Abstract
Immune system is equipped with mechanisms that downregulate hyperinflammation to avoid collateral damages. We have recently demonstrated that unprimed T cells downregulate macrophage TNF production through direct interaction with macrophages in the spleen during LPS enodotoxemia. Here, how T cell migration towards macrophages occurs upon LPS injection is still not clear. In this study, we demonstrate that secreted osteopontin (sOPN) plays a role in the T cell migration to initiate the suppression of hyperinflammation during endotoxemia. OPN levels in the splenic macrophage were upregulated 2 h after LPS treatment, while T cell migration towards macrophage was observed 3 h after treatment. Neutralization of sOPN and blockade of its receptor, integrin αv, significantly inhibited CD4+ T cell migration, and increased susceptibility to endotoxemia. Our study demonstrates that the sOPN/integrin αv axis, which induces T cell chemotaxis towards macrophage, is critical for suppressing hyperinflammation at the first three hours during endotoxemia.
Introduction
Inflammatory responses triggered by pro-inflammatory cytokines, such as tumor necrosis factor (TNF), work to eliminate microbial pathogens from hosts during infection. However, prolonged or excessive inflammation is harmful. Although innate immune cells are equipped with intrinsic inhibitory mechanisms to negatively control inflammation in innate immunity (1-5), we and others found that adaptive immune cells also suppress early innate inflammatory responses during endotoxemia or sepsis (6-9). In our previous study (9), we demonstrated that T cells, but not B cells, are recruited in the splenic red pulp to interact with F4/80+ red pulp macrophages (RPMs) and suppress macrophage TNF expression by a direct T cell-macrophage interaction during LPS endotoxemia (9). Red pulp in the spleen is rich with RPMs, but has scarce T cells. Once the cell interaction occurs, CD40L on the T cell surface ligates CD40 on the macrophage cell surface to initiate anti-inflammatory responses during LPS endotoxemia (9). Because these responses occur before T cell priming, T cell cognate antigens are not necessary to achive the suppression. This suggested that even unprimed T cells play a critical role in immune responses to protect hosts from collateral damages by hyperinflammation. In the study, we further demonstrated a molecular mechanism downstream of macrophage CD40, through which TNF expression by macrophages is downregulated (9). CD40 signaling in macropahges induces IRAK1 sumoylation and nuclear translocation in the presence of TRAF2 (9). Nuclear IRAK1 binds to the Il10 promoter in macrophages to induce expression IL-10, which reduces Tnfa mRNA stability to eventually downregulate TNFα production by macrophage (9). However, it was not clear how T cels migrate to splenic red pulp upon LPS treatment in order to interact with macrophages.
Osteopontin (OPN) is a glycosylated protein, expressed in various immune cells, including macrophages and dendritic cells (10). There are two isoforms of OPN, intracellular type of OPN (iOPN) and secreted type of OPN (sOPN) (11, 12). Both iOPN and sOPN are generally known to induce pro-inflammatory responses (12), but iOPN can inhibit hyperinflammation during LPS endotoxemia (9). Due to alternative translation initiation, the iOPN nascent protein does not have signal sequence; as a result, iOPN localizes in the cytoplasm instead of being secreted (11). iOPN plays a role as an adaptor molecule in signaling pathways downstream of innate immune receptors as well as cell motility, cytoskeletal rearrangement, and mitosis (12). Actually, iOPN is essential for the IRAK1 sumoylation upon CD40 signaling activation in macrophages (9), as mentioned above. In contrast to iOPN, sOPN is a secreted protein, and the majority of OPN studies focused on sOPN. sOPN is known to play a role in attracting immune cells (13). OPN contains a tripeptide Arg-Gly-Asp (RGD) integrin-binding motif; therefore, sOPN ligates integrins such as αvβ3, αvβ1, αvβ5, αvβ6, and α4β1(14-16). Integrins are involved in immune cell migration by mediating the rolling and firm adhesion process during an inflammatory response. In particular, the integrin αv plays a critical role on migration of CD4+ T cells in inflamed tissue (17).
In this study, we demonstrate that sOPN plays a critical role in initiating T cell recruitment for T-macrophage interactions in the spleen to inhibit hyperinflammation during an early stage of LPS endotoxemia. Integrin αv on T cells is the key receptor for sOPN detection to achieve the migration. RPMs were capable to produce sOPN in 2 h after LPS injection, while CD4+ T cells constitutively expressed integrin αv, suggesting the upregulation of sOPN by macrophages initiates T cell migration. CD4+ T cell migration towards macrophages was significantly inhibited by either OPN neutralizing antibody (Ab) or integrin αv blocking Ab. Inhibiting T cell migration toward macrophage by these antibodies significantly increased susceptibility for LPS endotoxemia. Therefore, production of sOPN during an early stage of endotoxemia is critical to protect hosts from TNFα-mediated hyperinflammation.
Material and Methods
Animals
C57BL/6 (B6) and B6 Spp1−/− mice were purchased from the Jackson Lab. Sex- (male or female) and age- (6 to 7 week old) matched animals were used for all the experiments. All mice were maintained in barrier facilities and used according to Duke University Institutional Guidelines. This study was approved by the Duke University Institutional Animal Care and Use Committee.
LPS endotoxemia and antibody neutralization
To induce endotoxemia, E. coli LPS (serotype 055:B5, Sigma-Aldrich) resuspended in PBS was intraperitoneally (i.p.) injected to mice (40 mg/kg). Some mice were i.p. treated with integrin αv antibody (Ab)(50 μg/mouse; Biolegend) or OPN Ab (20 μg/mouse; AF808, R&D Systems) 1h prior to or 4 h after LPS injection.
Confocal microscopy
Tisuse preparation, staining, and confocal analysis were performed as previously described (9). Brilliant Violet 421-conjugated CD4 Ab (Biolegend, 10043823), Alexa 647-conjugated F4/80 Ab (Biolegend, 123122), and OPN Ab (AKm2A1, Santacruz) were used for staining. CD4+ T cell number in the red pulp was evaluated with images from 5-10 spleen sections per mouse using the Fiji software by independent investigators in a blinded fashion.
Real-time quantitative PCR and ELISA
RPMs (F4/80+) and CD4+ T cells were isolated using microbeads from the spleen of naïve mice or mice treated with LPS (40mg/kg, i.p.). Total RNA was extracted from RPMs and CD4+ T cells (1×106 cells) with Trizol (Invitrogen). cDNA synthesis was performed using qScript cDNA SuperMix (Quanta). qPCR was performed using KAPA-SYBR-FAST (KAPA Bio Systems) with a thermocycler (Eppendorf). Relative expression of qPCR products was determined by using the ΔΔCt method with Actb mRNA as an internal control. Primers used for amplification were listed in Table S1. To evaluate sOPN protein levels, RPMs (1×106 cells/ml) were cultured in RPMI complete medium without stimulation for 3h and their supernatants were analyzed by ELISA, as previously described (10). Briefly, wells were coated with OPN Ab (AF808, R&D Systems) in coating buffer (0.1 M sodium carbonate, pH 9.5). Wells were blocked with 2% FBS in PBS for 1h RT. Detection was performed with biotinylated OPN Ab (BAF808, R&D Systems) and a secondary detection Ab (avidin-horseradish peroxidase Ab, BD Biosceience).
Chemotaxis assay
CD4+ T cells were obtained from spleens of LPS-injected mice or from naïve mice, and submitted for chemotaxis assays as previously described (18). Briefly, CD4+ T cells (106 cells/well) plated in upper chambers of Transwell (5 μm pore, Corning Costar). RPMs were isolated 2h after LPS injection, or from naïve mice, and cultured in the RPMI complete medium without any stimulation for 3h. RPM culture supernatant was added to lower chambers of Transwell. T cells were incubated for 5 hr at 37 °C in Transwell culture. Numbers of T cells migrated to the lower chamber were counted. OPN Ab (10 μg/ml) was added in a bottom chamber. CD4+ T cells were pre-incubated with integrin αv Ab (10 μg/ml) or integrin α4 Ab (10 μg/ml; Biolegend), and plated in upper chambers. Data are shown after subtracting numbers of T cells spontaneously migrated to the lower chamer (medium alone in the lower chamber) from those in test groups.
Statistical analysis
Statistical analyses for all figures except survival studies were performed using Student t tests. Survival studies were analyzed with the Gehan-Breslow-Wilcoxon test. The criterion of significance was set as p< 0.05. All the data showed normal distribution, and are expressed as the mean ± the standard error of the mean (SEM).
Results and Discussion
Upregulation of secreted osteopontin (sOPN) in splenic macrophage during LPS endotoxemia
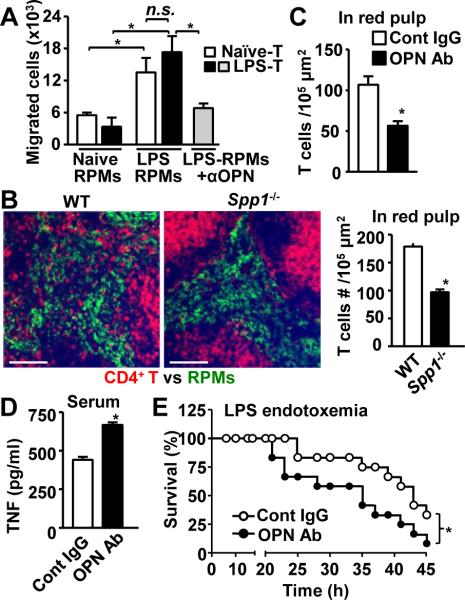
We have previously reported that LPS i.p. injection made CD4+ T cells migrate into the splenic red pulp, where macrophages were abundant, to initiate T cell-macrophage interaction and to negatively control hyperinflammation (9). However, it was not known what made T cells migrate towards macrophages during LPS endotoxemia. T cell migration occurs around 3 hr and subsides before 6 hr (9). Because sOPN plays a role in attracting immune cells as a ligand of various integrins (13), we evaluated sOPN producion during LPS endotoxemia. Serum OPN levels were increased 5-fold 2 h after i.p. LPS injection (Fig. 1A). Because LPS induces OPN expression in macrophages (19), we also evaluated OPN expression in RPMs. Levels of Spp1 (Opn) mRNA and secreted OPN (sOPN) were peaked at 1 h and 2 h after i.p. LPS injection with increase of 3-fold and 5-fold (Fig. 1B, C), respectively. To confirm the in situ distribution of OPN in the spleen, we carried out immunohistochemical analysis. OPN staining in the red pulp, but not T cell zone, was identified 2 hrs after LPS treatment (Fig. 1D). The data suggests that RPMs are a source of sOPN in the spleen.
Figure 1. Upregulation of secreted osteopontin (sOPN) in splenic macrophage during LPS endotoxemia.
(A) Serum OPN levels at indicated timepoints after LPS i.p. injection (40 mg/kg mouse weight). (B) Spp1 mRNA levels in RPMs isolated at indicated timepoints after LPS i.p. injection. (C) sOPN levels in supernatants of RPM culture. RPMs were isolated at indicated timepoints after LPS i.p. injection, and cultured for 3h in RPMI complete medium before harvesting supernatants. (D) Histological sections of spleens, isolated from naïve mice and LPS-injected mice (2 h after after i.p. injection), were stained to detect OPN (cyan), CD4+ T cells (CD4; red), and RPMs (F4/80; green). All the experiments are representatives from at least 2 similar experiments for each. *; p<0.05.
Requirment of sOPN for T cell migration and preventing hyperinflammation in endotoxemia
To confirm whether LPS-treated macrophages induce T cell migration via sOPN, we performed transwell migration assay. RPMs were isolated from mice 2 h after LPS i.p. injection or naïve mice, and cultured for 3 h in culture medium alone. Culture supernatant of RPMs were added to a bottom chamber of a transwell, and migration of CD4+ T cells in an upper chamber was evaluated. CD4+ T cell migration was enhanced through RPMs isolated from LPS-treated mice (LPS-Mϕ) but not by inducing T cell (LPS-T) migration ability per se (Fig. 2A). In addition, OPN neutralization in a bottom chamber abolished CD4+ T cell migration (Fig. 2A), suggesting that sOPN plays a critical role in attracting T cells.
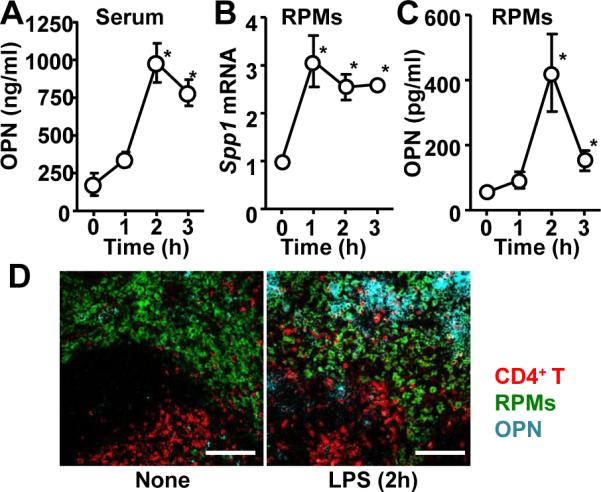
Figure 2. Requirement of sOPN for T cell migration and control of hyper-inflammation during LPS endotoxemia.
(A) Ex vivo migration assay. Splenic T cells and RPMs were isolated from naïve and mice 2 h post LPS injection. OPN neutralizing Ab was added to the bottom chambers in the indicated group. (B) Localization of CD4+ T cells (CD4; red) and RPMs (F4/80; green) in the spleen. Spleen was isolated from wild-type and Spp1−/− mice 3 h after LPS i.p. injection. Representative images (left panels) and results of quantitative analysis (right panel) are shown. Scale bars denote 100 μm. (C) Mice were i.p. treated with OPN Ab 1h prior to LPS injection, and spleens were harvested 3 h after LPS injection. T cell numbers were enumerated in images of the red pulp. Shown are average values of 10 sections/mouse from 3 mice. (D, E) LPS was i.p. injected into mice with (●) or without (○) OPN neutralization Ab i.p. injection 1h prior to LPS injection. n=12. Serum TNFα levels 6 h after injection (D) and survival (E) are shown. *; p<0.05 compared with control mice.
We next asked whether sOPN was required in T cell migration in vivo during LPS endotoxemia. Histological analysis showed that T cells in WT mice successfully migrated to the splenic red pulp 3 h after LPS injection, but T cell migration was significantly reduced in OPN-deficient (Spp1−/−) mice (Fig. 2B). Enumeration of T cell numbers in the red pulp confirmed the failure of T cell recruitment to the red pulp in Spp1−/− mice (Fig. 2B). Similar reduction of T cell numbers was also observed by in vivo OPN neutralizing Ab treatment (Fig. 2C). These findings suggest that sOPN is critical in T cell migration during LPS endotoxemia. Previously, we reported that the lack of T cell interaction with macrophages causes upregulation of macrophage TNF production, resulted in increased susceptibility in LPS endotoxemia (9). Indeed, mice treated with OPN neutralizing Ab upregulated serum TNFα level 6 h after LPS treatment (Fig. 2D), and showed earlier mortality than control IgG treated mice (Fig. 2E). These results suggested that OPN secreted by macrophages attracts T cells to achieve T cell-marophage interaction, which negatively controls hyper-inflammation in LPS endotoxemia. Therefore, sOPN also plays a host protective role in endotoxemia, but through a distict mechanism from the way iOPN does (9).
Integrin αV-mediated T cell migration into the splenic red pulp during enodotoxemia
Because integrins are sOPN receptors and involved in immune cell migration, we next evaluated roles of integrin αv and α4, two major receptors of sOPN, in T cell migration during LPS endotoxemia. First, Itgav mRNA was constitutively expressed in splenic CD4+ T cells before and after LPS injection, but Itga4 mRNA levels decreased after LPS injection (Fig. S1). Constitutive expression of integrin αv protein on the surface of CD4+ T cells was confirmed by flow cytometery (Fig. S1). To evaluate the functional involvement of integrin αv and α4 for T cell migration, we first performed transwell migration assay. Blocking integrin αv, but not α4, abolished CD4+ T cell migration toward RPMs (Fig. 3A), suggesting that integrin αv plays a critical role in T cell migration. We, then, treated mice with an integrin αv blocking Ab at two different time-points; 1 h prior to LPS injection and 4 h after LPS infection (Fig. 3B, C). Pre-LPS treatment with Ab significantly reduced T cell numbers in the red pulp, while post-LPS treatment with Ab did not have impact on T cell migration. The data suggests that integrin αv has to be blocked before sOPN comes to its effect.
Figure 3. Integrin αv on T cells is critical for T cell migration and resistance to enodotoxemia.
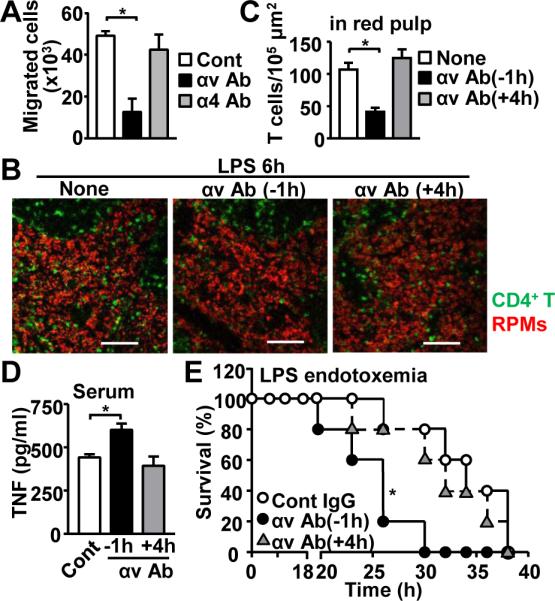
(A) Ex vivo T cell migration towards OPN. T cells were pre-treated with blocking Ab of either integrin αv or α4. (B-E) In vivo integrin αv Ab treatment during endotoxemia. Ab was i.p. administered either 1 h before or 4 h after LPS injection. Spleens were harvested at 6 h after LPS injection (B-D). Representative images of spleen (B) and results from T cell enumeration in red plup (C) are shown. Scale bars denote 100 μm. Serum TNFα levels 6 h after LPS injection (D). Mouse survival with (●, ▲) or without (○) integrin αv Ab (E). At least 5 mice/group. *; p<0.05 compared with control mice.
As direct interation between T cell and macrophage downregulates macrophage TNF expression (9), inhibition of T cell migration towards macrophages by OPN neutralization increased TNF expression, resulting in increased susceptibility to endotoxemia (Fig. 2D, E). Here, we sought the impact of integrin αv, an OPN receptor, in endotoxemia. Antibody-mediated blockade of αv integrin 1h prior to LPS injection increased serum TNFα levels and significantly increased susceptibility to endotoxemia (Fig. 3D, E). Congruent with the data showing no impact of integrin αv blockade 4h after LPS injection (Fig, 3B, C), integrin αv Ab treatment 4h after LPS injection did not alter serum TNF levels and host susceptibility (Fig. 3C, D).
Our findings here strongly suggest that integrin αv on the T cell surface contributes to T cell migration to macrophages in order to negatively control hyperinflammation by endotoxemia. Because extracellular matrix (ECM) such as collagen and fibronectin supports integrin αv-mediated T cell migration in inflamed tissues (17), ECM in spleen (20) may also support T cells migration in LPS-endotoxemia. Single nucleotide polymorphisms (SNPs) in human Itgav locus have been identified, and are associated with chronic hepatitis B infection (21), sickle cell disease (22), and rheumatoid arthritis (23). Although it is still not clear whether the SNPs have an impact on integrin αv expression on T cells, the SNPs in Itgav may be either a risk or protection factor for sepsis and endotoxemia.
In this study, we demonstrated the secreted OPN by splenic macrophages is detected by integrin αv on the T cell surface and attracts T cells towards macrophages at a very early stage (around 3h after treatment) of LPS enodotoxemia (Fig. 4). Our data does not rule out a possible involvement of sOPN produced in elsewhere other than the spleen. However, RPMs per se are the most proximate and plausible source of sOPN for splenic T cell migration towards RPMs. We previously reported that T cell-macrophage interaction suppresses macrophage TNF production through CD40 signaling, in which iOPN is involved (9). On the other hand, sOPN is dispensable in the CD40-mediated downregulation of macrophage TNF expression (9). Here, together with our previous study (9), we suggest distinct roles of sOPN and iOPN during early stages of endotoxemia: sOPN works first to attract T cells to macrophages, then iOPN works within macrophages to downregulate macrophage TNF expression (Fig. 4). OPN is largely known to induce proinflammatory responses. However, this study clearly demonstrated that OPN also functions to downregulate inflammation in the setting of the first several hours of endotoxemia, where both iOPN and sOPN participate to control hyperinflammation.
Figure 4. Roles of sOPN and iOPN during LPS endotoxemia.
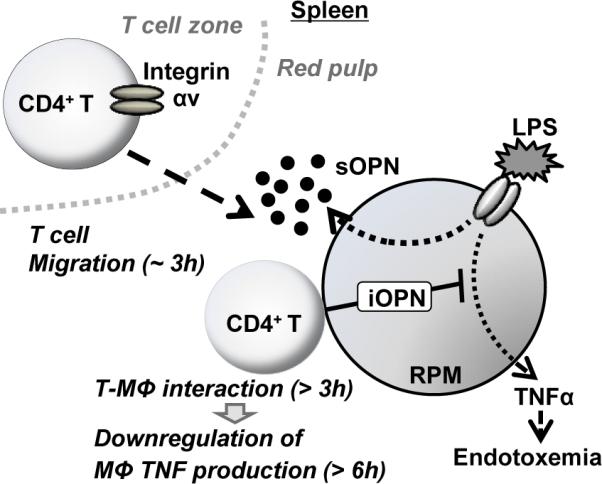
T cells and macrophages are separately localized in different zones in the spleen. OPN expression is upregulated by LPS in RPMs, and sOPN is secreted from LPS-stimulated RPMs in the first 3 h after LPS stimulation. sOPN is detected by integrin αv on the CD4+ T cell surface; and T cells start migrating towards macrophages. T cells then interact with macrophages to stimulate macrophage CD40 signaling pathway, in which intracellular osteopontin (iOPN) is essential for downregulation of macrophage TNF production (9).
Supplementary Material
Acknowledgments
We thank Mr. Jason Ashe for his technical help.
This work was supported by the National Institutes of Health (R01-AI088100 and R21- AI103584 to M.L.S.).
References
- 1.Kawagoe T, Takeuchi O, Takabatake Y, Kato H, Isaka Y, Tsujimura T, Akira S. TANK is a negative regulator of Toll-like receptor signaling and is critical for the prevention of autoimmune nephritis. Nature immunology. 2009;10:965–972. doi: 10.1038/ni.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heyninck K, Beyaert R. The cytokine-inducible zinc finger protein A20 inhibits IL-1-induced NF-kappaB activation at the level of TRAF6. FEBS letters. 1999;442:147–150. doi: 10.1016/s0014-5793(98)01645-7. [DOI] [PubMed] [Google Scholar]
- 3.Heyninck K, De Valck D, Vanden Berghe W, Van Criekinge W, Contreras R, Fiers W, Haegeman G, Beyaert R. The zinc finger protein A20 inhibits TNF-induced NF-kappaB-dependent gene expression by interfering with an RIP- or TRAF2-mediated transactivation signal and directly binds to a novel NF-kappaB-inhibiting protein ABIN. The Journal of cell biology. 1999;145:1471–1482. doi: 10.1083/jcb.145.7.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, Tanaka K, Kawai T, Tsujimura T, Takeuchi O, Yoshimori T, Akira S. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 5.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nature immunology. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim KD, Zhao J, Auh S, Yang X, Du P, Tang H, Fu YX. Adaptive immune cells temper initial innate responses. Nat Med. 2007;13:1248–1252. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly-Scumpia KM, Scumpia PO, Weinstein JS, Delano MJ, Cuenca AG, Nacionales DC, Wynn JL, Lee PY, Kumagai Y, Efron PA, Akira S, Wasserfall C, Atkinson MA, Moldawer LL. B cells enhance early innate immune responses during bacterial sepsis. The Journal of experimental medicine. 2011;208:1673–1682. doi: 10.1084/jem.20101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guarda G, Dostert C, Staehli F, Cabalzar K, Castillo R, Tardivel A, Schneider P, Tschopp J. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature. 2009;460:269–273. doi: 10.1038/nature08100. [DOI] [PubMed] [Google Scholar]
- 9.Inoue M, Arikawa T, Chen YH, Moriwaki Y, Price M, Brown M, Perfect JR, Shinohara ML. T cells down-regulate macrophage TNF production by IRAK1-mediated IL-10 expression and control innate hyperinflammation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:5295–5300. doi: 10.1073/pnas.1321427111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinohara ML, Jansson M, Hwang ES, Werneck MB, Glimcher LH, Cantor H. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17101–17106. doi: 10.1073/pnas.0508666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shinohara ML, Kim HJ, Kim JH, Garcia VA, Cantor H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7235–7239. doi: 10.1073/pnas.0802301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue M, Shinohara ML. Intracellular osteopontin (iOPN) and immunity. Immunologic research. 2011;49:160–172. doi: 10.1007/s12026-010-8179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uede T. Osteopontin, intrinsic tissue regulator of intractable inflammatory diseases. Pathology international. 2011;61:265–280. doi: 10.1111/j.1440-1827.2011.02649.x. [DOI] [PubMed] [Google Scholar]
- 14.Denhardt DT, Noda M. Osteopontin expression and function: role in bone remodeling. J Cell Biochem Suppl. 1998;30-31:92–102. [PubMed] [Google Scholar]
- 15.Gladson CL, Cheresh DA. Glioblastoma expression of vitronectin and the alpha v beta 3 integrin. Adhesion mechanism for transformed glial cells. The Journal of clinical investigation. 1991;88:1924–1932. doi: 10.1172/JCI115516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barry ST, Ludbrook SB, Murrison E, Horgan CM. Analysis of the alpha4beta1 integrin-osteopontin interaction. Exp Cell Res. 2000;258:342–351. doi: 10.1006/excr.2000.4941. [DOI] [PubMed] [Google Scholar]
- 17.Overstreet MG, Gaylo A, Angermann BR, Hughson A, Hyun YM, Lambert K, Acharya M, Billroth-Maclurg AC, Rosenberg AF, Topham DJ, Yagita H, Kim M, Lacy-Hulbert A, Meier-Schellersheim M, Fowell DJ. Inflammation-induced interstitial migration of effector CD4(+) T cells is dependent on integrin alphaV. Nature immunology. 2013;14:949–958. doi: 10.1038/ni.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue M, Williams KL, Gunn MD, Shinohara ML. NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:10480–10485. doi: 10.1073/pnas.1201836109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao W, Wang L, Zhang M, Wang P, Zhang L, Yuan C, Qi J, Qiao Y, Kuo PC, Gao C. NF-kappaB- and AP-1-mediated DNA looping regulates osteopontin transcription in endotoxin-stimulated murine macrophages. J Immunol. 2011;186:3173–3179. doi: 10.4049/jimmunol.1003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lokmic Z, Lammermann T, Sixt M, Cardell S, Hallmann R, Sorokin L. The extracellular matrix of the spleen as a potential organizer of immune cell compartments. Seminars in immunology. 2008;20:4–13. doi: 10.1016/j.smim.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Lee SK, Kim MH, Cheong JY, Cho SW, Yang SJ, Kwack K. Integrin alpha V polymorphisms and haplotypes in a Korean population are associated with susceptibility to chronic hepatitis and hepatocellular carcinoma. Liver international : official journal of the International Association for the Study of the Liver. 2009;29:187–195. doi: 10.1111/j.1478-3231.2008.01843.x. [DOI] [PubMed] [Google Scholar]
- 22.Elliott L, Ashley-Koch AE, De Castro L, Jonassaint J, Price J, Ataga KI, Levesque MC, Brice Weinberg J, Eckman JR, Orringer EP, Vance JM, Telen MJ. Genetic polymorphisms associated with priapism in sickle cell disease. British journal of haematology. 2007;137:262–267. doi: 10.1111/j.1365-2141.2007.06560.x. [DOI] [PubMed] [Google Scholar]
- 23.Jacq L, Garnier S, Dieude P, Michou L, Pierlot C, Migliorini P, Balsa A, Westhovens R, Barrera P, Alves H, Vaz C, Fernandes M, Pascual-Salcedo D, Bombardieri S, Dequeker J, Radstake TR, Van Riel P, van de Putte L, Lopes-Vaz A, Glikmans E, Barbet S, Lasbleiz S, Lemaire I, Quillet P, Hilliquin P, Teixeira VH, Petit-Teixeira E, Mbarek H, Prum B, Bardin T, Cornelis F, F. European Consortium on Rheumatoid Arthritis The ITGAV rs3738919-C allele is associated with rheumatoid arthritis in the European Caucasian population: a family-based study. Arthritis research & therapy. 2007;9:R63. doi: 10.1186/ar2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.