Abstract
The development of effective cancer vaccines remains an urgent, but as yet unmet, clinical need. This deficiency is in part due to an incomplete understanding of how to best invoke dendritic cells (DC) that are crucial for the induction of tumor-specific CD8+T cells capable of mediating durable protective immunity. In this regard, elevated expression of the transcription factor X-box-binding protein (XBP1) in DC appears to play a decisive role in promoting the ability of DC to cross-present antigens (Ag) to CD8+T cells in the therapeutic setting. Delivery of DNA vaccines encoding XBP1 and tumor Ag to skin DC resulted in increased IFN-α production by plasmacytoid DC (pDC) from skin/tumor draining lymph nodes (dLN) and the cross-priming of Ag-specific CD8+T cell responses associated with therapeutic benefit. Anti-tumor protection was dependent on cross-presenting Batf3+DC, pDC and CD8+T cells. CD103+DC from the skin/tumor dLN of the immunized mice appeared responsible for activation of Ag-specific naïve CD8+T cells, but were dependent on pDC for optimal effectiveness. Similarly, human XBP1 improved the capacity of human blood- and skin-derived DC to activate human T cells. These data support an important intrinsic role for XBP1 in DC for effective cross-priming and orchestration of Batf3+DC–pDC interactions, thereby enabling effective vaccine induction of protective anti-tumor immunity.
Keywords: XBP1, DC, Cancer Vaccines, Cross-priming, CD8+T cells
Introduction
Immunotherapies utilizing vaccines, antibodies, and T cells have the potential to (re)activate and optimize the body’s immune system to fight off cancer (1). Although vaccines are capable of eliciting robust, durable and protective tumor Ag-specific CD8+T effectors to limit tumor progression or disease recurrence, such approaches have typically resulted in only modest clinical efficacy to date (1–2). The limited efficacy may relate to the inability of current vaccine formulations to optimally invoke DC sub-populations in vivo, leading to inefficient induction (via DC-mediated cross-priming) and maintenance of tumor Ag-specific CD8+T cell responses (2–4).
Although drugs (e.g., chloroquine) that block endosomal and phagosomal acidification and the targeting of DC-specific receptors (e.g., DEC205, DNGR-1) for directed Ag uptake can improve the efficiency of DC-mediated cross-presentation, it has proven difficult to translate such findings into effective cancer vaccine formulations (4). An alternate strategy would be to (epigenetically) accentuate the ability of DC to mediate productive Ag-specific cross-priming via the use of DNA-based vaccines that represent an off-the-shelf, easily scalable treatment platform (5–7). Although several DNA vaccines have been licensed for veterinary use, current DNA vaccines have displayed only limited efficacy in humans (7), which may relate to their low efficiency in transfecting rare-event DC within vaccine sites in vivo. Furthermore, amongst all DC subsets, cross-presenting DC sub-populations (e.g., Batf3-dependent CD8α+ and CD103+ DC: Batf3+ DC) are preferred targets for cancer vaccine Ag uptake in both humans and mice (8–11). In addition, optimal DC-mediated cross-priming of CD8+T cells requires Type-1 IFN (12–14). Hence, an ideal vaccine would optimize the collaborative interaction of cross-presenting DC and Type-1 IFN-producing pDC in order to elicit and sustain robust tumor-specific CD8+T cell-mediated protective immunity.
The transcription factor XBP1 appears unique in its intrinsic ability to promote the differentiation, survival and function of DC subsets, including pDC and CD8α+DC (15–16). XBP1 synergizes with toll-like receptor (TLR) agonists to increase Type-1 IFN production and other inflammatory cytokines from various cells such as DC (17–20), and plays a critical role in the ability of humans to respond to vaccination against the influenza virus (21). Our data indicate ectopic delivery of XBP1 cDNA in a DNA-based vaccine formulation improves the ability of endogenous Batf3+DC and pDC to collaboratively orchestrate the cross-priming of therapeutic anti-tumor CD8+T cells in multiple clinically-relevant murine tumor models. These results support the prospective development of similar genetic vaccine approaches for the treatment of patients with cancer.
Materials and Methods
Mice and cell lines
C57BL/6 (B6)-wild type (WT), -Batf3−/−, -TLR3−/− and -Rag2/OT-I, BALB/c-WT and -Batf3−/− mice [female (f), 6–8 weeks (wks)] were purchased from JAX (Bar Harbor, ME) or Taconic (Rensselaer, NY). B6/129S-Batf3−/− mice were obtained through B6 mice backcrossed with 129S-Batf3−/− mice (8) for 5 generations. The inducible BrafV600E/Pten-driven melanoma model (22) was kindly provided by Dr. M. Bosenberg (Yale University). All mice were housed and bred in specific pathogen-free conditions in the University of Pittsburgh animal facility. All animal procedures were performed according to IACUC-approved protocols and in accordance with recommendations for the proper use and care of laboratory animals. Murine melanoma B16 (ATCC, Manassas, VA) and glioma GL26 cells were maintained in DMEM (IRVINE Scientific, Santa Ana, CA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT), 2 mmol/l glutamine (Invitrogen, Carlsbad, CA) and 1xantibiotic/antimycotic solution (Sigma, St Louis, MO), and murine breast carcinoma 4T1.2-Neu cells were cultured in the aforementioned medium including G-418 (500 μg/ml) (Invitrogen) (23–24).
Plasmids
DNA encoding murine self/tumor Ag tyrosinase-related protein 2 (TRP2), rat oncoantigen Neu extracellular domain (NeuED) or human MAGEA3 was fused to human heat shock protein 70 (hsp70) (TRP2hsp70, NeuEDhsp70 or MAGEA3hsp70) were described previously (25–27). DNA encoding XBP1 driven by DC-specific CD11c promoter and TRP2hsp70 or NeuEDhsp70 driven by constitutive mammalian CMV promoter (XBP1/TRP2hsp70 or XBP1/NeuEDhsp70) were recently reported (23). DNA encoding human XBP1 and dominant-negative mouse XBP1 (dnXBP1) (15) were kindly provided by Dr. Glimcher (Weill Cornell Medical College). Human XBP1 shRNA (sc-38627-SH) and control shRNA DNA were purchased from Santa Cruz Biotech (Paso Robles, CA). All DNA were prepared using the endotoxin-free DNA purification kit (Qiagen).
DC generation and transfection
Murine bone marrow-derived DC
Bone marrow cells (2–3×106/ml) from naive BALB/c or B6 mice (f, 6–8 wks) were cultured in DC culture medium [RPMI1640 (IRVINE Scientific) supplemented with 10% FBS, 2 mmol/l glutamine, 1xantibiotic/antimycotic solution, recombinant mouse granulocyte-macrophage colony stimulating factor (GM-CSF) (1,000 U/ml) and IL-4 (1,000 U/ml) (PeproTech)] (23). Day 5–6, DC were purified using anti-mouse CD11c microbeads (Miltenyi Biotec, Auburn, CA). Purified DC (2–3x106) were untreated or transfected with 7 μg endotoxin-free DNA using Amaxa mouse DC Nucleofector kit (Lonza, Cologne, Germany) following the vendor’s instruction, and continually cultured in 1 ml DC culture medium for 2–3 days before analysis or immunization (24).
Human monocyte-derived DC (moDC)
Immature human moDC were generated from peripheral blood mononuclear cells (PBMC) obtained from adult healthy donors with written consent under an IRB-approved protocol in DC culture medium [AIM V medium (invitrogen) supplemented with rhuIL-4 (20ng/ml) (PeproTech) and clinical-grade rhuGM-CSF (Leukine®) (1,000 U/ml) (Bayer)] (24). Day 5–6, moDC (3x106) were untreated or transfected with 7μg endotoxin-free DNA using Amaxa Human DC Nucleofector kit (Lonza) following the vendor’s instruction, and continually cultured in 1 ml DC culture medium for 2 days before use.
Human skin-derived DC
Human skin from surgical discard was obtained under an IRB-approved protocol. Human skin epidermal/dermal explants were prepared from abdominal skins with a skin graft knife, and subsequently untreated or immunized by 0.6μm gold particles (BioRad) conjugated with DNA using a gene gun (GG) under sterile conditions (24). After vaccination, skins were cultured on a sterile steel mesh with the epidermal side up in AIM V medium supplemented with 1xantibiotic/antimycotic solution at 37°C in 5% CO2. Three days later, skin migratory DC were harvested from the culture medium and were stained with anti-HLA-DR-alexa flour 488, -CD14-brilliant violet 570, -CD11c-percep-cy5.5, and -CD141-APC or isotype (ISO) control antibodies (Ab) (BD Biosciences, eBioscience, Biolegend), then analyzed by flow cytometry using a BD LSRII (BD Biosciences). CD14+DC were isolated from harvested skin DC using The EasySep™ Human CD14 Positive Selection Kit (Stem Cell Technologies) per the vendor’s instruction.
Western blotting
Untreated and DNA-transfected DC were harvested, washed, and lysed using CelLytic M Cell Lysis Reagent (Sigma). Sec22b protein from equivalent numbers of DC (1.25x105) was collected and analyzed by standard western blot using mouse anti-Sec22b Ab (29-F7, Santa Cruz Biotec) as a probe. Mouse β-actin or GADPH detected by anti-mouse β-actin or GADPH Ab (Sigma) served as internal-loading controls. Immunoreactive protein bands were visualized using appropriate HRP-linked secondary Ab and SuperSignal West Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL) as described previously (23).
Analyses of DC subsets in skin/tumor dLN
Skin/tumor dLN CD8α+DC and CD103+DC function
B6 mice (f, 6–8 wks, 10 per group) were injected subcutaneously (s.c.) in the left flank with GL26 (1x106) tumor cells on day 0. On day 8, tumor-bearing mice were left untreated or they were vaccinated using a GG with XBP1/TRP2hsp70 DNA as previously described (23). After 3 days, single-cell suspensions of pooled skin/tumor dLN were incubated with anti-CD16/32 (BD Biosciences) on ice for 15 minutes before being stained using anti-CD11c-APC, -CD8α-pacific blue, -CD205-percep-cy5.5, -CD103-PE, and CD11b-FITC Ab (BD Biosciences, eBioscience, Biolegend). CD8α+DC (CD11chiCD8α+CD205+) and CD103+DC (CD11chiCD8α−CD103+CD11blo/−) were then sorted using a BD FACSAria High Speed Cell Sorter (BD Biosciences). Sorted CD8α+DC or CD103+DC (2x104) were co-cultured with TRP2-specific CD8+T cells (1x105, provided kindly by Dr. Hurwitz at NCI) isolated from murine TRP2180–188 TCR transgenic (Tg) B6 mice (28) in 200 μl RPMI1640 containing 10% (v/v) FBS for 3 days. IFN-γ in the culture supernatants was measured by ELISA (BD Biosciences).
IFN-α production by pDC from skin/tumor dLN
BALB/c mice (f, 6–8 wks, 5 per group) were s.c. injected with 4T1.2-Neu (2x104) tumor cells on day 0. Eight days later, tumor-bearing mice were left untreated or they were vaccinated using a GG with XBP1 or Aghsp70 (NeuEDhsp70 or TRP2hsp70 as GG-mediated vaccination controls) DNA. After 3 days, pDC were isolated from pooled skin/tumor dLN using a mouse pDC isolation kit (Miltenyi). Isolated pDC (2x104) were cultured in 200 μl RPMI1640 supplemented with 10% (v/v) FBS for 24 hours without stimulation. IFN-α in the culture supernatants was measured by ELISA (eBioscience).
Ag cross-presentation
Untreated or DNA-transfected DC (2x105) in 200 μl DC culture medium were pulsed with endoGrade® OVA proteins (Hyglos GmbH, Germany) (100μg/ml). After an overnight incubation, DC (2x104) were gently washed with PBS and then co-cultured with freshly-isolated OT-I (1x105) T cells from B6-Rag2/OT-I mice in a total volume of 200 μl RPMI1640 supplemented with 10% (v/v) FBS for 3 days. IFN-γ in the culture supernatants was determined by ELISA.
T cell activation
Naïve mouse T cell activation
Untreated or XBP1/TRP2hsp70 or control DNA-transfected DC (6×104) were co-cultured with syngeneic CD8+T cells (3×105) isolated from splenocytes of naïve B6 mice using anti-mouse CD8 microbeads (Miltenyi Biotec) in 200 μl RPMI 1640 containing 10% (v/v) FBS for 3 days. 3H-thymidine (1 μCi/well; Du Pont/New England Nuclear, Boston, MA) was added during the last 16–18 hours of culture. 3H-thymidine incorporation was then measured using a scintillation counter (Packard, Meriden, CT) (23). In another set of experiments, untreated or XBP1/NeuEDhsp70 or control DNA-transfected DC (2x104) were co-cultured with syngeneic naïve splenic T cells (1x105) purified from naïve BALB/c mice using a mouse T cell isolation kit (R&D Systems) in 200 μl RPMI1640 media containing 10% (v/v) FBS for 5 days. IFN-γ in the culture supernatants were determined by ELISA.
Human T cell activation
Untreated or DNA-transfected moDC (2x104) were co-cultured with autologous human T cells (1x105) isolated from peripherl blood using a human T cell isolation kit (Miltenyi) in a total volume of 200 μl human T cell culture medium [IMDM supplemented with L-glutamine, penicillin, streptomycin, and nonessential amino acids (Invitrogen) and 10% (v/v) human AB serum (Cellgro)] (24). On day 6 of DC-T cell co-culture, T cells were then re-stimulated with MAGEA3hsp70-transfected autogolous moDC (2x104) for 6 days. Skin total DC, CD14+DC or CD14−DC subsets (1x105) were co-cultured with allogeneic human T cells (5x105) for 5 days. In all cases, human IFN-γ in the culture supernatants was determined by ELISA (BD Biosciences).
Therapeutic melanoma (TRP2)-specific CD8+T cell responses
BrafV600E/Pten-driven melanoma was developed by inducing oncogene BrafV600E expression with 4-hydroxytamoxifen (4-HT) (H6278, Sigma) in B6-Tyr-CreERT2BrafCAPtenlox/lox mice with correct genotype (presence of Tyr-CreERT2, BrafCA, and homozygous Ptenlox/lox) (22). BrafV600E/Pten-driven melanoma (~3mm)-bearing mice (2–3/group) were left untreated or they were vaccinated using a GG with XBP1/TRP2hsp70 DNA on days 0, 7 and 14 as described previously (23). On day 60, CD8+T cells were purified from splenocytes and skin/tumor dLN using anti-mouse CD8 microbeads. Purified CD8+T cells (2×105) were co-cultured with syngeneic DC (4×104) transfected with TRP2hsp70 DNA or pulsed by BrafV600E/Pten-driven melanoma lysates (irrelevant NeuEDhsp70 DNA-transfected and 4T1.2-Neu lysate-pulsed DC as Ag- or -tumor-specific controls) in 200 μl RPMI 1640 supplemented with 10% (v/v) FBS for 3 days. IFN-γ in the culture supernatants was determined by ELISA.
Vaccine models using transplantable tumors
DC vaccination
BALB/c or B6 mice (f, 6–8 wks, 3 per group) were inoculated s.c. with exponentially growing 4T1.2-Neu (2x104) in the 4th mammary fat pad; or GL26 (1x106) in the right flank on day 0 (24). On day 8, tumor-bearing mice were randomized into cohorts and left untreated or treated by intraperitoneal (i.p.) immunization with DC (2.5x105) in 100 μl of endotoxic-free 1xPBS (Sigma).
DNA vaccination
B6-WT, -Batf3−/−, TLR3−/− mice or B6/129S-Batf3−/− mice (f, 6–8 wks, 3–5 per group) were left untreated or they were vaccinated using a GG with XBP1/TRP2hsp70 DNA on days 1, 7 and 14. Day 21, mice were inoculated s.c. with exponentially growing B16 (5x104) melanoma cells. B6-WT or -Batf3−/− mice (f, 6–8 wks, 5 per group) were inoculated s.c. with GL26 (1x106) on day 0. GL26-bearing mice were the randomized into cohorts and left untreated or they were vaccinated using a GG with XBP1/TRP2hsp70 DNA on days 9 and 14. BALB/c-WT or -Batf3−/− mice (f, 6–8 wks, 3–5 per group) were inoculated s.c. with 4T1.2-Neu (2x104) on day 0. When primary 4T1.2-Neu reached ~3mm, tumor-bearing mice were randomized and left untreated or they were vaccinated using a GG with XBP1/NeuEDhsp70 or control DNA 1–3 times (as indicated in figure legends). In some experiments, pDC were depleted by i.p. injection of 200 μg anti-mouse pDC Ab (120G8; BioX Cell, West Lebanon, NH) 1 day before, on the day of, and 1 day after vaccination.
Treatment of genetically engineered BrafV600E/Pten-driven melanoma mouse model of human disease
4-HT induced melanomas in B6-Tyr-CreERT2BrafCAPtenlox/lox mice (22) were allowed to progress to a mean tumor size of ~3mm, at which time the melanoma-bearing mice were randomized into cohorts of 3–4 mice exhibiting a comparable mean tumor size. Mice were then left untreated or they were vaccinated using a GG with XBP1/TRP2hsp70 DNA on days 0, 7 and 14. To deplete CD8 T cells, anti-mouse CD8 mAb (53–6.7) (200 μg/injection) was injected i.p. 1 day before, on the day of, 1 and 3 day after first vaccination, and the once a week thereafter. Specific T cell depletion (>95%) was verified by flow cytometry monitoring of peripheral blood lymphocytes isolated by tail venipuncture.
Tumor and animal survival monitoring
Primary tumor area was determined every 3 days as the product of orthogonal measurements determined using a digital slide calipers (Fisher Scientific, Pittsburgh, PA). Mice were euthanized when tumor exceeded 10 mm in mean diameter. On day 30 after 4T1.2-Neu inoculation, mice were euthanized and harvested lungs were fixed with Bouin’s solution (Sigma) for enumeration of 4T1.2-Neu metastatic foci. Bone marrow was also isolated from these animals for quantitation of site-specific metastases (26).
Statistical analysis
Data were analyzed for statistical significance using a Student’s t-test (immune assays, tumor size and foci) (Graph Pad Prism version 6) or the Log rank test (Graph Pad Prism version 6) as indicated in text. Animal survival is reported in Kaplan-Meier Survival (i.e. time-to-euthanasia) Curves. P<0.05 is considered to be statistically significant. * P<0.05; ** P<0.01; *** P<0.001.
Results
XBP1 potentiates the therapeutic anti-tumor efficacy of ex vivo generated DC vaccines
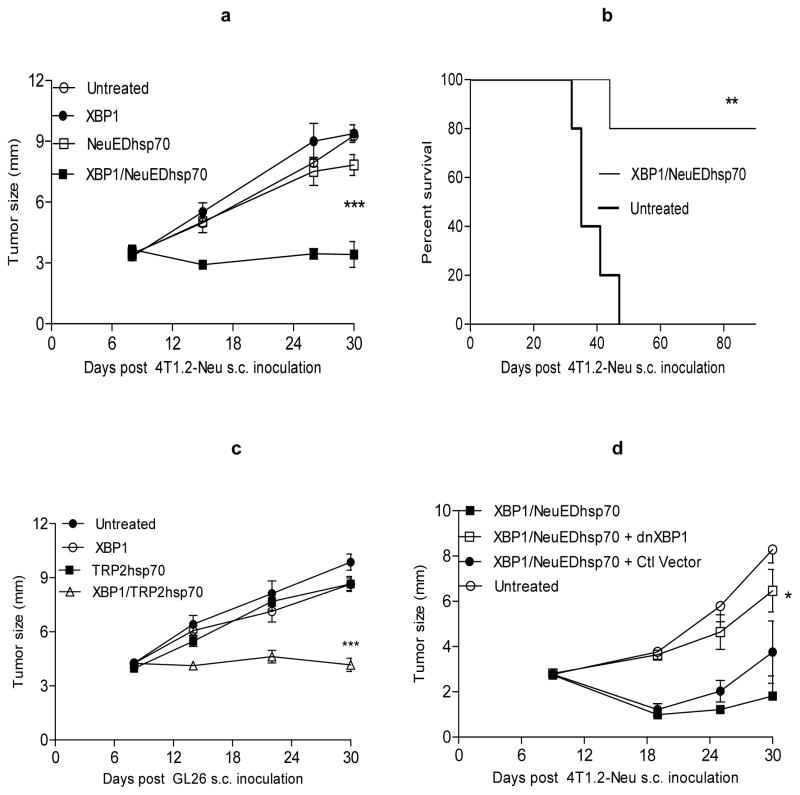
Vaccines implementing ex vivo generated autologous/syngeneic DC have been extensively evaluated, but have thus far resulted in only modest clinical efficacy in the cancer setting (1–2). Similarly, we observed that even DC genetically engineered to express tumor Ag (e.g., NeuED or TRP2) fused to hsp70 (to activate and promote enhanced cross-priming of T cells by DC; ref. 29) did not elicit therapeutic immunity against clinically-relevant 4T1.2-Neu breast carcinomas or GL26 (which intrinsically express TRP2) in vivo (Fig. 1a–c; Table I). To improve the immune-stimulatory capacity of DC-based vaccines, we have recently developed DC genetically modified to overexpress the transcriptional activator XBP1 in addition to Aghsp70 (XBP1/Aghsp70 DC) and showed that these DC display enhanced survival and an augmented T cell stimulatory phenotype (e.g., increased expression/production of CD86, IL-15Rα, IL-6, TNF-α; ref. 23). We now show that XBP1/Aghsp70 DC readily activates Ag-specific naïve CD8+T cells in vitro (Fig. S1a–b), and more importantly, when applied as a single immunization, small numbers (2.5x105) of these cells rapidly and efficiently elicit durable therapeutic immunity against established 4T1.2-Neu breast carcinomas or GL26 gliomas (Fig. 1a–c). Using a dominant-negative form of XBP1 (dnXBP1) which inhibits XBP1 activity but does not affect cell viability and growth (15), we further showed that antagonism of XBP1 function in XBP1/Aghsp70 DC abrogates their therapeutic efficacy in vivo (Fig. 1d).
Figure 1. XBP1 plays a decisive role in improving therapeutic DC vaccine potency.
a–c) XBP1 improves therapeutic DC vaccine efficacy. DC were untreated or transfected with XBP1/NeuEDhsp70, XBP1/TRP2hsp70 or control DNA. BALB/c or B6 mice were inoculated with 4T1.2-Neu (a–b) or GL26 (c) on day 0. On day 8, tumor-bearing mice were randomized into cohorts and left untreated or they were vaccinated once with the indicated DC populations.
d) Inhibition of XBP1 activity reduces XBP1-potentiated therapeutic DC vaccine efficacy. DC were transfected with XBP1/NeuEDhsp70 in the absence or presence of dnXBP1 or control (Ctl) vector. Therapeutic vaccination in 4T1.2-Neu-bearing mice was then performed as in (a).
Tumor growth was then monitored, reported and statistically-analyzed as outlined in Materials and Methods. Data from two independent experiments are shown.
Table I.
XBP1 DC-targeting DNA vaccine reduces tumor spontaneous bone marrow metastases
| DNA vaccines | Mice with bone marrow metastases/Mice used in experiments |
|---|---|
| XBP1 | 10/15 |
| NeuEDhsp70 | 8/15 |
| XBP1/NeuEDhsp70 | 1/15 |
| Untreated | 6/10 |
XBP1-based ex vivo generated DC and in vivo DC-targeting DNA vaccines elicit therapeutic anti-tumor immunity against spontaneous lung and bone marrow metastases
Activated onco-antigen rat Neu-expressing 4T1.2-Neu tumor cells efficiently spread to distant organ sites (e.g., bone marrow, lung) after initial implantation in the mammary fat pad (26). To determine the therapeutic anti-tumor benefit of DC engineered to overexpress XBP1 in vitro or in vivo (ref. 23), we employed to treatment regimens to 4T1.2-Neu-bearing female mice: i.) ex vivo generated DC were coordinately transduced to express XBP1 and Aghsp70 prior to administration as a vaccine, and ii.) DNA encoding XBP1 driven-off a CD11c-promoter (XBP1 DC-targeting, ref. 23) and Aghsp70 were delivered into the skin using a biolistic GG where in situ expression would be restricted to dermal DC. In both cases, we observed vaccine-associated therapeutic benefit against both primary 4T1.2-Neu tumors (Fig. 1; ref. 23) and spontaneous metastases of 4T1.2-Neu to the lung and bone marrow (Fig. 2; Table I–II).
Figure 2. XBP1-potentiated in vitro generated DC and in vivo DC-targeting DNA vaccines elicit therapeutic anti-tumor immunity against spontaneous lung metastases.
4T1.2-Neu-bearing mice (mammary fat pad) were left untreated or they were cutaneously vaccinated with XBP1/NeuEDhsp70 DNA on days 8, 15 and 22, or with i.p. with XBP1/NeuEDhsp70 cDNA-engineered syngeneic DC 8 days after tumor cell inoculation. On day 30 after tumor inoculation, mice were sacrificed and lungs were fixed with Bouin’s solution for enumeration of 4T1.2-Neu metastatic foci. Data from three (a) or two (b) independent experiments are depicted and statistically analyzed as described in Materials and Methods.
Table II.
XBP1 DC vaccine decreases tumor spontaneous bone marrow metastases
| DC vaccines | Mice with bone marrow metastases/Mice used in experiments |
|---|---|
| Untreated | 6/6 |
| XBP1 | 5/6 |
| NeuEDhsp70 | 4/6 |
| XBP1/NeuEDhsp70 | 0/6 |
The therapeutic efficacy of XBP1 DC-targeting DNA vaccines is dependent on skin Batf3+DC and TLR3-mediated signaling in vivo
In a previous publication, we demonstrated that skin immunization with DNA constructs directing XBP1 expression in dermal CD11c+DC leads to locoregional increases in the frequency and absolute numbers of CD8α+DC in vaccine site (skin)-draining LN (ref. 23). Batf3−/− mice are deficient in cross-presenting CD8α+DC and CD103+DC, which are required for the spontaneous priming of tumor-specific CD8+T cell responses in vivo, but these mice otherwise exhibit normal T cell and (alternate) DC subset phenotypes (8, 12–13). We noted that anti-tumor immunity elicited by skin immunization with XBP1 DC-targeting DNA vaccines was abrogated in Batf3−/− mice in the B16 melanoma, 4T1.2-Neu breast carcinoma and GL26 glioma model systems (Fig. 3a–d). Furthermore, we determined that TLR3 (expressed at high levels on CD8α+DC and CD103+DC subsets; ref. 30–31) was also critical to the anti-tumor efficacy of this vaccination approach since this treatment regimen was ineffective in tumor-bearing TLR3−/− recipient mice (Fig. S2a).
Figure 3. Batf3+DC and pDC are required for the induction of protective anti-tumor immunity by XBP1 DC-targeting Aghsp70 DNA vaccination.
a–d) XBP1/Aghsp70 cDNA vaccines targeting dermal DC elicits anti-tumor immunity dependent on host cross-presenting Baft3+DC. a) B6-WT or Batf3−/− mice were untreated or vaccinated with XBP1/TRP2hsp70 or control DNA on days 1, 7 and 14. On day 21, mice were challenged s.c. with B16 melanoma cells. b) BALB/c-WT or -Batf3−/− mice were inoculated with 4T1.2-Neu breast carcinoma cells on day 0. On day 8, tumor-bearing mice were randomized into cohorts of comparable mean tumor size and left untreated or they were vaccinated once (on day 8 post-tumor inoculation). c–d) B6-WT or -Batf3−/− mice were inoculated with GL26 glioma cells on day 0. On day 9 after tumor inoculation, the mice were randomized into cohorts of comparable mean tumor size and left untreated or they were vaccinated on days 9 and 14 (post-tumor inoculation).
e) XBP1 DC-targeting DNA vaccination enhances IFN-α production by pDC from skin/tumor dLN. 4T1.2-Neu-bearing mice were left untreated or they were vaccinated with XBP1 or Aghsp70 (NeuEDhsp70 or TRP2hsp70 as GG-mediated vaccination) cDNA by gene gun. After 3 days, pDC were isolated from pooled skin/tumor dLN and were cultured in vitro without stimulation for 24 hours. IFN-α in the culture supernatants was measured by ELISA.
f) DNA vaccination-elicited anti-tumor immunity depends on pDC. 4T1.2-Neu-bearing BALB/c mice were vaccinated cutaneously with XBP1/NeuEDhsp70 cDNA on days 7 and 14 post-tumor inoculation. pDC were depleted in vivo by i.p. injection of anti-mouse pDC Ab 1 day before, on the day of, and 1 day after vaccination. Control mice received isotype-matched Ig.
Data from two independent experiments are depicted and statistically analyzed in each case.
Effective therapeutic skin immunization with XBP1 DC-targeting DNA vaccines requires IFN-α-producing pDC
pDC are the major source for type I IFN, which is required for the effective cross-priming of CD8+T cells (12–14), and the prevalence of these APC was noted in 4T1.2-Neu and GL26 tumors growing progressively s.c. (Fig. S3). We observed that cutaneous delivery of XBP1 DC-targeting DNA vaccines promoted IFN-α production by pDC isolated from skin/tumor dLN (Fig. 3e), and that depletion of pDC in tumor-bearing mice before, during and after vaccination led to reduced therapeutic efficacy (Fig. 3f). Notably, the presence of XBP1 cDNA was required in the DNA vaccination, since IFN-α was not produced at elevated levels by pDC isolated from tumor-bearing mice vaccinated with Aghsp70 (NeuEDhsp70 or TRP2hsp70) DNA vaccines (Fig. 3e) that were poorly protective (ref. 23).
XBP1 DC-targeting TRP2hsp70 DNA vaccines promote robust Type-1 melanoma (TRP2)-specific CD8+T cell responses that are therapeutic in BrafV600E/Pten-driven melanoma models
The genetically engineered BrafV600E/Pten-driven melanoma model faithfully recapitulates human disease (22) and provides a model that may reliably predict the comparative clinical efficacy of interventional strategies designed for patients with melanoma. Cutaneous immunization with XBP1 DC-targeting TRP2hsp70 DNA vaccines resulted in the development of durable melanoma (TRP2)-specific CD8+T cell responses in association with suppressed tumor growth (Fig. 4a–c). Ab-depletion of CD8+T cells in vivo abrogated the vaccine-associated treatment benefits, supporting the critical anti-tumor activity of these effector cells (Fig. 4b).
Figure 4. XBP1 DC-targeting TRP2hsp70 DNA vaccination induces durable therapeutic melanoma (TRP2)-specific CD8+T cell-mediated immunity against endogenous BrafV600E/Pten−/− melanoma.
a–b). Melanomas were induced in B6-Tyr-CreERT2 BrafCA Ptenlox/lox by treatment with 4-HT and allowed to progress to a mean tumor size of ~3mm, at which point, melanoma-bearing mice were randomized into cohorts of 3–4 mice with each cohort exhibiting a comparable mean tumor size. Animals were then left untreated or they were vaccinated using by GG with XBP1/TRP2hsp70 cDNA on therapy days 0, 7 and 14. To deplete CD8+T cells, anti-mouse CD8 mAb was injected i.p. 1 day before, on the day of, 1 and 3 days after first vaccination, and then once a week thereafter. Tumor growth (a) and animal survival (i.e. time to euthanasia; panel b) were then monitored.
c) Therapeutic vaccination was performed (a–b). On day 60, mice were sacrificed and CD8+T cells were purified from splenocytes and tumor dLN. Purified CD8+T were co-cultured with syngeneic DC transfected by TRP2hsp70 DNA or pulsed by BrafV600E/Pten-driven melanoma lysates (NeuEDhsp70 DNA-transfected or 4T1.2-Neu lysate-pulsed DC as Ag- or tumor-specific controls) as described in Materials and Methods. IFN-γ in the culture supernatants was then determined by ELISA.
Data from two (CD8 depletion, CD8+T cell responses) to four (tumor growth and animal survival) independent experiments are depicted.
XBP1 enhances the intrinsic Ag cross-presentation capacity of DC
Engineered XBP1 overexpression in DC enhanced Sec22b expression (Figs. 5a, S1c), which plays a key role in Ag cross-presentation mediated by DC (32). In particular, we observed that elevated XBP1 in DC improved cross-priming of Ag-specific naïve CD8+T cells in the well-defined OVA and OT-I (OVA-specific CD8+T cells) system (Figs. 5b, S1d). In the clinically-relevant more physiological poorly-immunogenic self/tumor Ag TRP2 and TRP2-specific CD8+T cells from TCR Tg mice setting, XBP1 improved the ability of TRP2hsp70 DC in cross-priming of TRP2-specific naïve CD8+T cells (Fig. 5c). Antagonism of XBP1 activity (by inclusion of dnXBP1 DNA constructs) prevented Sec22b up-regulation in DC and mitigated the cross-priming of Ag-specific naïve CD8+T cells (Fig. 5a–c).
Figure 5. XBP1 enhances Ag cross-presentation by DC.
a) XBP1 upregulates DC expression of Sec22b, which can be blocked by co-transfection with dnXBP1 cDNA. Equivalent whole proteins from untreated or DNA-transfected DC were analyzed by western blot using anti-Sec22b Ab and GADPH as an internal control. Data are representative of three independent experiments providing similar results.
b) Inhibiting XBP1 activity (via co-transfection with dnXBP1 cDNA) in DC reduces XBP1-improved Ag cross-presentation. Untreated or DNA-transfected DC were pulsed with endoGrade® OVA proteins. After overnight incubation, DC were extensively washed and co-cultured with naïve OT-I for 3 days.
c) XBP1 potentiates TRP2hsp70 DC to activate self/tumor Ag TRP2-specific naïve CD8+T cells. Untreated or DNA-transfected DC were co-cultured with TRP2-specific naïve CD8+T cells for 5 days.
d) DNA vaccination with XBP1/TRP2hsp70 cDNA targeting dermal DC enhances the capacity of CD103+DC from skin/tumor dLN to activate TRP2-specific naïve CD8+T cells which is abrogated by in vivo depletion of pDC. GL26-bearing mice were left untreated or they were vaccinated with XBP1/TRP2hsp70 cDNA +/− pDC depletion as described in Materials and Methods. After 3 days, CD8α+DC and CD103+DC were sorted from single-cell suspensions of pooled skin/tumor dLN and co-cultured with TRP2-specific naïve CD8+T cells for 5 days.
IFN-γ (b–d) in the culture supernatants was measured by ELISA. Data from two independent experiments is depicted and analyzed as outlined in Materials and Methods
CD103+DC isolated from tumor-bearing mice immunized with XBP1 DC-targeting TRP2hsp70 DNA vaccines are competent to cross-prime TRP2 specific naïve CD8+T cells in a pDC-dependent manner
Skin migratory CD103+DC (not LN-resident CD8α+DC) isolated from the skin/tumor dLN of GL26-bearing mice treated with XBP1/TRP2hsp70 DNA vaccines effectively activated self/tumor Ag TRP2-specific naïve CD8+T cells from TCR Tg mice (Fig. 5d). However, Ab-depletion of pDC in tumor-bearing mice prior to cutaneous DNA immunization diminished the capacity of resident CD103+DC to activate TRP2-specific naïve CD8+T cells (Fig. 5d).
Human XBP1 enhances the immunogenicity of human blood- and skin-derived DC
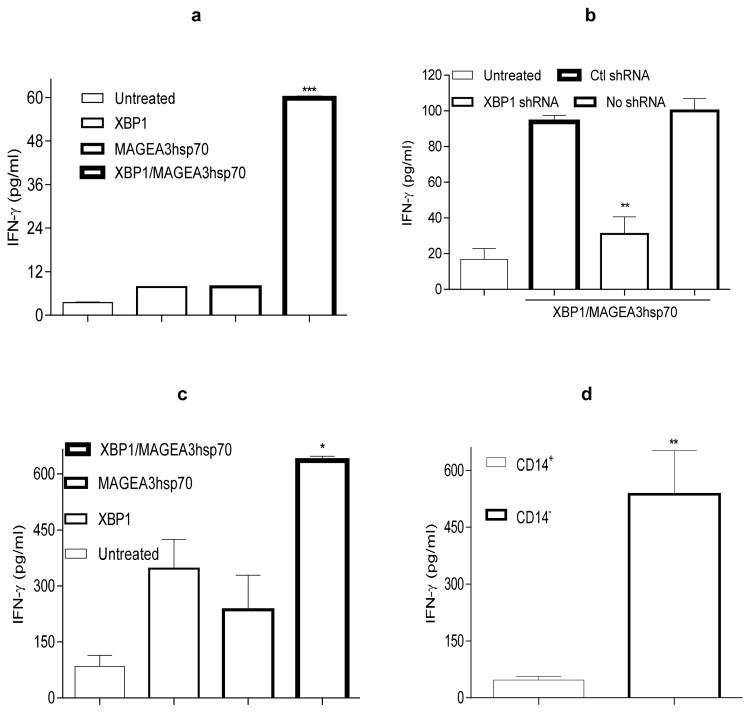
Human blood-derived moDC engineered by human XBP1 secreted elevated levels of pro-inflammatory cytokines (IL-6 and TNF-α; Fig. S4a) and strongly enhanced the ability of DC co-transduced with MAGEA3hsp70 cDNA to activate autologous T cells, which was abrogated by inclusion of XBP1 shRNA (Fig. 6a–b). DC emigrating from human skin explants genetically-immunized with human XBP1 and MAGEA3hsp70 cDNA augmented activation of human T cells when compared to comparable DC emigrating from explants vaccinated with XBP1 or MAGEA3hsp70 cDNA alone (Fig. 6c). Interestingly, CD14−DC (but not CD14+DC) were uniquely capable of activating human T cells in vitro (Fig. 6d). These results support the notion that ectopic XBP1 gene engineering of human (blood/skin) DC may be expected to similarly improve their ability to promote superior anti-tumor T cell responses as a consequence of active, specific immunization in patients with cancer.
Figure 6. XBP1 improves human blood- and skin-derived DC functions.
a) XBP1 potentiates moDC to activate autologous human T cells. Human moDC were generated from PBMC isolated from healthy adult donors. On day 6 of culture, moDC were left untreated or they were transfected with human XBP1 cDNA, MAGEA3hsp70 cDNA or XBP1 + MAGEA3hsp70 cDNA. After 2 additional days, DC were co-cultured with autologous human T cells. On day 6 of the DC-T cell co-culture, T cells were re-stimulated with MAGEA3hsp70-transfected autologous moDC for an additional 6 days.
b) Silencing of human XBP1 in moDC abrogates XBP1-potentiated T cell activation. MoDC were transfected with XBP1/MAGEA3hsp70 cDNA +/− human XBP1 shRNA or Ctl shRNA DNA, and then used to stimulate autologous human T cells as in (a).
c–d) XBP1 gene insertion potentiates skin-derived DC to activate human T cells in vitro. Skin DC, CD14+DC or CD14−DC were harvested as tissue migrated cells from control skin explants or matched skins receiving GG delivered XBP1 +/− MAGEA3hsp70 cDNA. These DC were then co-cultured with allogeneic human T cells for 5 days.
IFN-γ in the culture supernatants was measured by ELISA. Data from two (a, c) or three (b, d) independent experiments is reported and statistically analyzed per Materials and Methods.
Discussion
Despite the recent successes reported for immunotherapies integrating adoptive T cell transfer or the administration of immune checkpoint inhibitors in cancer patients, rates for durable clinical benefit remain modest. Means by which to improve the frequency of durable responses likely include the development of novel combination immunotherapies, including vaccine approaches. To date, however, single-modality vaccine strategies, including those based on patient DC have proven disappointing in their anti-tumor efficacy. Such impotence may, at least in part, relate to the inefficient delivery of vaccine Ag to (and appropriate activation of) resident DC populations within sites of tissue vaccination responsible for the optimal cross-priming of protective (Type-1) CD8+T cell responses in vivo (2–4).
DC taking up, processing and presenting exogenous Ag (i.e., cross-presenting) must be activated (e.g., increased costimulatory molecules and pro-inflammatory cytokines) to favor cross-priming (2–4). Conventional ex vivo generated DC have been most commonly employed in human cancer vaccine trials, however, these cells are not specialized for efficient cross-presentation of (vaccine) Ag to autologous CD8+T cells. Hence, the use of professional cross-priming DC or the ability to engineer conventional DC to reproducibly acquire cross-priming capacity would be envisioned to yield superior cell-based vaccine strategies in the cancer setting. XBP1 as a transcription factor controls expressions of many cell type- or condition-specific target genes (17, 19). To establish the influence of XBP1 on both mouse and human DC, DC were not intentionally activated with DC maturation factors (e.g., LPS, cytokines) (Figs. 1–2, 5–6, ref. 23). “Poor” (suboptimal) control DC (i.e., untreated or XBP1 DC without Ag, Aghsp70 DC without XBP1) did not effectively activate T cells and elicit anti-tumor immunity (Figs. 1–2, 5–6, ref. 23). These data indirectly support the finding that XBP1 may improve DC functions (e.g., cross-presentation, anti-tumor effect). In this regard, we believe that XBP1 plays a decisive intrinsic role in DC-mediated cross-priming, with even a single i.p. immunization with small numbers of cultured XBP1/Aghsp70 cDNA-engineered DC proving sufficient to elicit durable therapeutic anti-tumor immunity in our several clinically relevant murine tumor models.
XBP1 as an unfolded protein response-associated factor regulates endoplasmic reticulum structure and function and promotes the production and secretion of proteins (e.g. vaccine Ag) and DC survival (15, 17). Whether the observed XBP1-enhanced effects (e.g. Ag cross-presentation) are due to impact of XBP1 on DC survival and effects on UPR will be investigated in the future to further define the underlying mechanism(s).
XBP1 alone might not provide a signal that activate DC, and Aghsp70 might function synergistically with XBP1-initiated signals to promote DC activation (e.g., increased CD86, IL-15Rα, IL-6, TNF-α) (ref. 23). XBP1-overproducing DC alone did not elicit antitumor responses through i.p. immunization, probably due to the lack of tumor Aghsp70 (Figs. 1–2, Table II, ref. 23). How is it possible that there was no therapeutic effect of DC expressing tumor Aghsp70 in this single immunization with small numbers of DC (Fig. 1)? Presumably, XBP1 constitutive expression in those DC might lead to some effects. Does XBP1 overexpression in DC regulate key molecule(s) that can improve DC function(s)? Elevated XBP1 led to up-regulation of Sec22b (a vesicle trafficking protein and key DC Ag cross-presentation regulator; ref. 32) and promoted cross-presentation of Ag (Figs. 5, S1c–d). By utilizing DC-specific XBP1-deficient mice, recent data show that XBP1 controls cross-presentation by CD8α+DC (16). These results suggest the previously unknown function of XBP1 in cross-presentation by DC, but still one is left hanging as to what the essential features of XBP1 in DC will be. XBP1 constitutive expression in DC did not enhance Sec22b expression (Fig. 5). Why elevated XBP1 is required to promote Sec22b expression? It is possible that a strong signal (i.e., sufficient amounts) of XBP1 in DC may be required to directly target Sec22b gene via binding to its promoter leading to enhanced Sec22b expression. Possible binding sites of both mouse and human XBP1 on the promoter region of Sec22b gene were predicated (Fig. S1e). To define the direct effect of XBP1 on cross-presentation illustrates the sufficiency of XBP1 to augment DC-mediated cross-priming of T cells, it is worthwhile to investigate whether XBP1 regulates other cross-presentation related gene expressions, enhances Sec22b to recruitment of endoplasmic reticulum components to DC-phagosomes that are required for cross-presentation (32), and improves the number of peptide/MHC complexes delivered to the surface of the DC.
DNA vaccines offer a potential for large scale vaccine platform, leading to a great practical promise for a high-impact in cancer immunotherapy but have limited ability to activate self/tumor Ag-specific CD8+T cells against the Ag-expressing native tumors. Melanoma self Ag TRP2-based DNA vaccines cannot elicit antitumor in particular therapeutic immunity in syngeneic B6 mice (25, 33). Cross-presenting DC are preferred targets for cancer vaccines due to their ‘superior’ ability to cross-present Ag (9). pDC are the major source for type I IFN, which is required in cross-presenting DC function (12–14). DNA vaccines that enable to harness cross-presenting DC and pDC may lead to potent antitumor CD8+T cell immunity.
Skin immunization with DC-targeting XBP1 DNA vaccines resulted in increased CD8α+DC frequency and absolute numbers in the skin dLN, transgene expression in DC from the skin dLN, and CD8+T cell-dependent therapeutic immunity to B16, GL26 and 4T1.2-Neu (ref. 23). Skin harbors multiple DC subsets with different specialized functions and is the most accessible and ideal site for a vaccination (34). Understanding the contribution of distinct skin DC subsets in the vaccine to the induction of effective tumor Ag-specific T cell immunity will provide important mechanistic insights and clinical implications for development of effective tumor vaccines.
Type I IFN promotes DC survival, maturation and cross-presentation in CD103+DC (12–13, 35–37) and is naturally-produced by rare populations of pDC in normal skin (38). Elevated infiltration of the skin by pDC occurs after cutaneous trauma/injury (39) or in the case of cutaneous tumor progression (Fig. S3). Notably, our novel XBP1-based DNA vaccines increased IFN-α production by pDC in the skin/tumor dLN, which was required for optimal cross-priming of therapeutic CD8+T cells by skin-derived Batf3+DC (i.e., CD103+DC) in our murine tumor models. IFN-α production by pDC and DNA vaccine efficacy was dramatically lower in the absence of XBP1 cDNA, supporting the need for its inclusion in optimally effective DNA-based vaccinations.
129S- or B6/129S-Batf3−/− (B6 backcrossed with 129S-Batf3−/− for 5 generations) mice are CD8α+ and CD103+DC deficiency but have normal T cells and remaining DC subsets (8) (Fig. S2b–c). CD8α+DC and CD103+DC are required for in vivo priming of spontaneous tumor-specific CD8+T cells (12–13). XBP1 DC-targeting DNA vaccine-elicited anti-tumor immunity was abrogated in B6/129-Batf3−/− mice (Fig. S2d), suggesting that Batf3-dependent CD8α+ and/or CD103+DC are required for the vaccine-induced antitumor immunity. But which DC subset may play a decisive role in the skin immunization remains unknown. Batf3 is crucial for the development of the CD103+DC in B6-Batf3−/− (B6 backcrossed with 129S-Batf3−/− for at least 12 generations) (40). The number of CD8α+DC in skin dLN of B6-Batf3−/− mice is normal even though their cross-presentation ability is partially impaired (40). XBP1 DC-targeting DNA vaccine-elicited anti-tumor immunity depended on Batf3-dependent CD103+DC in skin dLN in both prevention (Fig. 3a) and therapeutic (Fig. 3b–d) settings, indicating that skin migratory CD103+DC are critical to the anti-tumor immunity induced by the XBP1 DC-targeting DNA vaccines skin immunization.
Our data support the key role of Batf3-dependent CD103+DC in effective cross-priming of protective anti-tumor immunity. But cross-presenting DC in skin/tumor dLN may present secreted tumor Aghsp70 from langerhans cells and/or dermal DC that take up the vaccine in the immunization sites and secrete Aghsp70 in the skin tumor dLN. Also, in skin immunization sites keratinocytes may take up the vaccine and secrete tumor Aghsp70 which may travel to the skin/tumor dLN where it is engulfed by CD103+DC and cross-presented to CD8+T cells. Alternatively, keratinocyte-secreted Aghsp70 may be in situ taken up by skin-resident DC (e.g., CD103+DC) and subsequently cross-present it to the skin-resident CD8+T cells or migrate to the skin/tumor dLN for cross-priming of CD8+ T cells. It also remains unclear CD103+DC deficiency-dampened cross-priming and anti-tumor responses due to a defect in cross-presentation or to others (e.g., IL-12 production by CD103+DC). Furthermore, although pDC are prime producers of IFN-α, and monocytes in vivo are a significant source for IFN-α (41), we have not yet formally established the in vivo cell source critical to the anti-tumor efficacy associated with effective Ag cross-presentation to therapeutic T cells. It will also clearly be of great prospective interest to determine whether ectopic XBP1 transactivates additional molecules associated with Type I IFN (from pDC, monocytes) responsiveness and the effective MHC I cross-presentation of Ag to CD8+T cells by CD103+DC.
The comparative success of our XBP1/Aghsp70 DNA-based vaccine strategies across a range of tumor models, however, this approach predicated on a single tumor Ag is unlikley to provide optimal clinical benefit to cancer patients harboring tumors composed of a heterogeneous population of cells that vary in target Ag and MHC I Ag processing machinery expression and tumor Ag loss during tumor progression. It will likely be important to integrate multiple tumor Ag into the DNA vaccine formulation, including private mutational Ag that have become increasingly recognized for their importance in determining clinical outcomes resulting from immunotherapy (42). Vaccine-modulated DC that can acquire broad tumor-associated Ag including tumor-derived debris, exosomes and soluble Ag and cross-present multiple tumor Ag including the private mutational Ag may have the greatest odds of promoting effective immunity. Efficient cross-presentation of Ag by XBP1 DC may explain the robust tumor (not just the vaccine Ag)-specific CD8+T cell responses (Fig. 4c). It is expected that vaccine-induced T cell responses will have to promote corollary rounds of endogenous tumor Ag cross-presentation to expand the protective anti-tumor CD8+T cell repertoire (i.e., epitope spreading; ref. 43) to most effectively address the logistic issue of tumor heterogeneity. In addition, the functional avidity of vaccine-induced T cells is anticipated to be critical to clinical outcome, with low-avidity T cells failing to recognize tumor cells in vivo and high-avidity T cells being preferentially tolerized within the immunosuppressive tumor microenvironment (44). Whether XBP1/Aghsp70 gene engineered DC condition moderate-to-high avidity Ag-specific CD8+T cells that are refractory to tumor-induced anergy or apoptosis is currently being evaluated in our laboratory.
From a translational perspective, we were also able to demonstrate that (CD14− but not CD14+) DC emigrating from human skin explants vaccinated with human XBP1 and MAGEA3hsp70 cDNA (but not XBP1 or MAGEA3hsp70 cDNA alone) activated robust Type-1 T cells in vitro. Any contribution of langerhans cells will be determined by examining the capacity of these cells emigrating from these immunized human skin explants to activate human T cells. Among CD14−DC emigrating from skin are the recently discovered human CD141+DC (HLA-DR+CD14−CD11clo/−CD141hi) subset (Fig. S4b), which appear to represent the human equivalents of mouse skin CD103+DC (10–11), the key cross-presenting DC for development of therapeutic immunity after immunization with our novel vaccine. These preliminary findings in human specimens provide enthusiasm for future translation of our novel genetic vaccine approach into pilot clinical trials for the treatment of patients with cancer.
In summary, the novel finding has the substantial implications for cancer vaccine development even though the defined mechanism(s) by which XBP1 makes DC “better” has yet been fully established. The demonstrations of XBP1 function in both mouse and human DC and this novel vaccination potency in highly clinically-relevant distinct tumor models including the stringent BrafV600E/Pten-driven melanoma will support its clinical translation.
Supplementary Material
Acknowledgments
Financial support: This work was supported by the Department of Dermatology at University of Pittsburgh School of Medicine (ZY) and NIH grants R01CA108813 and R01CA108813-04S2 (ZY), P50CA121973, R01AI076060 and R01EB01277 (LDF).
We are indebted to L. Glimcher (Weill Cornell Medical College) for sharing plasmids, M. Bosenberg (Yale University) for providing the inducible BrafV600E/Pten-driven melanoma model, A.A. Hurwitz (National Cancer Institute) for providing mouse TRP2-specific CD8+T cells, and D. Falkner (University of Pittsburgh) for assisting in flow cytometry.
Abbreviations used in this article
- DC
dendritic cells
- XBP1
X-box-binding protein
- Ag
antigens
- pDC
plasmacytoid DC
- dLN
draining lymph nodes
- TLR
toll-like receptor
- WT
wild type
- TRP2
tyrosinase-related protein 2
- NeuED
Neu extracellular domain
- hsp70
heat shock protein 70
- PBMC
peripheral blood mononuclear cells
- moDC
monocyte-derived DC
- shRNA
short hairpin RNA
- GG
gene gun
- Tg
transgenic
- 4-HT
hydroxytamoxifen
- ER
endoplasmic reticulum
- UPR
unfolded protein response
Footnotes
Authors’ contributions: YZ, GC, ZL, ST, JZ and CDC performed the experiments; KMM provided the critical material; YZ, GC, ZL, ST, WJS, LDF and ZY analyzed the data; WJS edited the manuscript; and ZY supervised the study and wrote the manuscript. The last two authors share the senior authorship.
The authors declare no conflict of interest.
References
- 1.Rosenberg SA. Entering the mainstream of cancer treatment. Nat Rev Clin Oncol. 2014;11:630–632. doi: 10.1038/nrclinonc.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palucka K, Banchereau J. Dendritic-Cell-Based Therapeutic Cancer Vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurts C, Robinson BWS, Knolle PA. Cross-priming in health and disease. Nat Rev Immunol. 2010;10:403–414. doi: 10.1038/nri2780. [DOI] [PubMed] [Google Scholar]
- 4.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 5.Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nat Rev Cancer. 2008;8:108–120. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- 6.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008;9:776–88. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pol J, Bloy N, Obrist F, Eggermont A, Galon J, Hervé Fridman W, Cremer I, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: DNA vaccines for cancer therapy. OncoImmunology. 2014;3:e28185. doi: 10.4161/onci.28185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shortman K, Health WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 10.Poulin LF, Reyal Y, Uronen-Hansson H, Schraml BU, Sancho D, Murphy KM, Håkansson UK, Moita LF, Agace WW, Bonnet D, Reis e Sousa C. DNGR-1 is a specific and universal marker of mouse and human Batf3-dependent dendritic cells in lymphoid and nonlymphoid tissues. Blood. 2012;119:6052–6062. doi: 10.1182/blood-2012-01-406967. [DOI] [PubMed] [Google Scholar]
- 11.Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, Singh Wasan P, Wang X-N, Malinarich F, Malleret B, Larbi A, Tan P, Zhao H, Poidinger M, Pagan S, Cookson S, Dickinson R, Dimmick I, Jarrett RF, Renia L, Tam J, Song C, Connolly J, Chan JKY, Gehring A, Bertoletti A, Collin M, Ginhoux F. Human Tissues ContainCD141hi Cross-Presenting Dendritic Cells with Functional Homology to Mouse CD103+ Nonlymphoid Dendritic Cells. Immunity. 2012;37:60–73. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuertes MB, Kacha AK, Kline J, Woo S-R, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for anti-tumor CD8+ T cell responses through CD8α+ dendritic cells. J Exp Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, Murphy KM, Schreiber RD. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spadaro F, Lapenta C, Donati S, Abalsamo L, Barnaba V, Belardelli F, Santini SM, Ferrantini M. IFN-α enhances cross-presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood. 2012;119:1407–1417. doi: 10.1182/blood-2011-06-363564. [DOI] [PubMed] [Google Scholar]
- 15.Iwakoshi NN, Pypaert M, Glimcher LH. The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J Exp Med. 2007;204:2267–2275. doi: 10.1084/jem.20070525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osorio F, Tavernier SJ, Hoffmann E, Saeys Y, Martens L, Vetters J, Delrue I, De Rycke R, Parthoens E, Pouliot P, Iwawaki T, Janssen S, Lambrecht BN. The unfolded-protein-response sensor IRE-1α regulates the function of CD8α+ dendritic cells. Nat Immunol. 2014;15:248–57. doi: 10.1038/ni.2808. [DOI] [PubMed] [Google Scholar]
- 17.Todd DJ, Lee A-H, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8:663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 18.Smith JA, Turner MJ, Hong D, Delay ML, Colbert RA. Endoplasmic reticulum stress and the unfolded protein response are linked to synergistic IFN-β induction via X-box binding protein 1. Eur J Immunol. 2008;38:1194–1203. doi: 10.1002/eji.200737882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinon F, Chen X, Lee A-H, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu F, Yu X, Wang H, Zuo D, Guo C, Yi H, Tirosh B, Subjeck JR, Qiu X, Wang XY. ER stress and its regulator X-box binding protein-1 enhance polyIC induced innate immune response in dendritic cells. Eur J Immunol. 2011;41:1086–1097. doi: 10.1002/eji.201040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, Means AR, Kasturi SP, Khan N, Li G-M, McCausland M, Kanchan V, Kokko KE, Li S, Elbein R, Mehta AK, Aderem A, Subbarao K, Ahmed R, Pulendran B. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol. 2011;12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, You MJ, DePinho RA, McMahon M, Bosenberg M. BrafV600E cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian S, Liu Z, Donahue C, Falo LD, Jr, You Z. Genetic Targeting of the Active Transcription Factor XBP1s to Dendritic Cells Potentiates Vaccine-induced Prophylactic and Therapeutic Anti-tumor Immunity. Mol Ther. 2012;20:432–442. doi: 10.1038/mt.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Tian S, Liu Z, Zhang J, Zhang M, Bosenberg MW, Kedl RM, Waldmann TA, Storkus WJ, Falo LD, Jr, You Z. Dendritic Cell-derived Interleukin-15 Is Crucial for Therapeutic Cancer Vaccine Potency. OncoImmunology. 2014;3:10, e959321. doi: 10.4161/21624011.2014.959321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JH, Chen J, Majumder N, Lin H, Falo LD, Jr, You Z. ‘Survival gene’ Bcl-xl potentiates DNA-raised anti-tumor immunity. Gene Ther. 2005;12:1517–1525. doi: 10.1038/sj.gt.3302584. [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, Majumder N, Lin H, Chen J, Falo LD, Jr, You Z. Enhanced immunity by NeuEDhsp70 DNA vaccine is needed to combat an aggressive spontaneous metastatic breast cancer. Mol Ther. 2005;11:941–949. doi: 10.1016/j.ymthe.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Rollins L, Gu Q, Chen SY, Huang XF. A Mage3/Heat Shock Protein70 DNA vaccine induces both innate and adaptive immune responses for the anti-tumor activity. Vaccine. 2010;28:561–570. doi: 10.1016/j.vaccine.2009.09.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh V, Ji Q, Feigenbaum L, Leighty L, Hurwitz AA. Melanoma Progression Despite Infiltration by In vivo-primed TRP-2-Specific T cells. J Immunother. 2009;32:129–139. doi: 10.1097/CJI.0b013e31819144d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malyshev I. Immunity, Tumors and Aging: The Role of Hsp70. Springer; 2013. [DOI] [Google Scholar]
- 30.Jelinek I, Leonard JN, Price GE, Brown KN, Meyer-Manlapat A, Goldsmith PK, Wang Y, Venzon D, Epstein SL, Segal DM. TLR3-Specific Double-Stranded RNA Oligonucleotide Adjuvants Induce Dendritic Cell Cross-Presentation, CTL Responses, and Antiviral Protection. J Immunol. 2011;186:2422–2429. doi: 10.4049/jimmunol.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicole Desch A, Randolph GJ, Murphy K, Gautier EL, Kedl RM, Lahoud MH, Caminschi I, Shortman K, Henson PM, Jakubzick CV. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell–associated antigen. J Exp Med. 2011;208:1789–1797. doi: 10.1084/jem.20110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cebrian I, Visentin G, Blanchard N, Jouve M, Bobard A, Moita C, Enninga J, Moita LF, Amigorena S, Savina A. Sec22b Regulates Phagosomal Maturation and Antigen Crosspresentation by Dendritic Cells. Cell. 2011;147:1355–1368. doi: 10.1016/j.cell.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Kim JH, Majumder N, Lin H, Watkins S, Falo LD, Jr, You Z. Induction of therapeutic anti-tumor immunity by in vivo administration of a lentiviral vaccine. Human Gene Ther. 2005;16:1255–1266. doi: 10.1089/hum.2005.16.1255. [DOI] [PubMed] [Google Scholar]
- 34.Heath WR, Carbone FR. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat Immunol. 2013;14:978–985. doi: 10.1038/ni.2680. [DOI] [PubMed] [Google Scholar]
- 35.Honda K, Sakaguchi S, Nakajima C, Watanabe A, Yanai H, Matsumoto M, Ohteki T, Kaisho T, Takaoka A, Akira S, Seya T, Taniguchi T. Selective contribution of IFN-α/β signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc Natl Acad Sci USA. 2003;100:10872–10877. doi: 10.1073/pnas.1934678100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, Tough DF. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–3271. doi: 10.1182/blood.v99.9.3263. [DOI] [PubMed] [Google Scholar]
- 37.Helft J, Manicassamy B, Guermonprez P, Hashimoto D, Silvin A, Agudo J, Brown BD, Schmolke M, Miller JC, Leboeuf M, Murphy KM, García-Sastre A, Merad M. Cross-presenting CD103+ dendritic cells are protected from influenza virus infection. J Clin Invest. 2012;122:4037–4047. doi: 10.1172/JCI60659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wollenberg A, Wagner M, Günther S, Towarowski A, Tuma E, Moderer M, Rothenfusser Simon, Wetzel S, Endres S, Hartmann G. Plasmacytoid dendritic cells: a new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J Invest Dermatol. 2002;119:1096–1102. doi: 10.1046/j.1523-1747.2002.19515.x. [DOI] [PubMed] [Google Scholar]
- 39.Gregorio J, Meller S, Conrad C, Di Nardo A, Homey B, Lauerma A, Arai N, Gallo RL, DiGiovanni J, Gilliet M. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921–2930. doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chopin M, Allan RS, Belz GT. Transcriptional regulation of dendritic cell diversity. Front Immunol. 2012;3:26. doi: 10.3389/fimmu.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansmann L, Groeger S, von Wulffen W, Bein G, Hackstein H. Human monocytes represent a competitive source of interferon-alpha in peripheral blood. Clin Immunol. 2008;127:252–264. doi: 10.1016/j.clim.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Tran E, Turcotte S, Gros A, Robbins PF, Lu Y-C, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, Parkhurst MR, Yang JC, Rosenberg SA. Cancer Immunotherapy Based on Mutation-Specific CD4+ T Cells in a Patient with Epithelial Cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corbière V, Chapiro J, Stroobant V, Ma W, Lurquin C, Lethé B, van Baren N, Van den Eynde BJ, Boon T, Coulie PG. Antigen spreading contributes to MAGE vaccination-induced regression of melanoma metastases. Cancer Res. 2011;71:1253–1262. doi: 10.1158/0008-5472.CAN-10-2693. [DOI] [PubMed] [Google Scholar]
- 44.Zhu Z, Singh V, Watkins SK, Bronte V, Shoe JL, Feigenbaum L, Hurwitz AA. High-Avidity T Cells Are Preferentially Tolerized in the Tumor Microenvironment. Cancer Res. 2013;73:595–604. doi: 10.1158/0008-5472.CAN-12-1123. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.