Abstract
Numerous virulence factors expressed by C. neoformans (C. neo) modulate host defenses by promoting non-protective Th2-biased adaptive immune responses. Prior studies demonstrate that the HSP70 homologue, Ssa1, significantly contributes to serotype-D C. neo virulence through the induction of laccase, a Th2-skewing and CNS-tropic factor. In the current study, we sought to determine whether Ssa1 modulates host defenses in mice infected with a highly virulent serotype A (serA) strain of C. neo (H99). To investigate this, we assessed pulmonary fungal growth, CNS dissemination, and survival in mice infected with either H99, an SSA1-deleted H99 strain (Δssa1), and a complement strain with restored SSA1 expression (Δssa1::SSA1). Mice infected with the Δssa1 strain displayed substantial reductions in lung fungal burden during the innate phase (days 3 and 7) of the host response whereas less pronounced reductions were observed during the adaptive phase (day 14) and mouse survival increased only by 5 days. Surprisingly, laccase activity assays revealed that Δssa1 was not laccase-deficient, demonstrating that H99 does not require Ssa1 for laccase expression, which explains the CNS tropism we still observed in the Ssa1-deficient strain. Lastly, our immunophenotyping studies showed that Ssa1 directly promotes early M2 skewing of lung mononuclear phagocytes during the innate, but not the adaptive phase of the immune response. We conclude that Ssa1’s virulence mechanism in H99 is distinct and laccase-independent. Ssa1 directly interferes with early macrophage polarization, limiting innate control of C. neo, but ultimately has no effect on cryptococcal control by adaptive immunity.
Introduction
Cryptococcus neoformans is a ubiquitous opportunistic fungal pathogen and a leading cause of fatal mycosis. Immunocompromised patients, especially when T-cell deficient, are at high risk of cryptococcosis (1, 2). Dissemination of fungus from the lungs, a primary site of infection, to the central nervous system is debilitating and accompanied by high mortality (3). The primary immune response to C. neoformans consists of an early innate (afferent phase) response where the microbe is only temporarily contained by pulmonary phagocytes (4, 5). Subsequently, a developing adaptive response (efferent phase) is orchestrated by specific CD4+ T helper cells and its outcome depends on either protective or non-protective Th bias. Protective Th1/Th17-type responses lead to progressive clearance of the organism (6), while a non-protective Th2 response is associated with fungal persistence and CNS dissemination (7–12). These differential effects of T-cell driven responses are attributed to differential effects of Th1 and Th2 cytokines on macrophage M1/M2 polarization (8, 9, 11, 13–15). Type 1 cytokines (IFNγ and TNFα) upregulate inducible nitric oxide synthase (iNOS), the enzyme that generates fungicidal NO from L-arginine and, accordingly, an important marker of M1 polarization (4, 10, 14, 16–20). In contrast, cytokines induced during the non-protective Th2 response (IL-4, IL-10, and IL-13) promote unfavorable M2 polarization (9, 19, 21–24) marked by the induction of Arginase1 (Arg1) that consumes L-arginine without yielding fungicidal NO (reviewed in (25)). Consequently, M2 macrophages support intracellular survival and growth of C. neoformans, promoting fungal persistence (9, 11).
Several cryptococcal virulence factors have been shown to interfere with macrophage polarization indirectly (i.e. by promoting non-protective Th2 response). For example, the cryptococcal virulence factors urease and laccase promote undesirable Th2 polarization and corresponding M2 macrophage polarization (15, 26, 27). However, these factors do not affect the early containment of C. neoformans, but instead promote extensive fungal growth during the efferent phase of the immune response that coincides with robust Th2 polarization (15, 27). Another group of virulence-associated genes including a phosphatidylinositol 4-kinase (PIK1), a ubiquitin-like protein (RUB1), and a cation ATPase transporter (ENA1), promote cryptococcal survival in the host’s tissues and thus contribute to early fungal growth. However, the overall level of virulence conferred by each of these factors correlates with their effects on the adaptive immune response polarization and subsequent macrophage polarization status during the efferent phase of host response (28).
The mammalian heat shock proteins 70 (Hsp70) function both as intracellular chaperones and as extracellular proteins that can be detected by the innate immune system. Some pathogens express Hsp70 homologues that unfavorably modulate the host immune response, including Mycobacterium tuberculosis (29) and Candida albicans (30, 31). These microbial Hsp70 have been linked to suppression of dendritic cells and induction of the non-protective pro-M2 cytokine IL-10. Cryptococcal Hsp70 homologue, Ssa1, is secreted as a component of extracellular vesicles destined for the capsule (32). Accordingly, Ssa1 is an immunodominant antigen for antibody production in both mice and humans (33–36). While microbial Hsp70 proteins show strong effects on the innate immune system and thus are likely to affect macrophages, cryptococcal Ssa1 also serves another role as a DNA-binding protein in C. neoformans that is a co-activator of cryptococcal laccase expression in serotype D (serD) C. neoformans JEC21 (37). However, it is unknown whether Ssa1 plays a similar role in the more prevalent and typically more virulent serotype A of C. neoformans and whether, in addition to its ability to regulate laccase expression, Ssa1 could interfere with the early control of C. neoformans.
In the current study, we report that Ssa1 expressed by serotype A (serA) strain of C. neoformans is a virulence-associated factor that uniquely promotes early fungal growth and an early M2-shift in pulmonary macrophage activation during the innate phase of the immune response. These effects are laccase independent since cryptococcal laccase expression in C. neoformans H99 (serotype A) is independent of Ssa1. This is the first report that identifies Ssa1 as a cryptococcal virulence factor that directly affects early macrophage polarization, and identifies crucial differences in the regulation of laccase expression by serA and serD cryptococcal strains.
Materials and Methods
Mice
6–8 week old female Balb/c mice were obtained from Jackson Labs and were housed at the Veterinary Medicine Unit at the Ann Arbor Veterans Administration Hospital. Mice were aged to 8–10 weeks old at the time of infection. At the time of data collection, mice were humanely euthanized by CO2 inhalation followed by severance of the portal vein. All experiments were approved by the University Committee on the Use and Care of Animals and the Veterans Administration Institutional Animal Care and Use Committee.
C. neoformans
C. neoformans serotype A strain H99 (ATCC 208821) was recovered from 10% glycerol frozen stocks stored at −80°C. C. neoformans mutants H99-Δssa1 and H99-Δssa1::SSA1 were recovered from 10% glycerol frozen stocks stored at −80°C for experiments. Cultures were grown at 37°C in Sabouraud dextrose broth (1% Neopeptone, 2% dextrose, Difco, Detroit, MI) on a shaker. When cultures reached mid-log phase growth (day 3 for H99, day 5 for H99-Δssa1 and H99-Δssa1::SSA1 when cultured from freezer stocks, at which point all strains had similar growth kinetics), an aliquot of culture was washed in sterile non-pyrogenic saline (Travenol, Deerfield, IL), counted on a hemocytometer, and diluted to 3.3 × 105 yeast cells/ml in sterile non-pyrogenic saline.
Generation of serotype A Δssa1 mutant and Δssa1::SSA1 complement strain
To make the deletion construct, a pair of PCR primers, which correspond to an internal sequence within the ORF of SSA1 (5′-TTCCATCACTCGTGCCCGA-3′ and 5-′ TATACAT GTCGTGTCACAGAC-3′), was used to amplify a 1.3 kb SSA1 genomic fragment. The PCR fragment was cloned into the pCR2.1 TA cloning vector (Invitrogen), and a 1.3 kb fragment of the cryptococcal selection marker URA5 was PCR amplified from plasmid pURA5g2 as described (38), and inserted into a unique AgeI site. The recovered plasmid (pSSA1URA5-1) was digested with EcoRI, and the deletion construct was gel purified and transformed into a C. neoformans H99 ura5 strain described previously (39) by electroporation (BioRad). Transformants were screened by PCR, and disruption of SSA1 was confirmed by Southern blot using the indicated restriction enzymes and the product was hybridized with a 32P-labelled 1.5 kb PCR-amplified fragment of SSA1 that was used above to produce the SSA1 deletion construct. For complementation of the Δssa1 mutant, a 4.5 kb SSA1 PCR fragment of wild-type DNA was amplified using 5′-GCCGCCCTGCAGTGAGGTTGATGTGCCTTTCC-3′ and 5′-GCCGCCATCGATATGTCCGAAAGACTATCCGGA-3 ′, digested with the appropriate restriction enzymes and ligated into compatible sites of pBS-Hyg (pBluscript containing the hygromycin B-resistant gene (40)). The plasmid (pSSA1Hyg-2) was digested with NotI and transformed into the Δssa1 mutant by electroporation and inoculated onto asparagine agar plates containing hygromycin. The transformant was characterized by Southern blot for SSA1 and Western blot for Ssa1 and laccase expression analysis. Retention of the SSA1 deletion in the complemented strain was also confirmed by Southern blot. Furthermore, genomic insertion of the wild-type SSA1-HgR construct was confirmed by Southern blot of uncut DNA hybridized with the HgR gene (data not shown).
Intratracheal inoculation of C. neoformans
Mice were anesthetized with intraperitoneal injection of ketamine/xylazine (100/6.8 mg/kg body weight) and were secured onto a clean foam board. Hair was removed from over the trachea and skin was sterilized with iodine and ethanol. A small incision was made over the trachea, and the underlying muscle and glands were separated to expose the trachea. A 30-gauge needle was inserted into the trachea and 30 μl (104 CFU) of the washed yeast (3.3 × 105 yeast cells/ml in sterile non-pyrogenic saline) were injected intratracheally from a 1-ml tuberculin syringe fitted to a stepper pipette. After inoculation, the incision was closed with cyanoacrylate adhesive, and mice were kept warm and monitored during recovery from anesthesia.
Lung leukocyte isolation
At the time of data collection, lungs were perfused with 3 ml sterile nonpyrogenic saline, removed, washed in RPMI 1640, and enzymatically dispersed as previously described (41, 42). Briefly, excised lungs from each mouse were minced with scissors and digested enzymatically at 37°C for 30 min in 5 ml digestion buffer (RPMI 1640, 5% FBS, penicillin and streptomycin [Invitrogen, Grand Island, NY]; 1 mg/ml collagenase A [Roche Diagnostics, Indianapolis, IN]; and 30 mg/ml DNase [Sigma]). The cell suspension and tissue fragments were further dispersed by repeated aspiration through the bore of a 10-ml syringe and centrifuged. Erythrocytes in the cell pellets were lysed by addition of 3 ml NH4Cl buffer (0.829% NH4Cl, 0.1% KHCO3, and 0.0372% Na2EDTA, pH 7.4) for 3 min followed by a 10-fold excess of RPMI 1640. Cells were resuspended and a second cycle of syringe dispersion and filtration through a sterile 100-mm nylon screen (Nitex, Kansas City, MO) was performed. The filtrate was centrifuged for 25 min at 1500 × g in the presence of 40% Percoll (Sigma) in complete RPMI 1640 (RPMI 1640, 5% FBS, 10 U/ml penicillin and streptomycin [Invitrogen, Grand Island, NY], 1X sodium pyruvate, 1X glutamax, 1X non-essential amino acids, 2-mercaptoethanol) with no brake to separate leukocytes from cell debris and epithelial cells. Leukocyte pellets were resuspended in 5 ml complete RPMI 1640 media and enumerated on a hemocytometer after dilution in trypan blue (Sigma).
Lung CFU assay
For determination of fungal burden in the lungs, 100 μl was removed from the enzymatically dispersed lungs prior to centrifugation and 10-fold dilutions were plated in duplicate on Sabouraud dextrose agar plates. For determination of brain CFU, brains from mice in the survival study were removed at the time of death and homogenized in 2 ml sterile mili-Q water. 10-fold dilutions were then plated in duplicate on Sabouraud dextrose agar plates. Colonies were counted after 48 h of growth at room temperature, and CFU was calculated on a per-organ basis.
Survival study
6–7 mice were infected intratracheally with 104 CFU of the wild type H99, H99-Δssa1 or H99-Δssa1::SSA1 strains as described above. Mice were monitored daily for survival, and moribund animals were humanely euthanized and survival data recorded.
Assessment of laccase expression
Cryptococcus neoformans strains H99 or H99-Δssa1 were isolated from the brains of infected mice at the time of death. The brain isolates and the original inoculum were plated on asparagine salts agar (40 g Bacto agar, 4 g Asparagine, 2 g MgSO4, 12 g KH2PO4, 12 g glucose, 4 mg thiamine, 4 mM polyphenol per liter in ddH2O). Melanin production, indicative of laccase expression, results in brown to black-colored colonies, while deficiency in laccase expression results in pale colonies when incubated for 3–7 days at room temperature.
Histology
Lungs were instilled with 1 ml of 10% neutral buffered formalin, excised, immersed 10% in neutral buffered formalin and embedded in paraffin as described previously (20). 5 μm sections were cut and stained with H&E and mucicarmine counterstain. Sections were analyzed with light microscopy and microphotographs were taken using Digital Microphotography system DFX1200 with ACT-1 software (Nikon, Tokyo, Japan).
Preparation and enumeration of lung leukocytes
Following enzymatic dispersal of the lungs, 50,000 cells were cytospun onto glass slides, fixed, stained with Wright-Giemsa stain, and dried. Monocytes, eosinophils, neutrophils and lymphocytes were counted as described previously (23).
Fluorescence microscopy
Lungs were inflated and prepared as previously described (43). Sectioning and staining were performed as follows: Serial frozen tissue sections were cut at a thickness of 10 μm and fixed at −20°C in acetone for 10 min. Tissue sections were rehydrated in 70% ethanol for 5 min and washed in PBS for 3 min. Sections were blocked in normal rabbit or rat serum, depending on the species in which the primary staining antibodies used were generated. Antibodies used were rabbit anti-mouse Arg-1 (Santa Cruz Biotechnology, Santa Cruz, CA), rat anti-mouse CD206 (macrophage mannose receptor, AbD Serotec, Raleigh, NC), and rabbit anti-mouse inducible nitric oxide synthase (iNOS, Axxora, San Diego, CA). Primary antibodies were detected using Alexa 488-conjugated goat anti-rat IgG or goat anti-rabbit IgG secondary antibodies (Invitrogen, Carlsbad, CA). Tissue sections were incubated overnight at 4°C with primary Abs diluted in species-specific serum (3% in PBS) at pre-optimized concentrations. Subsequently, the sections were washed five times in Tris-NaCl-Tween 20 (TNT) buffer solution for 3 min each. Sections were then incubated with secondary Abs for 30 min at room temperature. Slides were washed five times in TNT buffer for 3 min each, once in PBS containing 1% Triton X-100 to minimize background fluorescence (5 min), and given a final wash in TNT buffer (3 min). Sections were then mounted with FluorSave reagent (Calbiochem, La Jolla, CA) containing 0.3 μM DAPI (Molecular Probes, Eugene, OR). Fluorescence was visualized with a Leica DMR epifluorescence microscope (Leica Microsystems, Wetzlar, Germany). Images were acquired using a cooled Spot RT charge-coupled device camera (Diagnostic Instruments, Sterling Heights, MI). Imaging acquisition was done using the auto exposure setting within the software package and evaluating each specimen using these consistent conditions, and imaging analysis was performed using IPLab v4.08 (BD Biosciences).
Flow cytometry
For flow cytometry experiments, Abs were purchased from BioLegend (San Diego, CA), including rat anti-murine CD16/CD32 (Fc block), rat anti-murine CD45 conjugated to allophycocyanin, hamster anti-murine CD11c conjugated to Pacific blue, rat anti-murine CD11b conjugated to allophycocyanin-Cy7, rat anti-murine Ly6G conjugated to PE-Cy7, rat anti-murine CD3 or CD19 conjugated to PerCPCy5.5, rat anti-murine CD206 conjugated to FITC, and rat anti-mouse Galectin-3 or MHC class II (2A/IE) conjugated to PE.
Enzymatically dispersed lungs were counted and stained extracellularly, then fixed, permeabilized, and stained intracellularly as previously described (15), then run on an LSR II flow cytometer using FACSDiva software (BD Biosciences, San Jose, CA) and analyzed further using FlowJo software (Tree star, San Carlos, CA). Gating for lung myeloid cells proceeded as follows: the CD45+ cells were identified, lymphocytes were removed (CD19+/CD3+), neutrophils were removed (Ly6G+/CD11b+), eosinophils were excluded (SSChigh/CD11cint), and then CD11c+ cells were analyzed for expression of activation markers in the FITC and PE channels.
Real-Time PCR
Cells were spun down and directly resuspended in 1 ml Trizol reagent (Life Technologies, Inc., Gaithersburg, MD) in polypropylene tubes. Samples were allowed to incubate at room temperature, and 200 μl chloroform per 1 ml Trizol was added to them. Samples were spun at 10,000 rpm for 15 min, the aqueous phase was transferred into fresh tubes, and equal volumes of isopropanol were added. Samples were then incubated at −20°C for 90 minutes to precipitate the RNA and centrifuged again as described above. Pellets were washed with 1 ml of 70% ethanol and centrifuged. RNA was resuspended in nuclease-free water (Ambion). The yield and purity of the RNA were determined spectrophotometrically at 260 and 280 nm. cDNA was synthesized using QuantiTect Reverse Transcription Kit (Qiagen) using 1 μg RNA according to the manufacturer’s instructions. cDNA was quantified with SYBR Green– based detection using an MX 3000P system (Stratagene, La Jolla, CA) according to the manufacturer’s protocols. Forty cycles of PCR (94°C for 15 seconds followed by 60°C for 30 seconds and 72°C for 30 seconds) were performed on a cDNA template. The primers used are described in Table 1. The mRNA levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels and relative expression shown as % of GAPDH.
Table 1.
qRT-PCR primer sequences
| Gene | Forward/sense primer (5′ -3′) | Reverse/anti-sense primer (5′ -3′) |
|---|---|---|
| GAPDH | TATGTCGTGGAGTCTACTGGT | GAGTTGTCATATTTCTCGTGG |
| IFNγ | CTACCTCAGACTCTTTGAAGTCT | CAGCGACTCCTTTTCCGCTT |
| TNF-α | CCTGTAGCCCACGTCGTAGC | AGCAATGACTCCAAAGTAGACC |
| IL-4 | CTGACGGCACAGAGCTATTGA | TATGCGAAGCACCTTGGAAGC |
| IL-13 | GCCAGCCCACAGTTCTACAGC | GAGATGTTGCTCAGCTCCTCA |
| iNOS | TTTGCTTCCATGCTAATGCGAAAG | GCTCTGTTGAGGTCTAAAGGCTCCG |
| Fizz1 | GGTCCCAGTGCATATGGATGAGACCATAGA | CACCTCTTCACTCGAGGGACAGTTGGCAGC |
| Arg1 | CAGAAGAATGGAAGAGTCAG | CAGATATGCAGGGAGTCACC |
| CD206 | CTCTGTTCAGCTATTGGACGC | CGGAATTTCTGGGATTCAGCTTC |
| Gal-3 | TTTCAGGAGAGGGAATGATGTT | TCTTCATCCGATGGTGTGACTG |
Statistical analysis
All values are reported as means ± SEM. Continuous ratio scale data were evaluated by unpaired Student’s t-test (for comparison between two samples) or by ANOVA (for multiple comparisons) with post hoc analysis using Student’s t-test with Bonferroni adjustments. Non-parametric analysis was performed using the Kruskal-Wallis, Kolmogorov-Smirnov, or Mann-Whitney tests. Survival study comparisons were performed using Kaplan-Meier analysis. Statistical calculations were performed on a PC computer using GraphPad Prism version 6.00 for Windows (GraphPad Software, San Diego, CA). Statistical difference was accepted at p < 0.05.
Results
Cryptococcal Ssa1 expression contributes to pulmonary virulence of C. neoformans serotype A in a mouse model
The Hsp70 homologue Ssa1 has been shown to be required for cryptococcal laccase expression and thus to contribute significantly to the virulence composite of C. neoformans serD strain JEC21 (37). However, the function of virulence-associated genes between serD and the more commonly isolated serA may differ when it comes to clinical impact (44, 45). In this study, we sought to determine the role of Ssa1 in the virulence of C. neoformans serA using the wildtype strain H99 and its mutants with targeted manipulations of the SSA1 gene: (Δssa1) the deletant strain, and Δssa1 complemented with the functional SSA1 gene (Δssa1::SSA1). Deletant and complement strains were generated using an analogous protocol as described previously for serD (37). Both deletion and restoration of the SSA1 gene were confirmed by PCR and Southern blot analysis (data not shown). The deletant and complement strains of H99 did not exhibit demonstrable growth differences from the parent strain H99 organism in vitro. Subsequently, BALB/c mice were infected intratracheally with 104 CFU of wild type C. neoformans H99, Δssa1, or Δssa1::SSA1, and pulmonary fungal growth was assessed in the lungs at 3, 7, and 14 days post infection (dpi). Substantial decreases in fungal load were observed at 3 and 7 dpi in the lungs of mice infected with Δssa1 compared to both Δssa1::SSA1- and H99-infected mice; however, beyond day 7 Δssa1 acquires strong logarithmic growth rate displayed by both Ssa1-expressing strains (Fig. 1A), consistent with Ssa1 expression promoting early growth of C. neoformans in the infected lungs during the afferent phase of the immune response.
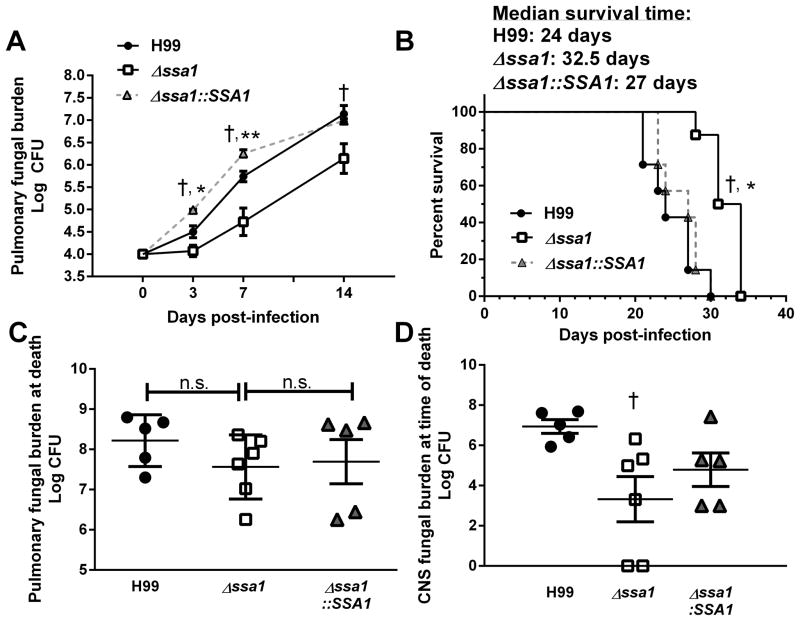
Figure 1.
Cryptococcal SSA1 gene deletion delays fungal growth in the lungs and the CNS dissemination, and improves survival of infected mice. (A) Mice were infected with 104 cells of wild type (WT) C. neoformans H99 (serotype A), SSA1-gene deleted mutant Δssa1, or complement strain Δssa1::SSA1 on an H99 background. Pulmonary fungal burdens were quantified on 3, 7, or 14 days post infection (dpi). Mice infected with Δssa1 had significantly reduced pulmonary fungal burden relative to H99 at 3, 7, and 14 dpi and had significantly reduced pulmonary fungal burden relative to Δssa1::SSA1 at 3 and 7dpi. Note the faster growth rates of the WT and complement strains at 0–7 dpi, and parallel growth rates for all 3 strains beyond day 7. Data represent pooled average CFU per lung or brain from separate matched experiments; n=13 mice per group per time point for H99 and Δssa1 and n=4 for Δssa1::SSA1. (B) Survival study was performed on 7–8 mice per group. Infection with Δssa1 conferred a modest but significant survival advantage compared to H99 and Δssa1::SSA1. (C, D) Post-mortem CFU assay was performed on homogenized lung (C) and brain (D) from mice in the survival study. Pulmonary fungal burden did not differ significantly between any strains at the time of death, while fungal burden in the brain was significantly decreased in Δssa1-infected mice. († p < 0.01 relative to H99, * p < 0.05 relative to Δssa1::SSA1, ** p < 0.01 relative to Δssa1::SSA1).
To further assess the virulence properties of the Δ ssa1 strain, we performed a comparative survival study in mice infected with H99, Δssa1 and Δssa1::SSA1. Mice infected with H99 or Δssa1::SSA1 succumbed to infection by 31 and 30 dpi, respectively, while mice infected with Δssa1 survived up to 35 dpi (Fig. 1B). The median survival time for mice infected with H99 andΔssa1::SSA1 was 24 and 27 days respectively. In contrast, the median survival for mice infected with Δ ssa1 displayed a small but statistically significant extension of survival out to 32.5 days. Interestingly, the fungal burden from the lungs at the time of death was not significantly different between the H99 and Δ ssa1-infected mice (Fig. 1C), indicating that SSA1 deletion resulted in a modest attenuation of H99 virulence.
Ssa1 expression in serotype A is not required for brain dissemination or laccase expression
Since H99 is highly neurotropic, we harvested brain tissue to assess microbial dissemination to the central nervous system (CNS) post-mortem during the survival study. As expected, H99 disseminated in high titers into the brain in 100% of the infected mice, as did the complemented strain Δ ssa1::SSA1. Surprisingly, the Δssa1 mutant also disseminated to the brain in all the infected mice, albeit in reduced numbers relative to H99 (Fig. 1D). This contrasted with our previously published work with theΔlac1 strains in both JEC21 and H99 backgrounds, which consistently demonstrated that laccase-deficient C. neo did not disseminate into the CNS (27, 46). Thus, the current data strongly suggested that the Δssa1 strain was capable of expressing laccase.
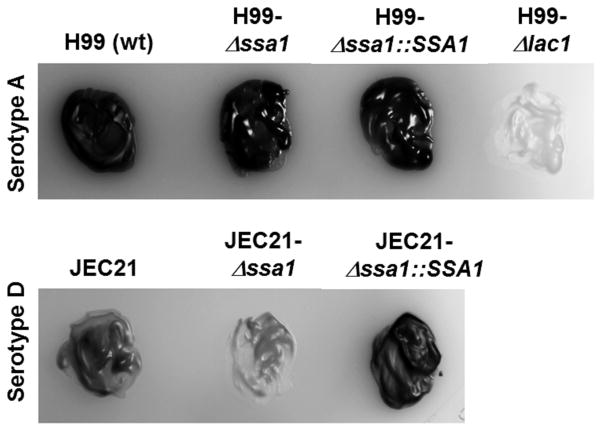
To test this hypothesis, we re-isolated Δssa1 from the brains of mice that succumbed to infection and tested them for completeness of the SSA1 deletion and for laccase activity. We found complete deletion of the SSA1 transcript by qPCR both in the originalΔssa1 strain used for infection and in theΔssa1 isolated from the brains of mice from the survival study (data not shown), showing that the H99-Δssa1 mutant did not revert to WT H99 in the infected mice. As a readout for laccase activity, we assessed melanin synthesis by serotype A strains H99, Δssa1, Δssa1::SSA1, and Δlac1 (Fig. 2, top panel, left to right) and by the serotype D strains JEC21 WT (JEC21-WT), SSA1-deficient (JEC21-Δssa1), and complement (JEC21-Δssa1::SSA1) (Fig. 2, bottom panel, left to right) in cultures on asparagine-norepinephrine agar. In serotype A, H99, Δssa1, and Δ ssa1::SSA1 strains displayed robust melanin pigmentation consistent with laccase activity, while the Δlac1 strain lacked any pigmentation (Fig. 2, top panel, left to right). In serotype D, the JEC21-WT strain displayed modest pigmentation, while the JEC1-Δ ssa1 strain completely lacked pigmentation (Fig. 2 bottom panel, middle) consistent with published studies (37), showing that laccase expression in JEC21 (a serD strain) requires Ssa1 while laccase expression in H99 (a serA strain) does not require Ssa1. Thus, apart from the differential role of Ssa1 in laccase regulation between strain H99 (serA) and JEC1 (serD), we demonstrated Ssa1’s role as a virulence factor independent of its role in laccase expression in the H99 strain.
Figure 2.
Cryptococcal SSA1 deletion suppresses laccase activity in a serotype D JEC21-Δssa1 mutant but not in a serotype A Δssa1 mutant. Laccase activity was determined by melanization of C. neoformans colonies cultured for 48 h in YPD media at 37°C, then plated on asparagine salts agar with norepinephrine, and incubated at room temperature for 5 days. Note strong melanin pigmentation displayed by WT serotype A strain H99, the Δssa1 mutant in an H99 background, and the corresponding Δssa1::SSA1 complemented serotype A, and the absence of pigmentation in the laccase-deficient mutant (Δlac) in an H99 background. Also note the pigmentation in the serotype D WT strain JEC21 and corresponding Δssa1::SSA1 but the minimal pigmentation displayed by the Δssa1 strain on JEC21 background.
Cryptococcal Ssa1 expression accelerates but does not significantly alter the development of lung and brain pathologies
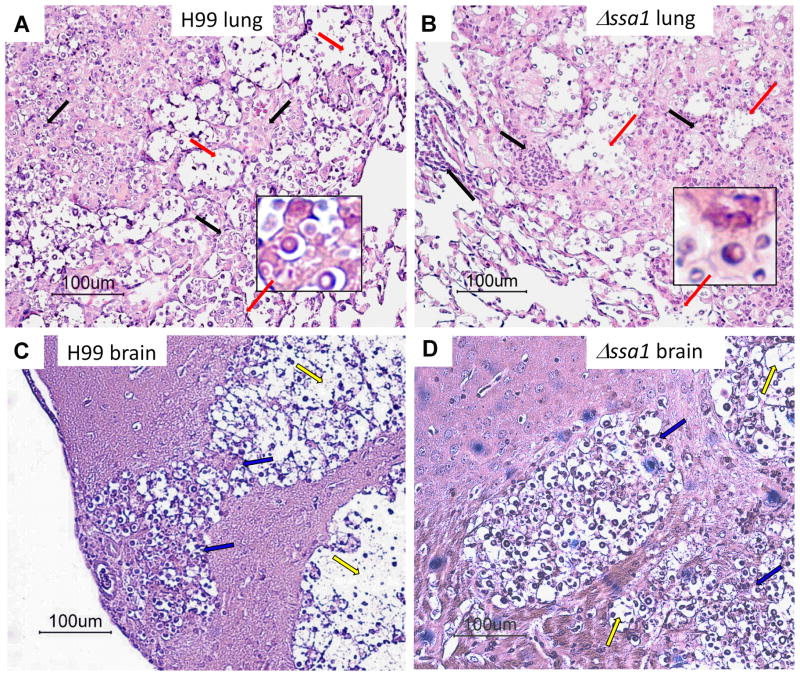
Next, we sought to determine the effect of cryptococcal Ssa1 expression on lung pathology and the extent of the inflammatory response via histopathological examination of lungs from mice infected with H99 or the Δssa1 strain at 21 dpi. H99 and Δssa1-infected mice showed similar leukocyte accumulation in the alveolar spaces of lungs at 21 dpi (Fig. 3A and B). Both strains induced severe lung pathology by 21 dpi, with organisms growing in widespread areas of the lungs. Inflammatory infiltrates were present in the infected areas, but many fungi resided within the alveolar space unaccompanied by inflammatory cells, indicating that both strains can evade the inflammatory response. We also noted areas of dissemination of the Δssa1 organisms to uninfected lung areas, consistent with the somewhat delayed kinetics of fungal growth but overall progressive nature of pulmonary infection with Δssa1.
Figure 3.
Deletion of cryptococcal SSA1 results in delayed but similar type lung and CNS pathology. Lungs and brains of mice infected with H99 (A, C, respectively) and Δssa1 (B, D, respectively) strains were isolated at week 3. Tissues were preserved in formalin, processed for histology, and cut and stained with mucicarmine to visualize C. neoformans. Lungs were counterstained with H&E. Representative images taken at 20x magnification with 100x magnification insets for lung histology images. A and B) Inflammatory infiltrates (black arrows) are present in both H99 and Δssa1 strains. Note the cryptococci (red arrows, 100x) residing in the alveolar space and the presence of inflammatory cell infiltrates in H99- and Δssa1-infected lungs. C and D) Cryptococcal mucoid cysts infiltrating and displacing brain tissue. Note the infiltrating inflammatory cells at the periphery and cocci and cell debris in the middle of the cysts (blue arrows), where normal brain tissue has eroded (yellow arrows) in both H99 and Δssa1 strains.
To further establish that Δssa1 was capable of invading the brain and inducing CNS pathology, we performed histological analysis of brain sections of mice infected with H99 or Δssa1. Our data demonstrate that at the time of death, Δssa1 induced CNS pathology similar to that of the H99. Both H99 and Δssa1 induced similarly-sized CNS lesions with evidence of modest inflammatory infiltrates at the periphery surrounding large central areas occupied mostly by C. neoformans and debris (Fig. 3C and D).
Cryptococcal Ssa1 expression affects pulmonary leukocyte populations during the innate but not the adaptive phase of the immune response in the infected lungs
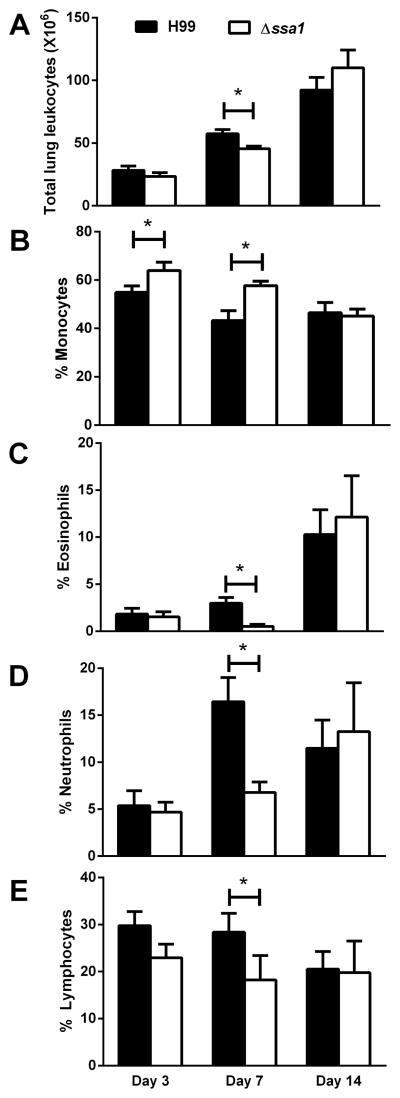
Collectively, data from our assessment of fungal burden, CNS dissemination, survival, and histopathology suggested that cryptococcal Ssa1 expressed by SerA C. neoformans affected the innate phase of the immune response more prominently than the adaptive phase. To determine the effect of Ssa1 on host defenses, we quantified and further characterized leukocyte populations in the lungs of mice infected with H99 or Δssa1 at 3, 7, and 14 dpi. Leukocytes from enzymatically dispersed lungs were evaluated by differential cell counts. Both H99- and Δssa1-infected mice accumulated similar numbers of leukocytes at 3 and 14 dpi, but the Δssa1 strain showed slight but significant decreases of leukocyte numbers at 7 dpi (Fig. 4A), indicating that cryptococcal SSA1 expression may contribute to the magnitude of the inflammatory response at the peak of innate inflammation. A leukocyte subset analysis (performed by visual inspection of cytospins) identified changes in frequencies of pulmonary leukocytes, specifically at the early time points. Relative to H99 infected mice, the Δssa1-infected lungs accumulated a higher proportion of mononuclear phagocytes (Fig. 4B) and a much lesser proportion of granulocytes (eosinophils and neutrophils) (Fig. 4C–D), indicating that cryptococcal Ssa1 contributed to the development of innate inflammation, especially its granulocytic component. In contrast, no changes in magnitude or frequency of pulmonary leukocyte subsets were observed on day 14, providing a clue that the adaptive response did not significantly differ between the H99- and Δssa1-infected mice. Thus, deletion of cryptococcal gene SSA1 altered the composition of lung leukocyte populations during the innate but not adaptive phase of the immune response.
Figure 4.
Deletion of cryptococcal SSA1 resulted in a similar magnitude but distinct polarization of early but not late inflammatory responses in the infected lungs. Lung leukocytes were isolated and characterized by microscopy at 3, 7, and 14 dpi from C. neoformans-infected lungs. Total leukocyte numbers in the lungs were equivalent in H99 versus Δssa1-infected lungs with the exception of a small but significant decrease on day 7 in the Δssa1-infected lungs (A). Monocytes (B), eosinophils (C), neutrophils (D) and lymphocytes (E) were identified by morphology and staining characteristics. Monocyte frequency was significantly increased at 3 and 7 dpi in Δssa1-infected mice, while eosinophil and neutrophil frequency was significantly decreased at 7 dpi in Δssa1-infected mice relative to H99-infected mice, and lymphocyte frequency never differed significantly between the two strains at any time points. N=5–11 mice per time point per strain, representing 3 separate matched experiments. *p < 0.05 compared to H99.
Cryptococcal Ssa1 promotes M2 macrophage polarization during the innate but not adaptive phase of the immune response to cryptococcal infection
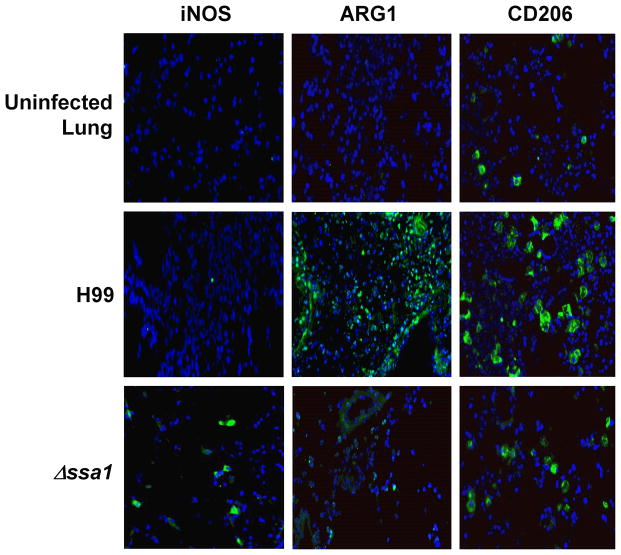
Pulmonary control of C. neoformans depends on macrophage polarization status: M1 (classical activation) is protective and M2 (alternative activation) is non-protective. Thus, we next sought to determine whether the early Ssa1-induced impairment in pulmonary fungal clearance was linked to differential M1/M2 polarization. First, we assessed early M1/M2 macrophage polarization markers in H99 and Δssa1-infected lungs using immunohistochemistry. Due to low levels of inflammation during the first days of infection (unpublished observations), lung sections were analyzed at 8 dpi, a time point that still largely represents an innate/afferent phase of the immune response. In lung sections obtained from mice infected with H99, we observed clusters of cells highly expressing Arg1 and mannose receptor (CD206), but very few cells positive for inducible nitric oxide synthase (iNOS) (Fig. 5), consistent with presence of M2 macrophages in the H99-infected lungs. In contrast, we observed fewer CD206 and Arg1-positve cells and higher number of cells expressing iNOS in lung sections obtained from mice infected with Δssa1, suggesting more predominant M1 polarization in the absence of cryptococcal Ssa1.
Figure 5.
Cryptococcal SSA1 expression promotes the induction of crucial M2 proteins ARG1 and CD206 while preventing induction of M1 protein iNOS in the lungs. Defrosted lung sections taken at 8 dpi were stained with DAPI (blue) to show nuclei, and antibodies for M1 activation marker iNOS (left panel), M2 activation marker Arginase (Arg1, middle panel) and M2 activation marker mannose receptor (CD206, right panel). Secondary FITC conjugated antibodies (green) were used to visualize immunoreactive proteins. Note the significant induction of Arg1 and CD206 and the absence of iNOS staining in H99-infected lung sections. In contrast, iNOS protein can be detected in Δssa1-infected sections, while the M2 markers Arg1 and CD206 show strikingly less immunoreactivity in Δssa1-infected lungs. Sections were imaged at 40X magnification.
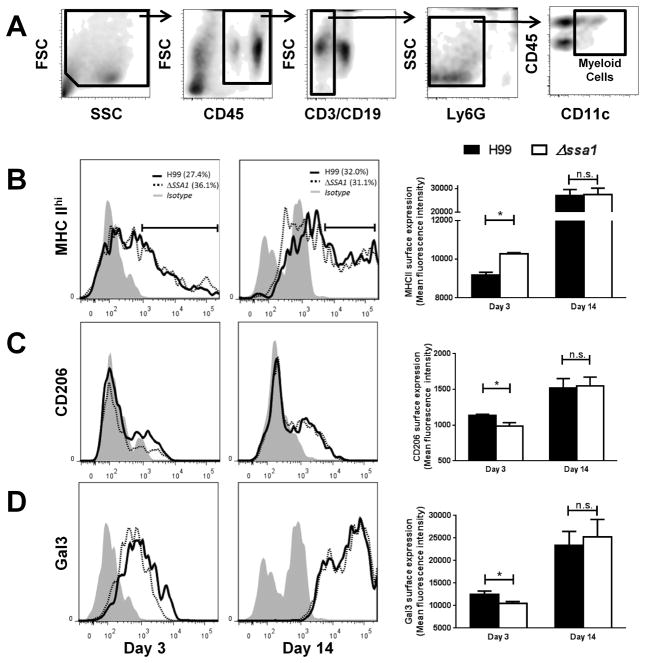
To evaluate these differences more quantitatively and to evaluate the M1/M2 status of pulmonary mononuclear phagocytes at the earlier and later time points, we next performed flow cytometry analysis on leukocytes populations obtained from H99- and Δssa1-infected lungs at 3 and 14 dpi. We measured expression of MHC class II (high expression consistent with M1 polarization) and CD206 and Galectin 3 (high expression consistent with M2 polarization) on CD11c+ populations of lung leukocytes which, following exclusion of T cells, B cells, neutrophils, and eosinophils (Fig. 6A, also see Materials and Methods) (47–49) are highly enriched for resident alveolar macrophages as well as monocyte-derived exudate macrophages and dendritic cells. These cell subsets represent fully differentiated mononuclear phagocytes, which are all capable of M1/M2-type polarization as well as killing of C. neoformans. Results showed significant differences in the expression levels of all of these markers by CD11c+ myeloid cells between H99 and Δssa1-infected lungs at 3 dpi. Surface expression of MHC class II (MHC II) was increased (Fig 6B) while surface expression of CD206 and Galectin 3 (Gal3) was decreased (Fig 6C–D) at 3 dpi in Δssa1-infected lungs compared to those infected with H99, consistent with enhanced M1 polarization in Δssa1-infected lungs and more pronounced M2 polarization in H99-infected lungs on 8 dpi. In contrast, the surface expression levels of these markers in H99 and Δssa1-infected groups were equivalent at 14 dpi (Fig. 6B–D), demonstrating similar M1/M2 polarization profiles of these mononuclear cells during the adaptive phase of the immune response in the presence and absence of cryptococcal Ssa1.
Figure 6.
Cryptococcal Ssa1 increases surface expression of M2 activation markers and decreases surface expression of MHCII during the innate but not adaptive phase of the immune response in the infected lungs. Flow cytometric analysis of leukocyte populations from infected mouse lungs used the gating scheme in (A) to sequentially gate out debris, non-immune cells, lymphocytes, and granulocytes and then we selected the CD11c+ subset of this population. M1 activation marker MHC class II (B) and M2 activation markers Gal3 (C) and CD206 (D) surface expression was assessed at 3 and 14 dpi. Note the significant increase in MHCII and decrease in CD206 and Gal3 surface staining in Δssa1-infected mice at 3 dpi, but a resolution of these differences by 14 dpi. Representative histograms were selected from two separate, matched experiments. N = 5 per treatment per time point. Combined mean fluorescence intensity plots represent high-expressing cells (MHC II) or positively staining cells (CD206 and Gal3), and reflect the cumulative phenotype. * p < 0.05 using Student’s t-test.
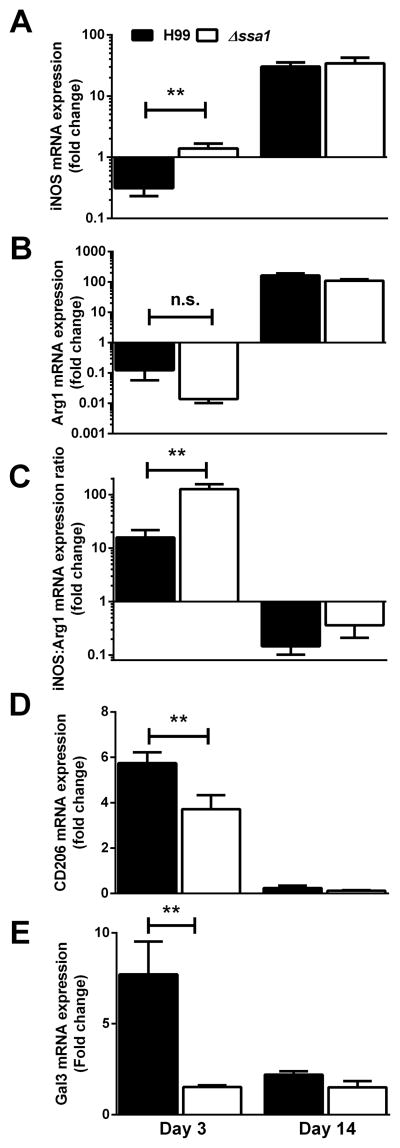
To further assess and verify our findings, additional M1/M2 markers and M1/M2-associated cytokines were analyzed by quantifying mRNA expression in adherence-purified macrophages from lungs of mice at 3 and 14 dpi with H99 and Δssa1. At 3 dpi, macrophages from Δssa1-infected lungs induced significantly more transcript for M1 activation gene iNOS (Fig. 7A) and less transcript for M2 activation genes Arg1 (Fig. 7B), Gal3 (Fig. 7D), CD206 (Fig. 7E) and FIZZ (not shown) at 3 dpi than did those infected with H99. Further, the iNOS:Arg expression ratio was M1-skewed, with Δssa1-infected lung macrophages having a higher expression ratio, compared to a lower expression ratio (indicating a less M1- and more M2-polarized phenotype) in the H99-infected lung macrophages. Consistent with our flow cytometry readouts, at 14 dpi none of these genes were differentially expressed, nor were gene expression ratios differentially skewed between H99 and Δssa1- infected groups. Collectively, these data indicate that cryptococcal SSA1 expression by SerA promotes M2 polarization during the innate, but not the adaptive, phase of the immune response to cryptococcal infection.
Figure 7.
Cryptococcal Ssa1 increases induction of macrophage M2 activation markers and decreases induction of M1 activation marker iNOS during the early but not the late phase of lung infection in mice. Macrophages were isolated from infected mouse lung leukocyte preparations by adherence selection. iNOS (A), Arg1 (B), the iNOS/Arg1 expression ratio (C), CD206 (D) and Gal3 (E) mRNA transcript levels were assessed by qPCR. Note the increase in iNOS in Δssa1-infected mouse macrophages relative to H99-infected mouse macrophages at day 3 but not at day 14. Arg1 is not significantly different between the macrophages at day 3. However, a paired iNOS/Arg1 ratio (C) where an individual mouse’s macrophage iNOS mRNA fold change was divided by its own Arg1 mRNA fold change yielded highly significant M1-skewed signature in macrophages from Δssa-infected mice but a far lower ratio in H99-infected mouse macrophages at day 3, but not at day 14, where both Δssa1- and H99-infected mice had M2-skewed macrophages. Also note the decrease in expression of both M2 activation markers CD206 and Gal3 in Δssa1- infected mice relative to H99-infected mice at day 3, but not at day 14. N = 8 from 2 separate experiments. * p < 0.05, ** p < 0.01 using Student’s t-test.
Cryptococcal SSA1 expression modulates innate but not adaptive phase pulmonary type 2 cytokine responses in infected lungs
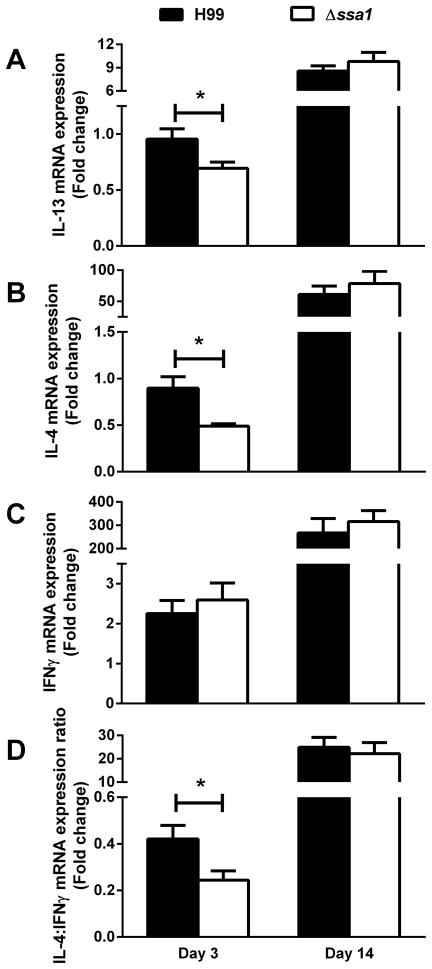
We next investigated the cytokine mRNA expression levels by leukocytes obtained from the lungs of H99- and Δssa1-infected mice. Cryptococcal SSA1 deletion resulted in decreased expression of non-protective Th2 cytokines IL-4 and IL13 at day 3 but not day 14 (Fig. 8A and B), indicating that cryptococcal Ssa1 acted as an early inducer of type 2 cytokine production. The expression of protective Th1-driving IFNγ was not significantly different between the groups (Fig. 8C). However, the IL-4:IFNγ ratio, shown to be a good indicator of type 1 versus type 2 response balance, was decreased in the absence of Ssa1 at 3 dpi (Fig. 8D), demonstrating that the early cytokine balance of type 1/type 2 cytokines was shifted towards type 2 in the presence of cryptococcal Ssa1. Consistent with the similar outcomes of lung pathology and leukocyte populations at the later time point, we did not observe differences in Th1/Th2 polarizing cytokine expression by leukocytes from H99 versus Δssa1-infected lungs on day 14 (Fig 8A–D). Thus, cryptococcal Ssa1 expression modulates innate but not adaptive phase pulmonary type 2 cytokine responses in the infected lung.
Figure 8.
Cryptococcal SSA1 expression results in increased pro-Th2 cytokine induction and increased IL4:IFNγ regulation ratio in pulmonary leukocytes from infected mice during innate phase of the immune response to C. neoformans. Leukocyte preparations from H99 and Δssa1- infected mouse lungs were processed for mRNA quantification of IL-13 (A), IL-4 (B), IFNγ (C) and for the IL-4:IFNγ induction ratio (D) at 3 and 14 dpi. Note the decrease in non-protective cytokines IL-4 and IL-13, along with the decreased IL-4:IFNγ induction ratio in the leukocytes obtained from Δssa1-infected mice relative to the H99-infected mice at day 3, followed by progressive but no longer differential cytokine induction in the lungs of Δssa1- and H99-infected mice on day 14. N = 5/time point/group from 2 separate experiments. * p < 0.05 using Student’s t-test.
Cryptococcal SSA1 expression promotes alternative activation markers and down-regulates classical activation markers during C. neoformans-macrophage interaction in vitro
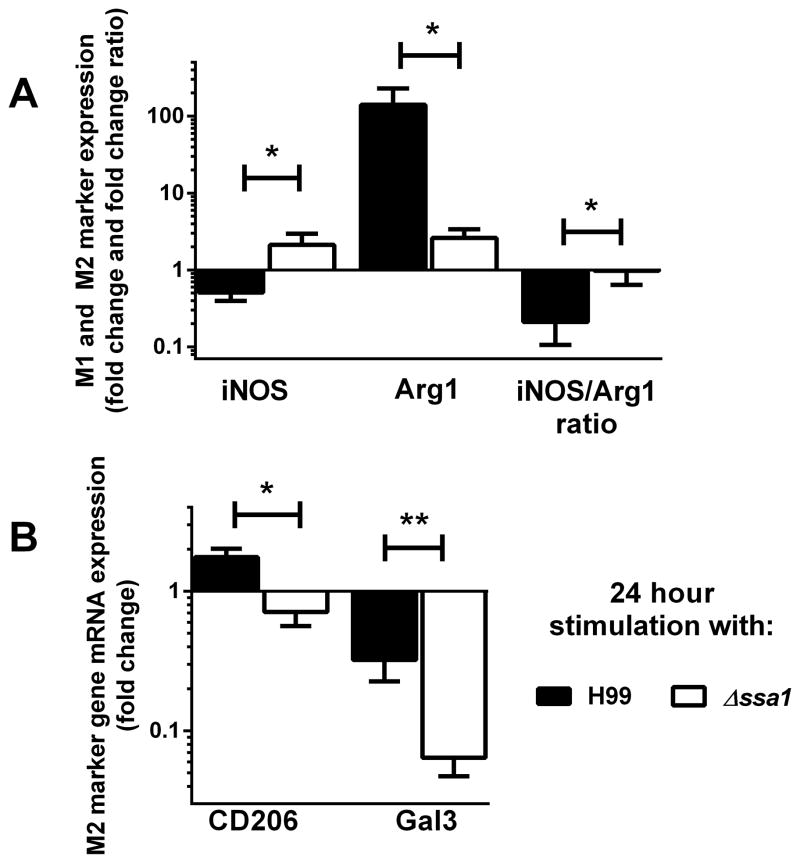
To determine if SSA1 expression is sufficient to directly promote M2 polarization of macrophages, we assessed the M1/M2 polarization response of bone marrow-derived macrophages (BMM) following direct stimulation with C. neoformans H99 or Δssa1 in vitro. BMMs were harvested after stimulation with H99 or Δssa1 for 24h and analyzed for expression of M1 and M2-associated genes by qPCR. Expression of M1 activation marker iNOS was increased, while expression of M2 activation markers Gal3 and CD206 (Fig. 9b) as well as Fizz1 (data not shown) was decreased in macrophages stimulated with Δssa1 compared to those stimulated with H99 (Fig. 9) as early as 24h post-treatment. Further, the iNOS:Arg expression ratio, a broadly accepted readout of M1/M2 polarization balance, was heavily skewed towards M1 in the Δssa1-treated macrophages compared to those stimulated with H99. Thus, these findings demonstrate that cryptococcal Ssa1 directly promotes M2 polarization in vitro.
Figure 9.
In vitro stimulation with an SSA1-expressing cryptococcus is sufficient to decrease M1 and increase M2 activation markers in bone marrow derived macrophages. mRNA was extracted from bone marrow-derived macrophages stimulated with live H99 or Δssa1 C. neo for 24 hours and qPCR was performed for M1 activation marker iNOS (A) and M2 activation markers Arg1 (A), CD206 (B) and Gal3 (B). iNOS/Arg1 expression ratio (A) was also prepared. Note the increase in iNOS expression and iNOS/Arg1 ratio and the decreases in Arg1, CD206, and Gal3 expression in the Δssa1-infected macrophages relative to the H99-infected macrophages. N=6–9 from three separate experiments. * p < 0.05, ** p < 0.01 using Student’s t-test.
Discussion
Here, we demonstrate that the cryptococcal Hsp70 homologue Ssa1 plays a unique role as a virulence factor that interferes with the innate immune response by promoting early M2 activation of pulmonary macrophages, thereby interfering with the first line of defense against cryptococcal infection. This is the first demonstration of a cryptococcal (fungal) virulence factor that works in this fashion (i.e. that significantly modulates innate host defenses, while leaving the development of the adaptive immunity intact). Our studies also reveal that in contrast with previously known functions of Ssa1 in C. neoformans serotype D, Ssa1 does not regulate cryptococcal laccase expression in strain H99 (serotype A strain). Differences in laccase gene regulation between strain H99 and strain JEC21 could be related to differences between serotypes A and D or other differences between H99 and JEC21 unrelated to serotype. As a whole, this work significantly advances our understanding of cryptococcal factors’ interactions with the mechanisms of host defenses in the infected host.
Our studies identified Ssa1 as a cryptococcal factor that promotes early alternative activation of pulmonary macrophages and other CD11c+ mononuclear phagocytes during the innate phase of the immune response to cryptococcal infection. This conclusion is based on the following observations obtained by infecting mice with Ssa1-deficient SerA C. neoformans (relative to mice infected with WT H99): 1) a significant early reduction in pulmonary fungal burden; 2) repression of alternative activation-associated genes and surface expression of M2 markers by macrophages and the entire population of CD11c+ mononuclear cells; 3) increased abundance of iNOS protein expression and MHCIIhi cells in the lungs during the innate phase of the immune response. The subsequent in vitro data show that deletion of cryptococcal SSA1 resulted in down-regulation of M2 markers and up-regulation of M1 markers when bone marrow derived macrophages were stimulated with C. neoformans Δssa1 versus H99. These data indicate that cryptococcal Ssa1 in serotype A strain H99 directly affects macrophage polarization pathways, promoting M2 and suppressing M1 pathways in infected macrophages. To our knowledge, this is the first cryptococcal factor known to affect macrophage polarization this early during the infection and in such a direct fashion.
The unique timing of the effects that Ssa1 induces in the host sets it apart from other virulence factors studied in similar systems (15, 26, 27, 46, 50). Observed differences in macrophage polarization state in the presence and absence of Ssa1 during C. neoformans infection (expression of M2 markers CD206, Arg1, and Gal3 and M1 markers iNOS and MHC class II at both the mRNA and the protein level) were significant on days 3 and 7 post-infection. However, by day 14 these differences in macrophage polarization in H99-WT and H99- Δssa1 infected lungs resolved completely (Figures 6, 7, and 8), consistent with the similar phenotypes of the adaptive immune response polarization (Fig. 5). Consequently, the fungal growth rate in the lungs is not inhibited beyond the early time points and we observe only a temporary delay in pulmonary fungal burdens, CNS dissemination, and infected mouse mortality in the absence of cryptococcal SSA1 expression. Together, these data demonstrate that Ssa1 is a virulence factor that selectively interferes with the innate immune response without significant effects on generation of the adaptive immune response.
This mechanism of the immunomodulatory effects of Ssa1 contrasts with previously reported effects of other virulence factors that modulate the immune responses. These factors, such as urease (15, 26, 50), phospholipase (46, 51), and laccase (27) promote the development of non-protective Th2 immunity and subsequent M2 polarization and confer a fungal growth advantage late during the infection. It is also in contrast with other factors, such as PIK1, RUB1, ENA1 (28), and VAD1 (52), which confer growth advantages during both the innate and adaptive phase of the immune response but nevertheless predominantly modulate the cytokine environment during the efferent phase of the immune response in C. neoformans-infected lungs. In contrast, Ssa1 directly modulates macrophage-C. neoformans interactions only during the innate phase, with little to no effect on the development of adaptive immunity. In an attempt to specifically address the mechanism of Ssa1-dependent effects on macrophages, our laboratory has performed studies using mice deficient in the SRA receptor, which we originally thought to be the major receptor for the Ssa1 protein (53). Through our investigation, we have found that it is possible that some effects of Ssa1 on macrophages could be induced by SRA; however, the data were not very clear, hinting that multiple receptors and signaling pathways were likely to be involved.
Our novel findings regarding the virulence effects of cryptococcal Ssa1 also have other important implications for understanding cryptococcal interactions with the host’s immune system. First, significant modulation of the innate immune response and early fungal load by a virulence factor is not always sufficient to induce downstream changes in the phenotype of the adaptive immune response. Second, our findings show that the direct effect of a virulence factor on macrophage polarization can eventually be overcome by the extrinsic effects of cytokines induced by the cells of the adaptive immune system. Potential mechanisms for this counter-intuitive finding could include a) contribution of other virulence factors that do not affect innate control but are known to skew the adaptive immune response, such as laccase and urease (15, 27), which are expressed by Δssa1, or b) possible differential effects of Ssa1 on dendritic cells, responsible for priming and perpetuating the adaptive response. Together, these data further support our view that cytokine environment manipulation could be a promising therapeutic strategy that may override many aspects of cryptococcal virulence and host deficiencies leading to improper M1/M2 polarization status within the infected host.
The present study also highlights a critical difference in Ssa1’s contribution to the virulence composite between two strains that belong to serA and serD, which is linked to their differential regulation of cryptococcal laccase expression. We initiated our work based on an assumption that SSA1 deletion in H99 would deprive the fungus of virulence properties associated directly with Ssa1 as well as the virulence properties of laccase. This was based on studies in C. neoformans JEC21 (serD), which demonstrated that Ssa1 is required for laccase expression (37). Laccase is an enzyme required for melanin production in C. neoformans (54–56) and a virulence factor essential for cryptococcal CNS colonization (27, 46). Accordingly, the Δssa1 strain on a JEC21 background showed a profound suppression of melanin production and a profound defect in CNS invasion (37). The SSA1 deletion in strain H99 did not reproduce these effects, as H99-Δssa1 successfully disseminated from lungs into the CNS, induced CNS pathology, and expressed comparable melanin pigmentation to the parent H99 strain (Fig. 1, 2 and 3). Likewise, SSA1 deletion in H99 did not induce the immunological consequences of laccase deletion, which were predominantly observed during the efferent phase of the immune response (27). These differences in the regulation of laccase expression between H99 (serA strain) and JEC21 (serD strain) allowed us to define a laccase-independent role of Ssa1 as a standalone virulence factor and also elucidated a novel and crucial difference in virulence gene regulation between C. neoformans strains. In addition to previously-observed differences in regulation of cryptococcal cytochrome c oxidase subunit 1 (COX1) (44), our data underscores major differences in gene regulation among C. neoformans strains. While it is yet unknown how this impacts the spectra of host susceptibilities and pathogenicity of serotypes A and D, our studies provide evidence of differential regulation of crucial virulence-associated genes between representative strains of these serotypes. If the observed differences in virulence gene regulation between strains H99 (serA) and JEC21 (serD) proved to be consistent between all or most serotype A and D strains, our findings would help to explain differential epidemiology between serotypes, similarly to what has been found for two cryptococcal species- C. neoformans and C. gatti- in recent studies (57, 58).
Finally, while our studies focused on C. neoformans, we believe they have broader implications for host interactions with pathogen-derived Hsp70. Other pathogens have been shown to employ Hsp70 as virulence factor/modulator of innate responses including Mycobacterium tuberculosis (29, 59), Toxoplasma gondi (60, 61) and Candida sp. (30). While the mechanism of the Ssa1-induced effects on the host and on pathogen’s virulence appear to be different in each group of pathogens, these previously published studies combined with our present work support broader importance of Hsp70 family proteins as “stand alone” virulence factors and immune response modulators.
In summary, our findings provide novel insights regarding the immunomodulatory role of cryptococcal Ssa1 and we identify important gene regulation differences between two major strains of serotypes A and D. We have shown that Ssa1 promotes early M2 macrophage polarization and that this form of immune modulation improves fungal growth during the innate phase of the immune response. Yet our data also emphasizes that the immunophenotype of the adaptive immune response ultimately defines the outcome of infection. Furthermore, our studies reveal that immune modulation and the propensity for CNS dissemination mediated by Ssa1 may vary between serotypes and depends on whether or not laccase expression is induced.
Acknowledgments
We would like to acknowledge the significant contributions of Fuyuan Wang, Yanmei Zhang, Amos Adler and Jeremy Dayrit in the preliminary phase of this project. We would like to thank Seagal Tannenbaum, Christian Love, Eric Woosung Cho, Stuart Zeltzer, Jacob Carolan, Nicole Potchen, and Enze Xing for their technical assistance.
Footnotes
A.J.E. was supported by T32 Immunology training grant T32AI007413 and University of Michigan Rackham Fellowship. The laboratories of M.A.O. and J.J.O. were supported by Merit Review Awards from the Biomedical Laboratory Research &Development Service, Department of Veterans Affairs grants 1I01BX000656 (M.A.O.) and BX002120-01 (J.J.O.), and the Training Program in Pulmonary Diseases Grant T32-HL07749-19 (M.J.D.). This research was supported in part by the intramural research program of the NIH, NIAID (P.R.W.). F.L.W. Jr. and S.E.H. were supported by grant 2RO1 AI071752 from the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) and a grant from the Army Research Office of the Department of Defense (W911NF-11-1-0136). Work of undergraduate students was supported by UM Undergraduate Research Opportunity Program.
References
- 1.Chuck SL, Sande MA. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. The New England journal of medicine. 1989;321:794–799. doi: 10.1056/NEJM198909213211205. [DOI] [PubMed] [Google Scholar]
- 2.Pappas PG, Perfect JR, Cloud GA, Larsen RA, Pankey GA, Lancaster DJ, Henderson H, Kauffman CA, Haas DW, Saccente M, Hamill RJ, Holloway MS, Warren RM, Dismukes WE. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2001;33:690–699. doi: 10.1086/322597. [DOI] [PubMed] [Google Scholar]
- 3.Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. Aids. 2007;21:2119–2129. doi: 10.1097/QAD.0b013e3282a4a64d. [DOI] [PubMed] [Google Scholar]
- 4.Huffnagle GB, Lipscomb MF. Cells and cytokines in pulmonary cryptococcosis. Research in immunology. 1998;149:387–396. doi: 10.1016/s0923-2494(98)80762-1. discussion 512–384. [DOI] [PubMed] [Google Scholar]
- 5.Huffnagle GB, Toews GB, Burdick MD, Boyd MB, McAllister KS, McDonald RA, Kunkel SL, Strieter RM. Afferent phase production of TNF-alpha is required for the development of protective T cell immunity to Cryptococcus neoformans. J Immunol. 1996;157:4529–4536. [PubMed] [Google Scholar]
- 6.Mody CH, Tyler CL, Sitrin RG, Jackson C, Toews GB. Interferon-gamma activates rat alveolar macrophages for anticryptococcal activity. Am J Respir Cell Mol Biol. 1991;5:19–26. doi: 10.1165/ajrcmb/5.1.19. [DOI] [PubMed] [Google Scholar]
- 7.Muller U, Stenzel W, Piehler D, Grahnert A, Protschka M, Kohler G, Frey O, Held J, Richter T, Eschke M, Kamradt T, Brombacher F, Alber G. Abrogation of IL-4 receptor-alpha-dependent alternatively activated macrophages is sufficient to confer resistance against pulmonary cryptococcosis despite an ongoing T(h)2 response. International immunology. 2013;25:459–470. doi: 10.1093/intimm/dxt003. [DOI] [PubMed] [Google Scholar]
- 8.Hardison SE, Ravi S, Wozniak KL, Young ML, Olszewski MA, Wormley FL., Jr Pulmonary infection with an interferon-gamma-producing Cryptococcus neoformans strain results in classical macrophage activation and protection. Am J Pathol. 2010;176:774–785. doi: 10.2353/ajpath.2010.090634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller U, Stenzel W, Kohler G, Werner C, Polte T, Hansen G, Schutze N, Straubinger RK, Blessing M, McKenzie AN, Brombacher F, Alber G. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J Immunol. 2007;179:5367–5377. doi: 10.4049/jimmunol.179.8.5367. [DOI] [PubMed] [Google Scholar]
- 10.Arora S, Hernandez Y, Erb-Downward JR, McDonald RA, Toews GB, Huffnagle GB. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J Immunol. 2005;174:6346–6356. doi: 10.4049/jimmunol.174.10.6346. [DOI] [PubMed] [Google Scholar]
- 11.Arora S, Olszewski MA, Tsang TM, McDonald RA, Toews GB, Huffnagle GB. Effect of cytokine interplay on macrophage polarization during chronic pulmonary infection with Cryptococcus neoformans. Infection and immunity. 2011;79:1915–1926. doi: 10.1128/IAI.01270-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB, Olszewski MA. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. mBio. 2013;4:e00264–00213. doi: 10.1128/mBio.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stenzel W, Muller U, Kohler G, Heppner FL, Blessing M, McKenzie AN, Brombacher F, Alber G. IL-4/IL-13-dependent alternative activation of macrophages but not microglial cells is associated with uncontrolled cerebral cryptococcosis. The American journal of pathology. 2009;174:486–496. doi: 10.2353/ajpath.2009.080598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen GH, Olszewski MA, McDonald RA, Wells JC, Paine R, 3rd, Huffnagle GB, Toews GB. Role of granulocyte macrophage colony-stimulating factor in host defense against pulmonary Cryptococcus neoformans infection during murine allergic bronchopulmonary mycosis. The American journal of pathology. 2007;170:1028–1040. doi: 10.2353/ajpath.2007.060595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osterholzer JJ, Surana R, Milam JE, Montano GT, Chen GH, Sonstein J, Curtis JL, Huffnagle GB, Toews GB, Olszewski MA. Cryptococcal urease promotes the accumulation of immature dendritic cells and a non-protective T2 immune response within the lung. The American journal of pathology. 2009;174:932–943. doi: 10.2353/ajpath.2009.080673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Classen A, Lloberas J, Celada A. Macrophage activation: classical versus alternative. Methods in molecular biology. 2009;531:29–43. doi: 10.1007/978-1-59745-396-7_3. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez Y, Arora S, Erb-Downward JR, McDonald RA, Toews GB, Huffnagle GB. Distinct roles for IL-4 and IL-10 in regulating T2 immunity during allergic bronchopulmonary mycosis. J Immunol. 2005;174:1027–1036. doi: 10.4049/jimmunol.174.2.1027. [DOI] [PubMed] [Google Scholar]
- 19.Chen GH, McNamara DA, Hernandez Y, Huffnagle GB, Toews GB, Olszewski MA. Inheritance of immune polarization patterns is linked to resistance versus susceptibility to Cryptococcus neoformans in a mouse model. Infection and immunity. 2008;76:2379–2391. doi: 10.1128/IAI.01143-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Wang F, Tompkins KC, McNamara A, Jain AV, Moore BB, Toews GB, Huffnagle GB, Olszewski MA. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. The American journal of pathology. 2009;175:2489–2500. doi: 10.2353/ajpath.2009.090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gratchev A, Kzhyshkowska J, Utikal J, Goerdt S. Interleukin-4 and dexamethasone counterregulate extracellular matrix remodelling and phagocytosis in type-2 macrophages. Scandinavian journal of immunology. 2005;61:10–17. doi: 10.1111/j.0300-9475.2005.01524.x. [DOI] [PubMed] [Google Scholar]
- 22.Huffnagle GB, Lipscomb MF, Lovchik JA, Hoag KA, Street NE. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. Journal of leukocyte biology. 1994;55:35–42. doi: 10.1002/jlb.55.1.35. [DOI] [PubMed] [Google Scholar]
- 23.Jain AV, Zhang Y, Fields WB, McNamara DA, Choe MY, Chen GH, Erb-Downward J, Osterholzer JJ, Toews GB, Huffnagle GB, Olszewski MA. Th2 but not Th1 immune bias results in altered lung functions in a murine model of pulmonary Cryptococcus neoformans infection. Infection and immunity. 2009;77:5389–5399. doi: 10.1128/IAI.00809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traynor TR, Kuziel WA, Toews GB, Huffnagle GB. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J Immunol. 2000;164:2021–2027. doi: 10.4049/jimmunol.164.4.2021. [DOI] [PubMed] [Google Scholar]
- 25.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nature reviews. Immunology. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 26.Cox GM, Mukherjee J, Cole GT, Casadevall A, Perfect JR. Urease as a virulence factor in experimental cryptococcosis. Infection and immunity. 2000;68:443–448. doi: 10.1128/iai.68.2.443-448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu Y, Davis MJ, Dayrit JK, Hadd Z, Meister DL, Osterholzer JJ, Williamson PR, Olszewski MA. Immune modulation mediated by cryptococcal laccase promotes pulmonary growth and brain dissemination of virulent Cryptococcus neoformans in mice. PloS one. 2012;7:e47853. doi: 10.1371/journal.pone.0047853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He X, Lyons DM, Toffaletti DL, Wang F, Qiu Y, Davis MJ, Meister DL, Dayrit JK, Lee A, Osterholzer JJ, Perfect JR, Olszewski MA. Virulence factors identified by Cryptococcus neoformans mutant screen differentially modulate lung immune responses and brain dissemination. The American journal of pathology. 2012;181:1356–1366. doi: 10.1016/j.ajpath.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motta A, Schmitz C, Rodrigues L, Ribeiro F, Teixeira C, Detanico T, Bonan C, Zwickey H, Bonorino C. Mycobacterium tuberculosis heat-shock protein 70 impairs maturation of dendritic cells from bone marrow precursors, induces interleukin-10 production and inhibits T-cell proliferation in vitro. Immunology. 2007;121:462–472. doi: 10.1111/j.1365-2567.2007.02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez-Ribot JL, Alloush HM, Masten BJ, Chaffin WL. Evidence for presence in the cell wall of Candida albicans of a protein related to the hsp70 family. Infection and immunity. 1996;64:3333–3340. doi: 10.1128/iai.64.8.3333-3340.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun JN, Solis NV, Phan QT, Bajwa JS, Kashleva H, Thompson A, Liu Y, Dongari-Bagtzoglou A, Edgerton M, Filler SG. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS pathogens. 2010;6:e1001181. doi: 10.1371/journal.ppat.1001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodrigues ML, Nimrichter L, Oliveira DL, Nosanchuk JD, Casadevall A. Vesicular Trans-Cell Wall Transport in Fungi: A Mechanism for the Delivery of Virulence-Associated Macromolecules? Lipid insights. 2008;2:27–40. doi: 10.4137/lpi.s1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kakeya H, Udono H, Ikuno N, Yamamoto Y, Mitsutake K, Miyazaki T, Tomono K, Koga H, Tashiro T, Nakayama E, Kohno S. A 77-kilodalton protein of Cryptococcus neoformans, a member of the heat shock protein 70 family, is a major antigen detected in the sera of mice with pulmonary cryptococcosis. Infection and immunity. 1997;65:1653–1658. doi: 10.1128/iai.65.5.1653-1658.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kakeya H, Udono H, Maesaki S, Sasaki E, Kawamura S, Hossain MA, Yamamoto Y, Sawai T, Fukuda M, Mitsutake K, Miyazaki Y, Tomono K, Tashiro T, Nakayama E, Kohno S. Heat shock protein 70 (hsp70) as a major target of the antibody response in patients with pulmonary cryptococcosis. Clinical and experimental immunology. 1999;115:485–490. doi: 10.1046/j.1365-2249.1999.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaturvedi AK, Weintraub ST, Lopez-Ribot JL, Wormley FL., Jr Identification and characterization of Cryptococcus neoformans protein fractions that induce protective immune responses. Proteomics. 2013;13:3429–3441. doi: 10.1002/pmic.201300213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young M, Macias S, Thomas D, Wormley FL., Jr A proteomic-based approach for the identification of immunodominant Cryptococcus neoformans proteins. Proteomics. 2009;9:2578–2588. doi: 10.1002/pmic.200800713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang S, Hacham M, Panepinto J, Hu G, Shin S, Zhu X, Williamson PR. The Hsp70 member, Ssa1, acts as a DNA-binding transcriptional co-activator of laccase in Cryptococcus neoformans. Molecular microbiology. 2006;62:1090–1101. doi: 10.1111/j.1365-2958.2006.05422.x. [DOI] [PubMed] [Google Scholar]
- 38.Salas SD, Bennett JE, Kwon-Chung KJ, Perfect JR, Williamson PR. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. The Journal of experimental medicine. 1996;184:377–386. doi: 10.1084/jem.184.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erickson T, Liu L, Gueyikian A, Zhu X, Gibbons J, Williamson PR. Multiple virulence factors of Cryptococcus neoformans are dependent on VPH1. Molecular microbiology. 2001;42:1121–1131. doi: 10.1046/j.1365-2958.2001.02712.x. [DOI] [PubMed] [Google Scholar]
- 40.Cox GM, Toffaletti DL, Perfect JR. Dominant selection system for use in Cryptococcus neoformans. Journal of medical and veterinary mycology: bi-monthly publication of the International Society for Human and Animal Mycology. 1996;34:385–391. [PubMed] [Google Scholar]
- 41.Olszewski MA, Huffnagle GB, McDonald RA, Lindell DM, Moore BB, Cook DN, Toews GB. The role of macrophage inflammatory protein-1 alpha/CCL3 in regulation of T cell-mediated immunity to Cryptococcus neoformans infection. J Immunol. 2000;165:6429–6436. doi: 10.4049/jimmunol.165.11.6429. [DOI] [PubMed] [Google Scholar]
- 42.Olszewski MA, Huffnagle GB, Traynor TR, McDonald RA, Cook DN, Toews GB. Regulatory effects of macrophage inflammatory protein 1alpha/CCL3 on the development of immunity to Cryptococcus neoformans depend on expression of early inflammatory cytokines. Infection and immunity. 2001;69:6256–6263. doi: 10.1128/IAI.69.10.6256-6263.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardison SE, Herrera G, Young ML, Hole CR, Wozniak KL, Wormley FL., Jr Protective immunity against pulmonary cryptococcosis is associated with STAT1-mediated classical macrophage activation. J Immunol. 2012;189:4060–4068. doi: 10.4049/jimmunol.1103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toffaletti DL, Del Poeta M, Rude TH, Dietrich F, Perfect JR. Regulation of cytochrome c oxidase subunit 1 (COX1) expression in Cryptococcus neoformans by temperature and host environment. Microbiology. 2003;149:1041–1049. doi: 10.1099/mic.0.26021-0. [DOI] [PubMed] [Google Scholar]
- 45.Ngamskulrungroj P, Chang Y, Roh J, Kwon-Chung KJ. Differences in nitrogen metabolism between Cryptococcus neoformans and C. gattii, the two etiologic agents of cryptococcosis. PloS one. 2012;7:e34258. doi: 10.1371/journal.pone.0034258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noverr MC, Williamson PR, Fajardo RS, Huffnagle GB. CNLAC1 is required for extrapulmonary dissemination of Cryptococcus neoformans but not pulmonary persistence. Infection and immunity. 2004;72:1693–1699. doi: 10.1128/IAI.72.3.1693-1699.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osterholzer JJ, Chen GH, Olszewski MA, Curtis JL, Huffnagle GB, Toews GB. Accumulation of CD11b+ lung dendritic cells in response to fungal infection results from the CCR2-mediated recruitment and differentiation of Ly-6Chigh monocytes. J Immunol. 2009;183:8044–8053. doi: 10.4049/jimmunol.0902823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osterholzer JJ, Milam JE, Chen GH, Toews GB, Huffnagle GB, Olszewski MA. Role of dendritic cells and alveolar macrophages in regulating early host defense against pulmonary infection with Cryptococcus neoformans. Infection and immunity. 2009;77:3749–3758. doi: 10.1128/IAI.00454-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osterholzer JJ, Chen GH, Olszewski MA, Zhang YM, Curtis JL, Huffnagle GB, Toews GB. Chemokine receptor 2-mediated accumulation of fungicidal exudate macrophages in mice that clear cryptococcal lung infection. The American journal of pathology. 2011;178:198–211. doi: 10.1016/j.ajpath.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olszewski MA, Noverr MC, Chen GH, Toews GB, Cox GM, Perfect JR, Huffnagle GB. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. The American journal of pathology. 2004;164:1761–1771. doi: 10.1016/S0002-9440(10)63734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cox GM, McDade HC, Chen SC, Tucker SC, Gottfredsson M, Wright LC, Sorrell TC, Leidich SD, Casadevall A, Ghannoum MA, Perfect JR. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Molecular microbiology. 2001;39:166–175. doi: 10.1046/j.1365-2958.2001.02236.x. [DOI] [PubMed] [Google Scholar]
- 52.Qiu J, Olszewski MA, Williamson PR. Cryptococcus neoformans growth and protection from innate immunity are dependent on expression of a virulence-associated DEAD-box protein, Vad1. Infection and immunity. 2013;81:777–788. doi: 10.1128/IAI.00821-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qiu Y, Dayrit JK, Davis MJ, Carolan JF, Osterholzer JJ, Curtis JL, Olszewski MA. Scavenger receptor A modulates the immune response to pulmonary Cryptococcus neoformans infection. J Immunol. 2013;191:238–248. doi: 10.4049/jimmunol.1203435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williamson PR. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. Journal of bacteriology. 1994;176:656–664. doi: 10.1128/jb.176.3.656-664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu X, Gibbons J, Garcia-Rivera J, Casadevall A, Williamson PR. Laccase of Cryptococcus neoformans is a cell wall-associated virulence factor. Infection and immunity. 2001;69:5589–5596. doi: 10.1128/IAI.69.9.5589-5596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eisenman HC, Mues M, Weber SE, Frases S, Chaskes S, Gerfen G, Casadevall A. Cryptococcus neoformans laccase catalyses melanin synthesis from both D- and L-DOPA. Microbiology. 2007;153:3954–3962. doi: 10.1099/mic.0.2007/011049-0. [DOI] [PubMed] [Google Scholar]
- 57.Schoffelen T, Illnait-Zaragozi MT, Joosten LA, Netea MG, Boekhout T, Meis JF, Sprong T. Cryptococcus gattii induces a cytokine pattern that is distinct from other cryptococcal species. PloS one. 2013;8:e55579. doi: 10.1371/journal.pone.0055579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saijo T, Chen J, Chen SC, Rosen LB, Yi J, Sorrell TC, Bennett JE, Holland SM, Browne SK, Kwon-Chung KJ. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. mBio. 2014;5:e00912–00914. doi: 10.1128/mBio.00912-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Retzlaff C, Yamamoto Y, Hoffman PS, Friedman H, Klein TW. Bacterial heat shock proteins directly induce cytokine mRNA and interleukin-1 secretion in macrophage cultures. Infection and immunity. 1994;62:5689–5693. doi: 10.1128/iai.62.12.5689-5693.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiss LM, Ma YF, Takvorian PM, Tanowitz HB, Wittner M. Bradyzoite development in Toxoplasma gondii and the hsp70 stress response. Infection and immunity. 1998;66:3295–3302. doi: 10.1128/iai.66.7.3295-3302.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mun HS, Aosai F, Norose K, Chen M, Hata H, Tagawa YI, Iwakura Y, Byun DS, Yano A. Toxoplasma gondii Hsp70 as a danger signal in toxoplasma gondii-infected mice. Cell stress & chaperones. 2000;5:328–335. doi: 10.1379/1466-1268(2000)005<0328:tghaad>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]