Abstract
Mycoplasma pneumoniae (Mp) is an extracellular pathogen that colonizes mucosal surfaces of the respiratory tract and is associated with asthma exacerbations. Previous reports demonstrate that surfactant protein-A (SP-A) binds live Mp and mycoplasma membranes (MMF) with high affinity. Humans express a repertoire of single amino acid genetic variants of SP-A that may be associated with lung disease, and our findings demonstrate that allelic differences in SP-A2 (Gln223Lys) affect the binding to MMF. We show that SP-A−/− mice are more susceptible to MMF exposure and have significant increases in mucin production and neutrophil recruitment. Novel humanized-SP-A2 transgenic mice harboring the hSP-A2 223K allele exhibit reduced neutrophil influx and mucin production in the lungs, when challenged with MMF, compared to SP-A−/− mice. Conversely, mice expressing hSP-A2 223Q have increased neutrophil influx and mucin production that is similar to SP-A−/− mice. Using tracheal epithelial cell cultures, we show that enhanced mucin production to MMF occurs in the absence of SP-A, and is not dependent upon neutrophil recruitment. Increased phosphorylation of the epidermal growth factor receptor (EGFR) was evident in the lungs of MMF-challenged mice when SP-A was absent. Pharmacologic inhibition of EGFR prior to MMF challenge dramatically reduced mucin production in SP-A−/− mice. These findings suggest a protective role for SP-A in limiting MMF-stimulated mucin production that occurs through interference with EGFR mediated signaling. The SP-A interaction with the EGFR signaling pathway appears to occur in an allele specific manner that may have important implications for SP-A polymorphisms in human diseases.
Introduction
Surfactant protein-A (SP-A) is a highly oligomeric protein component of pulmonary surfactant that belongs to the collagen domain containing-C type lectin (collectin) superfamily. Members of the collectin family typically contain an N-terminal collagen like domain and a C-terminal carbohydrate recognition domain (CRD). The CRD binds a variety of ligands, including pathogen-derived carbohydrate moieties in a Ca2+-dependent reaction. For numerous pathogens, SP-A binding to cell surface glycans results in enhanced phagocytosis. (1-3).
Human SP-A is encoded by two genes designated, SP-A1 and SP-A2 (4-6). Several recent studies have identified associations of specific SP-A alleles with infant wheezing (7), tuberculosis (8), respiratory distress syndrome (9, 10), RSV infections (11), COPD (12) and susceptibility to ozone (13). Additionally, we have shown that SP-A extracted from asthma subjects has decreased binding affinity for Mycoplasma pneumoniae (Mp) as compared to SP-A extracted from normal controls and that asthmatic SP-A is defective at abrogating Mp-induced Muc5AC expression (14).
Mycoplasma pneumoniae is an extracellular human pathogen that is frequently the causative agent for “walking pneumonia.” Mp is classified as a Mollicute, and can cause a variety of airway diseases including bronchiolitis, bronchitis and bronchiectasis and has recently been associated with asthma exacerbations (15-18). Mycoplasmas are characterized by their unusually small size (0.15-0.3 m in diameter) and absence of a cell wall, which renders them resistant to many antibiotics. Mycoplasmas contain a tri-layered cell membrane, composed of lipoprotein, glycolipid and lipoglycan components that are antigenic and capable of inducing a host pathogenic response (19, 20).
Although mycoplasmas lack the lipopolysaccharides found in gram-negative bacteria, they express several cell surface ligands capable of interacting with SP-A. One class of high affinity ligands for SP-A is comprised of disaturated phosphatidylglycerols (21). Kannan et al have also identified a specific membrane protein, MPN372, which also binds SP-A with high affinity (22). SP-A binding to Mp inhibits the growth of the organism (21). Thus, evaluation of mycoplasma membrane offers an opportunity to evaluate specific interaction with SP-A.
In previous studies using WT and SP-A−/− mice, we have shown that SP-A is protective against live Mp infection (23). Since SP-A binds Mp and acts as an opsonin, we sought to determine if the phenotypes observed in Mp-infected SP-A−/− mice were due to the increased pathogen burden, or innate recognition of pathogen-derived material. To address this issue, we utilized a preparation of MMF instilled into WT and SP-A−/− mice to determine the role of SP-A in regulating the inflammatory response to membrane components, in the absence of Mp colonization.
Our findings show that SP-A−/− mice have enhanced mucin production and neutrophil recruitment at 12 hrs after challenge with MMF, whereas WT mice have negligible mucin production. We also discovered that genetic variation in SP-A2 at position 223, which is associated with multiple lung diseases (7, 24), leads to dramatic differences in binding to MMF. We generated humanized knock-in transgenic mice that lack mouse SP-A, but express hSP-A2 with either Gln or Lys at position 223. Mice that express the Gln223 variant, which binds MMF poorly, have an enhanced response to MMF, as compared to those expressing the Lys223 variant. Using mouse tracheal epithelial cell cultures grown at air-liquid interfaces (ALI), we were able to determine the effect of SP-A in regulation of MMF-induced Muc5AC was independent of neutrophil recruitment. In further studies, we show that MMF challenge in SP-A−/− mice results in enhanced phosphorylation of the EGFR receptor on airway epithelial cells and that pharmacologic inhibition of EGFR results in attenuated mucin production following MMF stimulation. Taken together these findings provide novel insight into how genetic alterations in human SP-A2 at position 223 can alter binding affinity for a stimulus and thereby mediate the host response by reducing EGF receptor signaling and mucin production.
Materials and Methods
Generation of SP-A humanized transgenic mice
Site-specific knock-in transgenic mice harboring alleles for human SP-A, were generated with assistance of the Duke BAC core and Transgenics Shared Resource at Duke University, which is currently funded by CA014236 to the Duke Cancer Institute. Two targeting vectors were created that contained the sequence for human SP-A2 only differing at codons for amino acid 223, to give rise to either glutamine or lysine variants. The targeting vectors were linearized and underwent homologous recombination at the mouse endogenous SP-A locus. Positively targeted ES cells were verified by PCR using primers against human SP-A (Forward: CTA TCG CCT TCT TGA CGA GTT CTT C; Reverse: GCT TGA AAG GTA GTT ACA GGG TCT TAC), which amplifies the 2.5 Kb short arm of the targeting vector and (Forward: GAG CCA AGT TCC TCA CAG CAG AAG; Reverse: AGA CTG CCT TGG GAA AAG CGC CTC CCC), which amplifies the 9.5 Kb long arm of the targeting vector. Verified ES cells were micro-injected into pseudo-pregnant females and the offspring were genotyped for the presence of the Neo cassette. Positive offspring were then crossed to SP-A−/− mice to generate colonies of hemizygous SP-A2 223Q/- and SP-A2 223K/-, which were genotyped for human SP-A2 (Forward: TGC CTG CGA AGT GAA GGA C; Reverse: GTT CCT GTC ATT CCA CTG C). The amplified region yields a 658 bp product in gene-targeted mice.
Experimental mice
Mice were maintained in pathogen-free housing at Duke University. SP-A−/− mice were obtained (25) and crossed onto the C57BL/6 background after which they were bred in-house. Wild-type (WT) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Sex-matched female mice were used for experiments and were approximately 6-8 weeks of age. Mice were anesthetized by isoflourane inhalation, and given 5 μg of MMF in 50 μl of sterile saline via oropharyngeal delivery. At the desired time point, mice were euthanized and lavaged with PBS (0.1 mM EDTA) and lung tissue was obtained for further analysis. For analysis of mucin production, mice were harvested either at 16 or 24 hrs post MMF exposure as noted in the text for each experiment; for p-EGFR staining, mice were harvested 12 hrs after MMF exposure. Some mice were given an EGFR inhibitor, AG1478 (Selleckchem), via ip injection 30 minutes prior to receiving oropharyngeal delivery of MMF. AG1478 was solubilized in DMSO and diluted to a concentration of 10 mg/kg mouse weight in sterile saline. The vehicle control was DMSO diluted in sterile saline.
Production of recombinant SP-A2
The human cDNAs encoding for SP-A2: [91A, 223Q] and [91A, 223K] were constructed in a PEE14 vector under control of a hCMV-MIE promoter (21,26). The plasmids were purified in large scale and used to transfect Freestyle HEK-293 cells using 293-fectin, which ensures mammalian oligosaccharide patterns and sufficient hydroxylation of prolines within the collagen-like domain. After transfection, the cells were grown in suspension culture in serum free-Freestyle 293 Expression Medium. At 48hrs after transfection, the culture supernatants were harvested by centrifugation and filter sterilized. The supernatants were brought to 85% saturation with ammonium sulfate and the crude protein pellet was harvested by centrifugation, and subsequently dialyzed against 5 mM Tris-Cl pH 7.4. The dialyzed protein preparation was adjusted to 10 mM CaCl2 and applied to a mannose sepharose affinity column. The affinity column was washed with 10 volumes of binding buffer and the protein was eluted with 7 mM EDTA, 5mM Tris-Cl pH 7.4. The protein eluate was concentrated and EDTA was removed using a Centricon JumboSep apparatus with a 10kDa cutoff filter. Endotoxin was removed from the protein using a Mustang E adsorption filter. The endotoxin content of the final preparation was determined using the Limulus Amebocyte Lysis. Aliquots of the protein were stored at −20C prior to use. This affinity purification procedure, which requires the C-type lectin activity of the protein be functional (27) ensures the isolated SP-A proteins are properly folded and active.
MMF preparation and binding assay
Membrane fractions from M. pneumoniae were prepared as previously described (26). Briefly, Mp (strain FH, American Type Culture Collection 15531) was grown for 5 days at 37°C in polystyrene flasks containing 100 ml of SP-4 medium. After 5 days, adherent and non-adherent Mp cells were collected by scraping and centrifugation at 8,000 g for 15 min at 4°C. The pellet was washed twice with PBS by centrifugation and resuspended in PBS. The mixture was then layered on a discontinuous sucrose gradient (60%, 52%, 48%, and 40%) and centrifuged at 10,000 rpm at 4°C for 30 min. Cells were recovered from the 48–52% interface, mixed with PBS, and centrifuged at 8,000 g at 4°C for 15 min. The purified Mp pellet was resuspended in PBS and mixed with 3 volumes of distilled water and incubated on ice for 30 min. The cells were probe sonication on ice for a total of 2 min, using 30-s sonication and 30-s cooling cycles. Polymyxin B (100 μg/ml) was added to the lysed Mp cells and centrifuged at 100,000 g at 4°C for 1 hr. The pellet was washed twice with polymyxin B and centrifuged at 100,000 g at 4°C for 1 hr. The final Mp membrane pellet was resuspended in PBS, homogenized, and used as MMF.
The binding of purified SP-A to MMF was measured as previously described (21). MMFs (100 ng protein/well) were adsorbed to microtiter wells in 0.1 mM NaHCO3, pH 9.6, at 4° C overnight. Following adsorption, the wells were blocked with buffer A (20 mM Tris pH 7.4, 150 mM NaCl and 5mM CaCl2) containing 2% BSA for 1 hour at 37°C. SP-A at various concentrations was added to each well in buffer A with 2% BSA. The binding reaction was performed for 2 hr at 37°C. The bound SP-A was detected by ELISA using 5 μg/ml HRP conjugated primary antibody. Orthophenylenediamine (1 mg/ml) was used as the color development reagent for detecting the bound horseradish peroxidase conjugated antibody.
Mouse tracheal cell isolation
Mice were euthanized and tracheas were removed by dissection and immediately placed in Ham’s F-12 medium on ice. Excess connective tissue, muscle, vasculature and nerves were removed from the trachea and a longitudinal incision was made exposing the mucosal lining. The tracheas were then placed in 10 ml of Ham’s F-12 medium containing 0.1% protease solution (Sigma) and incubated for 45 minutes at 37° C. The protease activity was stopped using 3 ml of FBS. The tracheas were then transferred to a petri dish containing Ham’s F-12 medium and the mucosal linings were scrapped with a gel-loading pipette tip. The cells were then collected and transferred to a 15 ml conical tube and centrifuged (900rpm, 5 minutes, 4°C). The medium supernatant was removed and the cell pellet was resuspended in 5ml Versene (Gibco/Life Technologies) for 15 minutes at 37° C. 10% FBS Ham’s F-12 medium was added to the tube and centrifuged (900pm, 5 minutes, 4° C). The supernatant was removed and the cell pellet was resuspended in 6 ml of 10% FBS Ham’s F-12 medium.
Culture media and supplements
DMEM-Ham’s F-12 medium was used for both the harvest and culture of mouse tracheal epithelial cells (MTECs). The culture medium was supplemented with 250 ng/ml amphotericin B solution (Hyclone), 20 ng/ml cholera toxin (LIST biological), 104 μg/ml bovine pituitary extract (Lonza), 5 μg/ml insulin (Sigma), 5 μg/ml human apo-transferrin (Sigma), 0.1uM dexamethasone (Sigma), 5ng/ml mouse epidermal growth factor (Sigma), 0.01 μM retinol (Sigma), 20 U/ml nystatin (Sigma), 50 μg/ml gentamicin. Fetal bovine serum (Atlanta Biologicals) was used to prepare 10% and 5% serum rich media used at different steps in the air-liquid interface culture protocol.
In vitro culture of mouse tracheal epithelial cells (MTECs)
Costar® Transwell® (12 mm, 0.4 μm pores) 12-well plates were used to culture the MTECs according to methods previously described (28). The polyester membrane was coated with 300 μg/ml rat tail collagen in 0.02 N glacial acetic acid at room temperature for 1 hour. The membranes were then washed with PBS and conditioned with Ham’s F-12 medium for 1 hour. The media was removed from the apical and basolateral sides and 1 ml of 10% FBS culture media was added to the basolateral part of each well. All of the cells were plated evenly between the 12 wells in 500 μl of 10% FBS culture media. The plate was incubated at 37° in an air-5% CO2 atmosphere for 72 hours, without changing the medium. After the initial seeding period of 72 hours, the medium on both the apical and basal side of the membrane was replaced every other day. When the cells reached 80% confluence (6-8 days) the culture medium was changed to 5% FBS and replaced daily, only on the basolateral side establishing an air-liquid interface (ALI). When the cells reached full confluence, the medium was changed to serum-free Ham’s F-12 culture medium and replaced daily. The cells were maintained at ALI for 14 days.
RT-PCR
Mouse tissues and mouse tracheal epithelial cells were collected into 1 ml of TRI Reagent® (Sigma). Ribonucleic acid (RNA) was isolated using the standard TRI reagent/chloroform extraction method. The RNA was quantified using a Nanodrop® Spectrophotometer ND-1000. Complementary deoxyribonucleic acid (cDNA) was synthesized from 1 μg of total RNA using Bio-Rad™ cDNA Synthesis kit. Real-time polymerase chain reactions (RT-PCR) were performed using Bioline 2x SensiFAST SYBR no-ROX mix. The samples were analyzed for expression levels of mouse MUC5AC using forward and reverse primers specific to the gene (forward: 5′ GAG GGC CCA GTG AGC ATC TCC 3′, reverse: 5′ TGG GAC AGC AGC AGT ATT CAG T 3′). The relative levels of expression obtained were normalized to the mammalian housekeeper gene Cyclophilin using primers specific to the gene (forward: 5′ AGC ACT GGA GAG AAA GGA TTT GG 3′, reverse: 5′ TCT TCT TGC TGG TCT TGC CAT T 3′).
Histological analysis
Mice were euthanized by CO2 asphyxiation. Left lung lobes were excised, removed and fixed in 10% buffered formalin phosphate (Fisher Scientific). After 3-5 days the lung lobes were transferred from formalin to 70% ethanol. The lobes were paraffin embedded, cut to 4 μm, mounted on a slide, and stained with periodic acid-Schiff (PAS) to detect mucin production in the lungs. Slides were blind-coded and scored on a scale of 0 (no visible PAS stain) – 5 (PAS stain is present in all visible airways and mucus plugs are present) as previously described (29).
Immunohistochemistry
Paraffin embedded sections were stained according to standard IHC protocols using either a human SP-A antibody (Abcam, ab51891) or a murine anti-phospho-EGFR antibody (Cell Signaling, Tyr1173; 53A5; #4407). For IHC staining, slides were deparaffinized in Xylene and ethanol according to standard procedures. Antigen retrieval was carried out in 0.05% Trypsin (5 min), after which slides were incubated in 3% H202 for 10 min to block peroxidase activity. For p-EGFR staining, sections were blocked for 30 min with 5% donkey serum in TBS-T and incubated with primary antibody at 1:50 dilution (P-EGFR, Cell Signaling Tyr1173) overnight at 4° C, followed by secondary antibody (SignalStain Boost HRP Rabbit, Cell Signaling) for 30 min at room temperature. The isotype control (Rabbit monoclonal IgG DA1E, Cell Signaling) was used at the same concentration, and staining was carried out using the same conditions as for the specific antibody staining. Sections were visualized by VectorRed and hematoxylin staining according to manufacturers’ instructions. For hSP-A staining, sections were blocked using the mouse-on-mouse (MOM kit, Vector laboratories) immunodetection kit according to manufacturer’s instructions and the primary antibody hSP-A (abcam, cat number ab51891) was added at 1:3000 dilution for 30 min. Antibody reactivity was visualized using DAB substrate (Vector laboratories) and counterstained with hematoxylin.
P-EGFR positive staining was assessed by using a color thresholding method in ImageJ (version 1.47t, NIH). Ten to fifteen non-overlapping photomicrographs of the large airways were taken at 20x magnification to cover most of the tissue on the slide. For stain specificity of P-EGFR in the epithelial compartment, the lung tissue around the airways was erased in Photoshop (Adobe). For staining assessment, two macro scripts were designed to analyze the ratio of the antibody positive tissue and the total tissue. To avoid a biased quantification each macro was used for all the photomicrographs. The same macros also generated two downstream images representing the extracted areas to validate the specificity of the P-EGFR staining and the total tissue area. The final values were calculated as a percentage of the P-EGFR positive and total tissue area in microns.
Statistics
All statistical analysis was done using Prism software (GraphPad, version 5b). Data comparisons were analyzed for significance using either Student’s T-test or ANOVA with Tukey’s post-hoc analysis for multi-group comparisons and reported if *p<0.05 and **p<0.01.
Results
MMF induces neutrophil influx into mouse lung that is enhanced in the absence of SP-A
WT and SP-A−/− mice were challenged with MMF at varying concentrations (0.05 - 5 μg protein/mouse) and the inflammatory response was assessed after 24 hrs of stimulation. At a dose of 5 μg per mouse, we observed macrophage and neutrophil infiltration into the compartment recovered in bronchoalveolar lavage at 24 hrs. As shown in Fig. 1A, the macrophage population increased approximately 2-fold in response to MMF, and was not significantly affected by the presence of SP-A. MMF resulted in neutrophil recruitment in the presence of WT SP-A; however, neutrophil recruitment to MMF was approximately 5-fold greater in the absence of SP-A (Fig. 1B). These data demonstrate that SP-A can regulate the pulmonary response to pro-inflammatory constituents present in mycoplasma membranes.
Figure 1. Inflammatory responses to MMF in WT and SP-A−/− mice.
WT and SP-A−/− mice were challenged with 5 μg MMF and A) macrophages and B) neutrophils were quantified by cytospin analysis of BAL fluid 24 hrs after challenge. C) Sections of lung were scored for PAS staining at 12 and 24 hrs after MMF challenge. D) Representative PAS and E) Muc5AC staining for WT and SP-A−/− MMF challenged mice 24 hrs post challenge as compared to saline controls. *p<0.05, **p<0.01, ***p<0.001 by student’s T-test, n=3 experiments.
SP-A null mice produce more mucus in response to MMF challenge
WT and SP-A−/− mice were challenged with MMF and lungs were harvested for analysis 12 and 24 hrs after the challenge. PAS staining, which provides histological evidence for mucin production, was evident at 12 hrs, and more pronounced by 24 hrs in SP-A−/− mice (Fig. 1C,D). Little, if any, PAS positive staining was observed in WT mice or in saline treated mice. Specific staining for Muc5AC, a key component of mucus, was enhanced in MMF challenged SP-A−/− mice, whereas very little Muc5AC was detected in either saline controls or in MMF challenged WT mice (Fig. 1E). These data demonstrate that the presence of SP-A can have an inhibitory effect on the production of mucus in response to mycoplasma membrane components.
Genetic variation in SP-A2 alters binding to MMF
SP-A isolated from humans has been shown to bind live Mp via two distinct mechanisms: by recognition of disaturated phosphatidylglycerols (21) and through a specific binding to the protein Mpn372 (22). Unlike mouse SP-A, human SP-A is encoded by two genes designated, SP-A1 and SP-A2 (4-6). Since the majority of pathogens that interact with SP-A, bind the carbohydrate recognition domain (CRD) of the protein, we focused upon specific amino acid variants of SP-A2 that occur at position 223 within the CRD. The recombinant human isoforms of SP-A2 223Q and SP-A2 223K were examined for interaction with solid phase mycoplasma membranes. As shown in Fig. 2A, SP-A2 223K bound MMF with high affinity, similar to our positive control SP-A isolated from subjects with alveolar proteinosis (APP SP-A). In contrast, SP-A2 223Q failed to show significant binding to MMF. This differential binding of SP-A2 isoforms to Mp membranes provided an ideal system for testing the biological importance of this recognition process in regulating responses to the organism in vitro and in vivo.
Figure 2. SP-A binds to MMF in an isoform specific manner.
A) Human SP-A isoforms were examined for interaction with solid phase mycoplasma membranes. APP denotes the protein purified from alveolar proteinosis patients which is used as a positive control. High affinity binding was detected by ELISA. B) MMF was prepared from a mutant strain of Mp that lacks the SP-A binding protein (MMFΔ372) and was compared to WT MMF preparations in the ability to up-regulate Muc5AC RNA expression in WT and SP-A−/− mice. *p<0.05, **p<0.01 by student’s T-test, n=10 mice/group; 2 experiments combined.
Since SP-A is known to bind with high affinity to disaturated phosphatidylglycerols and Mpn372 (21, 22), we sought to determine which of these interactions could play a role in MMF regulation of Muc5AC expression. Membrane fractions were prepared from normal Mp (WT MMF) and from Mp lacking the binding protein (MMFΔ372) and subsequently used to induce Muc5AC expression in the lungs of WT and SP-A−/− challenged mice. Compared to SP-A deficient mice, WT mice showed reduced expression of Muc5AC in response to MMF regardless of whether the binding protein was present in the MMF preparation (Fig. 2B). This finding support that the SP-A binding protein in MMF is dispensible and suggests that SP-A binding to disaturated phosphatidylglycerols present in MMF may be regulating the host response.
Generation of humanized SP-A2 transgenic mice
In order to advance our mechanistic understanding of the role of SP-A allelic diversity in regulating innate immunity in the human lung, we have generated site-targeted transgenic mice harboring human SPA2 alleles (SPA2 223K and SPA2 223Q) expressed from the endogenous mouse locus (Fig. 3A). Mice bearing the human transgene were back-crossed onto the SPA−/− background to yield experimental animals SP-A2 223K/− and SP-A2 223Q/−. Targeted mice express human SP-A and no mouse SP-A, as detected by RT-PCR and by ELISA (~70 pg/ml lavage) (Fig. 3B). The humanized transgenic mice expressed hSP-A at much lower levels than we anticipated; in the range of pg/ml. Murine SP-A is normally expressed in the range of μg/ml. We were unable to detect hSP-A at this low level by Western of lavage fluid due to limitations of the antibody. However, we did detect hSP-A expression using more sensitive IHC methods. Tissue sections obtained from human lung biopsy were used as a positive control for hSP-A staining (Fig. 3C). No hSP-A was detected in sections from WT (Fig. 3D) or SP-A−/− (Fig. 3E) mice. Mice harboring the human SP-A2 transgenes gave positive reactions to the antibody in the alveolar regions and in alveolar macrophages (which are known to internalize SP-A) for both of the SP-A223K/− (Fig. 3F) and SP-A223Q/− (Fig. 3G) mice.
Figure 3. Generation of humanized SP-A transgenic mice.
A) Site targeted knock-in mice that express either SP-A2 223K or SP-A2 223Q were created by BAC recombineering into the endogenous mouse locus of C57BL/6 ES cells. B) Murine SP-A (mSP-A) and human SP-A2 (hSP-A2) expression was determined in targeted mice by RT-PCR of lung tissue and by ELISA of BALs. RT-PCR was standardized to the housekeeper, Cyclophilin. C) Staining by IHC for human SP-A in human lung tissue (positive control), D) WT murine distal airway (negative control), E) SP-A−/− murine distal airway (negative control), F) SP-A2 223K targeted mice alveolar macrophages and distal airway, G) SP-A2 223Q targeted mice alveolar macrophages and distal airway. All pictures were taken at 40× magnification.
Genetic Variation in SP-A2 results in differential responses to MMF
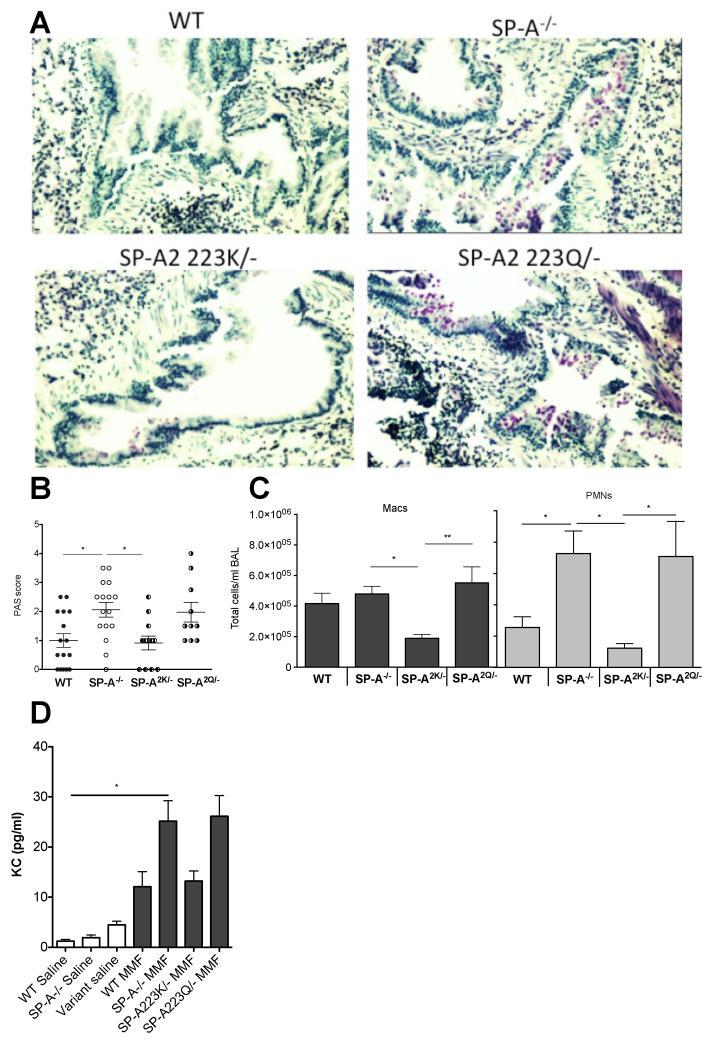
The humanized SP-A2 mice that express either SP-A2 223K, or SP-A2 223Q were challenged with MMF and compared to WT and mice to determine the immune response after 16 hrs. Mice that express SP-A2 223K had little PAS stain, similar to WT challenged mice. PAS stain was significantly more pronounced in SP-A2 223Q/− mice, similar to SP-A−/− mice (Fig. 4A,B). Similarly, mice that express SP-A2 223Q had enhanced neutrophil recruitment and increased KC in response to MMF challenge (Fig. 4C,D). Thus, the SP-A2 223Q transgenic mice essentially phenocopied the SP-A−/− mice. Conversely, the SP-A2 223K mice were protected from enhanced neutrophil influx and had lower KC levels when compared to the SP-A2 223Q variant, and thus appeared more like WT mice.
Figure 4. Differential immune responses to MMF in humanized mice.
A) Representative PAS stained lung sections from MMF challenged mice 16 hrs post challenge. B) Blinded scoring of PAS stained sections from 3 independent experiments. ANOVA p<0.01; Tukey’s post hoc *p<0.05. C) Macrophages (Macs) and neutrophils (PMNs) and from BAL of MMF challenged mice in the respective genotypes were determined by cytospin analysis. Macs-ANOVA p<0.01; Tukey’s post hoc *p<0.05, **p<0.01; PMNs-ANOVA p<0.01; Tukey’s post hoc *p<0.05. D) KC was detected by ELISA from the cell-free lavage of saline and MMF challenged mice. ANOVA p<0.001; Tukey’s post hoc *p<0.05. n=3 experiments for B-D.
MMF regulation of Muc5AC expression occurs in the absence of neutrophils
We next sought to determine if MMF induction of mucin production and Muc5AC expression in the absence of SP-A binding was due to enhanced neutrophil recruitment. MTECs from WT and SP-A−/− mice were cultured at an air-liquid interface (ALI) and stimulated with MMF. RT-PCR analysis of MMF challenged MTECs revealed that these cells responded much like the whole lung in the production of Muc5AC. Similar to what we observed in MMF-challenged WT mice (Fig. 2B), MMF-challenge of WT MTECs also resulted in decreased Muc5AC transcript as compared to vehicle treated controls (Fig. 5A). Likewise, in MMF-challenged MTECs from SP-A−/− mice, Muc5AC transcript was elevated over vehicle controls (Fig. 5A) similar to our in vivo results (Fig. 2B). Additionally, MTECs derived from SP-A−/− mice had significantly greater Muc5AC transcript after MMF challenge as compared to MTECs derived from WT mice. Interestingly, MTECs from WT mice produced an SP-A transcript as detected by RT-PCR and secreted a band that reacted with an anti-SP-A antibody by Western analysis (Fig. 5B,C). The reactive band, which appears to be present as a non-glycosylated dimer approximately 50kDa in size (30), is not detected in apical supernatants from SP-A−/− MTEC cultures. KC levels were significantly elevated in the basolateral supernatant compartment after MMF stimulation, which is shown as a fold-increase over vehicle stimulated controls. Additionally, significantly more KC was secreted from the SP-A−/− derived MTECS compared to those grown from WT mice (Fig. 5D). The increased KC produced in the cultures derived from SP-A−/− mice is a likely mechanism driving the increased neutrophil recruitment in SP-A deficient mice observed in Fig. 4C. The above findings obtained with ALI cultures demonstrate that the enhanced mucin production observed in SP-A−/− mice is independent of the recruitment of neutrophils.
Figure 5. Differential regulation of Muc5AC in MMF stimulated mouse tracheal epithelial cell cultures.
MTECs were harvested from respective genotypes of mice and grown at ALI for two weeks after confluence at which point they were stimulated with vehicle or MMF for 16 hrs. RNA was extracted from transwell inserts and RT-PCR was performed to examine Muc5AC expression. A) MTECS from WT mice have significant repression of Muc5AC transcript, whereas MTECs from SP-A−/− mice have significantly enhanced Muc5AC transcript following MMF exposure. n=10 transwells per condition from two separate experiments. ANOVA p<0.001; Tukey’s post hoc *p<0.05, ***p<0.001. B) SP-A RNA expression from WT and SP-A−/− MTECs by RT-PCR. ***p<0.001. C) Approximately 50 kDa band corresponds to a non-glycosylated form of SP-A that was secreted from WT MTECs as detected by Western of apical supernatants. D) KC was detected by ELISA from the basolateral supernatants of MMF challenged MTECs derived from WT and SP-A−/− mice and is presented as fold over their respective non-stimulated control wells to eliminate cell plating and confluence variability. **p<0.01 compared to WT MMF. n=10 transwells per condition from two separate experiments.
The epidermal growth factor receptor has enhanced phosphorylation in SP-A−/− mice when challenged with MMF
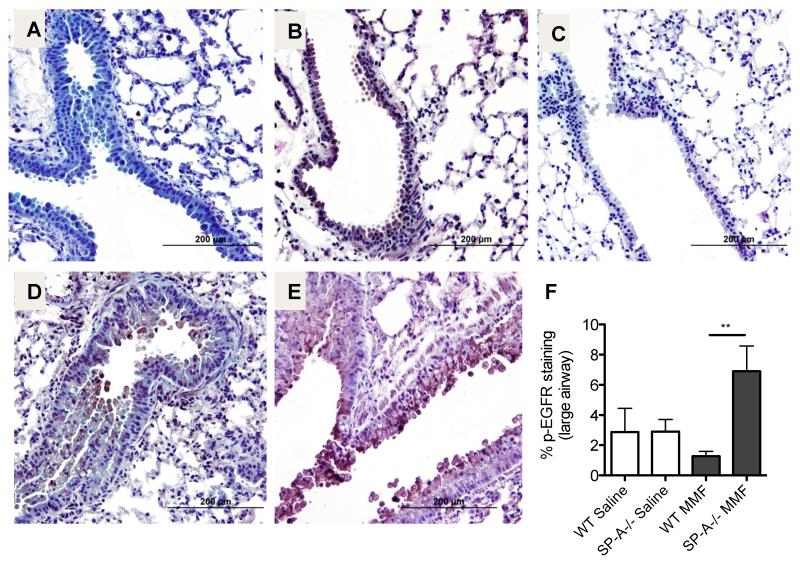
The EGFR is activated by a variety of ligands and undergoes a transformation from an inactive monomer, to an active homodimer. This dimerization leads to autophosphorylation and stimulation of tyrosine kinase pathways that initiate several signaling pathways including those involved in mucin production (31, 32) and IL-8 (or murine KC) production (33). Sections from saline treated WT (Fig. 6A) and SP-A−/− (Fig. 6B) mice and MMF-challenged WT (Fig. 6D) and SP-A−/− (Fig. 6E) mice were stained for p-EGFR. Little staining was evident in the isotype control (Fig. 6C) or in the WT saline or MMF-challenged mice sections. However, MMF exposed lung sections from mice lacking SP-A had increased staining for p-EGFR as compared to SP-A sufficient (WT) MMF challenged mice (Fig. 6E,F).
Figure 6. pEGFR expression is enhanced post MMF challenge.
IHC was performed for detection of p-EGFR 12 hrs post MMF or saline challenge. A) WT saline challenged, B) SP-A−/− saline challenged, C) isotype control D) WT MMF challenged, E) SP-A−/− MMF challenged. F) Computer generated quantitative analysis of the % of the large airway positive for p-EGFR staining. **p<0.01, n=3-4 per group.
Inhibition of EGFR in SP-A−/− mice results in decreased mucin production
We next sought to determine if EGFR signaling is necessary for MMF-induced mucin production when SP-A is absent. SP-A−/− mice were treated with the EGFR inhibitor, AG1478, prior to instillation of MMF and compared to vehicle treated and MMF challenged SP-A−/− mice. SP-A−/− mice challenged with MMF and treated with vehicle had comparable levels of PAS and pEGFR staining as previously detected in SP-A−/− mice challenged with MMF (Fig. 7A,C). However, the MMF challenged SP-A−/− mice that received treatment with the EGFR inhibitor (AG1478) prior to challenge had significantly decreased PAS staining compared to the vehicle controls (Fig. 7B,E). Additionally, SP-A−/− mice treated with the EGFR inhibitor had decreased Muc5AC RNA as compared to vehicle treated SP-A−/− mice (Fig. 7F) supporting the conclusion that inhibition of EGFR protects from MMF induced Muc5AC up-regulation and mucin production.
Figure 7. Inhibition of EGFR attenuates mucus in MMF challenged mice.
SP-A−/− mice were given AG1478 or vehicle prior to challenge with MMF. Representative PAS stained lung histology of vehicle A) versus AG1478 B) treated mice that were challenged with MMF 16 hrs post challenge. P-EGFR staining of vehicle C) versus AG1478 D) treated mice that were challenged with MMF. E) Blinded scoring of PAS stained lung sections. n=2 experiments with 11 vehicle and 10 AG1478 mice. **p<0.01. F) Muc5AC expression by RT-PCR from lung tissue of MMF challenged mice in the presence of absence of EGFR inhibition. **p<0.01, n=2 experiments.
Discussion
Previous studies have demonstrated that SP-A binds to a variety of pathogens and aides in their phagocytosis by alveolar macrophages. Our studies provide insight into a novel role for SP-A by binding to non-infectious membranes derived from pathogenic Mp and protecting from enhanced mucin production by limiting EGFR signaling. Additionally, we show that a specific genetic allelic variant in SP-A2 (position Glu223Lys), which is commonly found in the population, can alter the ability of SP-A to bind to membrane components derived from infectious Mp and as a consequence reduces the protective affect of SP-A in mounting a host defense response. In further studies, we determined that MMF stimulated mucin production occurred in the absence of neutrophil recruitment and that the protective affect of SP-A was likely occurring at the level of the epithelial cell. Using an EGFR inhibitor, we were able to ablate the enhanced mucin production observed in MMF challenged SP-A−/− mice. Taken together these finding suggest that when SP-A is absent (as in the SP-A−/− mice) or has a lower binding affinity for MMF (as in the SP-A223Q/− mice) that MMF will cause increased p-EGFR signaling that results in enhanced mucin production.
Mycoplasma pneumoniae is a well-known human pathogen that is a causative agent of community acquired “walking” pneumonia, acute bronchitis and asthma exacerbations. In many of these conditions, mucin production is thought to be an important factor in the pathogenesis of disease. While management of Mp infections is typically achieved by use of either macrolides, tetracyclines or fluoroquinolones, many studies into the mechanisms of Mp pathogenicity have demonstrated that immune stimulatory factors are inherent in the cell membrane and can also persist as secreted factors, both of which can stimulate immune responses independent of Mp viability. In fact, secreted community acquired respiratory distress syndrome (CARDS) toxin from M. pneumoniae can lead to ciliostasis, increased tissue permeability and cell death (34, 35). Interestingly, CARDs toxin has also been shown to demonstrate high affinity binding to SP-A (22). Typically, Mp only expresses CARDS toxin during infection of a live host and expresses poorly in in vitro culture systems (36). Therefore, the MMF stimuli used for our studies is unlikely to contain CARDS toxin and the differences in response observed between SP-A223Q and SP-A223K is likely due to the ability to interact with and bind the lipid components in the membrane preparation. Additionally, WT mice challenged with MMF derived from a CARDs deficient strain of Mp (lacks Mpn372) also had reduced Muc5AC expression at similar levels to MMF challenged WT mice (Fig. 2b). These findings suggest that the ability of SP-A to bind to Mp membrane lipid components, rather than the CARDs protein, is responsible for the protective mechanisms against mucin production.
MMF, which is composed of non-live Mp membranes that include lipoproteins and glycolipids, has been demonstrated by Chmura et al to stimulate IL-8 secretion from human bronchial epithelial cells (19). IL-8 in humans, the equivalent of murine KC, is a potent recruiter of neutrophils. SP-A−/− and SP-A223Q/− mice had significantly more neutrophil recruitment and KC levels in BAL in response to MMF challenge as compared to WT and SP-A223K/− mice. Since SP-A−/− and SP-A223Q/− mice also demonstrated enhanced mucin production during MMF challenge, and neutrophils are known to contribute to mucin production via release of elastase (37), we sought to determine if the enhanced mucin production was dependent on the presence of neutrophils by use of MTEC cultures. We suspected that if mucin production was tied to neutrophil recruitment then in the absence of neutrophil recruitment in the MTEC system, Muc5AC would fail to increase to MMF stimulation as compared with the saline controls. However, we observed that while the MTECs derived from SP-A−/− mice had significantly increased Muc5AC expression in MMF challenged as compared to vehicle treated cells, MTECs from WT MMF challenged mice had significantly repressed Muc5AC expression compared to vehicle controls. This unexpected finding led us to investigate SP-A expression in the WT MTECs. We were able to detect SP-A, as a band approximately 50 KDa, from the apical supernatants of the MTEC cultures that were derived from WT mice. No SP-A was detected from the SP-A−/− apical supernatants. While we were surprised to discover that SP-A was synthesized and secreted from MTECs during the month long growth and differentiation process, the size of the SP-A (~50 KDa) has previously been detected and is suspected to be a non-glycosylated dimer (30).
The finding that Muc5AC is repressed during MMF challenge of WT MTECs suggests the possibility that SP-A is inducing a negative regulatory pathway that would lead to the repression of Muc5AC. Additionally, the MTECs derived from SP-A−/− mice suggest that when SP-A is absent, MMF is capable of causing Muc5AC up-regulation in the absence of neutrophils. Interestingly, previous studies have suggested that live Mp signals almost exclusively through TLR-2 for induction of Muc5AC (38). While previous work shows that MMF stimulates IL-8 production in a manner independent of TLR-2, the major receptor involved in the inflammatory response to MMF was not defined. Our own experiments using TLR-2 deficient mice challenged with MMF supported the notion that MMF was likely signaling independently of TLR-2 (data not shown).
Non-live mycoplasma derived components are comprised of a variety of proteins and lipids and have been shown to possess antigen activity (39). Since many of the MMF components could signal through non-TLRs, we chose to focus on those non-TLR receptors that are already known to regulate mucin production. Staining by IHC for p-EGFR revealed that MMF challenge up-regulates this signal in the large airways when SP-A is absent. EGFR has long been established an important receptor that regulates mucin production through signaling of various ligands both endogenous and exogenous. Lipoteichoic acid, while absent from mycoplasmas, is found in the cell walls of many pathogens and has been shown to induce mucus via EGFR. Our findings of MMF-EGFR signaling is supported by recent work by Hao et al, which demonstrates that live Mp enhances mucin production by activating the EGFR signaling pathway (40). While they also report increased IL-6, a ligand for EGFR, after Mp challenge, our analysis found similarly increased IL-6 levels between WT and SP-A−/− MMF challenged mice as compared to vehicle mice. Since IL-6 was increased in both groups of mice post MMF challenge when compared to vehicle treated mice and there was only mucin production in the SP-A−/− mice, there remains a possibility that SP-A may alter the ability of IL-6 to signal via the EGFR thus leading to protection from mucin production. Future studies will examine whether either SP-A modifies IL-6 dependent signaling or MMF lipoproteins are capable of inducing mucin production through the EGFR directly.
It remains possible that SP-A could directly bind the EGFR and interfere with signaling. While SP-D has been shown to bind EGFR via lectin activity and reduce EGF-mediated signaling (41), we were unable to detect SP-A/EGFR direct binding (data not shown). While we do identify that SP-A is important in regulating MMF induced mucin production by limiting EGFR signaling, we recognize the effect may be limited to the ability of SP-A to bind the MMF thus halting further downstream signaling events.
The importance of SP-A binding to a myriad of pathogens has been well documented over the last 20 years. However, we are only beginning to understand the complex nature of specific allelic variants of SP-A and how they affect human lung disease. Our findings show for the first time that genetic alteration in one amino acid of human SP-A2 at position Q223K (1A0 versus 1A3) can dramatically impair SP-A2 from binding the pathogen (MMF), which consequently leads to a heightened innate response that was not significantly different than when SP-A is absent altogether. These findings highlight the importance of functional SP-A in the host response and provide new insight into the functional role of genetic variation of human SP-A in airways disease.
Acknowledgements
We would like to thank Gary Kucera and Cheryl Bock for assistance with generating the SP-A humanized mice, Dr. Duncan Krause (University of Georgia) for the generous gift of the mutant strain Mpn372 and Drs. W. Michael Foster, and Loretta Que for helpful discussions.
Footnotes
This work was supported by NIH funding: HL-111151 (Ledford) and AI81672 (Kraft, Hollingsworth, Voelker).
References
- 1.LeVine AM, Gwozdz J, Stark J, Bruno M, Whitsett J, Korfhagen T. Surfactant protein-A enhances respiratory syncytial virus clearance in vivo. J Clin Invest. 1999;103:1015–1021. doi: 10.1172/JCI5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LeVine AM, Kurak KE, Wright JR, Watford WT, Bruno MD, Ross GF, Whitsett JA, Korfhagen TR. Surfactant protein-A binds group B streptococcus enhancing phagocytosis and clearance from lungs of surfactant protein-A-deficient mice. Am J Respir Cell Mol Biol. 1999;20:279–286. doi: 10.1165/ajrcmb.20.2.3303. [DOI] [PubMed] [Google Scholar]
- 3.Sano H, Kuroki Y. The lung collectins, SP-A and SP-D, modulate pulmonary innate immunity. Molecular immunology. 2005;42:279–287. doi: 10.1016/j.molimm.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 4.White RT, D Damm, J Miller, K Spratt, J Schilling, S Hawgood, B Benson, B Cordell. Isolation and characterization of the human pulmonary surfactant apoprotein gene. Nature. 1985;317:361–363. doi: 10.1038/317361a0. [DOI] [PubMed] [Google Scholar]
- 5.Floros J, Steinbrink R, Jacobs K, Phelps D, Kriz R, Recny M, Sultzman L, Jones S, Taeusch HW, Frank HA, et al. Isolation and characterization of cDNA clones for the 35-kDa pulmonary surfactant-associated protein. J Biol Chem. 1986;261:9029–9033. [PubMed] [Google Scholar]
- 6.Katyal SL, Singh G, Locker J. Characterization of a second human pulmonary surfactant-associated protein SP-A gene. Am J Respir Cell Mol Biol. 1992;6:446–452. doi: 10.1165/ajrcmb/6.4.446. [DOI] [PubMed] [Google Scholar]
- 7.Pettigrew MM, Gent JF, Zhu Y, Triche EW, Belanger KD, Holford TR, Bracken MB, Leaderer BP. Respiratory symptoms among infants at risk for asthma: association with surfactant protein A haplotypes. BMC medical genetics. 2007;8:15. doi: 10.1186/1471-2350-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Floros J, Lin HM, Garcia A, Salazar MA, Guo X, DiAngelo S, Montano M, Luo J, Pardo A, Selman M. Surfactant protein genetic marker alleles identify a subgroup of tuberculosis in a Mexican population. The Journal of infectious diseases. 2000;182:1473–1478. doi: 10.1086/315866. [DOI] [PubMed] [Google Scholar]
- 9.Floros J, Fan R, Matthews A, DiAngelo S, Luo J, Nielsen H, Dunn M, Gewolb IH, Koppe J, van Sonderen L, Farri-Kostopoulos L, Tzaki M, Ramet M, Merrill J. Family-based transmission disequilibrium test (TDT) and case-control association studies reveal surfactant protein A (SP-A) susceptibility alleles for respiratory distress syndrome (RDS) and possible race differences. Clinical genetics. 2001;60:178–187. doi: 10.1034/j.1399-0004.2001.600303.x. [DOI] [PubMed] [Google Scholar]
- 10.Floros J, Fan R, Diangelo S, Guo X, Wert J, Luo J. Surfactant protein (SP) B associations and interactions with SP-A in white and black subjects with respiratory distress syndrome. Pediatrics international: official journal of the Japan Pediatric Society. 2001;43:567–576. doi: 10.1046/j.1442-200x.2001.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Saleeby CM, Li R, Somes GW, Dahmer MK, Quasney MW, DeVincenzo JP. Surfactant protein A2 polymorphisms and disease severity in a respiratory syncytial virus-infected population. The Journal of pediatrics. 2010;156:409–414. doi: 10.1016/j.jpeds.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 12.Guo X, Lin HM, Lin Z, Montano M, Sansores R, Wang G, DiAngelo S, Pardo A, Selman M, Floros J. Polymorphisms of surfactant protein gene A, B, D, and of SP-B-linked microsatellite markers in COPD of a Mexican population. Chest. 2000;117:249S–250S. doi: 10.1378/chest.117.5_suppl_1.249s-a. [DOI] [PubMed] [Google Scholar]
- 13.Huang W, Wang G, Phelps DS, Al-Mondhiry H, Floros J. Human SP-A genetic variants and bleomycin-induced cytokine production by THP-1 cells: effect of ozone-induced SP-A oxidation. Am J Physiol Lung Cell Mol Physiol. 2004;286:L546–553. doi: 10.1152/ajplung.00267.2003. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Voelker DR, Lugogo NL, Wang G, Floros J, Ingram JL, Chu HW, Church TD, Kandasamy P, Fertel D, Wright JR, Kraft M. Surfactant protein A is defective in abrogating inflammation in asthma. Am J Physiol Lung Cell Mol Physiol. 2011;301:L598–606. doi: 10.1152/ajplung.00381.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697–728. doi: 10.1128/CMR.17.4.697-728.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraft M, Cassell GH, Henson JE, Watson H, Williamson J, Marmion BP, Gaydos CA, Martin RJ. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med. 1998;158:998–1001. doi: 10.1164/ajrccm.158.3.9711092. [DOI] [PubMed] [Google Scholar]
- 17.Kraft M, Cassell GH, Pak J, Martin RJ. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782–1788. doi: 10.1378/chest.121.6.1782. [DOI] [PubMed] [Google Scholar]
- 18.Kraft M, Hamid Q. Mycoplasma in severe asthma. J Allergy Clin Immunol. 2006;117:1197–1198. doi: 10.1016/j.jaci.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Chmura K, Bai X, Nakamura M, Kandasamy P, McGibney M, Kuronuma K, Mitsuzawa H, Voelker DR, Chan ED. Induction of IL-8 by Mycoplasma pneumoniae membrane in BEAS-2B cells. Am J Physiol Lung Cell Mol Physiol. 2008;295:L220–230. doi: 10.1152/ajplung.90204.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rawadi G, Roman-Roman S. Mycoplasma membrane lipoproteins induced proinflammatory cytokines by a mechanism distinct from that of lipopolysaccharide. Infect Immun. 1996;64:637–643. doi: 10.1128/iai.64.2.637-643.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piboonpocanun S, Chiba H, Mitsuzawa H, Martin W, Murphy RC, Harbeck RJ, Voelker DR. Surfactant protein A binds Mycoplasma pneumoniae with high affinity and attenuates its growth by recognition of disaturated phosphatidylglycerols. J Biol Chem. 2005;280:9–17. doi: 10.1074/jbc.M411570200. [DOI] [PubMed] [Google Scholar]
- 22.annan TR, Provenzano D, Wright JR, Baseman JB. Identification and characterization of human surfactant protein A binding protein of Mycoplasma pneumoniae. Infect Immun. 2005;73:2828–2834. doi: 10.1128/IAI.73.5.2828-2834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ledford JG, Lo B, Kislan MM, Thomas JM, Evans K, Cain DW, Kraft M, Williams KL, Wright JR. Surfactant protein-A inhibits mycoplasma-induced dendritic cell maturation through regulation of HMGB-1 cytokine activity. J Immunol. 2010;185:3884–3894. doi: 10.4049/jimmunol.1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrera-Ramos E, Lopez-Rodriguez M, Ruiz-Hernandez JJ, Horcajada JP, Borderias L, Lerma E, Blanquer J, Perez-Gonzalez MC, Garcia-Laorden MI, Florido Y, Mas-Bosch V, Montero M, Ferrer JM, Sorli L, Vilaplana C, Rajas O, Briones M, Aspa J, Lopez-Granados E, Sole-Violan J, de Castro FR, Rodriguez-Gallego C. Surfactant protein A genetic variants associate with severe respiratory insufficiency in pandemic influenza A virus infection. Crit Care. 2014;18:R127. doi: 10.1186/cc13934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korfhagen TR, Bruno MD, Ross GF, Huelsman KM, Ikegami M, Jobe AH, Wert SE, Stripp BR, Morris RE, Glasser SW, Bachurski CJ, Iwamoto HS, Whitsett JA. Altered surfactant function and structure in SP-A gene targeted mice. Proc Natl Acad Sci U S A. 1996;93:9594–9599. doi: 10.1073/pnas.93.18.9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiba H, Pattanajitvilai S, Evans AJ, Harbeck RJ, Voelker DR. Human surfactant protein D (SP-D) binds Mycoplasma pneumoniae by high affinity interactions with lipids. J Biol Chem. 2002;277:20379–20385. doi: 10.1074/jbc.M201089200. [DOI] [PubMed] [Google Scholar]
- 27.Kuroki Y, Mason RJ, Voelker DR. Pulmonary surfactant apoprotein A structure and modulation of surfactant secretion by rat alveolar type II cells. J Biol Chem. 1988;263:3388–3394. [PubMed] [Google Scholar]
- 28.Lankford SM, Macchione M, Crews AL, McKane SA, Akley NJ, Martin LD. Modeling the airway epithelium in allergic asthma: interleukin-13-induced effects in differentiated murine tracheal epithelial cells. In Vitro Cell Dev Biol Anim. 2005;41:217–224. doi: 10.1290/0502012.1. [DOI] [PubMed] [Google Scholar]
- 29.Ledford JG, Mukherjee S, Kislan MM, Hollingsworth JW, Wright JR. Eosinophil peroxidase-mediated eosinophil clearance of Mycoplasma pneumoniae is negatively regulated by Surfactant Protein-A. In Revisions for PlosOne [Google Scholar]
- 30.Khubchandani KR, Snyder JM. Surfactant protein A (SP-A): the alveolus and beyond. FASEB J. 2001;15:59–69. doi: 10.1096/fj.00-0318rev. [DOI] [PubMed] [Google Scholar]
- 31.Burgel PR, Nadel JA. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax. 2004;59:992–996. doi: 10.1136/thx.2003.018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeyama K, Dabbagh K, Lee HM, Agusti C, Lausier JA, Ueki IF, Grattan KM, Nadel JA. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci U S A. 1999;96:3081–3086. doi: 10.1073/pnas.96.6.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakanaga T, Nadel JA, Ueki IF, Koff JL, Shao MX. Regulation of interleukin-8 via an airway epithelial signaling cascade. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1289–1296. doi: 10.1152/ajplung.00356.2006. [DOI] [PubMed] [Google Scholar]
- 34.Kannan TR, Baseman JB. ADP-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogens. Proc Natl Acad Sci U S A. 2006;103:6724–6729. doi: 10.1073/pnas.0510644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardy RD, Coalson JJ, Peters J, Chaparro A, Techasaensiri C, Cantwell AM, Kannan TR, Baseman JB, Dube PH. Analysis of pulmonary inflammation and function in the mouse and baboon after exposure to Mycoplasma pneumoniae CARDS toxin. PLoS One. 2009;4:e7562. doi: 10.1371/journal.pone.0007562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kannan TR, Musatovova O, Balasubramanian S, Cagle M, Jordan JL, Krunkosky TM, Davis A, Hardy RD, Baseman JB. Mycoplasma pneumoniae Community Acquired Respiratory Distress Syndrome toxin expression reveals growth phase and infection-dependent regulation. Mol Microbiol. 2010;76:1127–1141. doi: 10.1111/j.1365-2958.2010.07092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voynow JA, Young LR, Wang Y, Horger T, Rose MC, Fischer BM. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. Am J Physiol. 1999;276:L835–843. doi: 10.1152/ajplung.1999.276.5.L835. [DOI] [PubMed] [Google Scholar]
- 38.Kraft M, Adler KB, Ingram JL, Crews AL, Atkinson TP, Cairns CB, Krause DC, Chu HW. Mycoplasma pneumoniae induces airway epithelial cell expression of MUC5AC in asthma. Eur Respir J. 2008;31:43–46. doi: 10.1183/09031936.00103307. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu T, Kida Y, Kuwano K. Mycoplasma pneumoniae-derived lipopeptides induce acute inflammatory responses in the lungs of mice. Infect Immun. 2008;76:270–277. doi: 10.1128/IAI.00955-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hao Y, Kuang Z, Jing J, Miao J, Mei LY, Lee RJ, Kim S, Choe S, Krause DC, Lau GW. Mycoplasma pneumoniae Modulates STAT3-STAT6/EGFR-FOXA2 Signaling To Induce Overexpression of Airway Mucins. Infect Immun. 2014;82:5246–5255. doi: 10.1128/IAI.01989-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasegawa Y, Takahashi M, Ariki S, Asakawa D, Tajiri M, Wada Y, Yamaguchi Y, Nishitani C, Takamiya R, Saito A, Uehara Y, Hashimoto J, Kurimura Y, Takahashi H, Kuroki Y. Surfactant protein D suppresses lung cancer progression by downregulation of epidermal growth factor signaling. Oncogene. 2014 doi: 10.1038/onc.2015.266. [DOI] [PubMed] [Google Scholar]