Abstract
Ischemia and reperfusion (I/R) injury following liver transplantation (LTx) is an important problem that significantly impacts clinical outcomes. Interferon regulatory factor-1 (IRF-1) is a nuclear transcription factor that plays a critical role in liver injury. Our objective was to determine the immunomodulatory role of IRF-1 during I/R injury following allogeneic LTx. IRF-1 was induced in liver grafts immediately after reperfusion in both human and mouse LTx. IRF-1 contributed significantly to I/R injury as IRF-1 KO grafts displayed much less damage assessed by serum alanine aminotransferase (ALT) and histology. In vitro, IRF-1 regulated both constitutive and induced expression of IL-15, as well as IL-15Rα mRNA expression in murine hepatocytes and liver dendritic cells (DC). Specific knockdown of IRF-1 in human primary hepatocytes gave similar results. In addition, we identified hepatocytes as the major producer of soluble IL-15/IL-15Rα complexes in the liver. IRF-1 KO livers had significantly reduced NK, NKT and CD8+T cell numbers, while rIL-15/IL-15Rα restored these immune cells, augmented cytotoxic effector molecules, promoted systemic inflammatory responses, and exacerbated liver injury in IRF-1 KO graft recipients. These results indicate that IRF-1 promotes LTx I/R injury via hepatocyte IL-15/IL-15Rα production and suggest that targeting IRF-1 and IL-15/IL-15Rα may be effective in reducing I/R injury associated with LTx.
Introduction
Liver transplantation (LTx) is the established treatment for patients with end-stage liver disease and primary liver cancer. One of the major challenges limiting LTx in the United States is the shortage of donor organs (1). While >15,000 patients await LTx each year, <7,000 undergo the procedure according to data collected by Organ Procurement and Transplant Network. This critical organ shortage has led to use of extended criteria organs, such as those from elderly or non-heart beating donors, steatotic livers, or organs with extended cold storage time (2, 3).
Ischemia and reperfusion (I/R) injury remains one of the most understudied areas in organ transplantation, despite its clinical significance (3, 4). I/R injury following prolonged cold storage has a significant impact on early as well as late human liver transplant outcomes. Thus, prolonged cold ischemia time is not only associated with an increased incidence of re-transplantation and primary graft nonfunction during the first 2 weeks post-LTx (5), but also with increased mortality 3 and 12 months post-transplant (6). Whereas extended criteria donor organs are particularly susceptible to I/R injury (1–3), severe preservation injury leads to a higher incidence of rejection and lower survival rates among liver transplant recipients (7). Thus, there is a pressing need to elucidate molecular mechanisms of I/R injury following LTx and to develop effective therapeutic strategies. Prevention of I/R injury is likely to improve the outcome of LTx and potentially expand the limited donor pool by allowing wider use of marginal organs.
Interferon regulatory factor-1 (IRF-1) is a ubiquitous nuclear transcription factor identified originally as a regulator of the human IFN-β gene (8). It is expressed constitutively at low basal levels in various organs, as well as in a variety of immune cell types, including NK cells, T cells (9), macrophages (10) and dendritic cells (DC) (11–13). IRF-1 also regulates the development of certain lymphocyte subsets. Thus, previous reports have shown that IRF-1-deficient mice display severe NK and NKT cell deficiency in the liver, spleen and thymus (14, 15). There is also evidence that CD8+T cells are reduced in the thymus, peripheral blood, spleen and lymph nodes of these mice (16, 17). Some of these studies suggest that lack of inducible IL-15, especially in bone marrow (BM) stromal cells, is responsible for the deficiency of NK cells in IRF-1-deficient mice (14, 18, 19).
IL-15 is a pleiotropic cytokine in the common cytokine receptor γ-chain (γc) family that functions in the homeostasis and activation of innate and adaptive immune cells (19–21). The IL-15 gene is transcriptionally regulated by IRF-1 (8) and IRF-1 regulates the induction of IL-15 mRNA in BM cells in response to stimulation with LPS and IFN-γ in vitro (14, 15). IL-15Rα is a receptor subunit with which IL-15 forms a heterodimer complex (22). This IL-15/IL-15Rα complex binds to cells that express another heterodimeric receptor complex comprised of IL-2/IL-15Rβ and γc through which it signals (20). A recent study (22) indicates that this soluble IL-15/IL-15Rα complex is the only form of circulating soluble IL-15 in mouse and human serum. IL-15Rα also has the IRF responsive element sequence in its promoter region and overexpression of IRF-1 protein in COS-7 cells (monkey kidney fibroblast-like cells) can activate the IL-15Rα promoter (23). However, it remains unclear whether IRF-1 regulates IL-15 and IL-15Rα expression in hepatic immune and non-immune cells in the steady-state or under inflammatory conditions (24).
We have shown previously (25–29) that IRF-1 plays a critical role in various liver injury models. This includes hepatic warm I/R injury (25, 26), cold I/R injury during isograft LTx (27, 28), and immune-mediated liver injury (29). However, the precise mechanism by which IRF-1 regulates hepatic lymphocyte populations, and the role of these hepatic lymphocytes in mediating liver injury is poorly understood (30). Moreover, the role of IRF-1 in allogeneic LTx has not been defined. Thus, we examined the role of IRF-1 in hepatic lymphocyte homeostasis in the steady-state and in the pathogenesis of cold I/R injury using a clinically-relevant, mouse orthotopic allogeneic LTx model. Our novel findings suggest that IRF-1 regulates hepatic NK cell, NKT cell and CD8+T cell homeostasis via hepatocyte and DC production of IL-15/IL-15Rα complexes to promote proinflammatory cytokine production and the up-regulation of cytotoxic lymphocyte granules. These events contribute significantly to allograft liver I/R injury with prolonged cold ischemia time following allogeneic LTx.
Materials and Methods
IRF-1 staining of human liver allografts
Analysis of human liver allograft tissue (PRO10110393) and isolation of human primary hepatocytes (PRO012100076 and PRO08010372) were conducted under University of Pittsburgh Institutional Review Board protocols. Written informed consent was received from participants prior to inclusion in the study. Formalin-fixed, paraffin-embedded human liver allograft biopsy sections were obtained from 4 patients at two different time points (backtable and post-reperfusion (1–4 h)). 4 μm sections were deparaffinized, hydrated, and treated with citrated buffer for antigen retrieval. Sections were then blocked with avidin and biotin block kit (Vector Laboratories, Inc., Burlingame, CA). Staining was performed by sequential incubation cycles of rabbit anti-IRF-1 primary antibody (Santa Cruz Biotechnology, Inc., Dallas, TX), goat anti-rabbit biotinylated secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), ABC kit (Vector Laboratories, Inc.), and AEC Substrate kit (ScyTek Laboratories, Inc., Logan, UT). Sections were then counterstained with aqueous hematoxylin. Digital images of whole staining slides were obtained with MIRAX MIDI digital whole slide scanning system (Carl Zeiss Microimaging, Jena, Germany) and analyzed with Panoramic Viewer (3D Histech, Ltd, Ramsey, NJ).
Animals
Male 8- to 12-wk old wild-type C57BL/6 mice (IRF-1+/+; WT B6, H-2b), IRF-1 deficient mice (IRF-1−/−; IRF-1 KO, B6 background, H-2b), IL-15Rα deficient mice (IL-15Rα−/−; IL-15Rα KO, B6 background, H-2b) and C3H/HeJ (H-2k) mice were obtained from The Jackson Laboratory (Bar Harbor, Maine, USA). They were maintained in the specific pathogen-free animal facility at the University of Pittsburgh School of Medicine. All animal studies were approved by University of Pittsburgh IACUC protocol # 14023395; PHS assurance #A3187-01 and in accordance with criteria outlined in Guide for the Care and Use of Laboratory Animals, a publication of the National Institutes of Health.
Orthotopic liver transplantation (LTx)
Liver harvesting and orthotopic liver transplantation without hepatic artery reconstruction were performed as described (31, 32), with minor modifications. WT B6 or IRF-1 KO mice were used as liver donors. The grafts were perfused with 5ml University of Wisconsin (UW) solution via the inferior vena cava, stored for 24 h at 4°C, and then implanted orthotopically into C3H/HeJ recipients. The transplant recipients were euthanized 3 and 6 h after reperfusion to obtain blood and liver tissue.
Isolation of hepatocytes and non-parenchymal cells (NPC)
Hepatocytes and NPC were isolated from normal liver and liver grafts using the collagenase digestion method, as described (28, 33). The initial cell suspension was filtered using a cell strainer (70 μm, BD Bioscience, San Jose, CA) and hepatocytes and NPC separated by low speed centrifugation (x5; 45 g for 5 min).
Real-time RT-PCR
Total RNA was isolated using RNeasy Mini Kits (Qiagen Inc., Valencia, CA) and reverse transcribed into cDNA using RNA to cDNA EcoDry™ Premix (Double Primed) (Clontech Laboratories, Inc., Mountain View, CA). mRNA expression was quantified, as described (33), by SYBR Green RT-PCR with StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA). Expression of each gene was normalized to β-actin mRNA or GAPDH mRNA for mouse and human studies respectively and calculated with respect to normal liver tissue. Primer sequences are provided in Supplemental Table 1.
Confocal immunofluorescence imaging
Immunofluorescence staining and imaging of IRF-1 in mouse liver tissue was performed as described (26). Primary antibody was rabbit anti-IRF-1 (Santa Cruz Biotechnology, Inc.). A secondary antibody (goat anti-rabbit Cy3; Jackson ImmunoResearch Laboratories, Inc.) was applied with F-actin counterstain (Alexa Fluor 488 Phalloidin; Life Technologies, Grand Island, NY).
Circulating IL-15/IL-15Rα levels
IL-15/IL-15Rα levels in serum or culture supernatants were measured by mouse IL-15/IL-15R ELISA (eBioscience, Inc., San Diego, CA).
In vitro culture assays
Different liver cell populations (hepatocytes, bulk liver NPC [NPC], liver NPC depleted of CD11c+ DC [NPC-CD11c] and CD11c+ DC alone [CD11c]) were isolated from WT or IRF-1 KO mice and equal numbers of cells (5×106 cells/ml) incubated at 37 °C for 6 or 24 h in media alone or with LPS (30μg/ml) and IFN-γ (100U/ml). CD11c+ DC were purified as described (32, 34).
Soluble IL-15/IL-15Rα complex preparation and in vivo administration
Human rIL-15 and r mouse IL-15Rα Fc were purchased from R&D Systems (Minneapolis, Minnesota, USA) and IL-15/IL-15Rα complexes prepared as described (35, 36). To assess the function of soluble IL-15/IL-15Rα in vivo, IRF-1 KO mice were given 2.5 μg IL-15 and 15 μg IL-15Rα Fc in 200μl PBS i.p. 4 days before cell isolation for flow cytometry or donor liver harvest for LTx.
Flow cytometry
Mouse cell surface molecule and intracellular IFN-γ and FoxP3 staining was performed as described (32). Liver NPC were treated with FcγR-blocking rat anti-mouse CD16/32 mAb (2.4G2) to prevent non-specific Ab binding. For cell surface staining, the NPC were incubated for 30 min with FITC-, phycoerythrin (PE)-, allophycocyanin (APC) -, Pacific Blue-, or PE-cyanin (Cy)7-conjugated mAbs to detect expression of CD3 (145-2C11), CD4 (GK1.5), CD8 (53-6.7), CD11c (HL3), CD45 (30-F11), H2-Kb (AF6-88.5), NK1.1 (PK136) (BD Biosciences, San Diego, CA), CD19 (eBio 1D3, eBioscience, San Diego, CA) and F4/80 (BM8, BioLegend, San Diego, CA). For intracellular cytokine staining, cells were fixed with 4% paraformaldehyde and permeabilized using 0.1% saponin, then stained with anti-mouse IFN-γ Ab (XMG1.2) (BioLegend). For Foxp3 staining, cells were fixed and permeabilized using Foxp3 Fix Permkit (eBioscience) and stained with anti-Foxp3 mAb (FJK-16s) (eBioscience). Appropriate Ig isotype controls were obtained from BD PharMingen (San Diego, CA). Flow cytometry was performed using a LSR Fortessa flow cytometer (BD Biosciences) and data were analyzed using FlowJo software (version 7.6; TreeStar, Inc., Ashland, OR).
Assessment of liver Injury
The extent of hepatic injury after I/R was determined by measuring serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels 3 and 6 h after reperfusion, as described (33). Liver tissue was obtained from graft recipients immediately after euthanasia, fixed in 10% formalin, embedded in paraffin, then sectioned and stained with H&E and TUNEL as described (28). Liver injury was assessed by Suzuki scores (37) in a “blinded” fashion.
Cytokine measurements
Serum cytokine levels (IFN-γ, IL-6, and TNFα) were measured by cytometric bead array (BD Bioscience).
MTT assay
Hepatocytes (WT B6 or IRF-1 KO) were cultured with or without IFN- γ (1000 U/ml) for 24 h when their viability was assessed by MTT assay (Roche Diagnostics, Mannheim, Germany) following the manufacturer’s instructions.
Human primary hepatocyte culture
Human hepatocytes were isolated from histologically normal, surgically-resected liver tissue, as described (32) using a three-step collagenase perfusion technique and density-gradient centrifugation. They were cultured in media containing LPS (30μg/ml) and IFN-γ (250U/ml) and harvested 0, 1, 3, 6, or 24 h after stimulation for RNA isolation.
Hepatocyte transfection using adenovirus vector
A replication-deficient adenoviral vector (human adenovirus type 5, dE1/E3) containing the IRF-1 microRNA hairpin structure (Ad-IRF-1-shRNA) (Vector Biolabs, Philadelphia, PA) was prepared as described (29). The IRF-1 microRNA hairpin sequence is: TGCTGTTGACAGTGAGCGACCTGGCTAGAGATGCAGATTATAGTGAAGCCACAGAT GTATAATCTGCATCTCTAGCCAGGGTGCCTACTGCCTCGGA. The target sequences are: Homo sapiens IRF-1 mRNA (NM_002198.1) 3′-untranslated region (UTR) 227–245; Mus musculus IRF-1 mRNA (NM_008390.1) 3′-UTR 235–253; Rattus norvegicus IRF-1 mRNA (NM_012591.1) 3′-UTR 227–245. Human primary hepatocytes were transfected with Ad-IRF-1-shRNA or control vector (Ad-SCR-ShRNA) for 48 h. The cells were then stimulated with LPS (30μg/ml) and IFN-γ (250U/ml) for 6 h before harvesting for RNA isolation and functional analysis.
Statistical analysis
Data are presented as means ± 1 SEM. Comparisons between groups were performed by using Student’s ‘t’ test. Non-parametric data (Suzuki score) were analyzed with the Mann-Whitney U-test. A log rank test was performed to assess differences in graft survival. A probability value of p<0.05 was considered significant.
Results
IRF-1 is up-regulated in human LTx tissue after reperfusion
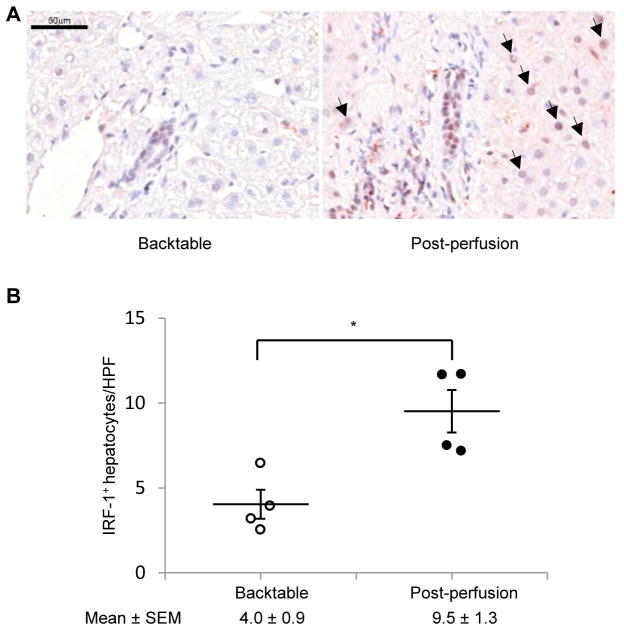
To determine if IRF-1 expression is up-regulate d in human liver allografts after reperfusion, we performed IRF-1 immunohistochemistry on liver tissue specimens obtained from 4 human LTx patients (cold ischemia time range 8 to 16 h) at two time points, before perfusion (backtable) and immediately after reperfusion (post-perfusion). The liver pathologist grading the IRF-1 expression was blinded to the groups. As shown in Figure 1A, IRF-1 was strongly induced and mainly localized to nuclei of hepatocytes in samples obtained post-perfusion, whereas the expression was minimal to negligible in backtable samples. The number of IRF-1 positive hepatocytes increased significantly post-perfusion compared to backtable (Figure 1B).
FIGURE 1. IRF-1 is induced in human liver allografts following reperfusion.
(A) Immunohistochemical staining of IRF-1 (red color) in human liver allograft biopsy samples obtained at backtable and post-perfusion. Representative images of four similar experiments are shown. Scale bar: 50 μm. Arrows show IRF-1 positive cells. (B) Incidences of IRF-1 positive hepatocytes per high power field (x400) were quantified (means ± SEM; 15–20 fields per patient, n=4 patients each time point); *p<0.05.
IRF-1 is up-regulated in both hepatocytes and non-parenchymal cells (NPC) after allogeneic LTx with extended cold storage time
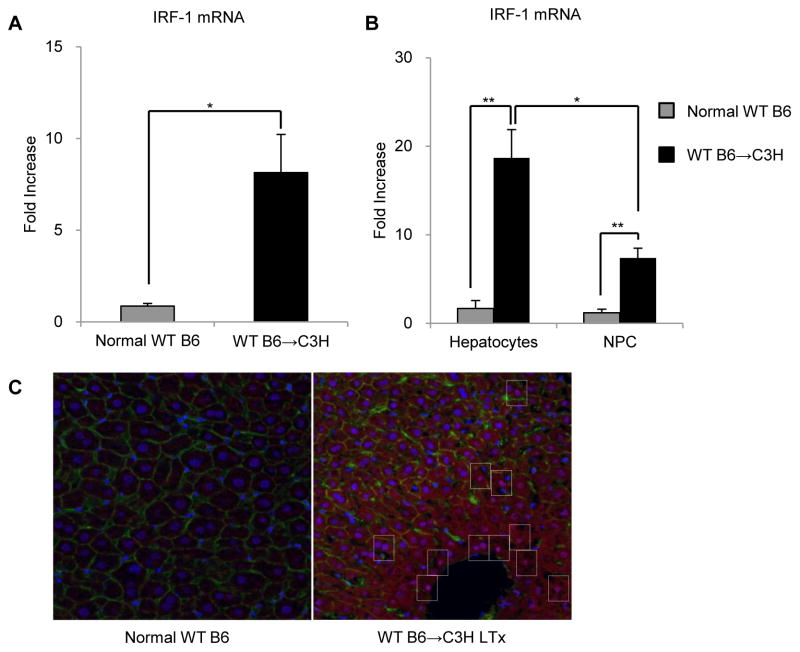
To determine the level of IRF-1 expression in liver grafts after mouse allogeneic (WT B6→C3H) LTx with extended cold storage (24 h preservation), we first performed RT-PCR of whole liver tissue collected 6 h after reperfusion. As we have shown previously with mouse syngeneic LTx (28), IRF-1 mRNA was markedly and significantly up-regulated (8-fold) compared with normal liver tissue (Figure 2A). Both hepatocytes and NPC isolated from the grafts 6 h after reperfusion exhibited marked elevations in IRF-1 expression. Hepatocytes showed significantly greater induction of IRF-1 mRNA (18-fold) than NPC (7-fold) (Figure 2B). Immunofluorescence staining of liver grafts 6 h after reperfusion confirmed that IRF-1 protein expression was significantly induced following LTx compared to normal liver and localized mainly to hepatocyte nuclei (Figure 2C). These data show that both IRF-1 mRNA and protein are up-regulated significantly after allogeneic LTx and that IRF-1 is expressed by both hepatocytes and NPC, with greater induction in hepatocytes.
FIGURE 2. IRF-1 is induced in both hepatocytes and non-parenchymal cells (NPC) following allogeneic liver transplantation in mice.
(A) Whole liver tissue IRF-1 mRNA expression 6 h after reperfusion determined by real-time RT-PCR (n=3); *p<0.05. (B) Hepatocyte and liver NPC IRF-1 mRNA expression 6 h after reperfusion determined by real-time RT-PCR. Data shown are fold increases compared to normal WT B6 hepatocytes or liver NPC, respectively (n=3); **p<0.01, *p<0.05. (C) Immunofluorescence staining of IRF-1 (red color, using anti-IRF-1 and Cy3 goat anti-rabbit), DAPI (blue color), and actin (green color, using Alexa Fluor 488 Phalloidin) in normal WT B6 livers and WT B6 liver allografts 6 h after reperfusion. Representative images (x400) of three similar experiments are shown. Dotted rectangles show IRF-1 positive cells.
IL-15 and IL-15Rα expression is up-regulated in an IRF-1-dependent manner in both hepatocytes and NPC during LTx cold I/R injury
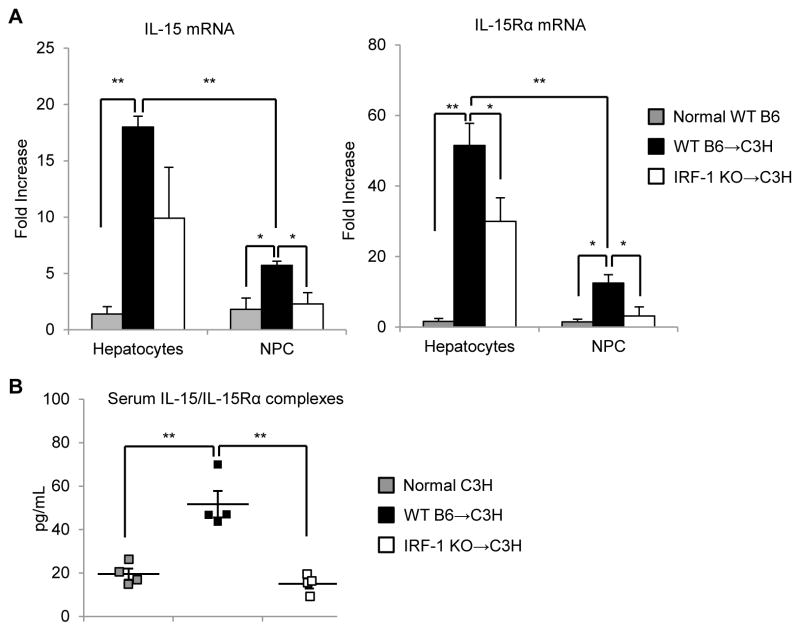
Although IRF-1 is known to induce the expression of both IL-15 and IL-15Rα by BM cells or COS-7 cells in vitro (14, 15, (23), it is unclear whether it induces IL-15 and/or IL-15Rα in the liver during cold I/R injury associated with allogeneic LTx. We observed that both IL-15 and IL-15Rα mRNA expression were induced significantly in hepatocytes 6 h after reperfusion in WT B6→C3H LTx compared to normal hepatocytes; IL-15 mRNA was up-regulated >15-fold and IL-15Rα mRNA increased >50-fold compared to normal WT hepatocytes (Figure 3A). Hepatic NPC obtained from WT B6→C3H allografts also displayed significantly up-regulated IL-15 and IL-15Rα mRNA expression after reperfusion compared to normal WT NPC (Figure 3A). However, the degree of IL-15 and IL-15Rα mRNA induction was significantly greater in hepatocytes compared to NPC. We then used IRF-1 KO donor liver allografts to determine if IRF-1 was involved in promotion of IL-15 and IL-15Rα expression in the liver after LTx. Expression of both IL-15 and IL-15Rα mRNA was reduced significantly in hepatocytes and NPC from IRF-1 KO→C3H compared to WT→C3H grafts (Figure 3A). These data suggest that IRF-1 indeed regulates the induction of both IL-15 and IL-15Rα mRNA in hepatocytes, as well as in liver NPC after LTx. We next measured circulating IL-15/IL-15Rα protein complexes in sera collected from graft recipients 6 h after reperfusion. Levels were elevated significantly in WT→C3H recipients compared to normal C3H mice (Figure 3B), while IRF-1 KO→C3H recipients showed significantly reduced levels of circulating IL-15/IL-15Rα complexes compared to WT→C3H recipients. These data indicate that donor-expressed IRF-1 regulates not only IL-15 and IL-15Rα mRNA expression in the liver, but also circulating IL-15/IL-15Rα complexes in host serum 6 h after LTx.
FIGURE 3. IL-15 and IL-15Rα expression is induced in hepatocytes and NPC in an IRF-1-dependent manner during liver transplantation I/R injury.
(A) Hepatocyte and liver NPC expression of IL-15 and IL-15Rα mRNA 6 h after liver reperfusion determined by real-time RT-PCR. Data shown are fold increases compared to normal WT B6 hepatocytes or liver NPC, respectively (n=3); **p<0.01, *p<0.05. (B) Concentration of IL-15/IL-15Rα complexes in serum 6 h after reperfusion determined by ELISA (n=4); **p<0.01.
Hepatocytes are the major source of soluble IL-15/IL-15Ra complexes among liver cell populations
There is evidence that CD11c+ DC are important producers of circulating IL-15 in endotoxin shock (38) and CpG-induced immune activation (39), based on studies in CD11c-diphtheria toxin receptor transgenic (DTR tg) mice. Other reports indicate that liver parenchymal cells can express IL-15 and IL-15Rα (40) and that they are equally responsible as hematopoietic cells in promoting hepatic NK and NKT cell development in the steady-state (41). Although previous studies have shown IL-15 production by human whole liver tissue (42) or human hepatocellular carcinoma cells (43), it remains unclear which cell population in the liver is the principal producer of soluble IL-15/IL-15Rα complexes. Based on our findings and earlier published observations (38–43), we hypothesized that both liver DC and hepatocytes might be the main producers of secreted IL-15/IL-15Rα complexes in the liver. To verify our hypothesis, we isolated different liver cell subsets and cultured these cells (5×106 cells/ml) with or without LPS and IFN-γ stimulation for 24 h. As shown in Figure 4, hepatocytes secreted significantly higher amounts of soluble IL-15/IL-15Rα complexes compared to bulk liver NPC, liver NPC depleted of DC (NPC-CD11c+), or DC (CD11c+) alone. Among liver NPC, DC constituted one of main producers of IL-15/IL-15Rα complexes, since secreted IL-15/IL-15Rα complexes decreased significantly when DC were removed from NPC (38 ± 6% decrease in media alone group and 57 ± 6% decrease with LPS and IFN-γ group). Interestingly, the degree of soluble IL-15/IL-15Rα production induced varied among different liver cell populations. Thus, in response to LPS and IFN-γ, induction was most pronounced in DC (3.7-fold), compared to hepatocytes (1.2-fold), bulk NPC (1.5-fold), or NPC-CD11c (1.0-fold) (Figure 4).
FIGURE 4. Hepatocytes are the principal producers of IL-15/IL-15Rα complexes among different liver cell populations.
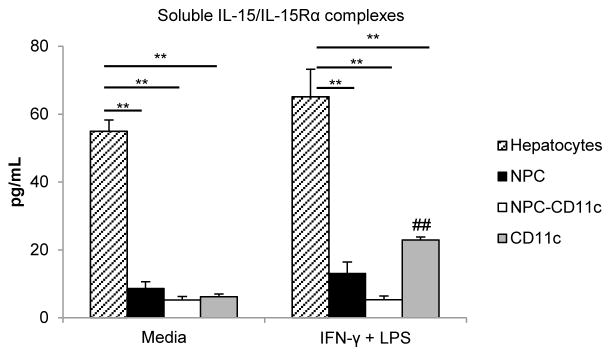
Concentrations of IL-15/IL-15Rα complexes in supernatants of cultures containing equal numbers (5×106 cells/ml) of different liver cell populations isolated from WT B6 mice (hepatocytes, bulk liver NPC [NPC], liver NPC depleted of DC [NPC-CD11c] and CD11c+ DC alone [CD11c]). Cells were incubated for 24 h with media alone or with culture media containing LPS (30μg/ml) and IFN-γ (100U/ml). Data are means obtained from 3 independent experiments; **p<0.01 vs hepatocytes; ## p<0.01 stimulated CD11c vs CD11c with media alone.
IRF-1 regulates basal and induced expression of IL-15 and IL-15Rα mRNA and IL-15/IL-15Rα secretion by mouse primary hepatocytes and liver DC
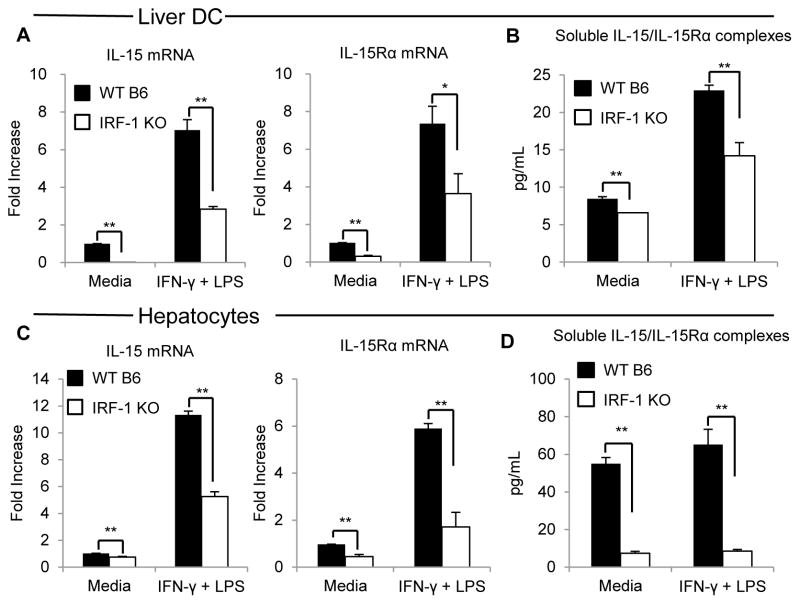
Although previous studies (14, 23) have indicated that IL-15 and IL-15Rα gene sequences both contain IRF-1 binding elements, it remains unclear whether IRF-1 regulates IL-15 and/or IL-15Rα expression equally in liver hematopoietic and parenchymal cells under steady-state and inflammatory conditions (24). Thus, we isolated primary hepatocytes and DC from mouse liver to determine whether IRF-1 could transcriptionally regulate IL-15 and IL-15Rα expression, as well as IL-15/IL-15Rα complex secretion by these cell populations. First, liver DC isolated from WT B6 or IRF-1 KO mice were cultured with or without IFN-γ and LPS for 6 h to determine their constitutive and induced IL-15 and IL-15Rα mRNA expression. As shown in Figure 5A, WT liver CD11c+ DC up-regulated both IL-15 and IL-15Rα mRNA markedly and (~7-fold) in response to stimulation with IFN-γ and LPS. The magnitude of basal and stimulated IL-15/IL-15Rα mRNA expression was diminished in liver DC lacking IRF-1. We then cultured WT or IRF-1 KO liver DC, with or without IFN-γ and LPS stimulation for 24 h, and measured secreted IL-15/IL-15Rα complexes in the culture supernatants. WT liver DC secreted significantly higher levels of IL-15/IL-15Rα complexes compared with IRF-1 KO liver DC, with or without IFN-γ and LPS stimulation (Figure 5B). As shown in Figure 5C and 5D, similar findings were seen using mouse primary hepatocytes. These results indicate that IRF-1 transcriptionally regulates not only constitutive expression but also induced expression of IL-15 and IL-15Rα in both DC and primary hepatocytes isolated from mouse liver.
FIGURE 5. IRF-1 regulates IL-15 and IL-15Rα mRNA expression and the secretion of IL-15/IL-15Rα complexes by liver DC and hepatocytes.
(A) Liver DC expression of IL-15 and IL-15Rα mRNA 6 h after the start of culture in either media alone or media containing LPS (30μg/ml) and IFN-γ (100U/ml) determined by real-time RT-PCR. Liver DC were isolated from WT mice or IRF-1 KO mice. Data are shown as fold increases compared to WT liver DC with media alone; **p<0.01, *p<0.05. (B) Concentrations of IL-15/IL-15Rα complexes in supernatants of cultures containing equal numbers (5×106 cells/ml) of liver DC isolated from WT or IRF-1 KO mice. DC were incubated for 24 h with either media alone or media containing LPS (30μg/ml) and IFN-γ (100U/ml). Data are representative of 3 independent experiments; **p<0.01. (C) Hepatocyte expression of IL-15 and IL-15Rα mRNA 6 h after the start of culture in either media alone or with media containing LPS (30μg/ml) and IFN-γ (100U/ml) determined by real-time RT-PCR. Hepatocytes were isolated from WT or IRF-1 KO mice. Data are fold increases compared to WT hepatocytes with media alone; **p<0.01. (D) Concentrations of IL-15/IL-15Rα complexes in supernatants of cultures containing equal numbers (5×106/ml) of hepatocytes isolated from WT or IRF-1 KO mice. Hepatocytes were incubated for 24 h with either media alone or media containing LPS (30μg/ml) and IFN-γ (100U/ml). Representative of 3 independent experiments; **p<0.01.
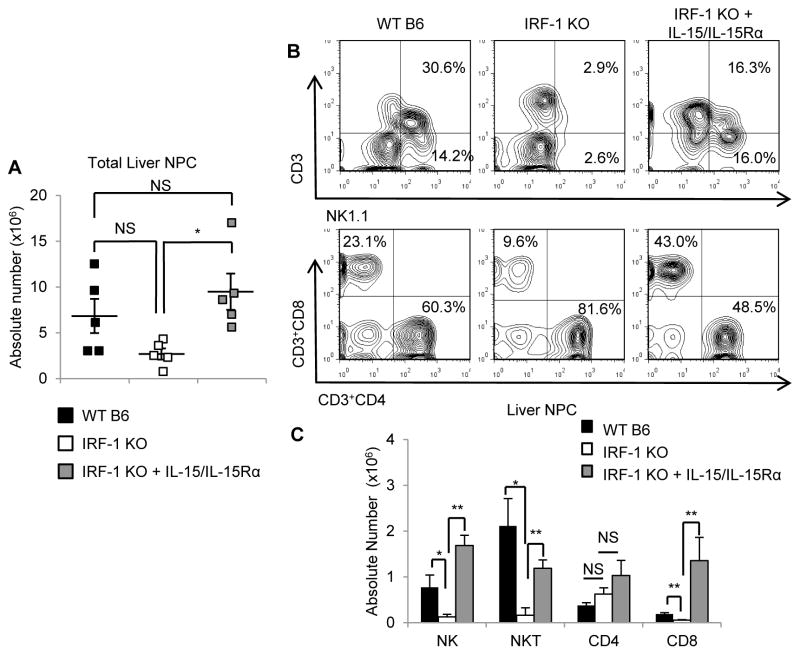
IRF-1-deficient mice lack hepatic NK, NKT and CD8+T cells in the steady-state, while systemic administration of soluble IL-15/IL-15Rα complexes restores these hepatic lymphocyte populations
Although previous studies (14, 15) have shown that IRF-1 KO mice are deficient in hepatic NK and NKT cells, it is not clear whether other hepatic lymphocyte populations, such as CD8+T cells or CD4+CD25+ regulatory T cells (Treg), that are known to be regulated by IRF-1 in other organs (17, 44), are also affected in the liver. It is also unknown whether deficiency of certain hepatic lymphocytes in IRF-1 KO mice can be restored by systemic administration of IL-15/IL-15Rα complexes. To answer these questions, we determined the absolute numbers of hepatic lymphocyte subsets isolated from normal WT B6, untreated IRF-1 KO, and IRF-1 KO mice given IL-15/IL-15Rα complexes systemically. When compared to WT livers, IRF-1 KO livers showed reduced absolute numbers of total liver NPC (Figure 6A). There were no significant differences in the absolute numbers of macrophages, DC, B cells or Treg between livers of WT and IRF-1 KO mice (data not shown) whereas absolute numbers of NK, NKT, and CD8+T cells were all reduced significantly in IRF-1 KO compared to WT livers (Figure 6B, 6C). There were no differences in the absolute numbers of CD4+ T cells between WT and IRF-1 KO livers (Figure 6C). IL-15/IL-15Rα complex administration increased the total number of liver NPC significantly in IRF-1 KO mice. This number was similar to the total liver NPC number in WT mice (Figure 6A). As shown in Figure 6C, soluble IL-15/IL-15Rα complex administration increased the absolute numbers of NK, NKT and CD8+T cells, but not CD4+T cells in IRF-1 KO mice. These data suggest that the deficiency of NK, NKT, and CD8+T cells in the livers of IRF-1 KO mice is due to reduced IL-15/IL-15Rα expression and that systemic administration of IL-15/IL-15Rα complexes can restore these hepatic lymphocytes in IRF-1 KO mice in vivo.
FIGURE 6. IRF-1 regulates NK, NKT, and CD8+T cell numbers in the liver in the normal steady-state via IL-15/IL-15Rα complex production.
(A) Absolute numbers of total liver non-parenchymal cells (NPC) isolated from normal WT B6 mice, IRF-1 KO mice, or IRF-1 KO mice given soluble IL-15/IL-15Rα complexes protein 4 days before cell isolation (means ± SEM; n=5 mice per group); *p<0.05. (B) Representative data (n=5 independent experiments) of liver-resident lymphocyte frequencies represented as a percentage of hepatic CD45+ cells. (C) The bar graph represents absolute numbers of each lymphocyte subset (means ± SEM; n=5 mice per group); *p<0.05, **p<0.01.
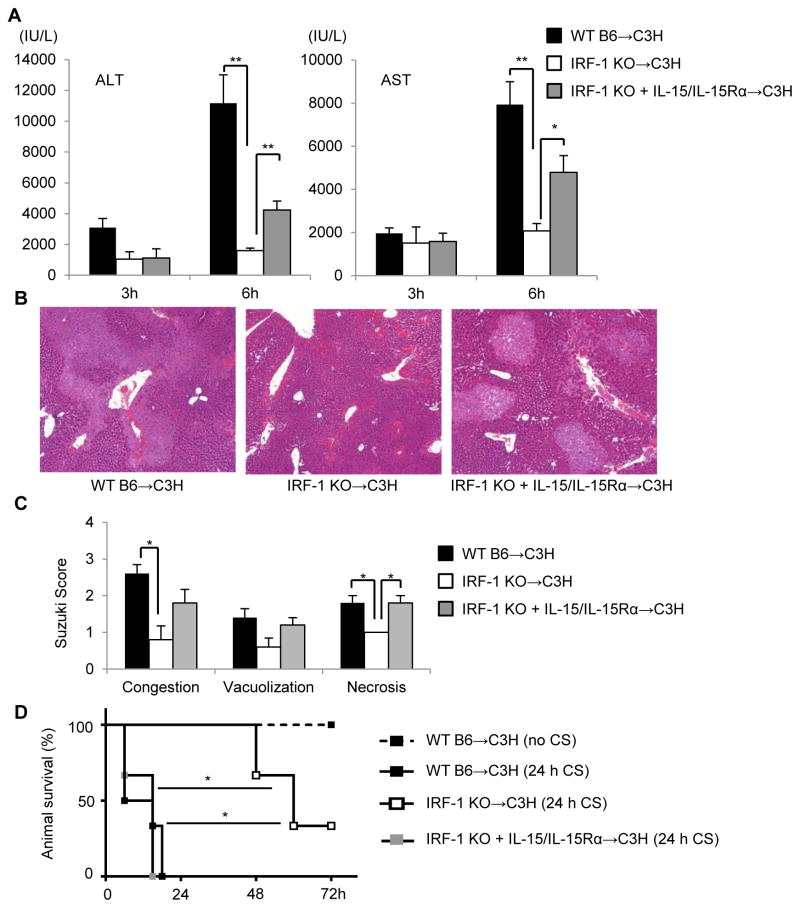
IRF-1 KO liver allografts are protected against cold I/R injury, whereas pretreatment with soluble IL-15/IL-15Rα complexes partially reverses the protective effect
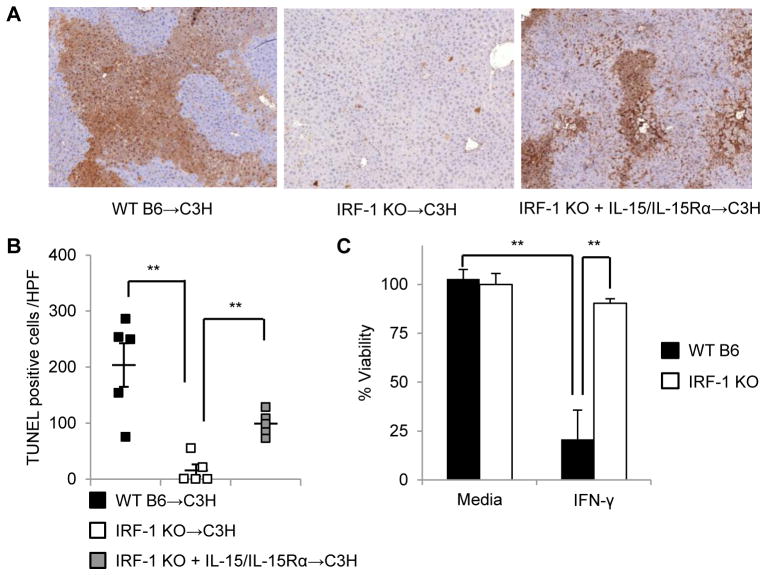
Previous studies have shown that liver-resident lymphocytes, including NK (45), NKT (46), and CD4+Tcells (47) play important roles in the pathogenesis of hepatic partial warm I/R injury (3). Although partial warm I/R injury and cold I/R injury following LTx may share common mechanism(s) (3), these do not always correlate between the two conditions (48, 49). Few studies have investigated the role of donor-derived lymphocytes during cold I/R injury after LTx (33, 50), and it remains uncertain how liver-resident NK, NKT and CD8+T cells regulated by IRF-1 via IL-15/IL-15Rα complexes might impact the outcome of LTx-induced cold I/R injury. Thus, we performed allogeneic LTx following 24 h extended cold storage using donor livers from WT B6, IRF-1 KO or IRF-1 KO mice pretreated with soluble IL-15/IL-15Rα complexes and C3H recipients. As shown in Figure 7A, IRF-1 KO grafts were markedly protected compared to WT grafts after 6 h reperfusion, as evidenced by significantly reduced levels of serum ALT and AST. Histological analysis confirmed significantly lower Suzuki scores for congestion and necrosis in IRF-1 KO compared to WT grafts (Fig 7B, 7C). However, when IRF-1 KO liver grafts pretreated with soluble IL-15/IL-15Rα complexes were transplanted into C3H recipients, protection was reversed, as demonstrated by significantly elevated serum ALT and AST levels and worse liver injury and Suzuki scores compared to IRF-1 KO grafts (Figure 7A, 7B, 7C).
FIGURE 7. IRF-1 mediates liver cold I/R injury via IL-15/IL-15Rα complex production.
(A) Liver allograft recipients’ serum ALT and AST levels 3 and 6 h after reperfusion (means ± SEM; n=3–5 transplants per group); *p<0.05, **p<0.01. (B) Representative histology 6 h after reperfusion showing significantly reduced allograft damage in IRF-1 KO liver recipients and partial restoration of injury by IRF-1 KO donor exposure to IL-15/IL-15R complexes. (C) Liver injury assessed by Suzuki score (means ± SEM; n=5 transplants per group); *p<0.05. (D) Survival rates of recipients receiving liver grafts from WT B6 without extended cold storage (CS) (no CS) (n=3), WT B6 with 24 h CS (24h CS) (n=6), IRF-1 KO with 24 h CS (n=3), or IRF-1 KO pretreated with IL-15/IL-15Rα with 24 h CS (n=3); *p<0.05, log rank test.
Survival of IRF-1 KO donor liver allografts is prolonged but this effect is reversed by soluble IL-15/IL-15Rα complexes
We further tested liver graft survival to determine whether IRF-1 deficiency in the allograft could prolong host survival in our extended cold storage model. Transplantation of WT grafts without extended cold storage resulted in 100% survival, while WT grafts with 24 h extended cold storage did not survive beyond 24 h (Fig 7D). In contrast, recipients of IRF-1 KO grafts showed significantly improved survival compared to WT grafts. However, when IRF-1 KO grafts were pretreated with soluble IL-15/IL-15Rα complexes, the recipients had markedly shortened survival.
IL-15Rα KO liver allografts are protected against cold I/R injury
We also compared liver injury between recipients of WT and IL-15Rα KO allografts to test whether the effects observed in IRF-1 KO mice could be reproduced with allografts deficient in IL-15Rα. Compared to recipients that received WT grafts, those that received IL-15Rα KO liver grafts were significantly protected 6 h after reperfusion with significantly lower ALT and AST levels (ALT 30,827 ± 1,988 vs. 18,967 ± 1,810 IU/L, AST 13,067 ± 965 vs. 8,353 ± 392 IU/L, respectively; n = 3 transplant/group, p<0.05)
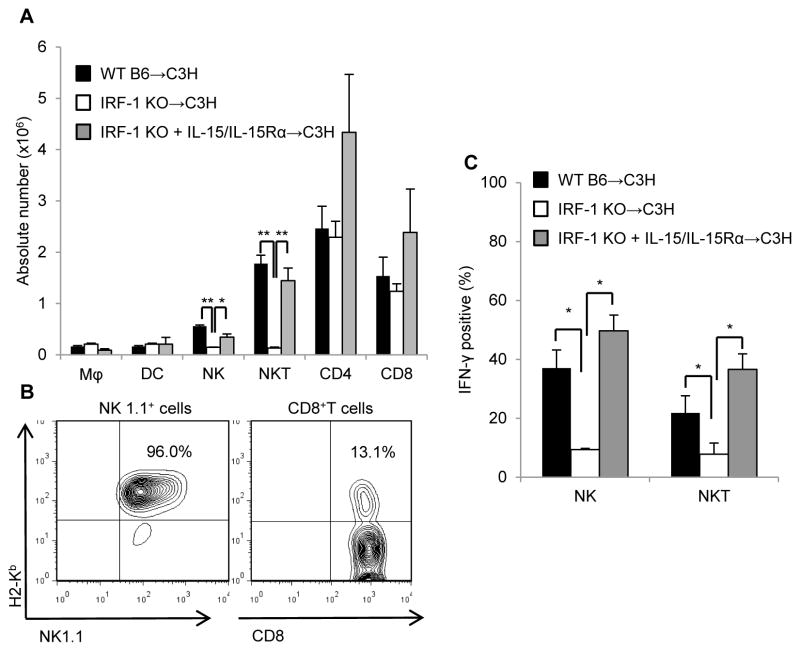
IRF-1 KO liver allografts exhibit diminished donor-derived NK1.1+ cells, cytotoxic effector molecules, and pro-inflammatory cytokines that are restored by pretreatment with soluble IL-15/IL-15Rα complexes
Flow cytometric analysis of intragraft mononuclear cells 6 h after reperfusion showed markedly decreased NK and NKT cell numbers in IRF-1 KO compared to WT grafts (Figure 8A). By contrast, no significant differences in absolute numbers of macrophages, DC, or CD4+T cells were observed. There was also no significant difference in the absolute numbers of CD8+T cells between WT and IRF-1 KO grafts, despite the significant difference observed in non-transplanted livers (Figure 6C). We hypothesized that this discrepancy might be due to differences in population dynamics between hepatic NK1.1+ and CD8+ cells. Thus, we next determined the proportions of these donor- and recipient-derived lymphocyte populations. As shown in Figure 8B, 6 h after reperfusion, the vast majority (approximately 95%) of graft NK 1.1+ cells were donor-derived (H2b+), whereas CD8+T cells were predominantly recipient-derived graft-infiltrating cells (H2b−).
FIGURE 8. IRF-1 regulates donor-derived NK and NKT cells and their IFN-γ production via IL-15/IL-15Rα complexes.
(A) Absolute numbers of various leukocyte populations isolated from liver allografts 6 h post-transplant. Total liver NPC were isolated from WT, IRF-1KO or IL-15/IL-15R-treated IRF-1KO liver recipients (means ± SEM; n=3 mice per group); *p<0.05, **p<0.01. (B) Representative data (n=3 independent experiments) showing donor-derived (H2b+) NK1.1+ and CD8+T cell frequencies as percentages of hepatic CD45+ cells isolated from liver graft recipients. (C) Incidences of IFN-γ+ NK and NKT cells in liver allografts of the 3 transplant groups (means ± SEM; n=3 mice per group); *p<0.05.
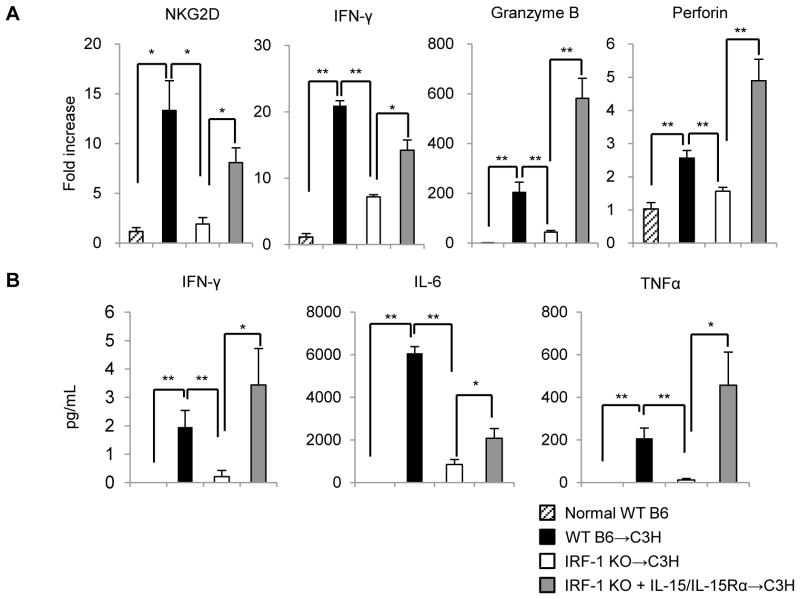
We then determined IFN-γ+ populations among NK cells and NKT cells and found that IRF-1 KO grafts had significantly reduced incidences of these cells (Figure 8C). Notably, donor pretreatment with soluble IL-15/IL-15Rα complexes restored both the absolute number of NK 1.1+ cells and their IFN-γ positivity. Consistent with the reduction in absolute numbers of NK and NKT cells in IRF-1 KO livers 6 h after reperfusion, gene transcripts for cytotoxic lymphocyte-activating receptor NKG2D, IFN-γ and cytotoxic mediators (granzyme B, perforin) were all reduced significantly in IRF-1 KO grafts compared with WT grafts (Figure 9A). However, donor pretreatment with soluble IL-15/IL-15Rα complexes significantly reversed expression of each of these cytotoxic effector molecules in IRF-1 KO grafts.
FIGURE 9. IL-15/IL-15Rα complexes augment cytotoxic effector molecule expression in liver allografts and promote systemic inflammatory responses in allograft recipients.
(A) Expression of cytotoxic molecules in liver grafts 6 h after LTx (means ± SEM; n=3 mice per group); *p<0.05, **p<0.01. (B) Corresponding systemic (serum) IFN-γ, IL-6, and TNFα levels determined by cytokine bead array (means ± SEM; n=5 transplants per group); *p<0.05, **p<0.01.
As shown in Figure 9B, circulating levels of proinflammatory cytokines (IFN-γ, IL-6, and TNFα) in serum were also reduced substantially in recipients of IRF-1 KO grafts compared with those given WT livers. However, donor pretreatment with IL-15/IL-15Rα complexes fully (IFN-γ, TNFα) or partially restored (IL-6) serum cytokine levels in recipients of IRF-1 KO grafts.
Pretreatment of IRF-1 KO grafts with IL-15/IL-15Rα complexes partially reverses hepatocyte cell death
We next examined whether the increase in cytotoxic effector molecules and proinflammatory cytokines observed with soluble IL-15/IL-15Rα complexes correlated with the extent of hepatocyte cell death assessed by TUNEL staining. As shown in Figure 10A and 10B, IRF-1 KO grafts exhibited significantly reduced incidences of TUNEL+ hepatocytes compared with WT grafts, while treatment of IRF-1KO donors with IL-15/IL-15Rα complexes partially reversed the effect. This partial reversal of TUNEL+ hepatocytes in grafts from IRF-1 KO donors given IL-15/IL-15Rα complexes was consistent with the differences in liver injury between the groups, as determined by serum transaminase levels (Figure 7A). We speculated that this partial rather than full restoration of hepatocyte cell death and liver injury might reflect IRF-1 KO hepatocyte resistance to cell death. Thus, IRF-1 is a crucial mediator of apoptosis (28) and IRF-1 KO primary hepatocytes are resistant to apoptosis induced by IFN-α (51) or IFN-γ (52). To confirm whether IRF-1 KO primary hepatocytes were resistant to cell death induced by IFN-γ, we examined the viability of primary hepatocytes from WT or IRF-1 KO mice exposed to IFN-γ in vitro. After incubation with IFN-γ for 24 h, the viability of WT hepatocytes decreased substantially (~80%), whereas IRF-1 KO hepatocytes remained viable and demonstrated resistance to IFN-γ-induced cell death (Figure 10C).
FIGURE 10. IL-15/IL-15Rα complexes promote hepatocyte cell death in liver allografts.
(A) Representative TUNEL-stained sections of liver grafts from WT, IRF-1KO or IL-15/IL-15Rα-treated IRF-1KO donors (B) Incidences of hepatocytes undergoing cell death (TUNEL+ cells) per high power field (x200) in the 3 transplant groups were quantified (means ± SEM; n=5 transplants per group); **p<0.01. (C) Hepatocyte viability determined by MTT assay. Hepatocytes isolated from WT or IRF-1 KO mice were cultured in media with or without IFN-γ (1000U/ml) for 24 h; **p<0.01.
Human primary hepatocytes express IRF-1, IL-15 and IL-15Rα and IRF-1 gene silencing reduces the expression of both IL-15 and IL-15Rα
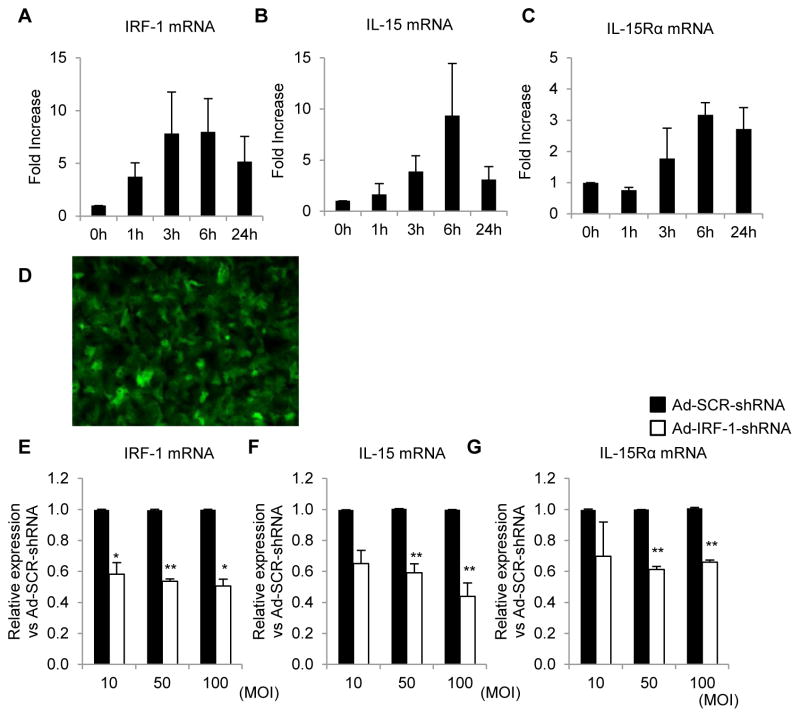
We next assessed the expression of IRF-1, IL-15, and IL-15Rα in human primary hepatocytes isolated from histologically normal, surgically-resected tissue. In response to LPS and IFN-γ stimulation, IRF-1 mRNA expression was up-regulated within 1 h and peaked at 3 h (Figure 11A). These data are consistent with our earlier observations (25, 28) using mouse or rat primary hepatocytes. Induction of IL-15 and IL-15Rα occurred within 3 h of stimulation and peaked 6 h after stimulation (Figure 11B, 11C). To determine whether IRF-1 directly regulated IL-15 and IL-15Rα expression in human primary hepatocytes, we used an adenoviral (Ad) vector containing a microRNA designed to specifically knock down IRF-1 expression (Ad-IRF1-shRNA) (29). Gene delivery efficiency was determined by GFP expression of infected hepatocytes and was >90% (Figure 11D). The Ad-IRF1-shRNA vector effectively knocked down both basal (data not shown) and LPS- and IFN-γ-induced IRF-1 expression (Figure 11E) in human primary hepatocytes compared to an Ad vector containing a scrambled (SCR) shRNA (Ad-SCR-ShRNA). Gene-silencing of IRF-1 also significantly diminished both basal (data not shown) and induced expression of IL-15 and IL-15Rα (Figure 11F, 11G), thus confirming an important role for IRF-1 in regulating human hepatocyte IL-15 and IL-15Rα expression.
FIGURE 11. IRF-1 regulates IL-15 and IL-15Rα expression by human primary hepatocytes.
(A–C) Time courses of human primary hepatocyte IRF-1 (A), IL-15 (B) and IL-15Rα mRNA (C) expression by real-time RT-PCR. The hepatocytes were cultured in media containing LPS (30μg/ml) and IFN-γ (250U/ml). Data are fold increases compared to hepatocytes without treatment (means ± SEM; n=3 independent experiments). (D–G) Analysis of human primary hepatocyte IRF-1 (E), IL-15 (F), and IL-15Rα mRNA expression (G) by real-time RT-PCR following transfection with Ad-IRF-1-shRNA or control vector (Ad-SCR-ShRNA) for 48 h. Gene transfection efficiency was confirmed by GFP expression in hepatocytes (D). The cells were then stimulated with LPS (30μg/ml) and IFN-γ (250U/ml) for 6 h before harvesting for analysis. Data are shown as relative expression vs Ad-SCR-ShRNA (means ± SEM; n=3 independent experiments); *p<0.05, **p<0.01. MOI = multiplicity of infection.
Discussion
The IRF family of transcription factors consists of nine members in mammals and plays a central role in the regulation of hematopoietic cell development, innate and adaptive immune responses, and oncogenesis (53). IRF-1, the first member of this family to be identified, is expressed constitutively at low levels in a variety of cell types, including both parenchymal and non-parenchymal cells. It is strongly induced by type I and II IFN, LPS, viral infection, double-stranded RNA and by various cytokines, such as TNFα, IL-1β and IL-6 (12, 54). IRF-1 exhibits remarkably diverse functions as it targets a variety of genes including iNOS, IL-12p40, and caspase 1 that are involved in anti-bacterial responses, Th1-type immunity and the induction of apoptosis (8). We have shown previously (27, 28) that IRF-1 plays a central role in promoting hepatocyte apoptosis following cold I/R injury in syngeneic mouse or rat orthotopic liver transplantation. However, the immunomodulatory role of hepatic IRF-1 in the steady-state or during cold I/R injury following allogeneic LTx is not well understood. Here, we demonstrate that IRF-1 KO donor allografts are markedly protected (approximately 90%) from cold I/R injury compared to WT allografts. Our novel findings indicate that this protective effect is due in large part to reduced expression of IL-15/IL-15Rα complexes in the IRF-1 KO grafts and consequent, diminished innate immune responses and hepatic lymphocyte insufficiency during cold I/R injury.
The liver is a unique organ that receives blood supply containing food and microbial products from the intestine via the portal vein (55–57). Its immune cell constituency is enriched in NK cells, NKT cells and CD8+T cells compared to other parenchymal organs (58–60) and previous studies have suggested that the hepatic microenvironment can support the maintenance of a unique lymphocyte repertoire (42, 57, 58). However, the exact mechanism of hepatic lymphocyte homeostasis is not fully understood. To our knowledge, no previous studies have examined the relative contribution of different liver cell populations to the production of IL-15/IL-15Rα complexes (24). Thus, we examined both parenchymal and non-parenchymal cells as potential sources of soluble IL-15/IL-15Rα complexes within the liver. Our study provides evidence, for the first time, that hepatocytes are the major producers of soluble IL-15/IL-15Rα complexes in both the steady-state (without stimulation) and under inflammatory conditions in vitro (IFN-γ and LPS stimulation). We also found that IRF-1 transcriptionally regulates not only the induced expression, but also the constitutive expression of both IL-15 and IL-15Rα mRNA in hepatocytes, as well as in liver DC. Additionally, we have confirmed that IRF-1 is important for basal and induced production of IL-15/IL-15Rα complex protein by both hepatocytes and liver DC. Importantly, using human primary hepatocyte cultures we have confirmed that these findings in mice also apply to human cells. Specific knockdown of IRF-1 allowed us to demonstrate that IRF-1 controls both basal and induced IL-15 and IL-15Rα mRNA expression in human primary hepatocytes.
These findings provide new insights into our understanding of IL-15 biology. Based on BM chimera experiments, a traditional view has been that IRF-1 expression by radiation-resistant BM stromal cells is important for the induction of IL-15 expression and subsequent maturation of NK cell precursors in the BM (8, 12, 14, 18, 19). However, these studies did not exclude the possibility that other radio-resistant parenchymal cells, such as hepatocytes, might also play an important role in development of lymphocytes within an organ such as the liver. Evidence has accumulated that parenchymal cells, including hepatocytes, can express both IL-15 and IL-15Rα protein (40) and thus provide a microenvironment favorable to T cell survival and CD8+T differentiation (61). Indeed, we found that hepatocytes could secrete significantly higher amounts of IL-15/IL-15Rα complex protein than various immune cells, including DC, a commonly-studied source of IL-15 (38, 39). Interestingly, our finding that hepatocytes are the major source of soluble IL-15/IL-15Rα complexes correlates well with a report (62) that identified hepatocytes as a new source of IL-7, another member of the common cytokine-receptor γc family, that prolongs the survival of naïve and memory CD4+ and CD8+ T cells. Our findings are also consistent with those of a recent study (63) showing that IRF-1 regulates the induced expression of IL-15Rα by Huh7 (human hepatocellular carcinoma cell line) cells and that IRF-2, another member of the IRF family, controls basal expression of both IL-7 and IL-15Rα. The latter study shows that IRF-2 is significantly down-regulated in the liver in hepatitis C virus (HCV) infection and subsequently impaired expression of IL-7 and IL-15Rα in hepatocytes may explain poor HCV-specific CD8+T cell responses, inability to eliminate HCV and perpetuation of infection (63).
Considering that hepatocytes constitute the majority (60–80%) of the total cell population in the liver (57), the present findings and these earlier reports together suggest that IRF-1 expression by hepatocytes may contribute significantly to the development and homeostasis of NK, NKT, and CD8+T cells in the liver through soluble IL-15/IL-15Rα complex production. Notably, lymphocytes isolated from livers of normal IRF-1 KO mice showed significantly reduced numbers of NK, NKT, and CD8+ T cells compared to those from livers of WT B6 mice. When IRF-1 KO mice were treated with soluble IL-15/IL-15Rα complexes in vivo, absolute numbers of hepatic NK, NKT, and CD8+ T cells became similar to those in WT B6 mice. These results suggest that the reduced cell numbers in IRF-1 KO livers are due to diminished expression of IL-15/IL-15Rα complexes and that IRF-1 KO hematopoietic cells retain the ability to develop and proliferate in response to exogenous soluble IL-15/IL-15Rα complexes. Our results are consistent with similar in vitro observations made using IRF-1 KO BM cells and rIL-15 (14). They do not, however, delineate the relative contributions of BM stromal cells and liver parenchymal cells to the development of NK, NKT, and CD8+T cells in the liver. Generation of a mouse strain with hepatocyte-specific deletion of IRF-1 may be useful in addressing this issue.
Our in vivo results also show that IL-15 and IL-15Rα mRNA is up-regulated in liver allograft hepatocytes and NPC in an IRF-1-dependent manner during I/R injury following extended cold storage. IL-15 and IL-15Rα expression was significantly higher in hepatocytes than NPC, suggesting that hepatocytes may also be the major producers of IL-15 and IL-15Rα in vivo. Circulating IL-15/IL-15Rα complex levels were increased significantly in WT but not IRF-1 KO allograft recipients 6 h post reperfusion. These results indicate that the predominant source of circulating IL-15/IL-15Rα complexes is the allograft, rather than host-derived cells. Normal IL-15/IL-15Rα complex levels in IRF-1 KO liver recipients were associated with significantly improved survival, reduced liver injury, less TUNEL+ hepatocytes, decreased numbers of graft NK and NKT cells, and reduced expression of cytotoxic effector molecules (NKG2D, granzyme B, perforin), and pro-inflammatory cytokines (IFN-γ, IL-6, TNFα). When IRF-1 KO livers were exposed to soluble IL-15/IL-15Rα complexes, cytotoxic molecules and proinflammatory cytokines were increased significantly and liver injury and hepatocyte cell death partially restored and recipient survival shortened. These findings indicate that exogenous IL-15/IL-15Rα complexes can promote the development and proliferation of immune cells in the liver and restore innate immune responses in IRF-1 KO liver recipients, which in turn, affects the viability of hepatocytes during liver transplant I/R injury. These findings from in vivo experiments are in accord with a previous report that showed proinflammatory effects of exogenous IL-15 in the liver; daily administration of rIL-15 for 7 days to healthy, WT mice significantly increased hepatic expression of proinflammatory cytokines (IFN-γ and TNFα), the number of inflammatory foci, and caspase-3 positive cells in the liver (40). We further tested whether the proinflammatory effects of soluble IL-15/IL-15Rα complexes in IRF-1 KO could be reproduced in IL-15Rα KO allografts. Recipients of IL-15Rα KO grafts were significantly protected compared to those that received WT transplants, as indicated by significantly lower ALT and AST levels. These results suggest that deficiency of IL-15Rα in donor liver grafts is indeed protective and supports the effects observed in IRF-1 KO mice. These findings from our study are also consistent with previous reports that IL-15 promotes the survival (64, 65) and activation of NK1.1+ cells (66, 67) and CD8+T cells (68) and up-regulates NKG2D(69), granzyme B, perforin (70) and IFN-γ (36, 71, 72) in various models of inflammation and infection. We hypothesized that the partial restoration of liver injury and hepatocyte cell death observed in recipients of IRF-1 KO livers treated with IL-15/IL-15Rα complexes was due to the resistance of IRF-1 KO hepatocytes to apoptosis, as described (28, 51, 52). Indeed, we found that IRF-1 KO hepatocytes were more resistance to cell death in response to IFN-γ compared to WT hepatocytes. These results indicate that IRF-1 regulates innate immune responses via soluble IL-15/IL-15Rα complexes and plays a significant role in the pathogenesis of LTx cold I/R injury.
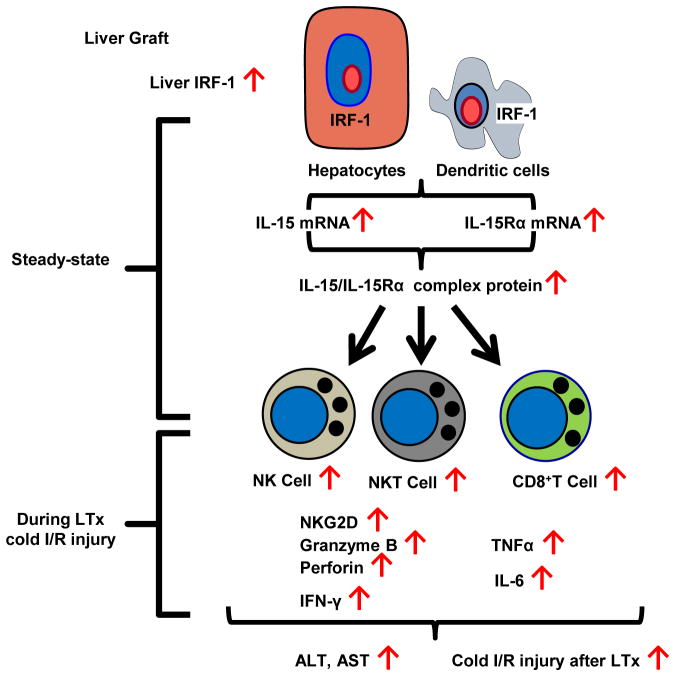
In conclusion, our study provides new evidence that IRF-1 regulates the homeostasis of NK, NKT and CD8+T cells in the liver through IL-15/IL-15Rα production by liver parenchymal and non-parenchymal cells and also contributes to liver injury during liver allograft cold I/R through innate immune cell activation. Findings from the present study and their interpretation are summarized in Fig 12. Our novel observations suggest that targeting of IRF-1 and/or IL-15/IL-15Rα may be an effective approach to reducing I/R injury associated with LTx.
FIGURE 12. Model depicting the proposed role of IRF-1 in hepatic lymphocyte homeostasis and innate immune cell activation during liver transplant cold I/R injury.
IRF-1 in hepatocytes and DC is crucial in regulating both IL-15 and IL-15Rα mRNA expression and subsequent production of soluble IL-15/IL-15Rα complex by these cells. Hepatocytes are the principal producers of soluble IL-15/IL-15Rα complexes among different liver cell populations in both the steady-state and under inflammatory conditions (LTx cold I/R injury with 24 h of cold storage). Soluble IL-15/IL-15Rα complexes contribute to the homeostasis of hepatic NK, NKT, and CD8+T cells and the activation of these cells during LTx cold I/R injury determined by increased cytotoxic effector molecule expression in the liver graft (NKG2D, Granzyme B, Perforin, IFN-γ) and systemic proinflammatory markers in the recipient’s serum (IFN-γ, TNFα, IL-6). Serum ALT and AST levels significantly increase, reflecting worsened liver injury as a result of these inflammatory responses.
Supplementary Material
Acknowledgments
We thank Miriam Freeman, Nicole Martik-Hays, and Kimberly Ferrero for their assistance.
Financial Support: This work was supported by National Institutes of Health (NIH) contract grant HHSN276201200017C (to D.A.G.) and by NIH grant P01 AI81678 (to A.W.T.). SY was supported by funds from Jichi Medical University, Shimotsuke, Tochigi, Japan. OY was supported by NIH grant T32 AI74490 (AWT).
Abbreviations used in this article
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BM
bone marrow
- DC
dendritic cell
- IRF-1
interferon regulatory factor 1
- I/R
ischemia and reperfusion
- LTx
liver transplantation
- NPC
non-parenchymal cells
- WT
wild-type
Footnotes
Disclosures: The authors have no financial conflict of interest.
References
- 1.Wertheim JA, Petrowsky H, Saab S, Kupiec-Weglinski JW, Busuttil RW. Major challenges limiting liver transplantation in the United States. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:1773–1784. doi: 10.1111/j.1600-6143.2011.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nickkholgh A, Weitz J, Encke J, Sauer P, Mehrabi A, Buchler MW, Schmidt J, Schemmer P. Utilization of extended donor criteria in liver transplantation: a comprehensive review of the literature. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2007;22(Suppl 8):viii29–viii36. doi: 10.1093/ndt/gfm654. [DOI] [PubMed] [Google Scholar]
- 3.Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nature reviews. Gastroenterology & hepatology. 2013;10:79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:1563–1569. doi: 10.1111/j.1600-6143.2011.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furukawa H, Todo S, Imventarza O, Casavilla A, Wu YM, Scotti-Foglieni C, Broznick B, Bryant J, Day R, Starzl TE. Effect of cold ischemia time on the early outcome of human hepatic allografts preserved with UW solution. Transplantation. 1991;51:1000–1004. doi: 10.1097/00007890-199105000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burroughs AK, Sabin CA, Rolles K, Delvart V, Karam V, Buckels J, O’Grady JG, Castaing D, Klempnauer J, Jamieson N, Neuhaus P, Lerut J, de Ville de Goyet J, Pollard S, Salizzoni M, Rogiers X, Muhlbacher F, Garcia Valdecasas JC, Broelsch C, Jaeck D, Berenguer J, Gonzalez EM, Adam R. 3-month and 12-month mortality after first liver transplant in adults in Europe: predictive models for outcome. Lancet. 2006;367:225–232. doi: 10.1016/S0140-6736(06)68033-1. [DOI] [PubMed] [Google Scholar]
- 7.Howard TK, Klintmalm GB, Cofer JB, Husberg BS, Goldstein RM, Gonwa TA. The influence of preservation injury on rejection in the hepatic transplant recipient. Transplantation. 1990;49:103–107. doi: 10.1097/00007890-199001000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annual review of immunology. 2001;19:623–655. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- 9.Galon J, Sudarshan C, Ito S, Finbloom D, O’Shea JJ. IL-12 induces IFN regulating factor-1 (IRF-1) gene expression in human NK and T cells. J Immunol. 1999;162:7256–7262. [PubMed] [Google Scholar]
- 10.Barber SA, Fultz MJ, Salkowski CA, Vogel SN. Differential expression of interferon regulatory factor 1 (IRF-1), IRF-2, and interferon consensus sequence binding protein genes in lipopolysaccharide (LPS)-responsive and LPS-hyporesponsive macrophages. Infection and immunity. 1995;63:601–608. doi: 10.1128/iai.63.2.601-608.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyamoto M, Fujita T, Kimura Y, Maruyama M, Harada H, Sudo Y, Miyata T, Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988;54:903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- 12.Lohoff M, Mak TW. Roles of interferon-regulatory factors in T-helper-cell differentiation. Nature reviews. Immunology. 2005;5:125–135. doi: 10.1038/nri1552. [DOI] [PubMed] [Google Scholar]
- 13.Gabriele L, Fragale A, Borghi P, Sestili P, Stellacci E, Venditti M, Schiavoni G, Sanchez M, Belardelli F, Battistini A. IRF-1 deficiency skews the differentiation of dendritic cells toward plasmacytoid and tolerogenic features. Journal of leukocyte biology. 2006;80:1500–1511. doi: 10.1189/jlb.0406246. [DOI] [PubMed] [Google Scholar]
- 14.Ogasawara K, Hida S, Azimi N, Tagaya Y, Sato T, Yokochi-Fukuda T, Waldmann TA, Taniguchi T, Taki S. Requirement for IRF-1 in the microenvironment supporting development of natural killer cells. Nature. 1998;391:700–703. doi: 10.1038/35636. [DOI] [PubMed] [Google Scholar]
- 15.Ohteki T, Yoshida H, Matsuyama T, Duncan GS, Mak TW, Ohashi PS. The transcription factor interferon regulatory factor 1 (IRF-1) is important during the maturation of natural killer 1.1+ T cell receptor-alpha/beta+ (NK1+ T) cells, natural killer cells, and intestinal intraepithelial T cells. The Journal of experimental medicine. 1998;187:967–972. doi: 10.1084/jem.187.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guy-Grand D, Vassalli P. Gut intraepithelial T lymphocytes. Current opinion in immunology. 1993;5:247–252. doi: 10.1016/0952-7915(93)90012-h. [DOI] [PubMed] [Google Scholar]
- 17.Matsuyama T, Kimura T, Kitagawa M, Pfeffer K, Kawakami T, Watanabe N, Kundig TM, Amakawa R, Kishihara K, Wakeham A, et al. Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development. Cell. 1993;75:83–97. [PubMed] [Google Scholar]
- 18.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annual review of immunology. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 19.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 20.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nature reviews. Immunology. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 21.Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Advances in immunology. 2005;86:209–239. doi: 10.1016/S0065-2776(04)86006-1. [DOI] [PubMed] [Google Scholar]
- 22.Bergamaschi C, Bear J, Rosati M, Beach RK, Alicea C, Sowder R, Chertova E, Rosenberg SA, Felber BK, Pavlakis GN. Circulating IL-15 exists as heterodimeric complex with soluble IL-15Ralpha in human and mouse serum. Blood. 2012;120:e1–8. doi: 10.1182/blood-2011-10-384362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mariner JM, Mamane Y, Hiscott J, Waldmann TA, Azimi N. IFN regulatory factor 4 participates in the human T cell lymphotropic virus type I-mediated activation of the IL-15 receptor alpha promoter. J Immunol. 2002;168:5667–5674. doi: 10.4049/jimmunol.168.11.5667. [DOI] [PubMed] [Google Scholar]
- 24.Huntington ND. The unconventional expression of IL-15 and its role in NK cell homeostasis. Immunology and cell biology. 2014;92:210–213. doi: 10.1038/icb.2014.1. [DOI] [PubMed] [Google Scholar]
- 25.Tsung A, Stang MT, Ikeda A, Critchlow ND, Izuishi K, Nakao A, Chan MH, Jeyabalan G, Yim JH, Geller DA. The transcription factor interferon regulatory factor-1 mediates liver damage during ischemia-reperfusion injury. American journal of physiology. Gastrointestinal and liver physiology. 2006;290:G1261–1268. doi: 10.1152/ajpgi.00460.2005. [DOI] [PubMed] [Google Scholar]
- 26.Dhupar R, Klune JR, Evankovich J, Cardinal J, Zhang M, Ross M, Murase N, Geller DA, Billiar TR, Tsung A. Interferon regulatory factor 1 mediates acetylation and release of high mobility group box 1 from hepatocytes during murine liver ischemia-reperfusion injury. Shock. 2011;35:293–301. doi: 10.1097/SHK.0b013e3181f6aab0. [DOI] [PubMed] [Google Scholar]
- 27.Kim KH, Dhupar R, Ueki S, Cardinal J, Pan P, Cao Z, Cho SW, Murase N, Tsung A, Geller DA. Donor graft interferon regulatory factor-1 gene transfer worsens liver transplant ischemia/reperfusion injury. Surgery. 2009;146:181–189. doi: 10.1016/j.surg.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueki S, Dhupar R, Cardinal J, Tsung A, Yoshida J, Ozaki KS, Klune JR, Murase N, Geller DA. Critical role of interferon regulatory factor-1 in murine liver transplant ischemia reperfusion injury. Hepatology. 2010;51:1692–1701. doi: 10.1002/hep.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao Z, Dhupar R, Cai C, Li P, Billiar TR, Geller DA. A critical role for IFN regulatory factor 1 in NKT cell-mediated liver injury induced by alpha-galactosylceramide. J Immunol. 2010;185:2536–2543. doi: 10.4049/jimmunol.1000092. [DOI] [PubMed] [Google Scholar]
- 30.Linfert D, Chowdhry T, Rabb H. Lymphocytes and ischemia-reperfusion injury. Transplant Rev (Orlando) 2009;23:1–10. doi: 10.1016/j.trre.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida O, Kimura S, Jackson EK, Robson SC, Geller DA, Murase N, Thomson AW. CD39 expression by hepatic myeloid dendritic cells attenuates inflammation in liver transplant ischemia-reperfusion injury in mice. Hepatology. 2013;58:2163–2175. doi: 10.1002/hep.26593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueki S, Castellaneta A, Yoshida O, Ozaki K, Zhang M, Kimura S, Isse K, Ross M, Shao L, Stolz DB, Thomson AW, Demetris AJ, Geller DA, Murase N. Hepatic B7 homolog 1 expression is essential for controlling cold ischemia/reperfusion injury after mouse liver transplantation. Hepatology. 2011;54:216–228. doi: 10.1002/hep.24360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bamboat ZM, Ocuin LM, Balachandran VP, Obaid H, Plitas G, DeMatteo RP. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. The Journal of clinical investigation. 2010;120:559–569. doi: 10.1172/JCI40008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroemer A, Xiao X, Degauque N, Edtinger K, Wei H, Demirci G, Li XC. The innate NK cells, allograft rejection, and a key role for IL-15. J Immunol. 2008;180:7818–7826. doi: 10.4049/jimmunol.180.12.7818. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Ohteki T, Tada H, Ishida K, Sato T, Maki C, Yamada T, Hamuro J, Koyasu S. Essential roles of DC-derived IL-15 as a mediator of inflammatory responses in vivo. The Journal of experimental medicine. 2006;203:2329–2338. doi: 10.1084/jem.20061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuwajima S, Sato T, Ishida K, Tada H, Tezuka H, Ohteki T. Interleukin 15-dependent crosstalk between conventional and plasmacytoid dendritic cells is essential for CpG-induced immune activation. Nature immunology. 2006;7:740–746. doi: 10.1038/ni1348. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki A, McCall S, Choi SS, Sicklick JK, Huang J, Qi Y, Zdanowicz M, Camp T, Li YX, Diehl AM. Interleukin-15 increases hepatic regenerative activity. Journal of hepatology. 2006;45:410–418. doi: 10.1016/j.jhep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Schluns KS, Nowak EC, Cabrera-Hernandez A, Puddington L, Lefrancois L, Aguila HL. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor alpha expression. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5616–5621. doi: 10.1073/pnas.0307442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golden-Mason L, Kelly AM, Doherty DG, Traynor O, McEntee G, Kelly J, Hegarty JE, O’Farrelly C. Hepatic interleuklin 15 (IL-15) expression: implications for local NK/NKT cell homeostasis and development. Clinical and experimental immunology. 2004;138:94–101. doi: 10.1111/j.1365-2249.2004.02586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamaji K, Nabeshima S, Murata M, Chong Y, Furusyo N, Ikematsu H, Hayashi J. Interferon-alpha/beta upregulate IL-15 expression in vitro and in vivo: analysis in human hepatocellular carcinoma cell lines and in chronic hepatitis C patients during interferon-alpha/beta treatment. Cancer immunology, immunotherapy : CII. 2006;55:394–403. doi: 10.1007/s00262-005-0005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fragale A, Gabriele L, Stellacci E, Borghi P, Perrotti E, Ilari R, Lanciotti A, Remoli AL, Venditti M, Belardelli F, Battistini A. IFN regulatory factor-1 negatively regulates CD4+ CD25+ regulatory T cell differentiation by repressing Foxp3 expression. J Immunol. 2008;181:1673–1682. doi: 10.4049/jimmunol.181.3.1673. [DOI] [PubMed] [Google Scholar]
- 45.Beldi G, Banz Y, Kroemer A, Sun X, Wu Y, Graubardt N, Rellstab A, Nowak M, Enjyoji K, Li X, Junger WG, Candinas D, Robson SC. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice. Hepatology. 2010;51:1702–1711. doi: 10.1002/hep.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. The Journal of experimental medicine. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen X, Wang Y, Gao F, Ren F, Busuttil RW, Kupiec-Weglinski JW, Zhai Y. CD4 T cells promote tissue inflammation via CD40 signaling without de novo activation in a murine model of liver ischemia/reperfusion injury. Hepatology. 2009;50:1537–1546. doi: 10.1002/hep.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klune JR, Tsung A. Molecular biology of liver ischemia/reperfusion injury: established mechanisms and recent advancements. The Surgical clinics of North America. 2010;90:665–677. doi: 10.1016/j.suc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Zhang M, Ueki S, Kimura S, Yoshida O, Castellaneta A, Ozaki KS, Demetris AJ, Ross M, Vodovotz Y, Thomson AW, BSD, Geller DA, Murase N. Roles of dendritic cells in murine hepatic warm and liver transplantation-induced cold ischemia/reperfusion injury. Hepatology. 2013;57:1585–1596. doi: 10.1002/hep.26129. [DOI] [PubMed] [Google Scholar]
- 50.Pommey S, Lu B, McRae J, Stagg J, Hill P, Salvaris E, Robson SC, d’Apice AJ, Cowan PJ, Dwyer KM. Liver grafts from CD39-overexpressing rodents are protected from ischemia reperfusion injury due to reduced numbers of resident CD4+ T cells. Hepatology. 2013;57:1597–1606. doi: 10.1002/hep.25985. [DOI] [PubMed] [Google Scholar]
- 51.Castellaneta A, Yoshida O, Kimura S, Yokota S, Geller DA, Murase N, Thomson AW. Plasmacytoid dendritic cell-derived IFN-alpha promotes murine liver ischemia/reperfusion injury by induction of hepatocyte IRF-1. Hepatology. 2014;60:267–277. doi: 10.1002/hep.27037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kano A, Haruyama T, Akaike T, Watanabe Y. IRF-1 is an essential mediator in IFN-gamma-induced cell cycle arrest and apoptosis of primary cultured hepatocytes. Biochemical and biophysical research communications. 1999;257:672–677. doi: 10.1006/bbrc.1999.0276. [DOI] [PubMed] [Google Scholar]
- 53.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annual review of immunology. 2008;26:535–584. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- 54.Geller DA, Nguyen D, Shapiro RA, Nussler A, Di Silvio M, Freeswick P, Wang SC, Tweardy DJ, Simmons RL, Billiar TR. Cytokine induction of interferon regulatory factor-1 in hepatocytes. Surgery. 1993;114:235–242. [PubMed] [Google Scholar]
- 55.Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47:729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 56.Corbitt N, Kimura S, Isse K, Specht S, Chedwick L, Rosborough BR, Lunz JG, Murase N, Yokota S, Demetris AJ. Gut bacteria drive Kupffer cell expansion via MAMP-mediated ICAM-1 induction on sinusoidal endothelium and influence preservation-reperfusion injury after orthotopic liver transplantation. The American journal of pathology. 2013;182:180–191. doi: 10.1016/j.ajpath.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 58.Crispe IN. The liver as a lymphoid organ. Annual review of immunology. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 59.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. Journal of leukocyte biology. 2009;86:513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swain MG. Natural killer T cells within the liver: conductors of the hepatic immune orchestra. Dig Dis. 2010;28:7–13. doi: 10.1159/000282059. [DOI] [PubMed] [Google Scholar]
- 61.Correia MP, Cardoso EM, Pereira CF, Neves R, Uhrberg M, Arosa FA. Hepatocytes and IL-15: a favorable microenvironment for T cell survival and CD8+ T cell differentiation. J Immunol. 2009;182:6149–6159. doi: 10.4049/jimmunol.0802470. [DOI] [PubMed] [Google Scholar]
- 62.Sawa Y, Arima Y, Ogura H, Kitabayashi C, Jiang JJ, Fukushima T, Kamimura D, Hirano T, Murakami M. Hepatic interleukin-7 expression regulates T cell responses. Immunity. 2009;30:447–457. doi: 10.1016/j.immuni.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 63.Larrea E, Riezu-Boj JI, Aldabe R, Guembe L, Echeverria I, Balasiddaiah A, Gastaminza P, Civeira MP, Sarobe P, Prieto J. Dysregulation of interferon regulatory factors impairs the expression of immunostimulatory molecules in hepatitis C virus genotype 1-infected hepatocytes. Gut. 2014;63:665–673. doi: 10.1136/gutjnl-2012-304377. [DOI] [PubMed] [Google Scholar]
- 64.Huntington ND, Puthalakath H, Gunn P, Naik E, Michalak EM, Smyth MJ, Tabarias H, Degli-Esposti MA, Dewson G, Willis SN, Motoyama N, Huang DC, Nutt SL, Tarlinton DM, Strasser A. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nature immunology. 2007;8:856–863. doi: 10.1038/ni1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Inoue S, Unsinger J, Davis CG, Muenzer JT, Ferguson TA, Chang K, Osborne DF, Clark AT, Coopersmith CM, McDunn JE, Hotchkiss RS. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J Immunol. 2010;184:1401–1409. doi: 10.4049/jimmunol.0902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ashkar AA, Reid S, Verdu EF, Zhang K, Coombes BK. Interleukin-15 and NK1.1+ cells provide innate protection against acute Salmonella enterica serovar Typhimurium infection in the gut and in systemic tissues. Infection and immunity. 2009;77:214–222. doi: 10.1128/IAI.01066-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zanoni I, Spreafico R, Bodio C, Di Gioia M, Cigni C, Broggi A, Gorletta T, Caccia M, Chirico G, Sironi L, Collini M, Colombo MP, Garbi N, Granucci F. IL-15 cis presentation is required for optimal NK cell activation in lipopolysaccharide-mediated inflammatory conditions. Cell reports. 2013;4:1235–1249. doi: 10.1016/j.celrep.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 68.Epardaud M, Elpek KG, Rubinstein MP, Yonekura AR, Bellemare-Pelletier A, Bronson R, Hamerman JA, Goldrath AW, Turley SJ. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer research. 2008;68:2972–2983. doi: 10.1158/0008-5472.CAN-08-0045. [DOI] [PubMed] [Google Scholar]
- 69.Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, Raulet DH, Lanier LL, Groh V, Spies T, Ebert EC, Green PH, Jabri B. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–366. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 70.Fehniger TA, Cai SF, Cao X, Bredemeyer AJ, Presti RM, French AR, Ley TJ. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26:798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 71.Strengell M, Matikainen S, Siren J, Lehtonen A, Foster D, Julkunen I, Sareneva T. IL-21 in synergy with IL-15 or IL-18 enhances IFN-gamma production in human NK and T cells. J Immunol. 2003;170:5464–5469. doi: 10.4049/jimmunol.170.11.5464. [DOI] [PubMed] [Google Scholar]
- 72.Fehniger TA, Yu H, Cooper MA, Suzuki K, Shah MH, Caligiuri MA. Cutting edge: IL-15 costimulates the generalized Shwartzman reaction and innate immune IFN-gamma production in vivo. J Immunol. 2000;164:1643–1647. doi: 10.4049/jimmunol.164.4.1643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.