Abstract
Thymic involution and the subsequent amplified release of autoreactive T cells increase the susceptibility toward developing autoimmunity, but whether they induce chronic inflammation with advanced age remains unclear. The presence of chronic low-level pro-inflammatory factors in elderly individuals (termed inflammaging) is a significant risk factor for morbidity and mortality in virtually every chronic age-related disease. To determine how thymic involution leads to the persistent release and activation of autoreactive T cells capable of inducing inflammaging, we used a FoxN1 conditional knockout (FoxN1-cKO) mouse model that induces accelerated thymic involution while maintaining a young periphery. We found that thymic involution leads to T cell activation shortly after thymic egress, which is accompanied by a chronic inflammatory phenotype consisting of cellular infiltration into non-lymphoid tissues, increased TNFα production and elevated serum IL-6. Autoreactive T cell clones were detected in the periphery of FoxN1-cKO mice. A failure of negative selection, facilitated by decreased expression of Aire rather than impaired regulatory T cell (Treg) generation, led to autoreactive T cell generation. Furthermore, the young environment can reverse age-related Treg accumulation in naturally aged mice, but not inflammatory infiltration. Together, these findings identify thymic involution and the persistent activation of autoreactive T cells as a contributing source of chronic inflammation (inflammaging).
Keywords: Aging, thymic involution, negative selection, regulatory T cells, autoimmunity, chronic inflammation
INTRODUCTION
Chronic inflammation is a ubiquitous feature of the aging process and implicated in virtually every age-related disease (1, 2). The term “inflammaging” describes the low-level, chronic, and systemic pro-inflammatory state that accompanies advanced age in the absence of infection (1). Even though clinical manifestations are not obvious, the presence of pro-inflammatory factors such as interleukin-6 (IL-6), tumor necrosis factor alpha (TNFα), interleukin-1 (IL-1), and C-reactive protein (CRP) are associated with the severity, incidence, and mortality of cardio vascular diseases such as atherosclerosis and myocardial infarction(3), neurodegenerative diseases such as Parkinson’s Disease(4) and Alzheimer’s Disease(1), and late-life cancers including colitis-associated colon cancer and hepatocellular carcinoma (5). IL-6 and TNFα are the most predictive inflammatory biomarkers and are highly correlated with “all-cause” morbidity and mortality in the elderly (6). Although the etiology of inflammaging is not fully understood, the source of pro-inflammatory factors is primarily attributed to the combination of cellular senescence induced senescence-associated secretory phenotype (SASP) and the persistent activation of immune cells (1, 2, 7).
The persistent activation of immune cells is hypothesized to result from chronic cell death and self-debris serving as damage-associated molecular patterns leading to innate immune cell activation (1, 8) or repeated life-long exposure to latent viral infections, such as cytomegalovirus (CMV) (9, 10), continuously activating both innate and adaptive immune responses. However, it is not known if thymic involution can lead to the persistent activation of T cells that are capable of inducing inflammaging.
The thymus is a primary lymphoid organ comprised of cortical and medullary thymic epithelial cells (cTECs and mTECs) responsible for the development of thymocytes and the generation of central immune tolerance towards self-tissues. Thymic driven central immune tolerance is accomplished through two mechanisms: the elimination of autoreactive T cell clones via the process of negative selection (11) and the generation of natural regulatory T cells (nTregs) via diverted differentiation (12, 13). These two processes depend on the T cell receptor (TCR): self-peptide-MHC avidity and signal strength, where a weak signal leads to thymocyte survival (14), a strong signal leads to clonal deletion (15) and a moderate signal plus cytokines (IL-2) leads to nTreg differentiation (16, 17). The efficiencies of negative selection and the differentiation of nTregs are dependent on the production and presentation of tissue-specific antigens (TSAs) on MHC, which is, in part, regulated by the autoimmune regulator gene (Aire) in mTECs (18–22).
The thymus undergoes a progressive and age-related involution attributed to the deterioration of the thymic microenvironment (23), which is made of an integrated three-dimensional meshwork of cTECs and mTECs, where TEC differentiation is regulated by the FoxN1 gene (24). It has been reported that defects in mTEC structure and the loss of Aire can affect the maintenance of central immune tolerance (25–27) by leading to the generation of fewer (28) or deficient nTregs (29), and thereby increasing the incidence of autoimmune disease. However, the mechanisms through which thymic involution impacts the two mechanisms of central tolerance (negative selection and nTregs) are not fully understood. Furthermore, whether thymic atrophy alone leads to the release of autoreactive T cells that become persistently activated immune cells and contribute to inflammaging remains unclear.
In this report, we focus on the involvement of thymic involution in inflammaging by utilizing a loxp-FoxN1-uCreERT conditional knockout (F-cKO), that induces accelerated thymic involution of a fully matured thymus while maintaining a young periphery (30), and naturally aged C57BL/6 mouse models. The F-cKO mouse model allows for the inducible deletion of FoxN1 after the thymus has fully matured, either by administering tamoxifen or the slow leakage of uCreERT, resulting in accelerated epithelial driven thymic atrophy that is comparable with thymic epithelium dysfunction observed in naturally aged C57BL/6 mice (24, 30). Although the slow leakage of uCreERT results in weak deletion of genomic FoxN1 at ~1 month of age (24), observable biological effects including the loss of FoxN1 expression, thymic involution, mTEC disruption, and thymic dysfunction do not become apparent until ~3–9 months of age (24) or until induced with the administration of tamoxifen (30). We demonstrate that thymic involution disrupts central immune tolerance and results in the release of autoreactive T cells to the periphery. Furthermore, shortly after thymic egress, these autoreactive T cells gain the activated immune cell phenotype and induce systemic low-grade inflammation that is indicative of inflammaging. Finally, we determined that the mechanism responsible for the thymic involution driven breakdown of immune tolerance results from perturbed negative selection and a reduction in the mTEC expression of Aire rather than defects in the generation of Tregs. Together, these results identify thymic involution as a contributing source of inflammaging and a potential therapeutic target for age-related chronic inflammation.
METHODS
Mice, Crossbreeding, and animal care
All animal experiments were in compliance with protocols approved by the Institutional Animal Care and Use Committee of the University of North Texas Health Science Center, in accordance with guidelines of the National Institutes of Health. Various gene manipulated mouse colonies (all on C57Bl/6 background) and their crossbreeding schemes are listed in supplemental Table-S1. They are the FoxN1 conditional knockout (cKO) (fx/fx-uCreERT mice with induced FoxN1 deletion via tamoxifen treatment: TM, termed “F-cKO”) (30); fx/fx-only (without uCreERT, same as wild-type “WT” in FoxN1 expression termed “FF-Ctr”(30); Rag2-GFP (green fluorescent protein) reporter; RAG−/− knockout; mOVA (Ovalbumin) transgenic (Tg); OT-II T cell receptor (TCR) transgenic; and autoimmunity regulator gene (Aire)−/− knockout mice. ~1–2 month old F-cKO mice display very faint deletion of FoxN1 exons 5&6 as detected by PCR, but do not differ from fx/fx-only control mice in FoxN1 expression, mTEC maturation, thymic size, etc (24). Following induced FoxN1 deletion via tamoxifen, ~1–2 month F-cKO mice display very strong deletion of FoxN1 exons 5&6 and undergo accelerated thymic involution (30). Mouse ages are indicated in each figure legend, defined young (1 – 2 months old) and aged (18 – 22 months old) groups. Aged WT mice were purchased from the National Institute on Aging.
Adoptive transfer
Erythrocyte-depleted spleen cells from young and aged WT mice, or young F-cKO mice, respectively, were intravenously (i.v.) injected through the retro-orbital route into young RAG−/− mice (2.5 × 107 cells per recipient mouse). Sixty days after the injection, the representative tissues (such as the lung, liver, salivary gland, as well as sera) of recipient RAG−/− mice were collected for analysis of inflammatory cell infiltration.
Thymic lobe kidney capsule transplantation
The surgical operation of the kidney capsule transplantation was performed as previously described (31). Intact newborn mouse thymic lobes of fx/fx-uCreERT and fx/fx-only with and without mOVA-Tg were directly transplanted into young host OT-II+ TCR-Tg mice. 3 days after the graft, the host mice were intraperitoneally (i.p.) injected with tamoxifen (TM;1mg/10g body weight/day) for 3 consecutive days to induce deletion of the FoxN1 gene. Two weeks after the last TM injection, the grafted thymi were isolated for FACS analysis of CD4 and CD8, as well as the TCR-Tg (Vα2Vβ5) marker.
Specific autoreactive T cell detection model: (IRBP) P2 immunization and P2-tetramer enrichment of IRBP specific T cells
The fx/fx-uCreERT (F-cKO) or fx/fx-only (FF-Ctr) mice (6 weeks old) were given 3x TM intraperitoneal (i.p.) injections to induce deletion of the FoxN1 gene. 4 weeks after the last TM injection, mice were immunized by subcutaneous injection of 100ug interphoto-receptor retinoid protein (IRBP, amino acids 294–306) P2 peptide emulsified in 100ul of complete Freund’s adjuvant (CFA). 10 days following immunization, cells from lymph nodes and spleen of the mice were harvested for IRBP-P2-IAb-tetramer (APC labeled) enrichment with anti-APC microbeads and MACS columns (Miltenyi Biotech), according to published protocols (32). Positively-selected cells were counted and then stained with antibodies for flow cytometry. P2-I-Ab tetramer was generated by the NIH Tetramer Core Facility and kindly provided by Dr. Mark Anderson (UCSF).
Flow cytometry assay
Single cell suspensions were prepared from the thymus and spleen of mice using a 70μm cell strainer. Spleen cells were erythrocyte-depleted with RBC lysing buffer (Sigma, Cat# R7757) and washed with staining buffer. Samples were then treated with Fc receptor blocking antibody 2.4G2. Samples were then stained with specific antibody of cell surface CD markers and or fixed with 2% PFA and permeabilized with Triton X100, as previously reported (33), followed by intracellular staining for Bim(cell signaling #2819s), Ki-67, Foxp3(ebioscience kit #12–5773–82) and Aire(ebioscience #50–5934–80). TECs were digested following previously published methods (33), and then stained with surface and intracellular antibodies. Fluorochrome conjugated antibodies (α:clone) CD4(GK1.5), CD8(53–6.7), CD44(IM7), Ki67(16A8), TNFα(MP6-XT22), CD25(PC61), TCR Vα2(B20.1), TCR Vβ5(MR9-4), CD45(30-F11), MHC-II(M5/114.15.2), Ly51(6C3), EpCAM(G8.8), and GITR(YGITR 765) were purchased from BioLegend. Flow cytometry was performed using a LSRII flow cytometer (BD Biosciences) and data were analyzed using Flow Jo software.
Lymphocyte isolation from non-lymphoid organs and flow cytometry based Treg analysis
Lymphocytes from non-lymphoid organs (liver, lung, and salivary gland) were isolated by two-layer density gradient centrifuge on Lympholyte-M (Cedarlane Labs #CL5031, Canada) at 2000 rpm for 15 min. The cells in between the two layers were collected for Treg cell staining. Thymocytes were isolated by cell strainer. Single cell suspensions were stained with fluorochrome-conjugated anti-mouse CD antibodies (BioLegend) Intracellular detection of FoxP3 with PE-conjugated anti-FoxP3 (clone FJK-16s, e-Bioscience) and detection of Bim with anti-Bim followed by secondary APC-conjugated anti-rabbit was performed on fixed and permeabilized cells via Cytofix/Cytoperm (e-Bioscience). Data were acquired and analyzed as above.
Treg suppressive function assay
fx/fx-uCreERT and fx/fx-only mice (3–4 months old) were treated with TM i.p. (F-cKO and FF-Ctr). 2–3 weeks following the final injection, spleens were harvested and passed through a cell strainer to obtain a single cell suspension. The samples were erythrocyte-depleted and washed, and then stained with anti-CD4 and anti-CD25. Cells were sorted on BD Influx Cell Sorter and collected into two groups: Treg (CD4+CD25+) or T effector (Teff, CD4+CD25−). 5 ×104 Teff: 2.5×104 Treg were co-cultured in 96-well U-bottom plate with: 5 ×104 irradiated APCs (splenocytes from C57Bl/6 mice), 1ug/ml anti-CD3ε, 2ug/ml anti-CD28, and RPMI-1640 medium totaling 100ul per well, for 72 hours. Proliferation of cells was determined using CellTiter 96 Aqueous One Solution Reagent (Progema, Cat#TB245) following the Progema protocol: adding 20ul solution per well for the last 2 hours of culture, and absorbance was measured at 490nm using an ELISA 96-well plate reader (BioTek ELx800).
H&E staining for visualizing lympho-infiltration
Following adoptive transfer into young RAG−/− mice, salivary glands were harvested and fixed, cut into 5 μm-thick sections, and stained with hematoxylin and eosin (H&E).
Statistics
For evaluation of group differences, the unpaired two-tailed Student’s t-test was used assuming equal variance. Differences were considered statistically significant at values of * p < 0.05; ** p < 0.01; *** p < 0.001.
RESULTS
Newly released T cells from the atrophied thymus acquire an activated immune cell phenotype
Recent evidence suggests that persistently activated immune cells may be a prime cause of age-related chronic inflammation, but the mechanisms leading to the chronic activation of immune cells are not fully understood (6, 9, 10). Utilizing our previously described loxp-FoxN1-uCreERT conditional knockout mouse model (F-cKO) for accelerated thymic involution (30), we sought to determine if thymic atrophy could lead to activated T cells without any additional stimuli. Administration of tamoxifen into ~1–2 month F-cKO allows for accelerated thymic involution that would not emerge from the slow leakage of uCreERT until ~3–9 months of age (24, 30). In order to differentiate T cells that have egressed from the atrophied thymus versus those that had egressed prior to thymic involution, we crossed our FoxN1-cKO mice with Rag2-GFP reporter mice with Rag2-promoter driven GFP expressing mice, which identifies newly generated T cells, or recent thymic emigrants (RTEs) (34–36). The GFP signal is turned on in thymocytes undergoing recombination-activating gene (RAG) dependent TCR recombination, and persists for up to two weeks following thymic egress (35).
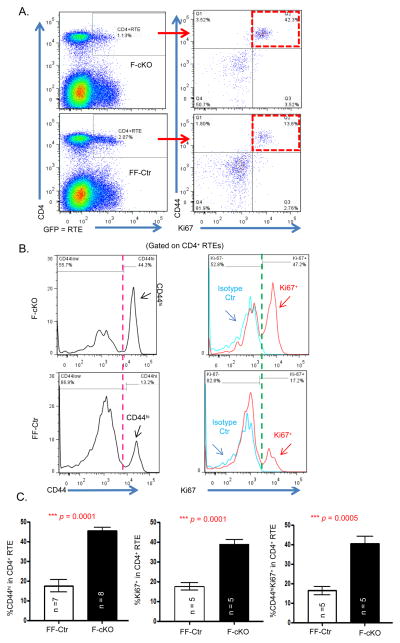
Generally, RTEs are not in a highly active state because they have yet to encounter their cognate antigens. As to be expected, using tamoxifen to conditionally knockout the FoxN1 gene in young fx/fx-uCreERT mice (F-cKO) to induce accelerated thymic atrophy (30) resulted in fewer CD4+ and CD8+ RTEs due to decreased thymic output (Fig. 1A, box in top-left panel, and supplemental Fig. S1A). However, within this reduced population of F-cKO RTEs, there was a higher proportion of CD44hi cells (Fig. 1B, left panels, and supplemental Fig. S1B), which represent antigen-experienced T cells (37), and these F-cKO RTEs were more proliferative (Fig. 1B and supplemental Fig. S1B, Ki67+ cells shown in red histograph in right panels). We further ascertained that the RTEs undergoing proliferation (Ki67+) were CD44hiRTEs (Figs. 1A, red boxes, and Fig. 1C, right panel). These mice are housed together in a clean environment and are specific pathogen free. Together, these results demonstrate that even in the absence of infection, CD4+ and CD8+ RTEs derived from the atrophied thymus take on an activated immune cell phenotype shortly after encountering the peripheral environment. Therefore, these activated T cells are potentially autoreactive T cells.
Figure 1. Newly released CD4+ T cells from the atrophied thymus acquired an activated immune cell phenotype.
F-cKO and FF-Ctr (fx/fx-uCreERT and fx/fx mice, respectively) were crossed with Rag2-GFP mice and then FoxN1fx/fx deletion was induced in 6-week-old adult mice with i.p. injected tamoxifen (TM). 14 days later, peripheral splenocytes were freshly isolated and stained with CD4, CD44, and Ki67 antibodies, and CD4+GFP+ cells were defined as CD4+ RTEs. (A) Representative dot plots show CD44hiKi67+ cell gates (red boxes) in CD4+ RTEs from F-cKO (top panels) and FF-Ctr control (bottom panels) mice. (B) Representative histograms of CD44hi (left panels) and Ki67+ (right panels) in CD4+ RTEs from F-cKO (top panels) and FF-Ctr control (bottom panels) mice. (C) Summarized results of % CD44hi, Ki67+, and CD44hiKi67+ double positive cells in CD4+ RTEs (from left to right panels). A Student t-test was used to determine statistical significance between groups. All data are expressed as mean ± SEM. Data are pooled from at least three independent experiments (n = animal numbers).
Thymic involution results in autoreactive T cells and chronic low-grade inflammation
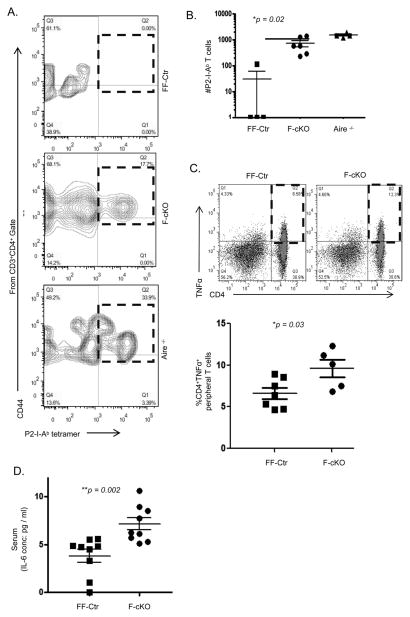
To verify whether activated RTEs derived from the atrophied thymus were responding to peripheral self-antigens, we employed an IRBP (Interstitial retinol-binding protein) immunization model to detect and amplify autoreactive T cell clones within a polyclonal T cell repertoire. IRBP is an eye protein expressed in mTECs as a tissue-specific antigen (TSA) under the control of the autoimmune regulator gene Aire (32, 38–40). Autoreactive T cells can be induced to incite autoimmune uveitis in Aire-deficient mice and detected in the spleen and lymph nodes following specific tetramer enrichment (41). In order to expand and detect autoreactive T cells, we first induced thymic involution through FoxN1-cKO. One month following induction of thymic involution, we immunized F-cKO, FF-Ctr, and Aire−/− (positive control) mice with an IRBP peptide-2 epitope (P2) and 10 days later pooled lymph nodes and spleen to enumerate CD4+ T cells reactive to the P2 peptide by P2-I-Ab tetramer enrichment. F-cKO mice showed a significant expansion of activated P2-I-Ab autoreactive T cells, which were mostly undetectable in littermate FF-Ctr controls (Figs. 2A and B). This likely explains the source of activated RTEs in our FoxN1-cKO thymic involution model as autoreactive T cell clones responding to peripheral self-antigens.
Figure 2. Autoreactive T cells and chronic low-grade inflammation following thymic involution.
One month following TM treatment for the conditional knockout of FoxN1, (A) F-cKO, FF-Ctr, and Aire−/− (positive control) mice were immunized with IRBP P2 peptide and lymphocytes were harvested 10 days later for flow cytometry following P2 tetramer enrichment of specific T cells. Plots are pre-gated on CD3+CD4+ events, and CD44hiP2-tetramer+ population is displayed in red boxes. (B) Calculated absolute number of P2 specific CD3+CD4+CD44hi peripheral T cells is shown in a bar format. (C) Freshly isolated splenocytes from FF-Ctr and F-cKO mice without immunization were co-stimulated with anti-CD28 & anti-CD3 ε in culture for 4.5 hrs, and then TNFα production was assessed by intracellular cytokine staining. (D) Sera from FF-Ctr and F-cKO mice in C were collected and IL-6 was measured by ELISA. A Student t-test was used to determine statistical significance between groups. All data are expressed as mean ± SEM. Data are pooled from three independent experiments (each symbol represents an animal).
Next, we wanted to know if the activated immune cell phenotype resulting from thymic involution was sufficient to induce an elevated pro-inflammatory state. We focused on the cytokines IL-6 and TNFα, which are the most indicative of poor prognosis in chronic age-related disease (2, 6). One month following induction of thymic involution, we observed a two-fold increase in the percentage of CD4+TNFα+ T cells in F-cKO mice compared to FF-Ctr littermate controls (Fig. 2C). Additionally, the concentration of IL-6 in the serum was found to be two-fold higher in F-cKO mice (Fig. 2D) compared to FF-Ctr littermate controls, implicating thymic involution in both local and systemic inflammation. This low-grade (just above baseline), but significant, inflammatory state found in the periphery of FoxN1-cKO mice is consistent with the conditions described in inflammaging.
We also found pathological changes in multiple organs, such as inflammatory cell infiltration in the liver, lung, pancreas, brain, and salivary glands of these FoxN1-cKO mice reported in our previously published manuscript (42), which confirms the presence of an autoreactive activated immune cell phenotype resulting from thymic involution, although neither dominant clinical manifestations nor full-blown autoimmune disease can be observed in these mice. Together, these results indicate that thymic involution alone is sufficient to induce a chronic and low-grade inflammatory state.
Conditional FoxN1 knockout impairs clonal deletion of single positive (SP) thymocytes resulting from defects in negative selection
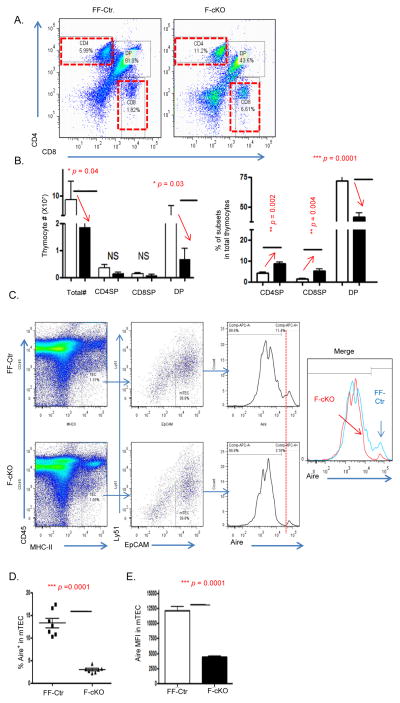
The detection of autoreactive T cells in the periphery of FoxN1-cKO mice indicates that thymic involution results in the impairment of central immune tolerance, disrupting either negative selection or the function/generation of nTregs. We first wanted to focus on the functional ability of the FoxN1-cKO atrophied thymus to execute negative selection and remove self-reactive TCRs from the developing TCR thymocyte pool. As we previously reported (30), conditional knockout of FoxN1 in TECs of young mice results in a significant decrease of total thymocyte number and causes thymic atrophy. We observed a dramatic reduction in the percentage and total cellularity of the CD4+CD8+ double positive (DP) thymocyte subpopulation (Figs 3A and B) in the F-cKO thymus compared to the FF-Ctr. Because of the highly stochastic nature of TCR rearrangement (43), one would presume that a diminished DP subpopulation would be accompanied by equally reduced CD4 and CD8 SP subpopulations. However, we found that the total numbers of CD4 and CD8 SP thymocytes were not reduced in the F-cKO thymus compared to the FF-Ctr, and the frequency of SP thymocytes is increased in the F-cKO atrophied thymus (Figs 3 A and B). The increased proportion of SP thymocytes suggests that the F-cKO cannot efficiently perform clonal deletion (negative selection) resulting from the loss of FoxN1 in TECs.
Figure 3. Clonal deletion of SP thymocytes was impaired in the FoxN1-cKO atrophied thymus.
(A and B) Five days after inducing FoxN1fx/fx deletion with TM in 6-week-old FF-Ctr and F-cKO mice, thymoyctes were freshly isolated for cell surface staining of CD4 vs. CD8. (A) Representative flow cytometry plots show CD4+ and CD8+ SP gates of F-cKO (right panel) and FF-Ctr control (left panel) mice. (B) Summarized results of absolute cell numbers per thymus (left panel) and % of each subpopulation in total thymocytes (right panel). Open bars represent FF-Ctr control mice, filled bars represent F-cKO mice. Each group contained seven animals. (C and D) Thymic epithelial cells were enzymatically digested from F-cKO and FF-Ctr thymi and intracellularly stained for Aire antibody. (C) Flow cytometry was used to gate on mTECs (CD45−, MHC-II+, EpCAM+, Ly51−), (D) Summarized results of the percentage of Aire+ mTECs, and (E) the mean fluorescent intensity of Aire+ mTECs. A Student t-test was used to determine statistical significance between groups. All data are expressed as mean ± SEM. Data are pooled from at least three independent experiments with a total of n = 7 animals per group.
We next attempted to investigate the mechanisms leading to the accumulation of SP thymocytes in the FoxN1-cKO thymus. Aire, which regulates expression of tissue-specific antigens, is one key factor in determining the efficiency of negative selection (21, 44). We previously published that Aire+ mTECs are reduced following the loss of FoxN1(42). Here, we further found that the reduction of Aire+ mTECs was not simply due to the reduction of total mTECs, but is actually due to a reduction in the frequency of Aire+ mTEC amongst all mTECs (Figs. 3C and D), as well as a reduction in the mTEC expression of Aire on a per cell basis, measured by mean fluorescent intensity (MFI) (Fig. 3E). This disruption in Aire expression following the loss of FoxN1 potentially perturbs negative selection by impairing the expression of Aire dependent self-antigens(42).
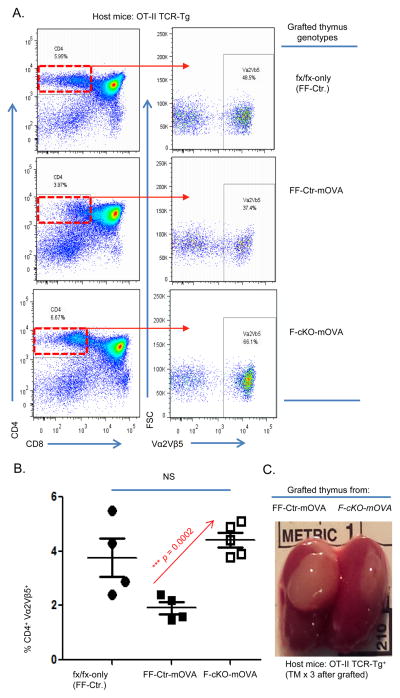
In order to find direct evidence that the loss of FoxN1 impairs the clonal deletion of autoreactive thymocytes, we utilized the OT-II-RIP-mOVA model of Aire dependent negative selection. RIP-mOVA+ mTECs express chicken ovalbumin as a mock self-antigen under the control of the rat insulin promoter by an Aire dependent mechanism (19, 45). OT-II bone marrow progenitors seed the kidney-grafted thymic lobes and gives rise to thymocytes with TCRs that are specific for ovalbumin, MHC-II restricted (CD4), and bind MHC:OVA complexes with a strong affinity. Thymocytes possessing TCRs that bind to MHC:OVA complexes with too strong of an affinity will be signaled to undergo clonal deletion. In our system, host mice do not receive any irradiation as in the conventional bone marrow transplantation method. Therefore, there is minimal risk for damage to the stromal cell niche that may affect antigen presentation. RIP-mOVA mice have been crossbred with FoxN1-cKO mice yielding F-cKO-mOVA, FF-mOVA, and FF-Ctr offspring. Fetal thymic lobes of equal size and cellularity (data not shown) were harvested from F-cKO-mOVA, FF-mOVA, and FF-Ctr newborns, and then transplanted under the kidney capsule of young adult OT-II transgenic mice. Three days after the transplantation surgery, i.p. injections of TM were administered to the OT-II host mice for 3 successive days in order to induce the FoxN1 conditional knockout in the kidney-grafted thymus. Two weeks after the final TM injection, the grafted thymic lobes were isolated for FACS analysis (Fig. 4C). As expected, OT-II hosts grafted with newborn thymic lobes from FF-Ctr mice that completely lack the mOVA transgene contain a normal proportion of OT-II specific CD4 SP thymocytes (CD4+CD8−Vα2Vβ5+) (Fig 4A top panel and 4B filled circles); when the OT-II progenitors seeded into the grafted FF-mOVA thymus that carries the mOVA transgene and wild-type levels of FoxN1 (not atrophied) then strong negative selection was observed in OT-II CD4 SP thymocytes (Fig 4A middle panel and 4B filled squares). However, when the OT-II progenitors seeded into the grafted F-cKO-mOVA thymus, which carries the mOVA transgene and the FoxN1-cKO induced thymic atrophy, the proportion of CD4+CD8−Vα2Vβ5+ cells was increased (Fig 4A bottom panel and 4B open squares) compared to FF-Ctr thymus, indicating that the OT-II CD4 SP thymocytes in F-cKO-mOVA thymus evade clonal deletion. These results provide direct evidence that the loss of FoxN1 and subsequent thymic involution perturbs negative selection leading to the survival of SP thymocytes that potentially recognize self-antigen.
Figure 4. mOVA expressing FoxN1-cKO thymi do not efficiently delete OT-II TCR transgenic thymocytes.
mOVA expressing mice were crossed with F-cKO and FF-Ctr to generate F-cKO-mOVA, FF-Ctr-mOVA, or FF-Ctr (without mOVA expression) genotypes. Newborn thymi of equal size from each genotype were grafted separately under the kidney capsule of OT-II host mice, which were then injected i.p. with TM to induce loxp-floxed FoxN1 deletion in the grafted thymi. Two weeks following kidney capsule transplantation, grafted thymi were harvested and stained with CD4, CD8, Vα2, and Vβ5 antibodies. (A) Representative dot plots of thymocytes from the grafted thymi gated on CD4 SP thymocytes (Left panels) and then the OT-II TCR+ (Vα2+Vβ5+) population (right panels) (B) Summarized results of the percentage of CD4SP-Vα2+Vβ5+ thymocytes of FF-Ctr (closed circle), FF-Ctr-mOVA (closed square) or F-cKO-mOVA (open square) grafted thymi. (C) Image of grafted thymus under OT-II kidney capsule two weeks post-surgery. A Student t-test was used to determine statistical significance between groups. All data are expressed as mean ± SEM. Data are pooled from three independent experiments (each symbol represents one animal).
Thymic involution does not impair the generation nor function of nTregs
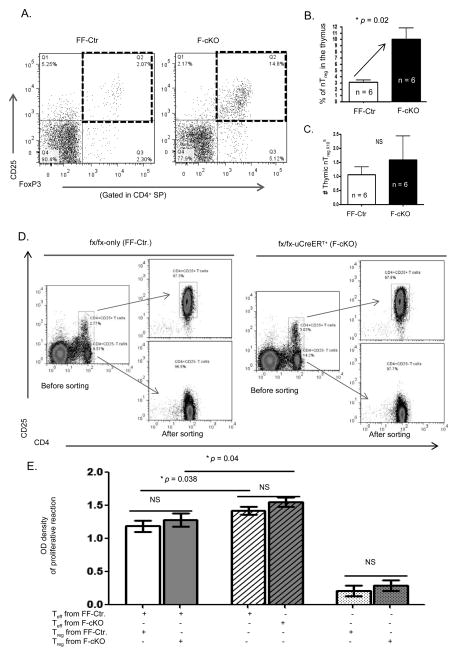
The manifestation of autoreactive inflammation in the FoxN1-cKO mouse model of accelerated thymic involution suggests a breakdown in the maintenance of central immune tolerance. We revealed measureable dysfunction in the process of negative selection in the FoxN1-cKO thymus. We next addressed whether FoxN1-cKO driven thymic involution alters the other arm of central immune tolerance: the generation of nTregs. Several reports have shown that the disruption of thymic medullary microstructure and a reduction in medullary size led to the generation of fewer or deficient CD4+ nTreg cells (29). Despite the severe depletion of overall thymic cellularity, there was not a significant difference (slightly increased) in the total numbers of thymic CD4+CD8−CD25+FoxP3+ nTregs (Figs. 5A and C) in the F-cKO thymus compared to FF-Ctr. Instead, the frequency of thymic nTregs was increased in the F-cKO mice (Fig. 5B). These findings suggest thymic involution does not impair the production of nTregs, and may actually be a contributing factor toward the age-related accumulation of Tregs (46).
Figure 5. Thymic involution does not impair the production nor function of nTregs.
Two – three weeks after inducing FoxN1fx/fx deletion with TM in F-cKO or FF-Ctr mice (3 – 4 months old) thymoyctes were freshly isolated for cell surface staining of CD4, CD8, CD25 and intracellular staining of FoxP3 (A, B, and C), or freshly isolated erythrocyte-depleted spleen cells were sorted for Treg-containing (CD4+CD25+) cells and T effector cells (CD4+CD25− Teffs) and then co-cultured to determine the suppressive function of Tregs on Teffs (D and E). (A) Representative dot plots show nTregs (CD4+CD25+FoxP3+) in the thymi of F-cKO and FF-Ctr control mice. Summarized results show the percentage (B), and absolute number (C) of thymic nTregs. (D) Representative dot plots before and after sorting of Treg and Teff cells from the spleen of F-cKO and FF-Ctr control mice. (E) Summarized results show suppressive function of Treg cells from F-cKO (dark bar) and FF-Ctr control mice (light bar), and proliferation of Teff cells from F-cKO (dark striped bar) and FF-Ctr control mice (light striped bar). A Student t-test was used to determine statistical significance between groups. All data are expressed as mean ± SEM. Data are pooled from three independent experiments with 6 animals/group (A, B, C) and 3 animals/group (D and E).
Irrespective to their numbers, Tregs must possess adequate suppressive capabilities in order to sustain immune tolerance. While many labs have shown that aged Tregs retain their suppressive function (46–49), others have reported a functional decline from thymic involution derived Tregs (29, 50). In order to determine if FoxN1-cKO-induced thymic atrophy impairs nTreg function, we tested the ability of peripheral Tregs derived from the FoxN1-cKO atrophied thymus to suppress the proliferation of effector T cells (Teff). Tregs (CD4+CD25+) were isolated from the spleens of either F-cKO or FF-Ctr mice, and Teffs (CD4+CD25−) were isolated from the spleens FF-Ctr mice (Fig. 5D). They were co-cultured in the presence of irradiated antigen presenting cells, and CD3ε and CD28 antibodies. We did not find any differences in the suppressive ability of Tregs coming from either F-cKO or FF-Ctr mice (Fig. 5E). Additionally, there were no measureable differences in the functional marker GITR (glucocorticoid-induced TNF receptor family-related gene) (12, 51) between F-cKO and FF-Ctr Tregs (Supplemental Fig. S2). These data suggest the progressive loss of FoxN1 and subsequent thymic involution does not impair the suppressive capacity of Tregs.
The peripheral environment rather than the involuted thymus determines the age-related accumulation of peripheral Tregs
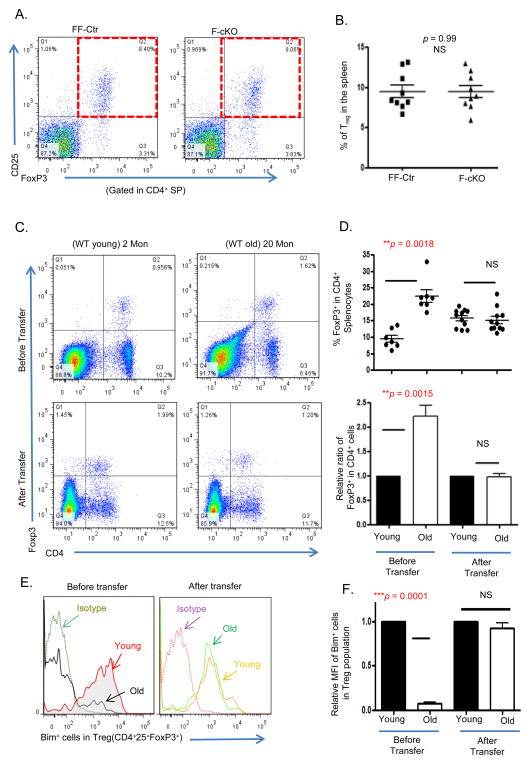
Natural aging induces the accumulation of Treg cells (the frequency is significantly increased in aged mice and humans) (46), which has been attributed to decreased levels of the pro-apoptotic gene Bcl2 family member Bim (52–54); however, whether thymic involution influences age-related accumulation of peripheral Tregs remains unclear. In Figs. 5A–C we observed that thymic involution increased the frequency of thymic nTregs, however this result did not appear to carry over into the periphery. We did not observe any peripheral accumulation, including splenic Tregs (Figs. 6A–B) and nonlymphoid (lung, liver, salivary gland) Tregs in the F-cKO mice (Supplemental Fig. S3).
Figure 6. The peripheral environment rather than the atrophied thymus determines the age-related accumulation of peripheral Tregs.
(A) Representative dot plots show the percentage of peripheral Tregs (CD4+CD25+FoxP3+) from the spleens of FF-Ctr and F-cKO mice. (B) Summarized results of % peripheral Tregs in FF-Ctr and F-cKO mice. (B – F) Young or aged erythrocyte-depleted spleen cells from WT mice were adoptively transferred into young RAG−/− mice. The peripheral Treg cells were analyzed before and 8 weeks after the transplantation via flow cytometry. (C) Representative dot plots show Treg gates before (Top panels) and after (Bottom panels) the transfer. (D) Top panel shows % FoxP3+ cells in CD4+ splenocytes; bottom panel shows relative ratio of FoxP3+ cells in CD4+ splenocytes. (E) Representative histogram of Bim+ Tregs in young and aged mice before and after the transfer. (F) Relative mean fluorescent intensity (MFI) of Bim within Treg cells before and after the transfer. A Student t-test was used to determine statistical significance between groups. All data are expressed as mean ± SEM. There is an (n = 5–11/group). Data are pooled from at least three independent experiments.
To better answer if thymic involution imparts any intrinsic changes onto nTregs that could lead to their peripheral accumulation, we performed an adoptive transplantation by infusing a pool of wild-type naturally aged (>18 months) T cells into young Rag2−/− mice, which lack T and B cells. Two months post-transfer, we found that the age-associated accumulation (frequency and relative ratio) of aged donor Treg cells was decreased, matching the levels of the young donor (6 week) wild-type control (Figs. 6C and D). Furthermore, the diminished expression of Bim observed in the aged Treg cell population was restored and equivalent to young mice (Fig. 6E). These results suggest that the peripheral microenvironment, rather than the thymus (Rag2−/− mice only have a rudimentary thymus), is responsible for the accumulation of Tregs with age. Moreover, the young peripheral microenvironment is capable of reducing a previously accumulated Treg cell pool.
Young microenvironment cannot reverse age-induced inflammatory infiltration
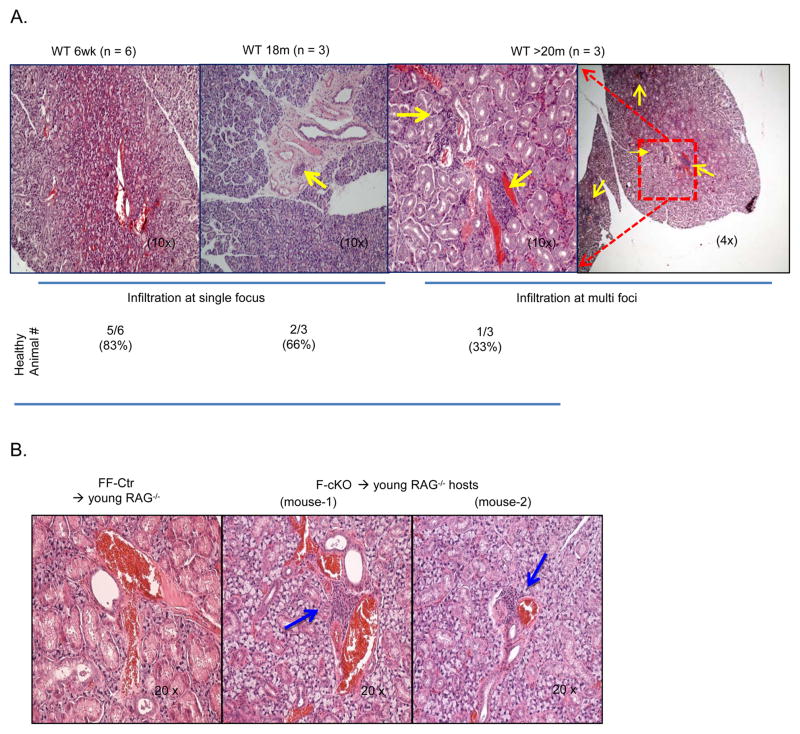
Our previous publications have shown that thymic involution leads to inflammatory infiltration in the lung, pancreas, liver, and most frequently in the salivary gland (42). However, we determined that the increased frequency of thymic nTregs does not persist into the periphery of FoxN1-cKO mice, and the age-related accumulation of peripheral Tregs can be reversed by the young microenvironment (Fig 6). Whether inflammatory infiltration is an intrinsic defect among T cells derived from an involuted thymus or dependent on age-related changes to the peripheral environment is unclear. To test whether the young microenvironment can protect against age-induced autoreactive T cells, we adoptively transferred either WT aged (>18 months) or WT young (<6 weeks) splenocytes into young Rag2−/− hosts. Two months after the infusion, we observed that young hosts infused with young splenocytes generally appeared healthy, but young hosts infused with aged splenocytes showed inflammatory infiltration around salivary blood vessels with severity that progressively worsened as the age of the donor increased. The 18 month donor was more likely to present with a single inflammatory foci, and hosts given >22 month donor cells were likely to display multi-foci infiltration (Fig. 7A).
Figure 7. Young microenvironment does not reverse age-induced inflammatory infiltration.
(A) Representative images of H&E stained salivary tissue slides from young RAG−/− mice, which were transplanted with splenocytes from donor young (6-week-old) and aged (18- or 20-month-old) C57Bl/6 WT mice for 8 weeks. Healthy refers to the absence of inflammatory infiltration. Single focus refers to one inflammatory infiltrating cluster being found in one tissue slide; Multi foci indicates that there were more than one inflammatory infiltrating cluster found in one tissue slide. Yellow arrows point out infiltrating clusters. n = animal numbers at the indicated age. (B) Representative images of H&E stained salivary tissue slides from young RAG−/− mice, which were transplanted with splenocytes from F-cKO (middle and right panels) and FF-Ctr control (left panel) mice for 8 weeks. Yellow arrows point out inflammatory infiltrating clusters. There were at least 5 animals in each group producing consistent results and data are representative of at least three independent experiments.
To eliminate the effect of peripheral age-related factors (thymus independent) on inflammatory infiltration, we infused a pool of T cells from young F-cKO or FF-Ctr splenocytes into young RAG−/− mice. Although FoxN1-cKO mice lack any peripheral age-related modifications not directly resulting from thymic involution, we found inflammatory cell infiltration in the salivary glands of the young Rag2−/− host mice infused with F-cKO (atrophied thymus) splenocytes, but infiltration was not observed in hosts infused with FF-Ctr (normal thymus) splenocytes (Fig. 7B).
Taken together, these results indicate that unlike the age-related accumulation of Tregs, inflammatory infiltration from the naturally aged or FoxN1-cKO atrophied thymus cannot be reversed by the young peripheral microenvironment. This indicates that inflammatory infiltration is an intrinsic defect in T cells imprinted during development in the involuted thymus.
DISCUSSION
Virtually every chronic age-related disease emerges under conditions of chronic inflammation, i.e. inflammaging, which provides a highly significant risk factor for both morbidity and mortality in the elderly. Although the characteristics of inflammaging, which describes a chronic systemic low-grade inflammation in the absence of overt/acute infection with advanced age, have been defined (7, 9, 55–57), the precise etiology and mechanisms responsible for inflammaging are largely unknown (1). Current knowledge of the etiology of inflammaging pinpoints SASP expressing senescent tissue cells(58) and the persistent activation of immune that likely arise from persistent latent viral infection (1). However, the predisposition towards autoimmunity in the elderly and the contribution of autoreactive T cells in generating a systemic inflammatory environment is widely overlooked in the prevention and treatment of inflammaging. In this study, we identified thymic involution resulting from the loss of FoxN1 as an additional source of activated immune cells (autoreactive T cells) that lead to a state of persistent and low-grade immunopathology with elevated pro-inflammatory cytokines. Newly generated T cells that have just exited the atrophied thymus of FoxN1-cKO mice adopt an activated and proliferative phenotype shortly after contacting the periphery, despite never encountering foreign antigen. Additionally, we were able to detect autoreactive T cells that specifically respond to the peripheral self-antigen IRBP in the periphery of FoxN1-cKO mice, which suggests T cell activation and elevated pro-inflammatory cytokines are the result of self-antigen stimulation. Interestingly, FoxN1-cKO mice do not develop overt autoimmune disease that would account for the elevated inflammatory milieu, but rather present with general and relatively mild infiltration in multiple non-lymphoid organs (42). Furthermore, it is unlikely that extrinsic aging-factors cause low-level T cell activation, as these inflammatory T cells cannot be rejuvenated by the young microenvironment. Since these inflammatory T cells do not encounter foreign antigen, they likely result from defects in the thymic driven generation and maintenance of immune tolerance.
Identification of activated autoreactive T cells in the periphery of FoxN1-cKO mice is evidence of defects in the maintenance of central immune tolerance. Here, we further identified impairment in the ability of the FoxN1-cKO involuted thymus to clonally delete OVA TCR transgenic thymocytes that recognize the endogenous RIP-mOVA antigen. mOVA expression in mTECs is under the control of the rat insulin promoter and dependent on Aire (19, 45). Complete knockout of the Aire gene (Aire−/−) disrupts negative selection and causes systemic autoimmune disease. Notably, even the partial loss of the Aire gene (heterozygous Aire+/−) leads to a drastic increase in islet-reactive T cells and progression towards diabetes in an insulin TCR transgenic mouse model (59). We previously showed mTEC structure disruption and an overall reduction in thymic Aire expression in the FoxN1-cKO mice (42). We report here that the percentage of Aire+ mTECs is diminished in FoxN1-cKO mice, and the expression of Aire in mTECs is decreased on a per cell basis. This implies that our observed negative selection impairment associated with thymic atrophy may result from the diminished expression of Aire in mTECs, which supports our previous finding of decreased tissue specific antigen expression in the FoxN1-cKO thymus (42). Although, there is evidence to support both Aire dependent (21, 44) and Aire independent (60) nTreg development, we did not observe any defects in the ability of the atrophied thymus to generate nTregs, despite the diminished expression of Aire in FoxN1-cKO thymi. It is possible that the low level of Aire present in the atrophied thymus is sufficient to support nTreg development, but is not sufficient enough to support the strong avidity necessary for the clonal deletion of autoreactive thymocytes. Additionally, it is possible that nTreg generation is maintained in the FoxN1-cKO thymus by Aire independent thymic dendritic cells (tDC) (22). We previously published that tDCs accumulation shifts from mTEC to cTEC regions in the atrophied thymus (42). However, further work needs to be done to determine if the loss of FoxN1 and thymic atrophy influences the functional ability of tDCs to facilitate negative selection.
It is unclear whether the loss of FoxN1 affects TCR avidity for MHC-self-peptide. The decreased negative selection with enhanced (at least unimpaired) generation of Tregs in the accelerated thymic involution model is probably better explained by an “avidity window” between negative selection (strong TCR signal strength) and deviation into the Treg lineage (moderate TCR signal strength) (61), i.e. diminishing the avidity of TCR for MHC-self-peptide should reduce the clonal deletion of negative selection and favor Treg cell induction in a specific cohort of MHC class II–restricted thymocytes.
In addition to negative selection, Tregs play a critical role in maintaining immune tolerance by inhibiting the activation of autoreactive T cells in the periphery. It has been reported that premature thymic aging, induced by a hypomorphic mutation in the FoxN1 gene (FoxN1Δ/Δ mice with a germline mutation) weakens the suppressive function of peripheral Tregs (50). However, this is contrary to our observation, which shows that Tregs derived from the FoxN1-cKO thymus can suppress the proliferation of FF-Ctr effector T cells just as efficiently as Tregs derived from the normal FF-Ctr thymus. Although CD4+CD25+ may not define all Tregs, our results are in agreement with others which show aged Foxp3-GFP Tregs maintain suppressive capabilities (47). Additionally, recent evidence suggests that there is an age-related accumulation of peripheral Tregs with increased suppressive function (46). Age-related Treg peripheral accumulation is suggested to result from decreased expression of the pro-apoptotic gene Bcl2 family member Bim (52–54). Here, we have shown peripheral Treg accumulation and diminished Bim expression in naturally aged wild-type mice can be normalized by the young peripheral microenvironment and are therefore not due to an intrinsic thymic atrophy-related defect, but potentially due to an extrinsic abnormality in the aged peripheral microenvironment. Although there are many age-related alterations to the peripheral environment including changes to circulating soluble factors, the stromal compartment, the hematopoietic compartment, and microbiota, the exact mechanisms signaling an age-related decrease in Bim and subsequent accumulation of Tregs remains unclear. Based on these findings, Treg accumulation and function do not appear to significantly contribute to thymic atrophy driven inflammaging.
Although direct anti-inflammatory interventions, such as the use of low dose aspirin or statins, to suppress, prevent, and alter the state of chronic inflammation hold great promise for treating multiple age-related diseases (1), targeting the sources of chronic inflammation may be the key to enhancing the prognosis of chronic age-related disease. Here, we have shown that in addition to developing a strategy for the elimination of senescent cells to suppress SASP (62), reduction or elimination of autoreactive T cells emigrated from the atrophied thymus is particularly promising to dampen age-related inflammation. We believe there are two strategies to do so. One is to rejuvenate the atrophied thymus to restore its function, including functional negative selection and functional generation of naïve T cells for TCR diversity. This rejuvenation of thymic involution will also improve immunosenescence, which is a major source of inflammaging (1). Removal of the atrophied thymus is another strategy that can be used to eliminate emigration of the harmful autoreactive T cells. This would likely require particular clinical criteria for the surgery in the elderly, such as ease of thymectomy (i.e. the patient is already having open chest surgery) or if the persistent chronic inflammation would significantly increase the morbidity of other diseases. While vaccination against persistent infections is another possible strategy to manage the persistent activation of immune cells, it does not address autoreactive immune cell activation.
A caveat to this study is the frequent use of our FoxN1-conditional knockout mouse model of accelerated thymic involution in place of a true natural age-related atrophied thymus from >18 month wild-type mice. Progressive thymic involution is an age-related alteration and its causes are almost certainly not due to only the loss of one gene. This complex and multifaceted nature of thymic involution is evidenced by the various processes implicated in age-related thymic atrophy including the involvement of other genes like ink4a (63), hormones (64), and adipocyte expansion (65–67). However, because thymic involution occurs alongside total body aging, separating the effects of thymic age from systemic aging (which includes the circulation of soluble factors, T cells, and dendritic cells back into the thymus) is extremely difficult, if not impossible, in an 18 month wild-type mouse. The strength of our FoxN1-conditional knockout model lies in the ability to induce the involution of a mature thymus while avoiding the effects of systemic aging. In fact, our model (both TM-induced and slow leakage of uCreERT) undergoes thymic involution including a loss of FoxN1+ TECs(24, 30), a decline in mature mTEC(24, 30, 42), thymic dendritic cell distribution (42), increased senescent clusters, and increased TAp63+ TECs (33) similar to 18 month C57BL/6 mice. Furthermore, naturally aged C57BL/6 mice and F-cKO splenocytes that were transferred into a young environment induced similar inflammatory infiltrates in the salivary gland (Fig. 7). Additionally, unlike the pre-natal FoxN1Δ/Δ mutation that blocks TEC maturation and reduces Treg function (50), our inducible FoxN1-cKO model of mature thymic atrophy maintains Treg suppressive capacity consistent with naturally aged Tregs (46). However, our FoxN1-cKO model undergoes an extremely accelerated thymic involution that is dissimilar to the 1–3% shrinkage per year observed in the naturally involuting thymus (68). Furthermore, similar to natural age-related thymic involution, the FoxN1-cKO model does not exhibit thymic regrowth that is seen in acute thymic involution following infection and pregnancy (67). However, the FoxN1-cKO model still does not fully recapitulate the physiology of the chronic and extremely gradual nature of natural age-related thymic involution. Therefore, the effects of rapid, as opposed to gradual, involution on defective negative selection and the emergence of chronic inflammation cannot be eliminated. Although the induced conditional knockout of FoxN1 and subsequent accelerated thymic involution in the fully mature thymus may not exactly mimic the naturally aged thymus, there are many molecular, morphological, and functional characteristics shared between the two. Therefore, the FoxN1-cKO model is useful to study thymic involution and is applicable for age-related thymic involution with careful consideration for the limitations of the animal model.
In summary, we have demonstrated that the steady release of autoreactive T cells resulting in tissue-specific inflammation following thymic involution contributes to low grade chronic inflammation, known as inflammaging. The observed inflammaging is the consequence of a T cell predisposition towards autoimmunity that arises from a decline in FoxN1 expression leading to the disruption of the steady-state thymic medullary compartment (24, 42, 69) and subsequent thymic involution. Thymic involution results in the release of autoreactive T cells that become activated shortly after reaching the periphery and produce low levels of inflammatory cytokines without causing overt autoimmune disease. The autoreactive T cells result from intrinsic defects in negative selection, rather than changes to the extrinsically maintained Treg cell pool. Our studies shed new light on the importance of targeting thymic involution in addition to improving the peripheral immune microenvironment as a potential treatment for eliminating inflammaging and ultimately reducing morbidity and mortality in chronic age-related disease.
Supplementary Material
Acknowledgments
Funding: This work was supported by NIAID/NIH grants R01AI081995 to D-M. S. and 3R01AI081995-03S1 to B. C.
We thank Dr. Mark Anderson (UCSF) for kindly providing IRBP peptide and tetramer, Dr. Rance Berg (UNTHSC) for critical reading of the manuscript, Dr. Xiangle Sun (UNTHSC) for flow cytometry technical support, and the NIH Tetramer Core Facility for providing tetramer reagent.
Abbreviations
- Aire
autoimmune regulator gene
- cKO
conditional knockout
- cTEC/mTEC
cortical/medullary thymic epithelial cells
- DP
double positive thymocytes
- F-cKO
FoxN1 conditional knockout
- fx
loxP-floxed-FoxN1 (FoxN1flox)
- OVA
Ovalbumi
- RTE
recent thymic emigrants
- SP
single positive thymocytes
- TM
tamoxifen
- Tg
transgenic
- Treg
regulatory T cells
- uCreERT
ubiquitous promoter-driven Cre-recombinase and estrogen-receptor fusion protein
- WT
wild type
Footnotes
Author declaration:
We have no competing financial interests.
Author contribution:
Conceived and designed the experiments: D-M.S. and B.C. Performed the experiments and analyzed the data: B.C., L.R., H.W., Wrote the paper: D-M.S. and B.C.
References
- 1.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(Suppl 1):S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 2.Howcroft TK, Campisi J, Louis GB, Smith MT, Wise B, Wyss-Coray T, Augustine AD, McElhaney JE, Kohanski R, Sierra F. The role of inflammation in age-related disease. Aging (Albany NY) 2013;5:84–93. doi: 10.18632/aging.100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arteriosclerosis, thrombosis, and vascular biology. 1999;19:2364–2367. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 4.Martinez TN, Chen X, Bandyopadhyay S, Merrill AH, Tansey MG. Ceramide sphingolipid signaling mediates Tumor Necrosis Factor (TNF)-dependent toxicity via caspase signaling in dopaminergic neurons. Molecular neurodegeneration. 2012;7:45. doi: 10.1186/1750-1326-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Annals of the rheumatic diseases. 2011;70(Suppl 1):i104–108. doi: 10.1136/ard.2010.140145. [DOI] [PubMed] [Google Scholar]
- 6.Varadhan R, Yao W, Matteini A, Beamer BA, Xue QL, Yang H, Manwani B, Reiner A, Jenny N, Parekh N, Fallin MD, Newman A, Bandeen-Roche K, Tracy R, Ferrucci L, Walston J. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. The journals of gerontology. Series A, Biological sciences and medical sciences. 2014;69:165–173. doi: 10.1093/gerona/glt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends in molecular medicine. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dall’Olio F, Vanhooren V, Chen CC, Slagboom PE, Wuhrer M, Franceschi C. N-glycomic biomarkers of biological aging and longevity: a link with inflammaging. Ageing research reviews. 2013;12:685–698. doi: 10.1016/j.arr.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Brunner S, Herndler-Brandstetter D, Weinberger B, Grubeck-Loebenstein B. Persistent viral infections and immune aging. Ageing research reviews. 2011;10:362–369. doi: 10.1016/j.arr.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nature reviews. Immunology. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer E. Negative selection--clearing out the bad apples from the T-cell repertoire. Nature reviews. Immunology. 2003;3:383–391. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunological reviews. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 13.Wirnsberger G, Hinterberger M, Klein L. Regulatory T-cell differentiation versus clonal deletion of autoreactive thymocytes. Immunol Cell Biol. 2011;89:45–53. doi: 10.1038/icb.2010.123. [DOI] [PubMed] [Google Scholar]
- 14.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annual review of immunology. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 15.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nature reviews. Immunology. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 17.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waterfield M, Khan IS, Cortez JT, Fan U, Metzger T, Greer A, Fasano K, Martinez-Llordella M, Pollack JL, Erle DJ, Su M, Anderson MS. The transcriptional regulator Aire coopts the repressive ATF7ip-MBD1 complex for the induction of immunotolerance. Nature immunology. 2014;15:258–265. doi: 10.1038/ni.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metzger TC, Anderson MS. Control of central and peripheral tolerance by Aire. Immunological reviews. 2011;241:89–103. doi: 10.1111/j.1600-065X.2011.01008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson MS, Su MA. Aire and T cell development. Curr Opin Immunol. 2010;23:198–206. doi: 10.1016/j.coi.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, Amit AS, Kang C, Geddes JE, Allison JP, Socci ND, Savage PA. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry JS, Lio CW, Kau AL, Nutsch K, Yang Z, Gordon JI, Murphy KM, Hsieh CS. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity. 2014;41:414–426. doi: 10.1016/j.immuni.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo J, Rahman M, Cheng L, Zhang S, Tvinnereim A, Su DM. Morphogenesis and maintenance of the 3D thymic medulla and prevention of nude skin phenotype require FoxN1 in pre- and post-natal K14 epithelium. J Mol Med. 2011;89:263–277. doi: 10.1007/s00109-010-0700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun L, Guo J, Brown R, Amagai T, Zhao Y, Su DM. Declining expression of a single epithelial cell-autonomous gene accelerates age-related thymic involution. Aging Cell. 2010;9:347–357. doi: 10.1111/j.1474-9726.2010.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallegos AM, Bevan MJ. Central tolerance: good but imperfect. Immunological reviews. 2006;209:290–296. doi: 10.1111/j.0105-2896.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 26.Fletcher AL, Seach N, Reiseger JJ, Lowen TE, Hammett MV, Scott HS, Boyd RL. Reduced thymic Aire expression and abnormal NF-kappaB2 signaling in a model of systemic autoimmunity. Journal of immunology. 2009;182:2690–2699. doi: 10.4049/jimmunol.0801752. [DOI] [PubMed] [Google Scholar]
- 27.Rucci F, Poliani PL, Caraffi S, Paganini T, Fontana E, Giliani S, Alt FW, Notarangelo LD. Abnormalities of thymic stroma may contribute to immune dysregulation in murine models of leaky severe combined immunodeficiency. Frontiers in immunology. 2011:2. doi: 10.3389/fimmu.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. The Journal of experimental medicine. 2005;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lomada D, Liu B, Coghlan L, Hu Y, Richie ER. Thymus medulla formation and central tolerance are restored in IKKalpha-/- mice that express an IKKalpha transgene in keratin 5+ thymic epithelial cells. Journal of immunology. 2007;178:829–837. doi: 10.4049/jimmunol.178.2.829. [DOI] [PubMed] [Google Scholar]
- 30.Cheng L, Guo J, Sun L, Fu J, Barnes PF, Metzger D, Chambon P, Oshima RG, Amagai T, Su DM. Postnatal tissue-specific disruption of transcription factor FoxN1 triggers acute thymic atrophy. The Journal of biological chemistry. 2010;285:5836–5847. doi: 10.1074/jbc.M109.072124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu X, Gui J, Dohkan J, Cheng L, Barnes PF, Su DM. Lymphohematopoietic progenitors do not have a synchronized defect with age-related thymic involution. Aging Cell. 2007;6:663–672. doi: 10.1111/j.1474-9726.2007.00325.x. [DOI] [PubMed] [Google Scholar]
- 32.Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nature protocols. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burnley P, Rahman M, Wang H, Zhang Z, Sun X, Zhuge Q, Su DM. Role of the p63-FoxN1 regulatory axis in thymic epithelial cell homeostasis during aging. Cell death & disease. 2013;4:e932. doi: 10.1038/cddis.2013.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu W, Nagaoka H, Jankovic M, Misulovin Z, Suh H, Rolink A, Melchers F, Meffre E, Nussenzweig MC. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 1999;400:682–687. doi: 10.1038/23287. [DOI] [PubMed] [Google Scholar]
- 35.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nature immunology. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 36.McCaughtry TM, Wilken MS, Hogquist KA. Thymic emigration revisited. The Journal of experimental medicine. 2007;204:2513–2520. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baaten BJ, Tinoco R, Chen AT, Bradley LM. Regulation of Antigen-Experienced T Cells: Lessons from the Quintessential Memory Marker CD44. Frontiers in immunology. 2012;3:23. doi: 10.3389/fimmu.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson G, Harman BC, Hare KJ, Jenkinson EJ. Microenvironmental regulation of T cell development in the thymus. Seminars in immunology. 2000;12:457–464. doi: 10.1006/smim.2000.0260. [DOI] [PubMed] [Google Scholar]
- 40.Taniguchi RT, DeVoss JJ, Moon JJ, Sidney J, Sette A, Jenkins MK, Anderson MS. Detection of an autoreactive T-cell population within the polyclonal repertoire that undergoes distinct autoimmune regulator (Aire)-mediated selection. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7847–7852. doi: 10.1073/pnas.1120607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeVoss J, Hou Y, Johannes K, Lu W, Liou GI, Rinn J, Chang H, Caspi RR, Fong L, Anderson MS. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. The Journal of experimental medicine. 2006;203:2727–2735. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia J, Wang H, Guo J, Zhang Z, Coder B, Su DM. Age-Related Disruption of Steady-State Thymic Medulla Provokes Autoimmune Phenotype via Perturbing Negative Selection. Aging Dis. 2012;3:248–259. [PMC free article] [PubMed] [Google Scholar]
- 43.Jung D, Alt FW. Unraveling V(D)J recombination; insights into gene regulation. Cell. 2004;116:299–311. doi: 10.1016/s0092-8674(04)00039-x. [DOI] [PubMed] [Google Scholar]
- 44.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nature immunology. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 45.Hubert FX, Kinkel SA, Davey GM, Phipson B, Mueller SN, Liston A, Proietto AI, Cannon PZ, Forehan S, Smyth GK, Wu L, Goodnow CC, Carbone FR, Scott HS, Heath WR. Aire regulates transfer of antigen from mTEC to dendritic cells for induction of thymic tolerance. Blood. 2011 doi: 10.1182/blood-2010-06-286393. [DOI] [PubMed] [Google Scholar]
- 46.Raynor J, Lages CS, Shehata H, Hildeman DA, Chougnet CA. Homeostasis and function of regulatory T cells in aging. Curr Opin Immunol. 2012;24:482–487. doi: 10.1016/j.coi.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, Belkaid Y, Chougnet C. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. Journal of immunology. 2008;181:1835–1848. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun L, Hurez VJ, Thibodeaux SR, Kious MJ, Liu A, Lin P, Murthy K, Pandeswara S, Shin T, Curiel TJ. Aged regulatory T cells protect from autoimmune inflammation despite reduced STAT3 activation and decreased constraint of IL-17 producing T cells. Aging Cell. 2012;11:509–519. doi: 10.1111/j.1474-9726.2012.00812.x. [DOI] [PubMed] [Google Scholar]
- 49.Decman V, Laidlaw BJ, Doering TA, Leng J, Ertl HC, Goldstein DR, Wherry EJ. Defective CD8 T cell responses in aged mice are due to quantitative and qualitative changes in virus-specific precursors. Journal of immunology. 2012;188:1933–1941. doi: 10.4049/jimmunol.1101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao S, Su DM, Manley NR. Atypical Memory Phenotype T Cells with Low Homeostatic Potential and Impaired TCR Signaling and Regulatory T Cell Function in Foxn1{Delta}/{Delta} Mutant Mice. Journal of immunology. 2007;179:8153–8163. doi: 10.4049/jimmunol.179.12.8153. [DOI] [PubMed] [Google Scholar]
- 51.Ono M, Shimizu J, Miyachi Y, Sakaguchi S. Control of autoimmune myocarditis and multiorgan inflammation by glucocorticoid-induced TNF receptor family-related protein(high), Foxp3-expressing CD25+ and CD25- regulatory T cells. Journal of immunology. 2006;176:4748–4756. doi: 10.4049/jimmunol.176.8.4748. [DOI] [PubMed] [Google Scholar]
- 52.Tsukamoto H, Clise-Dwyer K, Huston GE, Duso DK, Buck AL, Johnson LL, Haynes L, Swain SL. Age-associated increase in lifespan of naive CD4 T cells contributes to T-cell homeostasis but facilitates development of functional defects. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18333–18338. doi: 10.1073/pnas.0910139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsukamoto H, Huston GE, Dibble J, Duso DK, Swain SL. Bim dictates naive CD4 T cell lifespan and the development of age-associated functional defects. Journal of immunology. 2010;185:4535–4544. doi: 10.4049/jimmunol.1001668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chougnet CA, Tripathi P, Lages CS, Raynor J, Sholl A, Fink P, Plas DR, Hildeman DA. A major role for Bim in regulatory T cell homeostasis. Journal of immunology. 2011;186:156–163. doi: 10.4049/jimmunol.1001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005;579:2035–2039. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 56.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 57.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of ageing and development. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 58.Campisi J. Cellular senescence: putting the paradoxes in perspective. Curr Opin Genet Dev. 2011;21:107–112. doi: 10.1016/j.gde.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liston A, Gray DH, Lesage S, Fletcher AL, Wilson J, Webster KE, Scott HS, Boyd RL, Peltonen L, Goodnow CC. Gene dosage--limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. The Journal of experimental medicine. 2004;200:1015–1026. doi: 10.1084/jem.20040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 61.Hinterberger M, Aichinger M, Prazeres da Costa O, Voehringer D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nature immunology. 2010;11:512–519. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- 62.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, Johnson SM, Fedoriw Y, Rogers AB, Yuan H, Krishnamurthy J, Sharpless NE. Expression of p16(INK4a) prevents cancer and promotes aging in lymphocytes. Blood. 2011;117:3257–3267. doi: 10.1182/blood-2010-09-304402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, Blazar BR, Millar JL, Malin MA, Chidgey AP, Boyd RL. Activation of thymic regeneration in mice and humans following androgen blockade. Journal of immunology. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 65.Yang H, Youm YH, Dixit VD. Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age-related thymic involution. Journal of immunology. 2009;183:3040–3052. doi: 10.4049/jimmunol.0900562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, Lechler RI, Bloom SR. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. The Journal of clinical investigation. 1999;104:1051–1059. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dooley J, Liston A. Molecular control over thymic involution: from cytokines and microRNA to aging and adipose tissue. European journal of immunology. 2012;42:1073–1079. doi: 10.1002/eji.201142305. [DOI] [PubMed] [Google Scholar]
- 68.Goronzy JJ, Weyand CM. Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity - catalysts of autoimmunity and chronic inflammation. Arthritis research & therapy. 2003;5:225–234. doi: 10.1186/ar974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ortman CL, Dittmar KA, Witte PL, Le PT. Molecular characterization of the mouse involuted thymus: aberrations in expression of transcription regulators in thymocyte and epithelial compartments. Int Immunol. 2002;14:813–822. doi: 10.1093/intimm/dxf042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.