Abstract
Chronic muscle pain affects between 11–24% of the world’s population with the majority of people experiencing musculoskeletal pain at some time in their life. Acid sensing ion channels (ASICs) are important sensors of modest decreases in extracellular pH that occur within the physiological range. These decreases in extracellular pH occur in response to inflammation, fatiguing exercise,, and ischemia. Further, injection of acidic saline into muscle produces enhanced nociceptive behaviors in animals and pain in human subjects. Of the different types of ASICs, ASIC3 and ASIC1 have been implicated in transmission of nociceptive information from the musculoskeletal system. The current review will provide an overview of the evidence for ASIC3 and ASIC1 in musculoskeletal pain in both inflammatory and non-inflammatory models.
Keywords: pain, muscle, joint, inflammation, proton, acid, ASIC
1. Introduction
Chronic muscle pain affects between 11–24% of the world’s population with the majority of people experiencing musculoskeletal pain at some time in their life [1]. In the U.S. alone, such chronic pain is estimated to have an economic burden of over $600 billion dollars annually [1,2]. Pain of the musculoskeletal system is associated with reduced function and significant disability. Musculoskeletal pain can occur as a direct result of injury and is associated with injury and inflammation. Some inflammatory conditions persist, such as rheumatoid arthritis, and lead to long-lasting pain and disability. In most cases the acute injury resolves, but in some cases pain persists despite the lack of peripheral tissue injury or inflammation. Treatment of the pain associated with inflammatory and non-inflammatory pain may differ and depend on knowledge of the underlying mechanisms.
Non-inflammatory pain conditions include chronic widespread pain conditions such as fibromyalgia as well as more localized pain conditions such as non-specific neck and back pain or temporomandibular disorder. These conditions are commonly associated with muscle tenderness, resting pain, pain with movement, and significant disability without detectable tissue damage. On the other hand conditions such as osteoarthritis have clear peripheral joint degradation and synovial inflammation. However, often times the evidence of tissue damage does not match the pain and there is a wide variability in pain and disability [3]. Further, inflammatory arthritis conditions such as rheumatoid arthritis have clear joint inflammation that is associated with pain. Again the pain is variable and does not often match the extent of inflammation or joint destruction. Indeed, it is generally accepted that while peripheral nociceptors are critical to the development and maintenance of a variety of pain conditions, that there are central nervous system changes that may underlie some of the variability. Thus, there are a variety of different musculoskeletal pain conditions that each have a unique pathobiology that includes a role for nociceptors at the site of insult that can subsequently alter central nociceptive pathways.
Acid sensing ion channels (ASICs) are important sensors of decreases in extracellular pH [4] that occur within the physiological range. Of the different types of ASICs, ASIC3 and ASIC1 have been implicated in transmission of nociceptive information from the musculoskeletal system [5]. ASIC3 is found in primary afferent fibers innervating muscle and joint [6–9], including those expressing markers found in nociceptive afferents, e.g. substance P and calcitonin gene-related peptide [6–8,10]. ASIC1 is also found in primary afferent fibers that express nociceptive markers and has been implicated in nociceptive processing in the peripheral and central nervous system [5,11]. ASICs form heteromers in vivo, and dorsal root ganglia neurons (DRG) express both ASIC1 and ASIC3 [12,13]. In DRG innervating muscle, decreases in pH produce inward ASIC-like currents [14,15] and application of acidic solutions activates group IV unmyelinated muscle afferent fibers [16]. Further, injection of acidic saline into muscle produces enhanced nociceptive behaviors in animals [10,17]. Similarly, in human subjects, infusion of acidic buffer into the tibialis anterior tibialis muscle of the leg results in local pain at the site of infusion and referred pain at the ankle. In addition, these subjects report a decrease in pressure pain thresholds at both the site of infusion (primary hyperalgesia) and at the ankle (secondary hyperalgesia) [18]. In human subjects, decreases in pH occur in inflammatory conditions, in myofascial pain, and after fatiguing exercise [19–22]. These decreases in pH would be expected to activate ASICs on primary afferent fibers to produce pain. The current review will provide an overview of the evidence for ASIC3 and ASIC1 in musculoskeletal pain in both inflammatory and non-inflammatory models.
2. ASICs in non-inflammatory pain
To model non-inflammatory pain, our laboratory developed a model of persistent hyperalgesia without overt tissue damage [15,17,23]. This model is induced by two injections of acidic saline into one muscle, 5 days apart. Hyperalgesia develops within hours at the site of injection, but also occurs at remote sites in the contralateral muscle, in the skin of the paw, and in the viscera. Further, the hyperalgesia persists without overt damage to the tissue and persists for weeks beyond the initial decrease in extracellular pH (minutes) [17,24,25]. Associated with this widespread hyperalgesia, dorsal horn neurons show enhanced responsiveness to mechanical stimulation bilaterally and expansion of receptive fields to include the contralateral hindlimb [9]. Once developed, this hyperalgesia is reduced by blockade of neurotransmitters and inhibition of pathways in the central nervous system, but not by blockade of peripheral input including ASICs [15,17,26,27]. Thus, this model mimics conditions where pain persists after tissue healing and where pain is not associated with tissue damage such as fibromyalgia and non-specific back pain.
To test the role of ASICs in the initiation of the hyperalgesia, we initially examined the development of mechanical hyperalgesia of the paw in ASIC3−/− and ASIC1−/− mice using the non-inflammatory pain model. ASIC3−/− mice do not develop mechanical hyperalgesia of the paw after repeated acid injection when compared to wild-type controls; however, ASIC1−/− mice still develop the bilateral mechanical hyperalgesia of the paw [9]. Similarly, blockade of ASICs with the non-selective antagonist amiloride or A-317567, or with the selective ASIC3 antagonist APETx2 prevents the development of hyperalgesia [28,29]. The enhanced sensitivity to mechanical stimulation and the expanded receptive field of dorsal horn neurons that normally occurs after the second acid injection do not develop in ASIC3−/− mice [9]. These data suggest ASIC3 on primary afferent fibers is required for the development of widespread hyperalgesia and dorsal horn neuron sensitization after repeated acid injections into muscle.
On the other hand, once the bilateral hyperalgesia develops in this model, 24h after the second acid injection, blockade of ASICs with the non-selective antagonist A-317567 or ASIC3 with APETx2 has no effect on the existing hyperalgesia [15,28]. In parallel, patch clamp recordings from dorsal root ganglia cells innervating muscle, also 24h after the second acid injection, show no changes in responsiveness to acidic pH [15]. Thus, while decreases in extracellular pH activate muscle afferents during the induction of the non-inflammatory pain model, ASICs are not involved in maintaining the existing hyperalgesia in non-inflammatory pain conditions.
3. ASICs in Inflammatory pain
To model inflammatory musculoskeletal pain, carrageenan is injected into the gastrocnemius muscle or the knee joint [23] - this produces a unilateral inflammation that is initially associated with neutrophilic inflammation. Nociceptive behaviors after deep tissue inflammation include decreases in mechanical withdrawal thresholds, heat withdrawal latency of the paw, and withdrawal thresholds of the inflamed tissue (muscle or knee). The decreases in withdrawal thresholds and latency at the paw are considered secondary hyperalgesia and those at the inflamed joint are considered primary hyperalgesia. It is thought that primary hyperalgesia reflects enhanced activity of nociceptors and secondary hyperalgesia reflects enhanced activity of central neurons. However, the secondary hyperalgesia can certainly be initiated and maintained by continued nociceptive input from the site of injury – which is highly likely in inflammatory muscle and joint models.
ASICs play a clear role in inflammatory muscle and joint hyperalgesia, with ASIC3 and ASIC1 showing an interesting divergence. In ASIC3−/− mice, the decreased withdrawal thresholds of the paw do not occur after either muscle or joint inflammation [30,31]. However, ASIC3−/− mice still show decreases in latency to heat and decreases in withdrawal thresholds of deep tissue after muscle or joint inflammation [8,30,31]. In direct contrast, ASIC1−/− mice still develop decreases in withdrawal thresholds of the paw but do not develop decreases in withdrawal thresholds of the muscle or knee induced by muscle or joint inflammation, respectively [8,31]. In adult mice, knockdown of ASIC3 in DRG innervating muscle with a miRNA prevents the development of both paw and muscle hyperalgesia in mice with muscle inflammation [32]. After development of muscle inflammation, blockade of ASICs with the non-selective blockers or a selective ASIC3 antagonist reverses both the muscle and paw hyperalgesia [28,31,33]. After deep tissue inflammation there is an increase in ASIC2 and ASIC3 mRNA as well as ASIC3 protein expression in primary afferents innervating joint and CGRP-positive cells [7,8,31]. In DRG innervating muscle, there are enhanced ASIC-like currents to acidic pH 24h after induction of carrageenan inflammation [14]. Similar to injection of inflammatory mediators, eccentric exercise in which muscles are forced to lengthen during contraction produces local inflammation and hyperalgesia; this hyperalgesia is reduced by blockade of ASICs [33].
The data on inflammatory changes in muscle is mixed. There are no differences in inflammation between ASIC3+/+ and ASIC3−/− mice using a myeloperoxidase assay to measure neutrophilic inflammation and circumference measures to assess swelling [30]. On the other hand, using histological analysis, features associated with inflammation, granulomas and vasculitis, are less severe in ASIC3−/− mice when compared to ASIC3+/+ mice [34]
4. ASICs in inflammatory arthritis
Rheumatoid arthritis is an inflammatory joint disease associated with widespread inflammation, pain and stiffness. The passive collagen-induced arthritis model (CAIA) [23] is used to study rheumatoid arthritis since it produces distal and widespread inflammation and synovitis similar to rheumatoid arthritis. The CAIA model is also associated with enhanced sensitivity to nociceptive mechanical stimulation of cutaneous and deep tissue of the paw and reduced activity levels [35].
In the CAIA model, ASIC3−/− mice develop mechanical hyperalgesia of the ankle, but not the adjacent paw as compared to wild-type mice [35]; thus following a similar pattern to that observed after muscle or joint inflammation. The reduced activity levels observed in the CAIA model are also attenuated in ASIC3−/− mice. Thus, nociceptive behaviors in inflammatory arthritis appear to activate ASIC3.
Despite the decreases in hyperalgesia, the ASIC3−/− mice show enhanced inflammation as measured by ankle joint thickness, arthritis scores, and histological changes after induction of CAIA [35]. Specifically, there is enhanced proteoglycan destruction, synovial thickness, bone destruction and inflammatory infiltrations in ASIC3−/− mice when compared to wildtype mice [35]. In the ankle joints from animals with CAIA, there is increased production of the inflammatory mediators interleukin-6 and metalloproteinases 3 [35]. This enhanced inflammation is likely a result of the localization of ASIC3 to synoviocytes [8,36,37]. This is in contrast to an animal model of degenerative joint disease, i.e. osteoarthritis, induced by intraarticular injection of mono-iodoacetate into the knee joint, where blockade of ASIC3 in the OA joint with APETx2 protected the cartilage from damage [38]. Thus, there may be disease-specific and/or cell-specific ASIC3 modulation of joint tissues.
During the course of our studies we discovered that ASIC3 is located on Type B synoviocytes lining articular joints and there is mRNA and protein in cultured fibroblast-like synoviocytes (FLS) [8,36]. Acidic pH activates cultured FLS in a pH-dependent manner by increasing intracellular calcium, enhancing release of hyaluronan, and decreasing phosphorylation of ERK; these effects are significantly smaller in ASIC3−/− FLS [35–37]. Interestingly, when wild-type FLS are preincubated with IL-1β acidic pH produces synoviocyte cell death [35,37]. ASIC3−/− FLS show reductions in intracellular calcium, no decrease in p-ERK and no cell death [35,37] demonstrating a critical role for ASIC3 in mediating the cell death. Thus, ASIC3−/− mice with inflammatory arthritis show reduced pain and enhanced inflammation and joint degradation. These data suggest that ASIC3 activation on neuron produces pain that is designed to protect the joint by limiting usage, and that ASIC3 activation on synoviocytes produces cell death that protects the joint by limiting synovitis.
5. ASICs in models of ischemia, muscle fatigue, and postoperative pain
Ischemia, muscle fatigue, and muscle incision all produce decreases in pH in a range that is expected to activate ASICs. Muscle fatigue decreases pH, increases lactate [39], enhances hyperalgesia to muscle insult [40]. Application of fatigue metabolites to muscle produces pain in human subjects [41]. Muscle ischemia, induced by vascular occlusion, decreases pH to 6.7, and in DRG innervating muscle enhances the peak ASIC3-like currents, and increases the expression of ASIC3 in DRG [42,43]. ASIC3-mediated detection of ischemia may also contribute to incisional pain. Incision into the gastrocnemius muscle results in significant decreases in interstitial fluid pH that persists for days and is correlated with the development of mechanical hyperalgesia [44]. Further, blockade of ASIC3 function, either by the ASIC3-selective antagonist APETx2 or siRNA-mediated knockdown, prevents the development of post-incisional pain behaviors and thermal hyperalgesia. Interestingly, antagonism of ASIC1a by PcTx1 did not have this protective effect [45]. Thus, ASIC3 may also play a role in other conditions that result in decreases in pH in muscle including fatigue, ischemia and postoperative pain.
5. Summary
In summary ASICs play a unique role in musculoskeletal pain that depends on the model. Clearly, ASIC3 activation is necessary for development of widespread hyperalgesia in non-inflammatory pain models and may be a critical player in the transition from acute to chronic pain. On the other hand, in acute inflammatory models of muscle both ASIC1 and ASIC3 mediate hyperalgesia. In fact, non-specific blockade of ASICs or downregulation of ASIC3 in muscle afferents with a miRNA produces a robust reduction in both primary and secondary hyperalgesia. Thus, ASICs could be useful targets for modulating inflammatory muscle pain. Targeting ASICs in inflammatory arthritic conditions should be done with caution. Blockade of ASICs in a joint might reduce the hyperalgesia associated with inflammatory arthritis, but could enhance the inflammation through inhibition of ASICs on synoviocytes. On the other hand activation of ASICs on synoviocytes could be useful to limit synovitis associated with inflammatory arthritis. Lastly, ASICs contribute to ischemic pain and fatigue-induced pain.
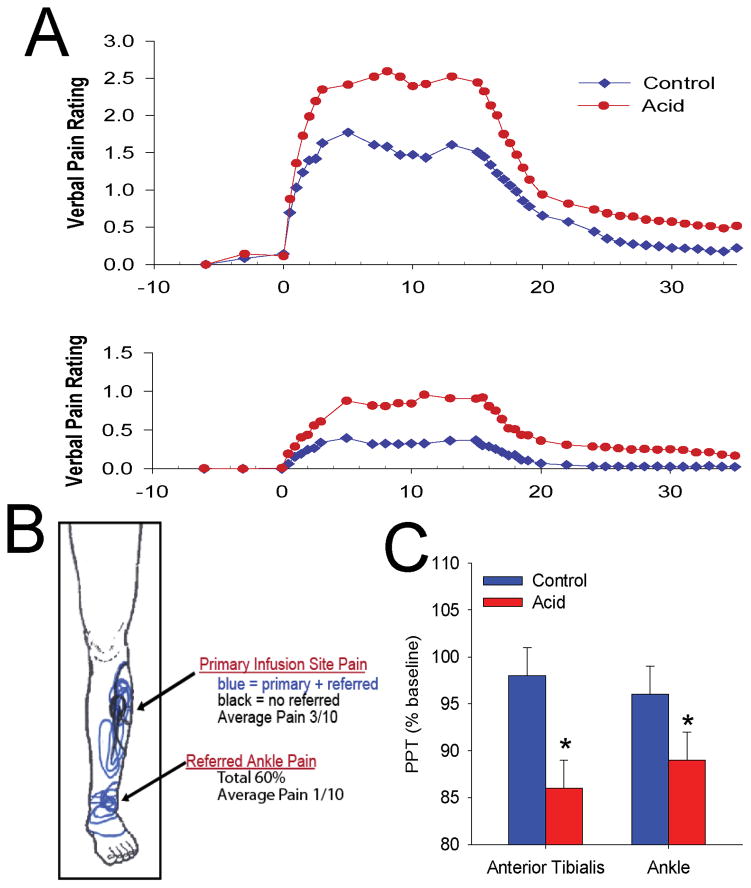
Figure 1.
This figure shows data from human subjects where pH 5.2 acidic buffer was infused into the tibialis anterior muscle and compared to saline controls. The infusion was started at time 9 and continued for 15 minutes. Pain ratings at the site of infusion and in the referred pain site (A), drawings of area pain felt by subject (B) and pressure pain thresholds at the site of infusion and in the referred pain site (C) were measured. A. Pain intensity ratings in the group infused with acidic buffer averaged around 2.5–3.0/10 during the infusion for the primary site of pain, and about 1/10 in the referred pain site at the ankle. B. The primary pain site occurred at the infusion site. A portion of the subjects, approximately 60%, had pain referred to the ankle, while 40% had only localized pain. C. Pain thresholds were significantly decreased from baseline during infusion of acidic buffer at both the site of infusion, primary hyperalgesia, and in the referred pain site, secondary hyperalgesia. Figures and data were redrawn from [18].
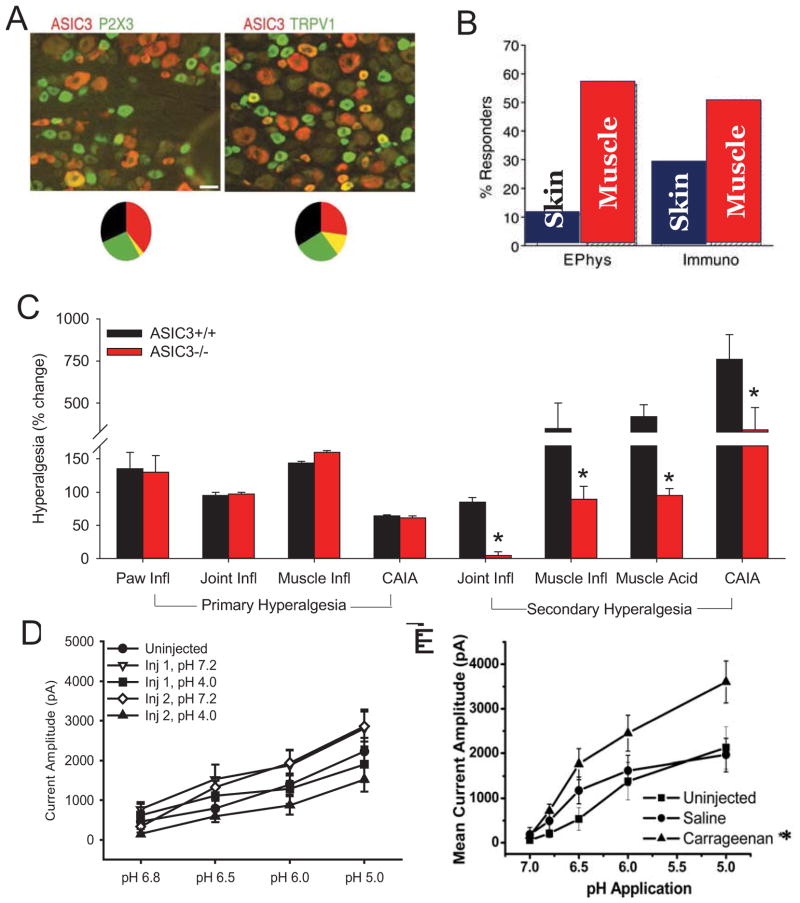
Figure 2.
A. Shows localization of ASIC3 (red) in dorsal root ganglia cells. ASIC3 is immunohistochemically stained and shows as red. The first panel also shows localization of P2X3 (green) and the second also shows localization of TRPV1 (green). The overlap between ASIC3 expression and either P2X3 or TRPV1 (yellow) is shown graphically in the pie chart shows the representation in terms of percentages. Notice minimal overlap with P2X3 and some overlap with TRPV1. Figure reproduced from [6]. B. Bar graphs show the percentage of dorsal root ganglia cells that express ASIC3 currents (Ephys) or protein (Immuno) retrogradely labeled from either the skin or the muscle. Notice that neurons innervating muscle have a higher percentage cells expressing ASIC3 than those innervating skin. Figure reproduced from [6]. C. Summary of the degree of hyperalgesia in different animal models of pain from ASIC3−/− mice when compared to ASIC3+/+ mice. Primary hyperalgesia was assessed by testing the site of the inflammation for response to mechanical stimulation, while secondary hyperalgesia was assessed by examining the responses of a site distant to the site of insult. Paw inflammation (Paw Infl) was induced by 3% carrageenan subcutaneously into the hindpaw and mechanical stimulation was applied to the inflamed paw. Joint inflammation (Joint Infl) and muscle inflammation (Muscle Infl) were induced by injection of 3% carrageenan into the knee joint or gastrocnemius muscle, respectively. Collagen-antibody-induced arthritis was induced by systemic administration of collagen type II antibodies and is used to mimic rheumatoid arthritis presenting with distal swelling and inflammation. Non-inflammatory hyperalgesia was induced by repeated acid injections into the gastrocnemius muscle ((Muscle Acid). Primary hyperalgesia in these deep tissue models was assessed by squeezing the joint (knee or ankle) or muscle with tweezers to obtain a withdrawal response from the site of injury. Secondary hyperalgesia in these models was assessed by examining mechanical sensitivity of the skin at the paw. No hyperalgesia is designated as a “0”. Notice that ASIC3−/− show no differences when measuring primary hyperalgesia in inflammatory models. On the other hand ASIC3−/− mice all show reduced hyperalgesia in tests examining secondary hyperalgesia in multiple musculoskeletal pain models. Data taken from multiple manuscripts [8,10,29,30,35]. D. Current amplitude to decreases doses of pH in dorsal root ganglia cells innervating muscle in the non-inflammatory hyperalgesia model. Responses were assessed 24h after the first injection of pH 4.0 saline into the muscle and 24h after the second injection of pH 4.0 saline into the muscle compared to controls injected with pH 7.2 and uninjected controls. No changes were observed in current amplitude in this model. Data reproduced with permission of Elsevier [15]. E. Current amplitude to decreased doses of pH in dorsal root ganglia innervating muscle 24h after induction of muscle inflammation with 3% carrageenan when compared to uninjected controls or controls injected with normal saline. Notice a significant increase in the current amplitude to decreasing doses of pH in the cells from animals with muscle inflammation. *, p<0.05. Figure reproduced from [14].
Highlights.
Injection of acidic saline into muscle produces enhanced nociceptive behaviors in animals and pain in human subjects.
ASIC1 and ASIC3 mediate the hyperalgesia of inflammatory and non-inflammatory musculoskeletal pain models in animals
ASIC3 is located in synoviocytes and promotes cell death and reduced inflammation in inflammatory arthritis models in mice
Acknowledgments
Funded by NIH AR063381 and AR061371 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Committee on Advancing Pain Research CaEIoM. Reliveing Pain in America: A blueprint for transforming prevention, care, education and research. Institute of Medicine of the National Academies; 2011. Ref Type: Report. [PubMed] [Google Scholar]
- 2.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Sluka KA, Berkley KJ, O’Connor MJ, Nicolella DP, Enoka RM, Boyan BD, et al. Neural and psychosocial contributions to sex differences in knee osteoarthritic pain. Biol Sex Differ. 2012;3:26. doi: 10.1186/2042-6410-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 5.Sluka KA, Winter OC, Wemmie JA. Acid-sensing ion channels: A new target for pain and CNS diseases. Curr Opin Drug Discov Devel. 2009;12:693–704. [PMC free article] [PubMed] [Google Scholar]
- 6.Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Molecular Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikeuchi M, Kolker SJ, Sluka KA. Acid-sensing ion channel 3 expression in mouse knee joint afferents and effects of carrageenan-induced arthritis. J Pain. 2009;10:336–342. doi: 10.1016/j.jpain.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeuchi M, Kolker SJ, Burnes LA, Walder RY, Sluka KA. Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. Pain. 2008;137:662–669. doi: 10.1016/j.pain.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- 10.Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, et al. The DRASIC Cation Channel Contributes to the Detection of Cutaneous Touch and Acid Stimuli in Mice. Neuron. 2001;32:1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 11.Olson TH, Riedl MS, Vulchanova L, Ortiz-Gonzalez XR, Elde R. An acid sensing ion channel (ASIC) localizes to small primary afferent neurons in rats. Neuroreport. 1998;9:1109–1113. doi: 10.1097/00001756-199804200-00028. [DOI] [PubMed] [Google Scholar]
- 12.Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, et al. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci U S A. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gautam M, Benson CJ. Acid-sensing ion channels (ASICs) in mouse skeletal muscle afferents are heteromers composed of ASIC1a, ASIC2, and ASIC3 subunits. FASEB J. 2013;27:793–802. doi: 10.1096/fj.12-220400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gautam M, Benson CJ, Sluka KA. Increased response of muscle sensory neurons to decreases in pH after muscle inflammation. N S. 2010;170:893–900. doi: 10.1016/j.neuroscience.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gautam M, Benson CJ, Ranier JD, Light AR, Sluka KA. ASICs do not play a role in maintaining hyperalgesia induced by repeated intramuscular acid injections. Pain Res Treat. 2012;2012:817437. doi: 10.1155/2012/817347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoheisel U, Reinohl J, Unger T, Mense S. Acidic pH and capsaicin activate mechanosensitive group IV muscle receptors in the rat. Pain. 2004;110:149–157. doi: 10.1016/j.pain.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 17.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle & Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Frey Law LA, Sluka KA, McMullen T, Lee J, rendt-Nielsen L, Graven-Nielsen T. Acidic buffer induced muscle pain evokes referred pain and mechanical hyperalgesia in humans. Pain. 2008;140:254–264. doi: 10.1016/j.pain.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldie I, Nachemson A. Synovial pH in rheumatoid knee-joints. I. The effect of synovectomy. Acta Orthop Scand. 1969;40:634–641. doi: 10.3109/17453676908989529. [DOI] [PubMed] [Google Scholar]
- 20.Goldie I, Nachemson A. Synovial pH in rheumatoid knee joints. II. The effect of local corticosteroid treatment. Acta Orthop Scand. 1970;41:354–362. doi: 10.3109/17453677008991521. [DOI] [PubMed] [Google Scholar]
- 21.Shah JP, Phillips TM, Danoff JV, Gerber LH. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol. 2005;99:1977–1984. doi: 10.1152/japplphysiol.00419.2005. [DOI] [PubMed] [Google Scholar]
- 22.Hood VL, Chubert C, Keller U, Muller S. Effect of systemic pH on pHi and lactic acid generation in exhaustive forearm exercise. Am J Physiol. 1988;255:F479–485. doi: 10.1152/ajprenal.1988.255.3.F479. [DOI] [PubMed] [Google Scholar]
- 23.Gregory NS, Harris AL, Robinson CR, Dougherty PM, Fuchs PN, Sluka KA. An Overview of Animal Models of Pain: Disease Models and Outcome Measures. Journal of Pain. 2013 doi: 10.1016/j.jpain.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miranda A, Peles S, Rudolph C, Shaker R, Sengupta JN. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126:1082–1089. doi: 10.1053/j.gastro.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Yokoyama T, Audette KM, Sluka KA. Pregabalin reduces muscle and cutaneous hyperalgesia in two models of chronic muscle pain in rats. J Pain. 2007 doi: 10.1016/j.jpain.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Tillu DV, Gebhart GF, Sluka KA. Descending facilitatory pathways from the RVM initiate and maintain bilateral hyperalgesia after muscle insult. Pain. 2008;136:331–339. doi: 10.1016/j.pain.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeSantana JM, da Cruz KM, Sluka KA. Animal models of fibromyalgia. Arthritis Res Ther. 2013;15:222. doi: 10.1186/ar4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karczewski J, Spencer RH, Garsky VM, Liang A, Leitl MD, Cato MJ, et al. Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br J Pharmacol. 2010;161:950–960. doi: 10.1111/j.1476-5381.2010.00918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sluka KA, Price MP, Wemmie JA, Welsh MJ. ASIC3, but not ASIC1, channels are involved in the development of chronic muscle pain. In: Dostrovsky JO, Carr DB, Koltzenburg M, editors. Proceedings of the 10th World Congress on Pain. 24. Seattle: IASP Press; 2003. pp. 71–79. [Google Scholar]
- 30.Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, et al. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129:102–112. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walder RY, Rasmussen LA, Rainier JD, Light AR, Wemmie JA, Sluka KA. ASIC1 and ASIC3 Play Different Roles in the Development of Hyperalgesia After Inflammatory Muscle Injury. J Pain. 2010;11:210–218. doi: 10.1016/j.jpain.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walder RY, Gautam M, Wilson SP, Benson CJ, Sluka KA. Selective Targeting of ASIC3 using miRNAs inhibits primary and secondary hyperalgesia following muscle inflammation. Pain. 2011;152:2348–2356. doi: 10.1016/j.pain.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujii Y, Ozaki N, Taguchi T, Mizumura K, Furukawa K, Sugiura Y. TRP channels and ASICs mediate mechanical hyperalgesia in models of inflammatory muscle pain and delayed onset muscle soreness. Pain. 2008;140:292–304. doi: 10.1016/j.pain.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Yen YT, Tu PH, Chen CJ, Lin YW, Hsieh ST, Chen CC. Role of acid-sensing ion channel 3 in sub-acute- phase inflammation. Mol Pain. 2009;5:1. doi: 10.1186/1744-8069-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sluka KA, Rasmussen LA, Edgar MM, O’Donnel JM, Walder RY, Kolker SJ, et al. Acid sensing ion channel 3 deficiency increases inflammation but decreases pain behavior in arthritis. Arthritis Rheum. 2013;65:1194–1202. doi: 10.1002/art.37862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolker SJ, Walder RY, Usachev Y, Hillman J, Boyle DL, Firestein GS, et al. ASIC3 expressed in Type B synoviocytes and chondrocytes modulates hyaluronan expression and release. Ann Rheum Dis. 2010;69:903–909. doi: 10.1136/ard.2009.117168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong W, Kolker SJ, Usachev Y, Walder RY, Boyle DL, Firestein GS, et al. Acid-sensing ion channel 3 decreases phosphorylation of extracellular signal-regulated kinases and induces synoviocyte cell death by increasing intracellular calcium. Arthritis Res Ther. 2014 doi: 10.1186/ar4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izumi M, Ikeuchi M, Ji Q, Tani T. Local ASIC3 modulates pain and disease progression in a rat model of osteoarthritis. J Biomed Sci. 2012;19:77. doi: 10.1186/1423-0127-19-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahlin K, Harris RC, Nylind B, Hultman E. Lactate content and pH in muscle obtained after dynamic exercise. Pflugers Arch. 1976;367:143–149. doi: 10.1007/BF00585150. [DOI] [PubMed] [Google Scholar]
- 40.Gregory NS, Gibson-Corley K, Frey-Law L, Sluka KA. Fatigue-enhanced hyperalgesia in response to muscle insult: Induction and development occur in a sex-dependent manner. Pain. 2013 doi: 10.1016/j.pain.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pollak KA, Swenson JD, Vanhaitsma TA, Hughen RW, Jo D, Light KC, et al. Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp Physiol. 2014;99:368–380. doi: 10.1113/expphysiol.2013.075812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xing J, Lu J, Li J. Acid-sensing ion channel subtype 3 function and immunolabelling increases in skeletal muscle sensory neurons following femoral artery occlusion. J Physiol. 2012;590:1261–1272. doi: 10.1113/jphysiol.2011.221788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Gao Z, Li J. Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am J Physiol Heart Circ Physiol. 2010;299:H1357–H1364. doi: 10.1152/ajpheart.00612.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woo YC, Park SS, Subieta AR, Brennan TJ. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiol. 2004;101:468–475. doi: 10.1097/00000542-200408000-00029. [DOI] [PubMed] [Google Scholar]
- 45.Deval E, Noel J, Gasull X, Delaunay A, Alloui A, Friend V, et al. Acid-sensing ion channels in postoperative pain. J NEUROSCI. 2011;31:6059–6066. doi: 10.1523/JNEUROSCI.5266-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]