Abstract
CD4+ germinal center (GC) T follicular helper (GC-Tfh) cells help B cells become long-lived plasma cells and memory cells. The transcriptional repressor BCL6 plays a key role in GC-Tfh formation by inhibiting the expression of genes that promote differentiation into other lineages. We determined whether BCOR, a component of a Polycomb repressive complex that interacts with the BCL6 BTB domain, influences GC-Tfh differentiation. T cell-targeted BCOR deficiency led to a substantial loss of peptide:MHCII-specific GC-Tfh cells following Listeria monocytogenes infection and a 2-fold decrease following immunization with a peptide in CFA. The reduction in GC-Tfh cells was associated with diminished plasma cell and GC B cell formation. Thus, T cell-expressed BCOR is critical for optimal GC-Tfh differentiation and humoral immunity.
Introduction
Germinal center (GC) T follicular helper (GC-Tfh) cells help B cells become long-lived plasma cells and memory B cells (1). The transcriptional repressor BCL6 promotes GC-Tfh formation by repressing the expression of transcription factors such as T-bet, GATA3, and RORγt required for the differentiation other effector cell lineages (2, 3). Repression by BCL6 depends on two domains, a middle repression domain 2 (RD2) domain and an N-terminal BTB domain (4, 5), which interact with corepressors. The RD2 domain can recruit the Metastasis-associated 3 (MTA3) corepressor (6), while the BTB domain can bind BCL6-interacting corepressor (BCOR), nuclear receptor corepressor (NCOR), or nuclear receptor corepressor 2 (SMRT) (7). BCOR potentiates transcriptional repression by BCL6 as part of a variant Polycomb complex, which may make epigenetic modifications that silence target genes (8).
The role, however, that these corepressors play in transcriptional repression by BCL6 in T cells is unclear. Mutation of the BCL6 RD2 domain leads to partial reduction in GC-Tfh differentiation (9). In contrast, it has been reported that GC-Tfh cell formation following sheep red blood cell immunization is normal in mice with a mutated BCL6 BTB domain (10), suggesting that none of the BTB-interacting corepressors are involved in GC-Tfh differentiation. It remained possible, however, that a defect was not detected in this experiment because relevant peptide:MHCII (p:MHCII)-specific T cells were not monitored. Indeed, in the accompanying study (11), Crotty and colleagues found that the BCL6 BTB domain contributes to GC-Tfh formation by viral p:MHCII-specific CD4+ T cells during acute infection. Here, we evaluated BCOR for its role in GC-Tfh formation. We found that BCOR deficiency in T cells led to a defect in p:MHCII-specific GC-Tfh cell formation that correlated with reduced formation of plasma cells and GC B cells. Therefore, BCOR was required for optimal GC-Tfh formation by p:MHCII-specific CD4+ T cells, perhaps through its capacity to interact with the BCL6 BTB domain.
Materials and Methods
Mice
The conditional Bcor allele (Bcorfl), which contains LoxP sites flanking Bcor exons 9 and 10, was generated by homologous recombination (Wamstad et al, manuscript in preparation). Cre-mediated deletion results in a premature stop codon and a null allele. Bcorfl/+ mice were backcrossed with C57BL/6NCr mice (NCI Frederick) for >6 (Fig. 1) or >10 generations (Fig. 3–4). B6.Cg-Tg(Lck-cre)3779Nik/J (The Jackson Laboratory) males were bred to Bcorfl/+ females to generate wild-type (WT;Bcor+/Y or Bcorfl/Y Lck-Cre−) and T cell BCOR-deficient (Bcorfl/Y Lck-Cre+) males. C57BL/6 (B6 mice) (The Jackson Laboratory) used in Fig. 2 were housed in specific pathogen-free conditions while other mice were housed in a conventional facility at the University of Minnesota. All experimental protocols were performed in accordance with guidelines of the University of Minnesota Institutional Animal Care and Use Committee and National Institutes of Health.
FIGURE 1. A substantial defect in GC-Tfh differentiation occurs after Lm infection in BCOR-deficient CD4+ T cells. WT and Bcorfl/Y Lck-Cre+ mice were infected with Lm bacteria. After 7 days, LLOp:I-Ab-specific CD4+ T cells were enriched from spleen and LNs using LLOp:I-Ab tetramer.
(A) B220− CD11b− CD11c− CD4+ T cells from LLOp:I-Ab tetramer-enriched samples with gates on CD44+ LLOp:I-Ab tetramer+ cells.
(B) Numbers of LLOp:I-Ab-specific cells in WT and Bcorfl/Y Lck-Cre+ mice.
(C) Identification of LLOp:I-Ab-specific (from gate in (A)) Th1, Tfh, and GC-Tfh cells based on PD-1 and CXCR5 expression.
(D) Percentages and (E) numbers of LLOp:I-Ab-specific Th1, Tfh, or GC-Tfh cells in WT and Bcorfl/Y Lck-Cre+ mice.
Pooled data from two independent experiments are shown. ** p < 0.01, *** p < 0.001.
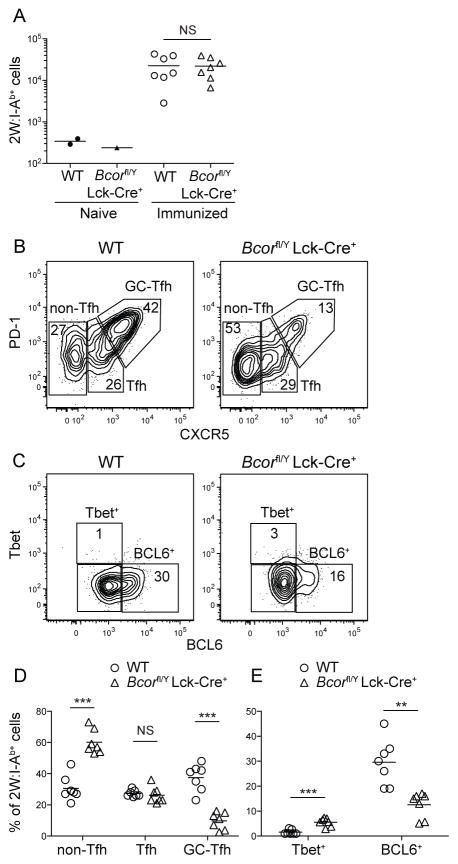
FIGURE 3. A partial defect in GC-Tfh differentiation occurs in BCOR-deficient CD4+ T cells after CFA immunization. 2W:I-Ab T cells were enriched from spleen and LNs of WT or Bcorfl/Y Lck-Cre+ mice using 2W:I-Ab tetramer 7 days after immunization with 2W-PE emulsified in CFA.
(A) Numbers of 2W:I-Ab CD4+ T cells in naive and immunized WT or Bcorfl/Y Lck-Cre+ mice.
(B) Identification of 2W:I-Ab-specific Th1, Tfh, and GC-Tfh cells based on PD-1 and CXCR5 expression.
(C) T-bet and BCL6 expression by 2W:I-Ab-specific CD4+ T cells.
(D) Percentages of 2W:I-Ab-specific non-Tfh, Tfh, or GC-Tfh cells in WT and Bcorfl/Y Lck-Cre+ mice.
(E) Percentages of 2W:I-Ab-specific T-bet+ or BCL6+ cells in WT and Bcorfl/Y Lck-Cre+ mice.
Pooled data from two independent experiments are shown. ** p < 0.01, *** p < 0.001.
FIGURE 4. Plasmablast and germinal center B cell formation is reduced in mice with BCOR-deficient T cells. PE-specific B cells were enriched from spleen and LNs of WT or Bcorfl/Y Lck-Cre+ mice 7 days after immunization with 2W-PE emulsified in CFA.
(A) CD90.2− CD11c− F4/80− Gr-1− PE-specific cells (PE B) (top panels) gated for B220int IgG [H+L]+ plasma cells (middle panels) or B220+ CD38+ GL7− naive/memory or CD38− GL7+ GC cells (bottom panels).
(B) Numbers of PE-specific B cells (top, left panel), plasma cells (top, right panel), GC B cells (bottom, left panel), and naive or memory B cells (bottom, right panel) in individual mice.
Results are from a single experiment. Similar results were obtained in a second experiment although the overall magnitude of the PE-specific B cell response was lower than in the first. * p < 0.05, ** p < 0.01.
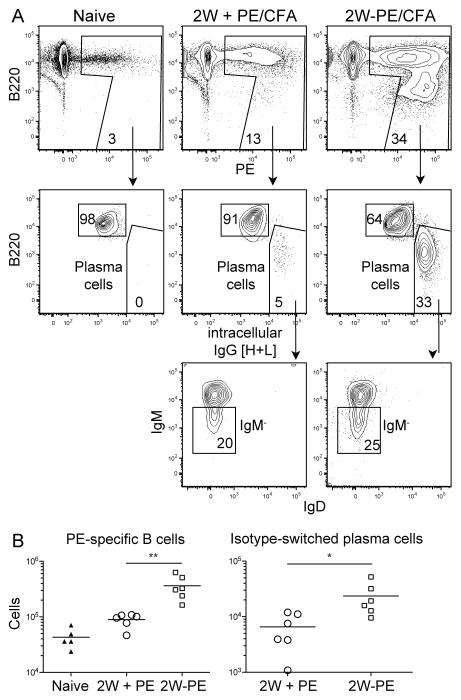
FIGURE 2. 2W:I-Ab-specific CD4+ T cells provide help for PE-specific B cells after 2W-PE/CFA immunization. PE-specific B cells were enriched from spleen and LNs of naive B6 mice or mice that were immunized for 7 days with 2W mixed with PE (unlinked) or 2W-PE emulsified in CFA.
(A) CD90.2− CD11c− F4/80− Gr-1− PE-specific cells (PE B) (top panels) gated for B220int IgG [H+L]+ plasma cells (middle panels) and B220int IgG [H+L]+ IgM− plasma cells (bottom panels).
(B) Numbers of PE-specific B cells (left panel) and IgM− plasma cells (right panel) in naive, PE/CFA immunized, or 2W-PE/CFA immunized mice.
Pooled data from two independent experiments are shown. ** p < 0.01, *** p < 0.001.
Infections and Immunizations
Mice were infected intravenously with 107 actA-deficient Listeria monocytogenes (Lm) bacteria expressing FliC peptide RFNSAITNLGN (12) or 2W peptide EAWGALANWAVDSA fused to chicken ovalbumin (13), or immunized by i.p. injection of 200 μl of 0.6 ug of 2W peptide conjugated to 25 μg of PE (2W-PE) emulsified in CFA (Sigma). The conjugate was formed by mixing biotinylated 2W peptide (Genscript) with streptavidin (SA)-PE (Prozyme) in a 4:1 ratio.
Cell Enrichment and Flow cytometry
Spleen and lymph node (LN) cells were divided for separate enrichments of CD4+ T cells and B cells. For p:MHCII-specific CD4+ T cell enrichment, cells were stained with CXCR5 (2G8; BD) and I-Ab tetramers containing listeriolysin O peptide NEKYAQAYPNVS (LLOp) or 2W peptide (14). Before PE-specific B cell enrichment, suspensions were incubated with dispase (Invitrogen), collagenase P (Roche), and DNase I (Roche) to release all B cell subsets. Enrichment was performed as previously described by mixing cell suspensions with 1 μg PE (Prozyme) (15). PE- or p:MHCII-bound cells were enriched using magnetic beads (Miltenyi) as previously described (14). Tetramer-enriched CD4+ T cells were stained with fluorochrome-labeled antibodies specific for B220 (RA3-6B2; all antibodies are from eBioscience unless otherwise indicated), CD11b (M1/70), CD11c (N418), CD44 (IM7), PD-1 (J43), CD90.2 (53-2.1), or CD4 (GK1.5; BD). For intracellular staining, cells were incubated in fixation/permeabilization buffer (eBioscience) and stained with fluorochrome-labeled anti-BCL6 (K112-91; BD) and anti-T-bet (4B10; Biolegend) antibodies. PE-enriched B cells were stained with fluorochrome-labeled antibodies against CD90.2 (53-2.1), CD11c (N418), F4/80 (BM8), and GR1 (RB6-8C5), CD38 (90), IgM (II/41), GL7 (GL-7), IgD (11-26c.2a; BD), and B220 (RA3-6B2; BD). Cells were fixed with 2% paraformaldehyde (Sigma) and stained with anti-IgG [H+L] (Life Technologies). Cells were analyzed on a Fortessa (Becton Dickinson) flow cytometer and analyzed with FlowJo (TreeStar).
Statistical analysis
Statistical tests were performed using Prism (Graphpad) software and p values were obtained using two-tailed unpaired t tests with a 95% confidence interval.
Results and Discussion
BCOR deficiency in T cells causes a defect in GC-Tfh differentiation
Given that BCOR, NCOR, and SMRT potentiate BCL6 repression (16–18), it was possible that one or several of these corepressors promotes Tfh formation. We assessed the role of BCOR using Bcorfl/Y Lck-Cre+ mice lacking BCOR specifically in CD4+ and CD8+ T cells. WT and Bcorfl/Y Lck-Cre+ mice were infected intravenously with an attentuated strain of Lm that expresses listeriolysin O. The recipient mice contained about 80 LLOp:I-Ab-specific CD44low naive CD4+ T cells before infection (13, 19). Lm infection of WT mice generated a large population of CD44high LLOp:I-Ab-specific CD4+ effector cells by day 7 (Fig. 1A–B). As shown in previous studies (13, 19–21), this population contained CXCR5− PD-1− Th1 cells, CXCR5low PD-1− Tfh cells, and CXCR5high PD-1+ GC-Tfh cells (Fig. 1C–E). BCOR-deficient LLOp:I-Ab-specific CD4+ T cells produced an even larger effector cell population than that generated by WT cells (Fig. 1B). The BCOR-deficient population had a higher fraction of Th1 cells, and a greater number of Th1 and Tfh cells (Fig. 1D–E) but a much lower fraction and number of GC-Tfh cells than the comparable WT population. Thus, BCOR expression in T cells is critical for GC-Tfh differentiation during Lm infection.
A caveat to these experiments was that BCOR-deficiency in CD8+ T cells, which play an important role in control of Lm bacteria (22), could have altered the infection in a way that indirectly affected GC-Tfh formation. We therefore examined the effects of BCOR deficiency on GC-Tfh differentiation during an immune response to a non-replicating Ag. WT and Bcorfl/Y Lck-Cre+ mice were immunized i.p. with a CFA emulsion containing PE or a 2W-PE conjugate. In the latter situation, PE-specific B cells that internalize 2W-PE through the BCR produce PE peptide:I-Ab and 2W:I-Ab complexes and could receive helper signals from either 2W:I-Ab- or PE peptide:I-Ab-specific T cells. PE-specific B cells underwent significantly more clonal expansion 7 days after immunization with 2W-PE/CFA than with PE/CFA (Fig. 2A–B). Thus, 2W:I-Ab-specific T cells were the main helpers of PE-specific B cells during the first week of the response when 2W-PE was the immunogen.
The effects of T cell-targeted BCOR deficiency on 2W:I-Ab-specific CD4+ T cells were then examined. Unlike after Lm infection, the total numbers of 2W:I-Ab-specific effector cells generated by 2W-PE immunization on day 7 were similar in WT and Bcorfl/Y Lck-Cre+ mice indicating that BCOR is not a regulator of T cell clonal expansion driven by a non-replicating Ag (Fig. 3A). BCOR-deficient 2W:I-Ab-specific effector cells, however, exhibited increased differentiation of CXCR5− non-Tfh cells and decreased differentiation of GC-Tfh cells relative to WT cells (Fig. 3B, D). Cells were also stained for intracellular T cell lineage-defining transcription factors to confirm a GC-Tfh defect. As shown in Fig. 3C, about 1% of the 2W:I-Ab-specific WT effector cells were T-bet+ BCL6− Th1 cells, while 30% were T-bet− BCL6+ GC-Tfh cells indicating that i.p. peptide priming in CFA is a poor Th1 and good Tfh stimulus on day 7 (Fig. 3B–E). The BCOR-deficient 2W:I-Ab-specific effector cell population contained about 3-fold more T-bet+ BCL6− Th1 cells and 2-fold less T-bet− BCL6+ GC-Tfh cells than the comparable population in WT mice. Therefore, BCOR was required for maximal GC-Tfh differentiation in response to a peptide Ag. The fact that BCOR was not essential indicates that some aspect of CFA priming, perhaps prolonged Ag presentation (23, 24), promotes BCOR-independent GC-Tfh formation.
A reduction in GC-Tfh cells due to BCOR deficiency correlates with defects in B cell activation
GC-Tfh cells help GC B cells become long-lived plasma cells and memory cells (1). We therefore assessed B cell activation in Bcorfl/Y Lck-Cre+ mice to see if the defect in GC-Tfh formation was associated with defects in B cell activation. As shown in Fig. 4A and B, PE-specific B cells in WT mice increased from about 50,000 cells on the day of immunization to about 400,000 cells on day 7 after injection of 2W-PE/CFA. The PE-specific B cell population consisted of plasma cells expressing large amounts of intracellular Ig heavy and light chains (IgG [H+L]) (Fig. 4A), B220high CD38+ GL7− naïve or memory B cells, and CD38− GL7+ GC B cells (Fig. 4A). PE-specific B cells in Bcorfl/Y Lck-Cre+ mice increased much less than WT cells after immunization and produced significantly fewer plasma cells and GC cells (Fig. 4B).
Our data show that BCOR deficiency hinders the differentiation of p:MHCII-specific GC-Tfh cells, which in turn reduces the formation of plasma cells and GC B cells. The recent report by Nance et al. (11) showing that the BCL6 BTB domain contributes to optimal GC-Tfh formation raises the likely possibility that the effects of BCOR on GC-Tfh differentiation depend on its interaction with the BCL6 BTB domain. It should be noted, however, that Huang et al. (10) found that GC-Tfh differentiation was induced normally by sheep red blood cells in mice with a mutated BCL6 BTB domain incapable of binding BCOR. It is therefore also possible that BCOR fosters GC-Tfh cell formation by a mechanism that depends on BCOR but not the BCL6 BTB domain. Such a mechanism could involve BCOR interaction with other known (25–29) or yet to be identified BCOR-binding transcription factors. Additional studies will be required to identify the molecular interactions that govern BCOR-dependent control of GC-Tfh formation during different immune responses.
Acknowledgments
We thank J. Walter and A. Quade for technical assistance and all members of the Jenkins Lab for helpful discussions.
This work was supported by National Institutes of Health Grants R01 AI039614, R01 AI103760, R37 AI027998 (MKJ), and R01 CA071540 (VJB), funds from the Minnesota Masonic Charities, and the University of Minnesota Office of the Vice President for Research.
Abbreviations
- B6
C57BL/6
- 2W-PE
2W peptide conjugated to phycoerythrin (PE)
- p:MHCII
peptide:MHC class II
- Lm
Listeria monocytogenes
- LN
lymph nodes
- LLOp
listeriolysin O peptide NEKYAQAYPNVS
References
- 1.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 2.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, Ellyard JI, Parish IA, Ma CS, Li QJ, Parish CR, Mackay CR, Vinuesa CG. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Chang CC, Ye BH, Chaganti RS, Dalla-Favera R. BCL-6, a POZ/zinc-finger protein, is a sequence-specific transcriptional repressor. Proc Natl Acad Sci U S A. 1996;93:6947–6952. doi: 10.1073/pnas.93.14.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seyfert VL, Allman D, He Y, Staudt LM. Transcriptional repression by the proto-oncogene BCL-6. Oncogene. 1996;12:2331–2342. [PubMed] [Google Scholar]
- 6.Fujita N, Jaye DL, Geigerman C, Akyildiz A, Mooney MR, Boss JM, Wade PA. MTA3 and the Mi-2/NuRD complex regulate cell fate during B lymphocyte differentiation. Cell. 2004;119:75–86. doi: 10.1016/j.cell.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Basso K, Dalla-Favera R. Roles of BCL6 in normal and transformed germinal center B cells. Immunol Rev. 2012;247:172–183. doi: 10.1111/j.1600-065X.2012.01112.x. [DOI] [PubMed] [Google Scholar]
- 8.Gearhart MD, Corcoran CM, Wamstad JA, Bardwell VJ. Polycomb group and SCF ubiquitin ligases are found in a novel BCOR complex that is recruited to BCL6 targets. Mol Cell Biol. 2006;26:6880–6889. doi: 10.1128/MCB.00630-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Gonzalez DG, Cote CM, Jiang Y, Hatzi K, Teater M, Dai K, Hla T, Haberman AM, Melnick A. The BCL6 RD2 domain governs commitment of activated B cells to form germinal centers. Cell Rep. 2014;8:1497–1508. doi: 10.1016/j.celrep.2014.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Hatzi K, Melnick A. Lineage-specific functions of Bcl-6 in immunity and inflammation are mediated by distinct biochemical mechanisms. Nat Immunol. 2013;14:380–388. doi: 10.1038/ni.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nance JP, Bélanger S, Johnston RJ, Takemori T, Crotty S. T follicular helper cell (TFH) differentiation is defective in the absence of Bcl6 BTB repressor domain function. J Immunol. 2015 doi: 10.4049/jimmunol.1500200. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johanns TM, Ertelt JM, Lai JC, Rowe JH, Avant RA, Way SS. Naturally occurring altered peptide ligands control Salmonella-specific CD4+ T cell proliferation, IFN-gamma production, and protective potency. J Immunol. 2010;184:869–876. doi: 10.4049/jimmunol.0901804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tubo NJ, Pagan AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, Huseby ES, Way SS, Jenkins MK. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell. 2013;153:785–796. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad KF, Melnick A, Lax S, Bouchard D, Liu J, Kiang CL, Mayer S, Takahashi S, Licht JD, Prive GG. Mechanism of SMRT corepressor recruitment by the BCL6 BTB domain. Mol Cell. 2003;12:1551–1564. doi: 10.1016/s1097-2765(03)00454-4. [DOI] [PubMed] [Google Scholar]
- 17.Ghetu AF, Corcoran CM, Cerchietti L, Bardwell VJ, Melnick A, Prive GG. Structure of a BCOR corepressor peptide in complex with the BCL6 BTB domain dimer. Mol Cell. 2008;29:384–391. doi: 10.1016/j.molcel.2007.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatzi K, Jiang Y, Huang C, Garrett-Bakelman F, Gearhart MD, Giannopoulou EG, Zumbo P, Kirouac K, Bhaskara S, Polo JM, Kormaksson M, MacKerell AD, Jr, Xue F, Mason CE, Hiebert SW, Prive GG, Cerchietti L, Bardwell VJ, Elemento O, Melnick A. A hybrid mechanism of action for BCL6 in B cells defined by formation of functionally distinct complexes at enhancers and promoters. Cell Rep. 2013;4:578–588. doi: 10.1016/j.celrep.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35:583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shulman Z, Gitlin AD, Targ S, Jankovic M, Pasqual G, Nussenzweig MC, Victora GD. T follicular helper cell dynamics in germinal centers. Science. 2013;341:673–677. doi: 10.1126/science.1241680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 23.Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, Sallusto F. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38:596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, Tangye SG. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33:241–253. doi: 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi WI, Jeon BN, Yoon JH, Koh DI, Kim MH, Yu MY, Lee KM, Kim Y, Kim K, Hur SS, Lee CE, Kim KS, Hur MW. The proto-oncoprotein FBI-1 interacts with MBD3 to recruit the Mi-2/NuRD-HDAC complex and BCoR and to silence p21WAF/CDKN1A by DNA methylation. Nucleic Acids Res. 2013;41:6403–6420. doi: 10.1093/nar/gkt359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon BN, Choi WI, Yu MY, Yoon AR, Kim MH, Yun CO, Hur MW. ZBTB2, a novel master regulator of the p53 pathway. J Biol Chem. 2009;284:17935–17946. doi: 10.1074/jbc.M809559200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeon BN, Kim MK, Choi WI, Koh DI, Hong SY, Kim KS, Kim M, Yun CO, Yoon J, Choi KY, Lee KR, Nephew KP, Hur MW. KR-POK interacts with p53 and represses its ability to activate transcription of p21WAF1/CDKN1A. Cancer Res. 2012;72:1137–1148. doi: 10.1158/0008-5472.CAN-11-2433. [DOI] [PubMed] [Google Scholar]
- 28.Koh DI, Choi WI, Jeon BN, Lee CE, Yun CO, Hur MW. A novel POK family transcription factor, ZBTB5, represses transcription of p21CIP1 gene. J Biol Chem. 2009;284:19856–19866. doi: 10.1074/jbc.M109.025817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korutla L, Wang P, Jackson TG, Mackler SA. NAC1, a POZ/BTB protein that functions as a corepressor. Neurochem Int. 2009;54:245–252. doi: 10.1016/j.neuint.2008.12.008. [DOI] [PubMed] [Google Scholar]