Abstract
Natural killer (NK) cells provide important host defense against viruses and can differentiate into self-renewing memory NK cells after infection, alloantigen stimulation, and cytokine stimulation. Here, we investigated the role of the IL-33 receptor ST2 in the differentiation of NK cells during mouse cytomegalovirus (MCMV) infection. Although ST2-deficient (Il1rl1−/−) Ly49H+ NK cells develop normally and differentiate into memory cells after MCMV infection, naïve and memory Il1rl1−/− Ly49H+ NK cells exhibited profound defects in MCMV-specific expansion, resulting in impaired protection against MCMV challenge. Additionally, IL-33 enhanced m157 antigen-specific proliferation of Ly49H+ NK cells in vitro. Thus, an IL-33-ST2 signaling axis in NK cells contributes to host defense against MCMV.
Introduction
Natural killer (NK) cells recognize abnormal or allogeneic cells by using a repertoire of receptors that regulates their activation and effector functions (1). NK cells have adaptive immune features, which include differentiation into self-renewing memory cells after exposure to mouse cytomegalovirus (MCMV), haptens, cytokines, and alloantigens (2-6). In humans, NKG2Chigh NK cells have been identified as memory NK cells based on their specific expansion and persistence for months following human cytomegalovirus infection (3, 7, 8). Mouse NK cells expressing Ly49H, which recognizes the m157 MCMV glycoprotein on infected cells (9), undergo clonal expansion, contraction, and differentiation into memory cells after MCMV infection (5). The DAP12 and DAP10 adaptor proteins and the co-stimulatory DNAM-1 receptor are required for optimal expansion of Ly49H+ NK cells and their differentiation into memory cells (5, 10). IL-12 is essential for both expansion and memory generation (11); however, the roles of other cytokines for adaptive immune responses mediated by NK cells are largely unknown.
IL-33, a member of the IL-1 cytokine family (12), is expressed constitutively by endothelial and epithelial cells and by fibroblastic reticular cells (FRCs) in secondary lymphoid organs (13). It is released upon tissue damage and necrosis, thus acting as an early inducer of inflammation, and exacerbates Th2 immune responses in mouse models of arthritis and asthma (14). Binding of IL-33 to its receptor ST2 induces MyD88-dependent activation of NF-κB and MAPK (14). ST2 is expressed on many immune cells (12, 15, 16), and the IL-33-ST2 signaling pathway augments IL-12-induced IFN-γ production by NK cells (15, 16). ST2 is essential for control of Coxsackievirus B5-induced pancreatitis by enhancing IFN-γ production by NK cells, which is associated with viral clearance (17). Here, we investigated the role of the IL-33-ST2 signaling axis in the adaptive immune response of NK cells during MCMV infection.
Materials and Methods
Mice and MCMV
C57BL/6 and congenic CD45.1+ C57BL/6 mice were purchased from the National Cancer Institute. C57BL/6 ST2-deficient (Il1rl1−/−) mice (18), generously provided by Dr. S. Akira, Osaka University, IL33-lacZ gene trap reporter (Il33−/−) mice (13), and Ly49H-deficient (Klra8−/−) mice (19), generously provided by Dr. S. Vidal, McGill University, and DAP12-deficient (Tyrobp−/−) mice (20) were maintained in accordance with the guidelines of the UCSF Institutional Animal Care and Use Committee. CD45.1+ WT and CD45.2+ Il1rl1−/− mixed BM chimeric mice were generated as described (5). Smith strain MCMV was prepared as described (6). Mice were infected by i.p. injection of 1-5 × 105 pfu.
NK cell enrichment and adoptive transfer
NK cells were enriched by antibody-coated magnetic bead selection or flow cytometry as described (10). Ten million splenocytes or 2 × 105 Ly49H+ NK cells from mixed BM chimeric mice were injected i.v. into Ly49H-deficient mice on the day before infection. In some experiments, splenocytes were labeled with 10 μM CellTrace Violet (Invitrogen).
Flow cytometry
Fc receptors were blocked with 2.4G2 mAb before staining with the indicated mAbs or isotype-matched control antibodies (BD Biosciences, eBioscience, or BioLegend). Samples were acquired on an LSRII (BD Biosciences) and analyzed with FlowJo software (Tree Star).
In vitro stimulation and proliferation of NK cells
One million splenocytes from mixed BM chimeric mice were incubated in 96-well tissue culture plates coated with anti-NKp46 (29A1.4) as described (5), or with 10 ng/ml mouse IL-12 plus 10 ng/ml mouse IL-18 (R&D Systems), 10 ng/ml PMA plus 1 μg/ml ionomycin (Sigma-Aldrich), or co-cultured with 1 × 105 RMA or m157-transfected RMA cells as described (6). One million CellTrace Violet-labeled splenocytes were co-cultured with 1 × 105 RMA or m157-transfected RMA cells (fixed in 1% paraformaldehyde) in the absence or presence of 25 ng/ml mouse IL-33 (R&D) and/or 10 ng/ml mouse IL-12 with 50 U/ml human IL-2 (NCI) for 4 days at 37° C.
Viral load
Ten thousand naïve or memory Ly49H+ NK cells were transferred separately into Ly49H-deficient or DAP12-deficient mice and infected with MCMV. Peripheral blood was collected on day 4 post-infection (pi), and the right lobe of liver and the spleen were homogenized in DMEM with 10% FCS on day 7 pi, and DNA was isolated from these specimens. The copy number of MCMV IE1 gene in blood, spleen, and liver was determined as described (10). The copy number of MCMV IE1 gene in the spleen was calculated for the whole organ and the copy number of MCMV IE1 in the liver was adjusted for weight of the tissue.
IL-33 in splenic stromal cells
Splenic stromal cells were prepared as described with minor modifications (21). Spleens were digested with 0.2 U/ml Dispase, 0.2 mg/ml collagenase D, and 0.1 mg/ml DNase I (Roche), and stromal cells were enriched by depletion with mAbs against CD4, CD8, CD11b, CD19, and Ter119, and magnetic separation with anti-rat IgG-coated beads (Qiagen). FRCs (CD31− gp38+), LEC-like cells (CD31+ gp38+), DN cells (CD31− gp38−), and BECs (CD31+ gp38−) were gated on 7-AAD− CD45− cells and purified by flow cytometry. The relative quantity of IL-33 transcripts was determined by q-RT-PCR analysis using primers: Actb: forward 5’-GGCTGTATTCCCCTCCATCG-3’; and reverse 5’-CCAGTTGGTAACAATGCCATGT-3’. Il33: forward 5’-TCCAACTCCAAGATTTCCCCG-3’; and 5’-reverse CATGCAGTAGACATGGCAGAA-3’.
Statistical methods
The student’s t test was used to compare results. The Mann–Whitney U test was used to compare MCMV viral titers. Error bars represent S.E.M.
Results
ST2 is dispensable for NK cell development
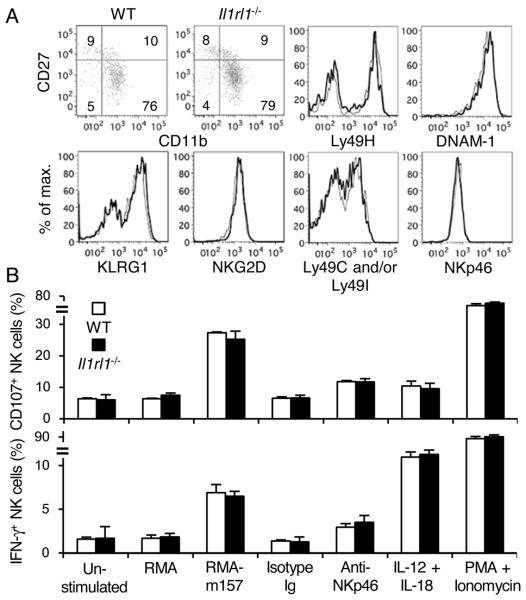
To determine whether an intrinsic lack of ST2 affects NK cell development and function, we reconstituted lethally irradiated recipient mice with CD45.1+ wild type (WT) and CD45.2+ Il1rl1−/− bone marrow (BM) cells and allowed NK cells to reconstitute in the recipient mice for more than 5 weeks. Equivalent frequencies of WT and Il1rl1−/− NK cells were detected in the blood, spleen, liver, and BM after reconstitution (data not shown). WT and Il1rl1−/− NK cells exhibited similar developmental stages, and equivalently expressed KLRG1, NKG2D, Ly49H, Ly49C and/or Ly49I, Ly49G2, DNAM-1, NKp46, NK1.1, IL-2Rα, IL-2Rβ, and IL-7Rα (Fig. 1A and data not shown). Similar frequencies of WT and Il1rl1−/− NK cells degranulated and produced IFN-γ when co-cultured with m157-transfected RMA cells, stimulated by crosslinking NKp46, cultured with IL-12 plus IL-18, and activated with PMA plus ionomycin (Fig. 1B). Similarly, NK cells in Il33−/− mice expressed NK receptors comparably to WT NK cells (data not shown).
Figure 1. Phenotype of Il1rl1−/− NK cells.
(A) Developmental stages as determined by expression of CD11b, CD27, and NK receptors on WT (bold lines) and Il1rl1−/− (thin lines) NK cells, gating on TCRβ− NK1.1+ lymphocytes in the blood from mixed BM chimeric mice. Data are representative of 3 experiments (n = 2-3 mice per experiment). (B) Degranulation and IFN-γ production of WT (open bars) and Il1rl1−/− (filled bars) NK cells after in vitro stimulation. Data are representative of 3 experiments (n = 3-4 in each stimulation).
ST2 enhances NK cell expansion during MCMV infection
WT and Il1rl1−/− Ly49H+ NK cells were isolated from BM chimeric mice, adoptively transferred into Ly49H-deficient recipient mice, and infected with MCMV. During the early phase of infection, donor WT and Il1rl1−/− Ly49H+ NK cells were activated equivalently, as evidenced by upregulation of CD69 on day 1.5 post-infection (pi) and KLRG1 on day 7 pi, and they produced similar amounts of IFN-γ on day 1.5 pi (Fig. 2A and 2B, and data not shown). A similar frequency of Il1rl1−/− Ly49H+ NK cells stained for annexin V in uninfected and infected recipient mice, comparably to WT Ly49H+ NK cells (Fig. 2C). However, Il1rl1−/− Ly49H+ NK cells proliferated less than WT Ly49H+ NK cells on day 3 pi (Fig. 2D and 2E). Consistent with the phenotype of Il1rl1−/− NK cells, Ly49H+ NK cells in Il33−/− mice activated, produced IFN-γ, and stained for annexin V early after infection comparably to WT mice (Fig. 2F-H). However, a smaller percentage of Ki67+ Ly49H+ NK cells was observed in Il33−/− mice compared with WT mice (Fig. 2I and 2J). These findings demonstrate that ST2 and IL-33 are dispensable for activation, IFN-γ production, and survival of NK cells, but augment expansion of Ly49H+ NK cells during MCMV infection.
Figure 2. ST2 enhances Ly49H+ NK cell proliferation during MCMV infection.
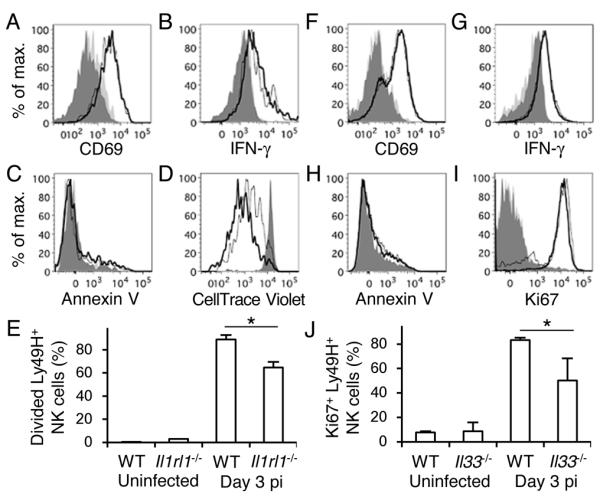
Ly49H+ NK cells (A and B) or CellTrace Violet-labeled splenocytes (C-E) from WT and Il1rl1−/− mixed BM chimeric mice were transferred into Ly49H-deficient mice, and infected with 1 × 105 pfu MCMV. (A) CD69 and (B) IFN-γ on day 1.5 pi, and (C) annexin V and (D) CellTrace Violet on day 3 pi of WT (bold lines) and Il1rl1−/− (thin lines) Ly49H+ NK cells in the spleen. Dark grey-shaded and light grey-shaded histograms represent naïve WT and Il1rl1−/− Ly49H+ NK cells, respectively. Data are representative of 2 experiments (n = 3 mice per experiment). (E) Percentages of divided Ly49H+ NK cells were quantified. Data are pooled from 2 experiments (n = 6 mice). (F-J) WT and Il33−/− mice were infected with 5 × 105 pfu MCMV. (F) CD69 and (G) IFN-γ on day 1.5 pi, (H) annexin V and (I) Ki67 on day 3 pi of WT (bold lines) and Il33−/− (thin lines) Ly49H+ NK cells in the spleen. Dark gray-shaded and light grey-shaded histograms represent naïve WT and Il33−/− Ly49H+ NK cells, respectively. Data are representative of 2 experiments (n = 2-4 per experiment). (J) Percentages of Ki67+ Ly49H+ NK cells were quantified. Data are pooled from 2 experiments (n = 6 mice). *p <0.05.
ST2 enhances expansion of naïve and memory Ly49H+ NK cells and control of MCMV
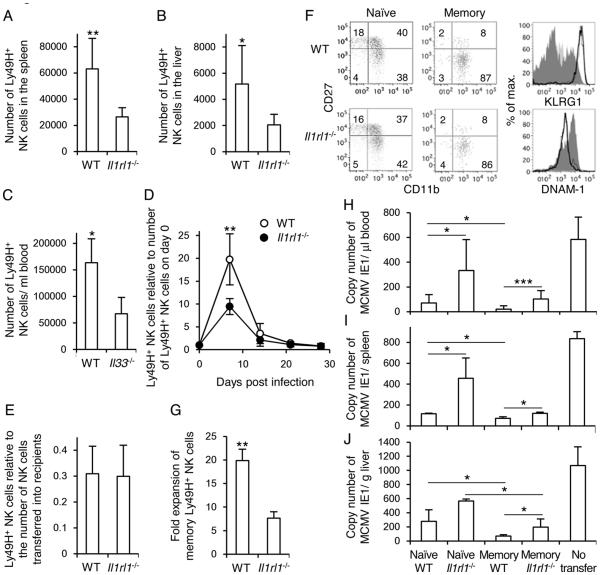
WT and Il1rl1−/− Ly49H+ NK cells from BM chimeric mice were adoptively transferred into Ly49H-deficient recipient mice and infected with MCMV. Il1rl1−/− Ly49H+ NK cells proliferated less than WT Ly49H+ NK cells in the spleen and liver at the peak of the NK cell response on day 7 pi (Fig. 3A and 3B). Consistent with the phenotype of Il1rl1−/− Ly49H+ NK cells, Ly49H+ NK cells in Il33−/− mice had a defect in proliferation after the infection compared with WT mice (Fig. 3C). WT and Il1rl1−/− Ly49H+ NK cells adoptively transferred into Ly49H-deficient recipient mice generated a long-lived subset in the blood and spleen 1 month after infection with MCMV (Fig. 3D and 3E), although Il1rl1−/− Ly49H+ NK cells showed pronounced impairment in expansion in the blood on day 7 pi (Fig. 3D). These Il1rl1−/− Ly49H+ NK cells displayed a memory-associated phenotype characterized as CD11b+ CD27− KLRG1high DNAM-1low, as described previously (5, 6, 10) (Fig. 3F). Memory Il1rl1−/− Ly49H+ NK cells that were isolated from infected mice and adoptively transferred into naïve Ly49H-deficient recipients also expanded poorly when re-challenged with MCMV (Fig. 3G). Moreover, both naïve and memory Il1rl1−/− Ly49H+ NK cells adoptively transferred into Ly49H-deficient mice or DAP12-deficient mice, which lack functionally competent Ly49H+ NK cells and are unable to control early replication of MCMV (5), showed a poor protective effect against MCMV challenge compared with WT Ly49H+ NK cells in the blood, spleen , and liver, consistent with the impaired proliferation of Il1rl1−/− Ly49H+ NK cells (Fig. 3H-J). Thus, ST2 is necessary not only for the optimal expansion of naïve and memory Ly49H+ NK cells during MCMV infection, but also for efficient control of MCMV.
Figure 3. ST2 and IL-33 augment expansion of naïve and memory Ly49H+ NK cells and control of MCMV.
(A and B) WT and Il1rl1−/− Ly49H+ NK cells from mixed BM chimeric mice were transferred into Ly49H-deficient mice and infected with 1 × 105 pfu MCMV. The number of Ly49H+ NK cells in the spleen (A) and liver (B) on day 7 pi is shown. Data are pooled from 2 experiments (n = 6 mice). (C) WT and Il33−/− mice were infected with 5 × 105 pfu MCMV. The number of Ly49H+ NK cells in the blood on day 7 pi is shown. Data are pooled from 2 experiments (n = 4 mice). (D and E) WT and Il1rl1−/− Ly49H+ NK cells were transferred into Ly49H-deficient mice and infected with MCMV. (D) The number of Ly49H+ NK cells in the blood is represented as the ratio relative to the number of Ly49H+ NK cells in the blood on day 0 (before infection). (E) Y-axis represents the number of memory Ly49H+ NK cells detected in the spleen on day 29 pi compared with the number of naïve Ly49H+ NK cells adoptively transferred into recipient mice on day 0. Data are pooled from 3 experiments (n = 8 mice). (F) Phenotype of WT and Il1rl1−/− memory Ly49H+ NK cells on day 29 pi in the spleen. Bold and thin lines represent memory WT and Il1rl1−/− Ly49H+ NK cells, respectively. Dark grey-shaded and light grey-shaded histograms represent naïve WT and Il1rl1−/− Ly49H+ NK cells, respectively. Data are representative of 2 experiments (n = 2-3 mice per experiment). (G) Memory Ly49H+ NK cells were isolated 29 days after infection, transferred into naïve Ly49H-deficient mice, and infected with 1 × 105 pfu MCMV. Expansion of memory Ly49H+ NK cells in the spleen on day 7 after secondary infection is represented as the fold expansion relative to the number of memory Ly49H+ NK cells detected in the spleen of mice adoptively transferred with memory Ly49H+ NK cells but not infected. Data are pooled from 3 experiments (n = 9 mice). *p <0.05, **p <0.01 vs. Il1rl1−/− or Il33−/−. (H-J) WT and Il1rl1−/− memory NK cells were purified 25–32 days after infection, and WT and Il1rl1−/− naïve NK cells were purified from BM chimeric mice. WT and Il1rl1−/− naïve and memory Ly49H+ NK cells were transferred separately into Ly49H-deficient or DAP12-deficient mice and infected with 1 × 105 pfu MCMV. The copy number of MCMV IE1 gene in the blood (H) on day 4 pi, and in the spleen (I) and liver (J) on day 7 pi was analyzed by q-PCR. (H) Data were pooled from 4 experiments (n = 7-11 mice per group) and (I and J) data were pooled from 2 experiments (n = 4 mice per group). *p <0.05, ***p <0.005.
Splenic stromal cells upregulate IL-33 during MCMV infection
FRCs, a stromal cell subset in secondary lymphoid organs, constitutively express IL-33, and IL-33 is upregulated in inflamed tissues (13). We investigated whether stromal cell subsets modulate expression of IL-33 during MCMV infection. Frequencies of splenic stromal cell subsets, as determined by staining CD45− cells with CD31 and gp38, did not change dramatically during MCMV infection (data not shown). However, FRCs, and lymphatic endothelial cell (LEC)-like cells, and double-negative (DN) cells, but not blood endothelial cells (BECs), highly upregulated IL-33 transcripts in the early course of infection (Fig. 4).
Figure 4. FRCs and LEC-like cells upregulate IL-33 during MCMV infection.
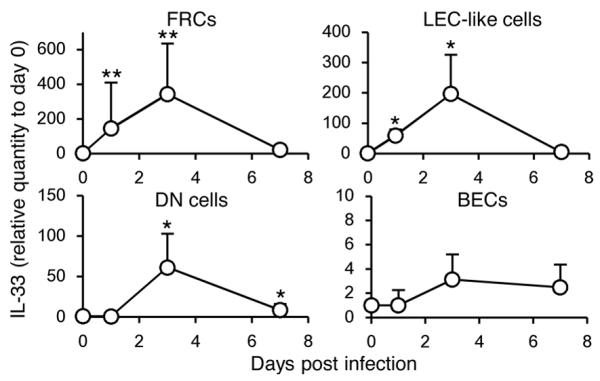
Splenic stromal cell subsets were purified and IL-33 transcripts were quantified by q-RT-PCR. Relative quantity of IL-33 transcripts in each stromal subset in infected mice with 5 × 105 pfu MCMV was represented as the ratio relative to the quantity in uninfected mice (day 0). Data were pooled from 3 experiments (n = 6 mice per day). *p <0.05, **p <0.01 vs. day 0.
IL-33 enhances m157-dependent proliferation of Ly49H+ NK cells
IL-33 synergizes with IL-12 for IFN-γ production by NK cells (15, 16). To address whether IL-33 has a direct effect on proliferation, WT and Il1rl1−/− Ly49H+ NK cells were co-cultured with RMA or m157-transfected RMA cells in the absence or presence of IL-12 and IL-33 with a low dose IL-2. IL-33 had little impact on antigen non-specific cell divisions of Ly49H+ NK cells induced by IL-2, or the m157-driven proliferation of Ly49H+ NK cells in the absence of IL-12 (Fig. 5A and 5B). On the other hand, in the presence of IL-12, IL-33 enhanced proliferation of WT Ly49H+ NK cells, but not Il1rl1−/− Ly49H+ NK cells (Fig. 5C and 5D).
Figure 5. IL-33 enhances m157-dependent proliferation of Ly49H+ NK cells.
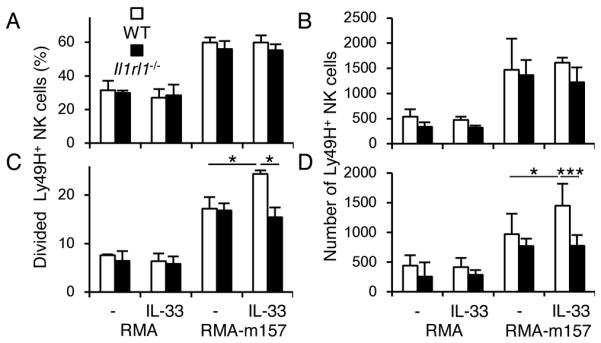
CellTrace Violet-labeled splenocytes from WT and Il1rl1−/− mixed BM chimeric mice were co-cultured with RMA or m157-transfected RMA cells in the absence or presence of IL-33 and/or IL-12. Percentages of divided Ly49H+ NK cells and the number of Ly49H+ NK cells in the cultures with or without IL-33 in the absence (A and B) or presence of IL-12 (C and D). Data were representative of 2 experiments (n = 3-4 in each stimulation). *p <0.05, ***p <0.005.
Discussion
The quantity of bioactive IL-33 is transcriptionally and post-translationally regulated (12, 14). IL-33 transcription is induced by poly I:C, an agonist of TLR3, which is essential in the innate immune defense against MCMV (22, 23). IL-33 is released by tissue damage or infection, and subsequently cleaved into the biological active form by proteases released during inflammation (12). A previous study using a MCMV strain encoding GFP has demonstrated that MCMV infects FRCs and spreads in the spleen within 48 h after infection (24). Interestingly, NK cells migrate to the white pulp of the spleen and are in contact with FRCs within the T cell area by 24 h after MCMV infection, prior to the expansion of Ly49H+ NK cells (25). Taken together, a high concentration of bioactive IL-33 at the local interface of NK cells with stromal cells would ensure efficient ST2 signaling in NK cells, which may contribute to the preferential expansion of Ly49H+ NK cells.
An IL-12-STAT4 signaling pathway is critical for both expansion and memory generation of NK cells in response to MCMV infection and alloantigen stimulation (6, 11). Unlike IL-12, the IL-33-ST2 signaling pathway augments the expansion of Ly49H+ NK cells, but is not required for differentiation into memory cells. Although IL-33 synergizes with IL-12 for IFN-γ production by NK cells in vitro (15, 16), production of IFN-γ by NK cells in vivo early after MCMV infection does not require IL-33 or ST2, indicating that other cytokines produced during infection might compensate. Moreover, previously we demonstrated that Ly49H+ NK cells do not require IFN-γ to undergo expansion during MCMV infection (11), suggesting that the robust proliferation of NK cells requires IL-12-dependents signals and is enhanced by IL-33-dependent signals, but not IFN-γ-mediated signaling. Both IL-12-deficient and STAT4-deficient Ly49H+ NK cells have a severe defect in expansion during MCMV infection (11), whereas an IL-33-ST2 signaling deficiency has a lesser impact. IL-18 and IL-1β, which are other members of the IL-1 cytokine family, are known to synergize with IL-12 for IFN-γ production by NK cells in vitro and in vivo (15, 16, 26). A recent study has demonstrated that an IL-18-IL-18R signaling axis is required for the optimal IFN-γ production, expansion, and memory differentiation of Ly49H+ NK cells during MCMV infection (27). The authors show that MyD88-deficient Ly49H+ NK cells exhibit the same defects as IL-18R-deficient Ly49H+ NK cells (27). In contrast, IL-1R-deficient Ly49H+ NK cells normally expand and differentiate into memory NK cells after the infection (27). In the present study, ST2-deficient Ly49H+ NK cells exhibit impairment in MCMV-specific expansion of naïve and memory Ly49H+ NK cells, but neither in IFN-γ production nor in differentiation into memory NK cells. Interestingly, IL-18R signaling is dispensable for the secondary expansion of memory Ly49H+ NK cells when re-challenged with MCMV. These results suggest that IL-33 is released by damaged cells in the early phase of MCMV infection and that ST2 signaling transiently enhances MyD88 signaling to augment the proliferation of naïve and memory Ly49H+ NK cells, whereas IL-18 more broadly impacts NK cell responses in the course of MCMV infection.
Our findings indicate that multiple cytokines and their downstream signaling pathways differentially modulate the adaptive immune features of NK cells. Further studies of spatiotemporal regulation of cytokine production, as well as the adaptor molecules through which cytokine receptors signal, will be required to understand fully the molecular mechanisms underlying the differentiation of memory NK cells.
Acknowledgments
We thank the Lanier laboratory, Mehrdad Matloubian, and Ari Molofsky (University of California, San Francisco) for comments and discussions and Dr. Rich Locksley for mice and reagents.
The work was supported by NIH grant AI068129. L.L.L. is an American Cancer Society Professor and the Japan Society for the Promotion of Science supports T.N.
Footnotes
Disclosures
The authors have no conflicting financial interests.
References
- 1.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 2.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL. Natural killer cells: walking three paths down memory lane. Trends Immunol. 2013;34:251–258. doi: 10.1016/j.it.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 5.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nabekura T, Lanier LL. Antigen-specific expansion and differentiation of natural killer cells by alloantigen stimulation. J Exp Med. 2014;211:2455–2465. doi: 10.1084/jem.20140798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Anasetti C, Weisdorf D, Miller JS. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol. 2012;189:5082–5088. doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Vergès S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang SM, Norris PJ, Nixon DF, Lanier LL. Expansion of a unique CD57?NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 10.Nabekura T, Kanaya M, Shibuya A, Fu G, Gascoigne NR, Lanier. LL. Costimulatory Molecule DNAM-1 Is Essential for Optimal Differentiation of Memory Natural Killer Cells during Mouse Cytomegalovirus Infection. Immunity. 2014 doi: 10.1016/j.immuni.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier. LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med. 2012;209:947–954. doi: 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cayrol C, Girard. JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014;31C:31–37. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, Girard. JP. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188:3488–3495. doi: 10.4049/jimmunol.1101977. [DOI] [PubMed] [Google Scholar]
- 14.Palmer G, Gabay C. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol. 2011;7:321–329. doi: 10.1038/nrrheum.2011.53. [DOI] [PubMed] [Google Scholar]
- 15.Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S, Gombert JM, Schneider E, Dy M, Gourdy P, Girard JP, Herbelin A. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur J Immunol. 2009;39:1046–1055. doi: 10.1002/eji.200838575. [DOI] [PubMed] [Google Scholar]
- 16.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith. DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 17.Sesti-Costa R, Silva GK, Proença-Módena JL, Carlos D, Silva ML, Alves-Filho JC, Arruda E, Liew FY, Silva. JS. The IL-33/ST2 pathway controls coxsackievirus B5-induced experimental pancreatitis. J Immunol. 2013;191:283–292. doi: 10.4049/jimmunol.1202806. [DOI] [PubMed] [Google Scholar]
- 18.Hoshino K, Kashiwamura S, Kuribayashi K, Kodama T, Tsujimura T, Nakanishi K, Matsuyama T, Takeda K, Akira S. The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J Exp Med. 1999;190:1541–1548. doi: 10.1084/jem.190.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fodil-Cornu N, Lee SH, Belanger S, Makrigiannis AP, Biron CA, Buller RM, Vidal. SM. Ly49h-deficient C57BL/6 mice: a new mouse cytomegalovirus-susceptible model remains resistant to unrelated pathogens controlled by the NK gene complex. J Immunol. 2008;181:6394–6405. doi: 10.4049/jimmunol.181.9.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakker AB, Hoek RM, Cerwenka A, Blom B, Lucian L, McNeil T, Murray R, Phillips LH, Sedgwick JD, Lanier. LL. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity. 2000;13:345–353. doi: 10.1016/s1074-7613(00)00034-0. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher AL, Malhotra D, Acton SE, Lukacs-Kornek V, Bellemare-Pelletier A, Curry M, Armant M, Turley. SJ. Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells. Front Immunol. 2011;2:35. doi: 10.3389/fimmu.2011.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polumuri SK, Jayakar GG, Shirey KA, Roberts ZJ, Perkins DJ, Pitha PM, Vogel. SN. Transcriptional regulation of murine IL-33 by TLR and non-TLR agonists. J Immunol. 2012;189:50–60. doi: 10.4049/jimmunol.1003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu KM, Pratt JR, Akers WJ, Achilefu SI, Yokoyama. WM. Murine cytomegalovirus displays selective infection of cells within hours after systemic administration. J Gen Virol. 2009;90:33–43. doi: 10.1099/vir.0.006668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grégoire C, Cognet C, Chasson L, Coupet CA, Dalod M, Reboldi A, Marvel J, Sallusto F, Vivier E, Walzer T. Intrasplenic trafficking of natural killer cells is redirected by chemokines upon inflammation. Eur J Immunol. 2008;38:2076–2084. doi: 10.1002/eji.200838550. [DOI] [PubMed] [Google Scholar]
- 26.Hunter CA, Chizzonite R, Remington. JS. IL-1 beta is required for IL-12 to induce production of IFN-gamma by NK cells. A role for IL-1 beta in the T cell-independent mechanism of resistance against intracellular pathogens. J Immunol. 1995;155:4347–4354. [PubMed] [Google Scholar]
- 27.Madera S, Sun. JC. Cutting Edge: Stage-Specific Requirement of IL-18 for Antiviral NK Cell Expansion. J Immunol. 2015;194:1408–1412. doi: 10.4049/jimmunol.1402001. [DOI] [PMC free article] [PubMed] [Google Scholar]