inhibitors exhibit anxiolytic-like activity in preclinical pharmacological models. Psychopharmacology (Berl) 2009;203:41–52. doi: 10.1007/s00213-008-1373-7. [DOI] [PubMed] [Google Scholar]
- 32.Pidoplichko VI, Aroniadou-Anderjaska V, Prager EM, Figueiredo TH, Almeida-Suhett CP, Miller SL, Braga MF. ASIC1a activation enhances inhibition in the basolateral amygdala and reduces anxiety. J Neurosci. 2014;34:3130–3141. doi: 10.1523/JNEUROSCI.4009-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price MP, Gong H, Parsons MG, Kundert JR, Reznikov LR, Bernardinelli L, Chaloner K, Buchanan GF, Wemmie JA, Richerson GB. Localization and behaviors in null mice suggest that ASIC1 and ASIC2 modulate responses to aversive stimuli. Genes, Brain and Behavior. 2014;13:179–194. doi: 10.1111/gbb.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziemann AE, Schnizler MK, Albert GW, Severson MA, Howard MA, 3rd, Welsh MJ, Wemmie JA. Seizure termination by acidosis depends on ASIC1a. Nat Neurosci. 2008;11:816–822. doi: 10.1038/nn.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan J, Edelmayer RM, Wei X, De Felice M, Porreca F, Dussor G. Dural afferents express acid-sensing ion channels: a role for decreased meningeal pH in migraine headache. Pain. 2011;152:106–113. doi: 10.1016/j.pain.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duan B, Wang YZ, Yang T, Chu XP, Yu Y, Huang Y, Cao H, Hansen J, Simon RP, Zhu MX, Xiong ZG, Xu TL. Extracellular spermine exacerbates ischemic neuronal injury through sensitization of ASIC1a channels to extracellular acidosis. J Neurosci. 2011;31:2101–2112. doi: 10.1523/JNEUROSCI.4351-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pignataro G, Simon RP, Xiong ZG. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain. 2007;130:151–158. doi: 10.1093/brain/awl325. [DOI] [PubMed] [Google Scholar]
- 38.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 39.Yang Z-J, Ni X, Carter EL, Kibler K, Martin LJ, Koehler RC. Neuroprotective effect of acid-sensing ion channel inhibitor psalmotoxin-1 after hypoxia–ischemia in newborn piglet striatum. Neurobiology of disease. 2011;43:446–454. doi: 10.1016/j.nbd.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arun T, Tomassini V, Sbardella E, de Ruiter MB, Matthews L, Leite MI, Gelineau-Morel R, Cavey A, Vergo S, Craner M, Fugger L, Rovira A, Jenkinson M, Palace J. Targeting ASIC1 in primary progressive multiple sclerosis: evidence of neuroprotection with amiloride. Brain. 2013;136:106–115. doi: 10.1093/brain/aws325. [DOI] [PubMed] [Google Scholar]
- 41.Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, Vincent A, Fugger L. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med. 2007;13:1483–1489. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- 42.Vergo S, Craner MJ, Etzensperger R, Attfield K, Friese MA, Newcombe J, Esiri M, Fugger L. Acid-sensing ion channel 1 is involved in both axonal injury and demyelination in multiple sclerosis and its animal model. Brain. 2011;134:571–584. doi: 10.1093/brain/awq337. [DOI] [PubMed] [Google Scholar]
- 43.Yin T, Lindley TE, Albert GW, Ahmed R, Schmeiser PB, Grady MS, Howard MA, Welsh MJ. Loss of Acid sensing ion channel-1a and bicarbonate administration attenuate the severity of traumatic brain injury. PLoS One. 2013;8:e72379. doi: 10.1371/journal.pone.0072379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazzuca M, Heurteaux C, Alloui A, Diochot S, Baron A, Voilley N, Blondeau N, Escoubas P, Gelot A, Cupo A, Zimmer A, Zimmer AM, Eschalier A, Lazdunski M. A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nat Neurosci. 2007;10:943–945. doi: 10.1038/nn1940. [DOI] [PubMed] [Google Scholar]
- 45.Walder RY, Gautam M, Wilson SP, Benson CJ, Sluka KA. Selective targeting of ASIC3 using artificial miRNAs inhibits primary and secondary hyperalgesia after muscle inflammation. Pain. 2011;152:2348–2356. doi: 10.1016/j.pain.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walder RY, Rasmussen LA, Rainier JD, Light AR, Wemmie JA, Sluka KA. ASIC1 and ASIC3 play different roles in the development of Hyperalgesia after inflammatory muscle injury. J Pain. 2010;11:210–218. doi: 10.1016/j.jpain.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ikeuchi M, Kolker SJ, Burnes LA, Walder RY, Sluka KA. Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. Pain. 2008;137:662–669. doi: 10.1016/j.pain.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diochot S, Baron A, Salinas M, Douguet D, Scarzello S, Dabert-Gay AS, Debayle D, Friend V, Alloui A, Lazdunski M, Lingueglia E. Black mamba venom peptides target acid-sensing ion channels to abolish pain. Nature. 2012;490:552–555. doi: 10.1038/nature11494. [DOI] [PubMed] [Google Scholar]
- 49.Escoubas P, De Weille JR, Lecoq A, Diochot S, Waldmann R, Champigny G, Moinier D, Menez A, Lazdunski M. Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem. 2000;275:25116–25121. doi: 10.1074/jbc.M003643200. [DOI] [PubMed] [Google Scholar]
- 50.Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem. 1997;272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- 51.Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- 52.Babini E, Paukert M, Geisler HS, Grunder S. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1). J Biol Chem. 2002;277:41597–41603. doi: 10.1074/jbc.M205877200. [DOI] [PubMed] [Google Scholar]
- 53.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4:869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- 54.Zhang P, Sigworth FJ, Canessa CM. Gating of acid-sensitive ion channel-1: release of Ca2+ block vs. allosteric mechanism. J Gen Physiol. 2006;127:109–117. doi: 10.1085/jgp.200509396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jing L, Chu XP, Jiang YQ, Collier DM, Wang B, Jiang Q, Snyder PM, Zha XM. N-glycosylation of acid-sensing ion channel 1a regulates its trafficking and acidosis-induced spine remodeling. J Neurosci. 2012;32:4080–4091. doi: 10.1523/JNEUROSCI.5021-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jing L, Jiang YQ, Jiang Q, Wang B, Chu XP, Zha XM. The interaction between the first transmembrane domain and the thumb of ASIC1a is critical for its N-glycosylation and trafficking. PLoS One. 2011;6:e26909. doi: 10.1371/journal.pone.0026909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kadurin I, Golubovic A, Leisle L, Schindelin H, Grunder S. Differential effects of N-glycans on surface expression suggest structural differences between the acid-sensing ion channel (ASIC) 1a and ASIC1b. Biochem J. 2008;412:469–475. doi: 10.1042/BJ20071614. [DOI] [PubMed] [Google Scholar]
- 58.Saugstad JA, Roberts JA, Dong J, Zeitouni S, Evans RJ. Analysis of the membrane topology of the acid-sensing ion channel 2a. J Biol Chem. 2004;279:55514–55519. doi: 10.1074/jbc.M411849200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sherwood T, Franke R, Conneely S, Joyner J, Arumugan P, Askwith C. Identification of protein domains that control proton and calcium sensitivity of ASIC1a. J Biol Chem. 2009;284:27899–27907. doi: 10.1074/jbc.M109.029009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li T, Yang Y, Canessa CM. Interaction of the aromatics Tyr-72/Trp-288 in the interface of the extracellular and transmembrane domains is essential for proton gating of acid-sensing ion channels. J Biol Chem. 2009;284:4689–4694. doi: 10.1074/jbc.M805302200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Passero CJ, Okumura S, Carattino MD. Conformational changes associated with proton-dependent gating of ASIC1a. J Biol Chem. 2009;284:36473–36481. doi: 10.1074/jbc.M109.055418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tolino LA, Okumura S, Kashlan OB, Carattino MD. Insights into the mechanism of pore opening of acid-sensing ion channel 1a. J Biol Chem. 2011;286:16297–16307. doi: 10.1074/jbc.M110.202366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang H, Yu Y, Li WG, Yu F, Cao H, Xu TL, Jiang H. Inherent dynamics of the acid-sensing ion channel 1 correlates with the gating mechanism. PLoS Biol. 2009;7:e1000151. doi: 10.1371/journal.pbio.1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bargeton B, Kellenberger S. The contact region between three domains of the extracellular loop of ASIC1a is critical for channel function. J Biol Chem. 2010;285:13816–13826. doi: 10.1074/jbc.M109.086843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paukert M, Chen X, Polleichtner G, Schindelin H, Grunder S. Candidate amino acids involved in H+ gating of acid-sensing ion channel 1a. J Biol Chem. 2008;283:572–581. doi: 10.1074/jbc.M706811200. [DOI] [PubMed] [Google Scholar]
- 66.Dürrnagel S, Kuhn A, Tsiairis CD, Williamson M, Kalbacher H, Grimmelikhuijzen CJ, Holstein TW, Gründer S. Three homologous subunits form a high affinity peptide-gated ion channel in Hydra. Journal of Biological Chemistry. 2010;285:11958–11965. doi: 10.1074/jbc.M109.059998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Golubovic A, Kuhn A, Williamson M, Kalbacher H, Holstein TW, Grimmelikhuijzen CJ, Gründer S. A peptide-gated ion channel from the freshwater polyp Hydra. Journal of Biological Chemistry. 2007;282:35098–35103. doi: 10.1074/jbc.M706849200. [DOI] [PubMed] [Google Scholar]
- 68.Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nature Cell Biology. 2000;2:703–708. doi: 10.1038/35036326. [DOI] [PubMed] [Google Scholar]
- 69.Muthal A, Mandhane S, Chopde C. Central administration of FMRFamide produces antipsychotic-like effects in rodents. Neuropeptides. 1997;31:319–322. doi: 10.1016/s0143-4179(97)90065-2. [DOI] [PubMed] [Google Scholar]
- 70.Yang HY, Fratta W, Majane EA, Costa E. Isolation, sequencing, synthesis, and pharmacological characterization of two brain neuropeptides that modulate the action of morphine. Proc Natl Acad Sci U S A. 1985;82:7757–7761. doi: 10.1073/pnas.82.22.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang Y, Luo L, Gustafson EL, Yadav D, Laverty M, Murgolo N, Vassileva G, Zeng M, Laz TM, Behan J, Qiu P, Wang L, Wang S, Bayne M, Greene J, Monsma F, Jr., Zhang FL. Identification and characterization of a novel RF-amide peptide ligand for orphan G-protein-coupled receptor SP9155. J Biol Chem. 2003;278:27652–27657. doi: 10.1074/jbc.M302945200. [DOI] [PubMed] [Google Scholar]
- 72.Hinuma S, Habata Y, Fujii R, Kawamata Y, Hosoya M, Fukusumi S, Kitada C, Masuo Y, Asano T, Matsumoto H, Sekiguchi M, Kurokawa T, Nishimura O, Onda H, Fujino M. A prolactin-releasing peptide in the brain. Nature. 1998;393:272–276. doi: 10.1038/30515. [DOI] [PubMed] [Google Scholar]
- 73.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–1737. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 74.Parhar I, Ogawa S, Kitahashi T. RFamide peptides as mediators in environmental control of GnRH neurons. Prog Neurobiol. 2012;98:176–196. doi: 10.1016/j.pneurobio.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 75.Allard M, Geoffre S, Legendre P, Vincent J, Simonnet G. Characterization of rat spinal cord receptors to FLFQPQRFamide, a mammalian morphine modulating peptide: a binding study. Brain Res. 1989;500:169–176. doi: 10.1016/0006-8993(89)90311-9. [DOI] [PubMed] [Google Scholar]
- 76.Gayton R. Mammalian neuronal actions of FMRFamide and the structurally related opioid Met-enkephalin-Arg6-Phe7. 1982. [DOI] [PubMed]
- 77.Kavaliers M. Calcium channel blockers inhibit the antagonistic effects of Phe-Met-Arg-Phe-amide (FMRFamide) on morphine- and stress-induced analgesia in mice. Brain Res. 1987;415:380–384. doi: 10.1016/0006-8993(87)90225-3. [DOI] [PubMed] [Google Scholar]
- 78.Raffa RB. The action of FMRFamide (Phe-Met-Arg-Phe-NH2) and related peptides on mammals. Peptides. 1988;9:915–922. doi: 10.1016/0196-9781(88)90141-6. [DOI] [PubMed] [Google Scholar]
- 79.Raffa RBH,J, Porreca F. Intrathecal FMRFamide (Phe-Met-Arg-Phe-NH2) induces excessive grooming behavior in mice. Neurosci Lett. 1986;65:94–98. doi: 10.1016/0304-3940(86)90126-6. [DOI] [PubMed] [Google Scholar]
- 80.Roumy M, Zajac J-M. Neuropeptide FF, pain and analgesia. European journal of pharmacology. 1998;345:1–11. doi: 10.1016/s0014-2999(97)01604-x. [DOI] [PubMed] [Google Scholar]
- 81.Askwith CC, Cheng C, Ikuma M, Benson C, Price MP, Welsh MJ. Neuropeptide FF and FMRFamide potentiate acid-evoked currents from sensory neurons and proton-gated DEG/ENaC channels. Neuron. 2000;26:133–141. doi: 10.1016/s0896-6273(00)81144-7. [DOI] [PubMed] [Google Scholar]
- 82.Perry SJ, Straub VA, Schofield MG, Burke JF, Benjamin PR. Neuronal expression of an FMRFamide-gated Na+ channel and its modulation by acid pH. J Neurosci. 2001;21:5559–5567. doi: 10.1523/JNEUROSCI.21-15-05559.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Allen NJ, Attwell D. Modulation of ASIC channels in rat cerebellar Purkinje neurons by ischaemia-related signals. J Physiol. 2002;543:521–529. doi: 10.1113/jphysiol.2002.020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Catarsi S, Babinski K, Seguela P. Selective modulation of heteromeric ASIC proton-gated channels by neuropeptide FF. Neuropharmacology. 2001;41:592–600. doi: 10.1016/s0028-3908(01)00107-1. [DOI] [PubMed] [Google Scholar]
- 85.Chen X, Kalbacher H, Grunder S. Interaction of acid-sensing ion channel (ASIC) 1 with the tarantula toxin psalmotoxin 1 is state dependent. J Gen Physiol. 2006;127:267–276. doi: 10.1085/jgp.200509409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sherwood TW, Askwith CC. Endogenous arginine-phenylalanine-amide-related peptides alter steady-state desensitization of ASIC1a. J Biol Chem. 2008;283:1818–1830. doi: 10.1074/jbc.M705118200. [DOI] [PubMed] [Google Scholar]
- 87.Xie J, Price MP, Wemmie JA, Askwith CC, Welsh MJ. ASIC3 and ASIC1 mediate FMRFamide-related peptide enhancement of H+-gated currents in cultured dorsal root ganglion neurons. J Neurophysiol. 2003;89:2459–2465. doi: 10.1152/jn.00707.2002. [DOI] [PubMed] [Google Scholar]
- 88.Deval E, Baron A, Lingueglia E, Mazarguil H, Zajac JM, Lazdunski M. Effects of neuropeptide SF and related peptides on acid sensing ion channel 3 and sensory neuron excitability. Neuropharmacology. 2003;44:662–671. doi: 10.1016/s0028-3908(03)00047-9. [DOI] [PubMed] [Google Scholar]
- 89.Xie J, Price MP, Berger AL, Welsh MJ. DRASIC contributes to pH-gated currents in large dorsal root ganglion sensory neurons by forming heteromultimeric channels. J Neurophysiol. 2002;87:2835–2843. doi: 10.1152/jn.2002.87.6.2835. [DOI] [PubMed] [Google Scholar]
- 90.Ostrovskaya O, Moroz L, Krishtal O. Modulatory action of RFamide-related peptides on acid-sensing ionic channels is pH dependent: the role of arginine. J Neurochem. 2004;91:252–255. doi: 10.1111/j.1471-4159.2004.02688.x. [DOI] [PubMed] [Google Scholar]
- 91.Frey EN, Pavlovicz RE, Wegman CJ, Li C, Askwith CC. Conformational changes in the lower palm domain of ASIC1a contribute to desensitization and RFamide modulation. PloS one. 2013;8:e71733. doi: 10.1371/journal.pone.0071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bilkei-Gorzo A, Erk S, Schürmann B, Mauer D, Michel K, Boecker H, Scheef L, Walter H, Zimmer A. Dynorphins regulate fear memory: from mice to men. The Journal of Neuroscience. 2012;32:9335–9343. doi: 10.1523/JNEUROSCI.1034-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sherwood TW, Askwith CC. Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity and acidosis-induced neuronal death. J Neurosci. 2009;29:14371–14380. doi: 10.1523/JNEUROSCI.2186-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen X, Kalbacher H, Grunder S. The tarantula toxin psalmotoxin 1 inhibits acid-sensing ion channel (ASIC) 1a by increasing its apparent H+ affinity. J Gen Physiol. 2005;126:71–79. doi: 10.1085/jgp.200509303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sherwood TW, Lee KG, Gormley MG, Askwith CC. Heteromeric acid-sensing ion channels (ASICs) composed of ASIC2b and ASIC1a display novel channel properties and contribute to acidosis-induced neuronal death. J Neurosci. 2011;31:9723–9734. doi: 10.1523/JNEUROSCI.1665-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bakalkin G, Watanabe H, Jezierska J, Depoorter C, Verschuuren-Bemelmans C, Bazov I, Artemenko KA, Yakovleva T, Dooijes D, Van de Warrenburg BP, Zubarev RA, Kremer B, Knapp PE, Hauser KF, Wijmenga C, Nyberg F, Sinke RJ, Verbeek DS. Prodynorphin mutations cause the neurodegenerative disorder spinocerebellar ataxia type 23. Am J Hum Genet. 2010;87:593–603. doi: 10.1016/j.ajhg.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Caudle RM, Mannes AJ. Dynorphin: friend or foe? Pain. 2000;87:235–239. doi: 10.1016/S0304-3959(00)00360-2. [DOI] [PubMed] [Google Scholar]
- 98.Watanabe H, Mizoguchi H, Verbeek DS, Kuzmin A, Nyberg F, Krishtal O, Sakurada S, Bakalkin G. Non-opioid nociceptive activity of human dynorphin mutants that cause neurodegenerative disorder spinocerebellar ataxia type 23. Peptides. 2012;35:306–310. doi: 10.1016/j.peptides.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 99.Vig PJ, Hearst SM, Shao Q, Lopez ME. Knockdown of Acid-Sensing Ion Channel 1a (ASIC1a) Suppresses Disease Phenotype in SCA1 Mouse Model. Cerebellum. 2014;13:479–490. doi: 10.1007/s12311-014-0563-6. [DOI] [PubMed] [Google Scholar]