Abstract
Introduction
Vasoactive Intestinal Peptide (VIP) is a 28-amino acid neuropeptide that belongs to the secretin-glucagon superfamily of peptides and has 68% homology with PACAP. VIP is abundantly expressed in the central and peripheral nervous system and in the gastrointestinal tract, where it exercises several physiological functions. Previously, it has been reported that VIP regulates feeding behavior centrally in different species of vertebrates such as goldfishes, chicken, and rodents. Additional studies are necessary to analyze the role of endogenous VIP on the regulation of appetite/satiety together with feeding behavior, metabolic hormone release, body mass composition and energy balance.
Aims
To elucidate the physiological pathways by which VIP regulates appetite/satiety, feeding behavior, metabolic hormones and body mass composition.
Methods
VIP deficient (VIP −/−) and age-matched wild-type (WT) littermates were weekly monitored from 5 to 22 weeks of age using a whole body composition EchoMRI analyzer. Food intake and feeding behavior were analyzed using the BioDAQ automated monitoring system. Plasma levels of metabolic hormones including active-ghrelin, GLP-1, leptin, PYY, pancreatic polypeptide (PP), adiponectin, and insulin were measured in fasting as well as in postprandial conditions.
Results
The genetic lack of VIP led to a significant reduction of body weight and fat mass and to an increase of lean mass as the mice aged. Additionally, VIP−/− mice had a disrupted pattern of circadian feeding behavior resulting in an abolished regular nocturnal/diurnal feeding. These changes were associated with an altered secretion of adiponectin, GLP-1, leptin, PYY and insulin in VIP−/− mice. Our data demonstrates that endogenous VIP is involved in the control of appetite/satiety, feeding behavior, body mass composition and in the secretion of six different key regulatory metabolic hormones.
Conclusions
Our data show that endogenous VIP is involved in the control of appetite/satiety, feeding behavior, body mass composition and in the secretion of six key regulatory metabolic hormones. VIP plays a key role in the regulation of body weight and mass composition phenotype by significantly enhancing body weight and fat mass accumulation. Therefore, VIP signaling is critical for the modulation of appetite/satiety and body mass phenotype and is suggested to be a target for future treatment of obesity.
INTRODUCTION
The gastrointestinal tract (GI) acts as a nutrient sensor in response to luminal stimuli, releasing gastrointestinal neurotransmitters and hormones (Woods, 1998; Dockray, 2004) which are considered to be the major peripheral regulators of appetite and satiety (Greenwood, 2011). Neuropeptides regulate important gastrointestinal functions such as motility, secretion, absorption and also they provide feedback to the central nervous system (CNS) to regulate appetite and feeding behavior.
Vasoactive Intestinal Peptide (VIP) is a highly conserved 28 amino-acid neuropeptide widely distributed in the CNS and in the GI tract neurons. VIP binds with equal high affinity to its G protein-coupled receptors VPAC1 and VPAC2 (Said, 1970; Vaudry; 2000). Physiologically, VIP plays an important role in a variety of gastrointestinal functions including mucosal ion transport, vasodilatation, gastric acid secretion, hemodynamic regulation, gastric and intestinal motility, sphincter relaxation, neuronal excitability and mucosal inflammatory immune responses (Bloom, 1973; Harmar, 2012; Vu, 2014). Initially discovered in the intestine and lung, VIP belongs to the glucagon/secretin family of peptides whose members also include GLP-1 and GLP-2, glucagon, gastric inhibitory peptide (GIP) and growth hormone releasing factor. In animal models, intracerebroventricular (ICV) injections of VIP have been shown to decrease food intake in vertebrates, including chicks and goldfishes, suggesting an anorexigenic role for VIP at the CNS level in the arcuate nucleus (ARC) (Tachibana, 2003; Matsuda, 2005). Recently, the development of a genetically engineered VIP deficient (VIP−/−) mouse model has allowed the characterization of VIP’s role in a variety of gastrointestinal functions (Cowell, 2003; Lelievre, 2007). To further understand the physiological role of VIP on the regulation of appetite/satiety and body composition we have utilized the same C57BL/6 murine model lacking VIP gene expression. The fact that VIP is abundant in the stomach, small and large intestine, all important areas for digestion and nutrient absorption, as well as in the hypothalamic ARC area (Inoue, 1984; Lam, 1991), led us to investigate the potential role of VIP in the regulation of energy balance and body composition.
Our data show that VIP−/− mice present a significantly reduced body weight and an altered body composition with decreased fat mass and increased lean mass. VIP−/− mice present a disrupted feeding behavior pattern together with significant alterations in plasmatic anorexigenic and orexigenic hormone levels in both fasting as well as in postprandial conditions.
METHODS
Animals
Male VIP neuropeptide deficient mice (VIP−/−) (5–21 weeks of age, backcrossed >12 generations to C57BL/6J mice) were developed and genotyped as previously described (Cowell, 2003). Age matched wild type (WT) littermates from the same colony were housed and fed ad libitum in a specific pathogen-free, sterile animal facility, under conditions of controlled illumination (12:12-hour light-dark cycle; lights from 06:00 to 18:00 hours) at the United States Department of Veteran Affairs Greater Los Angeles Healthcare System animal facility, according to the recommendations for animal use and welfare dictated by the Veterans Administration Institutional Animal Care and Use Committee.
Body weight composition analysis
After weaning, at 3–4 weeks of age, VIP−/− (n=8) and WT mice (n=8) were group-housed 4 in each cage and received free access to water and standard rodent diet (Prolab RMH 2500; LabDiet, St. Louis, MO). Mice weight and body composition were recorded at 5.5 week intervals starting at 5 weeks of age until 22 weeks of age. Body composition was assessed by nuclear magnetic resonance using an EchoMRI whole body composition analyzer (EchoMedical Systems, Houston, TX). Body fat and lean mass changes were calculated in terms of percentage of change from the first baseline measurement at 5.5 weeks of age.
Determination of feeding behavior
To study feeding behavior, the BioDAQ (Research Diets, Inc., New Brunswick, NJ) episodic food intake monitor system was used as previously described in details (Stengel, 2010). First, VIP−/− (n=12) and WT (n=16) mice, mice were habituated for 7 days to single housing and feeding using a purified standard rodent diet (D12450B, Research Diets, Inc.) in the BioDAQ cages. Food and water were provided ad libitum and food was replaced daily as the cages were examined for animal maintenance. After habituation, mouse food intake and body weight were measured. The BioDAQ monitoring of feeding behavior was recorded and the data collected for two consecutive days were averaged. The BioDAQ system allows for a continuous monitoring of meal patterns in undisturbed mice by using a low spill food hopper placed on an electronic balance that is attached to the cage. Total food intake (grams) was recorded at each two-hour interval period. Feeding intake data were measured for a 24 hour period and then separated into either dark phase (18:00-06:00) or light phase (06:00-18:00). Bouts are defined as individual feeding events and are recorded by changes in stable weight, considering the start time, duration and amount of food consumed. Feeding bouts separated by more than a 5 minutes interval are considered as a meal. Meal parameters are defined as consisting of a bout interval of at least 5 seconds, duration of 5 minutes and minimum of 0.02g consumed food. Analysis of meal parameters, calculated by the software provided by the manufacturer (BioDAQ Monitoring Software 2.2.02), consisted of number of meals (meal frequency), meal size (mg), meal duration (min) and inter-meal interval. Eating rate is defined as the meal size (mg) over the duration of the analyzed period (12-hr) and the satiety ratio is defined as the average of the inter-meal interval divided by the average meal size.
Metabolic hormone measurements
At the end of the EchoMRI experiments, blood plasma was obtained from VIP−/− (n=7) and WT (n=8) mice after overnight fasting (18:00-06:00 h) and in postprandial conditions consisting in overnight fasting (18:00-06:00 h) followed by re-feeding period (06:00-10:00 h), blood being collected 1 hour after feeding. The collected plasma were then added with a cocktail of protease inhibitors containing DPPIV (Millipore; Billerica, MA), Roche Complete Protease Inhibitor Cocktail (Roche), Aprotinin (Fisher Scientific), and Roche Pefabloc SC (AEBSF) (Roche) and stored frozen at −80C until assayed. Plasma levels of active ghrelin, glucagon-like peptide-1 (GLP-1), peptide YY (PYY), Insulin, Glucagon, and Leptin were analyzed in duplicate using the Milliplex MAP Mouse Metabolic Hormone Magnetic Bead Panel (Millipore, Billerica, MA). Adiponectin was measured in duplicate by EIA (Millipore).
Data Analysis
First, a mixed-effects regression model was conducted to compare each of the following feeding parameters (number of bouts, bout minutes, number of meals, meal minutes, food consumed during meals, inter-meal interval, satiety ratio, and eating rate) between mouse types. Factors included were period (light vs. dark), type of mouse (WT vs. VIP−/−), and a two-way interaction (period-by-mouse type). Each model also included a subject-level random effect to account for repeated observations within mice. An ANOVA model with the following factors: type of mouse (WT vs. VIP −/−), age group (5, 10.5, 16, and 21.5 weeks), and a two-way interaction (age by mouse type) was used to compare each of the body weight and composition parameters between two types of mice.
Second, two types of food intake questions were of interest: (1) whether the patterns of food consumption differed between WT and VIP−/− mice during the light and dark phases and (2) whether there were differences in cumulative food intake between mouse types at the end of dark and light phases. To study these questions simultaneously, we used a single model approach, i.e., piecewise mixed-effects model with mouse-level random effects, to estimate the rate of food consumption and the cumulative food consumed for each mouse type during each of the two phases.
Third, metabolic hormone parameters were analyzed using two-way ANOVA models with mouse type, condition (fasting vs. postprandial), and a two-way interaction term between mouse type and condition.
All analyses were conducted using SAS 9.4 software (Cary, NC, SAS Institute). The figures were generated using GraphPad Prism 6 Software (La Jolla, CA).
RESULTS
Decreased body weight, fat mass composition and increased lean mass composition in VIP−/− mice
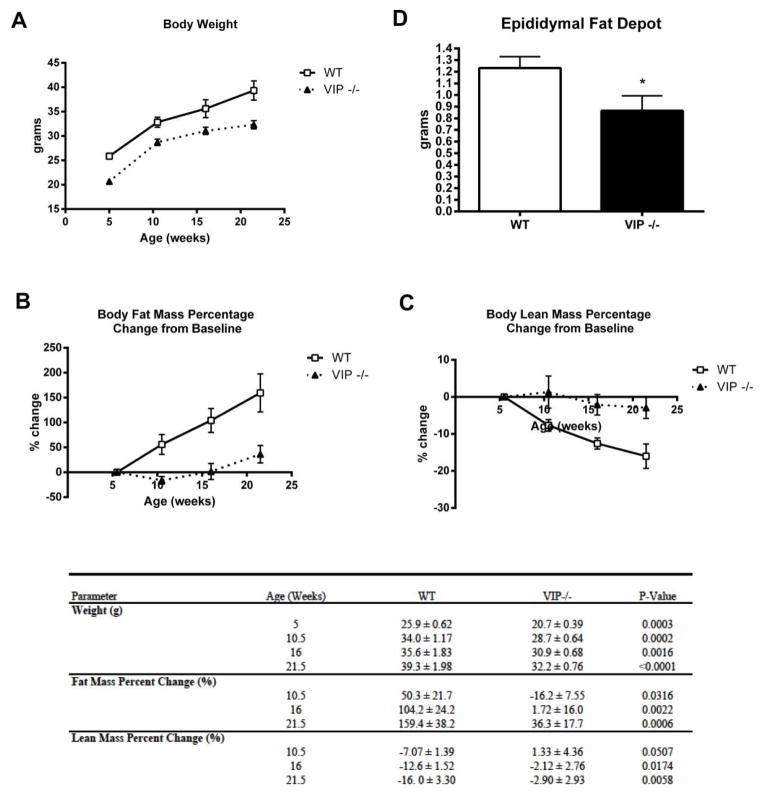
VIP−/− mice (n=8) fed a standard diet ad libitum for 21.5 weeks showed a significantly lower body weight, lower gain of fat mass and a reduced loss of lean mass throughout the study period compared to WT mice fed with the same diet (n=8) (Figure 1). VIP−/− mice had a consistently lower body weight average compared to WT mice throughout the 21.5 weeks of the study, beginning at five weeks of age (20.7 ± 0.39 vs. 25.9 ± 0.61 grams, p=0.0003) and at each 5.5 week interval measurement: at 10.5 weeks of age (28.7 ± 0.64 vs. 34.0 ± 1.17 grams, p=0.0002), at 16 weeks of age (30.9 ± 0.68 vs. 35.6 ± 1.83 grams, p=0.002) and finally at 21.5 weeks of age (32.2 ± 0.76 vs. 39.3 ± 1.98 grams, p<0.0001), as shown in Figure 1A.
Figure 1.
Vasoactive Intestinal Peptide deficient mice (VIP−/−) showed a lower body weight, fat mass and an increased lean mass. (A) VIP−/− mice had a consistently lower body weight average compared to WT mice throughout the 21.5 weeks of the study. (B) VIP−/− mice consistently demonstrated a lower tendency to accumulate body fat mass compared to WT mice beginning at 10.5 weeks of age through end of the study at 21.5 weeks of age. (C) VIP−/− mice showed a significant tendency as they aged to maintain their body lean mass compared to WT mice, as assessed at 10.5 weeks of age through the end of the study at 21.5 weeks of age. (D) VIP−/− mice had a significantly lower epididymal fat depot weight compared to WT mice.
Body fat and lean masses were analyzed as percentage of change from the first measurement obtained at 5.5 weeks of age, starting at 10.5 weeks of age and then at each 5.5 week interval as shown Figure 1B and 1C. VIP−/− mice consistently demonstrated a lower tendency to accumulate body fat mass, compared to WT mice: at 10.5 weeks of age (−16.2 ± 7.55 vs. 50.3 ± 21.7, p=0.032), at 16 weeks of age (1.72 ± 16.0 vs. 104.2 ± 24.2, p=0.002) and finally at 21.5 weeks of age (36.3 ± 17.7 vs. 159.4 ± 38.2, p=0.0006) (Figure 1B). Conversely, VIP−/− mice as they aged showed a significant tendency to maintain their body lean mass compared to WT mice, as assessed at 10.5 weeks of age (1.32 ± 4.36 vs. −7.07 ± 1.36, p=0.051), at 16 weeks of age (−2.12 ± 2.76 vs. −12.6 ± 1.52, p=0.017) and finally at 21.5 weeks of age (−2.90 ± 2.93 vs. −16.0 ± 3.30, p=0.006) (Figure 1C). At the end of the study, after the mice were euthanized, the collected epididymal fat depots had a significantly lower weight in VIP−/− mice compared to WT mice (1.23 ± 0.10 vs. 0.87 ± 0.13, p=0.044) (Figure 1D).
VIP plays a crucial role in regulating feeding behavior, appetite and satiety
Food consumption data of VIP−/− and WT mice were calculated in terms of 24 hours average values over 2 days of uninterrupted recording. The detailed time course of the cumulative food intake is illustrated in Figure 2. Results from the piecewise mixed-effects model indicated a disrupted feeding pattern observed in VIP−/− mice compared to WT mice, meaning that for WT mice food consumption rates were higher during the dark phase (0.44 ± 0.01 g per 2 hour; Table 1), whereas for VIP −/− mice the rates of food consumption were similar during both dark and light phases (0.21 ± 0.01 g per 2 hour; Table 1). Compared to their WT littermates, the VIP−/− mice ate less food during the dark phase (2.66 ± 0.06 vs. 1.24 ± 0.05, p < 0.0001, Table 1), but more food during the light phase (0.20 ± 0.06 vs. 1.24 ± 0.06, p < 0.0001, Table 1). Nonetheless, at the end of the 24 hours, VIP−/− mice still exhibited a significant reduction (approximately 15%) in cumulative food intake (2.48 ± 0.05 vs. 2.86 ± 0.06, p<0.0001).
Figure 2.
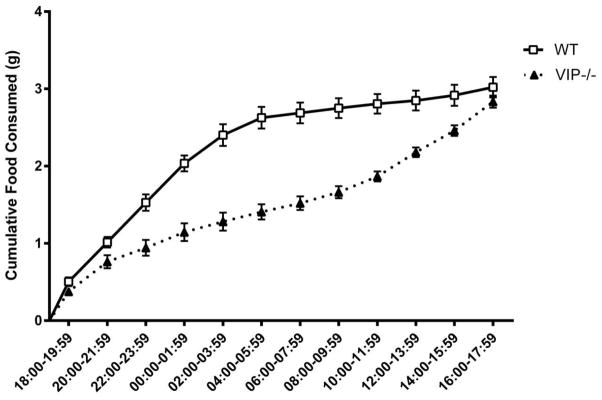
Food consumption data of VIP−/− and WT mice were calculated as 24 hours average values over 2 days of uninterrupted recording and a detailed time course of the cumulative food intake was calculated using 2 hour intervals.
Table 1.
Effects on Cumulative Food Intake From Piecewise Mixed-Effects Model
| Dark Phase | Light Phase | |
|---|---|---|
| Cumulative Food Intake | Mean (SE) | Mean (SE) |
| VIP−/− | 1.242 (0.052)1 | 1.240 (0.056)1 |
| WT | 2.655 (0.060)1 | 0.204 (0.064)2 |
| VIP−/− minus WT | −1.413 (0.080)1 | 1.037 (0.085)1 |
| Rate of Change (Slope) | Coefficient (SE) | Coefficient (SE) |
| VIP−/− | 0.207 (0.009)1 | 0.207 (0.009)1 |
| WT | 0.443 (0.010)1 | 0.034 (0.011)2 |
| VIP−/− minus WT | −0.236 (0.013)1 | 0.173 (0.014)1 |
p-value <0.0001,
p-value =0.002
Detailed feeding behavior parameters were analyzed at 12-hour intervals, during the dark as well as the light phase and summarized in Table 2. During the dark phase, VIP−/− reached the food hopper less frequently than WT mice, thus resulting in a significantly lower number of bouts (57.4 ± 6.1 vs. 86.0 ± 4.3, p=0.0001) and bout minutes (187 ± 31 vs. 274 ± 27 min, p=0.011). VIP−/− mice had significantly decreased meal size (1557 ± 160 vs. 2675 ± 134 mg, p<0.0001), lower meal frequency (6.11 ± 0.69 vs. 10.3 ± 0.99 meals, p=0.0007), lower meal duration (247 ± 36 vs. 373 ± 31 min, p=0.002), and lower eating rate (2.16 ± 0.22 vs. 3.72 ± 0.19 mg/min, p<0.0001). The inter-meal interval average was also significantly higher during the dark phase in VIP−/− compared to WT mice (113 ± 15.5 vs. 68.9 ± 9.6 min, p=0.035). Despite the significant difference in inter-meal intervals observed between the two types of mice, the difference in satiety ratio did not reach significance (414 ± 49 vs. 232 ± 30 min/mg food eaten). During the light phase, VIP−/− mice showed, as compared to WT, a significantly higher food intake and reached the food hopper significantly greater numbers of times resulting in higher number of bouts (37.1 ± 2.4 vs. 17.7 ± 3.4, p=0.005) and longer bout minutes (113 ± 8 vs. 37 ± 8, p=0.023). VIP−/− showed significantly increased meal size (1397 ± 87 vs. 336 ± 53 mg, p<0.0001), higher meal frequency (6.5 ± 0.5 vs. 2.9 ± 0.3 meals, p=0.003), longer time spent in meals (133 ± 7 vs. 41 ± 10 min, p=0.019) as well as a higher eating rate (1.94 ± 0.12 vs. 0.47 ± 0.07 mg/min, p<0.0001). The inter-meal interval average was significantly lower in VIP−/− compared to WT mice (67 ± 4 vs. 121 ± 17 min, p=0.014), corresponding to a significantly lower satiety ratio (319 ± 16 vs. 1308 ± 197 min/mg food eaten, p<0.0001).
Table 2.
Feeding intake microstructure in dark or light phase between WT vs. VIP−/− mice.
| Time | WT | Difference | VIP−/− | P-Value |
|---|---|---|---|---|
| Bouts (n) | ||||
| Dark Phase | 86 | 57.4 | −29 | 0.0001 |
| Light Phase | 17.7 | 37.1 | 19.5 | 0.0048 |
| Bout Minutes (min) | ||||
| Dark Phase | 274 | 187 | −86.8 | 0.0108 |
| Light Phase | 36.8 | 113 | 76 | 0.0234 |
| Meals (n) | ||||
| Dark Phase | 10.3 | 6.11 | −4.23 | 0.0007 |
| Light Phase | 2.87 | 6.5 | 3.62 | 0.0028 |
| Meal Minutes (min) | ||||
| Dark Phase | 372 | 247 | −126 | 0.0022 |
| Light Phase | 40.7 | 133 | 92.5 | 0.0185 |
| Meal (mg) | ||||
| Dark Phase | 2675 | 1557 | −1118 | <0.0001 |
| Light Phase | 336.2 | 1397 | 1060 | <0.0001 |
| Inter-meal Interval (min) | ||||
| Dark Phase | 68.9 | 113 | 44.4 | 0.0354 |
| Light Phase | 120 | 67 | −53.9 | 0.0135 |
| Satiety Ratio | ||||
| Dark Phase | 232 | 414 | 181 | 0.3273 |
| Light Phase | 1308 | 319 | −989 | <0.0001 |
| Eating Rate (mg/min) | ||||
| Dark Phase | 3.71 | 2.16 | −1.55 | <0.0001 |
| Light Phase | 0.467 | 1.94 | 1.47 | <0.0001 |
VIP regulates orexigenic and anorexigenic metabolic plasma hormone levels
In order to better evaluate the mechanisms by which VIP regulates feeding behavior, we measured the plasma levels of α-ghrelin, GLP-1, PYY, insulin, glucagon, leptin and adiponectin in both VIP−/− and WT mice during fasting as well as postprandial conditions. Compared to WT mice, VIP−/− mice had lower mean levels of orexigenic active ghrelin during fasting condition (241 ± 68 pg/mL vs. 448 ± 60 for VIP and WT −/−, respectively), but higher levels during postprandial conditions (Figure 3A). None of these differences reached statistical significant values. Anorexigenic GLP-1 levels were significantly increased in VIP−/− mice during fasting conditions (50.9 ± 14.5 vs. 21.3 ± 1.3 pg/mL, p=0.016) as well as during postprandial conditions (85.2 ± 7.8 vs. 33.5 ± 4.4 pg/mL, p=0.0002; Figure 3B). PYY was also significantly increased in VIP−/− mice during both fasting (271 ± 28 vs. 75 ± 28 pg/mL, p=0.0005) and postprandial conditions (268 ± 50 vs. 115 ± 27 pg/mL, p=0.004; Figure 3C). Strikingly, there was a significant up-regulation of plasma leptin levels during fasting conditions in VIP−/− compared to WT mice (4734 ± 1191 vs. 1357 ± 443 pg/mL, p=0.004 based on log scale), but no significant difference during postprandial conditions was observed (6311 ± 1496 vs. 8089 ± 1239 pg/mL) (Figure 3D). Insulin regulation was similar in both VIP−/− and WT mice during both fasting (328 ± 64 vs. 387 ± 84 pg/mL, p=0.97) and postprandial conditions (4708 ± 1531 vs. 3307 ± 871 pg/mL, p=0.28) as seen in Figure 3E. Glucagon levels were significantly increased in VIP−/− mice during postprandial (163 ± 47 vs. 52 ± 22 pg/mL, p=0.006) but not in fasting conditions (45.6 ± 12.1 vs. 20.1 ± 4.4 pg/mL) (Figure 3F). Finally, plasma adiponectin levels, measured during fasting conditions, were significantly lower in VIP−/− compared to WT mice (9.7 ± 0.4 vs. 11.6 ± 0.03 ng/mL, p=0.007; Figure 3G) whereas no significant differences were found in postprandial conditions in the different groups of mice.
Figure 3.
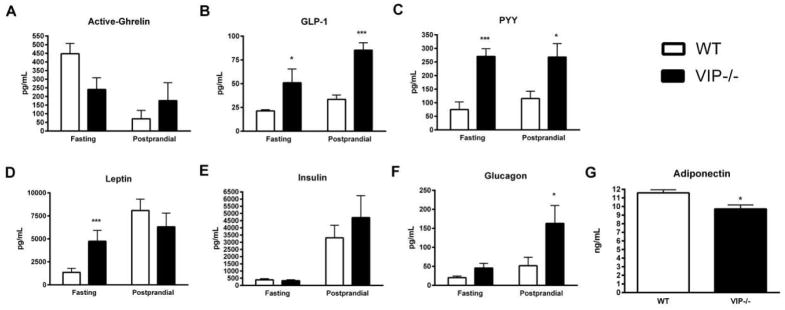
VIP regulates orexigenic and anorexigenic metabolic plasma hormone levels. (A) VIP−/− mice had lower levels of orexigenic active ghrelin during fasting condition, but higher levels during postprandial conditions. None of these data reached statistical significant value. (B) GLP-1 levels were significantly increased in VIP−/− mice during fasting as well as postprandial conditions. (C) PYY was significantly increased in VIP−/− mice during both fasting and postprandial conditions. (D) Plasma leptin levels were significantly up-regulated in VIP−/− during fasting as compared to WT mice, but no significant difference was recorded during postprandial conditions. (E) Insulin levels were similar in both VIP−/− and WT mice during both fasting and postprandial conditions. (F) Glucagon levels were significantly increased in VIP−/− mice only during postprandial conditions. (G) Plasma adiponectin levels, measured during fasting conditions, were significantly lower in VIP−/− as compared to WT mice.
DISCUSSION
Neuropeptides are released centrally in the hypothalamus and peripherally in the GI tract where they are known to be important regulators of digestive and immune functions. Recently, several neuromediators have been suggested to play an important role in the regulation of appetite/satiety, feeding behavior and also in energy balance and metabolism (Wang, 2007; Greenwood, 2011; Vu, 2011). The ARC neurons, containing neurotransmitter peptides associated with appetite signals (Woods, 1998), communicate with each other and with higher brain centers through released PACAP, neuropeptide Y, agouti-related peptide neurons and melanocortin-releasing neurons (Delgado, 1996; Barsh, 2000).
VIP neuropeptide is abundantly localized in the CNS as well as in the GI tract where it plays a key role in the regulation of many physiological functions such as mucosal ion transport, vasodilatation, hemodynamic regulation, gastric acid secretion, gastrointestinal motility, sphincter relaxation, neuronal excitability and mucosal inflammatory immune responses (Harmar, 2012). In this study we have characterized the overall physiologic role of endogenous VIP on the regulation of appetite and body phenotype by analyzing feeding behavior, body weight, body mass composition and metabolic hormone secretion, utilizing a C57BL/6 mice mouse model lacking VIP gene expression (Cowell, 2003).
Prior studies investigating the role of VIP on growth and development showed in VIP −/− mice or in WT mice treated with a VIP antagonist (Gressens, 2004) a reduced body weight and a delayed development, but the mechanism of this different body phenotype has not fully elucidated (Gozes, 1988; Hill, 1994; Girard, 2006; Lim, 2008). Therefore, in the current study we have utilized body NMR analysis to better evaluate the effects of VIP on body composition through accurate and sequential measurements of total body fat and lean masses in VIP−/− mice throughout the 22 weeks of our study as compared to WT controls. VIP−/− mice revealed a significantly reduced body fat mass and a preserved lean body mass as they aged compared to their WT littermates. At the end of the 22-week study, the VIP−/− mice showed a significantly leaner body profile. Additionally, the epididymal fat depots removed at the end of the study were significantly lower in VIP−/− compared to WT mice, providing further evidence of a deficit in fat mass accumulation. In previous studies ICV administration of VIP induced food intake decrease in several vertebrate species (Tachibana, 2003; Matsuda, 2005). Our study, on the other hand demonstrates that VIP−/− mice have lower body weight, an altered body composition together with an altered feeding behavior showing an increased food intake during the 12 hours light phase, and a decreased food intake during the 12 hours dark phase. Overall, our results support the hypothesis that the 15% decrease in food intake at the end of the 24 hour study period observed in VIP−/− mice, as compared to wild type littermates, can be explained by their reduced body size, slower growth rate and consequent lower metabolic needs. Recently, studies utilizing genome wide association analysis have strongly correlated VIP pathway genes to the development of fat mass accumulation and obesity (Liu, 2010). Analysis of these 500,000 SNPs from 1,000 citizens in the United Sates demonstrated that the VIP pathway is significantly associated with higher body fat mass accumulation (Liu, 2010). Therefore, our current findings support the hypothesis that VIP plays a major role in modulating body fat and lean mass composition changes that occur throughout lifetime and suggest that the an inhibition of VIP hormone can lead to lower body fat accumulation and higher lean mass maintenance.
VIP is a key molecule in mediating the synchronization of rhythms in clock gene expression (Cowell, 2003) and more recently accumulating evidence has revealed that core clock genes are important regulators of metabolism (Reppert, 2002). Several studies have demonstrated that circadian clocks are linked to obesity and body metabolism since they influence the physiologic functions of the endocrine, gastrointestinal, renal, hepatic systems and body temperature (Bass, 2010). Currently, studies in rodents and humans have suggested that the timing of food intake and energy balance plays an important role in the maintenance of a healthy metabolism and body phenotype (Green, 2008), but the published data are still very controversial. For example, Clock and Per2 mutant mice have similar disruptions in circadian behavior and feeding rhythms and eat during the night as well as the day period, have a tendency to develop an obese phenotype when fed with a high fat diet (Turek, 2005; Yang, 2009) in such a way showing an opposite phenotype compared to our VIP−/− mice. However, Clock and Per2 mutant mice when fed a regular diet failed to show a significant increase in body weight or fat mass as the VIP−/− in our current study. The higher lean body mass that we have observed in our VIP−/− mice parallels the gain of lean mass reported in Clock mutant mice fed with a regular diet (Turek, 2005). Conversely, mice lacking the circadian deadenylase gene Nocturin were observed to be resistant to diet-induced obesity, showing no reduction in food intake or activity, similarly to our studied VIP−/− mice (Green, 2007). In these models, the circadian system likely controls the expression of genes involved in the regulation of metabolic processes. Gene expression data from Nocturin deficient mice showed defects in lipids absorption and metabolism as suggested by the expression of the Pparγ gene, a principal regulator of adipogenesis in adipocytes and Srebp-1c, a transcription factor of several lipogenic genes. Similarly, Bmal1 a transcription factor known to regulate circadian rhythm, was shown to modulate adipogenesis and lipid metabolism in mature adipocytes (Shimba, 2005). Therefore VIP, as a key circadian mediator, could also similarly regulate adipogenesis and lipid metabolism, thus explaining the reduced adiposity observed in our VIP−/− mice.
VIP binds with high affinity to its VPAC1 and VPAC2 receptors (Harmar, 2012), which in turn would activate the signaling cascade leading to the metabolic changes responsible for the altered adipose tissue accumulation. In VIP−/− mice, there is no compensation by PACAP as determined using radioimmunoassay and RT-PCR (Girard, 2006). Through both VPAC1 and VPAC2, VIP is an important signaling mediator in adipocytes. Previously published in vitro data demonstrated that VPAC1 and VPAC2 are expressed on the murine preadipocyte NIH3T3-L1 cell line as well as on human and rat adipocytes (Alexander, 1995; Wei, 1996; Akesson, 2005). On fat cell membranes, VIP was found to be a potent stimulator of adenylate cyclase (Bataille, 1974) and through VPAC2 receptors it was shown to mediate lipolysis in primary rat adipocytes (Richter, 1989; Akesson, 2005). VPAC2 knockout male mice have been reported to have a leaner physical phenotype starting at 8 weeks of age with reduced body weight, length and reduced body fat in relationship to their body weight as they aged, as compared to their WT littermates (Asnicar, 2002). These body phenotypic characteristics reported in VPAC2−/− mice are similar to those that we have described in our VIP−/− mice in the current study. Previously published feeding behavior data in VPAC2−/− mice showed a significantly reduced amount of daily food intake, (Sheward, 2007; Bechtold, 2008), whereas in our VIP−/− mice we have found that the amounts of food intake were similar to their WT littermates. However, in the same publishes studies (Sheward, 2007; Bechtold, 2008) both VIP−/− and VPAC2−/− mice demonstrated an altered physiology with 3–4 hour advancement in their metabolic and feeding rhythms. Sheward et al. (Sheward, 2007) showed in VPAC2−/− mice liver, peripheral clock genes expression at feeding time even in absence of a functional SCN clock. This was confirmed in fasted rats by the administration of the VIP antagonist, [Lsy1, Pro2,5, Arg3,4,Tyr6]-VIP, which inhibited food intake-induced increase of plasmatic ACTH and corticosterone (Alexander, 1995). Conversely, VPAC1 receptors were not found to mediate lipolysis or significantly affect adipogenesis in vitro (Akesson, 2005; Sheward, 2007), but VPAC1 homozygous mutant knockout mice presented developmental delay and reduced body growth and weight, similar to our VIP−/− mice (Fabricius, 2011). However, a comprehensive study on body composition including lean and fat mass analysis has never been performed in VPAC1 knockout mice. In summary, even though both VPAC1 and VPAC2 receptors have been suggested to play an important role in promoting normal body weight gain and metabolism, the evidence accumulated through previously published data suggests that VIP could play a regulatory role in appetite, food intake and metabolism mainly through VPAC2 receptors.
GI hormones act within key areas of the hypothalamic ARC to regulate food intake and obesity (Williams, 2011). They are categorized as either anorexigenic hormones, which increase satiety and suppress appetite or orexigenic hormones that suppress satiety and increase appetite. Anorexigenic hormones include the gastrointestinal released GLP-1, PYY, cholesystokinin, PP (pancreatic polypeptide) (Dockray, 2004), the adipocytes-released leptin and the pancreatic β-cells-derived insulin. Orexigenic hormones include active-ghrelin, secreted by human gastric fundus P/D1 cells and pancreatic epsilon cells (Date, 2000). These combined hormones bind to their receptors in the GI tract and in the hypothalamus to control appetite, feeding, satiety, energy homeostasis and body mass composition. In the current study, we demonstrate that VIP is secreted in response to nutrient sensors and is essential in the regulation of anorexigenic and orexigenic hormones.
Previously, other authors demonstrated that plasma VIP concentrations increase following either a carbohydrate meal or water loading (Pedersen-Bjergaard, 1996), signaling a possible role for VIP in modulating appetite and food intake. Our data show that in VIP−/− mice, plasma levels of GLP-1, PYY, glucagon, adiponectin and leptin were altered in both fasting and post-prandial conditions, suggesting that VIP is a key regulator of metabolic hormones. The anorexigenic GLP-1 and PYY hormones were significantly higher in both fasting and postprandial conditions. GLP-1, a member of a subfamily that includes secretin, gastric inhibitory peptide (GIP) and VIP, is produced by intestinal L cells following food ingestion to stimulate glucose-dependent insulin secretion, β-cell growth and survival, as well as to inhibit glucagon release, gastric emptying and food intake (Marathe, 2013). For these hormonal effects, the GLP-1 system has been targeted (Garber, 2011) by using GLP-1 receptor agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors of GLP-1 degradation in order to establish new therapeutic approaches for treating type 2 diabetes and to promote satiety and body weight loss. Similarly PYY, secreted by the intestinal L cells in the gastrointestinal mucosa, is thought to be a satiety signal that reduces food intake (McGowan, 2004). VIP has been already described to stimulate PYY release in another animal model using isolated perfused rabbit distal colons (Ballantyne, 1993) and concurrently in our VIP−/− mice the levels of PYY were significantly more elevated than in WT. However, in our study the PYY plasmatic levels were unchanged in both fasting and postprandial conditions in VIP−/− mice, thus showing that a very important role for VIP in modulating PYY secretion. Also Martin et al. (Martin, 2010) have found GLP-1 and PYY plasma levels were altered in absence of VIP. Therefore, the higher levels of PYY in combination with GLP-1 and active-ghrelin could participate in the mechanisms involved in the altered appetite and phenotype observed in our VIP−/− mice. VIP receptors have been found to be highly expressed in the GI tract mucosa (Zimmerman, 1989). Other authors have reported that VIP can be released from the gastric fundus in rats (Boeckxstaens, 1992; D’Amato, 1992) and thereby can possibly regulate metabolic hormones secretion in the intestinal tract upon feeding. However, further studies are needed to fully identify the VIP receptors and pathways that are involved in GLP-1, PYY, glucagon, adiponectin and leptin release in the GI tract.
Additionally, in our study adipokines and glucagon levels were found significantly altered in VIP−/− mice in comparison to WT. The adipose tissue functions as an important endocrine organ to control energy homeostasis and metabolism through the secretion of adipokines, such as leptin and adiponectin (Yamauchi, 2001). VIP−/− mice had considerably higher plasma leptin compared to WT, despite their lean profile and lower body fat content. Leptin is secreted mainly by adipocytes but also by chief cells and P/D1 cells in the gastric mucosa to regulate food intake, appetite/satiety and energy expenditure (Bado, 1998; Friedman, 1998). Generally, the level of leptin in the peripheral blood is proportional to the adipose mass, as leptin plays a major physiologic function by informing the CNS about the amount of stored energy in order to restrain appetite and promote energy expenditure (Maffei, 1995; Considine, 1996). Therefore, our observed VIP−/− body lean phenotype and reduced fat mass content could be explained by the higher plasma leptin levels that would increase satiety and consumption of fat mass. The higher leptin levels found in VIP−/− mice in this study are similar to those observed by Martin et al. (Martin, 2010), but opposite to the levels observed in VPAC2−/− mice (Asnicar, 2002). Interestingly in VPAC2−/−, plasma leptin values were significantly lower compared to WT mice, despite the VPAC2−/− mice presented leaner phenotype and reduced body fat (Asnicar, 2002). Previously, PACAP was shown to mediate the anorexigenic effects of leptin centrally, not via VPAC1 or VPAC2 receptors, but only upon specific PAC1 receptor activation, as confirmed by the PAC1 specific antagonist PACAP6-38. Therefore, PACAP does not appear to directly mediate the effects of leptin through VPAC1 receptor, but through its high affinity PAC1 receptor. Therefore, VIP acting on VPAC1 could be an important regulator of leptin secretion, even though the involved receptor pathways need to be identified. Furthermore, adiponectin was found to be significantly higher in VIP−/− mice, in which most likely it could contribute to lipid and glucose metabolism alterations (Berg, 2002). Low adiponectin levels were demonstrated to be implicated in the development of insulin resistance in diet-induced models of obesity (Yamauchi, 2001). Our VIP−/− mice had no significant change in insulin levels, contrarily to those reported by Martin et al. (Martin, 2010), but they had significantly higher basal and postprandial levels of glucagon. However, previously VIP was shown to stimulate insulin secretion in a dose-dependent manner (Ahrén, 1981; Straub, 1996). Similarly, VIP was found to stimulate glucagon secretion in a dose-dependent manner in both normal and diabetic rats (Adeghate, 2000). In glucose homeostasis, Martin et al. (Martin, 2010) had shown that VIP−/− mice have significantly higher plasma glucose in both fasting and post-prandial conditions. Therefore, the VIP pathway could become in the future a target for the management of insulin and glycogen dysregulation.
In conclusion, this study to evaluate the effects of VIP genetic deficiency in the regulation of body phenotype in mice from 5 to 22 weeks, we report a significant reduction in body weight and fat mass accumulation. This altered body phenotype was associated with a significant up-regulation of leptin and a decrease in adiponectin plasma levels. Furthermore, VIP−/− mice showed a significantly altered feeding behavior structure. Finally, our data suggest that VIP acts as a nutrient sensor as levels of orexigenic and anorexigenic hormones were significantly altered in both fasting and postprandial conditions. Our current findings provide a new and more comprehensive picture of the overall VIP interplay between the circadian clock and the regulation of body composition, feeding behavior and metabolic hormones release. The regulation of appetite, food intake and metabolism is of major social importance given the worldwide increased prevalence of metabolic syndrome, overeating and obesity disorders, which are some of the major causes of morbidity and mortality in human patients. Our report concludes that VIP plays a crucial role in regulating body weight, fat and lean mass composition and some metabolic hormone secretion. However, future basic science studies are needed to elucidate the receptors and metabolic pathways by which VIP regulates lean body mass and adipocyte proliferation. In particular, an analysis of the genes involved in adipogenesis and lipid metabolism is necessary to fully highlight the mechanisms underlying the enhanced lean mass phenotype observed in VIP−/− mice. Additionally, pharmacological studies using VIP receptors antagonists should be developed to confirm and analyze in details the VIP pathways involved in adipocyte metabolism and feeding behavior. Our results highlight a novel key role for VIP in modulating appetite/satiety, feeding behavior and the release of the anorexigenic GLP-1, leptin, PYY, and insulin metabolic hormones, which ultimately can control energy homeostasis, body weight and mass composition. Finally, our data supports a key role for VIP in the control not only of appetite and feeding behavior but also in the age-dependent change of body phenotype, that includes body fat mass gain and lean mass loss, therefore suggesting that the VIP pathway may be an important target in the treatment of overeating and obesity disorders for the development of future clinical therapeutic protocols.
References
- Adeghate E, Ponery AS, Köves K. Distribution of vasoactive intestinal polypeptide and its effect on glucagon secretion from normal and diabetic pancreatic tissue fragments in rat. Ann N Y Acad Sci. 2000;921:434–7. doi: 10.1111/j.1749-6632.2000.tb07011.x. [DOI] [PubMed] [Google Scholar]
- Ahrén B, Lundquist I. Effects of vasoactive intestinal polypeptide (VIP), secretin and gastrin on insulin secretion in the mouse. Diabetologia. 1981;20(1):54–9. doi: 10.1007/BF00253818. [DOI] [PubMed] [Google Scholar]
- Akesson L, Ahrén B, Edgren G, Degerman E. VPAC2-R mediates the lipolytic effects of pituitary adenylate cyclase-activating polypeptide/vasoactive intestinal polypeptide in primary rat adipocytes. Endocrinology. 2005;146(2):744–50. doi: 10.1210/en.2004-0504. [DOI] [PubMed] [Google Scholar]
- Alexander LD, Evans K, Sander LD. A possible involvement of VIP in feeding-induced secretion of ACTH and corticosterone in the rat. Physiol Behav. 1995;58(2):409–13. doi: 10.1016/0031-9384(95)00058-q. [DOI] [PubMed] [Google Scholar]
- Asnicar MA, Köster A, Heiman ML, et al. Vasoactive intestinal polypeptide/pituitary adenylate cyclase-activating peptide receptor 2 deficiency in mice results in growth retardation and increased basal metabolic rate. Endocrinology. 2002;143(10):3994–4006. doi: 10.1210/en.2002-220354. [DOI] [PubMed] [Google Scholar]
- Bado A, Levasseur S, Attoub S, et al. The stomach is a source of leptin. Nature. 1998;394(6695):790–3. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- Ballantyne GH, Goldenring JR, Savoca PE, et al. Cyclic AMP-mediated release of peptide YY (PYY) from the isolated perfused rabbit distal colon. Regul Pept. 1993;47(2):117–26. doi: 10.1016/0167-0115(93)90415-5. [DOI] [PubMed] [Google Scholar]
- Barsh GS, Farooqi IS, O’Rahilly S. Genetics of body-weight regulation. Nature. 2000;404(6778):644–51. doi: 10.1038/35007519. [DOI] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349–54. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataille D, Freychet P, Rosselin G. Interactions of glucagon, gut glucagon, vasoactive intestinal polypeptide and secretin with liver and fat cell plasma membranes: binding to specific sites and stimulation of adenylate cyclase. Endocrinology. 1974;95(3):713–21. doi: 10.1210/endo-95-3-713. [DOI] [PubMed] [Google Scholar]
- Bechtold DA, Brown TM, Luckman SM, Piggins HD. Metabolic rhythm abnormalities in mice lacking VIP-VPAC2 signaling. Am J Physiol Regul Integr Comp Physiol. 2008;294(2):R344–51. doi: 10.1152/ajpregu.00667.2007. [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13(2):84–9. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- Bloom SR, Polak JM, Pearse AG. Vasoactive intestinal peptide and watery-diarrhoea syndrome. Lancet. 1973;2:14–16. doi: 10.1016/s0140-6736(73)91947-8. [DOI] [PubMed] [Google Scholar]
- Boeckxstaens GE, Pelckmans PA, De Man JG, Bult H, Herman AG, Van Maercke YM. Evidence for a differential release of nitric oxide and vasoactive intestinal polypeptide by nonadrenergic noncholinergic nerves in the rat gastric fundus. Arch Int Pharmacodyn Ther. 1992;318:107–15. [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, et al. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R939–49. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- D’Amato M, Currò D, Montuschi P, Ciabattoni G, Ragazzoni E, Lefebvre RA. Release of vasoactive intestinal polypeptide from the rat gastric fundus. Br J Pharmacol. 1992;105(3):691–5. doi: 10.1111/j.1476-5381.1992.tb09040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date Y, Kojima M, Hosoda H, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141(11):4255–61. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- Delgado M, Martinez C, Johnson MC, Gomariz RP, Ganea D. Differential expression of vasoactive intestinal peptide receptors 1 and 2 (VIP-R1 and VIP-R2) mRNA in murine lymphocytes. J Neuroimmunol. 1996;68:27–38. doi: 10.1016/0165-5728(96)00063-x. [DOI] [PubMed] [Google Scholar]
- Dockray G. Gut endocrine secretions and their relevance to satiety. Curr Opin Pharmacol. 2004;4(6):557–60. doi: 10.1016/j.coph.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Fabricius D, Karacay B, Shutt D, et al. Characterization of intestinal and pancreatic dysfunction in VPAC1-null mutant mouse. Pancreas. 2011;40(6):861–71. doi: 10.1097/MPA.0b013e318214c783. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34(Suppl 2):S279–84. doi: 10.2337/dc11-s231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard BA, Lelievre V, Braas KM, et al. Noncompensation in peptide/receptor gene expression and distinct behavioral phenotypes in VIP- and PACAP-deficient mice. J Neurochem. 2006;99(2):499–513. doi: 10.1111/j.1471-4159.2006.04112.x. [DOI] [PubMed] [Google Scholar]
- Gozes I, Schächter P, Shani Y, Giladi E. Vasoactive intestinal peptide gene expression from embryos to aging rats. Neuroendocrinology. 1988;47(1):27–31. doi: 10.1159/000124886. [DOI] [PubMed] [Google Scholar]
- Green CB, Douris N, Kojima S, et al. Loss of Nocturnin, a circadian deadenylase, confers resistance to hepatic steatosis and diet-induced obesity. Proc Natl Acad Sci U S A. 2007;104(23):9888–93. doi: 10.1073/pnas.0702448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134(5):728–42. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood HC, Bloom SR, Murphy KG. Peptides and their potential role in the treatment of diabetes and obesity. Rev Diabet Stud. 2011;8(3):355–68. doi: 10.1900/RDS.2011.8.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressens P, Hill JM, Paindaveine B, Gozes I, Fridkin M, Brenneman DE. Severe microcephaly induced by blockade of vasoactive intestinal peptide function in the primitive neuroepithelium of the mouse. J Clin Invest. 1994;94(5):2020–7. doi: 10.1172/JCI117555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Fahrenkrug J, Gozes I, et al. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br J Pharmacol. 2012;166(1):4–17. doi: 10.1111/j.1476-5381.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke Z, Ivanov TR, Bechtold DA, Dhillon H, Lowell BB, Luckman SM. PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J Neurosci. 2009;29(47):14828–35. doi: 10.1523/JNEUROSCI.1526-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM, Mervis RF, Politi J, et al. Blockade of VIP during neonatal development induces neuronal damage and increases VIP and VIP receptors in brain. Ann N Y Acad Sci. 1994;739:211–25. doi: 10.1111/j.1749-6632.1994.tb19823.x. [DOI] [PubMed] [Google Scholar]
- Inoue H, Shiosaka S, Sasaki Y, et al. Three-dimensional distribution of vasoactive intestinal polypeptide-containing structures in the rat stomach and their origins using whole mount tissue. J Neural Transm. 1984;59(3):195–205. doi: 10.1007/BF01250008. [DOI] [PubMed] [Google Scholar]
- Lam KS. Vasoactive intestinal peptide in the hypothalamus and pituitary. Neuroendocrinology. 1991;53(Suppl 1):45–51. doi: 10.1159/000125795. [DOI] [PubMed] [Google Scholar]
- Lelievre V, Favrais G, Abad C, et al. Gastrointestinal dysfunction in mice with a targeted mutation in the gene encoding vasoactive intestinal polypeptide: a model for the study of intestinal ileus and Hirschsprung’s disease. Peptides. 2007;28(9):1688–99. doi: 10.1016/j.peptides.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MA, Stack CM, Cuasay K, et al. Regardless of genotype, offspring of VIP-deficient female mice exhibit developmental delays and deficits in social behavior. Int J Dev Neurosci. 2008;26(5):423–34. doi: 10.1016/j.ijdevneu.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Guo YF, Zhang LS, et al. Biological pathway-based genome-wide association analysis identified the vasoactive intestinal peptide (VIP) pathway important for obesity. Obesity (Silver Spring) 2010;18(12):2339–46. doi: 10.1038/oby.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1(11):1155–61. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- Marathe CS, Rayner CK, Jones KL, Horowitz M. Glucagon-like peptides 1 and 2 in health and disease: a review. Peptides. 2013;44:75–86. doi: 10.1016/j.peptides.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Martin B, Shin YK, White CM, et al. Vasoactive intestinal peptide-null mice demonstrate enhanced sweet taste preference, dysglycemia, and reduced taste bud leptin receptor expression. Diabetes. 2010;59(5):1143–52. doi: 10.2337/db09-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Maruyama K, Nakamachi T, Miura T, Uchiyama M, Shioda S. Inhibitory effects of pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP) on food intake in the goldfish, Carassius auratus. Peptides. 2005;26(9):1611–6. doi: 10.1016/j.peptides.2005.02.022. [DOI] [PubMed] [Google Scholar]
- McGowan BM, Bloom SR. Peptide YY and appetite control. Curr Opin Pharmacol. 2004;4(6):583–8. doi: 10.1016/j.coph.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Pedersen-Bjergaard U, Høst U, Kelbaek H, et al. Influence of meal composition on postprandial peripheral plasma concentrations of vasoactive peptides in man. Scand J Clin Lab Invest. 1996;56(6):497–503. doi: 10.3109/00365519609088805. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–41. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Richter WO, Robl H, Schwandt P. Human glucagon and vasoactive intestinal polypeptide (VIP) stimulate free fatty acid release from human adipose tissue in vitro. Peptides. 1989;10(2):333–5. doi: 10.1016/0196-9781(89)90039-9. [DOI] [PubMed] [Google Scholar]
- Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169(3951):1217–8. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- Sheward WJ, Maywood ES, French KL, et al. Entrainment to feeding but not to light: circadian phenotype of VPAC2 receptor-null mice.Entrainment to feeding but not to light: circadian phenotype of VPAC2 receptor-null mice. J Neurosci. 2007;27(16):4351–8. doi: 10.1523/JNEUROSCI.4843-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimba S, Ishii N, Ohta Y, et al. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci U S A. 2005;102(34):12071–6. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Wang L, et al. Activation of brain somatostatin 2 receptors stimulates feeding in mice: analysis of food intake microstructure. Physiol Behav. 2010;101(5):614–22. doi: 10.1016/j.physbeh.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub SG, Sharp GW. Mechanisms of action of VIP and PACAP in the stimulation of insulin release. Ann N Y Acad Sci. 1996;805:607–12. doi: 10.1111/j.1749-6632.1996.tb17528.x. [DOI] [PubMed] [Google Scholar]
- Tachibana T, Saito S, Tomonaga S, et al. Intracerebroventricular injection of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibits feeding in chicks. Neurosci Lett. 2003;339(3):203–6. doi: 10.1016/s0304-3940(03)00017-x. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043–5. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52(2):269–324. [PubMed] [Google Scholar]
- Vu JP, Million M, Larauche M, et al. Inhibition of Vasoactive Intestinal Polypeptide (VIP) Induces Resistance to Dextran Sodium Sulfate (DSS)-Induced Colitis in Mice. J Mol Neurosci. 2014;52(1):37–47. doi: 10.1007/s12031-013-0205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu JP, Wang HS, Germano PM, Pisegna JR. Ghrelin in neuroendocrine tumors. Peptides. 2011;32(11):2340–7. doi: 10.1016/j.peptides.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HS, Oh DS, Ohning GV, Pisegna JR. Elevated serum ghrelin exerts an orexigenic effect that may maintain body mass index in patients with metastatic neuroendocrine tumors. J Mol Neurosci. 2007;33(3):225–31. doi: 10.1007/s12031-007-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Mojsov S. Tissue specific expression of different human receptor types for pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide: implications for their role in human physiology. J Neuroendocrinol. 1996;8(11):811–7. doi: 10.1046/j.1365-2826.1996.05191.x. [DOI] [PubMed] [Google Scholar]
- Williams KW, Elmquist JK. Lighting up the hypothalamus: coordinated control of feeding behavior. Nat Neurosci. 2011;14(3):277–8. doi: 10.1038/nn0311-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ, Porte D, Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280(5368):1378–83. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Yang S, Liu A, Weidenhammer A, et al. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology. 2009;150(5):2153–60. doi: 10.1210/en.2008-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman RP, Gates TS, Mantyh CR, et al. Vasoactive intestinal polypeptide receptor binding sites in the human gastrointestinal tract: localization by autoradiography. Neuroscience. 1989;31(3):771–83. doi: 10.1016/0306-4522(89)90440-5. [DOI] [PubMed] [Google Scholar]