Abstract
Extracellular brain pH fluctuates in both physiological and disease conditions. The main postsynaptic proton receptor is the acid-sensing ion channels (ASICs). During the past decade, much progress has been made on protons, ASICs, and neurological disease. This review summarizes the recent progress on synaptic role of protons and our current understanding of how ASICs contribute to various types of neuronal injury in the brain.
Keywords: Proton, acidosis, ASIC, neuronal injury, synaptic plasticity
1. Introduction
Acid signaling has attracted more and more attention in recent years. Protons and their receptor-the acid-sensing ion channels (ASICs) play important roles in physiology and neurological diseases. This current review focuses on the role of ASICs in the central nervous system (for a review of ASICs in the periphery, see Deval et al in this issue). We mainly discuss two major aspects of protons: the well established role of acidosis in neuronal injury, and the more recent progress on acid signaling and neural plasticity. For an overview of additional roles of ASICs, see other reviews (Abboud and Benson, 2015; Holzer, 2015; Omerbasic et al., 2014) in this special issue of Neuropharmacology
2. Expression of ASICs in the brain
ASIC1a, 2a and 2b are the major subunits present in the brain, with the mRNA of ASIC1a and ASIC2 expressed widely throughout the brain (Garcia-Anoveros et al., 1997; Price et al., 1996; Waldmann et al., 1997b; Waldmann et al., 1996). These mRNA expression data are consistent with the immunlocalizaiton studies (Coryell et al., 2009; Price et al., 2014; Wemmie et al., 2003). Some regions, e.g., amygdala, somatosensory cortex, posterior cingular cortex, dentate gyrus in hippocampus, show higher levels of ASIC1a expression than others. For a review of ASIC1a expression in the nervous system, see (Zha, 2013). One recent study performed a thorough immunolocalization analysis of ASIC2 in the brain, and compared it side-by-side with that of ASIC1a (Price et al., 2014). The result shows that the expression of ASIC2 and ASIC1a proteins overlaps in most brain regions, with synaptically dense regions exhibit high staining of both ASIC1a and ASIC2. Consistent with wide expression of both ASIC1a and ASIC2, acid-activated current recorded from brain neurons suggest the contribution from both ASIC1a and ASIC2 in most cases (Alvarez de la Rosa et al., 2002; Askwith et al., 2004; Baron et al., 2002; Chu et al., 2004; Sherwood et al., 2011).
ASICs function as trimers (Jasti et al., 2007) (also see Grunder 2015; Askwith 2015), and can form homomeric and heteromeric channels. Homomeric and heteromeric ASICs show different pH sensitivity (Table 1) and pharmacological properties {Zha, 2013 #1323}. Since three subunits form one functional channel, it is interesting to ask whether heteromeric ASICs favor a specific subunit stoichiometry. One recent study shows that, when expressed in Xenopus oocytes, ASIC1a and 2a heteromers have no special preference for a specific subunit stoichiometry (Bartoi et al., 2014). This result suggests that the subunit stoichiometry in ASIC heteromers is mainly determined by the expression level of the subunits. However, it remains unclear what is the relative subunit ratio of different ASICs in the brain. This question is interesting because it will speak to the relative proportion of homomeric and heteromeric channels in the brain, and will provide insight into subunit stoichiometry of ASIC1a/2 heteromeric channels in vivo. The latter can be an important consideration for pharmacological targeting of ASICs in brain neurons (see also Baron 2015).
Table 1.
pH50 of ASIC homomeric and heteromeric channels.
| Subunit composition | 1a | 1b | 2a | 2b | 3 |
|---|---|---|---|---|---|
| 1a | 5.8-6.8 | 5.8-6.3 | 5.4-6.1 | 6.2-6.4 | 6.3-6.7 |
| 1b | 5.8-6.1 | 4.9 | N/A | 6.3-6.7 | |
| 2a | 3.8-4.5 | 4.8 | 5.6-6.1 | ||
| 2b | N/A | 6.5 | |||
| 3 | 6.3-6.7 |
pH sensitivity data are based on the following literature: (Alijevic and Kellenberger, 2012; Askwith et al., 2004; Babini et al., 2002; Benson et al., 2002; Chen et al., 2005; Hattori et al., 2009; Hesselager et al., 2004; Poirot et al., 2004; Salinas et al., 2005; Sherwood et al., 2011; Sutherland et al., 2001; Waldmann et al., 1997a). ASIC4 is not included in this table because mammalian ASIC4 does not appear to contribute to acid-activated current.
Besides ASIC1a and ASIC2, some other ASIC family members are also present in the brain, but typically expressed in a more restricted pattern. For example, BLiNaC/BASIC, which stands for Brain Liver Intestine Na+ Channel (Schaefer et al., 2000), is expressed in unipolar brush cells in the ventral uvula and nodulus of the vestibulocerebellum (Boiko et al., 2014). ASIC3 is another subunit that is present in specialized structures in the brain (Babinski et al., 1999; Delaunay et al., 2012; Meng et al., 2009; Wang et al., 2014). The functional importance of these subunits in the brain has not been thoroughly studied.
3. Synaptic role of ASICs
The idea that protons can function as a neurotransmitter has existed for some time. However, it is only recently that we start to obtain evidence directly supporting this hypothesis. In this section, we summarize the recent development on protons as a neurotransmitter, and our current understanding of protons in synaptic physiology and structural remodeling, and the role of ASICs in fear and anxiety.
3.1. Proton-ASIC as a neurotransmitter-receptor pair
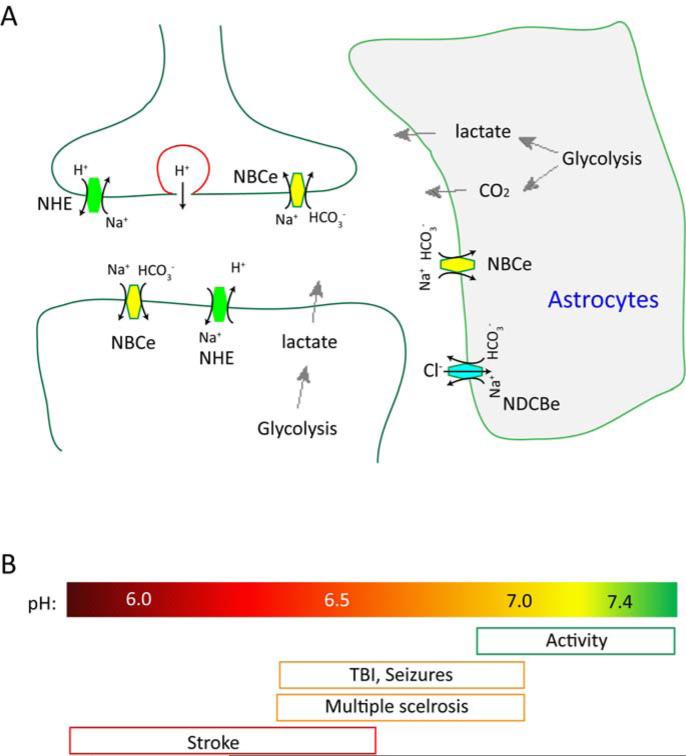
Extracellular acidification can occur during both physiological and disease conditions. Figure 1A illustrates the potential contributors to changes in extracellular proton concentrations. These include glycolysis in neurons, direct release from presynaptic vesicles, lactate and CO2 production from glial cells, and changed transporter activities in both neurons and glia (Deitmer, 2002; Du et al., 2014; Grichtchenko and Chesler, 1994; Highstein et al., 2014; Krishtal et al., 1987; Miesenbock et al., 1998; Obara et al., 2008; Siesjo, 1982; Siesjo and Wieloch, 1986; Voipio and Kaila, 1993). Many of the same machineries reside around synaptic region as well. Previous studies have assessed the magnitude of pH reduction at synaptic cleft. One approach is based on the fact that acidification inhibits voltage-gated calcium channels (VGCCs). Several groups measured the reduction in presynaptic Ca2+ current during neurotransmission, and back-calculated the reduction of synaptic cleft pH to be about 0.2-0.6 pH units (DeVries, 2001; Palmer et al., 2003; Vessey et al., 2005). As discussed below, this magnitude of pH reduction is big enough to regulate several ion channels.
Figure 1. Source of protons and the magnitude of pH changes in the brain.
(A) Illustration showing the major contributors to extracellular pH reduction in the brain. Increased glycolysis leads to lactate and CO2 production, which can acidify interstitial space. In addition, several exchangers actively regulate extracellular pH. Abbreviations: NBCe Na+/bicarbonate cotransporter; NDCBe Na+-dependent bicarbonate-chloride exchanger; NHE: Na+- H+ exchanger. (B) Illustration showing the magnitude of pH changes in various conditions. Note that neural activity can lead to both alkalinization and acidification.
A decrease in pH affects multiple proteins. Besides the above mentioned VGCCs, acidification also inhibits one important class of synaptic channels, the NMDA receptors, with a pKa of 6.9 (Tang et al., 1990; Traynelis and Cull-Candy, 1990). The effects on VGCCs and NMDA receptors are modulatory, and lead to an attenuation of synaptic transmission. In contrast, protons also directly gate several ion channels, including ASICs and TrpV1. However, TrpV1 does not start to open until pH 6.4, and its pH50 is about 5.4-5.7 (Ryu et al., 2007; Tominaga et al., 1998). Thus, TrpV1 is unlikely to act as a proton receptor in physiological pH ranges. ASICs are much more pH sensitive, can be activated at pH <= 7.0, and the pH50 values for ASIC1a and 3 are around 6.5 (see Table 1). These properties make ASICs a perfect candidate for sensing small to moderate pH reduction in the brain. The subcellular localization of ASICs further supports their role as a neuronal proton receptor (see (Zha, 2013). ASIC1a and ASIC2a show a preferential somatodendritic distribution (Zha et al., 2009; Zha et al., 2006). Moreover, both subunits are enriched in brain synaptosomes (Wemmie et al., 2002; Zha et al., 2009). Immunofluorescence staining further shows a preferential targeting of ASIC1a to dendritic spines (Jing et al., 2012). Consistent with their localization, acute pH 6 application induce [Ca2+]i rise in dendrites and dendritic spines, and this effect depends upon ASIC1a (Zha et al., 2009; Zha et al., 2006).
Although ASICs localize to dendritic region, early attempts to record ASIC-specific currents during neurotransmission have not been successful (Alvarez de la Rosa et al., 2003). Recently, several groups revisited this idea and have provided new evidence for protons to function as a neurotransmitter and regulate synaptic physiology (Beg et al., 2008; Du et al., 2014; Highstein et al., 2014; Kreple et al., 2014). In turtle lagena, the equivalent of vestibular system in mammals (Highstein et al., 2014), stimulated release from hair cells acidify the synaptic cleft at hair cell calyx and induces excitatory postsynaptic potential (EPSP). In amygdala, increased activity induces pH reduction at the cleft (Du et al., 2014). Moreover, ASIC1a activity contributes to EPSP in both amygdala and nucleus accumbens (Du et al., 2014; Kreple et al., 2014). Increased pH buffering attenuates the above acid-activated responses while decreased pH buffering has the opposite effect. A similar situation in the nematode shows that protons can function as a neurotransmitter and regulate muscle contraction (Beg et al., 2008).
Consistent with a role as postsynaptic proton receptor, ASICs regulate structural remodeling of synaptic sites. Overexpression of a human ASIC1a, either acutely or chronically, increases the density of dendritic spines in hippocampal slices (Zha et al., 2006). Conversely, ASIC2 deletion or acute knockdown of ASIC1a leads to spine reduction (Zha et al., 2009; Zha et al., 2006). Chronic knockout of ASIC1a, however, has no apparent effect on baseline spine density in organotypic hippocampal slices. In contrast, one recent study showed that deleting ASIC1a increases spine density in nuclear accumbens (Kreple et al., 2014). These data indicate that ASICs are important for spine remodeling, although the exact effect depends on the system studied. Previous studies have further examined the effect of ASIC1 deletion on baseline transmission. Deleting the ASIC1a gene has no effect on basal levels of GABA, AMPA and NMDA currents (Cho and Askwith, 2008; Wemmie et al., 2002), but alters the ratio of AMPA:NMDA current. ASIC deletion reduces the AMPA:NMDA ratio in a microisland hippocampal neuron culture but increases the ratio in nucleus accumbens neurons (Cho and Askwith, 2008; Kreple et al., 2014).
In summary, neural activity can acidify synaptic cleft and protons can function as a neurotransmitter. The pH sensitivity and dendritic distribution of ASICs put them in a perfect position to sense pH reduction around pH 7-6. Lastly, both electrophysiological recordings and morphological studies on spines support that ASICs are the main postsynaptic proton receptors in the brain.
3.2. ASICs and synaptic plasticity
While protons activate an inward current at postsynaptic cells, the amplitude of the proton-activated EPSCs is small, about 15-20 times less compared to that generated from glutamate receptors (Du et al., 2014; Highstein et al., 2014; Kreple et al., 2014). These data provide one explanation for why some previous attempts failed to detect a specific proton-activated component during neurotransmission (Alvarez de la Rosa et al., 2003). However, this component probably functions as a boosting mechanism for neurotransmission, and is important for the generation of long-term potentiation (LTP) in amygdala (Du et al., 2014). These results are consistent with a previous report, which shows a similar attenuation of LTP in ASIC1a null hippocampal slices (Wemmie et al., 2002). It is important to note that, while proton inhibits NMDA receptors directly, activation of ASIC1a and the resulting membrane depolarization could help relieve voltage-dependent blockade of NMDA receptors by Mg2+, thus facilitating the activation of NMDA receptors and the expression of LTP. Similar to the effect on LTP, deleting the ASIC1a gene reduces paired-pulse responses in microisland hippocampal cultures (Cho and Askwith, 2008). However, the effect on LTP depends on ASIC1a while that on paired-pulse facilitation is not sensitive to amiloride, suggesting that the effect on paired-pulse could be secondary to chronic changes in the knockout. In contrast to the above findings, one study using a CRE-mediated knockout of ASIC1a does not observe differences in hippocampal LTP between WT and ASIC1a knockout (Wu et al., 2013). These data suggest that ASICs can mediate proton signaling at synaptic sites, but the exact contribution of protons and ASICs to synaptic plasticity may vary, depending on the system and condition.
3.3. ASICs in fear and anxiety
The above studies implicate that ASICs regulate brain physiology. One interesting observation is that ASIC1a and ASIC2 exhibit high levels of expression in “fear structures” such as amygdala and bed nucleus of the stria terminalis (BNST) (Coryell et al., 2007; Price et al., 2014; Wemmie et al., 2003). These data suggest an important role of ASICs in fear and related behavior. Several previous reviews have covered this topic in detail (Sluka et al., 2009; Wemmie et al., 2013). Here, we summarize the key observations and highlight a possible difference between systems. In mice, deleting ASIC1a or inhibiting ASIC1a activity makes the animals less fearful to both conditioned and unconditioned fear. ASIC1a null mice exhibit a reduced acoustic startle, and a reduced freezing in response to CO2 and TMT, a predator odor (Coryell et al., 2008; Coryell et al., 2007; Ziemann et al., 2009). ASIC1a deletion also reduces learned fear (Wemmie et al., 2003). Viral-mediated expression of ASIC1a in the ASIC1a null mice in amygdala rescues conditioned but not unconditioned fear (Coryell et al., 2008). In another study, site-specific deletion of ASIC1a in BNST is sufficient to abolish CO2-induced freezing (Taugher et al., 2014). These data indicate that ASICs in different structures have differential effects on fear induced by specific modality.
Consistent with a role in fear, ASIC1a contributes to anxiety and depression-related behavior. In mice, deleting the ASIC1a gene or inhibiting ASIC1a shows an anti-depression effect in several assays, including open field test, tail suspension test and forced swim test (Coryell et al., 2008; Coryell et al., 2009). In contrast to the data from mice, injection of ammonium directly into amygdala in rat, which elicits ASIC-type current in these neurons, reduces center avoidance in the open field test and prolonged the time spent in the light compartment in the light/dark test (Pidoplichko et al., 2014). In addition, injecting Psalmotoxin-1 (PcTx1), a specific blocker of 1a-containing ASIC channels (Escoubas et al., 2000), into amygdala in rat increases center avoidance in the open field test. Thus, ASIC activation in rat has anxiolytic effect, which contrasts with the results obtained from the mice. These data indicate that ASICs are clearly important for fear and depression-type behavior, but the exact role they play may depend upon the paradigm.
The role of protons and ASICs in human brain function remains uncertain. In a recent study, Magnotta et al used magnetic resonance imaging to measure changes in proton concentration in the brain (Magnotta et al., 2012). The result shows that normal learning process is sufficient to induce acidification in the brain. Although the magnitude of pH changes in this study is unclear, the data suggests that acid signaling contributes to neural plasticity during normal learning process and possibly to long-term changes in various pathological conditions in human subjects.
4. ASICs and neuronal injury
Extracellular acidosis typically occurs in various injurious conditions in the brain. The range of pH reduction varies, from about pH 6.5 in multiple sclerosis to below pH 6.0 in severe ischemia (Figure 1B). As the main neuronal proton receptor in neurons, ASICs play a critical role in neuronal injury associated with acidosis. We will summarize the main findings in brain ischemia, multiple sclerosis, traumatic brain injury and Parkinson's disease.
4.1. Ischemic brain injury
Multiple mechanisms contribute to ischemia-induced brain injury. Excitotoxicity as a result of massive glutamate release is one main cause of early neurotoxicity (Lai et al., 2014). Following ischemiareperfusion, there is a large increase in reactive oxygen species accompanied by a large reduction in brain pH (Hertz, 2008; Siesjo et al., 1993). Brain pH can reduce to about pH 6.5 in a mild ischemia and to pH 6.0 or lower in severe ischemia (Xiong et al., 2007). These pH reductions are large enough for robust activation of ASIC1a containing channels (see Table 1). Indeed, ASIC1a plays a critical role in ischemia-induced neuronal injury. Deleting the ASIC1a gene protects mice from brain injury induced by middle cerebral artery occlusion (MCAO) (Pignataro et al., 2007; Xiong et al., 2007). Amiloride, a non-specific ASIC inhibitor, and PcTx1, have similar protective effect. The time window for this effect is long, with protection observed up to 5 hrs after MCAO (Pignataro et al., 2007). In addition, one previous study showed that activation of NMDA receptors, through the recruitment of calcium/calmodulin-dependent protein kinase II (CaMKII) signaling, increases ASIC1a current and potentiates ischemia-induced neuronal injury (Gao et al., 2005). Consistent with this result, the protective effect from ASIC inhibition is additive to that of inhibiting NMDA receptors (Mishra et al., 2011; Pignataro et al., 2007). These data suggest that targeting ASICs, especially when combined with other interventions, may be an effective therapeutic strategy for alleviating ischemia-induced brain injury.
It is interesting to note that some chemicals that are present in the brain and/or released during ischemia modulate ASIC channels. Lactate potentiates ASIC currents by “chelating”, thus reducing, the effective concentration of extracellular cations (Immke and McCleskey, 2001). Sperimine is one endogenous polyamine that is found at high concentrations in the brain. Spermine shifts the steady state inactivation of ASIC1a, probably due to a stabilization of the channel at resting state (Babini et al., 2002), and potentiates ischemia-induced injury in the brain (Duan et al., 2011). Inhibiting spermine synthesis with difluromethylornithine (DFMO) attenuated ASIC1a channel-mediated ischemic injury. Dynorphins are another class of endogenous peptides in the brain. Depending on the target, these peptides can be either protective or detrimental to neurons in disease (for a review, see (Hauser et al., 2005). Dynorphin reduces steady-state desensitization of ASIC1a and increases acid-activated current in brain neurons, with an EC50 of 26 nM (Sherwood and Askwith, 2009). In dissociated cultures, 1 μM Dynorphin potentiates acidosis-induced neuronal injury. The exact extracellular dynorphin concentration in the brain remains unclear and the consequence of ischemia on dynorphin levels remains controversial (Andrews et al., 1988; Cheung and Cechetto, 1995; Fried and Nowak, 1987). However, a rat brain, which has a density of ~1g/cm3 (Bishop and Wahlsten, 1999; Tajima et al., 1993), contains 10-100 fmol dynorphin per mg tissue (Andrews et al., 1988; Goldstein and Ghazarossian, 1980). Thus, the overall concentration of dynorphin in a rat brain is about 30-300 μM, which suggests that endogneous dynorphin can reach a level to potentiate ASIC currents. In reality, probably multiple endogenous factors work together to regulate ASIC function and acid-induced injury.
4.2. Multiple sclerosis
Multiple sclerosis (MS) is another type of central nervous system disorder associated with prolonged inflammation and acidification. In a mouse experimental autoimmune encephalomyelitis (EAE) model, pH in spinal cord was about pH 6.5-6.6 at 15 days post immunization (Friese et al., 2007), suggesting that there is a prolonged period of acidosis in this paradigm. In healthy axons, ASIC1a expression is relatively low (Vergo et al., 2011; Zha et al., 2009; Zha et al., 2006). However, in both an animal EAE model and human MS patients, inflammation leads to an increase of ASIC1a expression or trafficking to axons, as well as an upregulation of ASIC1a in oligodendrocytes (Arun et al., 2013; Vergo et al., 2011). Deleting the ASIC1a gene or inhibiting ASIC1a reduces EAE-induced axonal injury and improves the clinical score (Friese et al., 2007). Amiloride alleviates the severity of the disease, either when applied in the acute model or at the phase of first relapse (Vergo et al., 2011). These data indicate that ASICs mediate inflammation-induced axonal degeneration. A recent three-year clinical study followed up on a group of patient with progressive MS (Arun et al., 2013). Patients were imaged with MRI before, during and after a one-year long oral treatment with amiloride. The result shows that amiloride treatment significantly reduces the rate of brain atrophy, and improves scores reflecting axonal damage and myelin loss. While there are many issues that need to be addressed, this report is exciting because it is the first translational study that illustrates a promising neuroprotective effect of inhibiting ASICs.
4.3. Traumatic Neuronal Injury
Traumatic brain injury (TBI) and spinal cord injury (SCI) affect millions of people worldwide. Following the initial acute phase of injury, extensive neuronal injury can happen during the secondary phase. Multiple mechanisms contribute to the secondary injury observed following trauma. One important factor is the decrease in extracellular pH. Brain pH reduces to about 6.7-6.9 in rodent TBI models and human TBI patients (Gupta et al., 2004; Vink et al., 1987; Yin et al., 2013). Of note, these pH measurements were performed with Magnetic resonance spectroscopy, microelectrode or microdevice, and thus detect the pH changes of at the tissue level. Given that traumatic injuries typically lead to massive disruption of metabolism, it is conceivable that localized pH reduction at the cellular level can be more severe. In TBI patients, the degree of pH reduction is correlated with the outcome, with lower pH associated with higher lethality (Gupta et al., 2004; Timofeev et al., 2011; Zygun et al., 2004). In a mouse lateral fluid percussion injury model of TBI, attenuating pH reduction with bicarbonate alleviates neuronal injury (Yin et al., 2013). These observations suggest that the reduction in pH apparently is one causal factor of TBI-induced secondary injury. In a rat model of TSCI, ASIC1a expression was acutely increased in the penumbra region (Hu et al., 2011). In both the mouse TBI and rat TSCI models, inhibiting ASIC1a with amiloride or PcTx1 alleviates the severity of the injury (Hu et al., 2011; Yin et al., 2013). Together, these results indicate that ASICs play an important role in traumatic injury. Therefore, inhibiting or down-regulating ASICs can be a therapeutic approach to treat traumatic neuronal injuries.
4.4. Parkinson's Disease
Parkinson's Disease (PD) is common neurodegenerative disease that is characterized by motor impairment, mainly due to a loss of dopaminergic neurons in the substantia nigra (Dauer and Przedborski, 2003). However, the mechanisms that lead to the loss of dopaminergic neurons remain unclear. In PD, increased oxidative stress inhibits mitochondria function and disrupts energy metabolism (Mattson et al., 1999), which results in an increase in lactate production and acidosis (Koga et al., 2006). Neurons in substantia nigra express ASICs and exhibit typical ASIC-type current (Arias et al., 2008; Pidoplichko and Dani, 2006). In a mouse PD model induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), ASIC inhibition with amiloride or PcTx1 attenuates the reduction of immunoreactivity of tyrosine hydroxylase and dopamine transporter- both are markers for dopaminergic neurons (Arias et al., 2008). This result suggests that ASIC-mediated responses contribute to loss of dompaminergic neurons in PD. Since many other degenerative diseases are associated with pH reduction (Chu and Xiong, 2012), it is conceivable that inhibiting ASICs may have similar protective effects in neurodegenerative diseases in general.
5. Summary and speculation
Protons have emerged as an important signaling molecule in neurons. As illustrated in Figure 1A, multiple processes contribute to an increase in extracellular proton concentration. This leads to acidification and inhibition of some synaptic receptors, including NMDA receptor and VGCCs. While these typically dampens synaptic activity, the activation of ASIC family ion channels leads to increased calcium-dependent signaling and boosts neural activity. Depending on the magnitude and duration of acidification, this ASIC-dependent signaling can contribute to synaptic physiology and induce structural changes of the synaptic sites, or contribute to neuronal injury in various neurological disorders. This scheme, though simplified, highlights the importance of protons in both synaptic plasticity and brain injury. It will be interesting to determine what additional players are involved in acid signaling in neurons, and to determine the cross-talk between ASICs and other synaptic signaling pathways. Studies along this line will reveal a more dynamic picture of proton signaling, and will provide important insight into brain physiology and disease.
Highlights.
We discussed the general expression of ASICs in the brain.
We discussed the synaptic role of ASICs in the brain.
We discussed the role of ASICs in neuronal injury.
Acknowledgement
This work is partially supported by NIH R01NS066027, U54NS083932, NIMHD S21MD000101 (ZGX) and American Heart Association 13SDG13970009 (XMZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alijevic O, Kellenberger S. Subtype-specific Modulation of Acid-sensing Ion Channel (ASIC) Function by 2-Guanidine-4-methylquinazoline. J Biol Chem. 2012;287:36059–36070. doi: 10.1074/jbc.M112.360487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez de la Rosa D, Krueger SR, Kolar A, Shao D, Fitzsimonds RM, Canessa CM. Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system. J Physiol. 2003;546:77–87. doi: 10.1113/jphysiol.2002.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez de la Rosa D, Zhang P, Shao D, White F, Canessa CM. Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system. Proc Natl Acad Sci U S A. 2002;99:2326–2331. doi: 10.1073/pnas.042688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews BT, McIntosh TK, Gonzales MF, Weinstein PR, Faden AI. Levels of endogenous opioids and effects of an opiate antagonist during regional cerebral ischemia in rats. J Pharmacol Exp Ther. 1988;247:1248–1254. [PubMed] [Google Scholar]
- Arias RL, Sung ML, Vasylyev D, Zhang MY, Albinson K, Kubek K, Kagan N, Beyer C, Lin Q, Dwyer JM, Zaleska MM, Bowlby MR, Dunlop J, Monaghan M. Amiloride is neuroprotective in an MPTP model of Parkinson's disease. Neurobiol Dis. 2008 doi: 10.1016/j.nbd.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Arun T, Tomassini V, Sbardella E, de Ruiter MB, Matthews L, Leite MI, Gelineau-Morel R, Cavey A, Vergo S, Craner M, Fugger L, Rovira A, Jenkinson M, Palace J. Targeting ASIC1 in primary progressive multiple sclerosis: evidence of neuroprotection with amiloride. Brain. 2013;136:106–115. doi: 10.1093/brain/aws325. [DOI] [PubMed] [Google Scholar]
- Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem. 2004;279:18296–18305. doi: 10.1074/jbc.M312145200. [DOI] [PubMed] [Google Scholar]
- Abboud FM, Benson CJ. ASICs and Cardiovascular Homeostasis. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babini E, Paukert M, Geisler HS, Grunder S. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1). J Biol Chem. 2002;277:41597–41603. doi: 10.1074/jbc.M205877200. [DOI] [PubMed] [Google Scholar]
- Babinski K, Le KT, Seguela P. Molecular cloning and regional distribution of a human proton receptor subunit with biphasic functional properties. J Neurochem. 1999;72:51–57. doi: 10.1046/j.1471-4159.1999.0720051.x. [DOI] [PubMed] [Google Scholar]
- Baron A, Waldmann R, Lazdunski M. ASIC-like, proton-activated currents in rat hippocampal neurons. J Physiol. 2002;539:485–494. doi: 10.1113/jphysiol.2001.014837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoi T, Augustinowski K, Polleichtner G, Grunder S, Ulbrich MH. Acid-sensing ion channel (ASIC) 1a/2a heteromers have a flexible 2:1/1:2 stoichiometry. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1324060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg AA, Ernstrom GG, Nix P, Davis MW, Jorgensen EM. Protons act as a transmitter for muscle contraction in C. elegans. Cell. 2008;132:149–160. doi: 10.1016/j.cell.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci U S A. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop KM, Wahlsten D. Sex and species differences in mouse and rat forebrain commissures depend on the method of adjusting for brain size. Brain Res. 1999;815:358–366. doi: 10.1016/s0006-8993(98)01088-9. [DOI] [PubMed] [Google Scholar]
- Boiko N, Kucher V, Wang B, Stockand JD. Restrictive expression of Acid-sensing ion channel 5 (asic5) in unipolar brush cells of the vestibulocerebellum. PLoS One. 2014;9:e91326. doi: 10.1371/journal.pone.0091326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Kalbacher H, Grunder S. The tarantula toxin psalmotoxin 1 inhibits acid-sensing ion channel (ASIC) 1a by increasing its apparent H+ affinity. J Gen Physiol. 2005;126:71–79. doi: 10.1085/jgp.200509303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung RT, Cechetto DF. Neuropeptide changes following excitotoxic lesion of the insular cortex in rats. J Comp Neurol. 1995;362:535–550. doi: 10.1002/cne.903620408. [DOI] [PubMed] [Google Scholar]
- Cho JH, Askwith CC. Presynaptic Release Probability Is Increased in Hippocampal Neurons From ASIC1 Knockout Mice. J Neurophysiol. 2008;99:426–441. doi: 10.1152/jn.00940.2007. [DOI] [PubMed] [Google Scholar]
- Chu XP, Wemmie JA, Wang WZ, Zhu XM, Saugstad JA, Price MP, Simon RP, Xiong ZG. Subunit-dependent high-affinity zinc inhibition of acid-sensing ion channels. J Neurosci. 2004;24:8678–8689. doi: 10.1523/JNEUROSCI.2844-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu XP, Xiong ZG. Physiological and pathological functions of Acid-sensing ion channels in the central nervous system. Curr Drug Targets. 2012;13:263–271. doi: 10.2174/138945012799201685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell MW, Wunsch AM, Haenfler JM, Allen JE, McBride JL, Davidson BL, Wemmie JA. Restoring Acid-sensing ion channel-1a in the amygdala of knock-out mice rescues fear memory but not unconditioned fear responses. J Neurosci. 2008;28:13738–13741. doi: 10.1523/JNEUROSCI.3907-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell MW, Wunsch AM, Haenfler JM, Allen JE, Schnizler M, Ziemann AE, Cook MN, Dunning JP, Price MP, Rainier JD, Liu Z, Light AR, Langbehn DR, Wemmie JA. Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J Neurosci. 2009;29:5381–5388. doi: 10.1523/JNEUROSCI.0360-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell MW, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM, Price M, Schnizler MK, Wemmie JA. Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biol Psychiatry. 2007;62:1140–1148. doi: 10.1016/j.biopsych.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Deitmer JW. A role for CO(2) and bicarbonate transporters in metabolic exchanges in the brain. J Neurochem. 2002;80:721–726. doi: 10.1046/j.0022-3042.2002.00765.x. [DOI] [PubMed] [Google Scholar]
- Delaunay A, Gasull X, Salinas M, Noel J, Friend V, Lingueglia E, Deval E. Human ASIC3 channel dynamically adapts its activity to sense the extracellular pH in both acidic and alkaline directions. Proc Natl Acad Sci U S A. 2012;109:13124–13129. doi: 10.1073/pnas.1120350109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH. Exocytosed protons feedback to suppress the Ca2+ current in mammalian cone photoreceptors. Neuron. 2001;32:1107–1117. doi: 10.1016/s0896-6273(01)00535-9. [DOI] [PubMed] [Google Scholar]
- Du J, Reznikov LR, Price MP, Zha XM, Lu Y, Moninger TO, Wemmie JA, Welsh MJ. Protons and ASICs are a Neurotransmitter/Receptor Pair That Regulates Synaptic Plasticity in the Lateral Amygdala. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1407018111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B, Wang YZ, Yang T, Chu XP, Yu Y, Huang Y, Cao H, Hansen J, Simon RP, Zhu MX, Xiong ZG, Xu TL. Extracellular spermine exacerbates ischemic neuronal injury through sensitization of ASIC1a channels to extracellular acidosis. J Neurosci. 2011;31:2101–2112. doi: 10.1523/JNEUROSCI.4351-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoubas P, De Weille JR, Lecoq A, Diochot S, Waldmann R, Champigny G, Moinier D, Menez A, Lazdunski M. Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem. 2000;275:25116–25121. doi: 10.1074/jbc.M003643200. [DOI] [PubMed] [Google Scholar]
- Fried RL, Nowak TS., Jr. Opioid peptide levels in gerbil brain after transient ischemia: lasting depletion of hippocampal dynorphin. Stroke. 1987;18:765–770. doi: 10.1161/01.str.18.4.765. [DOI] [PubMed] [Google Scholar]
- Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, Vincent A, Fugger L. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med. 2007;13:1483–1489. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, Xu TL. Coupling between NMDA Receptor and Acid-Sensing Ion Channel Contributes to Ischemic Neuronal Death. Neuron. 2005;48:635–646. doi: 10.1016/j.neuron.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Garcia-Anoveros J, Derfler B, Neville-Golden J, Hyman BT, Corey DP. BNaC1 and BNaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proc Natl Acad Sci U S A. 1997;94:1459–1464. doi: 10.1073/pnas.94.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A, Ghazarossian VE. Immunoreactive dynorphin in pituitary and brain. Proc Natl Acad Sci U S A. 1980;77:6207–6210. doi: 10.1073/pnas.77.10.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichtchenko II, Chesler M. Depolarization-induced alkalinization of astrocytes in gliotic hippocampal slices. Neuroscience. 1994;62:1071–1078. doi: 10.1016/0306-4522(94)90344-1. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Zygun DA, Johnston AJ, Steiner LA, Al-Rawi PG, Chatfield D, Shepherd E, Kirkpatrick PJ, Hutchinson PJ, Menon DK. Extracellular Brain pH and Outcome following Severe Traumatic Brain Injury. J Neurotrauma. 2004;21:678–684. doi: 10.1089/0897715041269722. [DOI] [PubMed] [Google Scholar]
- Hattori T, Chen J, Harding AM, Price MP, Lu Y, Abboud FM, Benson CJ. ASIC2a and ASIC3 heteromultimerize to form pH-sensitive channels in mouse cardiac dorsal root ganglia neurons. Circ Res. 2009;105:279–286. doi: 10.1161/CIRCRESAHA.109.202036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Aldrich JV, Anderson KJ, Bakalkin G, Christie MJ, Hall ED, Knapp PE, Scheff SW, Singh IN, Vissel B, Woods AS, Yakovleva T, Shippenberg TS. Pathobiology of dynorphins in trauma and disease. Front Biosci. 2005;10:216–235. doi: 10.2741/1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L. Bioenergetics of cerebral ischemia: a cellular perspective. Neuropharmacology. 2008;55:289–309. doi: 10.1016/j.neuropharm.2008.05.023. [DOI] [PubMed] [Google Scholar]
- Hesselager M, Timmermann DB, Ahring PK. pH Dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem. 2004;279:11006–11015. doi: 10.1074/jbc.M313507200. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Holstein GR, Mann MA, Rabbitt RD. Evidence that protons act as neurotransmitters at vestibular hair cell-calyx afferent synapses. Proc Natl Acad Sci U S A. 2014;111:5421–5426. doi: 10.1073/pnas.1319561111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Duan B, Wang D, Yu Y, Li W, Luo H, Lu P, Lin J, Zhu G, Wan Q, Feng H. Role of acid-sensing ion channel 1a in the secondary damage of traumatic spinal cord injury. Ann Surg. 2011;254:353–362. doi: 10.1097/SLA.0b013e31822645b4. [DOI] [PubMed] [Google Scholar]
- Holzer P. Acid-Sensing Ion Channels in Gastrointestinal Function. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4:869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- Jing L, Chu XP, Jiang YQ, Collier DM, Wang B, Jiang Q, Snyder PM, Zha XM. N-glycosylation of acid-sensing ion channel 1a regulates its trafficking and acidosis-induced spine remodeling. J Neurosci. 2012;32:4080–4091. doi: 10.1523/JNEUROSCI.5021-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Mori A, Ohashi S, Kurihara N, Kitagawa H, Ishikawa M, Mitsumoto Y, Nakai M. H MRS identifies lactate rise in the striatum of MPTP-treated C57BL/6 mice. Eur J Neurosci. 2006;23:1077–1081. doi: 10.1111/j.1460-9568.2006.04610.x. [DOI] [PubMed] [Google Scholar]
- Kreple CJ, Lu Y, Taugher RJ, Schwager-Gutman AL, Du J, Stump M, Wang Y, Ghobbeh A, Fan R, Cosme CV, Sowers LP, Welsh MJ, Radley JJ, LaLumiere RT, Wemmie JA. Acid-sensing ion channels contribute to synaptic transmission and inhibit cocaine-evoked plasticity. Nat Neurosci. 2014;17:1083–1091. doi: 10.1038/nn.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal OA, Osipchuk YV, Shelest TN, Smirnoff SV. Rapid extracellular pH transients related to synaptic transmission in rat hippocampal slices. Brain Res. 1987;436:352–356. doi: 10.1016/0006-8993(87)91678-7. [DOI] [PubMed] [Google Scholar]
- Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol. 2014;115:157–188. doi: 10.1016/j.pneurobio.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Heo HY, Dlouhy BJ, Dahdaleh NS, Follmer RL, Thedens DR, Welsh MJ, Wemmie JA. Detecting activity-evoked pH changes in human brain. Proc Natl Acad Sci U S A. 2012;109:8270–8273. doi: 10.1073/pnas.1205902109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Pedersen WA, Duan W, Culmsee C, Camandola S. Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer's and Parkinson's diseases. Ann N Y Acad Sci. 1999;893:154–175. doi: 10.1111/j.1749-6632.1999.tb07824.x. [DOI] [PubMed] [Google Scholar]
- Meng QY, Wang W, Chen XN, Xu TL, Zhou JN. Distribution of acid-sensing ion channel 3 in the rat hypothalamus. Neuroscience. 2009;159:1126–1134. doi: 10.1016/j.neuroscience.2009.01.069. [DOI] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Mishra V, Verma R, Singh N, Raghubir R. The neuroprotective effects of NMDAR antagonist, ifenprodil and ASIC1a inhibitor, flurbiprofen on post-ischemic cerebral injury. Brain Res. 2011;1389:152–160. doi: 10.1016/j.brainres.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Obara M, Szeliga M, Albrecht J. Regulation of pH in the mammalian central nervous system under normal and pathological conditions: facts and hypotheses. Neurochem Int. 2008;52:905–919. doi: 10.1016/j.neuint.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Omerbasic D, Schuhmacher LN, Bernal Sierra YA, Smith ES, Lewin GR. ASICs and mammalian mechanoreceptor function. Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Palmer MJ, Hull C, Vigh J, von Gersdorff H. Synaptic cleft acidification and modulation of short-term depression by exocytosed protons in retinal bipolar cells. J Neurosci. 2003;23:11332–11341. doi: 10.1523/JNEUROSCI.23-36-11332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoplichko VI, Aroniadou-Anderjaska V, Prager EM, Figueiredo TH, Almeida-Suhett CP, Miller SL, Braga MF. ASIC1a Activation Enhances Inhibition in the Basolateral Amygdala and Reduces Anxiety. J Neurosci. 2014;34:3130–3141. doi: 10.1523/JNEUROSCI.4009-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoplichko VI, Dani JA. Acid-sensitive ionic channels in midbrain dopamine neurons are sensitive to ammonium, which may contribute to hyperammonemia damage. Proc Natl Acad Sci U S A. 2006;103:11376–11380. doi: 10.1073/pnas.0600768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignataro G, Simon RP, Xiong ZG. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain. 2007;130:151–158. doi: 10.1093/brain/awl325. [DOI] [PubMed] [Google Scholar]
- Poirot O, Vukicevic M, Boesch A, Kellenberger S. Selective regulation of acid-sensing ion channel 1 by serine proteases. J Biol Chem. 2004;279:38448–38457. doi: 10.1074/jbc.M407381200. [DOI] [PubMed] [Google Scholar]
- Price MP, Gong H, Parsons MG, Kundert JR, Reznikov LR, Bernardinelli L, Chaloner K, Buchanan GF, Wemmie JA, Richerson GB, Cassell MD, Welsh MJ. Localization and behaviors in null mice suggest that ASIC1 and ASIC2 modulate responses to aversive stimuli. Genes Brain Behav. 2014;13:179–194. doi: 10.1111/gbb.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. J Biol Chem. 1996;271:7879–7882. doi: 10.1074/jbc.271.14.7879. [DOI] [PubMed] [Google Scholar]
- Ryu S, Liu B, Yao J, Fu Q, Qin F. Uncoupling proton activation of vanilloid receptor TRPV1. J Neurosci. 2007;27:12797–12807. doi: 10.1523/JNEUROSCI.2324-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas M, Rash LD, Baron A, Lambeau G, Escoubas P, Lazdunski M. THE RECEPTOR SITE OF THE SPIDER TOXIN PcTx1 ON THE PROTON-GATED CATION CHANNEL ASIC1a. J Physiol. 2005 doi: 10.1113/jphysiol.2005.095810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L, Sakai H, Mattei M, Lazdunski M, Lingueglia E. Molecular cloning, functional expression and chromosomal localization of an amiloride-sensitive Na(+) channel from human small intestine. FEBS Lett. 2000;471:205–210. doi: 10.1016/s0014-5793(00)01403-4. [DOI] [PubMed] [Google Scholar]
- Sherwood TW, Askwith CC. Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity and acidosis-induced neuronal death. J Neurosci. 2009;29:14371–14380. doi: 10.1523/JNEUROSCI.2186-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood TW, Lee KG, Gormley MG, Askwith CC. Heteromeric acid-sensing ion channels (ASICs) composed of ASIC2b and ASIC1a display novel channel properties and contribute to acidosis-induced neuronal death. J Neurosci. 2011;31:9723–9734. doi: 10.1523/JNEUROSCI.1665-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjo BK. Lactic acidosis in the brain: occurrence, triggering mechanisms and pathophysiological importance. Ciba Found Symp. 1982;87:77–100. doi: 10.1002/9780470720691.ch5. [DOI] [PubMed] [Google Scholar]
- Siesjo BK, Katsura K, Mellergard P, Ekholm A, Lundgren J, Smith ML. Acidosis-related brain damage. Prog Brain Res. 1993;96:23–48. [PubMed] [Google Scholar]
- Siesjo BK, Wieloch T. Epileptic brain damage: pathophysiology and neurochemical pathology. Adv Neurol. 1986;44:813–847. [PubMed] [Google Scholar]
- Sluka KA, Winter OC, Wemmie JA. Acid-sensing ion channels: A new target for pain and CNS diseases. Curr Opin Drug Discov Devel. 2009;12:693–704. [PMC free article] [PubMed] [Google Scholar]
- Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci U S A. 2001;98:711–716. doi: 10.1073/pnas.011404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima A, Hans FJ, Livingstone D, Wei L, Finnegan W, DeMaro J, Fenstermacher J. Smaller local brain volumes and cerebral atrophy in spontaneously hypertensive rats. Hypertension. 1993;21:105–111. doi: 10.1161/01.hyp.21.1.105. [DOI] [PubMed] [Google Scholar]
- Tang CM, Dichter M, Morad M. Modulation of the N-methyl-D-aspartate channel by extracellular H+. Proc Natl Acad Sci U S A. 1990;87:6445–6449. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taugher RJ, Lu Y, Wang Y, Kreple CJ, Ghobbeh A, Fan R, Sowers LP, Wemmie JA. The bed nucleus of the stria terminalis is critical for anxiety-related behavior evoked by CO2 and acidosis. J Neurosci. 2014;34:10247–10255. doi: 10.1523/JNEUROSCI.1680-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Carpenter KL, Nortje J, Al-Rawi PG, O'Connell MT, Czosnyka M, Smielewski P, Pickard JD, Menon DK, Kirkpatrick PJ, Gupta AK, Hutchinson PJ. Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain. 2011;134:484–494. doi: 10.1093/brain/awq353. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Cull-Candy SG. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature. 1990;345:347–350. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- Vergo S, Craner MJ, Etzensperger R, Attfield K, Friese MA, Newcombe J, Esiri M, Fugger L. Acid-sensing ion channel 1 is involved in both axonal injury and demyelination in multiple sclerosis and its animal model. Brain. 2011;134:571–584. doi: 10.1093/brain/awq337. [DOI] [PubMed] [Google Scholar]
- Vessey JP, Stratis AK, Daniels BA, Da Silva N, Jonz MG, Lalonde MR, Baldridge WH, Barnes S. Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J Neurosci. 2005;25:4108–4117. doi: 10.1523/JNEUROSCI.5253-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink R, McIntosh TK, Weiner MW, Faden AI. Effects of traumatic brain injury on cerebral high-energy phosphates and pH: a 31P magnetic resonance spectroscopy study. J Cereb Blood Flow Metab. 1987;7:563–571. doi: 10.1038/jcbfm.1987.106. [DOI] [PubMed] [Google Scholar]
- Voipio J, Kaila K. Interstitial PCO2 and pH in rat hippocampal slices measured by means of a novel fast CO2/H(+)-sensitive microelectrode based on a PVC-gelled membrane. Pflugers Arch. 1993;423:193–201. doi: 10.1007/BF00374394. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997a;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997b;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Voilley N, Lauritzen I, Lazdunski M. The mammalian degenerin MDEG, an amiloride-sensitive cation channel activated by mutations causing neurodegeneration in Caenorhabditis elegans. J Biol Chem. 1996;271:10433–10436. doi: 10.1074/jbc.271.18.10433. [DOI] [PubMed] [Google Scholar]
- Wang XY, Yan WW, Zhang XL, Liu H, Zhang LC. ASIC3 in the cerebrospinal fluid-contacting nucleus of brain parenchyma contributes to inflammatory pain in rats. Neurol Res. 2014;36:270–275. doi: 10.1179/1743132813Y.0000000297. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr., Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23:5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr., Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Taugher RJ, Kreple CJ. Acid-sensing ion channels in pain and disease. Nat Rev Neurosci. 2013;14:461–471. doi: 10.1038/nrn3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PY, Huang YY, Chen CC, Hsu TT, Lin YC, Weng JY, Chien TC, Cheng IH, Lien CC. Acid-Sensing Ion Channel-1a Is Not Required for Normal Hippocampal LTP and Spatial Memory. J Neurosci. 2013;33:1828–1832. doi: 10.1523/JNEUROSCI.4132-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong ZG, Chu XP, Simon RP. Acid sensing ion channels--novel therapeutic targets for ischemic brain injury. Front Biosci. 2007;12:1376–1386. doi: 10.2741/2154. [DOI] [PubMed] [Google Scholar]
- Yin T, Lindley TE, Albert GW, Ahmed R, Schmeiser PB, Grady MS, Howard MA, Welsh MJ. Loss of Acid sensing ion channel-1a and bicarbonate administration attenuate the severity of traumatic brain injury. PLoS One. 2013;8:e72379. doi: 10.1371/journal.pone.0072379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha XM. Acid-sensing ion channels: trafficking and synaptic function. Mol Brain. 2013;6:1. doi: 10.1186/1756-6606-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha XM, Costa V, Harding AM, Reznikov L, Benson CJ, Welsh MJ. ASIC2 subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J Neurosci. 2009;29:8438–8446. doi: 10.1523/JNEUROSCI.1284-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha XM, Wemmie JA, Green SH, Welsh MJ. Acid-sensing ion channel 1a is a postsynaptic proton receptor that affects the density of dendritic spines. Proc Natl Acad Sci U S A. 2006;103:16556–16561. doi: 10.1073/pnas.0608018103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, 3rd, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygun DA, Steiner LA, Johnston AJ, Hutchinson PJ, Al-Rawi PG, Chatfield D, Kirkpatrick PJ, Menon DK, Gupta AK. Hyperglycemia and brain tissue pH after traumatic brain injury. Neurosurgery. 2004;55:877–881. doi: 10.1227/01.neu.0000137658.14906.e4. discussion 882. [DOI] [PubMed] [Google Scholar]