Abstract
The increased development of green low-carbon energy technologies that require platinum group metals (PGMs) and rare earth elements (REEs), together with the geopolitical challenges to sourcing these metals, has spawned major governmental and industrial efforts to rectify current supply insecurities. As a result of the increasing critical importance of PGMs and REEs, environmentally sustainable approaches to recover these metals from primary ores and secondary streams are needed. In this review, we define the sources and waste streams from which PGMs and REEs can potentially be sustainably recovered using microorganisms, and discuss the metal-microbe interactions most likely to form the basis of different environmentally-friendly recovery processes. Finally, we highlight the research needed to address challenges to applying the necessary microbiology for metal recovery given the physical and chemical complexities of specific streams.
Graphical abstract
Introduction
Biometallurgy is a term used to describe biotechnological processes that involve interactions between microorganisms and metals or metal-bearing minerals [1]. Biomining and bioremediation have been the two most studied branches in the biometallurgical field, and are employed worldwide at large scales. Biomining (or bioleaching) facilitates the extraction and recovery of metals from ores and waste materials [2] while bioremediation focuses on the removal or immobilization of hazardous contaminants such as radionuclides and heavy metals from contaminated sites [3]. However, biometallurgy has the potential to make novel contributions to a sustainable world that should rapidly eclipse current biomining and bioremediation applications. Indeed, worldwide changes in metal cycling have opened up a plethora of opportunities and challenges for biometallurgical technologies. More specifically, the central roles critical metals play in the emergent technologies needed to transition to e.g. a low carbon energy system, which is already driving expansions in secondary and urban mining, will only amplify demand to develop novel biometallurgy-based technologies to extract, separate, purify and recover critical metals [1]. Therefore, this overview focuses on metals whose supply has been deemed critical, reviewing possible sources and waste streams from which they can be recovered using microorganisms, the most promising microbe-metal interactions, and potential new biotechnologies on the horizon.
Selection of critical metals
Europe and the US are increasingly confronted with potential shortages of critical raw materials, i.e. materials for which the risk of supply shortage resulting in adverse impacts on the economy are high. The European Union defined a list of twenty critical raw materials that includes bulk metals, industrial minerals, platinum group metals (PGMs) and rare earth elements (REEs) [4]. One of the most powerful forces influencing their economic importance is the growing demand for these materials by emerging low-carbon energy technologies. In a similar review conducted by the US Department of Energy, five REEs (dysprosium, terbium, europium, neodymium and yttrium) were identified to be critical to the development of ‘clean’ emerging energy technologies [5]. These government reports along with further analyses [6,7] highlighted the inevitable need for metallurgical research to develop efficient methods to recover these critical materials, with specific focus on PGMs and REEs. The criticality of these materials is pushing society to expand capacity to mine and extract these materials from primary ores and concentrates as well as to optimize recovery and recycle from residues and scrap from pre-consumer products, end of life consumer goods, and landfilled waste streams [6,7]. We envision biotechnologies playing an important role in all of these activities.
The Potential for Biomining PGMs and REEs
Biomining of primary ores has mainly been practiced for copper, nickel and gold using bio-heap leaching or bio-oxidation in stirred tank bioreactors which are technologies that have been described in details [2]. The microbial processes that power current industrial biomining are the autotrophic utilization of sulfide and ferrous iron minerals [8]. The application of autotrophic biomining for PGMs and REEs, however, must overcome a number of challenges related to their source materials. REEs are typically mined as carbonates (bastnäsite) or phosphates (monazite and xentotime) from igneous and alkaline rocks, or as ions absorbed on clay minerals. To our knowledge, DNI Metals in Alberta Canada operates the only REE bio-heap leaching project. It seemed that DNI relies on the high content of polymetallic sulfides in the Buckton shale deposits to recover economically viable quantities of Sc, which occurs in a ‘metalized zone’ of the shale at ~5 g ton−1. In contrast to REEs, PGMs are generally mined from Ni or Cu deposits with Pt, Pd and Rh concentrations at 1–10 g ton−1 [9]. Most of these deposits are sulfide minerals, and Ni and Cu have been successfully biomined at full scale via heap leaching technologies. The PGM sulfides, however, are more stable than the base metal sulfides, and are therefore more difficult to oxidize [10]. For example, bioleaching of flotation concentrate from the Stillwater Complex, which contained pentlandite ((Fe,Ni)9S8), released Ni, but Pd as PdS, and other PGMs required further chemical leaching such as pressurized cyanidation [10]. Concentrates that had not undergone biological treatment, released none or very little PGMs, perhaps suggesting a role for bioleaching of PGMs if the process could be improved.
Besides proton-induced disassociation of metallic ores, metals can also be liberated from solid materials by ligand-induced solubilization. The broad diversity of solubilizing heterotrophic microorganisms, including yeasts, fungi and bacteria have been extensively described as well as the ligands they produce which are mainly organic acids such as citric, oxalic and gluconic acids [11–13]. To our knowledge, no industrial processes using heterotrophic microorganisms have been demonstrated. The probable reason for this is the continuous requirement for significant quantities of carbon and energy sources in the lixiviant (i.e. leaching solution) to support the growth and activity of the leaching microorganisms. This is in contrast with the autotrophic leaching which requires only small amounts of a few inexpensive inorganic nutrients. Although limited research has been performed on heterotrophic extraction of PGMs or REEs from primary ores using organic ligands, we believe that the high value of the product can justify the additional cost of heterotrophic bioleaching. This idea has been partially demonstrated by the BioHeap™ technology from Western Areas Ltd. that uses exogenous cultures acclimated to hypersaline and high temperature environments to conduct more effective bioleaching processes. It is not hard to imagine that similar technologies using heterotrophic microorganisms can be adopted to mine PGMs and REEs. By combining with other advantages from biometallurgy, such as cutting out froth flotation or ultrafine grinding steps with environmental advantages such as low carbon footprint, absence of noxious gases (sulfides, As) production and on-site treatment, biomining can make an interesting economic case under growing stringent regulations. It is envisioned that increased efforts in research can progress biomining of PGMs and REEs from primary ores significantly.
From Biomining to Biorecovery
A recent comprehensive review defined three major waste streams that present opportunities for effective metal recovery: (1) pre-consumer manufacturing scrap/residues; (2) recycling (urban mining) of end-of-life products; and (3) landfill mining of urban and industrial waste residues [6]. The first two waste streams have been the primary focus of both industrial attention and academic research targets because these waste streams are often found to have high contents of REEs and PGMs. Permanent magnets, nickel metal hybrid batteries, lamp phosphors, spent car catalysts and electric and electronic waste are the most interesting sources within the first two waste streams due to their high metals content and physical consistencies within each category. Consequently, more energy intensive pyrometallurgical techniques or chemical-intensive hydrometallurgical techniques can be used for high efficient metals recovery from these wastes and biometallurgical techniques seem to have very little utility for them currently. On the other hand, although urban and industrial waste residues contain much lower critical metal concentrations, their volumes can be enormous. Examples include, but are not limited to, bauxite mine residues, phosphogypsum, incinerator ash, metallurgy slags, acid mine drainage, and industrial and municipal wastewaters. Biometallurgical technologies are most likely to find their niches in recovering critical metals from these waste residues.
Liquid waste streams
Many wastewaters are currently being considered as energy or nutrient sources, and thus the research paradigm has shifted from simply organic and nutrient removal to resource recovery [14]. In contrast, metal recovery from wastewaters has hardly been considered. Wastewaters have not commonly been characterized for their PGM and REE content. This can be attributed to the fact that utilities and industries primarily focus on the metals included in environmental quality standards imposed by environmental legislation when monitoring the quality of their wastewaters. The few full-scale biotechnologies applied to waste streams from mining or metal refining industries today were primarily developed to remove toxic elements in order to comply with regulations (e.g. ABMet™ by General Electric; THIOTEQ™ by Paques technology).
Preliminary data and the scientific literature indicate that a large number of high volume waste streams can contain sufficient PGMs and REEs to warrant recovery from both an economic and an environmentally beneficial viewpoint [15]. For example, municipal wastewater has long been recognized as a source of PGMs, which comes from road dust present in stormwater run-off [16]. Deterioration of catalytic converters due to thermal and mechanical strain and to acid fume components leads to the emission of particles containing Pt and Pd at mg kg−1 concentrations. In addition, wastewaters from hospitals and dental clinics contain significant amounts of PGMs as well. For example, Pt can occur in hospital wastewater from administration of the anticancer drugs cisplatin and carboplatin [17–19]. Concentrations have been reported to range between 10–100 ng L−1 and 75 μg L−1 in the effluent of treatment plants receiving wastewater from hospitals and might occur in mg L−1 ranges in the urine of cancer patients [18,19]. In addition, these effluents contain low concentrations of Gd (up to 100 μg L−1) that is used in contrast media for magnetic resonance imaging [20,21]. Based on typical numbers of wastewater produced by hospitals (500 m3 d−1), and using the reported concentrations and the current metal prices (52129 $ kg−1 for Pt and 55 $ kg−1 for Gd), low recovery values of 2 $ d−1 Pt and 0.2 $ d−1 Gd can be calculated. Although this example shows that the recovery would only be beneficial from an environmental point of view, recent studies have shown that metal recovery from wastewater treatment plant sludges could be economically favorable due to the accumulation of multiple metals over time in the sludge [22].
Other potential sources for both PGMs and REEs include wastewaters from industrial activities in the pharmaceutical, fine chemical, electrochemical and glass sectors, from acid mine drainage and from extraction and separation processes in metal refineries. Water from geothermal resources has also been recognized as a potential source of PGM and REE metals [23]. During geothermal energy production, large volumes of brine are typically extracted from depth and discharged after cooling. During the extended time the water percolates through and is heated by crustal rocks, significant amounts of metals and minerals dissolve into the geothermal fluids. While the most abundant metals in these fluids are the alkali earth metals (e.g. Na+, Mg2+), geothermal fluids are a potential source of PGM and REE metals, including Pt, Pd, Nd, and Eu [24]. The geothermal fluids of the Salton Sea, one of most metal-rich water-bodies worldwide, contains ~225 mg L−1 Nd and ~300 mg L−1 Eu [25]. Based on the current metal prices, these fluids have a value of 0.2 $ L−1 which is significant given the massive volumes present. Historically, the high metal content of these brines posed a liability of geothermal power plants, as it has led to scaling [26]. Consequently, recovery of PMG and REE metals offers a means of improving the economic viability of geothermal energy. The central challenges posed by geothermal fluids are the need to withstand high temperatures (~70–150°C), the ~100–1000 times greater concentration of low-value metals over desired metals, and the presence of multiple REEs or PGMs.
Metals recovery from many of these fluids is complicated since the concentrations are dilute and a high specificity would be required to extract them from these aqueous streams. Similar to the nutrient recovery practices in wastewater treatment, research needs to focus on source collection of particular streams such as cancer patients’ urine. Examples from the chemical industry include the recovery of Pd used as a homogeneous catalyst in the manufacturing of acetaldehyde via the Wacker process, and the recovery of Pt from effluents containing hexahydroxy platinic acid from the production of autocatalyst washcoats.
Although researchers so far have mainly focused on PGM recovery from synthetic wastewaters using whole cells or products of microorganisms [15,27], a few studies have used real wastewater. Two studies reported the Pd recovery from metal refining [28] and catalytic converter production [29] waste streams by reductive precipitation on Desulfovibrio and Cupriavidus strains. Due to the higher concentrations of metals in these waste streams compared to hospital or municipal wastewaters, it becomes economically more interesting to recover these (metal values of 25 $ L−1 and 5 $ L−1 for the refining and converter study, respectively), even though the produced volumes are reasonably low. Ngwenya et al. demonstrated the uptake and reductive precipitation of Rh from a wastewater (PGM producer Anglo American Platinum) by a sulfate-reducing consortium and their extracted enzymes [30]. Finally, Ru recovery from a plating industry wastewater was described using selective adsorption on Rhodopseudomonas strains [31]. All of these streams contained high salt concentrations (40–300 g L−1) and a range of bacteriostatic metals such as Cu. Moreover, the solutions were highly acidic (pH<2) and the metal speciation was undefined. Therefore, the solutions were diluted or treated to lower the salt concentrations and increase the pH prior to the bioprocess. The complexity of the solutions might be the reason why most of the research in PGM/REE-microbe interactions has not yet made it into the field. It is clear that many research challenges remain in this area and that, once solved, a big improvement for PGM and REE biorecovery can be created.
Solid resource streams
As mentioned earlier, the biggest challenge related to solid waste residues is the effective liberation and separation of the valuable metals from the dominant minerals in these streams. Although pyro- or hydrometallurgy is currently more favorable than biometallurgy, active research efforts have been devoted into this area. Comparable to autotrophic bioleaching, direct heterotrophic leaching or chemical leaching in conjunction with biological recovery processes could be feasible routes. The latter has been successfully demonstrated for PGM recovery from pretreated end-of-life products [29,32,33]. In these studies, spent automotive catalysts and electronic scrap was shredded and ground into fine particles, then metals were extracted using strong mineral acids. The metal-containing leachates were often not very biocompatible given the low pH and high concentrations of toxic metals. Therefore, a dilution step or pH correction was required or direct contact between living biomass was avoided by using off gases of bacterial fermentation or pre-metallization of the biomass in order to obtain autocatalytic reduction of the PGMs [32]. The most remarkable result of this study was the selective and stepwise recovery of the Pd, Pt and Cu, and Au with almost complete recovery of each metal. The ability of a biometallurgy process to discriminate between PGMs represents a major advantage over traditional chemical recovery methods.
Heterotrophic leaching of PGMs and REEs from waste residues has been described to a lesser extent, even though this technology is more environmentally benign compared to mineral acids extraction processes. Citric acid seems to have the greatest potential given its demonstrated efficiency in extraction of heavy metals from many sources, including fly ash generated from municipal waste incineration, electronic scrap, and activated sludge [13,34,35]. Citric acid, which is largely produced by fungal fermentation processes, contains three carboxylic groups and one hydroxyl group that have been shown to form stable chelates with trivalent REEs [36]. REE recovery using citric acid was recently demonstrated using red mud or bauxite residues, the noxious by-product of the Bayer process for extracting aluminum from bauxite ore [37,38]. Filamentous fungi, identified as Penicillum tricolor strains were reported to produce citric acid and oxalic acid when provided glucose as carbon and energy source. Compared to using spent medium, live culture conditions are more interesting and challenging since the continuous acid production might compensate for the pH increase caused by the consumption of protons by iron oxides in the red mud, thus a constant low pH can be maintained to facilitate REEs extraction. On the other hand, excess metal ions might be toxic to the microorganism. It is important to note that oxalic acid is not an ideal lixiviant since it is known to form insoluble complexes with REEs [39], which would not be easily separated from red mud particles for downstream processes. From this point of view, it is important to steer the fermentation to citric acid. Further research on the efficacy in REE leaching using other organic acids, such as gluconic acid, itaconic acid or acetic acid is required. In addition, oxalic acid might be an effective way of selectively precipitating REEs from industrial aqueous waste streams from hydrometallurgical processes [40].
In addition to organic acids, another interesting microbial process for metal leaching is the use of cyanogenic cultures. This process for Au leaching has been demonstrated by using cyanide lixiviants microbially produced from glycine [41,42]. Until recently, the cyanide concentrations were considered too low for industrial up-scaling but metabolically-engineered C. violaceum strains doubled the cyanide production, which nearly doubled the Au recovery from electronics waste [43]. Similarly, it is reasonable to hypothesize that PGM recovery could also use cyanide based lixiviants (REEs are not solubilized with cyanide), but with elevated pressure and temperature to speed up the process. This process, however, would be achieved by using extremophiles or a two-step system that separates cyanide production from high pressure and temperature leaching.
Microbe-metal interactions
Naturally-occurring microbes can sorb a variety of PGMs, REEs and heavy metals, including Pt, Fe, Ni, Cu, Zn, Pb, Cu, Pd, Ag, Cd, Pt, Au, and Hg, with binding capacities typically on the order of 10−5 to 10−3 mol metal g−1 (dry wt) microbe (e.g., [44–46]). Thus, on a dry weight basis, the metal binding capacities of microbes compare favorably to the binding capacities of commercial ion exchangers. Biosorption of metals to microbes can involve a variety of processes, including absorption, ion exchange, complexation, and precipitation [45,46]. These processes are critically regulated by the chemical groups displayed on the extracellular surfaces of microbial cells, such as carboxyl, phosphoryl, hydroxyl, carbonyl, and thiol moities.
A limited number of studies of metabolically inactive microbes have demonstrated that sorption of metals onto microbes can be adequately predicted by thermodynamic surface complexation models [47,48]. In this type of model, the net equilibrium binding constant of a metal sorbed to a microbe surface is treated as a series of association reactions between the metal and surface-bound carboxylate, phosphoryl, and hydroxyl groups, each with a single equilibrium binding constant, pKa, and site density characteristic [48]. Variations among different microbial species within a given biome are then represented by different individual binding characteristic [49]. While this is clearly a simplification of the actual complexity of the microbial surface, this modeling approach has been shown to accurately predict uptake variations for a range of metal cations, including the REE Nd, as a function of both pH and binding constant [50].
Besides sorption on biomass, the reduction of PGM salts into metallic precipitates has been described extensively [27]. This process is thermodynamically unfavorable for REEs and typically their oxidation state remains unaffected in environmental conditions. Some studies attributed the facile microbial reduction of PGMs to the activity of cytochromes and hydrogenases [51,52]. The latter were shown to predominate in species with a versatile respiratory electron transport chain system such as Shewanella and Desulfovibrio [53–55]. Other authors showed that cell surface functional groups such as amines and carboxylic acids can capture Pd(II), after which chemical reduction can occur [56]. Metallophilic bacteria harbor numerous metal resistance gene clusters, enabling cell detoxification via a number of mechanisms such as complexation, efflux, or reductive precipitation [57]. Therefore, the resistance and PGM recovery mechanism might differ among different strains, and combinations of PGM responses are plausible. This highlights the need for more extensive use of high throughput molecular techniques, such as proteomics, (meta-) genomics, transcriptomics and metabolomics in this field.
Application of these molecular microbial tools greatly increased information about the composition of and interactions within microbial cultures in bio-mines and acid mine drainage [58–60]. Reith et al. used microarrays to demonstrate that Au detoxification by C. metallidurans was mediated by a combination of efflux, reduction and possible methylation of Au complexes [57]. These authors suggested that PGMs could have similar responses given their similar geochemical properties. Another recent study used a combination of density-based centrifugation, gel electrophoresis and mass spectroscopy to identify the high affinity proteins associated with Se precipitates, formed after reduction by Sulfurospirillum barnesii [61]. A similar process could be envisioned to determine the enzymes involved in PGM recovery. Hosseinkhani et al. sequenced indigenous microbial communities sampled from metal contaminated marine sediments and showed that high salt tolerant, culturable species such as Halomonas and Vibrio were able to reduce Pd in salt waters [62]. Potentially, these bacteria could be used for recovery of PGMs from metal refining or fine chemical industry waste streams that are often characterized by high salt and low pH conditions.
REE interaction with biogenic phosphates and specific transporter uptake are two recently described processes with potential for REE recovery from aqueous waste streams. Firstly, the sorption on, encapsulation into biogenic phosphate nanominerals and formation of REE-phosphates were demonstrated using biofilms of Serratia strains [63,64]. The formation of biogenic Ce phosphates was also shown to occur on the Mn-oxidizing bacteria Leptothrix and Pseudomonas [65]. Secondly, a recent discovery of an extremophile, acidophilic methanotrophic Methylacidiphilum fumariolicum SolV revealed that the function of a methanol dehydrogenase (MDH) in this bacterium strictly depends on the presence of REEs [66]. Analysis of the protein structure and alignment of amino acids sequences demonstrated that REE-binding motifs in this particular MDH are also present in other xoxF-type MDH enzymes, strongly suggesting that many MDHs are actually lanthanide-dependent. The crucial question is whether M. fumariolicum uses a transporter to actively concentrate REEs from its environment into its cell in order to ensure its MDH has sufficient supply of REEs. Presumably, by strategically modifying the protein structure, such as the amino acid residues of the binding sites in a binding protein from the transporter, both the specificity and binding efficiency of the protein towards its substrate could be improved [67,68]. Thus, one could potentially artificially evolve binding proteins in a REE-specific transporter to only recognize one particular REE so that the bacterium with the improved transporter could concentrate this REE in its cell. These approaches to optimizing microbial metal-recovery processes should be especially useful for developing methods to recover REEs from diluted aqueous waste streams.
Implications of extreme conditions for biometallurgy
The efficacy of biometallurgical recovery from liquid waste streams will largely depend on the matrix composition and chemical speciation of the targeted metal in the waste. The most attractive liquid waste streams often have extreme pH, high total dissolved solids and ionic strength, and can contain organics, solvents and undesired toxic metals.
PGM recovery methods are usually developed at the bench scale using idealized aqueous waste streams in which the targeted metal is present as a ‘free’ solvated ion. PGMs in waste streams, however, are anything but ‘free’, and instead occur as a trace component of nano-sized or micro-sized colloids, polymers, complexes with inorganic and organic ligands, or sorbed on suspended material in the waste. In general, a metal’s chemical speciation will be an important determinant of their reduction potential and uptake efficiency.
For example, Pt within the anticancer drugs cisplatin and carboplatin has been shown to undergo complex speciation changes in certain hospital wastewaters occurring both as a component of the original drug and as the anionic complexes [PtCl4]2− and [PtCl6]2− [17]. As a second example, the effect of Pd speciation on Pd reduction potential was recently studied using Geobacter species [69,70]. These studies showed how even seemingly simple waste stream properties such as solution pH and Pd concentration significantly affects chemical speciation, and as a result determines electrostatic interactions, ligand substitution and Pd reductions [69,70]. Surface complexation models and speciation-based kinetic rate laws for enzymatic reduction, as developed for other elements such as U, should be developed for PGMs in order to design and optimize biometallurgical recovery methods [71].
The effects of essential biological parameters (e.g. pH, ionic strength and temperature) on PGM and REE recovery can result from the competition between ions, changes in the activity of PGMs/REEs and/or microbial functional groups, or changes to the electrical double layer at the microbe-water interface. For example, the observed reduction in heavy metal uptake on Gram negative bacteria with increasing ionic strength has been attributed to changes in the activity of metal cations [72]. Although the effects of temperature on metal sorption on bacterial surfaces have not been extensively studied, the limited number of studies do point to potential challenges in applying microbial methods to recover metals from waste streams at elevated temperatures. Ginn and Fein showed that while Cd and Pb uptake onto Bacillus subtilis and Pseudomonas mendocina bacterial surfaces was not significantly affected as the temperature was increased between ambient and 80 °C, the distribution of Pb and Pb-hydroxy species on the bacterial surface and in solution did depend on temperature [73]. As a second example, in chloride-dominated industrial and geothermal fluids, increased stability of metal chloride complexes is the norm at higher temperatures [74], and hence, the current approach of extrapolating metal binding constants measured at ambient temperatures and low chloride concentrations to elevated process temperatures will result in overestimates of binding. The current body of research indicates that speciation changes would need to be explicitly accounted for in order to properly model recovery processes.
Conclusions
PGMs and REEs will only become more important given their increasing use in green technologies and their supply volatility. Closing the consumer cycle by recovering these metals from dilute and complex waste streams will become increasingly economically viable. In addition, increasingly stringent environmental regulations and constraints will drive demand for new methods to recycle and recover PGMs and REEs from waste streams, providing an ideal niche for biometallurgy. The advantages of microbial processes include specificity, energetics, and minimal creation of new waste. Current microbial processes face challenges associated with complex waste streams, toxicity and competing side reactions. Future biometallurgical research should take advantage of novel microbial and material characterization approaches to obtain a holistic understanding of microbial-metal interactions in order to develop effective bioprocesses to separate, recover and recycle critical metals.
Figure 1.
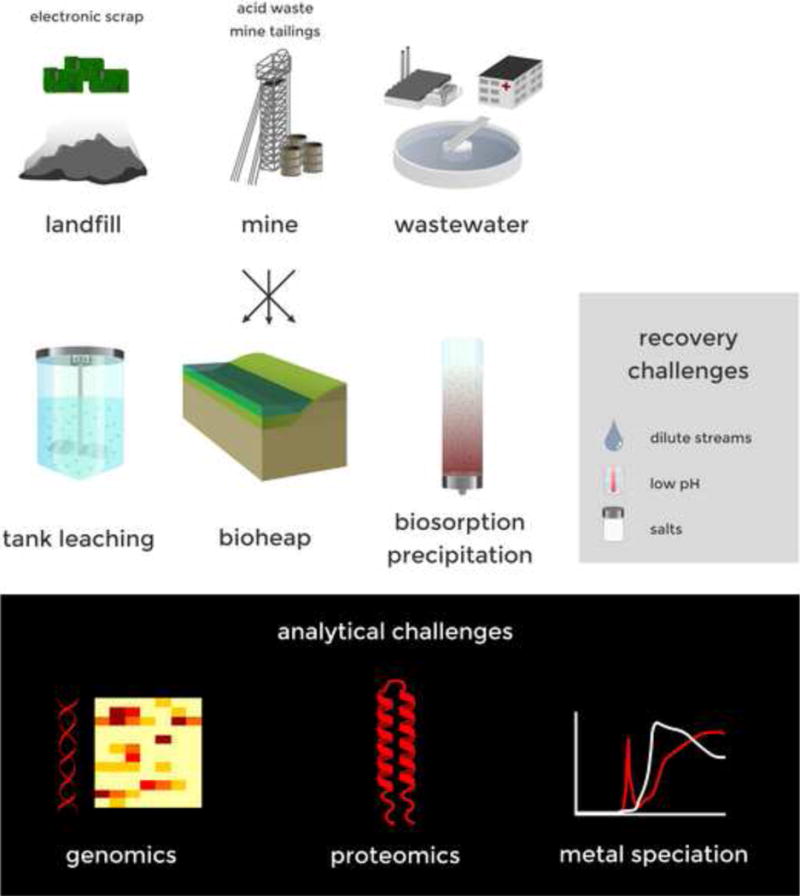
Schematic overview of the sources and waste streams from which PGMs and REEs can potentially be recovered using microorganisms, and of the research needed to address challenges to applying the necessary microbiology given the physical and chemical complexities of certain streams.
Research highlights.
The supply risk of PGMs and REEs drive biometallurgical technology development
Waste streams with low metal concentrations are niches for biometallurgy
The advantages of biometallurgy include specificity, energetics, and minimal waste
Challenges are associated with complex waste streams, toxicity and side reactions
Research should focus on novel microbial and material characterization approaches
Acknowledgments
WZQ acknowledges support of NIEHS (P42ES4705). TH (postdoctoral fellowship) is financially supported by the Fund of Scientific Research Flanders (FWO-Vlaanderen). JPF acknowledges support of NSF Environmental Engineering through grant CBET-1438278. The authors thank Tim Lacoere for the graphical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Recommended reading
- 1.Hennebel T, Boon N, Maes S, Lenz M. Biotechnologies for critical raw material recovery from primary and secondary sources: R&D priorities and future perspectives. New Biotechnology. :121–127. doi: 10.1016/j.nbt.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Johnson DB. Biomining-biotechnologies for extracting and recovering metals from ores and waste materials. Curr Opin Biotechnol. 2014;30:24–31. doi: 10.1016/j.copbio.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Gadd GM. Bioremedial potential of microbial mechanisms of metal mobilization and immobilization. Curr Opin Biotechnol. 2000;11:271–279. doi: 10.1016/s0958-1669(00)00095-1. [DOI] [PubMed] [Google Scholar]
- 4.European Commission. Critical raw materials for the EU – report of the Ad-hoc Working Group on defining critical raw materials. 2014 Available online at: http://ec.europa.eu/enterprise/policies/raw-materials/files/docs/crm-report-on-critical-raw-materials_en.pdf.
- 5.US Department of Energy. Critical Materials Strategy. 2011 Available online at: http://energy.gov/sites/prod/files/DOE_CMS2011_FINAL_Full.pdf 2011.
- 6*.Binnemans K, Jones PT, Blanpain B, Van Gerven T, Yang Y, Walton A, Buchert M. Recycling of rare earths: a critical review. J Clean Prod. 2013;51:1–22. A comprehensive review of the various industrial waste streams and potential pyrometallurgical or hydrometallurgical processes that are available to recover rare earth elements. [Google Scholar]
- 7.Binnemans K, Jones PT, Van Acker K, Blanpain B, Mishra B, Apelian D. Rare-Earth Economics: The Balance Problem. JOM. 2013;65:846–848. [Google Scholar]
- 8.Ehrlich HL. Technical potential for bioleaching and biobeneficiation of ores to recover base metals (other than iron or copper), platinum-group metals and silver. In: Rawlings DE, editor. Biomining. Springer Berlin Heidelberg; 1997. pp. 129–150. [Google Scholar]
- 9.Pina R, Gervilla F, Barnes SJ, Ortega L, Lunar R. Distribution of platinum-group and chalcophile elements in the Aguablanca Ni-Cu sulfide deposit (SW Spain): Evidence from a LA-ICP-MS study. Chem Geol. 2012;302:61–75. [Google Scholar]
- 10.Yopps DL, Baglin EG. Bacterial preoxidation of Stillwater Complex, MT, platinum-group metal flotation concentrate and recovery of plantinum-group metals by cyanidation and other leachants. U.S. Dept. of the Interior, Bureau of Mines; 1991. [Google Scholar]
- 11.Gadd GM. Fungal production of citric and oxalic acid: Importance in metal speciation, physiology and biogeochemical processes. Adv Microb Physiol. 1999;41:47–92. doi: 10.1016/s0065-2911(08)60165-4. [DOI] [PubMed] [Google Scholar]
- 12.Brandl H. Microbial leaching of metals. In: Rehm HJ, Reed G, editors. Biotechnology, vol.10 Special Processes. Wiley-VCH Verlag GmbH; 2008. pp. 191–224. [Google Scholar]
- 13*.Deng X, Chai L, Yang Z, Tang C, Wang Y, Shi Y. Bioleaching mechanism of heavy metals in the mixture of contaminated soil and slag by using indigenous Penicillium chrysogenum strain F1. J Hazard Mater. 2013;248–249:107–114. doi: 10.1016/j.jhazmat.2012.12.051. Heterotrophic bioleaching by using indigenous fungal species could be a feasible way to recover rare earth elements (here heavy metals) from solid wastes. Organic acids in conjunction with biogenic chelators could be efficient lixiviants in liberating rare earth elements from various solid waste streams. [DOI] [PubMed] [Google Scholar]
- 14.Gao H, Scherson YD, Wells GF. Towards energy neutral wastewater treatment: methodology and state of the art. Environ Sci-Proc Imp. 2014;16:1223–1246. doi: 10.1039/c4em00069b. [DOI] [PubMed] [Google Scholar]
- 15.De Corte S, Hennebel T, De Gusseme B, Verstraete W, Boon N. Bio-palladium: from metal recovery to catalytic applications. Microbial Biotechnol. 2012;5:5–17. doi: 10.1111/j.1751-7915.2011.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Commission E. Pollutants in urban waste water and sewage sludge. 2001. p. 273. Edited by. [Google Scholar]
- 17.Lenz K, Koellensperger G, Hann S, Weissenbacher N, Mahnik SN, Fuerhacker M. Fate of cancerostatic platinum compounds in biological wastewater treatment of hospital effluents. Chemosphere. 2007;69:1765–1774. doi: 10.1016/j.chemosphere.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 18.Lenz K, Mahnik SN, Weissenbacher N, Mader RM, Krenn P, Hann S, Koellensperger G, Uhl M, Knasmueller S, Ferk F, et al. Monitoring, removal and risk assessment of cytostatic drugs in hospital wastewater. Water Sci Technol. 2007;56:141–149. doi: 10.2166/wst.2007.828. [DOI] [PubMed] [Google Scholar]
- 19.Lenz K, Hann S, Koellensperger G, Stefanka Z, Stingeder G, Weissenbacher N, Mahnik SN, Fuerhacker M. Presence of cancerostatic platinum compounds in hospital wastewater and possible elimination by adsorption to activated sludge. Sci Total Environ. 2005;345:141–152. doi: 10.1016/j.scitotenv.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Telgmann L, Wehe CA, Birka M, Kunnemeyer J, Nowak S, Sperling M, Karst U. Speciation and isotope dilution analysis of gadolinium-based contrast agents in wastewater. Environ Sci Technol. 2012;46:11929–11936. doi: 10.1021/es301981z. [DOI] [PubMed] [Google Scholar]
- 21.Kummerer K, Helmers E. Hospital effluents as a source of gadolinium in the aquatic environment. Environ Sci Technol. 2000;34:573–577. [Google Scholar]
- 22.Westerhoff P, Lee S, Yang Y, Gordon GW, Hristovski K, Halden RU, Herckes P. Characterization, recovery opportunities, and valuation of metals in municipal sludges from U.S. wastewater treatment plants nationwide. Environ Sci Technol. 2015 doi: 10.1021/es505329q. [DOI] [PubMed] [Google Scholar]
- 23.Segneri B, R T, Deprizio J. Thirty-Ninth workshop on geothermal reservoir engineering February 24–26; Stanford University, Stanford, California. 2014. Geologic provenance of rare earth elements in the United States, and their potential collocation with geothermal resources. [Google Scholar]
- 24.Lo Y-C, Cheng C-L, Han Y-L, Chen B-Y, Chang J-S. Recovery of high-value metals from geothermal sites by biosorption and bioaccumulation. Bioresour Technol. 2014;160:182–190. doi: 10.1016/j.biortech.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Michard A. Rare earth element systematics in hydrothermal fluids. Geochim Cosmochim Acta. 1989;53:745–750. [Google Scholar]
- 26.Bakane PA. Thirty-eight workshop on geothermal reservoir engineering February 11–13; Stanford University, Stanford, California. 2013. Overview of extraction of minerals/metals with the help of geothermal fluids. [Google Scholar]
- 27.Hennebel T, De Corte S, Verstraete W, Boon N. Microbial production and environmental applications of Pd nanoparticles for treatment of halogenated compounds. Curr Opin Biotechnol. 2012;23:555–561. doi: 10.1016/j.copbio.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Gauthier D, Sobjerg LS, Jensen KM, Lindhardt AT, Bunge M, Finster K, Meyer RL, Skrydstrup T. Environmentally benign recovery and reactivation of palladium from industrial waste by using Gram negative bacteria. Chemsuschem. 2011;3:1036–1039. doi: 10.1002/cssc.201000091. [DOI] [PubMed] [Google Scholar]
- 29.Mabbett AN, Sanyahumbi D, Yong P, Macaskie LE. Biorecovered precious metals from industrial wastes: Single-step conversion of a mixed metal liquid waste to a bioinorganic catalyst with environmental application. Environ Sci Technol. 2006;40:1015–1021. doi: 10.1021/es0509836. [DOI] [PubMed] [Google Scholar]
- 30.Ngwenya N, Whiteley CG. Recovery of rhodium(III) from solutions and industrial wastewaters by a sulfate-reducing bacteria consortium. Biotechnol Prog. 2006;22:1604–1611. doi: 10.1021/bp060167h. [DOI] [PubMed] [Google Scholar]
- 31.Colica G, Caparrotta S, De Philippis R. Selective biosorption and recovery of ruthenium from industrial effluents with Rhodopseudomonas palustris strains. Appl Microbiol Biotechnol. 2012;95:381–387. doi: 10.1007/s00253-012-4053-9. [DOI] [PubMed] [Google Scholar]
- 32.Macaskie LE, Creamer NJ, Essa AMM, Brown NL. A new approach for the recovery of precious metals from solution and from leachates derived from electronic scrap. Biotechnol Bioeng. 2007;96:631–639. doi: 10.1002/bit.21108. [DOI] [PubMed] [Google Scholar]
- 33.Creamer NJ, Baxter-Plant VS, Henderson J, Potter M, Macaskie LE. Palladium and gold removal and recovery from precious metal solutions and electronic scrap leachates by Desulfovibrio desulfuricans. Biotechnol Lett. 2006;28:1475–1484. doi: 10.1007/s10529-006-9120-9. [DOI] [PubMed] [Google Scholar]
- 34.Bosshard PP, Bachofen R, Brandl H. Metal leaching of fly ash from municipal waste incineration by Aspergillus niger. Environ Sci Technol. 1996;30:3066–3070. [Google Scholar]
- 35.Krebs W, Brombacher C, Bosshard PP, Bachofen R, Brandl H. Microbial recovery of metals from solids. FEMS Microbiol Rev. 1997;20:605–617. [Google Scholar]
- 36.Wu S, Chang Z, Wang K, Xiong W. Preparation and thermal behaviour of rare earth citrate hydrates. J Therm Anal. 1995;45:199–206. [Google Scholar]
- 37*.Qu Y, Lian B. Bioleaching of rare earth and radioactive elements from red mud using Penicillium tricolor RM-10. Bioresour Technol. 2013;136:16–23. doi: 10.1016/j.biortech.2013.03.070. One of the first studies that used a waste stream (red mud) to study heterotrophic leaching for REE recovery. [DOI] [PubMed] [Google Scholar]
- 38.Ritter SK. Making the most of red mud. Chem Eng News. 2014;92:33–35. [Google Scholar]
- 39.Liu Z, Li M, Hu Y, Wang M, Shi Z. Preparation of large particle rare earth oxides by precipitation with oxalic acid. Journal of Rare Earths. 2008;26:158–162. [Google Scholar]
- 40.Larsson K, Binnemans K. Selective extraction of metals using ionic liquids for nickel metal hydride battery recycling. Green Chem. 2014;16:4595–4603. [Google Scholar]
- 41.Brandl H, Lehmann S, Faramarzi MA, Martinelli D. Biomobilization of silver, gold, and platinum from solid waste materials by HCN-forming microorganisms. Hydrometallurgy. 2008;94:14–17. [Google Scholar]
- 42.Faramarzi MA, Brandl H. Formation of water-soluble metal cyanide complexes from solid minerals by Pseudomonas plecoglossicida. FEMS Microbiol Lett. 2006;259:47–52. doi: 10.1111/j.1574-6968.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- 43**.Tay SB, Natarajan G, Rahim MNbA, Tan HT, Chung MCM, Ting YP, Yew WS. Enhancing gold recovery from electronic waste via lixiviant metabolic engineering in Chromobacterium violaceum. Sci Rep. 2014;3:1–7. doi: 10.1038/srep02236. This manuscript shows the improvement of heterotrophic leaching of precious metals from electronic scrap by metabolic engineering of the used microbes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vijayaraghavan K, Yun Y-S. Bacterial biosorbents and biosorption. Biotechnol Adv. 2008;26:266–291. doi: 10.1016/j.biotechadv.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Gadd GM. Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J Chem Technol Biotechnol. 2009;84:13–28. [Google Scholar]
- 46.Moriwaki H, Yamamoto H. Interactions of microorganisms with rare earth ions and their utilization for separation and environmental technology. Appl Microbiol Biotechnol. 2013;97:1–8. doi: 10.1007/s00253-012-4519-9. [DOI] [PubMed] [Google Scholar]
- 47.Fein JB, Boily JF, Yee N, Gorman-Lewis D, Turner BF. Potentiometric titrations of Bacillus subtilis cells to low pH and a comparison of modeling approaches. Geochim Cosmochim Acta. 2005;69:1123–1132. [Google Scholar]
- 48.Fein JB, Daughney CJ, Yee N, Davis TA. A chemical equilibrium model for metal adsorption onto bacterial surfaces. Geochim Cosmochim Acta. 1997;61:3319–3328. [Google Scholar]
- 49.Yee N, Fein J. Cd adsorption onto bacterial surfaces: A universal adsorption edge? Geochim Cosmochim Acta. 2001;65:2037–2042. [Google Scholar]
- 50.Fein JB, Martin AM, Wightman PG. Metal adsorption onto bacterial surfaces: Development of a predictive approach. Geochim Cosmochim Acta. 2001;65:4267–4273. [Google Scholar]
- 51.Ng CK, Sivakumar K, Liu X, Madhaiyan M, Ji L, Yang L, Tang C, Song H, Kjelleberg S, Cao B. Influence of outer membrane c-type cytochromes on particle size and activity of extracellular nanoparticles produced by Shewanella oneidensis. Biotechnol Bioeng. 2013;110:1831–1837. doi: 10.1002/bit.24856. [DOI] [PubMed] [Google Scholar]
- 52.Ng CK, Tan TKC, Song H, Cao B. Reductive formation of palladium nanoparticles by Shewanella oneidensis: role of outer membrane cytochromes and hydrogenases. RSC Adv. 2013;3:22498–22503. [Google Scholar]
- 53.De Windt W, Aelterman P, Verstraete W. Bioreductive deposition of palladium (0) nanoparticles on Shewanella oneidensis with catalytic activity towards reductive dechlorination of polychlorinated biphenyls. Environ Microbiol. 2005;7:314–325. doi: 10.1111/j.1462-2920.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- 54.De Windt W, Boon N, Van den Bulcke J, Rubberecht L, Prata F, Mast J, Hennebel T, Verstraete W. Biological control of the size and reactivity of catalytic Pd(0) produced by Shewanella oneidensis. Anton Leeuw Int J G. 2006;90:377–389. doi: 10.1007/s10482-006-9088-4. [DOI] [PubMed] [Google Scholar]
- 55.Deplanche K, Caldelari I, Mikheenko IP, Sargent F, Macaskie LE. Involvement of hydrogenases in the formation of highly catalytic Pd(0) nanoparticles by bioreduction of Pd(II) using Escherichia coli mutant strains. Microbiol-Sgm. 2010;156:2630–2640. doi: 10.1099/mic.0.036681-0. [DOI] [PubMed] [Google Scholar]
- 56.Rotaru A-E, Jiang W, Finster K, Skrydstrup T, Meyer RL. Non-enzymatic palladium recovery on microbial and synthetic surfaces. Biotechnol Bioeng. 2012;109:1889–1897. doi: 10.1002/bit.24500. [DOI] [PubMed] [Google Scholar]
- 57.Reith F, Etschmann B, Grosse C, Moors H, Benotmane MA, Monsieurs P, Grass G, Doonan C, Vogt S, Lai B, et al. Mechanisms of gold biomineralization in the bacterium Cupriavidus metallidurans. Proc Natl Acad Sci U S A. 2009;106:17757–17762. doi: 10.1073/pnas.0904583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ram RJ, VerBerkmoes NC, Thelen MP, Tyson GW, Baker BJ, Blake RC, Shah M, Hettich RL, Banfield JF. Community proteomics of a natural microbial biofilm. Science. 2005;308:1915–1920. [PubMed] [Google Scholar]
- 59.Tyson GW, Chapman J, Hugenholtz P, Allen EE, Ram RJ, Richardson PM, Solovyev VV, Rubin EM, Rokhsar DS, Banfield JF. Community structure and metabolism through reconstruction of microbial genomes from the environment. Nature. 2004;428:37–43. doi: 10.1038/nature02340. [DOI] [PubMed] [Google Scholar]
- 60.Valenzuela L, Chi A, Beard S, Orell A, Guiliani N, Shabanowitz J, Hunt DF, Jerez CA. Genomics, metagenomics and proteomics in biomining microorganisms. Biotechnol Adv. 2006;24:197–211. doi: 10.1016/j.biotechadv.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Lenz M, Kolvenbach B, Gygax B, Moes S, Corvini PFX. Shedding light on selenium biomineralization: proteins associated with bionanominerals. Appl Environ Microbiol. 2011;77:4676–4680. doi: 10.1128/AEM.01713-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hosseinkhani B, Hennebel T, Van Nevel S, Verschuere S, Yakimov MM, Cappello S, Blaghen M, Boon N. Biogenic nanopalladium based remediation of chlorinated hydrocarbons in marine environments. Environ Sci Technol. 2014;48:550–557. doi: 10.1021/es403047u. [DOI] [PubMed] [Google Scholar]
- 63.Handley-Sidhu S, Hriljac JA, Cuthbert MO, Renshaw JC, Pattrick RAD, Charnock JM, Stolpe B, Lead JR, Baker S, Macaskie LE. Bacterially produced calcium phosphate nanobiominerals: sorption capacity, site preferences, and stability of captured radionuclides. Environ Sci Technol. 2014;48:6891–6898. doi: 10.1021/es500734n. [DOI] [PubMed] [Google Scholar]
- 64**.Paterson-Beedle M, Jeong BC, Lee CH, Jee KY, Kim WH, Renshaw JC, Macaskie LE. Radiotolerance of phosphatases of a Serratia sp.: Potential for the use of this organism in the biomineralization of wastes containing radionuclides. Biotechnol Bioeng. 2012;109:1937–1946. doi: 10.1002/bit.24467. The study developed a novel mechanism based on biogenic phosphate bionanominerals to remove radionuclides but a similar technology has great potential to up-concentrate rare earth elements from dilute wastewater. [DOI] [PubMed] [Google Scholar]
- 65.De Gusseme B, Du Laing G, Hennebel T, Renard P, Chidambaram D, Fitts JP, Bruneel E, Van Driessche I, Verbeken K, Boon N, et al. Virus removal by biogenic cerium. Environ Sci Technol. 2010;44:6350–6356. doi: 10.1021/es100100p. [DOI] [PubMed] [Google Scholar]
- 66**.Pol A, Barends TRM, Dietl A, Khadem AF, Eygensteyn J, Jetten MSM, Op den Camp HJM. Rare earth metals are essential for methanotrophic life in volcanic mudpots. Environ Microbiol. 2014;16:255–264. doi: 10.1111/1462-2920.12249. The study harnessed the power of analytical chemistry, protein crystallography and bioinformatics to unambiguously demonstrate rare earth element-binding motifs in XoxF-type methanol dehydrogenases. The findings have both environmental and biometallurgical implications. [DOI] [PubMed] [Google Scholar]
- 67*.Kuroda K, Ueda M. Molecular design of the microbial cell surface toward the recovery of metal ions. Curr Opin Biotechnol. 2011;22:427–433. doi: 10.1016/j.copbio.2010.12.006. A very interesting review discussing recent progress in using cell surface engineered microorganisms to recover metal ions from aqueous solutions. Genetic engineering could play an important role in improving specificity and efficacy of the bioadsorption processes. [DOI] [PubMed] [Google Scholar]
- 68.Moriwaki H, Koide R, Yoshikawa R, Warabino Y, Yamamoto H. Adsorption of rare earth ions onto the cell walls of wild-type and lipoteichoic acid-defective strains of Bacillus subtilis. Appl Microbiol Biotechnol. 2013;97:3721–3728. doi: 10.1007/s00253-012-4200-3. [DOI] [PubMed] [Google Scholar]
- 69.Pat-Espadas AM, Razo-Flores E, Rene Rangel-Mendez J, Cervantes FJ. Reduction of palladium and production of nano-catalyst by Geobacter sulfurreducens. Appl Microbiol Biotechnol. 2013;97:9553–9560. doi: 10.1007/s00253-012-4640-9. [DOI] [PubMed] [Google Scholar]
- 70**.Pat-Espadas AM, Razo-Flores Ea, Rangel-Mendez JR, Cervantes FJ. Direct and Quinone-Mediated Palladium Reduction by Geobacter sulfurreducens: Mechanisms and Modeling. Environ Sci Technol. 2014;48:2910–2919. doi: 10.1021/es403968e. This study elucidates the mechanisms involved in the microbial reduction of Pd(II) to produce Pd(0) nanoparticles by Geobacter sulfurreducens. The authors describe the influence of pH on Pd speciation and availability of Pd ions, and thus on the preference for ligand-binding sites. Solute characteristics were found to greatly affect the bacterial Pd reduction. [DOI] [PubMed] [Google Scholar]
- 71**.Sheng L, Fein JB. Uranium reduction by Shewanella oneidensis MR-1 as a function of NaHCO3 concentration: Surface complexation control of reduction kinetics. Environ Sci Technol. 2014;48:3768–3775. doi: 10.1021/es5003692. These authors show that the extent and speciation of U on the bacterial cell envelope controls the kinetics of enzymatic U(VI) reduction by bacteria, and linked the rate of U(VI) reduction by bacteria to concentration of salts (NaHCO3) in solution. [DOI] [PubMed] [Google Scholar]
- 72.Borrok DM, Fein JB. The impact of ionic strength on the adsorption of protons, Pb, Cd, and Sr onto the surfaces of Gram negative bacteria: testing non-electro static, diffuse, and triple-layer models. J Colloid Interface Sci. 2005;286:110–126. doi: 10.1016/j.jcis.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 73.Ginn B, Fein JB. Temperature dependence of Cd and Pb binding onto bacterial cells. Chem Geol. 2009;259:99–106. [Google Scholar]
- 74.Yardley BW. 100th Anniversary Special Paper: metal concentrations in crustal fluids and their relationship to ore formation. Econ Geol. 2005;100:613–632. [Google Scholar]


