Abstract
We have produced and characterized two chimeric IgG1 monoclonal antibodies that bind different immunodominant epitopes on Vibrio cholerae lipopolysaccharide (LPS). MAb 2D6 IgG1 recognizes Ogawa O-polysaccharide antigen, while mAb ZAC-3 IgG1 recognizes core/lipid A moiety of Ogawa and Inaba LPS. Both antibodies were expressed using a Nicotiana benthamiana-based rapid antibody-manufacturing platform (RAMP) and evaluated in vitro for activities associated with immunity to V. cholerae, including vibriocidal activity, bacterial agglutination and motility arrest.
Keywords: cholera, antibodies, plant expression, vaccine
1. Introduction
Vibrio cholerae is the causative agent of cholera, an acute diarrheal disease that remains endemic in many parts of the world. Seven cholera pandemics have been recorded since 1817, with the 7th pandemic wave beginning in 1961 and continuing today. In addition to seasonal outbreaks in areas where V. cholerae is endemic, natural disasters such as the 2010 earthquake in Haiti highlights the continued potential for V. cholerae to cause mass casualties in regions that were previously cholera-free (Katz et al., 2013).
V. cholerae can be differentiated by serogroup, biotype, and serotype. The serogroups are defined by the surface lipopolysaccharide (LPS) which is composed of three parts: lipid A, core-polysaccharide, and O-polysaccharide (O-PS) (Chatterjee and Chaudhuri, 2003). Among the more than 200 serogroups of V. cholerae, which are identified by the O-PS portion of LPS, only two serogroups (O1and O139) have been shown to be capable of causing cholera epidemics. Within the O1 serogroup, there are two biotypes, classical and El Tor, which differ phenotypically and in disease severity. While the classical biotype was responsible for the first six cholera pandemics, the El Tor biotype is responsible for the ongoing seventh pandemic and has become the predominant circulating biotype worldwide (Bishop and Camilli, 2011; Harris et al., 2012). Among both biotypes, the two most prevalent serotypes of V. cholerae are Ogawa and Inaba, which differ by a 2-O-methyl group on the non-reducing terminal sugar of the O-PS that is present in Ogawa and absent in the Inaba serotype (Villeneuve et al., 1999).
There are currently two licensed oral cholera vaccines in use worldwide. Dukoral® is composed of a combination of whole cell killed V. cholerae O1 strains, representing both biotypes and Ogawa and Inaba serotypes, as well as the recombinant B subunit of cholera toxin (CTB). Shanchol™, contains representative strains of both O1 and O139 serogroups but lacks CTB (Bishop and Camilli, 2011). While the vaccines are safe, they are only moderately effective, in that there is a limited duration of immunity (<3 years), they require multiple doses, and they are not especially effective in young children, a population particularly vulnerable to disease. For these reasons, there are ongoing studies aimed at better understanding the serum and mucosal antibody responses to V. cholerae and then applying this information to vaccine development (Pasetti and Levine, 2012).
Serum LPS-specific IgG titers and vibriocidal activity are the two primary measures of immunity to V. cholerae. Both factors are important in the assessment of vaccine efficacy, though mucosal (not serum) antibodies are likely the principle mediators of intestinal immunity to V. cholerae (Winner et al., 1991; Apter et al., 1993; Harris et al., 2009; Johnson et al., 2012). A particular challenge associated with the analysis of LPS-specific serum antibody titers is the lack of a common IgG standard. Currently, serum antibody levels are compared to pooled human polyclonal antibody preparations from milk or sera (Qadri et al., 1999). Alternatively, baseline titers from healthy human controls are used as a reference, which can be problematic in areas where cholera is endemic and exposure to V. cholerae is common (Johnson et al., 2012). While these comparisons allow for relative antibody titer differences to be analyzed within a sample population, it limits comparisons across different clinical studies or vaccine trials. A universal human IgG antibody standard directed against one or more immunodominant epitopes on V. cholerae LPS would be of enormous benefit to the cholera research community.
Mouse monoclonal IgA antibodies (mAbs) 2D6 and ZAC-3 bind distinct immunodominant epitopes on V. cholerae LPS (Winner et al., 1991; Lullau et al., 1996; Wang et al., 1998). 2D6 IgA recognizes the Ogawa O-polysaccharide antigen defined by 2-O-methyl group on the non-reducing terminal sugar. ZAC-3 IgA recognizes the core/lipid A moiety of Ogawa and Inaba lipopolysaccharides and is thought to be similar to a number of other mAbs like 72.1 that have been shown to be protective in mice against experimental V. cholerae infection (Winner et al., 1991; Lullau et al., 1996; Wang et al., 1998; Dharmasena et al., 2009). In this study we produced chimeric mouse-human derivatives of mAbs 2D6 and ZAC-3 in which the VH and VL domains of each mAb were grafted onto a human IgG1 framework. The resulting chimeric antibodies were expressed in Nicotiana benthamiana-based rapid antibody-manufacturing platform (RAMP). We demonstrate that the chimeric IgG mAbs retain binding specificities and functional activities associated with the murine mAbs. Because RAMP enables ready, easy to scale antibody production, we propose that chimeric 2D6 and/or ZAC-3 IgG1 may prove to be valuable standards in cholera vaccine community.
2. Materials and methods
2.1 Bacterial strains and growth conditions
The O1 classical V. cholerae O395 strain was a gift from Dr. John Mekalanos (Harvard Medical School) (Mekalanos et al., 1979) and the V. cholerae O1 El Tor strain (C6706) was kindly provided by Dr. Fitnat Yildiz (University of California, Santa Cruz). Reference vaccine strain 9459 was obtained from the American Type Culture Collection (ATCC, Manassas, VA). Strains were grown in LB medium at 37°C with aeration (150 rpm) supplemented when necessary with ampicillin (100 μg/ml).
2.2 B cell hybridomas and production of chimeric IgG1 anti-V. cholerae mAbs
The 2D6 B cell hybridoma was obtained from Dr. Marian Neutra (Children's Hospital Boston). The ZAC-3 B cell hybridoma was obtained from Dr. Blaise Corthésy (CHUV, Switzerland). The hybridomas were maintained in RPMI medium supplemented with 10% fetal bovine serum (FBS) without antibiotics at 37°C in a 5% CO2-95% air atmosphere, as described (Forbes et al., 2008). The murine VL and VH domains of 2D6 and ZAC-3 were amplified by PCR from cDNA derived from the respective murine B cell hybridomas (Winner et al., 1991; Lullau et al., 1996). PCR amplicons were sequenced and consensus contigs for each domain were generated based on the Kabat and IMGT databases (Figure S1-2) (Lefranc, 2009). The codon-optimized VL and VH regions of each mAb were then synthesized commercially (GeneArt, LifeTechnologies, Grand Island, NY) and fused to human IgG1 and κ constant regions (O'Hara et al., 2012; Sully et al., 2014). Chimeric antibodies were expressed using Nicotiana benthamiana-based rapid antibody-manufacturing platform (RAMP) (Whaley et al., 2011). For this particular study, production was performed with a transgenic N. benthamiana line devoid of xylosyl transferase and fucosyl transferase activities, which results in transgenic immunoglobulins with glycans that are generally more homogeneous than those produced in mammalian cells (Schahs et al., 2007). Purity of the chimeric antibodies was assessed by HPLC (data not shown) and SDS-PAGE (Figure S3). Fab fragments were generated using the IgG Fab preparation kit (ThermoScientific, Rockford, IL) and visualized by SDS-PAGE and GelCode Blue (ThermoScientfic) (Figure S3). Concentration of Fab fragments was determined by NanoDrop (ThermoScientific, Wilmington, DE).
2.3 ELISAs for determining chimeric mAb specificity
Nunc Maxisorb F96 microtiter plates (ThermoFisher Scientific, Pittsburgh, PA) were coated overnight with poly-L-lysine (10 μg/ml). Overnight cultures of V. cholerae O395 (O1 classical Ogawa strain), C6706 (O1 El Tor Inaba strain) or ATCC 9459 (vaccine Inaba strain) were washed twice with PBS, and then applied (50 μl) to poly-L-lysine coated wells. Plates were subjected to centrifugation (1,500 rcf for 6 min) and cells were then fixed with 2% paraformaldehyde for 20 min. Residual paraformaldehyde was quenched by the addition of 0.1M glycine (50 μl) followed by incubation at room temperature for 30 min. Plates were blocked with 2% goat serum overnight at 4°C before being probed with the chimeric IgG mAbs. Horseradish peroxidase (HRP)-labeled goat anti-mouse IgA-specific polyclonal antibodies and goat anti-human IgG-specific polyclonal antibodies (SouthernBiotech, Birmingham, AL) were used as the secondary reagents. The ELISA plates were developed using the colorimetric detection substrate 3,3′,5,5′-tetramethylbenzidine (TMB; Kirkegaard & Perry Labs, Gaithersburg, MD) and were analyzed with a SpectroMax 250 spectrophotometer, with Softmax Pro 5.2 software (Molecular Devices, Sunnyvale, CA).
2.4 Bacterial Motility and Agglutination Assays
V. cholerae semi-solid agar motility assays were performed as described (Martinez et al., 2010). Briefly, V. cholerae O395 colonies grown on LB agar (supplemented with 100 μg/ml of streptomycin) were picked using sterile pipette tips and stabbed into 0.3% semi-solid agar containing mAbs or Fabs (9 μg/ml). Plates were incubated at 37°C for 8 h and the diameter of bacterial motility was measured. Bacterial agglutination assays were performed by mixing mid-log phase cultures of V. cholerae with two-fold dilutions of the mAbs in 96-well U-bottomed microtiter plates. The plates were incubated at 37°C for 1 h and bacterial agglutination was assessed visually.
2.5 Vibriocidal assays
The vibriocidal assays were performed as described (Son and Taylor, 2011). Briefly, V. cholerae O395 was grown to late exponential phase, washed with PBS, mixed with guinea pig complement serum (GPCS; Sigma-Aldrich, St. Lois, MO) and incubated on ice for 20 min. The V. cholerae/complement solution was then mixed 1:1 in a 96-well plate with PBS containing varying concentrations of antibody (1-100 μg/ml) and incubated at 37°C for 1 h. LB broth was added (200 μL/well) and the plate was incubated at 37°C for an additional 2 h. V. cholerae mixtures were then serially diluted, plated onto LB medium, incubated at 37°C and scored for CFUs the following day.
3. Results
3.1 Cloning and expression of chimeric 2D6 and ZAC-3 IgG1 mAbs
We generated chimeric IgG1 mAbs from two different well-characterized murine IgA mAbs, 2D6 and ZAC-3 (Table 1). The murine VL and VH domains of 2D6 and ZAC-3 were amplified by PCR from cDNA derived from the respective murine B cell hybridomas. Consensus contigs for each domain were generated based on the Kabat and IMGT databases (Figure S1-2). The murine variable domains were grafted onto human IgG1 frameworks, transformed into Agrobacterium tumefaciens and vacuum infiltrated and expressed in N. benthamiana. Plant tissue was harvested and the mAbs were extracted and purified on a protein A column. Purity was determined by HPLC and within the given chromatographic conditions, the 2D6 IgG1 and ZAC-3 IgG1 monomers were shown to be 92% and 91% pure, respectively (data not shown).
Table 1. Characteristics of murine and chimeric mAbs used in this study.
| mAb | Speciesa | Isotype | Target | Agglutination (μg/ml)b | Vibriocidalc |
|---|---|---|---|---|---|
| 2D6 | mouse | IgA | O-PS | 18 | no |
| chimeric | IgG1 | nd | yes | ||
| ZAC-3 | mouse | IgA | Lipid A/core | 0.25 | no |
| chimeric | IgG1 | nd | yes |
Chimeric antibodies were composed of a murine variable region grafted onto a human IgG1 constant region.
Lowest antibody concentration that induced visible agglutination of V. cholerae O395 after 2 hr incubation at 37°C, nd: none detected.
Late exponential phase V. cholerae O395 was pre-mixed with guinea pig serum and incubated in LB media containing 100 μg/ml of antibody. Bacterial samples were diluted and plated for CFUs. Vibriocidal activity was defined as ≥1 log decrease in CFU compared to control treatment.
3.2 Characterization of LPS-specific mAbs
To verify that chimeric mAbs retained specificity for LPS, we coated ELISA plates with purified V. cholerae Ogawa LPS and compared the binding of the 2D6 and ZAC-3 IgA mAbs to that of the IgG1 mAbs. Both 2D6 IgA and IgG1 recognized Ogawa LPS, though the chimeric derivative bound slightly less well than the murine IgA, suggesting that the Fc framework may affect the functionality of the variable domains (Figure 1A). The ZAC-3 IgG mAb, on the other hand, demonstrated considerably higher (∼3×) reactivity with Ogawa LPS than its murine counterpart (Figure 1A). To examine mAb reactivity with native LPS, the chimeric 2D6 and ZAC-3 antibodies were subjected to ELISAs in which plates were coated with whole cell V. cholerae classical Ogawa 395 biotype, El Tor Inaba biotype (C6706), or the Inaba strain used in the Dukoral® cholera vaccine (ATCC# 9459). As expected, 2D6 IgG1 recognized V. cholerae O395 but not the El Tor Inaba biotypes C6706 or 9459 to any appreciable degree except at very high mAb concentrations (Figure 1B-D). Conversely, ZAC-3 IgG1 (like its murine IgA counterpart) recognized all thee V. cholerae strains with an EC50 ranging between 0.14-0.84 μg/ml (Figure 1B-D). The relative avidity of ZAC-3 for V. cholerae O395, for example, was ∼100 times that of 2D6, suggesting that ZAC-3 may have considerably more utility than 2D6 as a standard.
Figure 1. Reactivity of chimeric 2D6 and ZAC-3 with purified LPS and whole cell V. cholerae.

2D6 (left panels) and ZAC-3 (right panels) IgA and IgG reactivity with (A) purified Ogawa LPS, (B) whole cell V. cholerae strain O395, (C) strain El Tor C6706 or (D) strain 9459. ELISAs were performed as described in the Materials and Methods. Sal4 and SyH7 were used as isotype mouse IgA and human IgG1 controls.
3.3 Biological characteristics of 2D6 and ZAC-3 IgG1 mAbs
Antibody-mediated agglutination of V. cholerae is a universal method to detect LPS-specific antibodies in serum or mucosal secretions (Gustafsson and Holme, 1985). To examine the capacity of chimeric 2D6 and ZAC-3 IgG to agglutinate V. cholerae, mid-log phase cultures of strain O395 were incubated for two hours at 37°C with two-fold dilutions of 2D6 and ZAC-3 IgA or IgG1 derivatives and then scored by visually for the appearance of macroscopic agglutination. We found that of 2D6 IgA and ZAC-3 IgA were each effective at inducing V. cholerae agglutination, although their relative potencies were notably different. Approximately 18 μg/ml of 2D6 IgA was required to promote V. cholerae O395 agglutination, whereas as little as 0.25 μg/ml ZAC-3 IgA was required (Figure 2A). Examination of the chimeric IgG1 derivatives of 2D6 or ZAC-3 indicated that neither was able to promote visible bacterial agglutination. These data demonstrate that even though 2D6 and ZAC-3 chimeric IgG1 mAbs recognize LPS in the context of the whole bacterial cells (as demonstrated by ELISA) they are unable to promote macroscopic agglutination of V. cholerae likely due to their monomeric forms (as compared to the polymeric forms of 2D6 and ZAC-3 IgA mAbs).
Figure 2. Effects on 2D6 and ZAC-3 on V. cholerae agglutination and motility.
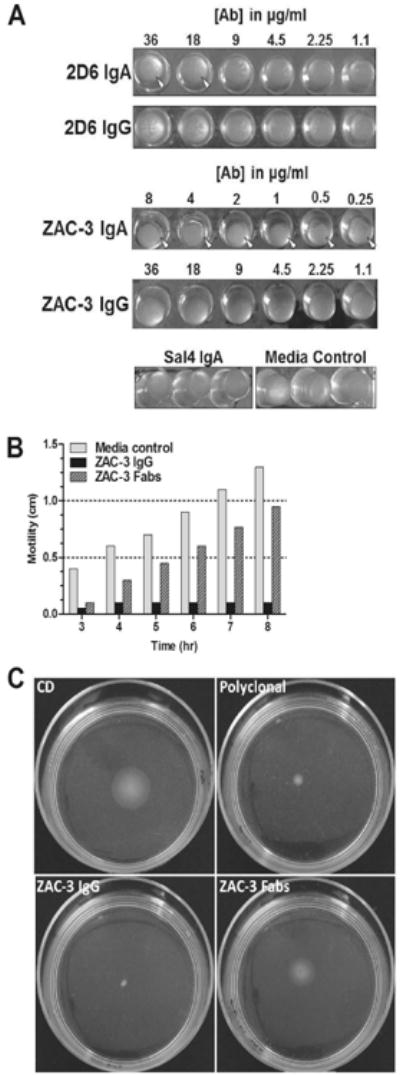
(A) Bacterial macro-agglutination assays: mid-log phase V. cholerae O395 was mixed with an equal volume of LB medium containing two-fold serial dilutions of Sal4 (control), 2D6 or ZAC-3 IgA or IgG mAbs and then seeded in U-bottom 96-well plates. Microtiter wells were monitored for the appearance of aggregated bacteria over a period of 2 hr. 2D6 IgA promoted the appearance of visible clumps at concentrations ≥18 μg/ml, whereas ZAC-3 IgA promoted agglutination at ≥0.25 μg/ml (arrowheads). Neither 2D6 nor ZAC-3 IgG produced visible agglutination at any concentration tested. (B and C) Semi-solid liquid motility assays: single colonies of V. cholerae O395 were stabbed into 0.3% semi-solid LB agar containing 9 μg/ml of ZAC-3 IgG1 or ZAC-3 Fab fragments. Plates were incubated at 37°C and the diameter of the bacterial spread was measured every 60 min. These data demonstrate that both ZAC-3 IgG and Fab fragments significantly reduced V. cholerae motility (P=0.0003, Student t test), although the Fab fragments effects were only apparent at the earliest time point.
Antibody-mediated motility arrest is another tool used to characterize serotype-specific responses to V. cholerae and therefore worth evaluating in the context of chimeric 2D6 and ZAC-3 IgG1, especially since we recently reported that 2D6 IgA arrests V. cholerae motility by at least two distinct mechanisms (K. Levinson, M. DeJesus and N. Mantis, in press). Using semi-solid agar to mimic the viscous environment of the intestinal lumen, we seeded V. cholerae O395 into LB agar containing either ZAC-3 IgG or ZAC-3 Fab fragments. The Fab fragments allowed us to examine the direct effects of antibody binding to the bacterial surfaces and flagella without being confounded by IgG-mediated bacterial cross-linking. As compared to the medium control, ZAC-3 IgG1 significantly inhibited V. cholerae motility at all time points measured (P=0.0003) (Figure 2B). In fact, ZAC-3 IgG was a more potent inhibitor of V. cholerae motility than the anti-LPS polyclonal antiserum (data not shown). ZAC-3 Fab fragments also significantly reduced V. cholerae motility at early 3 h, although this effect diminished with time (Figure 2B). Nonetheless, these data demonstrate that both direct binding and antibody valency may play a role in V. cholerae motility arrest.
Finally, because vibriocidal antibody titers are commonly used for clinical applications, we wanted to test whether the chimeric 2D6 and ZAC-3 IgG1 antibodies were able to promote complement-mediated killing of V. cholerae. As IgA antibodies are unable to bind complement proteins and activate the complement cascade, we used the 2D6 and ZAC-3 IgA antibodies as controls. Using a standard vibriocidal assay (Son and Taylor, 2011), we found that treatment of V. cholerae O395 with as little as 1 μg/ml of either 2D6 IgG or ZAC-3 IgG resulted in a log reduction in CFUs (Table 1; Figure S4). 2D6 and ZAC-3 IgG were also able to promote complement-mediated killing of V. cholerae El Tor strains C6706 and 9459, although higher concentrations (100 μg/ml) of antibody were needed to promote killing in the clinical strain. In contrast, comparable studies without complement or with murine 2D6 or ZAC-3 IgA mAbs had no effect on cell viability (data not shown). These results indicate that the chimeric ZAC-3 IgG is able to activate complement-mediated killing of V. cholerae.
4. Discussion
Currently, there are ongoing clinical trials aimed at assessing novel vaccines candidate platforms that may soon include OPS conjugate type antigens (Alam et al., 2014), as well as additional studies aimed at better understanding the human serum and mucosal antibody responses to V. cholerae. However the absence of a common human IgG antibody standard directed against one or more immunodominant epitopes on V. cholerae LPS makes it extremely difficult to directly compare results of one study to another. Current analysis relies on the use of pooled polyclonal human sera as a standard for ELISAs and vibriocidal activity that may differ from study site to study site (Jayasekera et al., 2008; Harris et al., 2009; Charles et al., 2014). To address this issue we have produced and characterized two chimeric IgG1 monoclonal antibodies that bind different immunodominant epitopes on V. cholerae LPS. 2D6 IgG1 recognizes Ogawa OPS antigen, while ZAC-3 IgG1 recognizes core/lipid A moiety of Ogawa and Inaba LPS. Both antibodies were successfully expressed using a N. benthamiana-based RAMP and evaluated in vitro for activities associated with immunity to V. cholerae, including, bacterial agglutination, motility arrest and vibriocidal activity. We found that ZAC-3 IgG1 was a far more potent antibody than 2D6 in terms of binding affinity, bacterial agglutination and motility arrest and is therefore likely the more useful of the two in terms of being used as a standard in the field. Moreover, the potential utility of ZAC-3 as a standard is augmented by the fact that it recognizes both Ogawa and Inaba biotypes, including the strains used in the current oral vaccines. Furthermore, the core/lipid A portion of V. cholerae LPS is conserved between the O1 and O139 serogroups. While this makes ZAC-3 and ideal standard for ELISAs examining serum responses to select vaccine strains, we recognize that ZAC-3's utility as a diagnostic reagent is limited because of its broad serogroup reactivity.
To our knowledge, these are the first human-murine chimeric antibodies made that retain specificity to known protective epitopes on V. cholerae. While we envision both of these antibodies to have utility as controls in vaccine development efforts, we believe ZAC-3 IgG1 holds the most potential. While mAb 2D6 binds specifically to the O1 classical Ogawa serotype, mAb ZAC-3 binds both V. cholerae O1 classical and El Tor biotypes as well as both Ogawa and Inaba serotypes. Further, ZAC-3 IgG1 binds with much higher affinity and has a greater overall avidity than mAb 2D6. ZAC-3 IgG1 was a potent inducer of V. cholerae agglutination and inhibitor of V. cholerae motility, both common and useful readouts for V. cholerae diagnostic studies. Both mAbs retained vibriocidal capacity, though ZAC-3 was better able to activate the complement cascade, with vibriocidal levels comparable to that of polyclonal anti-V. cholerae LPS antiserum. Taken together, the results of our analysis highlight to use of both mAbs as controls in V. cholerae vaccine studies, with mAb ZAC-3 providing a broader and stronger antibody response.
One major advantage of these chimeric mAbs is the ability to produce them rapidly and at a large scale using the RAMP technology (Whaley et al., 2011). This technology utilizes the plant pathogen A. tumefaciens that when transformed with the antibody sequence of interest is used to infect the N. benthamiana. Infection with A. tumefaciens allows for the plant cells to fully express and assemble the glycosylated mammalian antibodies at a much faster rate and higher concentration than what is typically achieved with conventional cell-based methods. Traditional cell-based antibody production methods are time, reagent, and labor intensive to scale-up; conversely, once antibodies are able to be fully expressed in the RAMP platform, the production and purification of the antibodies can be done quickly and on a much larger scale. This antibody production process can be utilized in a number of settings, such as biodefense (Sully et al., 2014) and more recently, in the Ebola outbreak in West Africa (Qiu et al., 2014). The specificity of the chimeric mAbs combined with the efficient production platform would enable researchers to have easy access to sufficient quantities of antibody for use in their V. cholerae studies.
Finally, in an effort to assess the biological characteristics of the chimeric mAbs, we tested their capacity to induce V. cholerae motility arrest and agglutination. We found that both 2D6 and ZAC-3 IgA were capable of antibody-mediated cross-linking, but the chimeric IgG1 mAbs were not. This suggests that antibody valency is a critical feature of pathogen agglutination and clearance both in our in vitro studies and perhaps in vivo during infection. Motility is also an important component of V. cholerae pathogenesis and we found that both ZAC-3 IgG and Fab fragments were capable of significantly reducing V. cholerae motility in semi-solid medium within hours, confirming an observation that we recently made with 2D6 IgA (K. Levinson, M. DeJesus, and N. Mantis, in press). This demonstrated that ZAC3's effects on motility are not solely attributable to antibody cross-linking and points to additional potential roles in antibody-pathogen interaction and mediating protection. Taken together, this study highlights the utility of the chimeric mAbs as IgG1 standards for surrogate measures of antibody response to V. cholerae and can be used to better understand mechanisms of how anti-LPS antibody can promote protective immunity.
Supplementary Material
Highlights.
Ongoing cholera vaccine trials lack available standards for serum antibody analysis
We produced two chimeric IgG1 monoclonal antibodies using a Nicotiana benthamiana-based system
2D6 IgG1 recognizes the immunodominant Ogawa O-polysaccharide antigen
ZAC-3 IgG1 recognizes core/lipid A moiety of Ogawa and Inaba lipopolysaccharides
2D6 and ZAC-3 will serve as important reagents for the cholera research community
Acknowledgments
We thank Drs. Blaise Corthésy (Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland) and Marian Neutra (Children's Hospital Boston) for graciously providing the ZAC-3 and 2D6 B cell hybridomas. We thank Dr. John Mekalanos (Harvard Medical School) and Dr. Fitnat Yildiz (UC Santa Cruz) for providing the V. cholerae strains used in this study. This work was supported in part by NIH grants HD061916 and GM082978 to NJM. KL was supported by the Wadsworth Center's Biodefense and Emerging Infectious Diseases training grant (5T32AI055429-08; McDonough).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam MM, Bufano MK, Xu P, Kalsy A, Yu Y, Freeman YW, Sultana T, Rashu MR, Desai I, Eckhoff G, Leung DT, Charles RC, LaRocque RC, Harris JB, Clements JD, Calderwood SB, Qadri F, Vann WF, Kovac P, Ryan ET. Evaluation in mice of a conjugate vaccine for cholera made from Vibrio cholerae O1 (Ogawa) O-specific polysaccharide. PLoS Negl Trop Dis. 2014;8:e2683. doi: 10.1371/journal.pntd.0002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apter FM, Michetti P, Winner LSd, Mack JA, Mekalanos JJ, Neutra MR. Analysis of the roles of antilipopolysaccharide and anti-cholera toxin immunoglobulin A (IgA) antibodies in protection against Vibrio cholerae and cholera toxin by use of monoclonal IgA antibodies in vivo. Infect Immun. 1993;61:5279–85. doi: 10.1128/iai.61.12.5279-5285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AL, Camilli A. Vibrio cholerae: lessons for mucosal vaccine design. Expert review of vaccines. 2011;10:79–94. doi: 10.1586/erv.10.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles RC, Hilaire IJ, Mayo-Smith LM, Teng JE, Jerome JG, Franke MF, Saha A, Yu Y, Kovac P, Calderwood SB, Ryan ET, LaRocque RC, Almazor CP, Qadri F, Ivers LC, Harris JB. Immunogenicity of a killed bivalent (O1 and O139) whole cell oral cholera vaccine, Shanchol, in Haiti. PLoS Negl Trop Dis. 2014;8:e2828. doi: 10.1371/journal.pntd.0002828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee SN, Chaudhuri K. Lipopolysaccharides of Vibrio cholerae. I. Physical and chemical characterization. Biochim Biophys Acta. 2003;1639:65–79. doi: 10.1016/j.bbadis.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Dharmasena MN, Krebs SJ, Taylor RK. Characterization of a novel protective monoclonal antibody that recognizes an epitope common to Vibrio cholerae Ogawa and Inaba serotypes. Microbiology. 2009;155:2353–64. doi: 10.1099/mic.0.025726-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes SJ, Eschmann M, Mantis NJ. Inhibition of Salmonella enterica serovar typhimurium motility and entry into epithelial cells by a protective antilipopolysaccharide monoclonal immunoglobulin A antibody. Infect Immun. 2008;76:4137–44. doi: 10.1128/IAI.00416-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B, Holme T. Rapid detection of Vibrio cholerae O:1 by motility inhibition and immunofluorescence with monoclonal antibodies. Eur J Clin Microbiol. 1985;4:291–4. doi: 10.1007/BF02013655. [DOI] [PubMed] [Google Scholar]
- Harris AM, Bhuiyan MS, Chowdhury F, Khan AI, Hossain A, Kendall EA, Rahman A, Larocque RC, Wrammert J, Ryan ET, Qadri F, Calderwood SB, Harris JB. Antigen specific memory B-cell responses to Vibrio cholerae O1 infection in Bangladesh. Infect Immun. 2009 doi: 10.1128/IAI.00369-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. Cholera. Lancet. 2012;379:2466–76. doi: 10.1016/S0140-6736(12)60436-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasekera CR, Harris JB, Bhuiyan S, Chowdhury F, Khan AI, Faruque AS, Larocque RC, Ryan ET, Ahmed R, Qadri F, Calderwood SB. Cholera toxin-specific memory B cell responses are induced in patients with dehydrating diarrhea caused by Vibrio cholerae O1. J Infect Dis. 2008;198:1055–61. doi: 10.1086/591500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RA, Uddin T, Aktar A, Mohasin M, Alam MM, Chowdhury F, Harris JB, LaRocque RC, Bufano MK, Yu Y, Wu-Freeman Y, Leung DT, Sarracino D, Krastins B, Charles RC, Xu P, Kovac P, Calderwood SB, Qadri F, Ryan ET. Comparison of immune responses to the O-specific polysaccharide and lipopolysaccharide of Vibrio cholerae O1 in Bangladeshi adult patients with cholera. Clin Vaccine Immunol. 2012;19:1712–21. doi: 10.1128/CVI.00321-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LS, Petkau A, Beaulaurier J, Tyler S, Antonova ES, Turnsek MA, Guo Y, Wang S, Paxinos EE, Orata F, Gladney LM, Stroika S, Folster JP, Rowe L, Freeman MM, Knox N, Frace M, Boncy J, Graham M, Hammer BK, Boucher Y, Bashir A, Hanage WP, Van Domselaar G, Tarr CL. Evolutionary dynamics of Vibrio cholerae O1 following a single-source introduction to Haiti. MBio. 2013;4 doi: 10.1128/mBio.00398-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefranc MP. Antibody databases: IMGT, a French platform of world-wide interest. Medecine sciences: M/S. 2009;25:1020–3. doi: 10.1051/medsci/200925121020. [DOI] [PubMed] [Google Scholar]
- Lullau E, Heyse S, Vogel H, Marison I, von Stockar U, Kraehenbuhl JP, Corthesy B. Antigen binding properties of purified immunoglobulin A and reconstituted secretory immunoglobulin A antibodies. Journal of Biological Chemistry. 1996;271:16300–9. doi: 10.1074/jbc.271.27.16300. [DOI] [PubMed] [Google Scholar]
- Martinez RM, Jude BA, Kirn TJ, Skorupski K, Taylor RK. Role of FlgT in anchoring the flagellum of Vibrio cholerae. J Bacteriol. 2010;192:2085–92. doi: 10.1128/JB.01562-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos JJ, Collier RJ, Romig WR. Enzymic activity of cholera toxin. II. Relationships to proteolytic processing, disulfide bond reduction, and subunit composition. J Biol Chem. 1979;254:5855–61. [PubMed] [Google Scholar]
- O'Hara JM, Whaley K, Pauly M, Zeitlin L, Mantis NJ. Plant-based expression of a partially humanized neutralizing monoclonal IgG directed against an immunodominant epitope on the ricin toxin A subunit. Vaccine. 2012;30:1239–43. doi: 10.1016/j.vaccine.2011.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasetti MF, Levine MM. Insights from natural infection-derived immunity to cholera instruct vaccine efforts. Clin Vaccine Immunol. 2012;19:1707–11. doi: 10.1128/CVI.00543-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri F, Ahmed F, Karim MM, Wenneras C, Begum YA, Abdus Salam M, Albert MJ, McGhee JR. Lipopolysaccharide- and cholera toxin-specific subclass distribution of B-cell responses in cholera. Clinical and diagnostic laboratory immunology. 1999;6:812–8. doi: 10.1128/cdli.6.6.812-818.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Wong G, Audet J, Bello A, Fernando L, Alimonti JB, Fausther-Bovendo H, Wei H, Aviles J, Hiatt E, Johnson A, Morton J, Swope K, Bohorov O, Bohorova N, Goodman C, Kim D, Pauly MH, Velasco J, Pettitt J, Olinger GG, Whaley K, Xu B, Strong JE, Zeitlin L, Kobinger GP. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schahs M, Strasser R, Stadlmann J, Kunert R, Rademacher T, Steinkellner H. Production of a monoclonal antibody in plants with a humanized N-glycosylation pattern. Plant Biotechnol J. 2007;5:657–63. doi: 10.1111/j.1467-7652.2007.00273.x. [DOI] [PubMed] [Google Scholar]
- Son MS, Taylor RK. Vibriocidal assays to determine the antibody titer of patient sera samples. Curr Protoc Microbiol. 2011;Chapter 6 doi: 10.1002/9780471729259.mc06a03s23. Unit6A 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sully EK, Whaley KJ, Bohorova N, Bohorov O, Goodman C, Kim do H, Pauly MH, Velasco J, Hiatt E, Morton J, Swope K, Roy CJ, Zeitlin L, Mantis NJ. Chimeric Plantibody Passively Protects Mice against Aerosolized Ricin Challenge. Clin Vaccine Immunol. 2014;21:777–82. doi: 10.1128/CVI.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve S, Boutonnier A, Mulard LA, Fournier JM. Immunochemical characterization of an Ogawa-Inaba common antigenic determinant of Vibrio cholerae O1. Microbiology. 1999;145(Pt 9):2477–84. doi: 10.1099/00221287-145-9-2477. [DOI] [PubMed] [Google Scholar]
- Wang J, Villeneuve S, Zhang J, Lei P, Miller CE, Lafaye P, Nato F, Szu SC, Karpas A, Bystricky S, Robbins JB, Kovac P, Fournier JM, Glaudemans CP. On the antigenic determinants of the lipopolysaccharides of Vibrio cholerae O:1, serotypes Ogawa and Inaba. J Biol Chem. 1998;273:2777–83. doi: 10.1074/jbc.273.5.2777. [DOI] [PubMed] [Google Scholar]
- Whaley KJ, Hiatt A, Zeitlin L. Emerging antibody products and Nicotiana manufacturing. Hum Vaccin. 2011;7 doi: 10.4161/hv.7.3.14266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner Ld, Mack J, Weltzin R, Mekalanos JJ, Kraehenbuhl JP, Neutra MR. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infection & Immunity. 1991;59:977–82. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


