Abstract
Zebrafish (Danio rerio) have been used as a model organism to explore the genetic basis for responsiveness to addictive drugs like cocaine. However, very little is known about how the physiological response to cocaine is mediated in zebrafish. In the present study electrocardiograms (ECG) were recorded from adult zebrafish treated with cocaine. Treatment with cocaine resulted in a bell-shaped dose response curve with a maximal change in heart rate seen using 5mg/L cocaine. Higher doses resulted in a higher percentage of fish showing bradycardia. The cocaine-induced tachycardia was blocked by co-treatment with propranolol, a ß-adrenergic antagonist, but potentiated by co-treatment with phentolamine, a α-adrenergic antagonist. Co-treatment with atropine, a classic cholinergic antagonist, had no effect on cocaine-induced tachycardia. Cocaine treatment of adult fish changed the ECG of treated fish, inducing a dose-dependent increase in QT interval after adjusting for heart rate (QTc), while not affecting the PR or QRS intervals. The acute effects of cocaine on heart rate were examined in 5-day old embryos to see if zebrafish might serve as a suitable model organism to study possible links of embryonic physiological response to subsequent adult behavioral response to the drug. Cocaine treatment of 5-day old zebrafish embryos also resulted in a bell-shaped dose response curve, with maximal tachycardia achieved with 10mg/L. The response in embryonic fish was thus comparable to that in adults and raises the possibility that the effects of embryonic exposure to cocaine on the developing cardiovascular system can be effectively modeled in zebrafish.
Keywords: zebrafish, cocaine, heart rate, electrocardiogram, adrenergic compounds
Introduction
Despite progress made in drug education and treatment cocaine use in the US remains high. In a survey taken by the National Institutes of Health (NIH) as many as 0.6% of all people surveyed had taken cocaine within the previous month of 2010 (Volkow, 2010). This provides the basis for a conservative estimate that as many as 2-6 million people older than 12 used the drug regularly as recently as 2010. Owing to the detrimental physiological effects of the drug, particularly on the cardiovascular system, cocaine is an important factor in emergency room (ER) visits. The Drug Abuse Warning Network (DAWN) reported that in 2011 cocaine was involved in over 40% of the 1.25 million drug-related ER visits, the highest percentage among illicit drugs (DAWN, 2011). Approximately half of the cocaine-related ER patients reported cardiovascular symptoms including chest pain, tachycardia, arrhythmias, myocardial infarction, and heart failure (Maraj et al., 2010).
The cardiovascular effects of cocaine have been extensively studied and reviewed (Kloner et al., 1992; Schwartz et al., 2010). The general consensus is that cocaine has two basic physiological actions that affect heart rate; a positive effect caused by increasing sympathetic nervous system output and a negative effect caused by inhibition of voltage-gated ion channels in cardiac muscle. Acute low doses of cocaine increase heart rate and blood pressure, achieved in part through blockade of the norepinephrine transporter at sympathetic synapses, and also by increased central sympathetic output (Gillis et al., 1995; Vongpatanasin et al., 1999). Both mechanisms increase stimulation of cardiac ß-adrenergic receptors in the myocardium and conducting system. Cocaine also induces alterations of the ECG, including interval length and rhythm (Hale et al., 1989). The pressor response to cocaine involves an increases in stimulation of α-adrenergic receptors in the vasculature that increase vasoconstriction, peripheral resistance, and blood pressure (Poon and va den Buuse, 1998; Tella et al., 1990). At higher concentrations cocaine also exhibits anesthetic properties by blocking voltage sensitive and ligand-activated sodium channels (Przywara and Dambach, 1989). Thus at higher doses, depending on the preparation, cocaine can actually slow heart rate by slowing excitation of cardiac myocytes.
The acute effects of cocaine have been most thoroughly investigated in animal models, given the inherent difficulties of human studies. In animals, the investigator can control subject history, genetic background, effective dosage and environment more carefully. Studies on the effects of cocaine on the cardiovascular system have complemented studies on cocaine addiction, for example, suggesting possible treatments for cocaine-induced cardiovascular problems (Menon et al., 2007). Very little is known about how individual cardiovascular response to cocaine might reflect vulnerability to addiction, with few studies conducted on model species (Ruth et al., 1988).
The zebrafish (Danio rerio) has been used as a model organism for a number of studies related to complex human behaviors, including behavioral sensitivity to cocaine (Darland and Dowling, 2001; Klee et al., 2012; Mathur and Guo, 2010). These fish are easy to maintain in the laboratory, breed prodigiously, and produce large clutches of eggs, which facilitates the use of forward genetic analysis in finding genes related to various biological processes (Darland and Dowling, 2001; Stainier et al., 1996). The zebrafish is also a vertebrate and shares many early developmental pathways and processes with mammals, including humans. While the brain of fish and mammals are distinctly different in many ways, in the extent of the cerebral cortex for example, their common development implies certain functional consistencies in adults. For example, because of common pathways generating the monoaminergic pathways in the brain the zebrafish has become an accepted model for addiction studies (Klee et al., 2012).
Similarly, the cardiovascular system of adult fish is fundamentally different than that of mammals in that it has a single atrium and ventricle and has no pulmonary circuit. However, the two share commonalities in development, structure, function and regulation (Hu et al., 2001). Thus, the zebrafish has emerged as a valuable system for understanding congenital defects of the heart (Hassel et al., 2008; Stainier et al., 1996), and the effects of certain toxins and drugs on cardiovascular regulation (Chaudhari et al., 2013; Milan et al., 2006; Milan et al., 2003). The effects of cocaine on the zebrafish cardiovascular system are unknown. Our goal was to provide a description of the effects of cocaine on the zebrafish heart rate to aid in understanding the interplay between the physiological and behavioral responses to cocaine. Pre-exposure to cocaine during neural development in other species can promote vulnerability to addiction-related behavior later in adulthood (Rocha et al., 2002). If there is a link between the physiological and behavioral responses to cocaine then the physiological response during embryonic development might predict subsequent adult behavior. We therefore also wanted to investigate the acute effects of cocaine on heart rate in embryonic zebrafish.
MATERIALS AND METHODS
Fish husbandry
All adult zebrafish used in these studies were 6-8 months old and housed in the University of North Dakota zebrafish facility. The fish were maintained on a 14-10 light-dark cycle at 28.5°C on stand-alone racks (Pentair Aquatic Habitats, Apopka FL). Fish were fed twice a day with artemia in the morning and pellet (Pentair Aquatic Habitats, Apopka FL) in the afternoon. All drug dosage-response experiments involved single clutches of wild-type fish bred on the system. Experiments on zebrafish embryos 5-days post-fertilization (dpf) were conducted on single clutches bred from the same stock of fish used in the adult experiments.
Electrocardiogram (ECG) recordings
ECG were performed essentially as described by others (Chaudhari et al., 2013; Milan et al., 2006; Sun et al., 2009). Zebrafish were sedated in tricaine as described by others (Westerfield, 2007), but with some differences in drug preparation. A 40μg/L solution of tricaine (tricaine, methanesulfonate, or MS-222, Sigma), dissolved in fish water (RO water with 0.2g/L Instant Ocean and 0.1g/L sodium bicarbonate). The fish were transferred ventral-side up to a sponge dampened with tricaine/water solution. A peristaltic pump was used to ventilate the gills, to maintain tricaine sedation, and to deliver other drugs. After removing a few ventral scales from the desired insertion positions, two 29-gauge microelectrodes (AD Instruments, Colorado Springs, CO) were inserted into the isoproterenol zebrafish to a depth of approximately 1mm. Using micromanipulators (AD Instruments) the positive electrode was placed on the midline approximately1.5-2mm posterior to the chevron created by attachment of the opercular flaps and just anterior (≈1 mm) to the spot where the beating heart caused an obvious protrusion (Figure 1A). The negative electrode was placed approximately 3-4mm posterior to the positive and the ground electrode was inserted into the sponge 1-2 cm away, lateral to the other electrodes. Placement of the electrodes was adjusted until an obvious ECG signal was detected (usually detected within 30 seconds). The pump was then shut off and the mouthpiece removed to minimize noise and a 45-60 second baseline trace was recorded. The pump was restarted to clear tricaine and then reinserted into the fish’s mouth, but the inlet water source was switched to administer other drugs dissolved in fish water. One minute of perfusion provided sufficient administration of cocaine to alter heart rate. The pump was then shut off again, the mouthpiece removed, and a second recording was made. Electrical signals detected by the electrodes were amplified and translated by a PowerLab data acquisition unit using LabChart 7.2.1 software (AD instruments, Colorado Springs, CO). Recordings were made in the range of 0-10 mV. Digital filters limiting frequency range to 8-40 Hz were applied and an averaging algorithm provided by the software was used to smooth the trace.
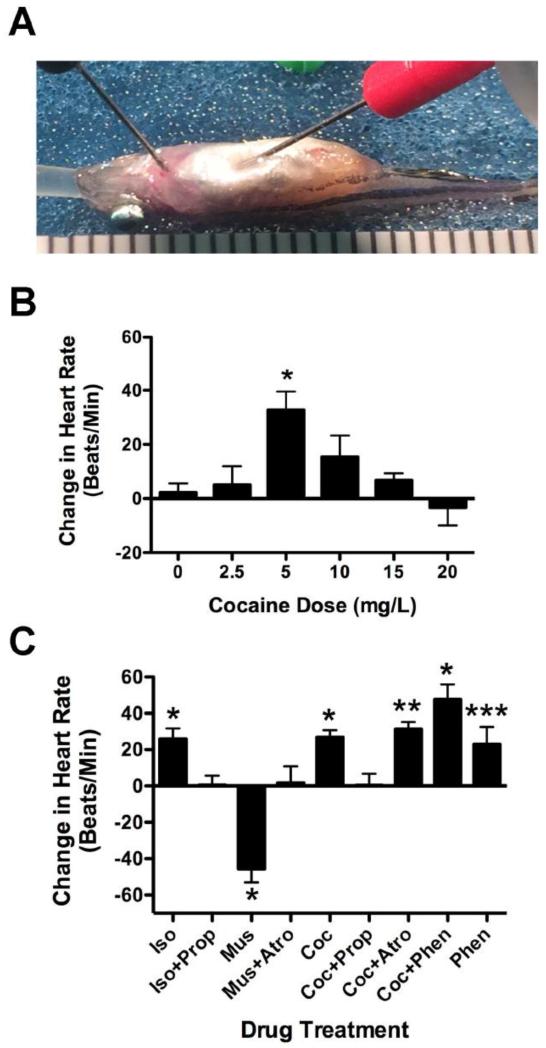
Figure 1. Cocaine affects heart rate in adult zebrafish in a dose dependent fashion and does so via action at ß-adrenergic receptors.
Figure 1A shows a typical ECG prep of an adult zebrafish (see methods for details). The graduations below the fish are 1mm. Figure 1B shows results from a representative experiment looking at the change in heart rate caused by different doses of cocaine. The dose response was bell-shaped with 5mg/L cocaine inducing a significant increase in heart rate above untreated fish and fish treated with 20mg/L (*p < 0.01, n = 5). Figure 1C shows the change in heart rate resulting from co-treatment of 5mg/L cocaine with adrenergic and cholinergic antagonists. Cocaine (5 mg/L, Coc), isopreretonol (20μM, Iso), phentolamine (20μM, Phen) induced tachycardia while muscarine (50μM, Mus) induced bradycardia (*p < 0.01, **p < 0.02, ***p < 0.05). Co-treatment with propranolol (20μM Prop) blocked cocaine- and isopreteronol-induced tachycardia. Co-treatment with atropine (20μM Atro) did not block cocaine-induced tachycardia, but did block muscarine-induced bradycardia. Phentolamine significantly potentiated cocaine-induced tachycardia (ANOVA, p < 0.02, F = 3.806, df = 50).
Experiments measuring the effects of drug treatment on adult zebrafish
To avoid possible confounds of multiple treatment, all fish used in these experiments were treated with drug only one time. Each fish was measured for baseline heart rate followed by treatment with drug. Thus, separate groups of fish were used for each condition. The data shown in Figure 1B is from a single dose response experiment in which all recordings were performed in a single day using 0, 2.5, 5, 10, 15, and 20mg/L cocaine (5 fish for each dose). Additional earlier experiments were performed, increasing the total number of fish tested at each dose to a minimum of 15. After the baseline measurement, fish were perfused with water containing drug for two minutes. Additional studies were conducted using co-treatment with agonists or antagonists for adrenergic and cholinergic receptors including, 20μM isoproterenol, 50μM muscarine, 20μM npropranolol, 20μM atropine, and 20μM phentolamine (Sigma). All agonist concentrations used were previously verified to elicit a statistically significant change in heart rate in earlier dose response experiments (data not shown). Antagonist concentrations were chosen based on the ability to completely block agonist responses in earlier dose response experiments (data not shown). We then combined several of these with cocaine in a series of experiments shown in Figure 1C.
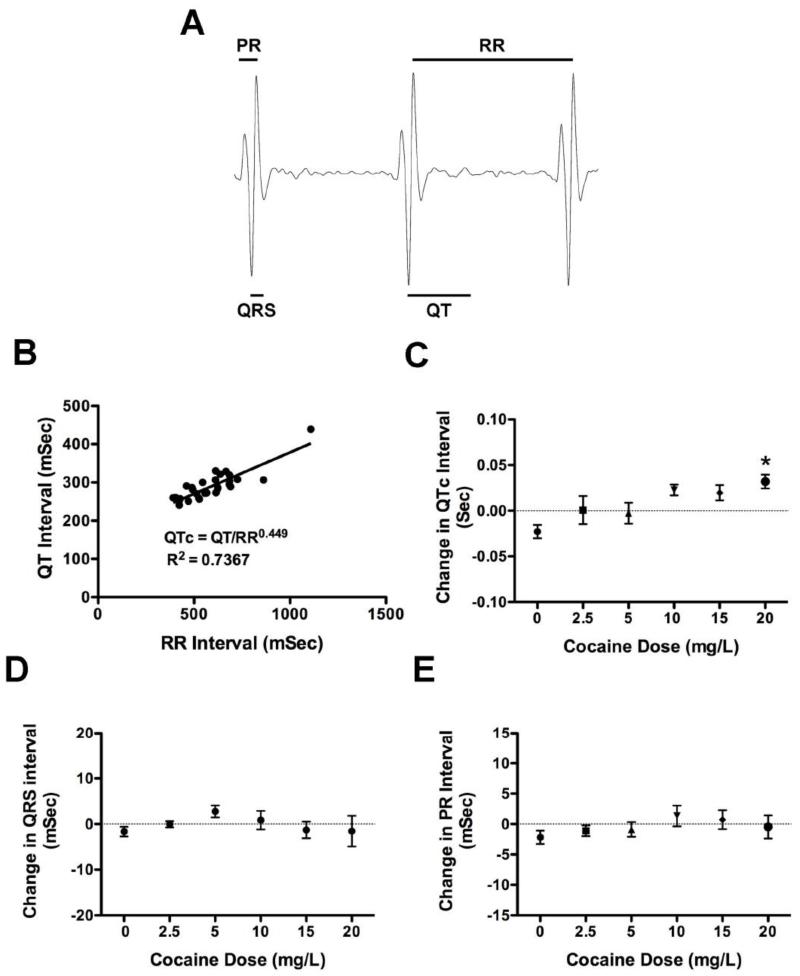
RR, PR, QRS and QT intervals were determined for the same experiment as shown in Figure 1B. In this experiment the recordings were all performed during the same trial and all 30 fish gave reliable P and T-waves in the ECG. Many fish from the other earlier experiments gave reliable T-waves, and similar results to those shown, but the trends were not statistically significant because of the lower number. Combining experiments was not optimal since the experiments were done under slightly different conditions (principally in placement of the electrodes, handling of the pump, and optimization of recording range) as we improved at the technique. Two investigators carried out all ECG analyses independently and blinded to conditions with near identical results. QTc was determined to correct for QT differences caused by increased heart rate, essentially as described by others (Chaudhari et al., 2013; Milan et al., 2006). Least means square analysis was used to determine the equation and correlation coefficient shown for the line in Figure 2B.
Figure 2. Cocaine affects the QTc interval of adult zebrafish relative to tricaine treatment.
Figure 2A shows a typical recording from an adult fish with the PR, QRS, QT and RR intervals outlined. In Figure 2B the QT intervals from 30 baseline recordings of fish in one representative experiment (the same as in Figure 1) are plotted as the least means square regression function of the preceding RR. The resulting best-fit line was defined by the equation QT=QTc × RR0.449. Figure 2C shows the changes in QTc for all 30 fish in the experiment. There is a dose dependent increase in QTc with cocaine treatment with a significant difference between untreated fish and fish treated with 20mg/L cocaine (*p < 0.01, F = 3.536 and df = 29). Figures 2D and 2E show the change in PR and QRS interval with cocaine treatment respectively. No significant differences were found between treatment groups.
Experiments measuring the effect of cocaine on embryonic heart rate
Embryonic heart rate was measured essentially as described by others (Hoage et al., 2012). Embryos 5 dpf were immobilized in 2% methylcellulose that contained 0, 2.5, 5, 10, 15, or 20mg/L cocaine. In order to minimize the possible local dilution of cocaine by the addition of water, the fish were then transferred to a second dish of methylcellulose containing the same concentration of drug for imaging. Care was used to manipulate the embryos as little as possible and to minimize dilution of cocaine during transfer to methylcellulose. Because of this, baseline readings for fish were not taken. Figure 3 then shows the average heart rates for the different treatment groups, rather than the difference between drug treatment and baseline reported for the adults. The fish were imaged using a Leica M165FC dissecting microscope equipped with a DFC310FX camera (North Central Instruments, Minneapolis MN). Minimizing resolution and increasing the binning profile to 8 × 8 maximized the frame rate of the recorded movies to 69 frames/sec, which was critical for accurate analysis given the fast heart rates of the embryos. Movies were played back in slow motion and the heartbeats were counted by inspection by two investigators.
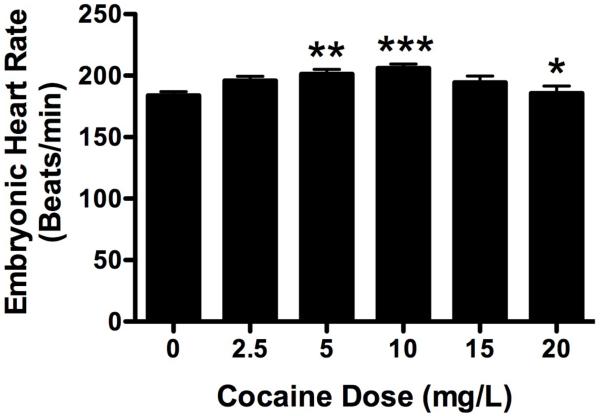
Figure 3. Cocaine affects heart rate in 5-day old zebrafish embryos in a dose dependent fashion.
Cocaine induced a bell-shaped dose response curve in heart rate similar to that seen in adults. Heart rates in fish treated with 5mg/L and 10mg/L cocaine were significantly higher than those of untreated controls (**p < 0.01, ***p < 0.005). Fish treated with 20mg/L had significantly lower heart rates than fish treated with 10mg/L (*p<0.02), but not 5mg/L.
Statistical analysis
All statistical analysis of cocaine dose response curves and when comparing cocaine doses was conducted using one-way analysis of variance (ANOVA), with Bonferroni’s post-test to the 95% confidence limits (GraphPad Prism). Experiments using combined treatment of adult fish with agonists with multiple drugs were done over several months; therefore statistical analysis was conducted using Mann-Whitney two-tailed t-tests comparing baseline heart rates to post treatment heart rates. ANOVA with Bonferroni’s test was used to examine the change in heart rate for the various drug treatments in which three conditions were compared. Linear regression by least means squares method was used to analyze the relationship between the QT and RR intervals. ANOVA with Bonferroni’s post-test was used to examine the change in QTc, PR and QRS for each cocaine dose. The ANOVA was also used to compare the responses of the different embryonic treatment groups.
RESULTS
The effect of cocaine on heart rate in adult zebrafish
Electrocardiograms were measured on zebrafish to determine the effect of cocaine on the function of the cardiovascular system. The baseline heart rate of an adult zebrafish tested under these conditions was 132.7 ± 19.8 beats per minute (bpm). The minimum baseline heart rate was 82 bpm, while the maximum was 190 bpm. Results in Figure 1 are reported as a difference between the heart rate after treatment and the baseline. Cocaine elevated heart rate in a bell-shaped dose dependent fashion (Figure 1B), with a maximal change in heart rate seen at 5mg/L that was significantly higher than baseline (p < 0.005, F = 4.558, n = 5 for each group). Higher and lower doses were effective at increasing heart rate in most fish, but at higher doses more fish showed a decrease in heart rate (26.5% for untreated fish, 12.5%, 0%, 21%, 26.7% and 37.5% for 2.5, 5, 10, 15 and 20mg/L respectively for all three experiments combined). Subsequent experiments to clarify the pharmacological mechanism of cocaine’s positive effect on heart rate in zebrafish were performed using 5mg/L cocaine.
Pharmacology underlying the cocaine response in adult zebrafish
Cocaine was co-administered with a number of compounds to determine its regulatory mechanisms, focusing on the tachycardia achieved with 5mg/L (Figure 1C). In preliminary experiments Isoproterenol, a ß-adrenergic agonist, stimulated heart rate above baseline in a dose dependent manner (data not shown). In Figure 1C we show results using 20μM isoproterenol, which stimulated tachycardia equivalent to that seen for 5mg/L cocaine (p < 0.005). When cocaine and isoproterenol were co-administered with propranolol (20μM), a widely used ß-receptor antagonist, heart rate was not raised above baseline (p < 0.96 for cocaine plus propranolol, and 0.49 respectively isoproterenol plus propranolol). When cocaine was co-administered with atropine, a muscarinic acetylcholine receptor antagonist; heart rate was still raised significantly above baseline to levels similar to that with cocaine alone (p < 0.02). The dose of atropine used was clearly effective because it did block the negative chronotropic effects of muscarine, the classic cholinergic agonist. In preliminary experiments we found that phentolamine, a widely used α-adrenergic antagonist, increased heart rate in a dose dependent fashion (data not shown) when administered alone. In Figure 1C, we show that 20μM was effective at increasing heart rate significantly (p < 0.05 when compared to baseline). Co-administration of phentolamine with cocaine, however, significantly raised heart rate to a level higher on average than for cocaine alone (p < 0.0001 when compared to baseline). In fact, when phentolamine and cocaine were co-administered the effects were approximately additive and ANOVA analysis with Bonferroni’s multiple comparison post-test showed that phentolamine co-treatment with cocaine was significantly higher than treatment with cocaine alone (p < 0.02, F = 3.806, df = 50).
The effect of cocaine on the QTc, PR and QRS intervals
To determine if cocaine has any effects on the ECG in zebrafish, the RR, QT, PR and QRS intervals were measured as indicated in Figure 2A. The average PR, QRS, QT and RR intervals for the baseline measurements for all 30 fish were 75.8 ± 5.9msec, 50.2 ± 6.9msec, 288 ± 36.9msec, and 558 ± 99.3msec respectively. The QT interval was plotted as a function of the RR interval. Least mean square analysis showed that the relationship was highly significant (R2 = 0.7357, p < 0.0001, F = 77.95, with df = 29), indicating that we were accurately assessing the QT interval. The equation for the best-fit line after transformation was: QTc = QT/RR0.449. QTc was calculated for both baseline and cocaine treatment using this equation and the differences between the two measurements at each dose are shown in Figure 2C. There was a dose dependent increase in QTc with cocaine treatment. The value at 20mg/L cocaine was significantly higher than that for untreated fish (p < 0.01, F = 3.54, and df = 29). In contrast, no significant changes were seen for either the PR and QRS intervals (Figures 2D and 2E).
The effects of cocaine on heart rate in 5-day old zebrafish embryos
We were also interested to see if the effect of cocaine on embryonic heart rate in embryos was comparable to that seen in adults. The heart rate of an untreated, unisoproterenol 5-day old zebrafish under these laboratory conditions was 188.3± 24.4 bpm, with a high value of 252 bpm and a low of 138 bpm. As in the adult, cocaine stimulated a bell-shaped dose response curve (Figure 3). Unlike the adult, maximal stimulation was at 10mg/L cocaine, with lower doses progressively increasing heart rate and higher doses decreasing heart rate (p < 0.0001, F = 5.420, df = 277). The maximal value at 10mg/L cocaine was significantly higher than that of untreated fish (p < 0.005) and fish treated with 20mg/L cocaine (p < 0.02). Treatment with 5mg/L cocaine also significantly increased heart rate above untreated controls (p < 0.01).
Discussion
It is well established that zebrafish are affected behaviorally by cocaine (Darland and Dowling, 2001; Darland et al., 2012), but there are no studies showing how the drug affects them physiologically. There are many studies in mammals, including humans, investigating cocaine’s acute effects on heart rate and blood pressure (Kloner et al., 1992). Results vary with the species used and whether the subjects were isoproterenol or conscious; however, the consensus is that cocaine’s effects on heart rate are dose dependent, causing tachycardia at lower doses and bradycardia at higher doses. This is comparable to what we saw in the present study and further validates using the zebrafish as a model system to study the effects of cocaine.
Cocaine-induced tachycardia at lower doses has been well studied in mammals and has both central and peripheral components (Kloner et al., 1992). The increase in heart rate is mediated by increased activity at sympathetic nerve terminals in the heart and vasculature. ß-adrenergic antagonists block the effects of cocaine on heart rate and blood pressure while ß-adrenergic antagonists block vasoconstrictor effects on blood pressure. The increases in heart rate and blood pressure are due, in part, to peripheral effects at the synapse by blockade of epinephrine reuptake, or possibly via sympathetic stimulation of epinephrine release from the adrenal medulla (Gillis et al., 1995; Tella et al., 1990). There is also considerable evidence that cocaine-induced cardiovascular effects are driven centrally by increasing sympathetic outflow from the brainstem (Menon et al., 2007; Poon and va den Buuse, 1998). Both central and peripheral mechanisms are undoubtedly important in mediating cocaine’s cardiovascular effects. We examined the pharmacology of the common endpoint, the adrenergic output at sympathetic terminals.
We tested whether the effects of adrenergic antagonists on cocaine-induced cardiovascular responses were the same in zebrafish as in mammals. The effect of co-treatment with propranolol suggests that cocaine-induced tachycardia is mediated by increased sympathetic activity possibly by increased sympathetic outflow, or by local increases in norepinephrine release. Conversely, the absence of an atropine effect suggests little parasympathetic impact on cocaine-induced tachycardia. We also have shown an increase in zebrafish heart rate using phentolamine, a widely used α-adrenergic antagonist. Phentolamine has been shown to lower blood pressure, but increase heart rate in other species (Gomes et al., 1978; Tella et al., 1990). The mechanism for elevating heart rate is not entirely certain, but it has been speculated that lower blood pressure triggers a baroreceptor reflex that increases heart rate, or that greater perfusion of the heart caused by vasodilation of coronary vasculature increases performance (Gomes et al., 1978; Tella et al., 1990). While zebrafish do very likely rely on luminal blood for oxygenation, they also have a well-developed coronary vasculature that feeds a compact layer of myocardium (Hu et al., 2001). It has been shown that phentolamine potentiates positive chronotropic effects of norepinephrine in isolated beating atria of carp (Temma et al., 1989). The baroreflex is present in teleost although its response to hypotension is less developed than other vertebrate groups and its significance remains somewhat controversial (Sandblom and Axelsson, 2005). The present study with phentolamine adds additional evidence for the hypotensive response in zebrafish. Co-treatment of phentolamine with cocaine in zebrafish resulted in an additive effect on heart rate. These results differ somewhat from that reported in other species, although possible ceiling effects on heart rate may have obscured an additive effect in these studies (Tella et al., 1990). Overall, the adrenergic pharmacology of the cocaine-induced cardiovascular response in zebrafish is very similar to that described for other species.
At higher doses cocaine is believed to slow heart rate by blocking voltage-gated ion channels in conducting fibers and cardiac muscle cells (Egashira et al., 1991; Przywara and Dambach, 1989). Blockade of these channels depresses depolarization and increases refractory time of ion channels, thus decreasing heart rate. We saw bradycardia at higher doses of cocaine in zebrafish consistent with what has been found in other species. This is likely responsible for the downward aspect of the bell-shaped response curve. The class I antiarrhythmic action of cocaine also reflects the observations made on the ECG waveforms. There are mixed reports in mammals in which the PR, QRS and QTc intervals are lengthened with higher doses of cocaine (reviewed by Kloner et al 1992). In the present study we measured the effect of cocaine on these intervals in zebrafish. The interval lengths measured are similar to those reported in other studies on adults (Chaudhari et al., 2013; Milan et al., 2006; Sun et al., 2009). An interesting difference between our recordings and that reported by others is the sharp hyperpolarization after the P-wave. The source of this is unknown and while it could affect interpretation of the Q wave, it appears in all baseline and post-cocaine recordings, therefore the relative changes in the intervals are still valid. QTc was increased by cocaine in a dose dependent manner, similar to what has been shown in mammals (Hale et al., 1989). Thus the positive and negative effects of cocaine are seen in zebrafish, validating their use as model organisms to study the effects of cocaine.
A potential caveat arises in the present studies by using tricaine sedation with cocaine treatment. The consensus in other studies recording ECGs in zebrafish sedated with tricaine suggest that the drug lengthens the ECG intervals, including QTc (Huang et al., 2010; Sun et al., 2009). Tricaine, like cocaine, blocks voltage-gated ion channels (Frazier and Narahashi, 1975). There is evidence for this effect in our study. For example, in Figure 2C, we show that water treated control fish show a QTc interval lower than the tricaine baseline. While the fish are still sedated, this suggests that tricaine lengthens the QTc interval relative to water alone. Cocaine progressively increases the QTc to levels above the tricaine baseline suggesting that the effects of the two drugs are additive to some degree. Therefore, by using tricaine we may have offset some of the cocaine-induced tachycardia. In one study, tricaine was shown to have a dramatic effect on heart rate, lowering it from approximately 150 bpm before sedation to 90 bpm afterwards (Huang et al., 2010). In our study the average heart rate (132.7±19.8 bpm) was comparable to the value recorded in paralyzed, unanesthatized fish (Chaudhari et al., 2013; Huang et al., 2010; Milan et al., 2006). Thus, while the effect of tricaine sedation on cocaine-induced tachycardia cannot be dismissed, we believe it was minimal.
The bell-shaped dose response to cocaine in adults was also seen in embryonic zebrafish heart rate (Figure 3). Significant increases above untreated embryos were seen at 5 and 10mg/L, the maximal response in the latter. Results are comparable to what were seen in adults although the dose resulting in maximal heart rate change in embryos was somewhat higher on average than that in adults. While it is possible that the adult is more sensitive to positive effects of cocaine the embryos may also be less responsive to the negative effects on heart rate associated with the drug. Further investigation might compare adult expression levels of ß-receptors and ion channel type expressed in the heart to those seen during embryonic heart development. This could then be coupled to pharmacological examination of the response. Of course, it is also possible that cell damage resulting from higher doses of cocaine causes the downswing of the embryonic dose response curve. Future studies might also examine possible cytotoxic affects of cocaine on the developing heart in zebrafish.
Our results also suggest a possible correlation between physiological and behavioral sensitivity to cocaine in zebrafish. Previous studies have shown that the dose response to cocaine in behavioral tests is also a bell-shaped (Darland and Dowling, 2001; Darland et al., 2012). The maximal rewarding effect in these behavioral studies was 10mg/L, while the maximal cardiovascular effects in the present study was 5mg/L (although many high responses were seen at 10mg/L). The connection between acute cardiovascular response and behavioral response has not been extensively studied. One report comparing the subjective high feeling and cardiovascular response in humans demonstrated that the two coincided, but that the peak subjective response preceded the peak cardiovascular effect (Newton et al., 2005). It is possible then that the maximal rewarding effect occurs at a different dose than that which elicits maximal tachycardia. A number of studies have demonstrated how rodent strains differ in locomotor behavioral sensitivity to cocaine, and that acute locomotor response reflects the tendency to develop drug self-administration (Thomsen and Caine, 2011). In contrast, very few studies have examined the correspondence of acute cardiovascular sensitivity to cocaine and reward-based learning in rodents. One study compared acute locomotor and cardiovascular responses to cocaine, suggesting that the two roughly correspond (Ruth et al., 1988). The prediction would be then that a different cardiovascular response to cocaine might reflect reward-based learning and thus perhaps indicate vulnerability to addiction. In zebrafish the peak behavioral response to cocaine is at a dose that corresponds to a less than maximal cardiovascular response. It is possible that cocaine-induced tachycardia feeds back negatively on the reward pathways in the brain as has been recently found in rats (Mejias-Aponte and Kiyatkin, 2012). Although beyond the scope of this study, it would be interesting to explore if this correspondence holds in fish strains that differ in behavioral sensitivity to the drug (Darland and Dowling, 2001).
Acknowledgements
We are grateful for funding support by a University of North Dakota (UND) faculty start-up package including North Dakota EPSCoR, College of Arts and Sciences, Vice President of Research, the UND Biology Department, and the Department of Pathology at the UND School of Medicine and Health Sciences. This work was also supported by NSF REU Site Grant 0851869 and NIH Grant P20GM103442/P20RR016471. This publication was also made possible by Grant number U261HS0045-04-01 from the Indian Health Service, with the support of the National Institutes of health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Indian Health Service or the National Institutes of Health. We would also like to acknowledge Diane C. Darland for critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chaudhari GH, Chennubhotla KS, Chatti K, Kulkarni P. Optimization of the adult zebrafish ECG method for assessment of drug-induced QTc prolongation. Journal of Pharmacological and Toxicological Methods. 2013;67:115–120. doi: 10.1016/j.vascn.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Darland T, Dowling JE. Behavioral screening for cocaine sensitivity in mutagenized zebrafish. Proceedings of the National Academy of Sciences. 2001;98:11691–11696. doi: 10.1073/pnas.191380698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darland T, Mauch JT, Meier EM, Hagan SJ, Dowling JE, Darland DC. Sulpiride, but not SCH23390, modifies cocaine-induce conditioned place preference and expression of tyrosine hydroxylase and elongation factor 1a in zebrafish. Pharmacology, Biochemistry and Behavior. 2012;103:157–167. doi: 10.1016/j.pbb.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWN . In: The DAWN Report: Emergency Department Visits Involving Illicit Drug Use among Males. S.A.a.M.H.S. Administration, editor. Center for Behavioral Health Statistics and Quality; Rockville, MD: 2011. [Google Scholar]
- Egashira K, Morgan KG, Morgan JP. Effects of Cocaine on Excitation-Contraction Coupling of Aortic Smooth Muscle from the Ferret. Journal of Clinical Investigation. 1991;87:1322–1328. doi: 10.1172/JCI115135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier D, Narahashi T. Tricaine (MS-222): effects on ionic conductances of squid axon membranes. European Journal of Pharmacology. 1975;33:313–317. doi: 10.1016/0014-2999(75)90175-2. [DOI] [PubMed] [Google Scholar]
- Gillis RA, Hernandez YM, Erzouki HK, Raczkowski VF, Mandal AK, Kuhn FE, Dretchen KL. Sympathetic nervous system mediated cardiovascular effects of cocaine are primarily due to a peripheral site of action of the drug. Drug and Alcohol Dependence. 1995;37:217–230. doi: 10.1016/0376-8716(94)01087-2. [DOI] [PubMed] [Google Scholar]
- Gomes C, Henning M, Persson B, Trolin G. Interaction of alpha- and beta-adrenergic receptor blocking agents: circulatory effects in the conscious rat. Clin. Exp. Hypertens. 1978;1:141–165. doi: 10.3109/10641967809068601. [DOI] [PubMed] [Google Scholar]
- Hale S, Lehman M, Kloner R. Electrocardiographic abnormalities after acute administration of cocaine in the rat. American Journal of Cardiology. 1989;63:1529–1530. doi: 10.1016/0002-9149(89)90024-6. [DOI] [PubMed] [Google Scholar]
- Hassel D, Scholz EP, Trano N, Friedrich O, Just S, Meder B, Weiss DL, Zitron E, Marquart S, Vogel B, Karle CA, Seemann G, Fishman MC, Katus HA, Rottbauer W. Deficient Zebrafish Ether-a-Go-GO-Related Gene Channel Gating Causes Short-QT Syndrome in Zebrafish Reggae Mutants. Circulation. 2008;117:866–875. doi: 10.1161/CIRCULATIONAHA.107.752220. [DOI] [PubMed] [Google Scholar]
- Hoage T, Ding Y, Xu X. Quantifying Cardiac Functions in Embryonic and Adult Zebrafish. Methods in Molecular Biology. 2012;843:11–20. doi: 10.1007/978-1-61779-523-7_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N, Yost J, Clark EB. Cardiac Morphology and Blood Pressure in Adult Zebrafish. The Anatomical Record. 2001;264:1–12. doi: 10.1002/ar.1111. [DOI] [PubMed] [Google Scholar]
- Huang W-C, Hsieh Y-S, Chen I-H, Wang C-H, Chang HW, Yang C-C, Ku T-H, Yeh S-R, Chuang Y-J. Combined Use of MS-222 (Tricaine) and Isoflurane Extends Anesthesia Time and Minimizes Cardiac Rhythm Side Effects in Adult Zebrafish. Zebrafish. 2010;7:297–304. doi: 10.1089/zeb.2010.0653. [DOI] [PubMed] [Google Scholar]
- Klee EW, Schneider H, Clark K, Cousin M, Ebbert J, Hooten M, Karpyak V, Warner D, Ekker S. Zebrafish: A Model for the Study of Addiction Genetics. Human Genetics. 2012;131:977–1008. doi: 10.1007/s00439-011-1128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloner R, Hale S, Alker K, Rezkalla S. The effects of acute and chronic cocaine use on the heart. Circulation. 1992;85:407–419. doi: 10.1161/01.cir.85.2.407. [DOI] [PubMed] [Google Scholar]
- Maraj S, Figueredo VM, Morris DL. Cocaine and the Heart. Clinical Cardiology. 2010;33:264–269. doi: 10.1002/clc.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur P, Guo S. Use of the zebrafish as a model to understand mechanisms of addiction and complex neurobehavioral phenotypes. Neurobiol. Dis. 2010;40:66–72. doi: 10.1016/j.nbd.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejias-Aponte CA, Kiyatkin EA. Ventral tegmental area neurons are either excited or inhibited by cocaine’s actions in the peripheral nervous system. Neuroscience. 2012;207:182–197. doi: 10.1016/j.neuroscience.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon DV, Wang Z, Fadel PJ, Arbique D, Leonard D, Li J-L, Victor RG, Vongpatanasin W. Central Sympatholysis as a Novel Countermeasure for Cocaine-Induced Sympathetic Acitivation and Vasoconstriction in Humans. Journal of the American College of Cardiology. 2007;50:626–633. doi: 10.1016/j.jacc.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Milan DJ, Jones IL, Ellinor PT, MacRae CA. In vivo recording of adult zebrafish electrocardiogram and assessment of drug-indued QT prolongation. American Journal of Physiology and Heart Circulation Physiology. 2006;291:H269–H273. doi: 10.1152/ajpheart.00960.2005. [DOI] [PubMed] [Google Scholar]
- Milan DJ, Peterson TA, Ruskin JN, Peterson RT, MacRae CA. Drugs That Induce Repolarization Abnormalities Cause Bradycardia in Zebrafish. Circulation. 2003;107:1355–1358. doi: 10.1161/01.cir.0000061912.88753.87. [DOI] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, II, Kalechstein AD, Liam N. Cocaine and methamphetamine produce different patterns of subjective and cardiovascular effects. Pharmacology, Biochemistry and Behavior. 2005;82:90–97. doi: 10.1016/j.pbb.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Poon J, va den Buuse M. Autonomic mechanisms in the acute cardiovascular effects of cocaine in conscious rats. European Journal of Pharmacology. 1998;363:147–152. doi: 10.1016/s0014-2999(98)00804-8. [DOI] [PubMed] [Google Scholar]
- Przywara DA, Dambach GE. Direct Actions of Cocaine on Cardiac Cellular Electrical Activity. Circulation Research. 1989;65:185–192. doi: 10.1161/01.res.65.1.185. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Mead AN, Kosofsky BE. Increased vulnerability to self-administer cocaine in mice prenataly exposed to cocaine. Psychopharmacology. 2002;163:221–229. doi: 10.1007/s00213-002-1140-0. [DOI] [PubMed] [Google Scholar]
- Ruth JA, Ullman EA, Collins AC. An Analysis of Cocaine Effects on Locomotor Activities and Heart Rate in Four Inbred Mouse Strains. Pharmacology, Biochemistry and Behavior. 1988;29:157–162. doi: 10.1016/0091-3057(88)90289-4. [DOI] [PubMed] [Google Scholar]
- Sandblom E, Axelsson M. Baroreflex mediated control of heart rate and vascular capacitance in trout. Journal of Experimental Biology. 2005;208:821–829. doi: 10.1242/jeb.01470. [DOI] [PubMed] [Google Scholar]
- Schwartz BG, Rezkalla S, Kloner RA. Cardiovascular Effects of Cocaine. Circulation. 2010;122:2558–2569. doi: 10.1161/CIRCULATIONAHA.110.940569. [DOI] [PubMed] [Google Scholar]
- Stainier DY, Fouquet B, Chen J-N, Warren KS, Weinstein BM, Meiler SE, Mohideen M-APK, Neuhauss SC, Solnica-Krezel L, Schier AF, Zwartruis F, Stemple DL, Malicki J, Driever W, Fishman MC. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development. 1996;122:285–292. doi: 10.1242/dev.123.1.285. [DOI] [PubMed] [Google Scholar]
- Sun P, Zhang Y, Yu F, Parks E, Lyman A, Wu Q, Ai L, Hu C-H, Zhou Q, Shung K, Lien C-L, Hsiai TK. Micro-Electrocardiograms to Study Post-Ventricular Amputation of Zebrafish Heart. Annals of Biomedical Engineering. 2009;37:890–901. doi: 10.1007/s10439-009-9668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tella SR, Schindler CW, Goldberg SR. The Role of Central and Autonomic Neural Mechanisms in the Cardiovascular Effects of Cocaine in Conscious Squirrel Monkeys. Journal of Pharmacology and Experimental Therapeutics. 1990;252:491–499. [PubMed] [Google Scholar]
- Temma K, Komazu Y, Shiraki Y, Kitazawa T, Kondo H. The roles of alpha- and beta-adrenoreceptors in chronotropic responses to norepinephrine in carp heart (Cyprinus carpio) Comparative Biochemistry and Physiology C. 1989;92:149–153. doi: 10.1016/0742-8413(89)90218-1. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Psychomotor stimulant effects of cocaine in rats and 15 mouse strains. Experimental Clinical Psychopharmacology. 2011;19:321–341. doi: 10.1037/a0024798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N. In: Cocaine: Abuse and Addiction. N.I.o.D. Abuse, editor. Bethesda, MD: 2010. [Google Scholar]
- Vongpatanasin W, Mansour Y, Chavoshan B, Arbique D, Victor RG. Cocaine Stimulates the Human Cardiovascular System via a Central Mechanism of Action. Circulation. 1999;100:407–412. doi: 10.1161/01.cir.100.5.497. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. Fifth ed. Eugene University of Oregon Press; Eugene Oregon: 2007. [Google Scholar]