Abstract
Background
Past work demonstrates that depressed individuals with suicidal thoughts or behaviors exhibit specific neuroanatomical alterations. This may represent a distinct phenotype characterized by specific findings on neuroimaging, but it is unclear if these findings extend to individuals with milder thoughts of death. We examined this question in outpatients with recurrent Major Depressive Disorder not receiving antidepressant treatment.
Methods
We examined 165 subjects: 53 depressed without thoughts of death, 21 depressed with thoughts of death, and 91 healthy comparison subjects. Participants completed 3T cranial MRI, including anatomical and diffusion tensor imaging acquisitions. Automated methods measured regional gray matter volumes in addition to cortical thickness. White matter analyses examined diffusion measures within specific fiber tracts and included voxelwise comparisons.
Results
After adjustment for multiple comparisons, the depressed group with thoughts of death did not exhibit differences in regional gray matter volume, but did exhibit reduced cortical thickness in frontoparietal regions and the insula. This depressed group with thoughts of death also exhibited widespread white matter differences in fractional anisotropy and radial diffusivity. These differences were observed primarily in posterior parietal white matter regions and central white matter tracts adjacent to the basal ganglia and thalamus.
Conclusions
Mild thoughts of death are associated with structural alterations in regions of the salience network, default mode network, and thalamocortical circuits. Further work is needed to understand the pathological basis of these findings.
Keywords: Depression, DTI, diffusion tensor imaging, MRI, suicide, insula, volumetric, anisotropy
1. INTRODUCTION
Suicide is one of the most devastating outcomes of Major Depressive Disorder (MDD). Although suicidal thoughts are seen in many neuropsychiatric conditions, individuals with depressive disorders are at particular risk, with reported prevalence of lifetime suicide risk between 2% to 7% (Bostwick and Pankratz, 2000, Bradvik et al., 2008). Importantly, suicidal thoughts are not unique to MDD and many patients with MDD never exhibit suicidal thoughts or behaviors. This suggests that, regardless of diagnosis, there may be distinct underlying neural differences that are associated with increased risk of suicidal thoughts and behavior.
Even in the absence of clear suicidal ideation, milder symptoms are common in MDD, including feelings of being better off dead or passively wishing for death. Such clinical presentations are common, as thoughts that life is not worth living occur in 15% of younger individuals seeking mental health treatment, with an additional 16% reporting thoughts of death or suicidal ideation (Scott et al., 2012). Comparable or higher rates are seen in older populations (Rurup et al., 2011, Scocco and De Leo, 2002). Despite the frequency of these milder symptoms, it is unclear if the neural differences observed in more severe suicidal ideation are also observed in individuals with milder symptoms.
Most work examining neural contributors to suicidality in MDD focused on severely affected individuals at high risk or with a history of suicide attempts. These studies identified widespread differences across frontal, temporal and parietal lobes (Hwang et al., 2010, Wagner et al., 2012). Suicidality is associated with smaller volumes of the anterior cingulate cortex (Wagner et al., 2011, Wagner, Schultz, 2012, Willeumier et al., 2011), insula (Soloff et al., 2012, Willeumier, Taylor, 2011), orbitofrontal cortex (OFC) (Monkul et al., 2007, Soloff, Pruitt, 2012), and dorsolateral prefrontal cortex (Wagner, Schultz, 2012). Reductions in perfusion and metabolism are also observed in many of these regions (Willeumier, Taylor, 2011). Suicidality is further associated with subcortical differences, including smaller volumes of the caudate and basal ganglia (Vang et al., 2010, Wagner, Koch, 2011) but larger volumes of the amygdala (Monkul, Hatch, 2007) and thalamus (Lopez-Larson et al., 2013). Suicidality in MDD is also associated with white matter alterations, with microstructural abnormalities reported in frontal white matter (Olvet et al., 2014) and the fronto-thalamic circuit, including the anterior limb of the internal capsule, right lentiform nucleus and OFC (Jia et al., 2013). Reduced corpus callosum volumes are also associated with suicide attempts in mood disorders (Cyprien et al., 2011, Matsuo et al., 2010, Nery-Fernandes et al., 2012). Jointly, this work suggests that structural alterations in a range of networks are involved in suicidal thoughts and behavior. Many of these regions are implicated more broadly in depression, yet may be involved differently or to a greater extent than observed in non-suicidal depression.
Clear suicidal ideation and suicidal behaviors are the severe end of this symptom spectrum, but milder symptoms including thoughts of death and passive wishes for death are common. It is unclear if the neuroanatomical findings observed in high-risk suicidal individuals are also present in individuals with milder thoughts and lower risk. In this study, we hypothesized that even milder thoughts of death would be associated with gray matter volumetric differences and white matter microstructural differences. We utilized this past literature to identify gray and white matter regions that we a priori expected to exhibit differences in depressed subjects reporting thoughts of death while also conducting whole-brain analyses. We then tested for group differences using both regional volumetric measures and voxel-based approaches.
2. METHODS
2.1 Participants and Assessments
Participants were outpatients at Duke University between the ages of 20 and 50 years. Depressed participants had a DSM-IV diagnosis of recurrent MDD, as assessed by the Mini-International Neuropsychiatric Interview (MINI, version 5.0) (Sheehan et al., 1998) and interview with a study psychiatrist (WDT). Following an approach used by others (Sheline et al., 1999), we used the Diagnostic Interview for Genetic Studies (Nurnberger et al., 1994) to identify the number of past major depressive episodes and the cumulative duration of depression, summing over all episodes. Corroborating information was obtained when possible.
Entry criteria included onset of first depressive episode before age 35 years and a Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) of 15 or greater. Entry criteria also specified no antidepressant use in the last month; however, most subjects reported no antidepressant use for at least three months or longer. Eligible control subjects had neither any lifetime history of psychiatric disorders nor any history of psychotropic medication use.
Exclusion criteria included other lifetime DSM-IV Axis I disorders including substance abuse or dependence, although anxiety symptoms occurring exclusively during depressive episodes were allowable. Subjects were excluded for Axis II disorders determined by the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) (First et al., 1997). Additional exclusion criteria included: history of psychosis, use of illicit substances in the last month, ECT in the last 6 months, a family history of bipolar disorder, any unstable medical condition, any history of neurological illness or head injury, or MRI contraindications. Participants who reported acute intent or plans for suicide were excluded and referred for emergent treatment.
The Duke University Medical Center Institutional Review Board approved this study. All participants provided informed consent. As data analyses were conducted at Vanderbilt University, the Vanderbilt University Institutional Review Board also approved these procedures.
Depressed participants were dichotomized based on the presence or absence of current thoughts of death or suicide as determined on the clinician-administered MINI. The MINI suicidality assessment includes 9 questions assigned differentially weighted scores. The majority of questions focus on the last 30 days with an additional question assessing any lifetime history of suicide attempts. Presence of thoughts of death was determined as a score of 1 or greater, with a score of 1 indicating thoughts of being better off dead or wishing for death. The score increases with thoughts of self-injury, suicidal intent, plan, or past attempts. A score of 0 indicates no thoughts of being better off dead.
2.2 MRI Acquisition
Cranial MRI was performed using the eight-channel parallel imaging head coil on a whole-body MRI system (Trio, Siemens Medical Systems, Malvern, PA). Parallel imaging was employed with an acceleration factor of 2. Duplicate T1-weighted image sets were acquired during the scan session using a sagittal MPRAGE sequence with TR/TE = 2300/3.46 msec, a 240 Hz/pixel bandwidth, a 256 × 256 matrix, a 240 mm diameter field-of-view, 160 slices with a 1.2 mm slice thickness, yielding an image with voxel sizes of 0.9 × 0.9 × 1.2mm. Similarly, two identical 20-direction diffusion-weighted acquisitions were acquired during the session using a single shot 2D diffusion tensor echo planar pulse sequence in the axial plane. Parameters included TR/TE = 10200/93 msec, a 1396 Hz/pixel bandwidth, a 256 mm diameter field of view, 75 slices with a 2mm slice thickness yielding an image with voxel sizes of 2 × 2 × 2mm, 2 signal averages, with 20 diffusion directions, each with a b-value of 1000 sec/mm2 plus an acquisition of b = 0 sec/mm2.
2.3 Volumetric MRI Analyses
For both volumetric and DTI analyses, we conducted both evidence-guided regional measures and atheoretical voxelwise group comparisons. All volumetric measures were calculated using FreeSurfer (version 5.1) software running in a high-performance Linux cluster environment. The FreeSurfer methods used to derive cortical and subcortical brain volumes have been previously described (Dale et al., 1999, Fischl et al., 2002, Fischl et al., 2004). Cortical parcellation used the Desikan-Killiany Atlas (Desikan et al., 2006); in each hemisphere, this method identified 33 cortical and 7 subcortical gray matter regions (nucleus accumbens, amygdala, caudate, hippocampus, pallidum, putamen, and thalamus). Intracranial volume was assessed using the method implemented in FreeSurfer. We visually inspected the data by overlaying the surfaces and subcortical segmentations over the T1 data. Individual slices in each orientation were assessed for errors. No manual corrections were needed.
In secondary analyses, we tested for differences in cortical thickness using Freesurfer’s QDEC module. This was an exploratory approach to test for differences not captured using our atlas-based comparisons. In this method, cortical thickness is computed as the shortest distance between any point on the pial surface and the gray/white boundary and vice-versa; these two values are averaged (Fischl and Dale, 2000). Maps were smoothed using the standard Gaussian kernel of 10mm. We used a general linear model (GLM) to test for differences in cortical thickness between diagnostic groups, including age as a nuisance variable. Correction for multiple comparisons was carried out using the Monte Carlo simulation method with a p < 0.05. Data were tested against an empirical null distribution of maximum cluster size by running 10,000 synthesized Gaussian noise simulations with an initial threshold of p < 0.05. Right and left hemispheres were tested separately.
2.4 White Matter Analyses
Diffusion tensor imaging (DTI) data were run through quality assurance tests (Lauzon et al., 2013) and corrected for distortion and head motion using the eddy current correction within FSL (Smith et al., 2004). The FSL Brain Extraction Tool removed non-brain tissue (Smith, 2002) and “dtfit” fit the diffusion tensor model within each voxel, creating fractional anisotropy (FA) and radial diffusivity (RD) maps, with RD calculated as the mean of the two smaller eigenvalues. Diffusion data were next processed using the Tract-Based Spatial Statistics (TBSS) module (Smith et al., 2006). All subjects’ FA data were aligned into common MNI space using the nonlinear registration tool FNIRT (Andersson et al., 2007a, b), which uses a b-spline representation of the registration warp field (Rueckert et al., 1999). Next, the mean FA image was created and thinned to create a mean FA skeleton that represents the center of all tracts common to the group. Each subject’s aligned FA data was then projected onto this skeleton. Similarly, each subject’s RD data were projected onto this skeleton using the same parameters. Similar to an approach used by others (Huang et al., 2012), to acquire quantifiable tract- and region-specific DTI measures, the FA and RD skeletons were parcellated with the JHU ICBM-DTI-81 white-matter label atlas (Wakana et al., 2007). We extracted the average FA and RD for the skeleton voxels in each region.
For voxelwise analysis of the RD and FA data, we used the general linear model (GLM) method included in the FSL tools. The significance threshold was set to p < 0.05 corrected for family-wise error using the Threshold-Free Cluster Enhancement method in FSL’s randomise tool. The TBSS analyses were performed for both the RD and FA maps, comparing controls vs. depressed individuals with thoughts of death vs. depressed individuals without thoughts of death. In all comparisons, we controlled for age and sex. Only clusters of 50 voxels or greater were considered significant.
2.5 Selection of a priori regions of interest
Selection of a priori volumetric regions for primary analyses was based on work that identified volumetric or metabolic differences related to suicidality in MDD. These included: the orbitofrontal cortex (OFC) (Mahon et al., 2012, Monkul, Hatch, 2007, Soloff, Pruitt, 2012), cingulate cortex (Amen et al., 2009, Wagner, Koch, 2011, Wagner, Schultz, 2012, Willeumier, Taylor, 2011), insula (Soloff, Pruitt, 2012, Willeumier, Taylor, 2011), amygdala (Monkul, Hatch, 2007), parahippocampus (Altshuler et al., 1990, Soloff, Pruitt, 2012), thalamus (Lopez-Larson, King, 2013) and basal ganglia (Vang, Ryding, 2010, Wagner, Koch, 2011, Willeumier, Taylor, 2011). Similarly, past work using both volumetric and DTI methods to examine white matter in suicidal individuals identified: the internal capsule (Jia, Wang, 2013), thalamic radiation (Jia, Wang, 2013, Lopez-Larson, King, 2013), cingulum bundle (Yurgelun-Todd et al., 2011), and the corpus callosum (Cyprien, Courtet, 2011, Matsuo, Nielsen, 2010, Nery-Fernandes, Rocha, 2012). We included the uncinate fasciculus as it connects with the orbitofrontal cortex, identified in gray matter analyses (Jia, Wang, 2013, Mahon, Burdick, 2012). Although differences in the ventromedial or dorsomedial PFC white matter are reported in suicidality (Olvet, Peruzzo, 2014), we did not include these regions as they do not clearly map onto specific tracts identified in our analysis method.
2.6 Statistical Analyses
All analyses were conducted using SAS 9.3 (Cary, NC). Given the large number of variables available through our image analysis techniques, we planned a two-step analysis strategy. For our primary approach, we examined the a priori regions from past literature. For these analyses, we used FDR (false discovery rate, implemented within SAS) to control for multiple comparisons within the volumetric and DTI data. In a secondary approach, we examined all remaining identified regions, again using FDR to control for multiple comparisons.
We tested for univariate differences among study groups (depressed/thoughts of death, depressed/no thoughts of death, and control) in demographic and clinical variables using chi-square tests for categorical variables and ANOVA for continuous variables, with subsequent post-hoc group comparisons using the least squared means approach. Group comparisons of imaging measures (volumetric or DTI) were conducted using mixed models (PROC MIXED). Imaging measures were dependent variables; for regions measured bilaterally, these were repeated measures with hemisphere included as an independent variable. Study group, age, sex, race (dichotomized as white or minority) were also included as independent variables. Total intracranial volume was included as an independent variable for volumetric analyses. When we observed statistically significant differences in the study group variable, we tested for differences between adjusted group means using the least squares means approach.
3. RESULTS
3.1 Sample Characteristics
We examined 165 subjects: 53 adults with depression but no suicidality or thoughts of death (DepNS), 21 with depression and suicidality or thoughts of death (DepSI) and 91 healthy comparison subjects (controls; Table 1). Depression severity by MADRS differed between groups, reflecting differences between the controls and both depressed groups (p<0.0001), but not between the depressed groups (p=0.0852). Although data were unavailable for 5 subjects, there was no statistically significant difference between the DepNS and DepSI groups in number of total depressive episodes (DepNS: 3.2, SD=2.0; DepSI: 2.9, SD=1.1; t=0.41, 67df, p = 0.6841) or lifetime estimate of depression duration in months (DepNS: 70.1, SD=62.7; DepSI: 72.2, SD=53.2; t=0.11, 67df, p = 0.9137). To better elucidate differences in depressive symptomatology between the depressed groups, we tested for univariate differences in each MADRS item. The only items with statistically significant group differences were Item 2 (Reported Sadness; DepNS 3.0, SD=0.8; DepSI 3.4, SD=0.6; 77 df, t = 2.59, p = 0.0115) and Item 10 (Suicidal Thoughts; DepNS 0.06, SD=0.2; DepSI 1.58, SD=0.7; 77 df, t = 11.20, p < 0.0001).
Table 1.
Demographic differences across diagnostic groups
| Control (N=91) | Dep/NS (N = 53) | Dep/SI (N = 21) | Test Statistic | p value | |
|---|---|---|---|---|---|
|
| |||||
| Age (years) | 29.9 (9.1) | 37.5 (8.9) | 33.5 (9.1) | F2,162 = 11.95 | < 0.0001 |
|
| |||||
| Sex, Female | 61.5% (56) | 77.3% (41) | 52.4% (11) | X2 = 5.53, 2df | 0.0631 |
|
| |||||
| Education (years) | 15.8 (2.0) | 15.3 (2.4) | 15.3 (2.7) | F2,162 = 0.89 | 0.4113 |
|
| |||||
| Race | Fisher’s exact | 0.4117 | |||
| • African-Am | 35.2% (32) | 26.4% (14) | 14.3% (3) | ||
| • White | 50.5% (46) | 54.6% (29) | 66.6% (14) | ||
| • Asian | 9.9% (9) | 7.6% (4) | 9.5% (2) | ||
| • Native Am | 0% (0) | 3.8% (2) | 4.8% (1) | ||
| • Multiracial | 4.4% (4) | 7.6% (4) | 4.8% (1) | ||
|
| |||||
| MADRS | 0.8 (1.1) | 23.3 (4.5) | 24.7 (4.2) | F2,162 = 1137.1 | < 0.0001 |
|
| |||||
| Intracranial Volume (ml) | 1158.8 (181.2) | 1179.7 (196.0) | 1236.9 (243.0) | F2,162 = 1.34 | 0.2654 |
Data presented as mean (standard deviation) for continuous variables or percentage (N) for categorical variables.
By definition, both the control group and DepNS group had scores of 0 on the MINI suicidality assessment, indicating no current thoughts of death and no history of past suicide attempts. The DepSI group had a mean suicidality score of 7.3 (SD=4.8, range = 1–19). Per the MINI, 12 subjects were “low” suicide risk (score 1–8), 8 were “moderate” (score 9–16) and 1 was “high” (score ≥ 17). In addition to endorsing at least current thoughts of death, 10 of the 21 DepSI participants endorsed a history of past suicide attempts. No subjects endorsed thoughts of self-harm or recent self-injurious behavior without also endorsing at least thoughts of or wishes for death.
3.2 Gray matter differences
For our primary volumetric analyses, we examined 12 Freesurfer-defined regions (Supplemental Table 1). After controlling for covariates, no regions significantly differed between study groups after FDR correction. In secondary analyses, we tested for group differences in the remaining Freesurfer-defined regions. Again, no regions significantly differed between study groups after FDR correction.
We then tested for differences in cortical thickness. Areas that differed between the two depressed groups (Figure 1) included regions in the left hemisphere frontal, temporal, and parietal lobes, with the largest cluster over the insula. No regions differed in the right hemisphere. There were no regions where cortical thickness significantly differed between the control group and either of the depressed groups.
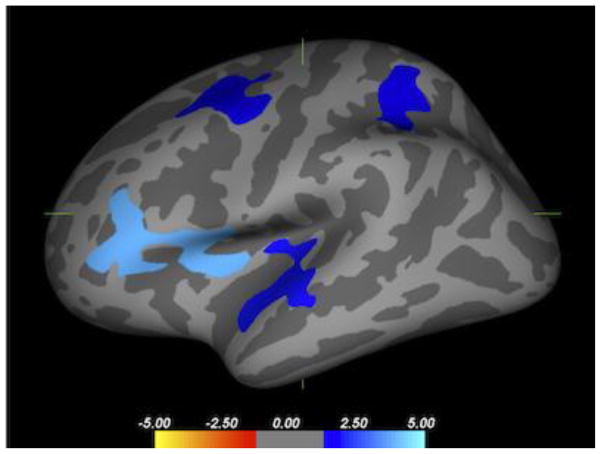
Figure 1. Difference in cortical thickness between depressed groups.
Map of regions in the left hemisphere where cortical gray matter thickness differs between suicidal and nonsuicidal depressed groups. Blue regions are those areas where cortical thickness is lower in the suicidal group, including the insula (p = 0.0001, peak MNI coordinates −33.5, 1.4, 6.5), caudal middle frontal gyrus (p=0.0169; peak −24.5, 4.1, 42.9), superior parietal gyrus (p=0.0448; peak −31.3, 46.7, 48.2) and superior temporal gyrus (p=0.0119, peak −52.6, −10.6, 0.4). There were no differences in cortical thickness in the right hemisphere and no regions where the nonsuicidal depressed cohort exhibited smaller cortical thickness.
3.3 White matter microstructural differences
After controlling for covariates, multiple a priori white matter measures exhibited significant group differences after FDR correction (Figure 2, Supplemental Table 2). These regions included the cingulum bundle adjacent to the hippocampus, the anterior thalamic radiation, the posterior limb of the internal capsule, and the superior and posterior corona radiata. Several additional regions exhibited DTI differences between groups at unadjusted p-values not surviving FDR correction, including the cingulum bundle adjacent to the cingulate gyrus, posterior thalamic radiation, and anterior limb of the internal capsule.
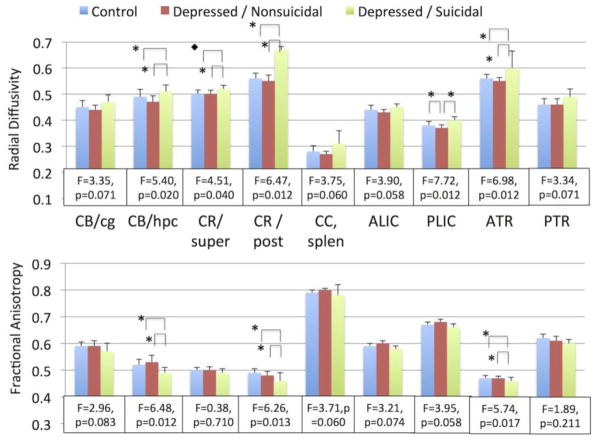
Figure 2. DTI measures by diagnostic group.
Mean measures of fractional anisotropy (FA) and radial diffusivity (RD) with standard deviations. After controlling for controlling for age, race, sex, and hemisphere, the presented tracts exhibited a significant difference between groups in either RD or FA at unadjusted p-values (Supplemental Table 2). Statistical data show F values for group variable and the FDR-corrected p-value, with 2 degrees of freedom for the variable and 158 for the model. Overall, suicidal depressed subjects tended to have higher RD and lower FA in these tracts. Statistically significant group comparisons for regions surviving FDR correction are shown with brackets, ◆ p < 0.05, * p < 0.01.
CB/cg – cingulum, cingulate gyrus; CB/hpc – cingulum, hippocampus region; CR/super – superior corona radiata; CR/post – posterior corona radiata; CC, splen – splenium of the corpus callosum; ALIC – anterior limb of the internal capsule; PLIC – posterior limb of the internal capsule; ATR – anterior thalamic radiation; PTR – posterior thalamic radiation.
We conducted pairwise comparisons for regions that significantly differed between groups prior to FDR correction (Figure 2, Supplemental Table 3). In all statistically significant comparisons, the DepSI group consistently exhibited higher RD and lower FA. Only one region – RD of the posterior internal capsule – exhibited a significant difference between the control and DepNS groups, with the DepNS group exhibiting lower RD in this region than the control group.
In secondary analyses we tested for group differences in tracts that we did not a priori plan to examine. After FDR correction, we did not observe any statistically significant differences.
Finally we conducted voxelwise group comparisons of the FA and RD white matter skeletons. We found multiple white matter regions exhibiting differences between the control and DepSI groups and between the DepNS and DepSI groups (Table 2, Figure 3). When compared with other groups, in statistically significant clusters the DepSI group exhibited lower FA and higher RD values. These clusters were widespread but primarily occurred in central white matter regions adjacent to the thalamus and in posterior regions, including the splenium of the corpus callosum and posterior corona radiata. No statistically significant differences were observed in either FA or RD between the control and DepNS groups.
Table 2.
Significant fractional anisotropy and radial diffusivity clusters obtained from the TBSS analyses.
| Comparison | DTI Measure | Cluster Maxima | MNI coordinates | Size | p value |
|---|---|---|---|---|---|
| Control > Depressed/Suicidal | FA | Corpus Callosum, Splenium | −10, 33, 24 | 6,529 | 0.020 |
| FA | R. Corticospinal Tract | 23, −20, 59 | 503 | 0.046 | |
| FA | R. Anterior Thalamic Radiation | 9, −4, 11 | 365 | 0.046 | |
| RD | L. Superior Longitudinal Fasciculus | −18, −6, 7 | 11,495 | 0.021 | |
| Depressed/Nonsuicidal > Depressed/Suicidal | FA | L. Post Corona Radiata | −26, −38, 23 | 17,533 | 0.010 |
| RD | R. Anterior Thalamic Radiation | 7, −5, −1 | 25,846 | 0.004 |
All clusters showed a reduced fractional anisotropy (FA) and elevated radial diffusivity (RD) in the suicidal group compared with other groups. Cluster size measured in voxels.
Abbreviations: MNI = Montreal Neurological Institute
Figure 3. Voxelwise comparison of DTI measures between groups.
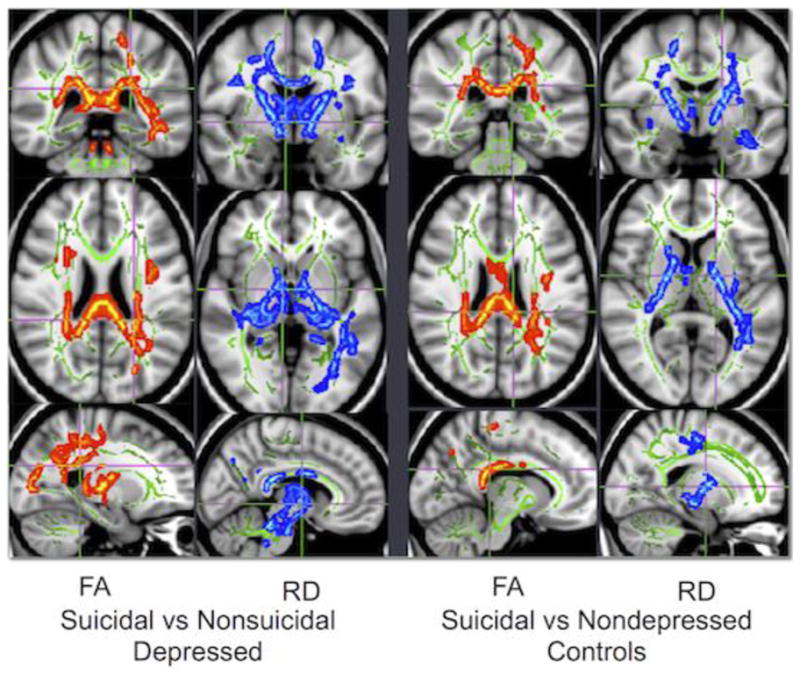
Comparisons between groups in whole-brain measures of FA (fractional anisotropy) and RD (radial diffusivity). Please refer to Table 2 for location of cluster maxima. Green voxels represent the white matter skeleton, red voxels are areas of lesser value in the suicidal group, and blue voxels are areas of greater value in the suicidal group. In these analyses, the suicidal group exhibited lower FA and higher RD in identified regions when compared with either the nonsuicidal depressed group or the control group. There were no white matter regions exhibiting significant differences in either FA or RD between the nonsuicidal depressed and nondepressed control groups.
4. DISCUSSION
When compared with individuals without thoughts of death, depressed individuals with thoughts of death exhibit widespread alterations in white matter microstructure. These findings were robust across central tracts and in parietal and temporal regions. In contrast, we observed no difference in regional gray matter volumes after FDR correction. However, analyses examining cortical thickness found statistically significant isolated differences in the left hemisphere, including the insula.
Our findings in the white matter are generally concordant with past reports in populations with more severe symptoms of suicidality and implicate fibers involved with multiple cortical, striatal, and limbic connections. Multiple factors may contribute to white matter alterations as detected by DTI. One possibility given the widespread nature of this study’s findings suggests that the DTI alterations may reflect underlying glial pathology. Alterations in astrocyte morphometry and function are associated with suicide (Ernst et al., 2011, Torres-Platas et al., 2011). Additionally, as RD is thought to be a marker of myelination (Song et al., 2002, Wei et al., 2013), our findings may reflect oligodendrocyte dysfunction and myelination deficits, which are themselves associated with suicide (Klempan et al., 2009a, Klempan et al., 2009b). The gray matter findings were more focal and limited. However, our analysis of cortical gray matter thickness supported past work identifying differences in the insula (Soloff, Pruitt, 2012, Willeumier, Taylor, 2011).
Jointly, these data provide important clues about neural circuits involvement in thoughts of death and suicidality. The insula is a component of the salience network and through connections with the amygdala and cingulate cortex may potentiate the neural response to negative stimuli (Hamilton et al., 2012, Seeley et al., 2007). The pulvinar nucleus of the thalamus is also involved in this network (Hamilton, Etkin, 2012), and observations of disruption in the internal capsule and thalamic radiations further support this network may be disrupted in individuals with suicidal thoughts. Additionally, the DTI findings in the parietal white matter regions potentially implicate the default mode network’s (DMN’s) posterior hub. This theory is supported by cortical thinning in the superior parietal gyrus and involvement of the parahippocampal gyrus, which is connected to the DMN (Greicius et al., 2003). The DMN is involved in self-referential thoughts; when coupled with dysfunction in the salience network, this may result in greater self-referential responses to negative stimuli. These theories require testing.
4.1 Comparison of depressed and control subjects without thoughts of death
Except for the posterior limb of the internal capsule, we did not observe any regions that exhibited significant differences between nonsuicidal depressed and control groups. This is in contrast to past studies reporting volumetric or DTI differences in MDD, as previously reviewed (Kempton et al., 2011), although recent large studies have also not found significant differences on DTI measures between depressed and nondepressed groups (Choi et al., 2013). This is an important negative finding, as our sample of 165 subjects (91 control and 74 depressed participants) is comparable in size to those of the largest previously published non-geriatric volumetric imaging studies examining MDD that reported samples ranging from 156 to 230 subjects (Frodl et al., 2008, Jessen et al., 2009, Meisenzahl et al., 2010). By examining a subpopulation with even mild thoughts of death, we may be teasing apart heterogeneity within the diagnosis of MDD that contributes significantly to past reports of volumetric and DTI differences. More broadly, the underlying heterogeneity within the diagnosis of MDD may contribute to the discrepancy between our lack of findings between the nonsuicidal depressed and control groups and other published reports. In the end, this supports the concept that volumetric differences on MRI are not seen in all individuals with MDD.
Age may also be a critical difference. These past larger studies reported mean ages between 45–54 years (Kempton, Salvador, 2011), while our sample was younger, with a mean age of 32.7 years. Although best studied in the hippocampus (Sheline, Sanghavi, 1999), volumetric differences observed in past studies may be related to depression duration and cumulative burden, which would be more observable with aging. This is supported by a meta-analysis that reported no difference in hippocampal volumes between individuals with and without MDD (McKinnon et al., 2009). However, upon limiting analyses by age, the authors found differences in hippocampal volume in midlife or older depressed adults, but not younger adults. The authors proposed that they observed an effect of recurrent depressive episodes on brain morphology, an effect that had not yet manifested in the younger cohort. If volumetric differences in MDD are related to a cumulative effect of depression over time, this could explain the lack of findings in our younger adult cohort.
4.2 Study Strengths and Limitations
The primary strength of the study is the overall large, well-defined sample. Our antidepressant-free depressed group is well characterized with recurrent depressive episodes. Moreover, our image analysis plan uses well-established methods and adjusts for multiple comparisons. However, the method used to acquire DTI data used 20-directions, which is less than modern techniques using 25–30 directions. Although the FDR approach is more conservative than some alternate methods for controlling for multiple comparisons, we desired to have a robust approach given the large number of comparisons examined. Using a less conservative method would not have substantially changed the overall study conclusions.
The primary limitation relates to the definitions used within the depressed group. The group reporting thoughts of death is the smallest of the three groups, raising the issue that the groups were disproportionate and may have been underpowered for some analyses. Additionally, thoughts of death were assessed with the MINI. Although a helpful tool, more robust instruments than the MINI are available, such as the Columbia-Suicide Severity Rating Scale (C-SSRS) (Posner et al., 2011). Our definition for this group was broad, leading to clinical heterogeneity and a range of severity within the group reporting thoughts of death. Moreover, approximately half of the group reporting thoughts of death reported a past history of suicide attempts, demonstrating the heterogeneity even in this sample. These clinical differences may be related to other differences in neurobiology that we could not elucidate in the current study. Additionally, although study participants were antidepressant-free for at least a month prior to enrollment, they were not antidepressant-naïve. Thus it is uncertain how antidepressant exposure months or even years earlier may have affected the study results.
4.3. Conclusions
Future studies should examine suicidal ideation as a phenotype both in MDD and across psychiatric diagnoses. Further work is needed in better-balanced samples to see if even milder thoughts of death as well as more severe symptoms of suicidality are related to common circuit deficits or common white matter abnormalities. Distinctions between individuals with thoughts of death and those with true suicidal ideation should be considered, examining whether there is progressive differences noted on imaging corresponding with the greater clinical severity. This question may lend itself to a dimensional approach as has been proposed for other symptoms in the NIMH’s Research Domain Criteria project.
Our findings should be further explored by examining the nature of the changes observed on DTI. It will be important to consider if these findings represent widespread white matter pathology, or changes that are limited to thalamocortical circuits or the default mode network. This could involve future neuroimaging studies examining other white matter measures but also longitudinal studies or autopsy studies examining persistence of white matter disease.
Supplementary Material
Highlights.
Depressed individuals with thoughts of death exhibit subtle differences in cortical thickness, primarily in the region of the insula.
There were no significant differences in subcortical volumes between depressed individuals with thoughts of death, depressed individuals without such thoughts, and nondepressed comparison subjects.
Depressed individuals with thoughts of death exhibited widespread alterations in white matter microstructure in central white matter tracts and posterior parietal regions.
Regions implicated are components of the salience network, default mode network, and thalamocortical circuits. Work is needed to determine if thoughts of death are associated with altered function of these circuits.
Acknowledgments
Funding: This project was supported by National Institute of Mental Health (NIMH) grant R01 MH077745. It was further supported by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences and conducted in part using the resources of the Advanced Computing Center for Research and Education at Vanderbilt University, Nashville, TN.
The authors would like to thank Mr. Dean Holbert and Ms. Carolyn Carson for their work on subject recruitment and assessment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altshuler LL, Casanova MF, Goldberg TE, Kleinman JE. The hippocampus and parahippocampus in schizophrenia, suicide, and control brains. Arch Gen Psychiatry. 1990;47:1029–34. doi: 10.1001/archpsyc.1990.01810230045008. [DOI] [PubMed] [Google Scholar]
- Amen DG, Prunella JR, Fallon JH, Amen B, Hanks C. A comparative analysis of completed suicide using high resolution brain SPECT imaging. J Neuropsychiatry Clin Neurosci. 2009;21:430–9. doi: 10.1176/jnp.2009.21.4.430. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. FMRIB Technical Report TR07JA12007a [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation. FMRIB Technical Report TR07JA22007b [Google Scholar]
- Bostwick JM, Pankratz VS. Affective disorders and suicide risk: a reexamination. Am J Psychiatry. 2000;157:1925–32. doi: 10.1176/appi.ajp.157.12.1925. [DOI] [PubMed] [Google Scholar]
- Bradvik L, Mattisson C, Bogren M, Nettelbladt P. Long-term suicide risk of depression in the Lundby cohort 1947–1997--severity and gender. Acta Psychiatr Scand. 2008;117:185–91. doi: 10.1111/j.1600-0447.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- Choi KS, Holtzheimer PE, Franco AR, Kelley ME, Dunlop BW, Hu XP, et al. Reconciling Variable Findings of White Matter Integrity in Major Depressive Disorder. Neuropsychopharmacology. 2013;39:1332–9. doi: 10.1038/npp.2013.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyprien F, Courtet P, Malafosse A, Maller J, Meslin C, Bonafe A, et al. Suicidal behavior is associated with reduced corpus callosum area. Biol Psychiatry. 2011;70:320–6. doi: 10.1016/j.biopsych.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Ernst C, Nagy C, Kim S, Yang JP, Deng X, Hellstrom IC, et al. Dysfunction of astrocyte connexins 30 and 43 in dorsal lateral prefrontal cortex of suicide completers. Biol Psychiatry. 2011;70:312–9. doi: 10.1016/j.biopsych.2011.03.038. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JB, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) Washington, D.C: American Psychiatric Press, Inc; 1997. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23 (Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Frodl T, Jager M, Born C, Ritter S, Kraft E, Zetzsche T, et al. Anterior cingulate cortex does not differ between patients with major depression and healthy controls, but relatively large anterior cingulate cortex predicts a good clinical course. Psychiatry Res. 2008;163:76–83. doi: 10.1016/j.pscychresns.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–38. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Fan X, Weiner M, Martin-Cook K, Xiao G, Davis J, et al. Distinctive disruption patterns of white matter tracts in Alzheimer’s disease with full diffusion tensor characterization. Neurobiol Aging. 2012;33:2029–45. doi: 10.1016/j.neurobiolaging.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JP, Lee TW, Tsai SJ, Chen TJ, Yang CH, Lirng JF, et al. Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and voxel-based morphometry. J Geriatr Psychiatry Neurol. 2010;23:171–84. doi: 10.1177/0891988710363713. [DOI] [PubMed] [Google Scholar]
- Jessen F, Schumacher A, von Widdern O, Guttenthaler V, Hofels S, Suliman H, et al. No association of the Val66Met polymorphism of the brain-derived neurotrophic factor with hippocampal volume in major depression. Psychiatr Genet. 2009;19:99–101. doi: 10.1097/YPG.0b013e32832080ce. [DOI] [PubMed] [Google Scholar]
- Jia Z, Wang Y, Huang X, Kuang W, Wu Q, Lui S, et al. Impaired frontothalamic circuitry in suicidal patients with depression revealed by diffusion tensor imaging at 3.0 T. J Psychiatry Neurosci. 2013;38:130023. doi: 10.1503/jpn.130023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton MJ, Salvador Z, Munafo MR, Geddes JR, Simmons A, Frangou S, et al. Structural Neuroimaging Studies in Major Depressive Disorder: Meta-analysis and Comparison With Bipolar Disorder. Arch Gen Psychiatry. 2011;68:675–90. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- Klempan TA, Ernst C, Deleva V, Labonte B, Turecki G. Characterization of QKI gene expression, genetics, and epigenetics in suicide victims with major depressive disorder. Biol Psychiatry. 2009a;66:824–31. doi: 10.1016/j.biopsych.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, ffrench-Mullen J, et al. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol Psychiatry. 2009b;14:175–89. doi: 10.1038/sj.mp.4002110. [DOI] [PubMed] [Google Scholar]
- Lauzon CB, Asman AJ, Esparza ML, Burns SS, Fan Q, Gao Y, et al. Simultaneous analysis and quality assurance for diffusion tensor imaging. PLoS ONE. 2013;8:e61737. doi: 10.1371/journal.pone.0061737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson M, King JB, McGlade E, Bueler E, Stoeckel A, Epstein DJ, et al. Enlarged thalamic volumes and increased fractional anisotropy in the thalamic radiations in veterans with suicide behaviors. Frontiers in psychiatry. 2013;4:83. doi: 10.3389/fpsyt.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon K, Burdick KE, Wu J, Ardekani BA, Szeszko PR. Relationship between suicidality and impulsivity in bipolar I disorder: a diffusion tensor imaging study. Bipolar Disord. 2012;14:80–9. doi: 10.1111/j.1399-5618.2012.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K, Nielsen N, Nicoletti MA, Hatch JP, Monkul ES, Watanabe Y, et al. Anterior genu corpus callosum and impulsivity in suicidal patients with bipolar disorder. Neurosci Lett. 2010;469:75–80. doi: 10.1016/j.neulet.2009.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatr Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- Meisenzahl EM, Seifert D, Bottlender R, Teipel S, Zetzsche T, Jager M, et al. Differences in hippocampal volume between major depression and schizophrenia: a comparative neuroimaging study. Eur Arch Psychiatry Clin Neurosci. 2010;260:127–37. doi: 10.1007/s00406-009-0023-3. [DOI] [PubMed] [Google Scholar]
- Monkul ES, Hatch JP, Nicoletti MA, Spence S, Brambilla P, Lacerda AL, et al. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Mol Psychiatry. 2007;12:360–6. doi: 10.1038/sj.mp.4001919. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Nery-Fernandes F, Rocha MV, Jackowski A, Ladeia G, Guimaraes JL, Quarantini LC, et al. Reduced posterior corpus callosum area in suicidal and non-suicidal patients with bipolar disorder. J Affect Disord. 2012;142:150–5. doi: 10.1016/j.jad.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–59. doi: 10.1001/archpsyc.1994.03950110009002. discussion 63–4. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Peruzzo D, Thapa-Chhetry B, Sublette ME, Sullivan GM, Oquendo MA, et al. A diffusion tensor imaging study of suicide attempters. J Psychiatr Res. 2014;51:60–7. doi: 10.1016/j.jpsychires.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–77. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–21. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Rurup ML, Deeg DJ, Poppelaars JL, Kerkhof AJ, Onwuteaka-Philipsen BD. Wishes to die in older people: a quantitative study of prevalence and associated factors. Crisis. 2011;32:194–203. doi: 10.1027/0227-5910/a000079. [DOI] [PubMed] [Google Scholar]
- Scocco P, De Leo D. One-year prevalence of death thoughts, suicide ideation and behaviours in an elderly population. Int J Geriatr Psychiatry. 2002;17:842–6. doi: 10.1002/gps.691. [DOI] [PubMed] [Google Scholar]
- Scott EM, Hermens DF, Naismith SL, White D, Whitwell B, Guastella AJ, et al. Thoughts of death or suicidal ideation are common in young people aged 12 to 30 years presenting for mental health care. BMC Psychiatry. 2012;12:234. doi: 10.1186/1471-244X-12-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Inventory (M.I.N.I.): the development and validation of a structured diagnostic interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;(Suppl 20):22–33. [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–43. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 (Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Pruitt P, Sharma M, Radwan J, White R, Diwadkar VA. Structural brain abnormalities and suicidal behavior in borderline personality disorder. J Psychiatr Res. 2012;46:516–25. doi: 10.1016/j.jpsychires.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Torres-Platas SG, Hercher C, Davoli MA, Maussion G, Labonte B, Turecki G, et al. Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology. 2011;36:2650–8. doi: 10.1038/npp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vang FJ, Ryding E, Traskman-Bendz L, van Westen D, Lindstrom MB. Size of basal ganglia in suicide attempters, and its association with temperament and serotonin transporter density. Psychiatry Res. 2010;183:177–9. doi: 10.1016/j.pscychresns.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Schachtzabel C, Schultz CC, Sauer H, Schlosser RG. Structural brain alterations in patients with major depressive disorder and high risk for suicide: evidence for a distinct neurobiological entity? Neuroimage. 2011;54:1607–14. doi: 10.1016/j.neuroimage.2010.08.082. [DOI] [PubMed] [Google Scholar]
- Wagner G, Schultz CC, Koch K, Schachtzabel C, Sauer H, Schlosser RG. Prefrontal cortical thickness in depressed patients with high-risk for suicidal behavior. J Psychiatr Res. 2012;46:1449–55. doi: 10.1016/j.jpsychires.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–44. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei PT, Leong D, Calabrese E, White L, Pierce T, Platt S, et al. Diffusion tensor imaging of neural tissue organization: correlations between radiologic and histologic parameters. The neuroradiology journal. 2013;26:501–10. doi: 10.1177/197140091302600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeumier K, Taylor DV, Amen DG. Decreased cerebral blood flow in the limbic and prefrontal cortex using SPECT imaging in a cohort of completed suicides. Translational psychiatry. 2011;1:e28. doi: 10.1038/tp.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Bueler CE, McGlade EC, Churchwell JC, Brenner LA, Lopez-Larson MP. Neuroimaging correlates of traumatic brain injury and suicidal behavior. The Journal of head trauma rehabilitation. 2011;26:276–89. doi: 10.1097/HTR.0b013e31822251dc. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.