Abstract
Migraine secondary to meningeal input is referred to extracranial regions innervated by somatic afferents that project to homologous regions in the trigeminal nucleus caudalis (TNC). Reported efficacy of extracranial botulinum toxin (BoNT) in treating migraine is surprising since a local extracranial effect of BoNT cannot account for its effect upon meningeal input. We hypothesize that intradermal BoNT acts through central transport in somatic afferents. Anesthetized C57Bl/6 mice (male) received unilateral supraorbital (SO) injections of BoNT-B (1.5 U/40 μl) or saline. 3 days later, mice received ipsilateral (ipsi) -SO capsaicin (2.5 μg/30 μl) or meningeal capsaicin (4 μl of 1mg/ml). Pre-treatment with ipsi-SO BONT-B i) decreased nocicsponsive ipsilateral wiping behavior following ipsi-SO capsaicin; ii) produced cleavage of VAMP in the V1 region of ipsi-TG and in TG neurons showing WGA after SO injection; iii) reduced expression of c-fos in ipsi-TNC following ipsi-SO capsaicin; iv) reduced c-fos activation and NK-1 internalization in ipsi-TNC secondary to ipsi-meningeal capsaicin; vi) SO WGA did not label dural afferents. We conclude that BoNT-B is taken up by peripheral afferents and transported to central terminals where it inhibits transmitter release resulting in decreased activation of second order neurons. Further, this study supports the hypothesis that SO BoNT exerts a trans-synaptic action on either the second order neuron (which receives convergent input from the meningeal afferent) or the terminal/TG of the converging meningeal afferent.
Keywords: botulinum toxin, migraine, referred pain, transcytosis, trans-synaptic
1. Introduction
Botulinum neurotoxins (BoNTs), in addition to being used in treating neuromuscular disorders, has shown clinical efficacy in the treatment of chronic migraine (Diener et al. 2010; Dodick et al. 2010; Grogan et al. 2013). To date, type A and B serotypes are the most commonly studied isoforms of this family. Although, BoNT-A has been clinically approved for treating chronic migraine, BoNT-B is reported to have a therapeutic effect similar to that of BoNT-A (Fadeyi and Adams 2002; Grogan et al. 2013). While the use of BoNT in migraine may only apply to a subpopulation of migraine patients, the clinical studies thus far point to the advantageous use of BoNT over other prophylactic strategies with respect to reduced side effects, long term effectiveness and tolerability in the treatment of migraine (Samton and Mauskop 2006). The mechanism whereby BoNT delivered into the cranial skin and musculature exerts its effect in migraine is yet to be understood.
BoNTs consist of heavy and light chains linked via a di-sulphide bridge. Heavy chain mediates binding of the toxin to the membrane and translocates the light chain to the cytosol where it cleaves soluble N-methylaleimide-sensitive attachment protein receptor (SNARE) proteins, this reduces transmitter exocytosis (Dong et al. 2007; Fischer and Montal 2007; Montecucco and Schiavo 1994). Current work shows that BoNTs alter SNAREs in motor neuron terminals, and in addition, they are also taken up by sensory neurons affecting primary afferent function at central and peripheral terminals. Ex vivo dorsal root ganglion (DRG) culture work and in vivo studies demonstrate cleavage of SNAREs and inhibition of amino acid (glutamate) and peptide (substance P (sP), CGRP) release (Durham et al. 2004; Meng et al. 2009). In vivo work has shown that spinal or intraplantar delivery of BoNTs (A & B) cleaves DRG SNAREs and suppresses small primary afferent transmitter release (Cui et al. 2004; Dolly et al. 2009; Huang et al. 2011; Marino et al. 2014). A growing body of data suggests that peripheral BoNTs may alter inflammatory and neuropathic pain states in animal models (Bach-Rojecky et al. 2010; Bach-Rojecky and Lackovic 2005; Cui et al. 2004; Marino et al. 2014; Matak et al. 2011) Park, et al, 2015) and importantly in humans (Gazerani et al. 2006; Liu et al. 2006; Piovesan et al. 2005; Ranoux et al. 2008; Ruiz and Bermejo 2008; Voller et al. 2003; Xiao et al. 2010; Yuan et al. 2009).
Migraine pain is believed to result from the activation of meningeal perivascular afferents with cell bodies in trigeminal ganglia (TG) which project centrally to trigeminal nucleus caudalis (TNC). The pain arising from activation of these afferents innervating the intracranial structures corresponds to the referred location of migraine headaches, e.g. supraorbital (SO), retrobulbar and occipital region (Ray and Wolff 1940). The afferent input from these intracranial structures to the central sites results in activation of second order trigeminal dorsal horn neurons in the ipsilateral TNC. Importantly, these nocisponsive neurons in TNC also appear to receive convergent input from somatic afferents, arising from the homologous regions of the head and face, thereby leading to the referral of the meningeally derived afferent input to the superficial supraorbital region (e.g. a classic referred visceral-somatic pain state) (Gebhart 2000; Sengupta 2009). Given this anatomic organization, how then does extracranially applied BoNT block nociceptive inputs arising from intracranial meningeal afferents?
Intramuscularly or cutaneously administered BoNTs reduce local intradermal capsaicin evoked flare and plasma protein extravasation in animal (Filipovic et al. 2012; Marino et al. 2014) and human models (Carmichael et al. 2010; Gazerani et al. 2006; Kramer et al. 2003; Tugnoli et al. 2007) reflecting a local block of terminal transmitter release. Current work indicates that intraplantar BoNT-A and BoNT-B serotypes are taken up and retrogradely transported in sensory afferents, where they cleave their target SNAREs in the ipsilateral DRG and in central (spinal) terminals (Bach-Rojecky and Lackovic 2009; Marino et al. 2014). This emphases an action of the peripherally delivered BoNTs on the central afferent terminal. Indication of a central effect was also seen in the trigeminal system that showed reversal of formalin evoked nocifensive behavior after blocking BoNT-A axonal transport using colchicine (Matak et al. 2011).
Based on this background, we hypothesized that BoNT delivered into the forehead (ophthalmic nerve distribution) would be transported to the central terminals via the TG where it would cleave its target SNAREs, block neurotransmitter release from those afferents and prevent TG neuron activation. As we have previously noted, (Ramachandran and Yaksh 2014), while these central effects are intriguing, this action cannot in and of itself explain how cutaneous BoNT can alter the input into the TNC from meningeal afferents. While it has long been presumed that the BoNTs do not undergo trans-synaptic movement, recent data from our group cast doubt on that thesis. We showed that the c-fos activation otherwise initiated bilaterally by intrathecal sP (acting on NK1 receptors located on 2nd order dorsal horn) is reduced in the dorsal horn ipsilateral to the paw that received intraplantar (IPLT) BoNT-B (Marino et al. 2014). Accordingly, we hypothesize that the intradermal BoNT effects may reflect a possible trans-synaptic action. Therefore, the present study has two principal aims: i) Does SO-BoNT-B cleave the target SNARE, i.e. vesicle associated membrane protein (VAMP), in TG and block TNC c-fos activation produced by SO capsaicin and ii) Does SO-BoNT-B cleave VAMP in TG neurons projecting to the meninges and block TNC sP release and c-fos activation otherwise produced by meningeal capsaicin. The present studies indeed show that SO BoNT is trafficked to the first division of the TG where it cleaves VAMP and prevents dorsal horn neurotransmitter release and second order neuron activation produced by both SO and meningeal capsaicin, and further, that this effect of SO BoNT may not be mediated by uptake through cranial collaterals of meningeal afferents. These novel observations suggest an important enabling mechanism for the effects of extracranial BoNT in migraine.
2. Materials and methods
2.1. Animals
Adult male C57B/l6 mice, 25–30 grams (Harlan Sprague Dawley Inc., Indianapolis, IN, USA), were housed in the vivarium for a minimum of 2 days before use, maintained on a 12/12-hour day-night cycle and given free access to food and water. All studies were carried out according to protocols approved by the Institutional Animal Care and Use Committee of the University of California, San Diego.
2.2. Drugs
Capsaicin was obtained from Sigma-Aldrich, St. Louis, MO, USA. A stock solution of 0.02 M was prepared in 90 % ethanol and further dilutions were prepared in saline. This vehicle was used for all the vehicle control experiment in this study. BoNT-B (Solstice Neurosciences, Louisville, KY, USA), Wheat germ agglutinin (WGA) tagged with Alexa Fluor 488 and 594 from Life Technologies, Carlsbad, CA, USA. BoNT-B solutions were prepared from stock solutions of 5000 U/mL, which were then serially diluted to the final concentration in 0.9% saline. All solutions were stored at 4°C and brought to room temperature prior to use.
2.3. WGA application in the supraorbital (SO) and meninges
To label projections from the SO region, and the meninges, mice were anesthetized (2.5% isoflurane, with 80% oxygen and 20% room air). For SO delivery, WGA 594 (30 μl of 5 %) was delivered above the orbit using a 29G needle. For meningeal delivery, the mouse was mounted in a stereotaxic holder. A midline incision was made on the skin and, using a dental drill, the right parietal bone (AP ~ −1.06 to −3.06, ML ~ 1.32 to 2.40) was slowly thinned, exposing the underlying dura. A small piece of Spongostane presoaked with 5ul of 1% WGA- 488 was laid on the dura and covered using a thin piece of parafilm (Parafilm “M”®, American National Can, Chicago, IL, USA). The edges of the Parafilm were sealed with bone wax to prevent the tracer from infiltrating the surrounding tissues. The incision was sutured and after two days of recovery, the WGA treated mice were deeply anesthetized with Beuthanesia © and transcardially perfused with saline followed by 4 % paraformaldehyde solution.
2.4. Supraorbital (SO) BoNT-B and supraorbital capsaicin
Mice were briefly anesthetized (as above) and received unilateral injections of BoNT-B (1.5 U/40 μl)) or vehicle (saline) in the SO region. Three days later, animals were injected with capsaicin (20 μL of 0.5 mM solution) into the ipsilateral SO region. Pain behavior was then quantified. Animals were perfused at 2 hrs post capsaicin injection and tissue was harvested for analysis of c-fos in the TNC.
2.5. Pain behavior
Capsaicin (20 μL of 0.5 mM solution) or vehicle were injected into the SO region of the mice induced nocisponsive behavior consisting of ipsilateral face wiping. Wiping frequency was tabulated for 30 min. Another group of mice that were pretreated with SO BoNT-B were also subjected to this behavioral testing. The wiping activity was manually counted and the observer was blinded as to the pre-treatment given to the mice.
2.6. Supraorbital BoNT and meningeal capsaicin
Mice were briefly anesthetized and received unilateral injections of BoNT-B (1.5 U/40 μl)) or vehicle (saline) in the SO region. Three days later, the animals were injected with capsaicin in the meninges. After a tiny incision was made on the skin above the right parietal bone, a 30 gauge needle was used to puncture the skull. The needle had a sleeve that allowed only 0.25 mm of the bevel to pass through the skull such that the capsaicin solution was distributed exclusively on the dura mater. This method was standardized using methylene blue dye to ensure the correct distribution of the solution before the actual experiments were performed, e.g. to ensure that the dural injection of capsaicin did not spread to the tissue outside the cranium. (See supplementary Fig. 1.). The needle was connected to a Hamilton syringe using a PE-10 catheter. Four μl of 0.35 μM capsaicin was injected into the meninges. Animals were perfused either at 15 min or 2 hrs for analysis of NK-1 internalization and c-fos, respectively, in TNC.
2.7. Supraorbital or meningeal application of WGA-Alexa 594 and supraorbital BoNTs
As previously described, mice were anesthetized and the skull was carefully drilled to expose the dura. Spongostan pre-soaked in 1% WGA-594 was placed on the dura and covered using bone wax and parafilm such that the skin was not exposed to the WGA. One day after placement of the tracer, the mice then received ipsilateral injection of BoNT in the SO region. Similarly, another set of mice received intradermal SO injections of WGA-594 which was followed in one day by SO BoNT injections. In both the sets, mice were allowed to recover for 3 days before transcardially perfusion. The trigeminal ganglion was analyzed for VAMP and WGA-594.
2.8. Immunohistochemistry
The previously exposed dura, trigeminal ganglia and TNC were collected following perfusion. After overnight fixation with PFA tissues were transferred to 30 % sucrose. Five days later TG and TNC were sectioned at 10 μm and 30 μm thickness, respectively. The TG sections were directly collected onto glass slides while TNC sections and dura were stored free-floating prior to staining.
2.8.1. WGA staining in TG and TNC
Since the WGA was already labeled with fluorophores, sections were washed with PBS-Triton x and mounted onto glass slides. The slides were then cover slipped and observed using an Olympus BX-51 fluorescence microscope (Olympus Optical, Tokyo, Japan).
2.8.2. c-fos (DAB immunostaining)
Free-floating sections prepared as above were incubated overnight at 25°C in rabbit anti-c-fos polyclonal antibody (1:20,000) (Calbiochem Inc., Darmstadt, Germany) and a goat anti rabbit biotinylated secondary antibody. Sections were then subject to the ABC method, and 3, 3′-Diaminobenzidine (DAB) was used as the chromogen to detect c-fos positive cells. An observer blinded to the treatment counted c-fos positive cells rostrocaudally in TNC (lamina I and II) using light microscopy. Total number of c-fos positive cells stained in the section were counted rostrocaudally. Cross-sections of TNC were cut, with each section having a thickness of 30 μm. Every 4th section was subjected to c-fos immunostaining. The Vi/Vc region was defined as the distance between 0 to 0.6 mm, Vc region between 0.6 mm to 1.5 mm and C1/C2 above 1.5 mm to 1.8 mm from obex. There were a minimum 2 to a maximum 3 sections taken between each division depending upon the quality of the sections. The number of c-fos positive cells were counted within these three region (Vi/Vc, Vc and C1/C2) in the ipsilateral and contralateral side to BoNT/capsaicin injections. The mean number of c-fos positive cells were calculated from the total number of cells/3 sections between each division from each animal.
2.8.3. Double labelling of VAMP and Neu N in TG
The slides containing TG sections were incubated overnight with rabbit anti-VAMP polyclonal antibody (1:1000, Abcam, Cambridge, MA) and mouse biotinylated anti-Neu N polyclonal antibody (1:300, Millipore, Temecula, CA). Sections were then incubated with Alexa 488 conjugated anti rabbit antibody (1:1000; Life Technologies, Grand Island, NY, USA) and streptavidin 595. Images of at least four sections/animal were captured under 20X magnification for control and BoNT-B treated mice using a fluorescence microscope. Branching of the trigeminal nerve in TG was used as a landmark to differentiate V1 (ophthalmic)/V2 (maxillary) from V3 (mandibular) neurons (Fig. 3B). The percentage of VAMP positive neurons was determined by calculating the total number of VAMP positive neurons/total number Neu N positive cells in each section with a total of at least 3 sections/animal. Following quantification, images used for figures were processed to enhance contrast for figure presentation only.
Figure 3. Effects of supraorbital BoNT-B on VAMP expression in the trigeminal ganglion (TG).
Schematic representation of experimental design where the mice were treated with SO BoNT-B (1.5 U) or vehicle and sacrificed 3 days later (A). Longitudinal section (10 μm thickness) of TG showing V1 (ophthalmic), V2 (maxillary) and V3 (mandibular) region labelled with Neu N. Representative images of TG labelled with Neu N (red) and VAMP (green) in the V1 region following vehicle (C–E) and BoNT-B treatment (F–H). Histogram depicts the percentage of VAMP positive neurons in V1 region of TG. Note loss of VAMP is an indirect measure of VAMP cleavage by BoNT-B (I). Representative images of TG labelled with Neu N (red) and VAMP (green) in the V1 (J–L) and V3 (M–O) region of the same animal following BoNT-B treatment. Histogram depicts the percentage of VAMP positive neurons that was analyzed by the presence of VAMP (measure of VAMP cleavage by BoNT-B) in Neu N positive cells in the V1 and V3 (P) region following BoNT-B SO administration, N=4. **P<0.01 as compared to vehicle, *P<0.05 as compared to V3. TG- trigeminal ganglion, BoNT-B- botulinum toxin B, VAMP- vesicle associated membrane protein. Scale bar 75 μm.
2.8.4. NK-1 internalization in TNC
To assess NK1r expression in the TNC and upper cervical dorsal horn, sections were incubated with rabbit anti-NK1r polyclonal antibody (1:1000, Millipore, Temecula, CA) for 72 hrs followed by goat anti-rabbit secondary antibody conjugated with Alexa Fluor 594 (Invitrogen, Eugene, OR, USA). Neurons positive for NK1r internalization were counted using a 60x oil-immersion objective lens. Internalization was assessed according to the standard of previous reports (Huang et al. 2011; Kondo et al. 2005; Marino et al. 2014). The filter set was Omega Optical XF100 to 2 Red Bandpass Filter (Omega Optical Inc., Brattleboro, VT, USA). Neuronal profiles that had 10 or more endosomes were considered to have internalized NK1rs (Bowden et al. 1994; Nazarian et al. 2008). The total number of NK1r immunoreactive neurons in lamina I/II, with and without NK1r internalization, was counted and used to calculate the percentage of neurons showing internalization. Counting was done without knowledge of treatments. Mean counts from 3 to 4 sections per animal were used.
2.8.5. Double labelling of VAMP and WGA 594 in TG
The slides containing TG sections were incubated overnight with rabbit anti-VAMP polyclonal antibody (1:1000) and followed by goat anti- rabbit Alexa 488 secondary antibody since the section already contained WGA 594 localized in the V1 region of TG. Images of at least four sections/animal were captured under 20X magnification in the V1 region of control and BoNT-B treated mice. Percentage of VAMP co-localized with WGA 594 was calculated by counting total VAMP co-localized with WGA/total WGA positive neurons.
2.8.6. Double labelling of CGRP and WGA 594
The free floating dura was incubated overnight with rabbit anti-CGRP (Sigma Aldrich, St. Louis, MO, USA) polyclonal antibody (1:1000) followed by goat anti- rabbit Alexa 488 secondary antibody. The presence of WGA 488 was analyzed in CGRP positive neurons.
2.9. Statistical Analysis
All values are presented as mean ± SEM. Behavioral data in Fig. 2 were analyzed using two way ANOVA followed by post hoc tests with Bonferroni corrections for multiple comparisons and VAMP data for the two groups were analyzed using two tailed unpaired t-tests and within the same group using two tailed paired t-tests. Data in Fig. 4, 5 and 6 were analyzed using one way ANOVA followed by post hoc tests with Bonferroni corrections for multiple comparisons. Differences reaching the level of P<0.05 were considered to be significant. Prism (GraphPad Prism software Inc., version 5.0, San Diego, CA, USA) was used for statistical analysis.
Figure 2. Effects of supraorbital BoNT-B on ipsilateral supraorbital capsaicin induced pain behavior.
Schematic representation of experimental design where the mice were treated with SO BoNT-B (1.5 U) or vehicle 3 days (3d) prior to ipsilateral SO capsaicin (20 μl of 0.5 mM) (A). Pain behavior measured by number of wipes following SO capsaicin injection (B), N=8. ***P<0.001.
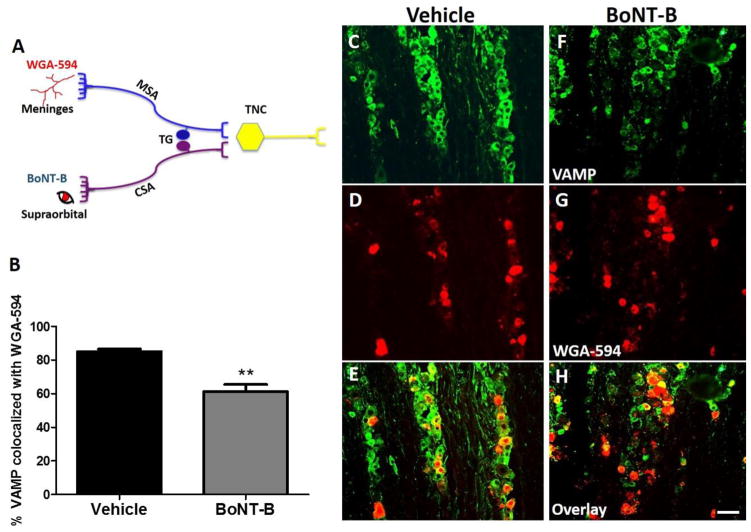
Figure 4. Percentage of VAMP positive neurons co-localized with WGA-594 (transganglionic tracer injected in SO) in V1 (ophthalmic) region of trigeminal ganglion (TG).
Schematic representation of experimental design where the mice were supraorbitally injected with WGA-594, 1 day prior to ipsilateral SO BoNT-B (1.5 U) or vehicle (A). Histogram depicts the percentage of VAMP positive neurons that was colocalized with the WGA-594 positive neurons (reterogradely labelled from sensory cutaneous afferents (CSA)) in V1 region of TG. Loss of VAMP was the measure of VAMP cleavage (B). Representative images of TG labelled with VAMP (green) and WGA -594 (red) in the V1 (C–H) region, N=3. *P<0.05 as compared to vehicle. BoNT-B- botulinum toxin B, VAMP- vesicle associated membrane protein, V1- ophthalmic region, WGA- wheat germ agglutinin. Scale bar 75 μm.
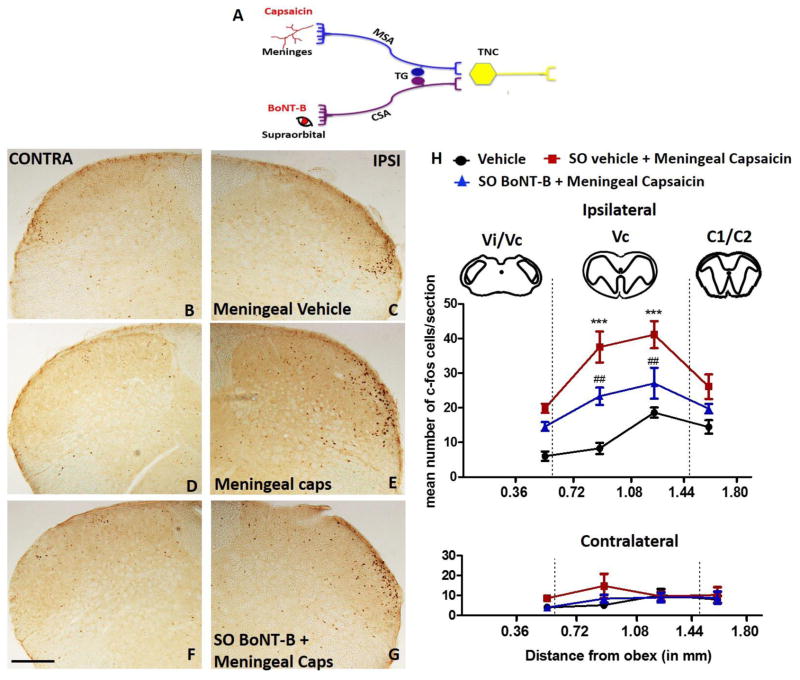
Figure 5. Effects of supraorbital BoNT-B on c-fos expression evoked by ipsilateral supraorbital capsaicin in the trigeminal nucleus caudalis (TNC).
Schematic representation of experimental design where the mice were treated with SO BoNT-B (1.5 U) or vehicle 3 days (3d) prior to ipsilateral SO capsaicin (20 μl of 0.5 mM) (A). Representative images of ipsi and contra TNC labelled with c-fos positive cells in the Vc region treated with SO vehicle (B, C), SO capsaicin (D, E) and SO BoNT-B pretreatment followed by ipsi SO capsaicin (F, G). Graph represents the number of c-fos positive neurons in the ipsilateral and contralateral TNC rostrocaudally between 0.36 mm to 1.80 mm caudal to obex at 2 hrs after SO vehicle or capsaicin and following the pre-treatment with BoNT-B (1.5 U. 3 days earlier) (H). N=5–9. * P<0.05, ***P<0.001 as compared to SO vehicle. ###P<0.001 as compared to SO capsaicin. BoNT-B- botulinum toxin B. Scale bar 100 μm.
Figure 6. Effects of supraorbital BoNT-B on c-fos expression evoked by ipsilateral meningeal capsaicin in the trigeminal nucleus caudalis (TNC).
Schematic representation of experimental design where the mice were treated with SO BoNT-B (1.5 U) or vehicle 3 days (3d) prior to ipsilateral meningeal capsaicin (4 μl of 0.35 μM) (A). Representative images of ipsi and contra TNC labelled with c-fos positive cells in the ventrolateral region treated with meningeal vehicle (B, C), meningeal capsaicin (D, E) and SO BoNT-B pretreatment followed by ipsi meningeal capsaicin (F, G). Graph represents the number of c-fos positive neurons in the ipsilateral and contralateral TNC rostrocaudally between 0.36 mm to 1.80 mm caudal to obex at 2 hrs after meningeal vehicle or capsaicin and following the pre-treatment with BoNT-B (1.5 U, 3 days earlier) (H). N=6. ***P<0.001 as compared to meningeal vehicle. ##P<0.01 as compared to meningeal capsaicin. Scale bar 100 μm.
3. Results
3.1. Meningeal and supraorbital projections
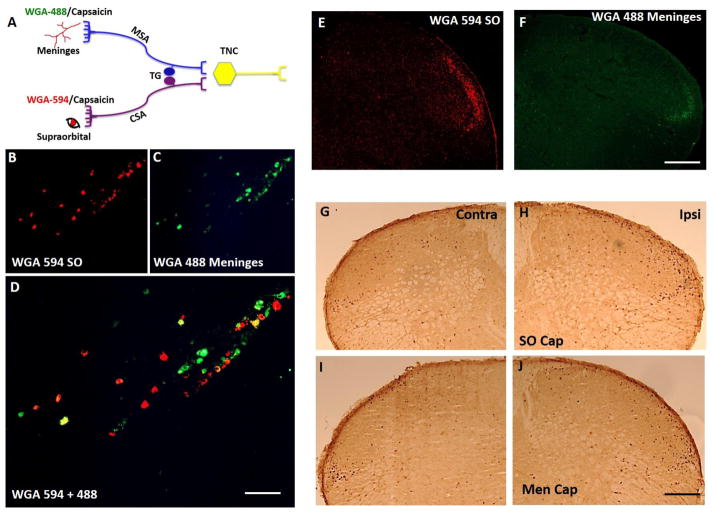
Retrograde labelling of cell bodies were observed in the ipsilateral TG following the application of trans-ganglionic tracers WGA-594 into the SO region and WGA-488 onto the meninges. The labelled cells were localized in the V1 region of the TG that gives rise to the ophthalmic nerves and both sets of afferent neurons were found in close proximity to each other in this region (Fig. 1A–D). The number of cell bodies stained with WGA 488 (from meninges) were fewer as compared to the number of cell bodies stained with WGA 594 (meninges), reflecting the relative density of fibers present in the respective receptive fields. WGA 488 and 594 positive fibers were also observed in TNC ipsilateral to the application site. Neurons stained with both tracers were located in lamina I and II of the ventrolateral segment of TNC (Vc) and upper cervical dorsal horn (C1/C2) (Fig 1E, F) confirming that the second order neurons in this region receive convergent inputs from the meninges and the extracranial SO region. However, the staining arising from the meninges (Fig. 1F) was weak and difficult to observe as compared to staining arising from cutaneous afferents (Fig. 1E). As suggested, above this low intensity may reflect the density of fibers in the injected areas.
Figure 1. Meningeal and supraorbital projections to TG and TNC.
Schematic representation of experimental design where transganglionic tracer wheat germ agglutinin (WGA) tagged to fluorophores 594 or 488 and capsaicin was applied to the supraorbital (SO) and/or the meninges (dura mater) (A). Localization of WGA flurophores in the trigeminal ganglion (TG) (B–D). Note that the cutaneous sensory afferent neurons and meningeal sensory afferent neurons lie in close proximity to each other in the V1 region of TG. The nerve fibers in the ventrolateral region along lamina I and II of trigeminal nucleus caudalis (TNC) showed localization of WGA fluorophores (E, F). Localization of c-fos expression in TNC following SO (G, H) and meningeal capsaicin (I, J), N=4. Note both of the fluorophores stained nerve fibers localized in the same region as c-fos suggesting that the nerve fibers converge around the same second order neurons. CSA- cutaneous sensory afferents, MSA- meningeal sensory afferents, Men cap – meningeal capsaicin. Scale bar 100 μm.
Injection of capsaicin into the SO resulted in activation of neurons in lamina I and II of the ventrolateral segment in TNC and in the C1 and C2 regions (Fig. 1A, G and H) as evidenced by increased incidence of c-fos (+) neurons. This region corresponds to the ophthalmic representation. Capsaicin injected into the meninges also activated c-fos neurons in the same TNC region (Fig. 1A, I and J), thus confirming that fibers from meninges and SO region project to the neurons in the same region.
3.2. Supraorbital capsaicin and supraorbital BoNT-B
3.2.1. Pain behavior
SO intradermal injections of capsaicin produced an ipsilateral forepaw wiping activity directed to the region above the eye as compared to SO vehicle injections. This was maximal by 4–6 min and lasted for approximately 10 min. Treatment with ipsilateral SO BoNT (1.5 U/40 μl) 3 days prior to the ipsi SO intradermal capsaicin injection significantly reduced the wiping frequency. A lower dose of BoNT-B (1 U) failed to produce a significant effect (Fig. 2A and B). Importantly, SO BoNT at the highest dose was not observed to alter motor function. Animals displayed normal grasping behavior as measured by a suspension test where the animals were required to grip onto the wire for at least 1 min (Marino et al. 2014). This test was performed in order to confirm that BoNT injected in the SO did not get systemic.
3.2.2. VAMP cleavage
BoNT-B inhibits neurotransmitter release by cleavage of VAMP, a SNARE protein present on the synaptic vesicle, which is involved in exocytosis. The VAMP antibody used in this study recognizes the intact molecule. Therefore, reduction of VAMP protein expression was used as a measure of VAMP cleavage. In control animals, VAMP expression was co-localized with NeuN (marker for neurons). Following pre-treatment with SO BoNT-B, NeuN positive neurons in the V1 (ophthalmic) region showed a reduction in VAMP expression. Thus, VAMP cleavage was significantly higher in the SO BoNT-B treated TG as compared to the saline treated animals (Fig. 3A, C–I). Further, this reduction was also significant as compared to the V3 (mandibular) region of the same ipsilateral TG (receiving input from the adjacent mandibular nerve distribution) (Fig. 3J–P). Here, V3 served as an internal control for V1, since BoNT was injected in the SO region that receives primary afferents arising from the ophthalmic region (i.e. V1) of TG (See Fig. 3B).
To further confirm the association of VAMP cleavage with afferents exposed to SO BoNT, we injected WGA 594 in the SO region 1 day prior to ipsi BoNT-B injection. Tissue from the BoNT-B treated animals showed a significant reduction in VAMP expression (i.e. more VAMP cleavage) in TG, WGA positive neurons (Fig. 4A–H). These findings suggest that BoNT-B was taken up by the cutaneous sensory afferents and transported retrogradely to the trigeminal sensory neurons. It should also be noted that when BoNT-B was given a day before WGA-594, tissue from these animals did not have tracer in their TG, suggesting that SNAREs are also important for endocytosis of the WGA, as previously reported (Zhang et al. 2013).
3.2.3. c-fos expression in TNC
Intradermal injection of capsaicin in the SO region activated nocisponsive neurons in the ipsi-TNC. Fos-positive cells were counted in lamina I and II starting from 0.36 mm to 1.80 mm caudally to the obex. Quantitative analysis showed a significant increase in the number of ipsi- c-fos positive neurons between 0.72 mm to 1.80 mm in the ventrolateral axis that represents V1 region as compared to both the contra- side and vehicle control animals (Fig. 5H). This increased c-fos expression was significantly reduced in animals pre-treated with ipsi- SO intradermal BoNT-B (1.5 U) (Fig. 5. A, B–G, H).
3.3 Supraorbital BoNT and meningeal capsaicin
3.3.1. c-fos expression in TNC
Meningeal application of capsaicin increased expression of c-fos in the superficial laminae between 0.72 and 1.44 mm in ventrolateral axis of ipsilateral TNC at 2 hrs post injection as compared to the vehicle (Fig. 6H). As shown earlier, this region also receives afferents from facial region including from the areas around the SO region. Thus, these data confirm the previous findings that second order neurons in ventrolateral segments of TNC receive inputs from both meninges and the ophthalmic region of the face. However, pre-treatment with ipsilateral SO BoNT-B reduced expression of c-fos positive neurons otherwise activated by meningeal capsaicin injection. This suggests a possible effect of BoNT given in the cutaneous SO afferents on the TNC excitation induced by meningeal afferent activation, either by an effect upon the second order TNC neurons or on the adjacent meningeal afferents (Fig. 6B–H).
3.3.2 NK-1 internalization in TNC
To determine whether the BoNT-B from the SO afferents had a transynaptic effect on second order neurons or on adjacent meningeal afferents, we analyzed the effect of SO BoNT on sP release in TNC as measured by NK-1 internalization evoked by meningeal capsaicin. A maximum of 4 to 5 NK-1 positive cells were observed in each section. In vehicle control animals, NK-1 positive cells are observed throughout lamina I and II in the TNC with the majority of neurons showing NK-1 immunoreactivity localized to the cytoplasmic membrane. Following the binding of substance P, the NK-1 receptor undergoes internalization with the immunoreactivity then localized in endosomes in the cytosol. Square in the fig. 7E and K represents the internalized NK-1 receptor neurons and circle represents non-internalized NK-1 receptor neurons. Following the capsaicin injection in the meninges, we observed increased NK-1 internalization in the ipsilateral TNC at 15 min, predominantly in the ventrolateral axis as compared to the contralateral side (Fig. 7C–H), suggesting that meningeal capsaicin induced release of sP from the meningeal central afferents. Pre-treatment with SO BoNT-B, 3 days prior to meningeal capsaicin injection reduced the incidence of NK-1 internalization in the second order neurons (Fig. 7I–N). This suggests that SO BoNT from the cutaneous afferents acts on meningeal afferents to block the release of sP from the meningeal afferents. This finding likely holds true for other transmitters as well (for e.g. CGRP, glutamate etc) (Dolly et al. 2009; Durham et al. 2004)
Figure 7. Effects of supraorbital BoNT-B on NK-1 receptor internalization in TNC induced by ipsilateral meningeal capsaicin.
Schematic representation of experimental design where the mice were treated with SO BoNT-B (1.5 U) or vehicle 3 days (3d) prior to ipsilateral meningeal capsaicin (4 μl of 0.35 μM) (A). Plot represents percentage of NK-1 positive neurons that are internalized (marker for substance P release) at 15 min in TNC following meningeal capsaicin alone or group pretreated with SO BoNT-B, 3 days prior to ipsilateral meningeal capsaicin (B). Representative images of a neuron in ipsilateral ventrolateral TNC with internalized NK-1 receptor after meningeal capsaicin (C–H) and a non-internalized neuron in the TNC pretreated with SO BoNT-B prior to meningeal capsaicin (I–N). Note that represents neurons that are NK-1 receptor internalized and represents non-internalized NK-1 positive neurons. Note that pre-treatment with ipsilateral SO BoNT/B significantly decreased NK-1 internalization in these neurons (N=4). Scale bar C–E, I–K 100 μm and F–H, L–N 25 μm. ***P<0.001 as compared to meningeal capsaicin. BoNT-B- botulinum toxin B, NK-1 neurokinin 1 receptor, caps – capsaicin, SO- supraorbital, N=4.
3.3.3. VAMP cleavage
In order to analyze if BoNT from the cutaneous afferents may undergo transcytotic movement to the meningeal afferents, we applied WGA - 594 in the meninges one day prior to injecting SO BONT-B and assessed for VAMP cleavage in those neurons (Fig. 8A). Interestingly, we observed a decrease of VAMP in those WGA-594 positive neurons in the TG (Fig. 8B–H). Therefore, we assume that BoNT-B injected into the SO region was taken up by the cutaneous afferents and transported to the TG and central terminals, where it may have undergone transcytosis either to the adjacent meningeal sensory neuron in TG or to adjacent meningeal afferents in TNC. However, the exact site and mechanism whereby such a transport may occur is unknown.
Figure 8. Percentage of VAMP positive neurons co-localized with WGA-594 (applied in meninges (dura mater)) in V1 region of trigeminal ganglion (TG).
Schematic representation of experimental design where WGA-594 was applied in the dura mater, 1 day prior to ipsilateral SO BoNT-B (1.5 U) (A). Histogram depicts the percentage of VAMP positive neurons that was colocalized with the WGA-594 positive neurons (reterogradely labelled from meningeal sensory afferents (MSA)) in V1 region of TG. Note that loss of VAMP was the measure of VAMP cleavage (B). Representative images of TG labelled with VAMP (green) and WGA -594 (red) in the V1 (C–H) region, N=3. **P<0.01 as compared to vehicle. BoNT-B- botulinum toxin B, VAMP- vesicle associated membrane protein, V1- ophthalmic region, WGA- wheat germ agglutinin. Scale bar 75 μm.
3.4. Meningeal collaterals
An important concern in these studies is whether SO BoNT directly accesses meningeal afferent terminals. Recent electrophysiological and tracing studies in rats report that afferent fibers of trigeminal nerve that innervate the meninges form functional collaterals that exit though cranial sutures and innervate extracranial muscles (Schueler et al. 2013). Therefore, we considered whether such meningeal collateral might account for the uptake of SO BONT-B as delivered in this study. However, injection of WGA-594 in the SO region using the protocol as described did not appear in the afferent fibers in the dura mater (Fig. 9A–C). In contrast, application of WGA 594 to the meninges in separate set of experiments resulted in heavy labeling of dural afferents. (Fig. 9D–F). The tissue was co-labeled with CGRP inorder to show the presence of nerve fibres around the region of MMA and to determine the presence of WGA in these fibres.
Figure 9. Labelling of nerve fibers in dura mater following WGA-594 (transganglionic tracer) applied in SO and meninges.

Representative image of nerve fibers close to MMA in the dura mater following SO WGA 594, tissue was co-stained with CGRP (A, B). Note that no dural fibers stained positively for WGA-594 (A), suggesting that meningeal collaterals do not project to the SO skin. Retrograde labelling of WGA-594 in the nerve fibers close to MMA was observed only after its application directly to the dura matter (C). This region is close to the site of WGA-594 applied in the meninges, N=3. Scale bar 50 μm.
It should be noted that so far this functional connections of collaterals has been shown only in the temporal and occipital pericranial muscles suggesting that these collaterals may innervate only the periosteum and muscles, and may not innervate the skin, the site where BoNT-B was injected in our study.
4. Discussion
The work reported herein highlights systematic attempts to illustrate action of BoNT-B in the trigeminal system utilizing the convergence - projection theory of referred pain. This approach allowed us to identify the possible mechanism of action of BoNT as summarized below:
BoNT is taken up by the afferents at the site of injection and is retrogradely transported to its cell body located in the TG as evidenced by the cleavage of VAMP in the V1 region of TG.
The transport of BoNT to the central terminals is supported by inhibition of neurotransmitter release from the central terminal thereby attenuating capsaicin induced c-fos activation in second order neurons
Further, BoNT injected in the SO region inhibits sP release (as measured by NK-1 internalization) and c-fos activation in the ipsilateral TNC evoked by meningeal capsaicin, indicating an effect of cutaneous BoNT on meningeal afferent input into the TNC.
4.1. Capsaicin induced trigeminal activation
Electrophysiological studies have demonstrated that trigeminal nociceptors, and nociceptive neurons in TNC respond to the transient receptor potential vanilloid receptor (TRPV1) agonist capsaicin injected into their receptive fields (Carstens et al. 1998; Gallar et al. 1993). The TRPV1 channels are present on both peripheral and central nerve terminals of dural and cutaneous trigeminal sensory neurons. Activation of these terminals by capsaicin mobilizes SNAREs to release glutamate and neuropeptides, like substance P and CGRP, from their peripheral and central nerve terminals (Numazaki and Tominaga 2004; Pingle et al. 2007). Therefore, we utilized capsaicin application into cutaneous (SO) and meningeal (dura mater) sites to evoke their respective activation of the TNC and evaluated the effects of cutaneous BoNT-B on the resulting release and 2nd order neuron activation. Such application of capsaicin yields strong nocifensive behavior focused at the SO injection site. Similar behavioral experiments after meningeal application were not accomplished given the need to have anesthesia to achieve the associated exposure.
4.2. Convergence of cutaneous and meningeal afferents
Considerable circumstantial evidence suggests that migraine pain arises from activation of meningeal perivascular afferents that project through TG to the TNC (Levy et al. 2007; Olesen et al. 2009; Strassman et al. 1996). It has long been recognized that migraine has properties in common with visceral referred pain, and depending on the site of intracranial stimulation, the referred pain appears to be localized to areas commonly associated with pain during the migraine attacks, e.g. supraorbital, retrobulbar and occipital region (Bartsch and Goadsby 2003; Piovesan et al. 2001). Several anatomical and electrophysiological studies have reported that nociresponsive neurons in TNC and upper cervical spinal cord dorsal horn (Morch et al. 2007; Strassman and Vos 1993) receive convergent input from afferents innervating the head and face regions to which the meningeally derived pain is referred (Burstein et al. 1998; KERR and OLAFSON 1961; Noma et al. 2008; Strassman et al. 1994). Consistent with these observations, we demonstrated this convergence by exposing the cutaneous and meningeal afferents to trans-ganglionic tracers (WGA-488 and 594, respectively).
4.3. Peripheral action and central transport of BoNT
The effect of peripheral BoNTs in treating pain conditions has been argued to be local, i.e. at the site of injection, by blocking the release of neurotransmitters from peripheral nerve endings that contributes to local inflammation and pain. In the present study, we showed that pre-treatment with SO BoNT reduced the ipsilateral wiping behavior in mice otherwise evoked by SO capsaicin, suggesting a local effect of BoNT-B. Similar local effects of BoNT-A have been reported on capsaicin evoked pain and flare in humans (Gazerani et al. 2006; Tugnoli et al. 2007) and in capsaicin induced plasma extravasation in animals (Marino et al. 2014). Several pre-clinical studies, have suggested that apart from their local effect, local sensory (and motor) terminals will not only take up local BoNTs, but move them in an active form via fast axonal transport to central terminals. Such transport has been demonstrated by movement of radiolabeled toxins as shown in early studies (Black and Dolly 1986; Habermann 1974; Wiegand et al. 1976); and by the cleavage of the respective SNARE protein in the dorsal root/TG or motor neurons in ventral horn (Antonucci et al. 2008; Aoki 2003; Lawrence et al. 2012; Marino et al. 2014; Matak et al. 2011; Restani et al. 2011; Restani et al. 2012; Simpson 2013). Analogous to these observations, we have shown that SO BoNT-B undergoes retrograde transport as evidenced by the reduction of VAMP in the ipsilateral TG and, is consistent with our previously reported findings, in DRG following intraplantar BoNT-B. In addition to this, we observed a reduction in c-fos expression in the ventrolateral segment of TNC and upper cervical dorsal horn. We have previously shown that intraplantar BoNT-B was able to inhibit substance P release from the ipsilateral central afferents evoked by intrathecal (spinal) capsaicin. This suggests an effect of peripheral BoNT upon ipsilateral central terminal. Long range effects of BoNT have also been evidenced by changes in the activity of CA1 pyramidal neurons after injection of BoNT-A into the contralateral hippocampus (Antonucci et al. 2008). Of critical importance, transport of catalytically active BoNTs occurs not only in the retrograde direction to the DRG, but also anterogradely to the central terminals. This property of BoNTs (A and B) has been demonstrated by detecting the presence of neurotoxin- cleaved SNAREs (increased SNAP-25 cleavage products and reduced VAMP) in the central terminals after peripheral BoNT injection (Marino et al. 2014; Matak et al. 2011) and these central effects can be blocked by treatment of the nerve with blockers of axon transport. One can question how BoNT reaches central terminals in an intact form? Studies in motor neurons have suggested that some of the BoNT may not end up in acidic endosomes where the Lc (light chain) component is cleaved and released to the cytoplasm. Full-length BoNT-A has been shown to undergo axonal retrograde transport in non-acidic organelles with speed profiles matching fast microtubule-dependent transport and largely overlapping with TeNT (tetanus neurotoxin) positive carriers in motor neurons (Restani et al. 2012a).
4.4. Trans-synaptic effects
While it has been argued that BoNTs do not undergo trans-synaptic movement, recent data also cast doubt on that thesis. We strikingly observed that SO BoNT-B could block the sP release from the meningeal afferent as measured by NK-1 receptor internalization in second order neurons and reduced c-fos activation of second order neurons otherwise evoked by meningeal capsaicin. Our data suggests the possibility of trans-synaptic transport of the BoNT from the cutaneous trigeminal afferent to either the second order neurons (which receives convergent input from the meningeal afferent) or the terminal of the converging meningeal afferent. These findings correspond well with our recent data with IPLT BONT-B, where we have shown that bilateral dorsal horn c-fos expression evoked by spinal SP (acting on post synaptic NK1 receptors, bypassing afferent terminals) is reduced in the dorsal horn ipsilateral to the paw which received IPLT BoNT-B (Marino et al. 2014). While such transynaptic movement is indeed controversial, increasing evidence suggests that it occurs in several neuronal systems: 1) Direct evidence supporting the hypothesis that BoNT-A is retrogradely transported in an active form and undergoes transcytosis in second-order neurons was provided by the detection of BoNT-A-cleaved SNAP-25 in cholinergic synapses in rat retina after injection of the neurotoxin in the superior colliculus (Antonucci et al. 2008). 2) In the trigeminal system, BONT-A injected in the temporomandibular joint inhibited plasma extravasation in the dura mater, clearly indicating the likelihood of transynaptic movement from mandibular afferents to meningeal afferents. (Filipovic et al. 2012). 3) Cleavage of SNAP-25 in the glial cells of spinal cord dorsal horn has been observed following injection of BoNT-A in the paw (Marinelli et al. 2012). Similar studies in humans (Marchand-Pauvert et al. 2013) also support this trans-synaptic action.
Further, the present study noted loss of VAMP protein in the TG cell bodies following BoNT-B that was reterogradely labeled with WGA 488 from the dura. This finding establishes that transport of BoNT occurred between neighboring afferents resulting in blockade of excitatory drive from meningeal afferents induced by capsaicin. However, these studies do not confirm if this transport occurred between adjacent cell bodies in the TG or between spatially contiguous terminals in TNC.
4.5. Meningeal afferent collaterals
A further complexity of migraine afferent circuitry is indicated by recent electrophysiological and tracing studies, which reveal that intracranial afferents to the meninges also have collaterals that leave the skull and innervate the pericranial muscles and periosteum (Schueler et al. 2013). It has been reported that extracranially administered BoNT-A inhibited mechanical sensitivity of intracranial meningeal nociceptors (Burstein et al. 2014). Taken together, these data raise the possibility that extracranial meningeal collaterals might account for the effect of SO BoNT-B on meningeal activation observed in our study. To evaluate such a possibility, we performed three experiments. i) We injected the tracer WGA-594 into the SO region and found no WGA-594 positive fibers in dura. ii) We demonstrated that scraping these suture lines did not alter the effects of SO BoNT on meningeal evoked release (data not shown). These observations suggests that uptake of BoNT-B by extracranial meningeal collaterals may be unlikely in this model. However, these results do not exclude the possibility that these meningeal collaterals may play a role in mediating BoNT effects.
Conclusion
In summary, the present study for the first time provides a possible trans-synaptic mechanism of action of BoNT by utilizing the vicerosomatic convergence model in the trigeminal system. The present study thus provides insights relevant to the development of therapies for migraine as well as to other debilitating craniofacial disorders.
Supplementary Material
Figure showing the SO and meningeal regions where capsaicin injections were performed (A). The skin isolated following methylene blue dye injections into the meninges through the skull showed no leakage of the dye outside the cranium (skin) (B). Note that methylene blue dye excites between 560 to 600 nm wavelengths. Another set of mice was used to produce a positive control for methylene blue dye leakage (red) tissue that was injected just below the skin (C). This was performed in order to compare leakage v/s non leakage tissue..
Highlights.
This study presents mechanism of action of BoNT-B using trigeminal sensitization migraine model
BoNT-B is taken up by peripheral afferents and transported to central terminals
Supraorbital BoNT-B exerts a trans-synaptic action on meningeal input into the nucleus caudalis.
This effect is either on the second order neuron or the terminal of the converging meningeal afferent.
Acknowledgments
These experiments were performed when the first author was supported as a fellow by the Migraine research foundation and DA02110. BoNT-B was gifted by Solstice Neurosciences. We would like to thank Shelly Malkmus, Marc Marino, Joanne Steinauer and Qinghao Xu for their technical assistance.
Abbreviations
- BoNT
botulinum toxin
- CGRP
calcitonin gene related peptide
- CSA
cutaneous sensory afferents
- DRG
dorsal root ganglion
- HC
heavy chain
- IPLT
intraplanatar
- LC
Light chain
- MSA
meningeal sensory afferents
- NK-1
Neurokinin
- SNARE
soluble N-methylaleimide-sensitive attachment protein receptor
- SNAP-25
synaptosomal associated protein
- sP
substance P
- TeNT
tetanus neurotoxin
- TRPV1
transient receptor potential vanilloid receptor
- TG
trigeminal ganglion
- TNC
trigeminal nucleus caudalis
- VAMP
vesicle associated membrane protein
- WGA
wheat germ agglutinin
Footnotes
Conflict of Interest
We declare that there is no conflict of financial interest with regard to or manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Antonucci F, Rossi C, Gianfranceschi L, Rossetto O, Caleo M. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28(14):3689–3696. doi: 10.1523/JNEUROSCI.0375-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki KR. Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache. 2003;43(Suppl 1):S9–15. doi: 10.1046/j.1526-4610.43.7s.3.x. [DOI] [PubMed] [Google Scholar]
- 3.Bach-Rojecky L, Lackovic Z. Antinociceptive effect of botulinum toxin type a in rat model of carrageenan and capsaicin induced pain. Croat Med J. 2005;46(2):201–208. [PubMed] [Google Scholar]
- 4.Bach-Rojecky L, Lackovic Z. Central origin of the antinociceptive action of botulinum toxin type A. Pharmacol Biochem Behav. 2009;94(2):234–238. doi: 10.1016/j.pbb.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Bach-Rojecky L, Salkovic-Petrisic M, Lackovic Z. Botulinum toxin type A reduces pain supersensitivity in experimental diabetic neuropathy: bilateral effect after unilateral injection. Eur J Pharmacol. 2010;633(1–3):10–14. doi: 10.1016/j.ejphar.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Bartsch T, Goadsby PJ. The trigeminocervical complex and migraine: current concepts and synthesis. Curr Pain Headache Rep. 2003;7(5):371–376. doi: 10.1007/s11916-003-0036-y. [DOI] [PubMed] [Google Scholar]
- 7.Black JD, Dolly JO. Interaction of 125I-labeled botulinum neurotoxins with nerve terminals. I Ultrastructural autoradiographic localization and quantitation of distinct membrane acceptors for types A and B on motor nerves. J Cell Biol. 1986;103(2):521–534. doi: 10.1083/jcb.103.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowden JJ, Garland AM, Baluk P, Lefevre P, Grady EF, Vigna SR, Bunnett NW, McDonald DM. Direct observation of substance P-induced internalization of neurokinin 1 (NK1) receptors at sites of inflammation. Proc Natl Acad Sci U S A. 1994;91(19):8964–8968. doi: 10.1073/pnas.91.19.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79(2):964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 10.Burstein R, Zhang X, Levy D, Aoki KR, Brin MF. Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: Therapeutic implications for migraine and other pains. Cephalalgia. 2014 doi: 10.1177/0333102414527648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmichael NM, Dostrovsky JO, Charlton MP. Peptide-mediated transdermal delivery of botulinum neurotoxin type A reduces neurogenic inflammation in the skin. Pain. 2010;149(2):316–324. doi: 10.1016/j.pain.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Carstens E, Kuenzler N, Handwerker HO. Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J Neurophysiol. 1998;80(2):465–492. doi: 10.1152/jn.1998.80.2.465. [DOI] [PubMed] [Google Scholar]
- 13.Cui M, Khanijou S, Rubino J, Aoki KR. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain. 2004;107(1–2):125–133. doi: 10.1016/j.pain.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Diener HC, Dodick DW, Aurora SK, Turkel CC, DeGryse RE, Lipton RB, Silberstein SD, Brin MF. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30(7):804–814. doi: 10.1177/0333102410364677. [DOI] [PubMed] [Google Scholar]
- 15.Dodick DW, Turkel CC, DeGryse RE, Aurora SK, Silberstein SD, Lipton RB, Diener HC, Brin MF. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50(6):921–936. doi: 10.1111/j.1526-4610.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- 16.Dolly JO, Lawrence GW, Meng J, Wang J, Ovsepian SV. Neuro-exocytosis: botulinum toxins as inhibitory probes and versatile therapeutics. Curr Opin Pharmacol. 2009;9(3):326–335. doi: 10.1016/j.coph.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Dong M, Tepp WH, Liu H, Johnson EA, Chapman ER. Mechanism of botulinum neurotoxin B and G entry into hippocampal neurons. J Cell Biol. 2007;179(7):1511–1522. doi: 10.1083/jcb.200707184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durham PL, Cady R, Cady R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: implications for migraine therapy. Headache. 2004;44(1):35–42. doi: 10.1111/j.1526-4610.2004.04007.x. [DOI] [PubMed] [Google Scholar]
- 19.Fadeyi MO, Adams QM. Use of botulinum toxin type B for migraine and tension headaches. Am J Health Syst Pharm. 2002;59(19):1860–1862. doi: 10.1093/ajhp/59.19.1860. [DOI] [PubMed] [Google Scholar]
- 20.Filipovic B, Matak I, Bach-Rojecky L, Lackovic Z. Central action of peripherally applied botulinum toxin type A on pain and dural protein extravasation in rat model of trigeminal neuropathy. PLoS One. 2012;7(1):e29803. doi: 10.1371/journal.pone.0029803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer A, Montal M. Single molecule detection of intermediates during botulinum neurotoxin translocation across membranes. Proc Natl Acad Sci U S A. 2007;104(25):10447–10452. doi: 10.1073/pnas.0700046104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallar J, Pozo MA, Tuckett RP, Belmonte C. Response of sensory units with unmyelinated fibres to mechanical, thermal and chemical stimulation of the cat’s cornea. J Physiol. 1993;468:609–622. doi: 10.1113/jphysiol.1993.sp019791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gazerani P, Staahl C, Drewes AM, Arendt-Nielsen L. The effects of Botulinum Toxin type A on capsaicin-evoked pain, flare, and secondary hyperalgesia in an experimental human model of trigeminal sensitization. Pain. 2006;122(3):315–325. doi: 10.1016/j.pain.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Gebhart GF. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications IV. Visceral afferent contributions to the pathobiology of visceral pain. Am J Physiol Gastrointest Liver Physiol. 2000;278(6):G834–G838. doi: 10.1152/ajpgi.2000.278.6.G834. [DOI] [PubMed] [Google Scholar]
- 25.Grogan PM, Alvarez MV, Jones L. Headache direction and aura predict migraine responsiveness to rimabotulinumtoxin B. Headache. 2013;53(1):126–136. doi: 10.1111/j.1526-4610.2012.02288.x. [DOI] [PubMed] [Google Scholar]
- 26.Habermann E. 125I-labeled neurotoxin from Clostridium botulinum A: preparation, binding to synaptosomes and ascent to the spinal cord. Naunyn Schmiedebergs Arch Pharmacol. 1974;281(1):47–56. doi: 10.1007/BF00500611. [DOI] [PubMed] [Google Scholar]
- 27.Huang PP, Khan I, Suhail MS, Malkmus S, Yaksh TL. Spinal botulinum neurotoxin B: effects on afferent transmitter release and nociceptive processing. PLoS One. 2011;6(4):e19126. doi: 10.1371/journal.pone.0019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.KERR FW, OLAFSON RA. Trigeminal and cervical volleys. Convergence on single units in the spinal gray at C-1 and C-2. Arch Neurol. 1961;5:171–178. doi: 10.1001/archneur.1961.00450140053005. [DOI] [PubMed] [Google Scholar]
- 29.Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, Yaksh TL. Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J Neurosci. 2005;25(14):3651–3660. doi: 10.1523/JNEUROSCI.0252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kramer HH, Angerer C, Erbguth F, Schmelz M, Birklein F. Botulinum Toxin A reduces neurogenic flare but has almost no effect on pain and hyperalgesia in human skin. J Neurol. 2003;250(2):188–193. doi: 10.1007/s00415-003-0971-x. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence GW, Ovsepian SV, Wang J, Aoki KR, Dolly JO. Extravesicular intraneuronal migration of internalized botulinum neurotoxins without detectable inhibition of distal neurotransmission. Biochem J. 2012;441(1):443–452. doi: 10.1042/BJ20111117. [DOI] [PubMed] [Google Scholar]
- 32.Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130(1–2):166–176. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu HT, Tsai SK, Kao MC, Hu JS. Botulinum toxin A relieved neuropathic pain in a case of post-herpetic neuralgia. Pain Med. 2006;7(1):89–91. doi: 10.1111/j.1526-4637.2006.00100.x. [DOI] [PubMed] [Google Scholar]
- 34.Marchand-Pauvert V, Aymard C, Giboin LS, Dominici F, Rossi A, Mazzocchio R. Beyond muscular effects: depression of spinal recurrent inhibition after botulinum neurotoxin A. J Physiol. 2013;591(Pt 4):1017–1029. doi: 10.1113/jphysiol.2012.239178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marinelli S, Vacca V, Ricordy R, Uggenti C, Tata AM, Luvisetto S, Pavone F. The analgesic effect on neuropathic pain of retrogradely transported botulinum neurotoxin A involves Schwann cells and astrocytes. PLoS One. 2012;7(10):e47977. doi: 10.1371/journal.pone.0047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marino MJ, Terashima T, Steinauer JJ, Eddinger KA, Yaksh TL, Xu Q. Botulinum toxin B in the sensory afferent: Transmitter release, spinal activation, and pain behavior. Pain. 2014;155(4):674–684. doi: 10.1016/j.pain.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matak I, Bach-Rojecky L, Filipovic B, Lackovic Z. Behavioral and immunohistochemical evidence for central antinociceptive activity of botulinum toxin A. Neuroscience. 2011;186:201–207. doi: 10.1016/j.neuroscience.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 38.Meng J, Ovsepian SV, Wang J, Pickering M, Sasse A, Aoki KR, Lawrence GW, Dolly JO. Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J Neurosci. 2009;29(15):4981–4992. doi: 10.1523/JNEUROSCI.5490-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montecucco C, Schiavo G. Mechanism of action of tetanus and botulinum neurotoxins. Mol Microbiol. 1994;13(1):1–8. doi: 10.1111/j.1365-2958.1994.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 40.Morch CD, Hu JW, Arendt-Nielsen L, Sessle BJ. Convergence of cutaneous, musculoskeletal, dural and visceral afferents onto nociceptive neurons in the first cervical dorsal horn. Eur J Neurosci. 2007;26(1):142–154. doi: 10.1111/j.1460-9568.2007.05608.x. [DOI] [PubMed] [Google Scholar]
- 41.Nazarian A, Christianson CA, Hua XY, Yaksh TL. Dexmedetomidine and ST-91 analgesia in the formalin model is mediated by alpha2A-adrenoceptors: a mechanism of action distinct from morphine. Br J Pharmacol. 2008;155(7):1117–1126. doi: 10.1038/bjp.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noma N, Tsuboi Y, Kondo M, Matsumoto M, Sessle BJ, Kitagawa J, Saito K, Iwata K. Organization of pERK-immunoreactive cells in trigeminal spinal nucleus caudalis and upper cervical cord following capsaicin injection into oral and craniofacial regions in rats. J Comp Neurol. 2008;507(3):1428–1440. doi: 10.1002/cne.21620. [DOI] [PubMed] [Google Scholar]
- 43.Numazaki M, Tominaga M. Nociception and TRP Channels. Curr Drug Targets CNS Neurol Disord. 2004;3(6):479–485. doi: 10.2174/1568007043336789. [DOI] [PubMed] [Google Scholar]
- 44.Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. The Lancet Neurology. 2009;8(7):679–690. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- 45.Pingle SC, Matta JA, Ahern GP. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb Exp Pharmacol. 2007;(179):155–171. doi: 10.1007/978-3-540-34891-7_9. [DOI] [PubMed] [Google Scholar]
- 46.Piovesan EJ, Kowacs PA, Tatsui CE, Lange MC, Ribas LC, Werneck LC. Referred pain after painful stimulation of the greater occipital nerve in humans: evidence of convergence of cervical afferences on trigeminal nuclei. Cephalalgia. 2001;21(2):107–109. doi: 10.1046/j.1468-2982.2001.00166.x. [DOI] [PubMed] [Google Scholar]
- 47.Piovesan EJ, Teive HG, Kowacs PA, Della Coletta MV, Werneck LC, Silberstein SD. An open study of botulinum-A toxin treatment of trigeminal neuralgia. Neurology. 2005;65(8):1306–1308. doi: 10.1212/01.wnl.0000180940.98815.74. [DOI] [PubMed] [Google Scholar]
- 48.Ramachandran R, Yaksh TL. Therapeutic use of botulinum toxin in migraine: mechanisms of action. Br J Pharmacol. 2014;171(18):4177–4192. doi: 10.1111/bph.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ranoux D, Attal N, Morain F, Bouhassira D. Botulinum toxin type A induces direct analgesic effects in chronic neuropathic pain. Ann Neurol. 2008;64(3):274–283. doi: 10.1002/ana.21427. [DOI] [PubMed] [Google Scholar]
- 50.Ray BS, Wolff HG. Experimental studies on headache; pain-sensitive structures of the head and their significance in headache. Archives of surgery. 1940;41:813–856. [Google Scholar]
- 51.Restani L, Antonucci F, Gianfranceschi L, Rossi C, Rossetto O, Caleo M. Evidence for anterograde transport and transcytosis of botulinum neurotoxin A (BoNT/A) J Neurosci. 2011;31(44):15650–15659. doi: 10.1523/JNEUROSCI.2618-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Restani L, Giribaldi F, Manich M, Bercsenyi K, Menendez G, Rossetto O, Caleo M, Schiavo G. Botulinum neurotoxins A and E undergo retrograde axonal transport in primary motor neurons. PLoS Pathog. 2012;8(12):e1003087. doi: 10.1371/journal.ppat.1003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruiz HC, Bermejo P. Botulinum toxin type A in the treatment of neuropathic pain in a case of postherpetic neuralgia. Neurologia. 2008;23(4):259–262. [PubMed] [Google Scholar]
- 54.Samton J, Mauskop A. Treatment of headaches with botulinum toxin. Expert Rev Neurother. 2006;6(3):313–322. doi: 10.1586/14737175.6.3.313. [DOI] [PubMed] [Google Scholar]
- 55.Schueler M, Messlinger K, Dux M, Neuhuber WL, De CR. Extracranial projections of meningeal afferents and their impact on meningeal nociception and headache. Pain. 2013;154(9):1622–1631. doi: 10.1016/j.pain.2013.04.040. [DOI] [PubMed] [Google Scholar]
- 56.Sengupta JN. Visceral pain: the neurophysiological mechanism. Handb Exp Pharmacol. 2009;(194):31–74. doi: 10.1007/978-3-540-79090-7_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simpson L. The life history of a botulinum toxin molecule. Toxicon. 2013;68:40–59. doi: 10.1016/j.toxicon.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 58.Strassman AM, Mineta Y, Vos BP. Distribution of fos-like immunoreactivity in the medullary and upper cervical dorsal horn produced by stimulation of dural blood vessels in the rat. J Neurosci. 1994;14(6):3725–3735. doi: 10.1523/JNEUROSCI.14-06-03725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384(6609):560–564. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- 60.Strassman AM, Vos BP. Somatotopic and laminar organization of fos-like immunoreactivity in the medullary and upper cervical dorsal horn induced by noxious facial stimulation in the rat. J Comp Neurol. 1993;331(4):495–516. doi: 10.1002/cne.903310406. [DOI] [PubMed] [Google Scholar]
- 61.Tugnoli V, Capone JG, Eleopra R, Quatrale R, Sensi M, Gastaldo E, Tola MR, Geppetti P. Botulinum toxin type A reduces capsaicin-evoked pain and neurogenic vasodilatation in human skin. Pain. 2007;130(1–2):76–83. doi: 10.1016/j.pain.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 62.Voller B, Sycha T, Gustorff B, Schmetterer L, Lehr S, Eichler HG, Auff E, Schnider P. A randomized, double-blind, placebo controlled study on analgesic effects of botulinum toxin A. Neurology. 2003;61(7):940–944. doi: 10.1212/01.wnl.0000086374.92906.6a. [DOI] [PubMed] [Google Scholar]
- 63.Wiegand H, Erdmann G, Wellhoner HH. 125I-labelled botulinum A neurotoxin: pharmacokinetics in cats after intramuscular injection. Naunyn Schmiedebergs Arch Pharmacol. 1976;292(2):161–165. doi: 10.1007/BF00498587. [DOI] [PubMed] [Google Scholar]
- 64.Xiao L, Mackey S, Hui H, Xong D, Zhang Q, Zhang D. Subcutaneous injection of botulinum toxin a is beneficial in postherpetic neuralgia. Pain Med. 2010;11(12):1827–1833. doi: 10.1111/j.1526-4637.2010.01003.x. [DOI] [PubMed] [Google Scholar]
- 65.Yuan RY, Sheu JJ, Yu JM, Chen WT, Tseng IJ, Chang HH, Hu CJ. Botulinum toxin for diabetic neuropathic pain: a randomized double-blind crossover trial. Neurology. 2009;72(17):1473–1478. doi: 10.1212/01.wnl.0000345968.05959.cf. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Z, Wang D, Sun T, Xu J, Chiang HC, Shin W, Wu LG. The SNARE proteins SNAP25 and synaptobrevin are involved in endocytosis at hippocampal synapses. J Neurosci. 2013;33(21):9169–9175. doi: 10.1523/JNEUROSCI.0301-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure showing the SO and meningeal regions where capsaicin injections were performed (A). The skin isolated following methylene blue dye injections into the meninges through the skull showed no leakage of the dye outside the cranium (skin) (B). Note that methylene blue dye excites between 560 to 600 nm wavelengths. Another set of mice was used to produce a positive control for methylene blue dye leakage (red) tissue that was injected just below the skin (C). This was performed in order to compare leakage v/s non leakage tissue..