Abstract
Importance
Polycyclic aromatic hydrocarbons are common carcinogenic and neurotoxic urban air pollutants. Toxic exposures, including air pollution, are disproportionately high in communities of color and frequently co-occur with chronic economic deprivation.
Objectives
We examined whether the association between child IQ and prenatal exposure to polycyclic aromatic hydrocarbons differed between groups of children whose mothers reported high vs. low material hardship during their pregnancy and through child age 5. We tested statistical interactions between hardships and polycyclic aromatic hydrocarbons, as measured by DNA adducts in cord blood, to determine whether material hardship exacerbated the association between adducts and IQ scores.
Design
Prospective cohort. Participants were recruited from 1998 to 2006 and followed from gestation through age 7 years.
Setting
Urban community (New York City)
Participants
A community-based sample of 276 minority urban youth
Exposure measure
Polycyclic aromatic hydrocarbon-DNA adducts in cord blood as an individual biomarker of prenatal polycyclic aromatic hydrocarbon exposure. Maternal material hardship self-reported prenatally and at multiple timepoints through early childhood.
Main outcome measure
Child IQ at 7 years assessed using the Wechsler Intelligence Scale for Children.
Results
Significant inverse effects of high cord PAH-DNA adducts on full scale IQ, perceptual reasoning and working memory scores were observed in the groups whose mothers reported a high level of material hardship during pregnancy or recurring high hardship into the child’s early years, and not in those without reported high hardship. Significant interactions were observed between high cord adducts and prenatal hardship on working memory scores (β=−8.07, 95% CI (−14.48, −1.66) and between high cord adducts and recurrent material hardship (β=−9.82, 95% CI (−16.22, −3.42).
Conclusion
The findings add to other evidence that socioeconomic disadvantage can increase the adverse effects of toxic physical “stressors” like air pollutants. Observed associations between high cord adducts and reduced IQ were significant only among the group of children whose mothers reported high material hardship. These results indicate the need for a multifaceted approach to prevention.
Keywords: air pollution, child IQ, prenatal exposure, PAH, adducts
1: Introduction
Exposure to polycyclic aromatic hydrocarbons (PAH) is prevalent in urban populations as a result of incomplete combustion of fossil fuels and other organic material. Specific sources of PAH include combustion of diesel, gasoline, coal, residential heating oil, tobacco smoke, and chargrilled or broiled foods1,2. There is growing evidence that exposures to ambient and indoor air pollutants have adverse effects on neurodevelopment and that such toxic exposures are disproportionately high in lower income communities of color3–6. These minority populations are also more likely to experience material hardship, an indicator of chronic economic stress, to live in lower quality housing, and to have inadequate educational and nutritional resources compared to higher income communities. Such socioeconomic stressors potentially compound or increase the effect of toxic environmental exposures7,8.
In addition to exerting epigenetic effects9, PAH bind covalently to DNA to form adducts, a widely used biomarker that reflects inter-individual variation in exposure, absorption, metabolic activation, and DNA repair, thereby providing an individual biologic dosimeter of an individual’s exposure to PAH. PAH-DNA adducts have previously associated with cancer in adults 10–12 and with adverse neurodevelopmental outcomes in children13–16.
The fetus is considered particularly susceptible to the effects of PAH exposure due to slower clearance of chemicals, underdeveloped detoxification and repair mechanisms, high rate of metabolic activity, and rapid growth during fetal development17,18. PAH are readily transferred across the placenta and the fetal blood brain barrier (reviewed in19,20).
Here we tested the hypothesis that the adverse effect of prenatal exposure to PAH, measured by PAH-DNA adducts in cord blood, would be greater among children whose mothers experienced material hardship during pregnancy and in the children’s early years compared to children whose mothers did not experience material hardship. Material hardship is a measure used to assess an individual’s unmet basic needs in the areas of food, housing, and clothing21. As has been shown with lead22, even in the absence of significant main effects, combined exposures to environmental toxicants and social stress can have significant impacts on neurodevelopment and therefore are of concern23–25. We focused on prenatal PAH exposure because of the extensive structural and cellular-level changes that occur during the prenatal period and prior studies that have suggested that prenatal exposure to PAH adversely affects cognitive development26,27. We evaluated both prenatal material hardship and that experienced continuously from pregnancy through childhood because prior studies have reported adverse effects of economic disadvantage and stress experienced during both developmental periods 28,29.
2: Methods
2.1: The Columbia Center for Children’s Environmental Health (CCCEH) cohort study
A complete description of the CCCEH cohort and study design appears elsewhere30. Briefly, African-American and Dominican women who resided in Washington Heights, Harlem, or the South Bronx in New York City (NYC) were recruited into a prospective cohort study between 1998 and 2006 through the local prenatal care clinics. To reduce the potential for confounding, enrollment was restricted to women who were non-active cigarette smokers in the age range of 18–35 years; non-users of other tobacco products or illicit drugs; free of diabetes, hypertension, or known HIV; and had initiated prenatal care by the 20th week of pregnancy. The Institutional Review Board of the New York Presbyterian Medical Center approved the study. The mothers provided informed consent for themselves and their children and children provided assent at age 7.
2.2: Personal interviews, home caretaking environment, and maternal intelligence
2.2.1: Prenatal interview
A 45-minute questionnaire administered by a trained bilingual interviewer during the last trimester of pregnancy elicited demographic information, residential history, health and environmental data such as active smoking (to confirm non-active smoking status as reported on the screening questionnaire) and exposure to environmental tobacco smoke (ETS). In the cohort, self-reported ETS exposure was positively correlated with cotinine measured in cord blood (r=0.44, p-value<0.0001). The questionnaire also elicited information on dietary PAH (consumption of broiled, fried, grilled or smoked meat) and information related to income and education.
2.2.2: Postnatal interviews and assessments
Postnatal interviews were administered in-person at 6 months and annually thereafter to determine changes in residence, ETS exposure, and health and environmental conditions. The PERI-D was also re-administered at those interviews31. At child age 3, the quality of the proximal caretaking environment was assessed using Caldwell and Bradley’s Home Observation for Measurement of the Environment (HOME)32. Maternal nonverbal intelligence was measured at child age 3 by the Test of Non-Verbal Intelligence-Third Edition (TONI-3)33, a 15-minute, language-free measure of general intelligence, relatively stable and free of cultural bias.
2.3: Material hardship
A measure of material hardship21, assessing the level of unmet basic needs in the areas of food, housing, and clothing, was obtained prenatally and at child age 6 months, and 1, 2, 3, and 5 years by asking: “In the past year has there been a time when you: 1. couldn’t afford to buy food?; 2. couldn’t afford a place to stay?; 3. couldn’t afford gas/electricity?; or 4. couldn’t afford clothing?” Each answer was dichotomized (yes/no). High prenatal hardship was defined by a positive answer to at least one of the four questions at the prenatal assessment, and recurrent hardship by a positive response to at least one of the questions at ≥ 50% of the prenatal period and postnatal visits.
2.4 Biomarker measurement and prenatal monitoring
At the time of delivery, umbilical cord blood and maternal blood were collected and transported within several hours of collection to the CCCEH Molecular Epidemiology Laboratory. The buffy coat, packed red blood cells, and plasma were separated and stored at −70°C. DNA adducts of the representative PAH, benzo[a]pyrene (B[a]P), were analyzed in extracted white blood cell DNA using a high performance chromatography (HPLC)/fluorescence method which detects B[a]P tetraols18,34. The adducts were dichotomized into detectable/nondetectable (high/low) because 57% of cord blood DNA samples had levels below the limit of detection (0.25 adducts per 108 adducts). Some children lacked data on cord DNA adducts due to inadequate quantity or quality of DNA (n=145).
PAH metabolites were measured in spot urine collected at child age 5 at the Centers for Disease Control and Prevention (CDC) using automated liquid-liquid extraction and gas chromatography/isotope dilution high-resolution mass spectrometry, as previously described35–37. Although a short-term biomarker (half-life of 6–35 hours)38, in conditions of chronic exposure the metabolites are considered a useful measure of exposure to PAH from all exposure sources and pathways35,36. To adjust for urinary dilution of the samples, specific gravity (SG) measurements were obtained using a handheld refractometer (PAL-10-S-P14643C0; TAGO, Bellevue, WA). Metabolite levels were adjusted for SG using the formula: freshweight metabolites for the subject*(mean SG-1)/(SG for that subject-1)39.
2.5: Outcomes
At child age 7 years, trained research workers administered the Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV), which consists of 15 subtests, 10 of which are core subtests used for the present study. Raw scores were converted into scaled scores based on a metric with a mean of 10 and standard deviation of 3 specific for the child’s age group as described in the WISC-IV manual. The scaled scores were used to derive four composite scores (working memory, perceptual reasoning, processing speed, and verbal comprehension). These four indices were summed to derive the composite score for full scale IQ. The average expected performance on the full scale IQ score on the WISC-IV is 100, with a standard deviation of 1540.
2.6: Statistical analysis
A total of 394 children had available data on cord adducts. Of these, 276 also had available data on material hardship and WISC outcomes. 27 subjects were missing data on one or more covariates identified as predictors of IQ in the present cohort (at p<0.1) or as reported in the literature. These covariates included maternal self-report of ETS exposure during pregnancy, maternal education (<high school; ≥high school), ethnicity, maternal intelligence (treated as a continuous variable), child sex, and quality of the early home caretaking environment assessed at child age 3 (treated as a continuous variable). We conducted multiple imputation for missing data on those covariates.
Adducts were treated as high/low (detectable/nondetectable). Stratified analyses assessed the effect of high cord adducts in the different strata of material hardship (high vs. low prenatal and recurrent vs. non-recurrent hardship, respectively). To determine if the effects of high cord adducts in different strata of material hardship are statistically different or not, we tested statistical interactions, a product term between adducts and material hardship (prenatal or recurrent), in a linear regression model, with the terms for adducts, material hardship and key covariates regressed on each WISC outcome. The interaction term betas and 95% confidence intervals (CI) were then examined to determine whether the associations between PAH and IQ within the low and high hardship strata were statistically different.
In separate models with a smaller number of subjects (n=230), we adjusted for postnatal PAH exposure, using the SG-adjusted level of PAH metabolites in child urine at age 5. In a further analyses (n=226), we adjusted for levels of the pesticide chlorpyrifos (CPF) and lead (n=156) in cord blood because these toxicants have been associated with lower working memory scores in our cohort41. Our analyses involved multiple comparisons; however to reduce the possibility of making a type II error42 we did not perform Bonferroni adjustment.
As in previous analyses43, to account for potential bias due to selection and loss to follow up, in post-hoc sensitivity analyses, we applied the inverse probability weighting (IPW) technique44–46. As before43, to model probability of staying in the study for each subject, we used a logistic model that included baseline variables for race/ethnicity, receipt of public assistance during pregnancy, high school education, college education, reported satisfaction with living conditions, cord adduct level, neighborhood poverty rate, Spanish language linguistic isolation, and indicator variables for missing data on these variables. Note that the missing data on covariates in the 27 subjects were first filled in with single imputation.
3: Results
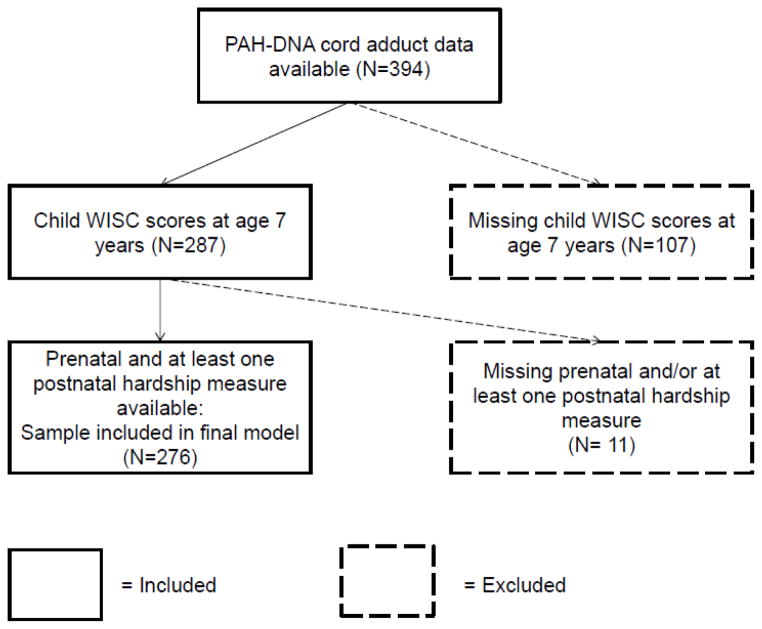
Socio-demographic and exposure characteristics of the sample included in the analysis are presented in Table 1. Within our fully enrolled cohort, there were 394 women with available cord adduct data. A consort diagram showing how we arrived at our final analyzed sample is shown in Figure 1. There were no significant differences between the children included in the analysis and those not included due to missing WISC outcome or cord adduct data (n=118), except that there was a higher percentage of African Americans in the included group (38% vs. 26%) (Supplemental Table S1). In the sample analyzed, the correlation between prenatal material hardship and cord adducts was not significant (r=−0.03, p=0.67) and high cord adducts was not correlated with PAH metabolites at age 5 years (r=0.04, p=0.52) (Supplemental Table S2).
Table 1.
Socio-demographic and exposure characteristics of subjects (n=276)a
| Variable | Mean | SD | range |
|---|---|---|---|
| Cord blood PAH-DNA adducts (% detectable) | 43.8 | ||
| PAH Metabolites at Age 5 [% > median]b | 47.0 | ||
| Sex (% female) | 53.6 | ||
| Maternal ETS (% reporting smoker in the home) | 33.3 | ||
| WISC Full Scale | 98.6 | 13.2 | (48, 133) |
| WISC Verbal Comprehension | 96.3 | 12.2 | (45, 134) |
| WISC Processing Speed | 101.1 | 15.2 | (62, 138) |
| WISC Perceptual Reasoning | 100.8 | 14.3 | (63, 137) |
| WISC Working Memory | 97.4 | 14.2 | (54, 135) |
| Maternal education (% ≥ high school education) | 63.4 | ||
| Ethnicity (% AA) | 38.0 | ||
| Maternal IQ (TONI) | 20.7 | 8.7 | (0, 43) |
| Prenatal hardship (% ≥1 hardships) | 42.8 | ||
| Recurrent up to 5 years (% ≥ 1 hardship, 50% of the time) | 39.9 | ||
| HOME inventory | 39.8 | 6.0 | (22, 52) |
Subjects have data on cord adducts and IQ test results at age 7.
Variable dichotomized at the median level for the entire population (8223.41 ng/m3)
Figure 1.
Subject selection for present analysis
As we had previously hypothesized, stratified analyses showed that the association between PAH-DNA adducts in cord blood and IQ measures were significant only among the children whose mothers reported high prenatal or recurrent material hardship (Table 2). Among the group with high prenatal hardship, children who had high levels of adducts in cord blood had a 5.81 point lower full scale IQ score, a 5.44 point lower perceptual reasoning score and a 6.67 point lower working memory score compared to children whose cord adducts were low (Table 2). The same significant relationships between adducts and IQ were not observed in the low material hardship group.
Table 2.
Association between cord blood PAH-DNA adducts on IQ at age 7, stratified by prenatal and recurrent material hardship (N=276)a
| Low Prenatal Material Hardship (N=158) | High Prenatal Material Hardship (N=118) | Interaction | Non-Recurrent Material Hardship (N=166) | Recurrent Material Hardship (N=110) | Interaction | |
|---|---|---|---|---|---|---|
| WISC components | βadducts (95% CI) | βadducts (95% CI) | βinteraction (95% CI) | βadducts (95% CI) | βadducts (95% CI) | βinteraction (95% CI) |
| Full Scale | −1.79 (−5.50, 1.93) | −5.81 (−10.35, −1.26)* | −4.66 (−10.43, 1.11) | −1.32 (−4.97, 2.33) | −6.63 (−11.28, −1.98)* | −5.59 (−11.37, 0.20) |
| Verbal Comprehension | −1.08 (−4.29, 2.14) | −3.36 (−7.61, 0.90) | −2.39 (−7.57, 2.80) | −0.79 (−3.98, 2.40) | −4.21 (−8.51, 0.09) | −3.13 (−8.32, 2.07) |
| Processing Speed | −3.59 (−8.21, 1.02) | −4.17 (−9.75, 1.41) | −0.97 (−8.09, 6.16) | −3.46 (−7.71, 0.79) | −4.02 (−10.13, 2.09) | −0.83 (−7.97, 6.30) |
| Perceptual Reasoning | −1.45 (−5.86, 2.95) | −5.44 (−10.27, −0.61)* | −4.66 (−11.20, 1.89) | −1.29 (−5.55, 2.96) | −5.66 (−10.71, −0.61)* | −4.74 (−11.32, 1.85) |
| Working Memory | 0.57 (−3.70, 4.85) | −6.67 (−11.38, −1.95)* | −8.07 (−14.48, −1.66)* | 1.24 (−3.13, 5.60) | −8.06 (−12.49, −3.63)* | −9.82 (−16.22, −3.42)* |
p-value<0.05
Adjusting for ETS, sex, maternal education, maternal intelligence, ethnicity, and the home caretaking environment.
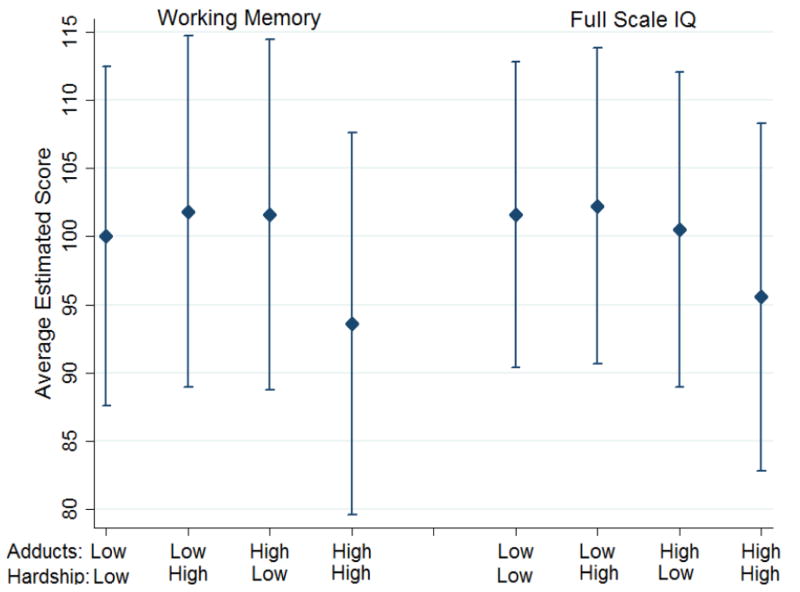
Similarly, after stratifying on recurrent hardship, adducts were significantly associated with full scale IQ, perceptual reasoning and working memory only within the high hardship group (Table 2, Figure 2). Among the group with recurrent hardship, children who had high levels of adducts in cord blood had a 6.63 point lower full scale IQ score, a 5.66 point lower perceptual reasoning score and a 8.06 point lower working memory score compared to children with low cord adducts. Statistically significant interactions between both prenatal (β=−8.07, 95% CI (−14.48, −1.66)) and recurrent (β=−9.82, 95% CI (−16.22,−3.42)) material hardship and cord adducts were observed on working memory scores (Table 2). Regarding the overall main effects of high cord adducts on cognitive outcomes, associations were uniformly inverse as hypothesized and significant for full scale IQ (β=−3.45, 95% CI (−6.35, −0.55)) and processing speed (β=−3.72, 95% CI (−7.28, −0.17)) and borderline for perceptual reasoning (β=−3.02, 95% CI (−6.30, 0.26)) (data not shown). We also conducted the stratified and interaction analyses in the subset with postnatal PAH exposure data available (n=230) (Supplemental Table S3). There was no difference in results before and after adjusting for postnatal PAH exposure, and therefore we did not include this variable in our final model.
Figure 2.
Full Scale IQ and Working Memory Scores in the low and high cord PAH-DNA adduct groups stratified by recurrent hardship (n=276)
In the subset with available cord plasma CPF data (n=226), CPF was significantly associated with working memory scores, after adjusting for cord adducts and stratified on prenatal and recurrent material hardship (data not shown). When the interaction model for cord adducts and recurrent hardship was adjusted for CPF, the interaction on working memory remained significant (β= −10.93, 95% CI (−17.94, −3.93)) and the interaction on full scale IQ and verbal comprehension became significant (β= −6.48, 95% CI (−12.76, −0.20) and β=−6.19, 95% CI (−10.83, −1.57), respectively). The same interactions on working memory and full scale IQ were seen when considering prenatal hardship (β= −13.01, 95% CI (−19.97, −6.04) and β=−7.23, 95% CI (−13.52, −0.94), respectively) (Supplemental Table S4). Cord adduct and CPF levels were not significantly correlated (r=−0.02, p=0.81); neither were cord adduct and lead levels (r=−0.06, p=0.48). Furthermore, after adjusting for prenatal lead exposure in a smaller subsample with available data (n=156), high cord adducts were significantly associated with working memory scores in both the high prenatal (β=−10.45, 95% CI (−16.87, −4.02) and recurrent material hardship (β=−9.46, 95% CI (−16.08, −2.83) groups. The corresponding interaction terms were also significant (Supplemental Table S5).
After repeating all analyses with IPW, the direction and magnitude of associations did not materially change, indicating that the results are not influenced by sample selection and loss to follow-up (Supplemental Table S6).
4: Discussion
This is the first report of an interaction between chronic socioeconomic stress and prenatal exposure to PAH, represented by PAH-DNA adducts in cord blood, on children’s IQ. Cord PAH-DNA adducts are a direct measure of the individual fetal dose of PAH integrating exposure over the past 3–4 months47.
The findings are of concern because, as has been shown with lead, even a modest decrease in IQ can impact lifetime earnings48,49. They are also consistent with studies showing modification of the neurotoxic effect of lead by social class22.
PAH are common urban pollutants and include known carcinogens and neurotoxicants such as B[a]P. B[a]P, considered a representative PAH, is highly correlated with the other 7 genotoxic PAH measured in prenatal air (r = 0.80–0.96, p = 0.001 except for dibenz[a,h]anthracene, r = 0.53, p < 0.001)14. Although PAH is ubiquitous in the urban environment, low-income communities are disproportionately exposed due to greater siting of heavily trafficked roadways, bus and truck depots, power plants and industrial boilers, and the higher prevalence of smokers in low-income households8,50.
This report adds to the growing literature on the vulnerability of the developing fetus and young child to the toxic effects of environmental pollutants17,18 as well as to socioeconomic disadvantage 51–53 (for review see54). Additional studies have observed similar decreases in IQ score as measured by the WISC-IV in response to environmental toxicants experienced prenatally and during early childhood as our present study55–58. Economic deprivation and related stress early in life have been linked to behavioral problems and lower IQ scores in children59. Cumulative poverty and hardship in the first year of life were associated with negative effects on cognitive function in childhood28.
The observed interaction between PAH (cord adducts) and material hardship is consistent with other reports that adverse social conditions can modify the neurotoxicity of environmental pollutants such as lead, traffic-related pollutants, and ETS22,55,60–62 (see63 for review).
A number of prior reports in other populations have indicated adverse neurodevelopmental effects of air pollution26,27,64,65. In the present NYC cohort, prenatal exposure to PAH, as measured by 48-hour prenatal air monitoring, was associated with delayed mental development at age 3 years30 and was associated with lower intelligence at age 5 years in both the NYC cohort and in a parallel Polish cohort26,27. In those reports, the association with PAH-DNA adducts was not examined.
Mechanisms underlying interactions between toxic pollutants and psychosocial factors are not well understood. However, chronic psychosocial stress is known to increase allostatic load that can impair individual resilience and ability to recover from toxic insults by interfering with normal functioning of protective toxicokinetic and toxicodynamic processes, resulting in elevated inflammatory tone63,66,67. Production of inflammatory mediators is also stimulated by physical toxicants68,69. Thus the two types of exposures could potentiate each other through common physiological pathways such as inflammation63,68,70,71. Research on the combined effect of maternal stress during pregnancy and prenatal air pollution in mice showed that these stressors act synergistically to induce neuroinflammation, leading to future neurobehavioral disorders29.
With respect to the harmful effects of prenatal PAH exposure, a number of additional pathways have been suggested including endocrine disruption72–74, binding to receptors for placental growth factors resulting in decreased exchange of oxygen and nutrients75, binding to the human Ah receptor to induce P450 enzymes76, DNA damage resulting in activation of apoptotic pathways77–79, oxidative stress due to inhibition of the brain antioxidant scavenging system80, epigenetic alterations affecting gene expression81,82 and/or altered expression of nuclear transcription factors that mediate the onset of neuronal cell differentiation19.
Our findings are consistent with those from other animal and human studies. Although the exposure in experimental laboratory studies is considerably higher than those in the NYC cohort, impaired memory has been observed in animals exposed gestationally to PAH at doses below those causing overt toxicological effects83,84. Differences in the medial temporal/memory composite have been significantly related to children’s socioeconomic status85.
Regarding the interpretation of our results, it is possible that PAH are equally toxic under conditions of low and high hardship, but that low hardship families have unmeasured resources positively affecting the health and development of children and buffering the adverse impact of PAH exposure86. Furthermore, those individuals experience material hardship may also have had a poor prenatal diet87,88 and exposure to other chemical toxicants, such as lead57,89, ETS55 and other air pollutants90–92, that also may account for the lower IQ seen in the children.
In addition, it is possible that the WISC-IV scores at age 7 are capturing manifestations other than child IQ. For example, Oliveras-Rentas et al., found that among a sample of high-functioning children with autism-spectrum disorder, scores on the WISC-IV were correlated with adaptive behavior as measured on the Vineland instrument and autism spectrum symptomology scores as measured on the Autism Diagnostic Observation Schedule (ADOS). This study did not see a significant correlation between WISC-IV scores and ADHD symptomology93. However, an additional study investigated clusters of IQ profiles as defined by the WISC-IV and found an association between processing speed and inattention among children diagnosed with ADHD. They concluded that WISC-IV scores may be helpful in predicting symptomology of children with ADHD94. In particular, working memory scores on the WISC may be affected by clinical behavior problems40.
We acknowledge a number of additional limitations. Although we have adjusted for the possible confounding effects of ETS, there is always the possibility that some residual confounding remains; and we did not have data on a measure of psychological stress. In addition, we excluded active smokers, illicit drug users, and women with preexisting disease, thereby limiting generalizability.
The strengths of the study include the longitudinal design and ability to account for a number of factors other than PAH exposure that are known to affect child neurobehavioral development via available biomarker and questionnaire data.
There is growing recognition of the continuing need to document interactions between adverse social conditions/psychosocial stressors and environmental toxicants and to understand the mechanisms involved for effective intervention63,95. The present results suggest the need for a multifaceted approach to reduce PAH exposure and alleviate material hardship in order to protect the developing fetus and young child. The approach could combine screening women early in pregnancy to identify those needing material support and policy interventions to reduce air pollution exposure in urban areas, especially low-income communities.
5. Conclusion
This study provides evidence that material hardship influences the effect of prenatal exposure to environmental PAH, measured by PAH-DNA adducts in cord blood, on child IQ. The associations between PAH on full scale IQ and working memory were seen mainly among the group of children whose mothers experienced material hardship during pregnancy and in the children’s early years.
PAH are widespread in urban environments worldwide, largely as a result of fossil fuel combustion. Their concentrations can be reduced using currently available pollution controls, greater energy efficiency, alternative energy sources, and regulatory intervention to remove highly polluting sources. These results add to prior data linking PAH to cognitive and behavioral problems in children and suggest the need for a multifaceted approach to prevention.
Supplementary Material
Highlights.
PAH-DNA adducts in cord blood provided an individual measure of prenatal exposure.
Material hardship in pregnancy and child’s early life proxied economic deprivation.
Adverse effects on child IQ at age 7 were seen only among mothers with hardship.
Interaction between high adducts and hardship on working memory was significant.
These results indicate the need for a multifaceted approach to prevention.
Acknowledgments
We thank Joanne Chin, MFA at the Columbia Center for Children’s Environmental Health, 722 W. 168th Street, New York, NY 10032, United States, for help in preparation of the manuscript. PAH metabolites were measured by Andreas Sjodin, PhD and colleagues at the Centers for Disease Control and Prevention (CDC), National Center for Environmental Health, Division of Laboratory Sciences, 4770 Buford Highway, Atlanta, Georgia 30341, United States.
Funding: Funding was provided by the National Institute for Environmental Health Sciences (NIEHS) and the U.S. Environmental Protection Agency (US EPA): NIEHS/EPA P01ES09600/R82702701, NIEHS/EPA P01ES09600/RD832141, NIEHS/EPA P01ES09600/RD834509, NIEHS R01ES08977. This publication was also made possible in part by US EPA grant RD832096, the John and Wendy Neu Family and the Blanchette Hooker Rockefeller Foundations, and the National Center for Advancing Translational Sciences (UL1TR000040).
Funding from all the institutions listed was used towards the design and conduct of the study; collection, management, analysis, and interpretation of the data; and the preparation, review, and approval of the manuscript. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Larsen RK, Baker JE. Source Apportionment of Polycyclic Aromatic Hydrocarbons in the Urban Atmosphere: A Comparison of Three Methods. Environ Sci Technol. 2003 May 01;37(9):1873–1881. doi: 10.1021/es0206184. [DOI] [PubMed] [Google Scholar]
- 2.Bostrom CE, Gerde P, Hanberg A, et al. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environmental health perspectives. 2002 Jun;110 (Suppl 3):451–488. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morello-Frosch R, Zuk M, Jerrett M, Shamasunder B, Kyle AD. Understanding The Cumulative Impacts Of Inequalities In Environmental Health: Implications For Policy. Health Affairs. 2011 May 1;30(5):879–887. doi: 10.1377/hlthaff.2011.0153. [DOI] [PubMed] [Google Scholar]
- 4.Mohai P, Lantz PM, Morenoff J, House JS, Mero RP. Racial and Socioeconomic Disparities in Residential Proximity to Polluting Industrial Facilities: Evidence From the Americans’ Changing Lives Study. American Journal of Public Health. 2009 Nov 1;99(S3):S649–656. doi: 10.2105/AJPH.2007.131383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jerrett M. Global Geographies of Injustice in Traffic-Related Air Pollution Exposure. Epidemiology. 2009;20(2):231–233. doi: 10.1097/EDE.0b013e31819776a1. doi:210.1097/EDE.1090b1013e31819776a31819771. [DOI] [PubMed] [Google Scholar]
- 6.Woodruff TJ, Parker JD, Kyle AD, Schoendorf KC. Disparities in exposure to air pollution during pregnancy. Environmental health perspectives. 2003 Jun;111(7):942–946. doi: 10.1289/ehp.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008 May;87(5):1107–1117. doi: 10.1093/ajcn/87.5.1107. [DOI] [PubMed] [Google Scholar]
- 8.Evans GW, Kantrowitz E. Socioeconomic status and health: the potential role of environmental risk exposure. Annu Rev Public Health. 2002;23:303–331. doi: 10.1146/annurev.publhealth.23.112001.112349. [DOI] [PubMed] [Google Scholar]
- 9.Herbstman J, Tang D, Zhu D, et al. Prenatal exposure to polycyclic aromatic hydrocarbons, Benzo[a]Pyrene-DNA adducts and genomic DNA methylation in cord blood. Environmental health perspectives. 2012 doi: 10.1289/ehp.1104056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rybicki BA, Rundle A, Savera AT, Sankey SS, Tang D. Polycyclic aromatic hydrocarbon-DNA adducts in prostate cancer. Cancer Res. 2004 Dec 15;64(24):8854–8859. doi: 10.1158/0008-5472.CAN-04-2323. [DOI] [PubMed] [Google Scholar]
- 11.Tang D, Santella RM, Blackwood A, et al. A molecular epidemiological case-control study of lung cancer. Cancer Epidemiol Biomarkers Prev. 1995;4(4):341–346. [PubMed] [Google Scholar]
- 12.Tang D, Cho S, Rundle A, et al. Polymorphisms in the DNA repair enzyme XPD are associated with increased levels of PAH-DNA adducts in a case-control study of breast cancer. Breast Cancer Res Treat. 2002;75(2):159–166. doi: 10.1023/a:1019693504183. [DOI] [PubMed] [Google Scholar]
- 13.Perera F, Li TY, Zhou ZJ, et al. Benefits of reducing prenatal exposure to coal burning pollutants to children’s neurodevelopment in China. Environ Health Perspect. 2008;116(10):1396–1400. doi: 10.1289/ehp.11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perera FP, Tang D, Wang S, et al. Prenatal Polycyclic Aromatic Hydrocarbon (PAH) Exposure and Child Behavior at age 6–7. Environmental health perspectives. 2012 Mar 22; doi: 10.1289/ehp.1104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perera FP, Wang S, Vishnevetsky J, et al. PAH/aromatic DNA adducts in cord blood and behavior scores in New York City children. Environmental health perspectives. 2011;119(8):1176–1181. doi: 10.1289/ehp.1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perera FP, Chang HW, Tang D, et al. Early-life exposure to polycyclic aromatic hydrocarbons and ADHD behavior problems. Plos One. 2014;9(11):e111670. doi: 10.1371/journal.pone.0111670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006 Dec 16;368(9553):2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 18.Perera FP, Tang D, Jedrychowski W, et al. Biomarkers in maternal and newborn blood indicate heightened fetal susceptibility to procarcinogenic DNA damage. Environ Health Perspect. 2004;112(10):1133–1136. doi: 10.1289/ehp.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hood DB, Nayyar T, Ramesh A, Greenwood M, Inyang F. Modulation in the developmental expression profile of Sp1 subsequent to transplacental exposure of fetal rats to desorbed benzo[a]pyrene following maternal inhalation. Inhal Toxicol. 2000 Jun;12(6):511–535. doi: 10.1080/089583700402897. [DOI] [PubMed] [Google Scholar]
- 20.Brown LA, Khousbouei H, Goodwin JS, et al. Down-regulation of early ionotrophic glutamate receptor subunit developmental expression as a mechanism for observed plasticity deficits following gestational exposure to benzo(a)pyrene. Neurotoxicology. 2007 Sep;28(5):965–978. doi: 10.1016/j.neuro.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer SE, Jencks C. Poverty and the Distribution of Material Hardship. The Journal of Human Resources. 1989;24(1):88–114. [Google Scholar]
- 22.Bellinger DC. Effect modification in epidemiologic studies of low-level neurotoxicant exposures and health outcomes. Neurotoxicology and teratology. 2000;22(1):133–140. doi: 10.1016/s0892-0362(99)00053-7. Review. [DOI] [PubMed] [Google Scholar]
- 23.Bellinger D, Leviton A, Waternaux C. Lead IQ and social class. Int J Epidemiol. 1989;18:180–185. doi: 10.1093/ije/18.1.180. [DOI] [PubMed] [Google Scholar]
- 24.Bellinger D, Leviton A, Waternaux C, Needleman H, Rabinowitz M. Longitudinal analyses of prenatal and postnatal lead exposure and early cognitive development. New Engl J Med. 1987;316:1037–1043. doi: 10.1056/NEJM198704233161701. [DOI] [PubMed] [Google Scholar]
- 25.Lansdown R, Yule W, Urbanowicz MA, Hunter J. The relationship between blood-lead concentrations, intelligence, attainment and behaviour in a school population: the second London study. International archives of occupational and environmental health. 1986;57(3):225–235. doi: 10.1007/BF00405790. [DOI] [PubMed] [Google Scholar]
- 26.Perera FP, Li Z, Whyatt R, et al. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics. 2009 Aug;124(2):e195–202. doi: 10.1542/peds.2008-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards SC, Jedrychowski W, Butscher M, et al. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and children’s intelligence at 5 years of age in a prospective cohort study in Poland. Environmental health perspectives. 2010 Sep;118(9):1326–1331. doi: 10.1289/ehp.0901070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoon I, Jones E, Cheng H, Maughan B. Family hardship, family instability, and cognitive development. J Epidemiol Community Health. 2012 Aug;66(8):716–722. doi: 10.1136/jech.2010.121228. [DOI] [PubMed] [Google Scholar]
- 29.Bolton JL, Huff NC, Smith SH, et al. Maternal Stress and Effects of Prenatal Air Pollution on Offspring Mental Health Outcomes in Mice. Environ Health Perspect. 2013 Jul 3; doi: 10.1289/ehp.1306560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perera FP, Rauh V, Whyatt RM, et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environmental health perspectives. 2006 Aug;114(8):1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dohrenwend B, Shrout P, Egri G, Mendelsohn F. Nonspecific psychological distress and other dimensions of psychopathology. Measures for use in the general population. Arch Gen Psychiatr. 1980;37:1229. doi: 10.1001/archpsyc.1980.01780240027003. [DOI] [PubMed] [Google Scholar]
- 32.Bradley RH. The Home Inventory: review and reflections. Adv Child Dev Behav. 1994;25:241–288. doi: 10.1016/s0065-2407(08)60054-3. [DOI] [PubMed] [Google Scholar]
- 33.Brown L, Sherbenou RJ, Johnsen SK. Test of nonverbal intelligence. 3. Bensenville, IL: Scholastic Testing Service, Inc; 1997. [Google Scholar]
- 34.Alexandrov K, Rojas M, Geneste O, et al. An improved fluorometric assay for dosimetry of benzo(a)pyrene diol-epoxide-DNA adducts in smokers’ lung: comparisons with total bulky adducts and aryl hydrocarbon hydroxylase activity. Cancer Res. 1992;52(22):6248–6253. [PubMed] [Google Scholar]
- 35.Li Z, Romanoff LC, Trinidad DA, et al. Measurement of urinary monohydroxy polycyclic aromatic hydrocarbons using automated liquid-liquid extraction and gas chromatography/isotope dilution high-resolution mass spectrometry. Anal Chem. 2006 Aug 15;78(16):5744–5751. doi: 10.1021/ac0606094. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Sandau CD, Romanoff LC, et al. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environmental research. 2008 Jul;107(3):320–331. doi: 10.1016/j.envres.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Miller RL, Garfinkel R, Lendor C, et al. Polycyclic aromatic hydrocarbon metabolite levels and pediatric allergy and asthma in an inner-city cohort. Pediatr Allergy Immunol. 2010 Mar;21(2 Pt 1):260–267. doi: 10.1111/j.1399-3038.2009.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jongeneelen FJ, van Leeuwen FE, Oosterink S, et al. Ambient and biological monitoring of cokeoven workers: determinants of the internal dose of polycyclic aromatic hydrocarbons. Br J Ind Med. 1990 Jul;47(7):454–461. doi: 10.1136/oem.47.7.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environmental health perspectives. 2004;112:1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wechsler D. Wechsler Intelligence Scale for Children® — Fourth Edition (WISC®-IV) Psychological Corporation; 2003. [Google Scholar]
- 41.Rauh V, Arunajadai S, Horton M, et al. 7-Year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environmental health perspectives. 2011;119(8):1196–1201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 43.Rundle A, Hoepner L, Hassoun A, et al. Association of childhood obesity with maternal exposure to ambient air polycyclic aromatic hydrocarbons during pregnancy. American journal of epidemiology. 2012;175:1161–1172. doi: 10.1093/aje/kwr455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000 Sep;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007 Oct;45(10):S103–S107. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 46.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004 Sep;15(5):615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 47.Mooney LA, Santella RM, Covey L, et al. Decline of DNA damage and other biomarkers in peripheral blood following smoking cessation. Cancer Epidemiol Biomarkers Prev. 1995 Sep;4(6):627–634. [PubMed] [Google Scholar]
- 48.Grosse SD. Association of Environmental and Resource Economists (AERE) Vol. 27. AERE; 2007. How Much Does IQ Raise Earnings? Implications for Regulatory Impact Analyses; p. 44. [Google Scholar]
- 49.Grosse SD, Matte TD, Schwartz J, Jackson RJ. Economic gains resulting from the reduction in children’s exposure to lead in the United States. Environmental health perspectives. 2002 Jun;110(6):563–569. doi: 10.1289/ehp.02110563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chuang JC, Callahan PJ, Lyu CW, Wilson NK. Polycyclic aromatic hydrocarbon exposures of children in low-income families. J Expo Anal Environ Epidemiol. 1999;9(2):85. doi: 10.1038/sj.jea.7500003. [DOI] [PubMed] [Google Scholar]
- 51.Laplante DP, Brunet A, Schmitz N, Ciampi A, King S. Project Ice Storm: prenatal maternal stress affects cognitive and linguistic functioning in 5 1/2-year-old children. J Am Acad Child Psy. 2008 Sep;47(9):1063–1072. doi: 10.1097/CHI.0b013e31817eec80. [DOI] [PubMed] [Google Scholar]
- 52.Singer L, Arendt R, Farkas K, Minnes S, Huang J, Yamashita T. Relationship of prenatal cocaine exposure and maternal postpartum psychological distress to child developmental outcome. Development and Psychopathology. 1997 Summer;9(3):473–489. doi: 10.1017/s0954579497001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sandman CA, Davis EP, Buss C, Glynn LM. Exposure to Prenatal Psychobiological Stress Exerts Programming Influences on the Mother and Her Fetus. Neuroendocrinology. 2012;95(1):8–21. doi: 10.1159/000327017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annu Rev Psychol. 2011;62:501–530. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rauh VA, Whyatt RM, Garfinkel R, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicology and teratology. 2004;26(3):373–385. doi: 10.1016/j.ntt.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wasserman GA, Liu X, Loiacono NJ, et al. A cross-sectional study of well water arsenic and child IQ in Maine school children. Environmental health: a global access science source. 2014;13(1):23. doi: 10.1186/1476-069X-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lanphear BP, Hornung R, Khoury J, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environmental health perspectives. 2005 Jul;113(7):894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bouchard MF, Chevrier J, Harley KG, et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year old children. Environmental health perspectives. 2011;119(8):1189–1195. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duncan GJ, Brooks-Gunn J, Klebanov PK. Economic deprivation and early childhood development. Child Dev. 1994 Apr;65(2 Spec):296–318. [PubMed] [Google Scholar]
- 60.Dietrich KN, Succop PA, Berger OG, Hammond PB, Bornschein RL. Lead exposure and the cognitive development of urban preschool children: the Cincinnati Lead Study cohort at age 4 years. Neurotoxicology and teratology. 1991 Mar-Apr;13(2):203–211. doi: 10.1016/0892-0362(91)90012-l. [DOI] [PubMed] [Google Scholar]
- 61.Winneke G, Kraemer U. Neuropsychological effects of lead in children: interactions with social background variables. Neuropsychobiology. 1984;11(3):195–202. doi: 10.1159/000118077. [DOI] [PubMed] [Google Scholar]
- 62.Clougherty JE, Levy JI, Kubzansky LD, et al. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environmental health perspectives. 2007 Aug;115(8):1140–1146. doi: 10.1289/ehp.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McEwen BS, Tucker P. Critical biological pathways for chronic psychosocial stress and research opportunities to advance the consideration of stress in chemical risk assessment. Am J Public Health. 2011 Dec;101 (Suppl 1):S131–139. doi: 10.2105/AJPH.2011.300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sram RJ, Benes I, Binkova B, et al. Teplice Program -- the impact of air pollution on human health. Environ Health Perspect. 1996;104(Suppl 4):699–714. doi: 10.1289/ehp.104-1469669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang D, Lee J, Muirhead L, et al. Molecular and neurodevelopmental benefits to children of closure of a coal burning power plant in China. Plos One. 2014;9(3):e91966. doi: 10.1371/journal.pone.0091966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McEwen BS. Protective and Damaging Effects of Stress Mediators. New England Journal of Medicine. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 68.Bierhaus A, Wolf J, Andrassy M, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003 Feb 18;100(4):1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saxon A, Diaz-Sanchez D. Diesel exhaust as a model xenobiotic in allergic inflammation. Immunopharmacology. 2000 Jul 25;48(3):325–327. doi: 10.1016/s0162-3109(00)00234-4. [DOI] [PubMed] [Google Scholar]
- 70.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007 Jul;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 71.Bierhaus A, Humpert PM, Nawroth PP. Linking stress to inflammation. Anesthesiol Clin. 2006 Jun;24(2):325–340. doi: 10.1016/j.atc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 72.Archibong AE, Inyang F, Ramesh A, et al. Alteration of pregnancy related hormones and fetal survival in F-344 rats exposed by inhalation to benzo(a)pyrene. Reprod Toxicol. 2002;16(6):801–808. doi: 10.1016/s0890-6238(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 73.Bui QQ, Tran MB, West WL. A comparative study of the reproductive effects of methadone and benzo(a)pyrene in the pregnant and pseudopregnant rat. Toxicology. 1986;42(2–3):195–204. doi: 10.1016/0300-483x(86)90009-0. [DOI] [PubMed] [Google Scholar]
- 74.Takeda K, Tsukue N, Yoshida S. Endocrine-disrupting activity of chemicals in diesel exhaust and diesel exhaust particles. Environ Sci. 2004;11(1):33–45. [PubMed] [Google Scholar]
- 75.Dejmek J, Solansky I, Benes I, Lenicek J, Sram RJ. The impact of polycyclic aromatic hydrocarbons and fine particles on pregnancy outcome. Environ Health Perspect. 2000;108(12):1159–1164. doi: 10.1289/ehp.001081159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manchester DK, Gordon SK, Golas CL, Roberts EA, Okey AB. Ah receptor in human placenta: stabilization by molybdate and characterization of binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin, 3-methylcholanthrene, and benzo(a)pyrene. Cancer Res. 1987;47:4861–4868. [PubMed] [Google Scholar]
- 77.Meyn MS. Ataxia-telangiectasia and cellular responses to DNA damage. Cancer Res. 1995;55:5991–6001. [PubMed] [Google Scholar]
- 78.Nicol CJ, Harrison ML, Laposa RR, Gimelshtein IL, Wells PG. A teratologic suppressor role for p53 in benzo[a]pyrene-treated transgenic p53-deficient mice. Nat Genet. 1995;10(2):181–187. doi: 10.1038/ng0695-181. [DOI] [PubMed] [Google Scholar]
- 79.Wood KA, Youle RJ. The role of free radicals and p53 in neuron apoptosis in vivo. J Neuroscience. 1995;15:5851–5857. doi: 10.1523/JNEUROSCI.15-08-05851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saunders CR, Das SK, Ramesh A, Shockley DC, Mukherjee S. Benzo(a)pyrene-induced acute neurotoxicity in the F-344 rat: role of oxidative stress. J Appl Toxicol. 2006 Sep-Oct;26(5):427–438. doi: 10.1002/jat.1157. [DOI] [PubMed] [Google Scholar]
- 81.Wilson VL, Jones PA. Inhibition of DNA methylation by chemical carcinogens in vitro. Cell. 1983 Jan;32(1):239–246. doi: 10.1016/0092-8674(83)90514-7. [DOI] [PubMed] [Google Scholar]
- 82.Perera F, Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reproductive Toxicology. 2011 Apr;31(3):363–373. doi: 10.1016/j.reprotox.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saunders CR, Ramesh A, Shockley DC. Modulation of neurotoxic behavior in F-344 rats by temporal disposition of benzo(a)pyrene. Toxicol Lett. 2002 Mar 24;129(1–2):33–45. doi: 10.1016/s0378-4274(01)00467-2. [DOI] [PubMed] [Google Scholar]
- 84.Wormley DD, Chirwa S, Nayyar T, et al. Inhaled benzo(a)pyrene impairs long-term potentiation in the F1 generation rat dentate gyrus. Cell Mol Biol (Noisy-le-grand) 2004 Sep;50(6):715–721. [PubMed] [Google Scholar]
- 85.Farah MJ, Shera DM, Savage JH, et al. Childhood poverty: Specific associations with neurocognitive development. Brain Res. 2006;1110(1):166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 86.Gershoff ET, Aber JL, Raver CC, Lennon MC. Income is not enough: incorporating material hardship into models of income associations with parenting and child development. Child Dev. 2007 Jan-Feb;78(1):70–95. doi: 10.1111/j.1467-8624.2007.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.George GC, Hanss-Nuss H, Milani TJ, Freeland-Graves JH. Food choices of low-income women during pregnancy and postpartum. Journal of the American Dietetic Association. 2005 Jun;105(6):899–907. doi: 10.1016/j.jada.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 88.Ashiabi GS, O’Neal KK. Children’s health status: examining the associations among income poverty, material hardship, and parental factors. Plos One. 2007;2(9):e940. doi: 10.1371/journal.pone.0000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brody DJ, Pirkle JL, Kramer RA, Flegal KM, Matte TD, Gunter EW. Blood lead levels in the US population. Phase I of the National Health and Nutrition Examination Survey (NHANES III, 1988 to 1991) JAMA. 1994;272:277–283. doi: 10.1001/jama.272.4.277. [DOI] [PubMed] [Google Scholar]
- 90.Wang S, Zhang J, Zeng X, Zeng Y, Wang S, Chen S. Association of traffic-related air pollution with children’s neurobehavioral functions in Quanzhou, China. Environmental health perspectives. 2009 Oct;117(10):1612–1618. doi: 10.1289/ehp.0800023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suglia SF, Gryparis A, Wright RO, Schwartz J, Wright RJ. Association of black carbon with cognition among children in a prospective birth cohort study. American journal of epidemiology. 2008 Feb 1;167(3):280–286. doi: 10.1093/aje/kwm308. [DOI] [PubMed] [Google Scholar]
- 92.Calderon-Garciduenas L, Mora-Tiscareno A, Ontiveros E, et al. Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain and cognition. 2008 Nov;68(2):117–127. doi: 10.1016/j.bandc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 93.Oliveras-Rentas RE, Kenworthy L, Roberson RB, 3rd, Martin A, Wallace GL. WISC-IV profile in high-functioning autism spectrum disorders: impaired processing speed is associated with increased autism communication symptoms and decreased adaptive communication abilities. Journal of autism and developmental disorders. 2012 May;42(5):655–664. doi: 10.1007/s10803-011-1289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thaler NS, Bello DT, Etcoff LM. WISC-IV profiles are associated with differences in symptomatology and outcome in children with ADHD. Journal of attention disorders. 2013 May;17(4):291–301. doi: 10.1177/1087054711428806. [DOI] [PubMed] [Google Scholar]
- 95.Hernandez LM. Genes, Behavior, and the social environment: moving beyond the nature/nurture debate. National Academy Press; 2006. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.