Abstract
Human PON1 is a HDL-associated lipolactonase capable of preventing LDL and cell membrane oxidation and is therefore considered to be atheroprotective. PON1 contributes to the antioxidative function of HDL and reductions in HDL-PON1 activity, prevalent in a wide variety of diseases with an inflammatory component, is believed to lead to dysfunctional HDL which can promote inflammation and atherosclerosis. However, PON1 is multifunctional and may contribute to other HDL functions such as in innate immunity, preventing infection by quorum sensing gram negative bacteria by destroying acyl lactone mediators of quorum sensing, and putative new roles in cancer development and the promotion of healthy ageing.
In this review we explore the physiological roles of PON1 in disease development, as well as PON1 gene and protein structure, promiscuous activities and the roles of SNPs and ethnicity in determining PON1 activity.
Keywords: paraoxonases, paraoxonase-1, HDL, LDL, atherosclerosis, oxidation, inflammation, quorum quenching, toxicology, ageing
1. INTRODUCTION
Human serum paraoxonase-1 (PON1) is a Ca2+ dependent high-density lipoprotein (HDL) associated lactonase capable of hydrolysing a wide variety of lactones (including a number of pharmaceutical agents), thiolactones, arylesters, cyclic carbonates and organophosphate pesticides, nerve gases such as sarin and soman, glucuronide drugs and oestrogen esters [1]. PON1 is synthesised mainly in the liver and secreted into the blood where it associates predominantly with HDL [2]. However immunohistochemical studies have indicated the presence of PON1 in a wide variety of mammalian tissues including humans [3, 4]. Whether this is due to local synthesis or to PON1 being transported to the tissues by HDL is unknown.
PON1 is currently classified as an aryldialkylphosphatase (EC 3.1.8.1) by the Enzyme Commission of the International Union of Biochemistry and Molecular Biology [5]. PON1 was first described in the 1940´s when Mazur reported an enzyme activity found in mammalian tissues which was capable of hydrolysing organophosphate pesticides [6]. Interestingly, PON1 is not found in the blood of birds, fishes and most reptiles. The enzymes were further classified by Norman Aldridge [7] as "A"- esterases (esterases capable of hydrolysing organophosphates, as opposed to "B"- esterases which are inhibited by organophosphates). However, the widespread use of paraoxon as substrate for the enzyme led to the, almost, universal adoption of the name paraoxonase.
PON1 is a glycoprotein of 354 amino acids and approximate molecular mass of 43KDa, it retains its hydrophobic signal sequence in the N-terminal region (with the exception of the initial methionine) which enables its association with HDL [5]. PON1 associates with a specific HDL sub-species which also contains apo A1 and clusterin. On ultracentrifugation, the majority of PON1 (and HDL antioxidant activity) resides on the small dense HDL3 subfraction [8].
The gene for PON1 is located between q21.3 and q22.1 on the long arm of chromosome 7 in humans (chromosome 6 in mice). An X-ray crystallography study has indicated the structure of PON1 to be a 6- bladed propeller, with a lid covering the active site passage and containing 2 Ca2+, one essential for activity and one essential for stability [9].
PON1 is the first discovered member of the paraoxonase (PON) multi-gene family which comprises 3 members, PON1, PON2 and PON3, the genes for which are located adjacent to each other [10]. The genes for all 3 members of the family are widely expressed in mammalian tissues [11], however, PON1 and PON3 are predominantly located in the plasma associated with HDL while PON2 is not found in the plasma but has a wide cellular distribution [12]. PON1, PON2 and PON3 all retard the proatherogenic oxidative modification of low-density lipoprotein (LDL) and cell membranes and are therefore considered to be antiatherogenic [13]. PON1 is now considered to be a major factor in the antioxidative activity of HDL [14].
Although many nutritional, life-style and pharmaceutical modulators of PON1 are known [15, 16], by far the biggest effect on PON1 activity levels, which can vary by over 40 fold between individuals, is through PON1 genetic polymorphisms [2]. The coding region PON1-Q192R polymorphism determines a substrate dependent effect on activity. Some substrates eg paraoxon are hydrolysed faster by the R- isoform while others such as diazoxon are hydrolysed more rapidly by the Q- isoform [2]. Both the coding region PON1-L55M and the promoter region PON1-T-108C polymorphisms are associated with different serum concentrations and therefore different activities. The 55L allele results in significantly higher PON1 mRNA and serum protein levels and therefore higher activity compared to the 55M allele [17].The -108C allele has greater promoter activity than the -108T allele which results in different serum activities [18]. Several other polymorphisms affect serum PON1 activity to a lesser extent [19].
The PON1-Q192R polymorphism also determines the efficacy with which PON1 inhibits LDL oxidation with the Q isoform being the most efficient and the R isoform least efficient [20, 21]. These observations resulted in a plethora of genetic epidemiological studies to link the PON1 polymorphisms with CHD presence to little or no effect, meta-analyses showing a marginal relationship at best [22].
2. PON1 PROTEIN STRUCTURE AND ENZYME ACTIVITY
PON1 is a glycoprotein of 354 amino acids and an approximate molecular mass of 43KDa. X-ray crystallography of recombinant PON1 has indicated a 6 bladed propeller structure. The mature protein retains its hydrophobic leader sequence (except the N-terminal methionine) allowing its association with HDL. The structure also has a unique active site lid which may modulate the association with HDL. The PON1 structure also contains 2 Ca2+, one at the base of the active site gorge (adjacent to a phosphate ion) which is involved in the catalytic mechanism. The other Ca2+ is believed to be involved in enzyme stability [9, 23].
The catalytic site is an oxy anion hole, as seen in secreted phospholipase A2. The catalytic mechanism involves a histidine-histidine (His) catalytic dyad. His-115 (4.1 Å from the catalytic Ca2+) acts as a general base to deprotonate a water molecule and generate the attacking hydroxyl radical. The His-134 acts as a proton shuttle to increase the basicity of His-115 [24, 25]. The active site also contains a functional polymorphic site at amino acid 192 (Q192R, glutamine/arginine). This polymorphism is important in that it determines a differential catalytic activity towards some, but by no means all substrates (see section 4).
Although the "natural" substrates of PON1 appear to be lactones [26], PON1 has a large number of promiscuous activities towards organophosphate triesters, arylesters, cyclic carbamates, glucuronides, oestrogen esters and thiolactones (see [27]). One group of potentially important lactone substrates are the statins. Statins inhibit cellular cholesterol synthesis and are widely used to reduce LDL-cholesterol to prevent cardiovascular diseases (mainly heart disease and stroke- the main causes of mortality and morbidity in industrialised nations). However, there are no studies published looking at the effect of PON1 on the efficacy of statin treatment. This is a major omission which requires addressing.
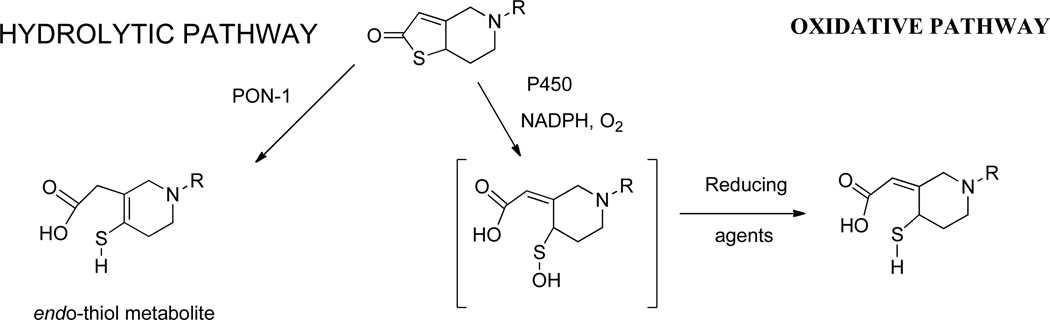
Clopidogrel inhibits platelet function and prevents thrombosis. Alongside aspirin it is used in the treatment of acute coronary syndrome and after percutaneous coronary intervention with stent implantation to improve outcomes [28]. Clopidogrel undergoes a 2 stage bioactivation process. It is firstly converted to 2-oxo-clopidogrel by hepatic cytochrome P-450 enzymes and then in the presence of excess GSH to the pharmacologically active thiol metabolite by oxidative opening of the thiolactone ring. In 2011, Boumann and colleagues suggested that PON1 was responsible for this 2nd step and that the wide range of efficacy found with Clopidogrel was due to different rates of activation by the PON1-192Q and R isoforms [29]. Unfortunately, there were many methodological problems associated with this study [30]. Subsequently, numerous clinical studies have failed to confirm these results [31] and detailed biochemical studies have suggested that PON1 can metabolise 2-oxo-clopidogrel by hydrolytic opening of the thiolactone ring but this leads to the pharmacologically inactive endo-thiol isomer (Figure 1) [32].
Figure 1.
Metabolism of 2-oxo-clopidogrel by oxidative and hydrolytic pathways.
Figure kindly provided by Prof Patrick Dansette, Universite de Paris Descartes
3. PON1 GENE STRUCTURE
The PON1 gene is localised to 7q21-q22 on chromosome 7 in humans (the proximal region of chromosome 6 in mice). The PON1 gene comprises approximately 26KB. The coding sequence comprises 9 exons with splice donor and acceptor sites typical for mammalian genes. There appears to be no canonical polyadenylation signal sequence. The 4th intron (of 8) contains a CA repeat, the length of which is polymorphic, allele lengths vary by up to 4 CA units. The most common allele had 17 repeats in a population of 17 individuals. The 5´ UTR has no canonical TATA box [10, 33].
The promoter region of the PON1 gene contains binding sites for sterol regulatory binding protein 2 (SREBP2) and specificity protein 1 (Sp-1), which putatively upregulate PON1 in the presence of statins. The arylhydrocarbon receptor and PPARs have also been reported to regulate the PON1 gene, however, binding sites remain elusive (See Section 5).
4. SINGLE NUCLEOTIDE POLYMORPHISMS (SNPs) OF THE PON1 GENE
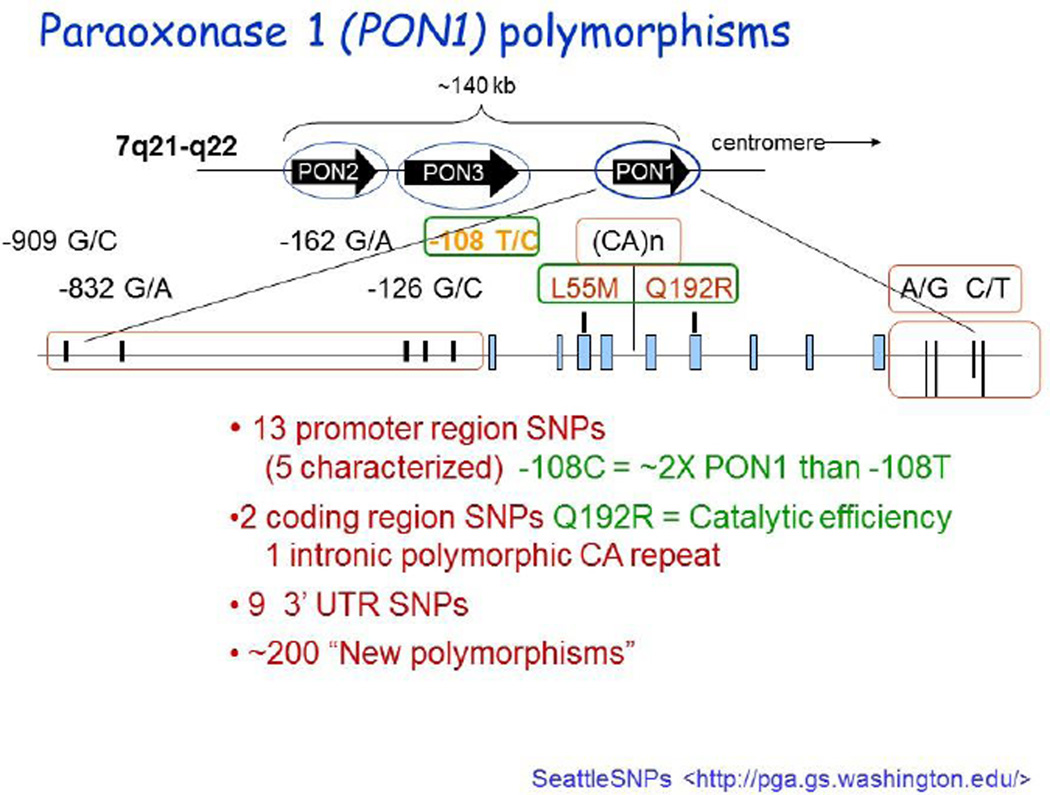
As eluded to previously the PON1 gene contains many SNPs. Many result in truncated, missense mutated or other wise altered forms of the enzyme affecting activity. It is worth noting that no null activity individuals have been reported so far. The effects of many of the SNPs on PON1 activity or concentration are unknown (for a comprehensive SNP list see http://pga.gs.washington.edu) (Figure 2).
Figure 2.
PON1 polymorphisms.
Figure kindly provided by Prof Clem Furlong, University of Washington
However, some of the more common SNPs functionally affect PON1 activity and/or concentration. The most commonly studied PON1 SNPs are the coding region Q192R and L55M and promoter region C-108T polymorphisms.
Although many nutritional, life-style and pharmaceutical modulators of PON1 are known [15, 16], by far the biggest effect on PON1 activity levels, which can vary by over 40 fold between individuals, is through PON1 genetic polymorphisms [5]. The coding region PON1-Q192R polymorphism determines a substrate dependent effect on activity. Some substrates eg paraoxon are hydrolysed faster by the R- isoform while others such as diazoxon are hydrolysed more rapidly by the Q- isoform [5]. Both the coding region PON1-L55M and the promoter region PON1-T-108C polymorphisms are associated with different serum concentrations and therefore activities. The 55L allele results in significantly higher PON1 mRNA and serum protein levels and therefore activity compared to the 55M allele [17].The −108C allele has greater promoter activity than the -108T allele which results in different serum activities [18]. Several other polymorphisms affect serum PON1 activity to a lesser extent [19].
The PON1-Q192R polymorphism also determines the efficacy with which PON1 inhibits LDL oxidation with the Q isoform being the most efficient and the R isoform least efficient [20, 21]. These observations resulted in a plethora of genetic epidemiological studies to link the PON1 polymorphisms with CHD presence to little or no effect, meta-analyses showing a marginal relationship at best with the Q192R polymorphism but none with the L55M or C-108T polymorphisms [22, 34].
PON1 SNPs have been shown to be linked to a number of other diseases including diabetic complications, various cancers, HIV, renal and hepatic diseases and macular degeneration [35]. Unfortunately the vast majority of these studies included only small numbers of subjects and are very prone to statistical error (See Section 7). There is an urgent need for large scale clinical epidemiological studies to confirm any relationships between PON1 SNPs and disease.
It is worth noting that the distribution of the PON1 polymorphisms varies with ethnicity. The frequency of the PON1-192R allele increases the further from Europe a population originates, the frequency in Caucasians of 15–30% increases to 70–90% in Far Eastern Oriental and Sub-Saharan African populations [36]. In the southern USA African-Americans are 5 times more likely to be RR than Caucasians [37]. In contrast the PON1-55M allele is much less frequent in Oriental and black African populations compared to Caucasians and are extremely rare/absent in some populations eg Thais [38]. These ethnic differences in SNP distribution can lead to large activity differences between populations [36].
5. REGULATION OF HUMAN PON1
The regulation of human PON1 has been the subject of some excellent recent reviews [39–42] and will be dealt with only briefly here. Factors known to affect PON1 such as inflammation, diabetes, smoking and diet have been described in detail previously [39–42] and will not be dealt with here.
In vitro, different chemicals appear to act through different receptors and/or signalling systems. For example, statins, glucose and quercetin cause PON1 translocation through SREBP2 and Sp1 binding to the PON1 promoter. Quercetin, resveratrol and aspirin on the other hand act through the aryl hydrocarbon receptor, while Berberine induced PON1 stimulation is through the JNK-c-JUN signalling cascade and pomegranate juice polyphenolics through the PPARγ-PKA-cAMP signalling pathway [39–42]. Other dietary factors such as curcumin, betanin, isothiocyanates, olive oil and liquorice polyphenolics are also inducers of PON1, by mechanisms awaiting discovery [14–16].
The statin studies referred to above were all of a short-term nature, however, we have reported recently that long term statin treatment is associated with a reduction in PON1 activity [43] further emphasising the need for PON1 statin efficacy studies.
In humans, neonatal PON1 activity is very low and progressively increases until reaching adult levels at 6 to 15 months of age. The mechanism(s) controlling this are unknown [44].
A single study has found dynamic variation in allele specific gene expression of the PON1 gene in human and mouse tissues [45]. Using human foetal tissues, Parker-Katirace et al analysed the expression of the human PON1 gene. Monoallelic or preferential allelic expression was found in 6 out of 7 liver samples and 4 of 4 pancreas samples. The PON1 gene did not show a parent of origin preference in allelic expression, however, dramatic variations were found in allele specific expression occurring throughout development. This indicates that the expression of PON1 alleles can be unequal and dynamic and could affect studies investigating genotype/disease relationships.
Micro RNAs (miRNA) are small RNAs of about 21 nucleotides which can bind to the 3´ UTR of target mRNA to post-transcriptionally regulate genes. miRNAs play a role in many biological processes including atherosclerosis. Liu et al [46] studied a C/T SNP at rs 3735590 in the 3´ UTR of the PON1 gene which is within a miRNA binding site. Subjects with CT or TT genotypes had a significant lower risk of ischaemic stroke and thinner carotid artery IMT than subjects with the CC genotype. 3´ UTR reporter studies indicated that in plasmid constructs containing the C allele miRNA-616 inhibited the expression of PON1, whereas miRNA-616 binding to constructs with the T allele was reduced and PON1 was overexpressed. This is the first study to show PON1 regulation by miRNA and also the first to show a disease protective effect of a 3´ UTR functional SNP.
It has recently been suggested that PON1 may be regulated in trans by an unknown gene found on chromosome 8 (p11, 21) [47]. This warrants further investigation in the context of PON1 involvement in disease.
Our knowledge of the mechanisms which regulate human PON1 in vivo are scant, at best. As this lack of knowledge hampers the development of small molecule pharmaceuticals to modulate PON1 in disease prevention, it needs to be urgently addressed.
6. PHYSIOLOGICAL ROLES OF HUMAN PON1
6.1 PON1 and Atherosclerosis
6.1.1 HDL and Atherosclerosis
The concentration of LDL is directly related to the risk of developing atherosclerosis [48]. The current theory to explain the development of atherosclerosis in the artery wall, states that the oxidation of LDL to produce proinflammatory/proatherosclerotic bioactive oxidised lipids is critical for its initiation and propagation [49–51]. On the other hand epidemiological studies have shown a strong inverse relationship between serum HDL cholesterol concentration and the development of atherosclerosis [52] indicating that HDL is atheroprotective. The mechanism of this protection has been the subject of intense research, with the majority directed at the central role of HDL in reverse cholesterol transport (RCT), the process of transporting excess cholesterol from peripheral tissues particularly arterial wall macrophages to the liver for disposal.
Recently, however, attention has turned to other pleiotropic mechanisms whereby HDL exerts its atheroprotective effects. These include antioxidative, anti-inflammatory, antiapoptopic, vasorelaxative and antithrombotic effects as well as promoting the normalisation of endothelial function and stimulating endothelial progenitor cell function [53].
Recently, however, a number of human pharmaceutical intervention studies with HDL raising agents failed to show clinical benefit. Unfortunately, one using the CETP inhibitor torcetrapib was terminated due to off target toxicity of the drug, while another with niacin had a flawed design while, more recently a 2nd CETP inhibitor study was prematurely terminated due to lack of efficacy [53]. A genetic Mendelian Randomisation study also failed to find a relationship between myocardial infarction and common genetic variants only associated with HDL-C levels [54]. Although these studies have cast some doubt on the "HDL Hypothesis", it is not known whether HDL function was affected in these studies and many more studies will be required before the hypothesis is cast aside.
6.1.2 Dysfunctional HDL
The concept of dysfunctional HDL first arose from observations that some individuals with high or normal HDL-C but low PON1 activity were susceptible to CHD development, while others with low HDL-C but high PON1 activity were not [55]. Since this time many studies have been performed to show how and why HDL becomes dysfunctional [reviewed in 54, 56]. Dysfunctionality of HDL can take the form of reduced cholesterol efflux capacity, but is most commonly measured by the loss of anti-inflammatory/anti-oxidative function. LDL added to endothelial cells in co-culture models is oxidised, inducing the production of monocyte chemotactic factors which increase monocyte binding and migration. The addition of HDL to the co-culture prevents the oxidation of the LDL and impairs the inflammatory response [57].
However, HDL from patients with a wide variety of diseases with an inflammatory component such as diabetes, rheumatoid arthritis, macular degeneration, psoriasis and many more does not inhibit monocyte chemotaxis but may actually increase it, thus this dysfunctional HDL is often pro-inflammatory, promoting inflammation and CHD development Likewise, HDL from patients with coronary artery disease or acute coronary syndrome (due to a deficiency in PON1 activity) is unable to activate endothelial cell eNOS and therefore unable to maintain normal endothelial cell function [58].
6.1.3 PON1 and Atherosclerosis
Normal HDL is able to retard the oxidation of LDL to prevent atherosclerosis development [59]. Several HDL associated proteins including PON1 contribute to this antioxidant of HDL. These have recently been reviewed [60, 61] and other than PON1 will not be dealt with here.
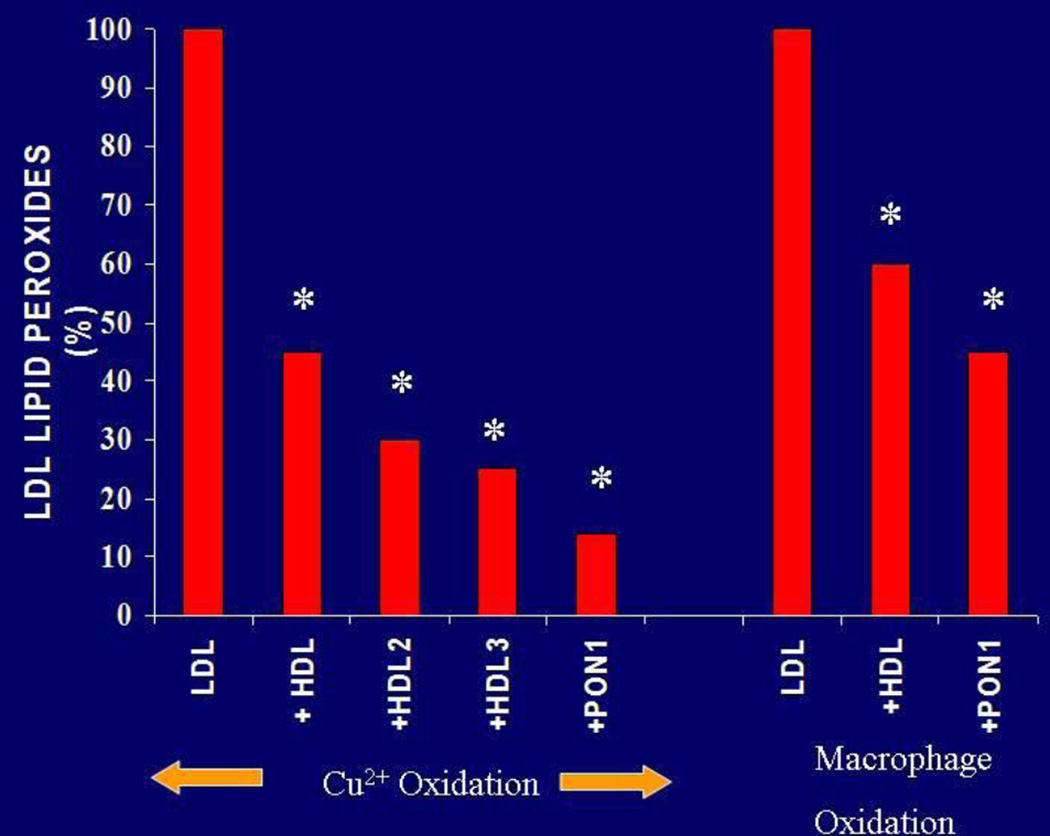
PON1 was first shown to retard the oxidation of LDL in vitro by Mackness et al [62, 63] (Figure 3), results which were subsequently confirmed by many other laboratories [20, 64–66] and extended to include HDL and cell membranes [67, 68]. In studies of macrophages in cell culture PON1 exhibits a variety of potentially atheroprotective properties such as reducing macrophage oxidative stress and the ability of macrophages to oxidise LDL, inhibit cholesterol synthesis and promote cholesterol efflux [12, 69].
Figure 3.
Inhibition of LDL oxidation catalysed by Cu2+ and by macrophages by PON1
The mechanism by which PON1 retards LDL oxidation is unproven but appears to involve the hydrolysis of the truncated oxidised fatty acids from phospholipid, cholesterylester and triglyceride hydroperoxides resulting in the production of lysophospholipids, cholesterol, diglyceride and oxidised fatty acids [60, 61, 70]. The lysophospholipids and oxidised fatty acids produced by PON1 are themselves potentially atherogenic but do not appear to be so when produced on HDL [71].
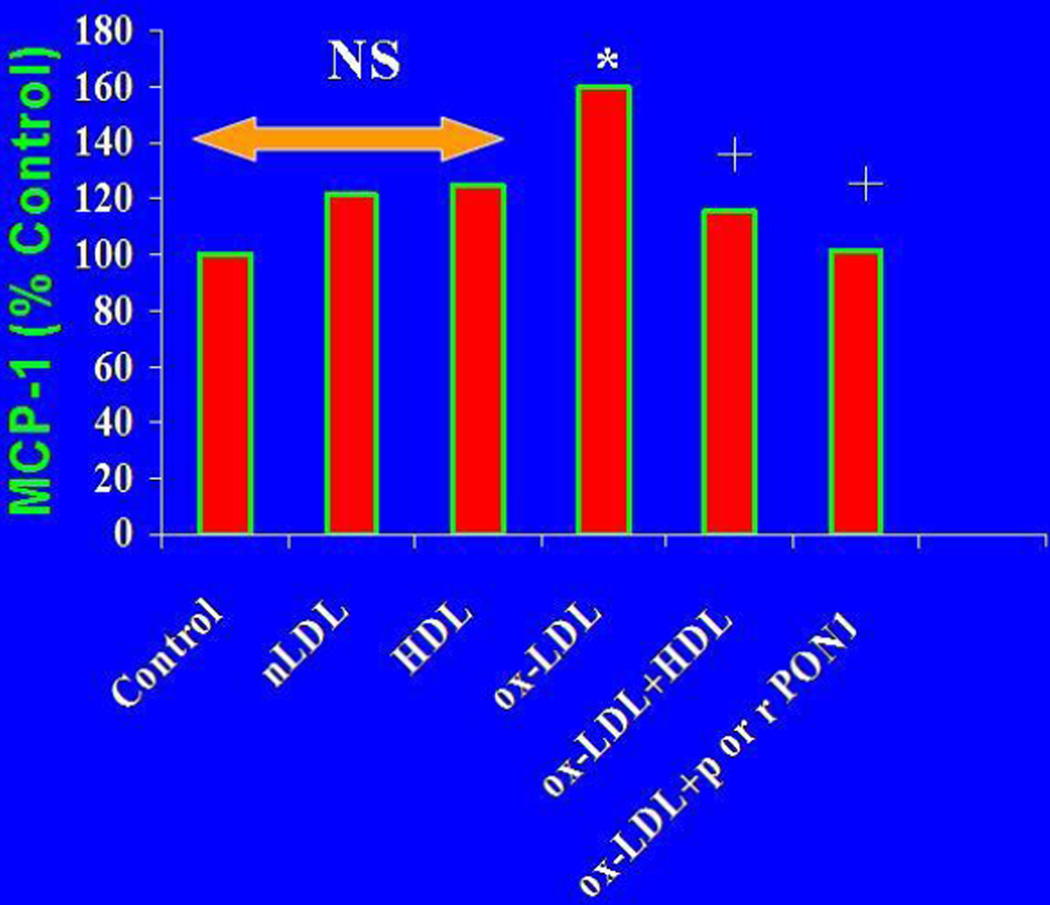
Unmetabolised lipid hydroperoxides are highly inflammatory, inducing the production of MCP-1 by arterial cells which attracts monocytes into the arterial intima at the very start of the atherosclerotic process [49–51]. In the presence of PON1, lipid hydroperoxide concentrations are reduced, MCP-1 production inhibited [57, 72] and the atherosclerotic process attenuated (Figure 4).
Figure 4.
Inhibition of ox-LDL mediated endothelial cell MCP-1 production by PON1
The transgenic or adenovirus mediated expression of human PON1 in various mouse models of atherosclerosis has been shown to retard or reverse atherosclerosis by mechanisms which include a reduction in circulating and aortic oxidised-LDL (ox-LDL), a reduction in macrophage oxidative stress and foam cell formation, an increase in reverse cholesterol transport and a normalisation of endothelial function [73–77].
Interestingly, in human aortas, immunostaining for PON1 progressively increases as atherosclerosis develops [78] and the presence of both PON1 and PON3 in aortic macrophages indicates a cellular protective effect of these enzymes [79]. Recently it has been shown that the expression of human PON1 can prevent diabetes development in mice through its antioxidant properties and the stimulation of beta-cell insulin release, suggesting a possible role for PON1 in insulin biosynthesis [80, 81].
A possible relationship between PON1 and atherosclerosis was first indicated by the discovery that patients who had a myocardial infarction had lower activity than matched controls. Low levels of serum PON1 have been consistently associated with susceptibility to CHD development in case-control studies. Several studies have previously shown prospectively that PON1 activity is a risk factor for CHD development independently of HDL concentration [82–84] including a study in Type 2 diabetes [85] although the finding is not universal [86, 87]. However, a recent meta-analysis of the relationship between PON1 activity and CHD susceptibility which studied 9853 cases and 11408 controls showed that on pooled analysis CHD patients had 19% lower PON1 activity than did controls (P< 10−5). The same results were found in all subgroup analyses including coronary stenosis, myocardial infarction, ethnicity, age and sample size amongst others [88]. Low PON1 concentration predicts cardiovascular mortality in haemodialysis patients [89].
Many studies have also investigated several PON1 polymorphisms as risk factors for CHD with positive associations being seen in some but not all studies. Meta analyses have shown at best a marginal significance of the PON1-Q192R polymorphism as a risk factor for CHD but no relationship of other PON1 polymorphisms and CHD [22, 23].
In a study of 3668 subjects undergoing elective coronary angiography without acute coronary syndrome each had serum paraoxon and phenylacetate hydrolysis measured and were followed for 3 years for major adverse cardiovascular events (MACE= death, MI, stroke). Low PON1 paraoxonase and arylesterase activities were both associated with increased MACE risk [90]. Low arylesterase activity predicted future development of MACE in both primary and secondary prevention cohorts and even reclassified some subjects into higher risk categories. A genome wide association study of SNPs in the PON1 gene associated with PON1 activity were not associated with MACE risk in an angiographic cohort of 2136, or with history of CHD or MI in the Coronary Artery Disease Genome Wide Replication and Meta-Analysis Consortium of some 80000 subjects [90]. These authors were therefore able to replicate the findings of several previous studies, (PON1 activity is an important determinant of CHD development, genotype is not [91, 92]) with far greater numbers of subjects.
It would appear therefore, on the balance of current evidence, that PON1 activity is atheroprotective. The putative mechanisms explaining this atheroprotection are shown in table 1. These mechanisms have been reviewed in some detail previously [60, 61] and only 3 of these studies will be described here.
Table 1.
Potential antiatherosclerotic mechanisms of PON1
| Mechanism | Reference |
|---|---|
| Prevention of LDL and cell membrane oxidation | 20, 60–65, 68 |
| Prevention of LDL Glycation | 95 |
| Prevention of diabetes development | 80, 81 |
| Reduction of macrophage oxidative stress | 73 |
| Promotion of macrophage RCT | 69, 73 |
| Normalisation of endothelial function | 58, 75 |
| Metabolism of homocysteine thiolactones+ | 96–98 |
| Prevention of LCAT oxidative inactivation | 99 |
| Disposal of toxic apoptosis products | 93 |
| Prevention of apoptosis | 94 |
| Reduction of monocyte macrophage inflammatory response | 101, 102 |
A physiological role for PON1 in the detoxication of homocysteine thiolactone (HCTL) has recently been challenged with the discovery that biphenyl hydrolase-like protein has a catalytic efficiency towards HCTL 7.7 × 104 M−1 s−1 greater than PON1 indicating it to be the physiological detoxicant [100].
HDL isolated from healthy individuals increases NO bioavailability (improving endothelial function) via interaction with the SR-B1 receptor when incubated with cultured endothelial cells. In contrast, HDL isolated from individuals with CHD contained more malondialdehyde, was less able to interact with SR-B1 and caused no increase in (and in some cases inhibited) NO bioavailability. Adding malondialdehyde to healthy HDL or using HDL from PON1 deficient mice blunted NO production. The reason for this difference between healthy and CHD HDL was due to much lower PON1 activity associated with CHD HDL and hence a lower ability to remove the malondialdehyde [58].
Enrichment of human monocyte/macrophages with unesterified cholesterol causes the release of highly procoagulent microvesicles (UCMV) via the induction of apoptosis [93]. MVs contain damage-associated molecular patterns (DAMPs) which are endogenous danger signals which stimulate an immune response. UCMV´s induce lymphocyte rolling and adhesion to post capillary venules in rats in vivo, and augment adhesion of human monocytes to mouse aortic explants and cultured human endothelial cells via induction of intercellular adhesion protein-1. UCMVs induce mitochondrial production of superoxide and peroxides and contain malondialdehyde-like peroxidised epitopes. The incubation of UCMVs with HDL or purified PON1 detoxifies the malondialdehyde DAMPs and prevents the immune response. Thus, this is a potentially novel atheroprotective role of PON1 but also has wider implications of PON1 as a non-inflammatory remover of apoptosis induced cellular debris [93].
Garcia-Heredia et al used a metabolomics approach to study the effects of Ox-LDL on cultured human endothelial cells [94]. Ox-LDL induced perturbations of carbohydrate and phospholipid metabolism, specifically in hexose metabolism, glycolysis and the tricarboxylic acid cycle, decreased phospholipid synthesis and increased phospholipid breakdown. These metabolic changes were associated with increased endothelial cell apoptosis. HDL containing PON1 largely reversed these metabolic changes and greatly decreased apoptosis, HDL with no PON1 was far less effective.
6.2 PON1 and other inflammatory diseases
Low serum PON1 is associated with many diseases with a large inflammatory component including diabetes mellitus, rheumatoid arthritis, systemic lupus erythromatosis, and various hepatic and renal diseases including renal failure, psoriasis and macular degeneration [reviewed in 35]. These diseases are also characterised by having dysfunctional HDL believed to be (but not proven to be) caused by the low PON1 activity [56] and increased rates of CHD.
At present it is not known whether the low PON1 in these diseases is mechanistically causative in the aetiology of these diseases or simply a consequence of disease presence. Large prospective epidemiological studies will be required to determine whether low PON1 is causal in disease development and to determine the future direction of PON1 research in these diseases either as a causal agent and potential therapeutic target or as a potential diagnostic tool.
5. PON1 and organophosphate toxicity
This is the subject of excellent recent reviews and will be dealt with only briefly here [103, 104].
Organophosphorus compounds (OPs) are widely used in both rural and urban settings as pesticides leading to widespread human exposure. OPs are activated in the body by the process known as oxidative desulphuration to produce the toxic oxon forms. Some but not all parent or activated OPs are PON1 substrates, of those that are (which include some of the most widely used including diazinon and chlorpyriphos (CP) oxons), most are hydrolysed at different rates by the PON1-Q and R isoforms. Therefore, the majority of studies in this area have concentrated on PON1 as a genetic determinant of OP toxicity [103]. Recently, however, studies have indicated that at environmentally relevant concentrations (nanomolar) of CPoxon, it is unlikely that PON1 genotypes would influence an individual's ability to hydrolyse CPoxon [105].
Animal studies have consistently shown that PON1 protects against OP toxicity. The administration of exogenous PON1 to rats and mice protects against OP toxicity and administration of the PON1 isoform that hydrolyses the OP at the greatest rate affords most protection [106]. PON1 knock-out mice are dramatically more susceptible to diazoxon and CPoxon toxicity and the administration of exogenous PON1 restores resistance to these OPs [77].
In contrast to animal studies, there have been relatively few studies on the role of PON1 in OP toxicity in humans. Military personnel deployed in the Persian Gulf War of 1990–91 were exposed to low levels of the OP nerve gas sarin and various OP insecticides as well as other chemical and biological agents [103]. In US veterans, low activity of the PON1-192Q isoform correlated better with neurological symptoms of Gulf War Illness (GWS) than did the PON1-192R isoform or PON1 genotype [107]. In UK deployed veterans, serum PON1 activity was 25–35% lower than in non-deployed veterans which was not due to differences in PON1 genotype [108]. However, neither PON1 activity nor genotype was associated with specific symptoms of illness. Further studies are required to show PON1 as a risk factor for GWS.
Several studies have investigated the role of PON1 in modulating the chronic central and peripheral nervous system abnormalities, so called "dippers flu", associated with exposure of sheep dippers to diazinon [109–111]. On the basis of the findings reported, it appears that diazinon has contributed to ill health in sheep dippers who had a lower capacity to detoxify diazoxon. Several other studies of OP exposed agricultural workers have indicated individuals with PON1 genotypes associated with low activity (Q/M) had a greater frequency of various indices of OP toxicity (chronic toxicity, genotoxicity, impaired thyroid function) [112–115]. Unfortunately in these studies exposure was to a large number of pesticides including OPs, not all of which were PON1 substrates, limiting the significance of the results.
Residential exposure to OPs (mainly diazinon and CP) was associated with an increase in Parkinson's disease risk in PON1-55M carriers and to an increased risk of brain tumours in children with low PON1 activity [116, 117]. Epidemiological studies also suggest that occupational exposure to OPs might increase the risk of Parkinson's disease [118]. In utero exposure to OPs of foetuses of mothers with low PON1 resulted in babies born with smaller head circumference and shorter gestational age [119, 120]. In children of OP exposed Mexican-American women living in California and children in New York exposed in utero, low PON1 was associated with poorer scores in various tests of mental development [121, 122].
Amyotrophic lateral sclerosis (ALS) is an adult onset progressive and fatal neurodegenerative disease with unknown aetiology. Recent evidence has, however, suggested a link between exposure to toxic environmental compounds including OPs and sporadic ALS [123]. Population studies have indicated an association of PON1 polymorphisms and ALS, however, the finding is inconsistent between populations [124–126]. However, in a large multi-population study, Wills et al were unable to find any reductions in PON1 OP hydrolysis associated with ALS [127], clearly demonstrating the problems in defining a role for PON1 in human toxicology.
Although available human studies provide some evidence that low PON1 activity may increase susceptibility to various adverse effects of some OPs, doubts remain, and further studies in which OP exposure is more carefully characterised and monitored are required. There is also a need to determine what effect low PON1 activity found in children has on OP toxicity.
6.4 PON1 and cancer
Although lower PON1 activity has been linked to the development of a wide variety of cancers [35] unfortunately, the majority of studies reported have included only small numbers of study subjects and cases and controls were inadequately matched (see Section 7), usually through the absence of matching for PON1 genotype. Both of these factors increase the statistical chance of any difference being found by accident. Much larger and matched studies are required. However, Saadat [128] conducted a meta-analysis of PON1 genetic polymorphisms and breast cancer susceptibility covering six eligible studies. The PON1-192R allele was associated with a decreased risk of breast cancer (OR= 0.57, 95% CI 0.49–0.67, P< 0.001). However, both PON1-55LM and PON1-55MM genotypes were associated with increased risk (OR= 1.32, 95% CI 1.10–1.58, P= 0.002 and OR= 2.16, 95% CI 1.75–2.68, P< 0.001 respectively). There was also a significant linear trend in risk associated 0, 1 and 2 PON1-55M alleles (X2= 54.2, P< 0.001).
Destructive bone disease is a common feature in patients with multiple myeloma. Dowling et al studied 111 patients with multiple myeloma and found that serum PON1 concentration was positively related to the degree of bone disease (more bone disease = higher PON1) indicating PON1 could be a diagnostic marker [129].
These studies offer some hope that rigorously conducted research may indicate a role for PON1 in the development or diagnosis of some cancers.
7. PON1 and quorum quenching
Quorum sensing (QS) is a bacterial cell to cell signalling system which controls the production of virulence factors in many pathological bacteria [130, 131]. QS is mediated by the production of autoinducers, small signal molecules which activate or repress gene expression when a minimal threshold concentration is reached [130, 131].
N-acyl homoserine lactones (AHLs) are used as autoinducers by many gram-negative pathogens such as Pseudomonas aeruginosa to control the expression of virulence factors [130, 131]. AHLs are degraded by lactonases which is termed quorum quenching and the production of these enzymes is an effective way to interfere with QS and prevent bacterial virulence [132].
Both murine and human PON1 are capable of hydrolysing AHLs and therefore of modulating P. aeruginosa QS [133]. Using a Drosophila melanogaster (which have no intrinsic PON1) model of infection, the transgenic expression of human PON1 protected Drosophila from P. aeruginosa lethality [134]. Further studies in this model have indicated that as well as quorum quenching, PON1 expression decreased superoxide anion levels and altered the expression of multiple genes related to oxidative stress. In addition, the profile of bacterial species colonising the Drosophila gut was dramatically altered [135]. These and other studies indicate that PON1 (and PON2 and PON3 which share these quorum quenching properties) could be useful tools in combating infections by QS bacteria warranting further studies.
6.6 PON1 and ageing
Several studies have shown a reduction in serum PON1 activity and consequent loss of protective functions of HDL, with advancing age [136, 137]. On the other hand, polymorphisms within the PON1 gene have been associated with successful ageing, leading to the suggestion that PON1 is a longevity associated protein [138]. One large meta-analysis, however, failed to find a relationship between the PON1-192 SNP and human longevity [139]. This study did not include PON1 activity measurements, which if activity falls with age regardless of genotype, would be a more relevant parameter. Further detailed studies are required.
In an exciting recent advance Lee et al [139] have investigated the effects of silencing the PON1 gene in cultured human dermal microvascular endothelial cells (HDMECs). The expression of endogenous PON1 was knocked down using small interfering RNA. The authors found that 1) cell viability was decreased, 2) the expression of cellular senescence biomarkers such as moesin and Rho-GDI were significantly decreased and 3) ageing of the HDMECs was triggered. The findings suggest that cellular PON1 has a functional role in cellular senescence and also functions as an ageing related protein. With an ageing world population, healthy ageing is an important goal, therefore further study into this new and potentially exciting function of PON1 is warranted.
7. ANALYTICAL CONSIDERATIONS
Due to the lack of a definitive "natural" substrate for PON1, a variety of non-physiological substrates are used to measure PON1 activity. Many of these substrates, particularly the organophosphates, are highly toxic e.g. paraoxon has a LD50 of 0.5 ppm on the same scale that HCN gas has an LD50 of 12 ppm, and not suitable for automated analysis systems. The use of different assay systems also makes comparisons between studies almost impossible. A number of relatively non-toxic substrates have been developed in Seattle [140] which could be used until a definitive PON1 substrate is developed.
One of the major problems afflicting the PON1 field is the proliferation of small case-control studies with inadequate subject matching [141]. Small (inadequate subject numbers) studies are inherently problematic in that they are very prone to Type II statistical error i.e there is a large probability that any difference between cases and controls are found simply by chance and/or error. Coupling small studies with inadequate matching almost guarantees this will happen. Because PON1 activity is largely determined by SNP distribution it is vital that cases and controls are matched for PON1 haplotype. Large properly matched epidemiological studies are required in many areas of PON1 research to verify PON1 involvement in particular diseases.
8. CONCLUSION
It appears increasingly obvious that serum PON1 contributes to the atheroprotective function of HDL by decreasing lipid peroxidation in a variety of diseases with an inflammatory component. Much more research has been and is being conducted into PON1 and atherosclerosis than into other diseases, although there is still a need to determine exactly how PON1 contributes to HDL atheroprotective function. Almost nothing is known regarding the PON1 substrates in atherosclerotic tissue, there is therefore an urgent need to uncover theses substrates (and those in other diseases in which PON1 is implicated) which should point to the molecular mechanism of action of PON1. It is also true to say that a definitive role for PON1 in protecting humans from the toxic effects of OP pesticides has not been proven despite decades of research. Research into other putative roles for PON1 in cancer development, bacterial infection and ageing are really in their infancy but could never the less prove fruitful and exciting avenues of research.
In conclusion, PON1 contributes to the antioxidative function of HDL and is atheroprotective. The putative role of PON1 in preventing bacterial infection may contribute to HDLs role in innate immunity. More basic and properly conducted clinical epidemiological investigations of all the possible functions of PON1 are required. Particularly, although much is known about genetic, nutritional, life-style and pharmacological factors which effect human serum PON1 activity levels in vivo and some information is available on receptors and signalling pathways affecting PON1 synthesis in vitro [16, 40, 41], almost nothing is known about the regulatory pathways which operate in humans in vivo nor is much information available on epigenetic factors or miRNAs which may contribute to normal regulation of PON1. There is, therefore, a large hole in our knowledge base which hampers the discovery of small molecule effectors of PON1 for therapeutic interventions.
HIGHLIGHTS.
Human paraoxonase 1 (PON1) protein and gene structure
PON1 single nucleotide polymorphisms
PON1 promiscuous activities
PON1 multiple roles in disease development
Acknowledgements
"This review and the corresponding Gene Wiki article are written as part of the Cardiac Gene Wiki Review series- a series resulting from a collaboration between the journal GENE, the Gene Wiki Initiative and the BD2K initiative. The Cardiac Gene Wiki Initiative is supported by National Institutes of Health (GM083924 and GM114833). Additional support for Gene Wiki Reviews is provided by Elsevier, the publisher of GENE. The authors thank Professor Patrick Dansette, University of Paris, Descartes for providing Figure 1 and Professor Clem Furlong, University of Washington for providing Figure 2.
The corresponding Gene Wiki entry for this review can be found at http://en.wikipedia.org/wiki/PON1.
ABBREVIATIONS
- ALS
amyotrophic lateral sclerosis
- CETP
cholesteryl-ester transfer protein
- CHD
coronary heart disease
- CPoxon
chlorpyriphos-oxon
- DAMPs
damage associated molecular patterns
- eNOS
endothelial nitric oxide synthase
- GSH
reduced glutathione
- GWS
Gulf war illness
- HDL
high-density lipoprotein
- HDL-C
HDL cholesterol
- LDL
low-density lipoprotein
- MACE
major adverse coronary events
- MCP1
monocyte chemotactic protein 1
- miRNA
microRNA
- NO
nitric oxide
- OP
organophosphate
- ox-LDL
oxidized LDL
- PON
paraoxonase
- PPAR
peroxisome proliferator activated protein
- QS
quorum sensing
- SNP
single nucleotide polymorphism
- Sp1
specificity protein 1
- SREBP2
sterol regulatory binding protein 2
- UCMV
unesterified cholesterol microvesicles
- UTR
untranslated region.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
There are no conflicts of interest
REFERENCES
- 1.Rajkovic MG, Rumora L, Barisic K. The paraoxonase 1, 2 and 3 in humans. Biochemia Medica. 2011;21:122–130. doi: 10.11613/bm.2011.020. [DOI] [PubMed] [Google Scholar]
- 2.Deakin S, Leviev I, Gomaraschi M, Calabresi L, Francesshini G, James RW. Enzymatically active paraoxonase-1 is located at the external membrane of producing cells and released by a high affinity, saturable, desorption mechanism. J Biol Chem. 2002;277:4301–4308. doi: 10.1074/jbc.M107440200. [DOI] [PubMed] [Google Scholar]
- 3.Marsillach J, Mackness B, Mackness M, Riu F, Beltran R, Joven J, Camps J. Immunohistochemical analysis of paraoxonases 1, 2 and 3 expression in normal mouse tissues. Free Rad Biol Med. 2008;45:146–157. doi: 10.1016/j.freeradbiomed.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigo L, Hernandez A, Lopez-Caballero JJ, Gil F, Pla A. Immunohistochemical evidence for the expression and induction of paraoxonase in rat liver, kidney, lung and brain tissue. Implications for its physiological role. Chem Biol Interact. 2001;137:123–137. doi: 10.1016/s0009-2797(01)00225-3. [DOI] [PubMed] [Google Scholar]
- 5.Mackness B, Durrington PN, Mackness MI. Human serum paraoxonase. Gen. Pharmac. 1998;31:329–336. doi: 10.1016/s0306-3623(98)00028-7. [DOI] [PubMed] [Google Scholar]
- 6.Mazur A. An enzyme in the animal organism capable of hydrolysing the phosphorus-fluorine bond of alkyl fluorophosphates. J Biol Chem. 1946;164:271–289. [PubMed] [Google Scholar]
- 7.Aldridge WN. Serum esterases 2- An enzyme hydrolysing diethyl p-nitrophenylphosphate (E600) and its identity with the A-esterase of mammalian sera. Biochem. J. 1953;53:117–124. doi: 10.1042/bj0530117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson WS, Silva RAGD, Chantepie S, Lagor WR, Chapman MJ, Kontush A. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters- Relevance to antioxidative function. Arteriioscler Thromb Vasc Biol. 2009;29:870–876. doi: 10.1161/ATVBAHA.109.186031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harel M, Aharoni, Gaidukov L, Brumshtein B, Khersonsky O, Mayeb R, et al. Structure and evolution of the serum paraoxonase family of detoxifying and antiatherosclerotic enzymes. Nature Structural & Mol. Biol. 2004;11:412–419. doi: 10.1038/nsmb767. [DOI] [PubMed] [Google Scholar]
- 10.Primo-Parma SL, Sorenson RC, Teiber J, La Du BN. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics. 1996;33:498–509. doi: 10.1006/geno.1996.0225. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Sanabria F, Rull A, Beltran-Debon R, Aragones G, Camps J, Mackness B, et al. Tissue distribution and expression of paraoxonases and chemokines in the mouse: the ubiquitous and joint localisation suggest a systemic and coordinated role. J Mol Histol. 2010;41:379–386. doi: 10.1007/s10735-010-9299-x. [DOI] [PubMed] [Google Scholar]
- 12.Aviram M, Rosenblat M. Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic Biol Med. 2004;37:1304–1316. doi: 10.1016/j.freeradbiomed.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 13.Reddy ST, Devarajan A, Bourquard N, Shih D, Fogelman AM. Is it just paraoxonase 1 or are other members of the paraoxonase gene family implicated in atherosclerosis? Curr Opin Lipidol. 2008;19:405–408. doi: 10.1097/MOL.0b013e328304b64e. [DOI] [PubMed] [Google Scholar]
- 14.Deakin SP, James RW. Genetic and environmental factors modulating serum concentrations and activities of the antioxidant enzyme paraoxonase-1. Clin Sci. 2004;107:435–447. doi: 10.1042/CS20040187. [DOI] [PubMed] [Google Scholar]
- 15.Costa LG, Vitalone A, Cole TB, Furlong CE. Modulation of paraoxonase activity. Biochem Pharmacol. 2005;69:541–550. doi: 10.1016/j.bcp.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 16.Schrader C, Rimbach G. Determinants of paraoxonase 1 status: genes, drugs and nutrition. Curr Med Chem. 2011;18:5624–5643. doi: 10.2174/092986711798347216. [DOI] [PubMed] [Google Scholar]
- 17.Leviev I, Negro F, James RW. Two alleles of the human paraoxonase gene produce different amounts of mRNA. An explanation for differences in serum concentrations of paraoxonase associated with the (Leu-Met54) polymorphism. Arterioscler Thromb Vasc Biol. 1997;17:2935–2939. doi: 10.1161/01.atv.17.11.2935. [DOI] [PubMed] [Google Scholar]
- 18.Leviev I, James RW. Promoter polymorphisms of human paraoxonase PON1 gene and serum paraoxonase activities and concentrations. Arterioscler Thromb Vasc Biol. 2000;20:516–521. doi: 10.1161/01.atv.20.2.516. [DOI] [PubMed] [Google Scholar]
- 19.Kim DS, Burt AA, Ranchalis JE, Richter RJ, Marshall JK, Eintracht JF, et al. Additional common polymorphisms in the PON gene cluster predict PON1 activity but not vascular disease. J Lipids. 2012;2012:476316. doi: 10.1155/2012/476316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aviram M, Billecke S, Sorenson R, Bisgaier C, Newton R, Rosenblat M, et al. Paraoxonase active site required for protection against LDL oxidation involves its free sulphydryl group and is different from that required for its arylesterase/paraoxonase activities: selective action of human paraoxonase alloenzymes Q and R. Arterioscl. Thromb. Vasc. Biol. 1998;10:1617–1624. doi: 10.1161/01.atv.18.10.1617. [DOI] [PubMed] [Google Scholar]
- 21.Mackness B, Mackness MI, Arrol S, Turkie W, Durrington PN. Effect of the human serum paraoxonase 55 and 192 genetic polymorphisms on the protection by high density lipoprotein against low density lipoprotein oxidative modification. FEBS Letts. 1998;423:57–60. doi: 10.1016/s0014-5793(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 22.Wheeler JG, Keavney BD, Watkins H, Collins R, Danesh J. Four paraoxonase gene polymorphisms in 11212 cases of coronary heart disease and 12786 controls: meta-analysis of 43 studies. Lancet. 2004;363:689–695. doi: 10.1016/S0140-6736(04)15642-0. [DOI] [PubMed] [Google Scholar]
- 23.Harel M, Brumshtein B, Meged R, Dvir H, Ravelli RBG, McCarthy A, et al. 3-D structure of serum paraoxonase 1 sheds light on its activity, stability, solubility and crystalizability. Arh Hig Rada Toksikol. 2007:347–353. doi: 10.2478/v10004-007-0028-0. [DOI] [PubMed] [Google Scholar]
- 24.Khersonsky O, Tawfik DS. The histidine 115-histidine 134 dyad mediates the lactonase activity of mammalian serum paraoxonases. J Biol Chem. 2006;281:7649–7656. doi: 10.1074/jbc.M512594200. [DOI] [PubMed] [Google Scholar]
- 25.Rosenblat M, Gaidukov L, Khersonsky O, Vaya J, Oren R, Tawfik DS, Aviram M. The catalytic histidine dyad of high-density lipoprotein associated serum paraoxonase-1 (PON1) is essential for PON1-mediated inhibition of low density lipoprotein oxidation and stimulation of macrophage cholesterol efflux. J Biol Chem. 2006;281:7657–7665. doi: 10.1074/jbc.M512595200. [DOI] [PubMed] [Google Scholar]
- 26.Khersonsky O, Tawfik DS. Structure-reactivity studies of serum paraoxonase PON1 suggest that its true native activity is lactonase. Biochemistry. 2005;44:6371–6382. doi: 10.1021/bi047440d. [DOI] [PubMed] [Google Scholar]
- 27.Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2 and PON3 are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005;46:1239–1247. doi: 10.1194/jlr.M400511-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Syed FA, Bett J, Walters DL. Anti-platelet therapy for Acute Coronary Syndrome: A review of currently available agents and what the future holds. Cardoivasc Hematol Disord Drug Targets. 2011 doi: 10.2174/187152911798347007. [DOI] [PubMed] [Google Scholar]
- 29.Bouman HJ, Schomig E, van Werkum JW, Velder J, Hackeng CM, Hirschhauser C, et al. Paraoxonase-1 is a major determinant of Clopidogrel efficacy. Nature Med. 2011;17:110–116. doi: 10.1038/nm.2281. [DOI] [PubMed] [Google Scholar]
- 30.Camps J, Joven J, Mackness B, Mackness M, et al. Paraoxonase-1 and clopidogrel efficacy. Clarifications needed. Nat Med. 2011;17:1041–1042. doi: 10.1038/nm.2386. (2011) [DOI] [PubMed] [Google Scholar]
- 31.Tresokosol D, Suktitipat B, Hunnangkul S, Kamkaew R, Poldee S, Tassaneetrithep B, Likidlilid A. Effects of cytochrome P450 2C19 and paraoxonase 1 polymorphisms on antiplatelet response to Clopidogrel therapy in patients with coronary artery disease. PLOS One. 9:e110188. doi: 10.1371/journal.pone.0110188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dansette PM, Rosi J, Bertho G, Mansuy D. Cytochromes P450 catalyze both steps of the major pathway of Clopidogrel bioactivation, whereas paraoxonase catalyzes the formation of a minor thiol metabolite isomer. Chem Res Toxicol. 2012;25:348–356. doi: 10.1021/tx2004085. [DOI] [PubMed] [Google Scholar]
- 33.Clendenning JB, Humbert R, Green ED, Wood C, Traver D, Furlong CE. Structural organisation of the human PON1 gene. Genomics. 1996;35:586–589. doi: 10.1006/geno.1996.0401. [DOI] [PubMed] [Google Scholar]
- 34.Wang M, Lang X, Zou L, Huang S, Xu Z. Four genetic polymorphisms of the paraoxonase gene and risk of coronary heart disease: A meta-analysis based on 88 casecontrol studies. Atherosclerosis. 2010;214:377–385. doi: 10.1016/j.atherosclerosis.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 35.Goswami B, Tayal D, Gupta N, Mallika V. Paraoxonase: A multifaceted biomolecule. Clin Chim Acta. 2009;410:1–12. doi: 10.1016/j.cca.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 36.La Du BN. Human serum paraoxonase/arylesterase. In: Kalow W, editor. Pharmacogenetics of Drug Metabolism. New York: Pergamon Press; 1992. pp. 51–91. [Google Scholar]
- 37.McDaniel CY, Dail MB, Wills RW, Chambers HW, Chambers JE. Paraoxonase 1 polymorphisms within a Mississippi USA population as possible biomarkers of enzyme activities associated with disease susceptibility. Biochem Genet. 2014;52:509–523. doi: 10.1007/s10528-014-9663-8. [DOI] [PubMed] [Google Scholar]
- 38.Phuntuwate W, Suthisisang C, Koanantabul B, Mackness MI, Mackness B. Paraoxonase 1 status in the Thai population. J. Hum. Genets. 2005;50:293–300. doi: 10.1007/s10038-005-0255-7. [DOI] [PubMed] [Google Scholar]
- 39.Durrington PN, Mackness B, Mackness MI. The hunt for nutritional and pharmacological modulators of paraoxonase. Arterioscler. Thromb. Vasc. Biol. 2002;22:1248–1250. doi: 10.1161/01.atv.0000027414.34728.1f. [DOI] [PubMed] [Google Scholar]
- 40.Costa LG, Giordano G, Furlong CE. Pharmacological and dietary modulators of paraoxonase 1 (PON1) activity and expression: the hunt goes on. Biochem Pharmacol. 2011;81:337–344. doi: 10.1016/j.bcp.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camps J, Marsillach J, Joven J. Pharmacological and lifestyle factors modulating serum paraoxonase-1 activity. Mini Rev Med Chem. 2009;9:911–920. doi: 10.2174/138955709788681591. [DOI] [PubMed] [Google Scholar]
- 42.Precourt L-P, Amre D, Denis M-C, Lavoie J-C, Delvin E, Seidman E, Levy E. The three-gene paraoxonase family: Physiologic roles, actions and regulation. Atherosclerosis. 2011;214:20–36. doi: 10.1016/j.atherosclerosis.2010.08.076. [DOI] [PubMed] [Google Scholar]
- 43.Mackness B, Marsillach J, Elkeles RS, Godsland IF, Feher MD, Rubens MB, et al. Paraoxonase-1 is not associated with coronary artery calcification in type 2 diabetes: Results from the PREDICT study. Dis Markers. 2012;33:101–112. doi: 10.3233/DMA-2012-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cole TB, Jampsa RL, Walter BJ, Arndt TL, Richter RJ, Shih DM, et al. Expression of human paraoxonase (PON1) during development. Pharmacogenetics. 2003;13:357–364. doi: 10.1097/00008571-200306000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Parker-Katiraee L, Bousiaki E, Monk D, Moore GE, Nakabayashi K, Scherer SW. Dynamic variation in allele-specific gene expression of Paraoxonase-1 in murine and human tissues. Hum Molecul Genet. 2008;17:3263–3270. doi: 10.1093/hmg/ddn222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu M-U, Liao Y-C, Lin R-T, Wang Y-S, Hsi E, Lin H-F, et al. A functional polymorphism of PON1 interferes with microRNA binding to increase the risk of ischemic stroke and carotid atherosclerosis. Atherosclerosis. 2013;228:161–167. doi: 10.1016/j.atherosclerosis.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 47.Nolan D, Kraus WE, Hauser E, Li Y-J, Thompson DK, Johnson J, et al. Genome-wide linkage analysis of cardiovascular disease biomarkers in a large, multigenerational family. PLOS ONE. 2013;8:e71779. doi: 10.1371/journal.pone.0071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durrington PN. Diagnosis and Management. 2nd Edition. London: Butterworth Heinemann; 1995. Hyperlipidaemia. [Google Scholar]
- 49.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol modifications of low-density lipoprotein that increase its atherogenicity. New Engl. J. Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 50.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chisholm GM, Penn MS. Oxidised lipoproteins and atherosclerosis. In: Fuster V, Ross R, Topol EJ, editors. Atherosclerosis and coronary artery disease. Philadelphia: Lippincott-Raven; 1996. pp. 129–149. [Google Scholar]
- 52.Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 53.Shah PK. Evolving concepts on benefits and risks associated with therapeutic strategies to raise HDL. Curr Opin Cardiol. 2010;25:603–608. doi: 10.1097/HCO.0b013e32833f0382. [DOI] [PubMed] [Google Scholar]
- 54.Fisher EA, Feig JE, Hewing B, Hazen SL, Smith JD. High-density lipoprotein function, dysfunction and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2012;32:2813–2820. doi: 10.1161/ATVBAHA.112.300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Navab M, Hama-Levy S, Van Lenten BJ, Fonarow GC, Carelinez CJ, Castellani LW, et al. A.M. Mildly oxidised LDL induces an increased apolipoprotein J/paraoxonase ratio. J. Clin. Invest. 1997;99:2005–2019. doi: 10.1172/JCI119369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fogelman AM. Mechanisms of disease: proatherogenic HDL- an evolving field. Nat Clin Pract Endocrinol Metab. 2006;2:504–511. doi: 10.1038/ncpendmet0245. [DOI] [PubMed] [Google Scholar]
- 57.Navab M, Imes SS, Hama SY, Hough GP, Ross LA, Bork RW, et al. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J. Clin. Invest. 1991;88:2039–2046. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest. 2011;121:2693–2708. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mackness MI, Abbott CA, Arrol S, Durrington PN. The role of high density lipoprotein and lipid-soluble antioxidant vitamins in inhibiting low-density lipoprotein oxidation. Biochem. J. 1993;294:829–835. doi: 10.1042/bj2940829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mackness B, Mackness M. The antioxidant properties of high-density lipoproteins in atherosclerosis. Panminerva Med. 2012;54:83–90. [PubMed] [Google Scholar]
- 61.Mackness M, Mackness B. Current aspects of paraoxonase-1 research. In: Komoda T, editor. The HDL Handbook- Biological functions and clinical implications. 2nd Edition. London: Academic Press; 2014. pp. 273–291. [Google Scholar]
- 62.Mackness MI, Arrol S, Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Letts. 1991;286:152–154. doi: 10.1016/0014-5793(91)80962-3. [DOI] [PubMed] [Google Scholar]
- 63.Mackness MI, Arrol S, Abbott CA, Durrington PN. Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atherosclerosis. 1993;104:129–135. doi: 10.1016/0021-9150(93)90183-u. [DOI] [PubMed] [Google Scholar]
- 64.Watson AD, Berliner JA, Hama SY, La Du BN, Fault KF, Fogelman AM, Navab M. Protective effect of high density lipoprotein associated paraoxonase - Inhibition of the biological activity of minimally oxidised low-density lipoprotein. J. Clin. Invest. 1995;96:2882–2891. doi: 10.1172/JCI118359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahmed Z, Ravandi A, Maguire GF, Emili A, Draganov D, La Du BN, et al. Apolipoprotein AI promotes the formation of phosphatidylcholine core aldehydes that are hydrolysed by paraoxonase (PON1) during high density lipoprotein oxidation with a peroxynitrite donor. J. Biol. Chem. 2001;276:24473–24481. doi: 10.1074/jbc.M010459200. [DOI] [PubMed] [Google Scholar]
- 66.Draganov DI, La Du BN. Pharmacogenetics of paraoxonase: a brief review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004;369:78–88. doi: 10.1007/s00210-003-0833-1. [DOI] [PubMed] [Google Scholar]
- 67.Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. J. Clin. Invest. 1998;101:1581–1590. doi: 10.1172/JCI1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deakin SP, Bioletto S, Bochaton-Piallat ML, James RW. HDL-associated paraoxonase-1 can redistribute to cell membranes and influence sensitivity to oxidative stress. Free Radic Biol Med. 2011;50:102–109. doi: 10.1016/j.freeradbiomed.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 69.Berrougui H, Loued S, Khalil A. Purified human paraoxonase-1 interacts with plasma membrane lipid rafts and mediates cholesterol efflux from macrophages. Free Rad Biol Med. 2012;52:1372–1381. doi: 10.1016/j.freeradbiomed.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 70.Tavori H, Aviram M, Khatib S, Musa R, Mannheim D, Karmeli R, Vaya J. Paraoxonase 1 protects macrophages from atherogenicity of a specific triglyceride isolated from human carotid lesion. Free Rad Biol Med. 2011;51:234–242. doi: 10.1016/j.freeradbiomed.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 71.Mackness MI, Durrington PN. High density lipoprotein, its enzymes and its potential to influence lipid peroxidation. Atherosclerosis. 1995;115:243–253. doi: 10.1016/0021-9150(94)05524-m. [DOI] [PubMed] [Google Scholar]
- 72.Mackness B, Hine D, Liu Y, Mastorikou M, Mackness M. Paraoxonase 1 inhibits oxidised LDL-induced MCP-1 production by endothelial cells. BBRC. 2004;318:680–683. doi: 10.1016/j.bbrc.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 73.Rozenberg O, Shih DM, Aviram M. Paraoxonase 1 (PON1) attenuates macrophage oxidative status: studies in PON1 transfected cells and in PON1 transgenic mice. Atherosclerosis. 2005;181:9–18. doi: 10.1016/j.atherosclerosis.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 74.Mackness B, Quarck R, Verreth W, Mackness M, Holvoet P. Human paraoxonase-1 overexpression inhibits atherosclerosis in a mouse model of metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:1545–1550. doi: 10.1161/01.ATV.0000222924.62641.aa. [DOI] [PubMed] [Google Scholar]
- 75.Guns P-J, Van Assche T, Verreth W, Fransen P, Mackness B, Mackness M, Holvoet P, Bull H. Paraoxonase 1 gene transfer lowers vascular oxidative stress and improves vasomotor function in apolipoprotein E-deficient mice with pre-existing atherosclerosis. Br. J. Pharmacol. 2008;153:508–516. doi: 10.1038/sj.bjp.0707585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tward A, Xia YR, Wang XP, Shi YS, Park C, Castellani LW, Lusis A, Shih DH. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation. 2002;106:484–490. doi: 10.1161/01.cir.0000023623.87083.4f. [DOI] [PubMed] [Google Scholar]
- 77.Shih DM, Gu L, Xia Y-R, Navab M, Li W-F, Hama S, Castellani LW, Furlong CE, Costa LG, Fogelman AM, Lusis AJ. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- 78.Mackness B, Hunt R, Durrington PN, Mackness MI. Increased immunolocalisation of paraoxonase, clusterin and apolipoprotein AI in the human artery wall with progression of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 1997;17:1233–1238. doi: 10.1161/01.atv.17.7.1233. [DOI] [PubMed] [Google Scholar]
- 79.Marsillach J, Camps J, Beltran-Debon R, Rull A, Aragones G, Maestre-Martinez C, et al. Immunohistochemical analysis of paraoxonases 1 and 3 in human atheromatous plaques. Eur J Clin Invest. 2011;41:308–314. doi: 10.1111/j.1365-2362.2010.02411.x. [DOI] [PubMed] [Google Scholar]
- 80.Rozenberg O, Shiner M, Aviram M, Hayek T. Paraoxonase 1 (PON1) attenuates diabetes development in mice through its antioxidative properties. Free Rad Biol Med. 2008;44:1951–1959. doi: 10.1016/j.freeradbiomed.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 81.Koren-Gluzer M, Aviram M, Meilin E, Hayek T. The antioxidant HDL-associated paraoxonase-1 (PON1) attenuates diabetes development and stimulates β-cell insulin release. Atherosclerosis. 2011;219:532–537. doi: 10.1016/j.atherosclerosis.2011.07.119. [DOI] [PubMed] [Google Scholar]
- 82.Mackness B, Durrington P, McElduff P, Yarnell J, Azam N, Watt M, Mackness M. Low paraoxonase activity predicts coronary events in the Caerphilly Prospective Study. Circulation. 2003;107:2775–2779. doi: 10.1161/01.CIR.0000070954.00271.13. [DOI] [PubMed] [Google Scholar]
- 83.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299:1265–1276. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Himbergen TM, van der Schouw YT, Voorbij HAM, van Tits LJH, Stalenhoef AFH, et al. Paraoxonase (PON1) and the risk for coronary heart disease and myocardial infarction in a general population of Dutch women. Atherosclerosis. 2008;198:408–414. doi: 10.1016/j.atherosclerosis.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 85.Ikeda Y, Inoue M, Suehiro T, Arii K, Kumon Y, Hashimoto K. Low human paraoxonase predicts cardiovascular events in Japanese patients with type 2 diabetes. Acta Diabetol. 2009;46:239–242. doi: 10.1007/s00592-008-0066-3. [DOI] [PubMed] [Google Scholar]
- 86.Troughton JA, Woodside JV, Yarnell JWG, Arveiler D, Amouyel P, Ferrieres J, et al. Paraoxonase activity and coronary heart disease in healthy middle-aged males: The PRIME study. Atherosclerosis. 2008;197:556–563. doi: 10.1016/j.atherosclerosis.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 87.Birjmohun RS, Vergeer M, Stroes ES, Sandhu MS, Ricketts SL, Tanck MW, et al. Both paraoxonase-1 genotype and activity do not predict the risk of future coronary artery disease; the EPIC- Norfolk prospective population study. PLOS One. 2009;4:e6809. doi: 10.1371/journal.pone.0006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang M, Lang X, Cui S, Zou L, Wang S, Wu X. Quantitative assessment of the influence of paraoxonase 1 activity and coronary heart disease. DNA Cell Biol. 2012;31:975–982. doi: 10.1089/dna.2011.1478. [DOI] [PubMed] [Google Scholar]
- 89.Ikeda Y, Suehiro T, Itahara T, Inui Y, Chikazawa H, Inoue M, et al. Human serum paraoxonase concentration predicts cardiovascular mortality in haemodialysis patients. Clin Nephrol. 2007;67:358–365. doi: 10.5414/cnp67358. [DOI] [PubMed] [Google Scholar]
- 90.Tang WHW, Hartiala J, Fan Y, Wu Y, Stewart AFR, Erdmann J, et al. Clinical and genetic association of serum paraoxonase and arylesterase activities with cardiovascular risk. Arterioscler Thromb Vasc Biol. 2012;32:2803–2812. doi: 10.1161/ATVBAHA.112.253930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mackness B, Davies GK, Turkie W, Lee E, Roberts DH, Hill E, et al. Paraoxonase status in coronary heart disease. Are activity and concentration more important than genotype? Arterioscler. Thromb. Vasc. Biol. 2001;21:1451–1457. doi: 10.1161/hq0901.094247. [DOI] [PubMed] [Google Scholar]
- 92.Jarvik GP, Rozek LS, Brophy VH, Hatsukami TS, Richter RJ, Schellenberg GD, Furlong CE. Paraoxonase (PON1) phenotype is a better predictor of vascular disease than is PON1192 or PON155 genotype. Arterioscl. Thromb. Vasc. Biol. 2000;20:2441–2447. doi: 10.1161/01.atv.20.11.2441. [DOI] [PubMed] [Google Scholar]
- 93.Liu M-L, Scalia R, Mehta JL, Williams KJ. Cholesterol-induced membrane microvesicles as novel carriers of damage-associated molecular patterns. Arterioscler Thromb Vasc Biol. 2012;32:2113–2121. doi: 10.1161/ATVBAHA.112.255471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garcia-Heredia A, Marsillach J, Rull A, Triguero I, Fort I, Mackness B, et al. Paraoxonase-1 inhibits oxidised low-density lipoprotein-induced metabolic alterations and apoptosis in endothelial cells: A nondirected metabolomics study. Med Inflamm. 2013 doi: 10.1155/2013/156053. ID 156053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Younis NN, Soran H, Charlton-Menys V, Sharma R, Hama S, Pemberton P, Elseweidy MM, Durrington PN. High-density lipoprotein impedes Glycation of low-density lipoprotein. Diabet Vasc Dis Res. 2013;10:152–160. doi: 10.1177/1479164112454309. [DOI] [PubMed] [Google Scholar]
- 96.Jakubowski H. Protein homocysteinylation: possible mechanisms underlying pathological consequences of elevated homocysteine levels. Faseb J. 1999;13:2277–2283. [PubMed] [Google Scholar]
- 97.Jakubowski H. Homocysteine thiolactone: metabolic origin and protein homocysteinylation in humans. J Nutr. 2000;130:377S–381S. doi: 10.1093/jn/130.2.377S. [DOI] [PubMed] [Google Scholar]
- 98.Jakubowski H. Calcium-dependent human serum homocysteine thiolactone hydrolase- a protective mechanism against protein s-homocysteinylation. J Biol Chem. 2000;275:3957–3962. doi: 10.1074/jbc.275.6.3957. [DOI] [PubMed] [Google Scholar]
- 99.Hine D, Mackness B, Mackness M. Co-incubation of PON1, APO A1 and LCAT increases the time HDL is able to prevent LDL oxidation. IUBMB Life. 2012;64:157–161. doi: 10.1002/iub.588. [DOI] [PubMed] [Google Scholar]
- 100.Marsillach J, Suzuki SM, Richter RJ, McDonald MG, Rademacher PM, MacCoss MJ, et al. Human valacyclovir hydrolase/biphenyl hydrolase-like protein is a highly efficient homocysteine thiolactonase. PLOS One. 2014;9:e110054. doi: 10.1371/journal.pone.0110054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rosenblat M, Volkova N, Ward J, Aviram M. Paraoxonase 1 (PON1) inhibits monocyte-to-macrophage differentiation. Atherosclerosis. 2011;219:49–56. doi: 10.1016/j.atherosclerosis.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 102.Aharoni S, Aviram M, Fuhrman B. Paraoxonase 1 (PON1) reduces macrophage inflammatory responses. Atherosclerosis. 2013;228:353–361. doi: 10.1016/j.atherosclerosis.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 103.Costa LG, Giordano G, Cole TB, Marsillach J, Furlong CE. Paraoxonase 1 (PON1) as a genetic determinant of susceptibility to organophosphate toxicity. Toxicology. 2013;307:115–122. doi: 10.1016/j.tox.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Costa LG, Cole TB, Jarvik GP, Furlong CE. Functional genomics of the paraoxonase (PON1) polymorphisms: effect on pesticide sensitivity, cardiovascular disease and drug metabolism. Ann Rev Med. 2003;54:371–392. doi: 10.1146/annurev.med.54.101601.152421. [DOI] [PubMed] [Google Scholar]
- 105.Coombes RH, Meek EC, Dail MB, Chambers HW, Chambers JE. Human paraoxonase 1 hydrolysis of nanomolar chlorpyriphos-oxon concentrations is unaffected by phenotype or Q192R genotype. Toxicol Letts. 2014;230:57–61. doi: 10.1016/j.toxlet.2014.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li WF, Costa LG, Richter RJ, Hagen T, Shih DM, Tward A, et al. Catalytic efficiency determines the in vivo efficacy of PON1 for detoxifying organophosphates. Pharmacogenetics. 2000;10:767–779. doi: 10.1097/00008571-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 107.Haley RW, Billecke S, La Du BN. Association of low PON1 type Q (type A) arylesterase activity with neurologic symptom complexes in Gulf War veterans. Toxicol Appl Pharmacol. 2000;157:227–233. doi: 10.1006/taap.1999.8703. [DOI] [PubMed] [Google Scholar]
- 108.Hotopf M, Mackness MI, Nikolau V, Collier DA, Curtis C, David A, et al. Paraoxonase in Gulf War veterans. J Occup Environ Med. 2003;45:668–675. doi: 10.1097/01.jom.0000071506.96740.39. [DOI] [PubMed] [Google Scholar]
- 109.Mackenzie Ross SJ, Brewin CR, Curran HV, Furlong CE, Abraham-Smith KM, Harrison V. Neuropsychological and psychiatric functioning in sheep farmers exposed to low levels of organophosphate pesticides. Neurotoxicol Teratol. 2010;32:452–459. doi: 10.1016/j.ntt.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cherry N, Mackness M, Mackness B, Dippnall M, Povey A. "Dippers´Flu" and its relationship to PON1 polymorphisms. Occ Environm Med. 2011;68:211–217. doi: 10.1136/oem.2009.052126. [DOI] [PubMed] [Google Scholar]
- 111.Mackness B, Durrington P, Povey A, Thomson S, Dippnall M, Mackness M, et al. Paraoxonase and susceptibility to organophosphorus poisoning in farmers dipping sheep. Pharmacogenetics. 2003;13:81–88. doi: 10.1097/00008571-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 112.da Silva J, Moraes CR, Heuser VD, Andrade VM, Silva FR, Kvitko K, et al. Evaluation of genetic damage in a Brazilian population occupationally exposed tp pesticides and its correlation with polymorphisms in metabolising genes. Mutagenesis. 2008;23:415–422. doi: 10.1093/mutage/gen031. [DOI] [PubMed] [Google Scholar]
- 113.Lee BW, London L, Poulauskis J, Myers J, Christiani DC. Association between human paraoxonase gene polymorphism and chronic symptoms in pesticide-exposed workers. J Occup Environ Med. 2003;45:118–122. doi: 10.1097/01.jom.0000052953.59271.e1. [DOI] [PubMed] [Google Scholar]
- 114.Lacasana M, Lopez-Flores I, Rodriguez-Barranco M, Aguilar-Garduno C, Blanco-Munoz J, Perez-Mendez O, et al. Interaction between organophosphate pesticide exposure and PON1 activity on thyroid function. Toxicol Appl Pharmacol. 2010;249:16–24. doi: 10.1016/j.taap.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 115.Hernández AF, Mackness B, Rodrigo L, López O, Pla A, Gil F, et al. Paraoxonase activity and genetic polymorphisms in greenhouse workers with long term pesticide exposure. Hum. Exp. Toxicol. 2003;22:565–574. doi: 10.1191/0960327103ht400oa. [DOI] [PubMed] [Google Scholar]
- 116.Manthripragada AD, Costello S, Cokburn MG, Bronstein JM, Ritz B. Paraoxonase 1, agricultural organophosphate exposure and Parkinson disease. Epidemiology. 2010;21:87–94. doi: 10.1097/EDE.0b013e3181c15ec6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Searles Nielsen S, Mueller BA, DeRoos AJ, et al. Risk of brain tumours in children and susceptibility to organophosphorus insecticide; the potential role of paraoxonase (PON1) Environ Hlth Perspecs. 2005;113:909–913. doi: 10.1289/ehp.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Searles Nielson S, Checkoway H, Zhang J, Hofmann JN, Keifer MC, Paulsen M, et al. Blood α-synuclein in agricultural pesticide handlers in central Washington State. Environ Res. 2015;136:75–81. doi: 10.1016/j.envres.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Berkowitz GS, Wetmur JG, Birman-Deych E, Obel J, Lapinski RH, Godbold JH, et al. In utero pesticide exposure, maternal paraoxonase activity and head circumference. Environ Health Perspect. 2004;112:388–391. doi: 10.1289/ehp.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Harley KG, Huen K, Schall RA, Holland NT, Bradman A, Barr DB, Eskenazi B. Association of organophosphate pesticide exposure and paraoxonase with birth outcome in Mexican American Women. PLOS One. 2011;6:e23923. doi: 10.1371/journal.pone.0023923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, Wolff SM. Prenatal exposure to organophosphates, paraoxonase 1 and cognitive development in childhood. Environ Health Perspec. 2011;119:1182–1188. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Eskenazi B, Huen K, Marks A, Harley KG, Bradman A, Barr DB, Holland NT. PON1 and neurodevelopment in children from the CHAMACOS study exposed to organophosphate pesticides in utero. Environ Health Perspec. 2010;118:1775–1781. doi: 10.1289/ehp.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Garliardi S, Abel K, Bianchi M, et al. Regulation of FMO and PON detoxication systems in ALS human tissues. Neurotox Res. 2012 doi: 10.1007/s12640-012-9356-1. [DOI] [PubMed] [Google Scholar]
- 124.Ricci C, Battistini S, Cozzi L. Lack of association of PON polymorphisms with sporadic ALS in an Italian population. Neurobiol Aging. 2011;32:552.e7–552.e13. doi: 10.1016/j.neurobiolaging.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 125.Slowik A, Tomik B, Wolkow PP, et al. Paraoxonase gene polymorphisms and sporadic ALS. Neurology. 2006;67:766–770. doi: 10.1212/01.wnl.0000219565.32247.11. [DOI] [PubMed] [Google Scholar]
- 126.Ticozzi N, LeClerc AL, Keagle PJ, et al. Paraoxonase gene mutations in ALS. Ann Neurol. 2010;68:102–107. doi: 10.1002/ana.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wills AM, Landers JE, Zhang H, et al. Paraoxonase 1 (PON1) organophosphate hydrolysis is not reduced in ALS. Neurology. 2008;70:929–934. doi: 10.1212/01.wnl.0000305956.37931.dd. [DOI] [PubMed] [Google Scholar]
- 128.Saadat M. Paraoxonase 1 genetic polymorphisms and susceptibility to breast cancer: a meta-analysis. Cancer Epidemiol. 2012;36:e101–e103. doi: 10.1016/j.canep.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 129.Dowling P, Hayes C, Ting KR, Hameed A, Meiller J, Mitsiades C, et al. Identification of proteins found to be significantly altered when comparing the serum proteome from Multiple Myeloma patients with varying degrees of bone disease. BMC Genomics. 2014;15:904. doi: 10.1186/1471-2164-15-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Camps J, Pujol I, Ballester F, Joven J, Simo JM. Paraoxonases as potential antibiofilm agents: Their relationship with quorum-sensing signals in gram-negative bacteria. Antimocrob Agents Chemother. 2011;55:1325–1331. doi: 10.1128/AAC.01502-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Simanski M, Babucke S, Eberl L, Harder J. Paraoxonase 2 acts as a quorum-quenching factor in human keratinocytes. J Invest Dermatol. 2012;132:2296–2299. doi: 10.1038/jid.2012.128. [DOI] [PubMed] [Google Scholar]
- 132.Teiber JF, Horke S, Haines DC, Chowdhary PK, Xiao J, Kramer JK, et al. Dominant role of paraoxonases in inactivation of the pseudomonas aeruginosa quorum-sensing signal N-(3-oxododecanoyl)-L-homoserine lactone. Infect Immun. 2008;76:2512–2519. doi: 10.1128/IAI.01606-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ozer EA, Pezzulo A, Shih DM, Chun C, Furlong C, Lusis AJ, et al. Human and murine paraoxonase 1 are host modulators of pseudomonas aeruginosa quorum-sensing. FEMS Microbiol Lett. 2005;253:29–37. doi: 10.1016/j.femsle.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 134.Stoltz DA, Ozer EA, Taft PJ, Barry M, Liu L, Kiss PJ, et al. Drosophila are protected from pseudomonas aeruginosa lethality by transgenic expression of paraoxonase-1. J Clin Invest. 2008;118:3123–3131. doi: 10.1172/JCI35147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pezzulo AA, Hornick EE, Rector MV, Estin M, Reisetter AC, Taft PJ, et al. Expression of human paraoxonase 1 decreases superoxide levels and alters bacterial colonisation in the gut of Drosophila melanogaster. PLOS One. 2012;7:e43777. doi: 10.1371/journal.pone.0043777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Seres I, Paragh G, Deschene E, Fulop T, Jr, Khalil A. Study of factors influencing the decreased HDL associated PON1 activity with aging. Exp Gerontol. 2004;39:59–66. doi: 10.1016/j.exger.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 137.Jaouad L, de Guise C, Berrougui H, Cloutier M, Isabelle M, Fulop T, et al. Age related decrease in high-density lipoproteins antioxidant activity is due to alteration in the PON1´s free sulphydryl groups. Atherosclerosis. 2006;185:191–200. doi: 10.1016/j.atherosclerosis.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 138.Lescai F, Marchegiani F, Franceschi C. PON1 is a longevity gene: results of a meta-analysis. Ageing Res Rev. 2009;8:277–284. doi: 10.1016/j.arr.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 139.Caliebe A, Kleindorp R, Blanche H, Christiansen L, Puca AA, Rea IM, et al. No or only population-specific effect of PON1 on human longevity: a comprehensive meta-analysis. Ageing Res Rev. 2010;9:238–244. doi: 10.1016/j.arr.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 140.Lee YS, Park CO, Noh JY, Jin S, Lee NR, Noh S, et al. Knockdown of paraoxonase 1 expression influences ageing of human dermal microvascular endothelial cells. Exp Dermatol. 2012;21:682–687. doi: 10.1111/j.1600-0625.2012.01555.x. [DOI] [PubMed] [Google Scholar]
- 141.Richter RJ, Jarvik GP, Furlong CE. Determination of paraoxonase 1 status without the use of toxic organophosphate substrates. Circ Cardiovasc Genet. 2008;1:147–152. doi: 10.1161/CIRCGENETICS.108.811638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Camps J, Mackness M, Mackness B, Marsillach J, Joven J. Serum paraoxonase-1 activity and genetic polymorphisms: common errors in measurement and interpretation of results. Clin Chem Lab Med. 2010;48:893–894. doi: 10.1515/CCLM.2010.156. [DOI] [PubMed] [Google Scholar]