Abstract
Resistance to BRAF inhibitors is a major clinical problem. Here we evaluate BI-847325, an ATP-competitive inhibitor of MEK and Aurora kinases, in treatment-naïve and drug-resistant BRAF-mutant melanoma models. BI-847325 potently inhibited growth and survival of melanoma cell lines that were both BRAF inhibitor naïve and resistant in 2D culture, 3D cell culture conditions and in colony formation assays. Western blot studies showed BI-847325 to reduce expression of phospho-ERK and phospho-histone 3 in multiple models of vemurafenib resistance. Mechanistically, BI-847325 decreased the expression of MEK and Mcl-1 while increasing the expression of the pro-apoptotic protein BIM. Strong suppression of MEK expression was observed after 48 h of treatment, with no recovery following >72 h of washout. siRNA mediated knockdown of Mcl-1 enhanced the effects of BI-847325, whereas Mcl-1 overexpression reversed this in both 2D cell culture and 3D spheroid melanoma models. In vivo, once weekly BI-847325 (70 mg/kg) led to durable regression of BRAF-inhibitor naive xenografts with no regrowth seen (>65 days of treatment). In contrast, treatment with the vemurafenib analog PLX4720 was associated with tumor relapse at >30 days. BI-847325 also suppressed the long-term growth of xenografts with acquired PLX4720 resistance. Analysis of tumor samples revealed BI-847325 to induce apoptosis associated with suppression of phospho-ERK, total MEK, phospho-Histone3 and Mcl-1 expression. Our studies indicate that BI-847325 is effective in overcoming BRAF inhibitor resistance and has long-term inhibitory effects upon BRAF-mutant melanoma in vivo, through a mechanism associated with the decreased expression of both MEK and Mcl-1.
Keywords: melanoma, BRAF, aurora kinase, trametinib, ERK, resistance
INTRODUCTION
Melanoma is the most lethal type of skin cancer whose incidence continues to increase worldwide (1, 2). Around 50% of melanomas harbor BRAF mutations, with 90% of these occurring at the 600 position, leading to constitutive kinase activation (3). It is known that BRAF V600E mutant melanomas are dependent on mitogen activated protein kinase (MAPK) signaling pathway for their proliferation, invasion and resistance to apoptosis (4–6). In the clinic, selective BRAF inhibitors such as vemurafenib and dabrafenib are associated with high levels of response, which are typically short-lived (7, 8). Most of the clinically validated resistance mechanisms reported to date involve the reactivation of MEK/ERK signaling and current strategies are focused upon combinations that vertically inhibit the MAPK signaling pathway. There is already evidence that the combination of a MEK inhibitor and a BRAF inhibitor (dabrafenib/trametinib and cobimetinib/vemurafenib) is associated with greater progression-free and overall survival compared to BRAF inhibitor alone (9, 10). Despite this, relapse and resistance also occurs in the majority of patients receiving the BRAF/MEK inhibitor combination and further therapeutic strategies are urgently needed (11, 12).
Cancer is characterized by uncontrolled cell growth and deregulation of the cell cycle (13). The mitotic cell cycle is controlled through the activity of protein-protein complexes such as the chromosomal passenger complex proteins including the Aurora kinases, survivin, borealin and INCENP (14). Increased expression of some of these, such as Aurora kinase B has been observed in many cancers, including melanoma (15, 16). In melanoma cells, expression of Aurora kinase B is controlled via the MAPK signaling pathway through the transcription factor FOXM1 (15). There is also evidence that melanoma cells with acquired BRAF inhibitor resistance have a greater dependency upon Aurora B (15). Aurora kinase A plays a major role in centrosome function and spindle assembly as well as cytokinesis and the telophase of mitosis (17). A potential role for Aurora kinase A in melanoma was demonstrated by the ability of the Aurora kinase A inhibitors MLN8054/ MLN8237 to reduce entry into mitosis, induce senescence and to inhibit the proliferation of patient-derived melanoma tumor xenografts (18). Aurora kinase inhibitors are currently being investigated both pre-clinically and clinically across multiple cancer types.
BI-847325 is a novel, ATP-competitive, orally available inhibitor of Aurora kinases and MEK. In in vitro studies, BI-847325 inhibited the activity of Xenopus laevis Aurora Kinase B with an IC50 of 3 nM; with IC50 values for human Aurora kinase A and Aurora kinase C being 25 and 15 nM, respectively. BI-847325 also inhibited human MEK1 and MEK2 with respective IC50 values of 25 and 4 nM. In a panel of 29 additional kinases that represented the diversity of the kinome tree, BI-847325 inhibited 7 enzymes at 1 µM by more than 50% (LCK, MAP3K8, FGFR1, AMPK, CAMK1D, RAF and TBK1). The only kinases inhibited significantly in isolated kinase assays at concentrations <100 nM were LCK (5 nM) and MAP3K8 (93 nM).
In the current study, we demonstrate BI-847325 to be highly effective at overcoming acquired BRAF inhibitor resistance mediated through multiple mechanisms in both cell lines and human melanoma mouse xenograft models. Our results reveal BI-847325 to have a novel mechanism of action involving the downregulation of both Mcl-1 and MEK.
Materials and Methods
Cell culture
The parental melanoma cell lines 1205Lu, WM793, WM39 and WM164 were a kind gift from Dr. Meenhard Herlyn (The Wistar Institute, Philadelphia, PA). Cell lines were genotyped for BRAF V600E mutations in (19). The M229, M229R, M249 and M249R were generated as described in Nazarian and colleagues (20). The A375 and RPMI7951 melanoma cell lines were purchased from American Type Culture Collection and A375R were a kind gift from Plexxikon Inc. The identity of each cell line was confirmed through STR validation analysis by Biosynthesis Inc (Lewisville, TX). Vemurafenib resistant cell lines 1205LuR, WM793R and WM164R were generated in (21). All the naive and intrinsically resistant cell lines were cultured in RPMI complete medium with 5% FBS. All the acquired resistant cell lines were cultured in RPMI complete medium with 5% FBS with the addition of vemurafenib at the following concentrations: M229R and M249R (2µM), A375R (2.5µM), WM793R and WM164R (2µM) and 1205LuR (3µM).
Cell proliferation assay
Cells were plated at a density of 2.5 × 103 cells per 100 µl and left to grow overnight before being treated with increasing concentrations of BI-847325 (Boehringer Ingelheim) for 72h. The metabolic activity was determined using Alamar blue reagent as per the manufacturer’s protocol.
Colony formation assay
Cells were grown overnight at a density of 1 × 104 cells per ml and treated with vehicle (dimethyl sulfoxide) or with 30 nM, 100 nM, 300 nM and 1 µM of BI-847325 (Boehringer Ingelheim). The medium and drug/vehicle was replaced every two weeks. After 4 weeks of treatment the colonies were stained with crystal violet dye, as described in (22). The percent relative clonogenic survival was determined using ImageJ software.
Flow cytometry
Cells were plated into 6 well plates at a density of 1 × 105 cells per ml and left to adhere overnight. Cells were treated with 10nM, 100nM and 1µM BI-847325 (Boehringer Ingelheim), 30nM trametinib (Selleck), 1µM VX680 (Selleck) for 48h. Annexin V and TMRM staining was done as described in (21).
Three-dimensional spheroid assay
3D melanoma spheroids were prepared using the liquid overlay method as described in (23) before being treated with vehicle (dimethyl sulfoxide) or 300nM – 3µM of BI-847325 (Boehringer Ingelheim) for 48h. Spheroids were washed, stained with Live/Dead viability stain (Invitrogen/ Life Technologies Corp.) and analyzed as described in (14). The percentage of dead cells was determined using ImageJ software.
Western blotting
Proteins extraction and blotting was performed as in (24). The primary antibodies for phospho-ERK, total-ERK, phospho-Histone 3, Mcl-1, BIM and total MEK, were from Cell Signaling Technology. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Sigma Aldrich) was used as a loading control to demonstrate even protein loading. The secondary antibodies; goat anti-rabbit IgG HRP and sheep anti-mouse IgG HRP were from Amersham/ GE Healthcare. For mouse xenograft experiments, tumor samples were harvested and immediately stored in RNAlater solution (Ambion) at 4°C before protein extraction.
RNA interference
1205Lu, 1025LuR, WM793, WM793R, WM793-Mcl-1 cells were plated at a cell density of 1 × 105 and left overnight to grow in complete RPMI medium with 5% FBS. The complete medium was replaced with Opti-MEM (Invitrogen). Cells were transfected with siRNA for BIM, Mcl-1, MEK 1 or MEK 2 (Cell Signaling) in complex with Lipofectamine 2000 (Invitrogen). For the Mcl-1 experiments 25nM of siRNA concentration was used and cells were transfected overnight. In the BIM studies, cells were transfected with 50nM siRNA overnight. For MEK1 and MEK2 studies, cells were transfected with 50nM siRNA for 4 h. Scrambled siRNAs were added as non-targeting control. Following the transfection time, cells were replaced with complete RPMI medium with 5% FBS and treated with 1µM BI-847325, 30nM trametinib, 1µM VX680 for 48h.
Quantitative Real-time PCR
Cells were treated with 1µM BI-847325 for 48h before RNA isolation. Total RNA was extracted using Qiagen’s RNeasy mini kit. Taqman Gene Expression Assays primer/probes used were: Hs00360961_m1 (MEK2), Hs01050896_m1 (Mcl-1) and P/N 4319413E (18S) for normalizing the data. Quantitative reverse transcriptase PCR reactions were carried out as per Molecular Genomics Core Facility protocol. For mouse xenograft experiments, tumor samples were harvested and stored in RNAlater solution (Ambion) at −80°C before RNA extraction.
Mcl-1 inducible cell line
WM793-Mcl-1 cells were a generous gift from Dr. Andrew Aplin (Kimmel Cancer Center, Philadelphia). Mcl-1 expression was induced by treating the cells with 100ng/ml doxycycline for 72h prior to treatment with 1µM BI-847325 for 48h.
Proteasome-Glo Chymotrypsin-like cell-based assay
1205Lu and 1205LuR cells were seeded in 96-well plates at a density of 7500 cells/ 50 µl per well and left overnight to grow before treatment with 1 µM BI-847325 or 300 nM, 1 µM and 3 µM MG-132 (Selleck) or BI 847325 and MG-132 in combination for 48h. Control cells were treated with vehicle (dimethyl sulfoxide). Proteasomal activity was measured by luminometry using Proteasome-Glo Chymotrypsin-like cell-based assay (Promega) as per the manufacturer’s protocol.
Mouse human melanoma xenograft experiments
BALB severe combined immune deficient (SCID) mice (Taconic) were subcutaneously injected with 2.5 × 106 cells. The tumors were grown to approximately 100mm3 size before initiation of dosing. Mice (n=5) were treated with 70mg/kg of BI-847325 or equivalent volume of vehicle (2-hydroxyethyl cellulose (Sigma Aldrich), polysorbate 80 (Fluka) with pH adjusted to 2.8 with 1M HCl (Sigma Aldrich)) 1 × per week by oral gavage. In parallel studies to assess the timeline of BRAF inhibitor failure, mice (n=5) were kept on a diet of chow containing 412mg PLX4720/ kg or control chow (Plexxikon). The mouse weights and tumor volumes (L × W2 × 0.523) were measured twice per week. On completion of the experiment (after < 24 h for PLX4720 treated and 48h for BI-847325 treated mice), the treated and control tumors collected were processed for Western Blotting and RNA extraction.
Immunohistochemical staining
Mice xenograft tissues were formalin-fixed and paraffin embedded. Slides were stained for cleaved caspase-3 as per the manufacturer’s protocol by Moffitt Tissue Core Research Histology services.
Statistical Analysis
All the data show mean of three independent experiments ± SEM. Results with p value ≤ 0.05 were considered as statistically significant.
RESULTS
BI-847325, a novel ATP-competitive MEK/Aurora kinase inhibitor, has prolonged growth inhibitory effects on BRAF-mutant and vemurafenib-resistant melanoma cells
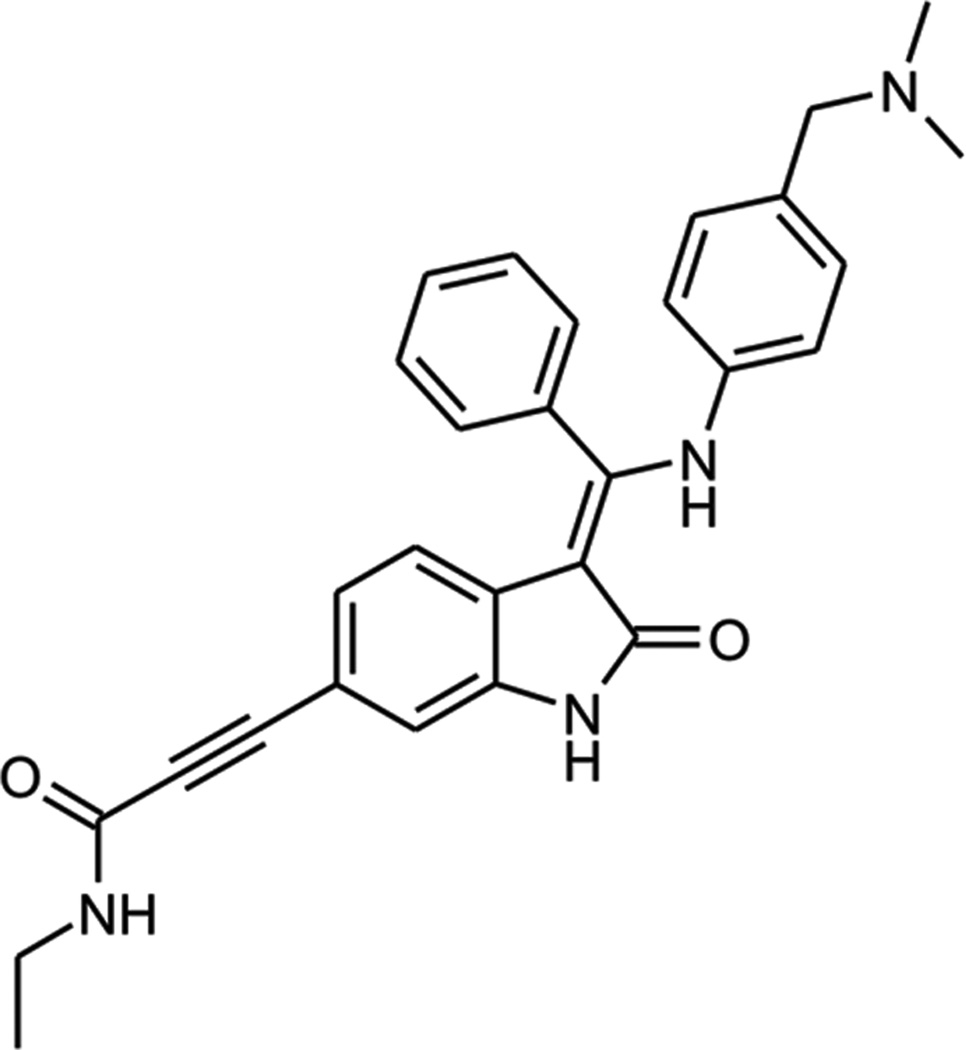
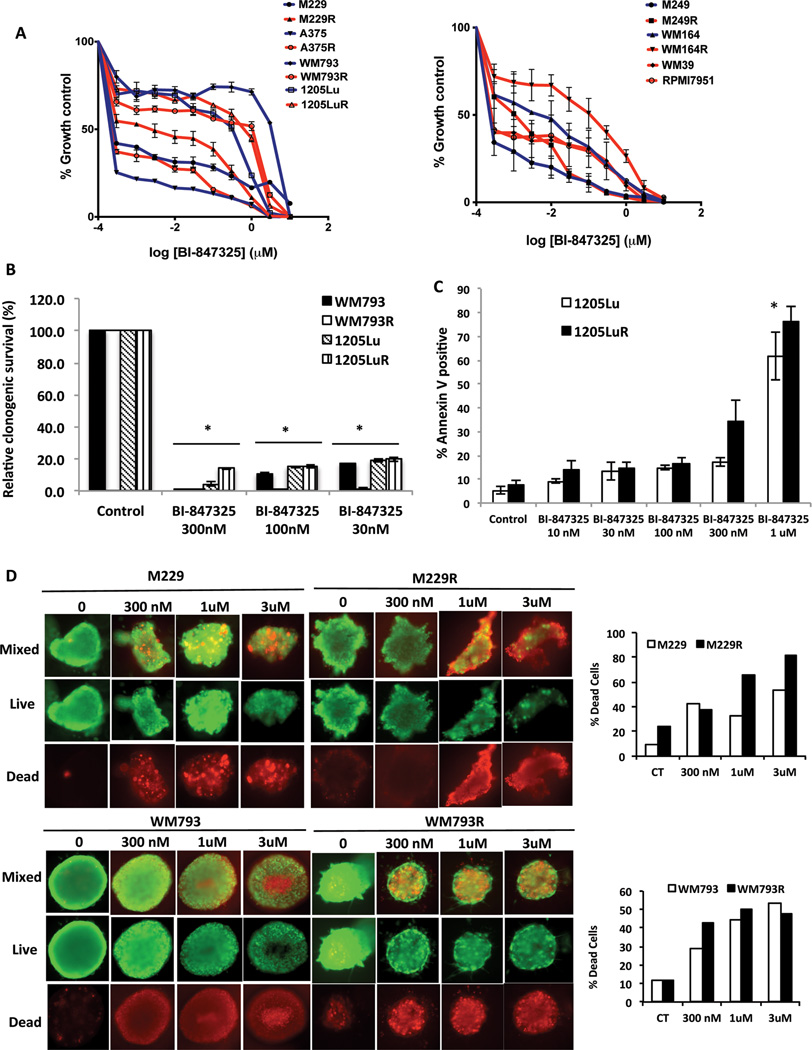
A panel of 14 BRAF-mutant melanoma cell lines (including some that had either acquired or intrinsic vemurafenib resistance: resistance mechanisms listed in Supplemental Table 1) was treated with BI-847325 (structure shown in Figure 1). Concentration-dependent decreases in cell growth were noted in all cell lines evaluated (Figure 2A). The IC50 values (Supplemental Table 2) demonstrate that vemurafenib resistant cell lines were less sensitive to BI-847325 compared to the naïve cell lines. Treatment of the cell line panel with BI-847325 (30–300 nM, 28 days) prevented colony formation in 6 BRAF-mutant melanoma cell lines (Figure 2B and Supplemental Figure 1A). The growth inhibitory effect of BI-847325 (48h, 1 µM) was also associated with apoptosis induction, with significant levels of cell death being seen at concentrations >100 nM (Figure 2C). Apoptosis induction was observed in melanoma cell lines that were both vemurafenib-naïve and resistant (Supplemental Figure 1B). BI-847325 was also cytotoxic (>300nM) in a 3D cell spheroid model of cell lines that were both vemurafenib naïve and resistant (Figure 2D).
Figure 1. Chemical structure of BI-847325.
Figure 2. The dual MEK and Aurora kinase inhibitor BI-847325 blocks the growth and survival of BRAF-mutant melanoma cell lines through induction of apoptosis.
A, growth curve showing the response of BRAF-mutant naïve and vemurafenib-resistant melanoma cell lines to BI-847325 treatment. Cells were treated with increasing concentrations of BI-847325 (1 nM to 30 µM) for 72h and analyzed by the Alamar blue assay. B, Quantitative analysis of percentage relative clonogenic survival after 4 weeks of BI-847325 treatment. Cell lines were treated with 30nM, 100nM and 300nM BI-847325 for 4 weeks, then fixed and stained with crystal violet. * P<0.05. C, BI-847325 induces apoptosis in BRAF-mutant and vemurafenib-resistant melanoma cell lines. Cells were treated with BI-847325 (10nM – 1µM) for 48h and stained with Annexin V. Induction of apoptosis was measured by flow cytometry. * P<0.05. D, BI-847325 induces cell death in 3D organotypic cell cultures of BRAF-mutant melanoma cell lines. Cultures were treated with BI-847325 (300nM–3µM; 48h) and stained with ethidium bromide and calcein-AM. Green stain indicates live cells and red stain indicates dead cells. The bar graphs (right) indicate quantitative analysis of the percent dead cells following treatment with BI-847325 for 48h.
BI-847325 induces apoptosis by reducing Mcl-1 expression
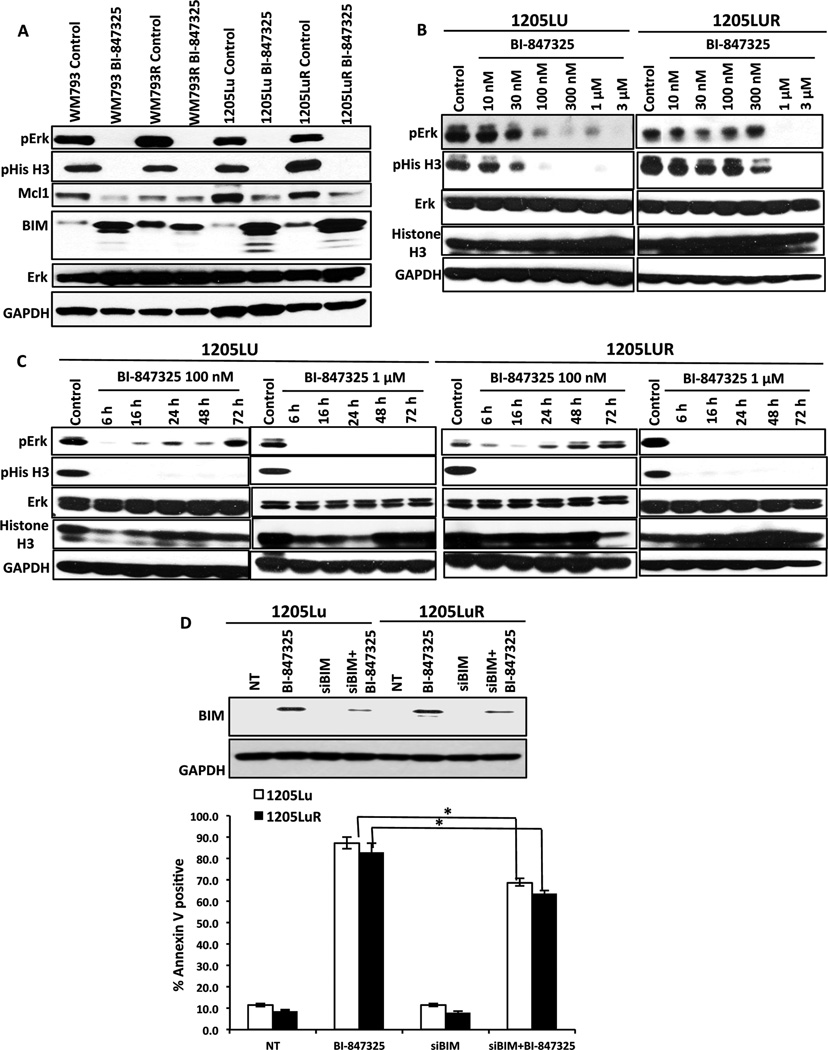
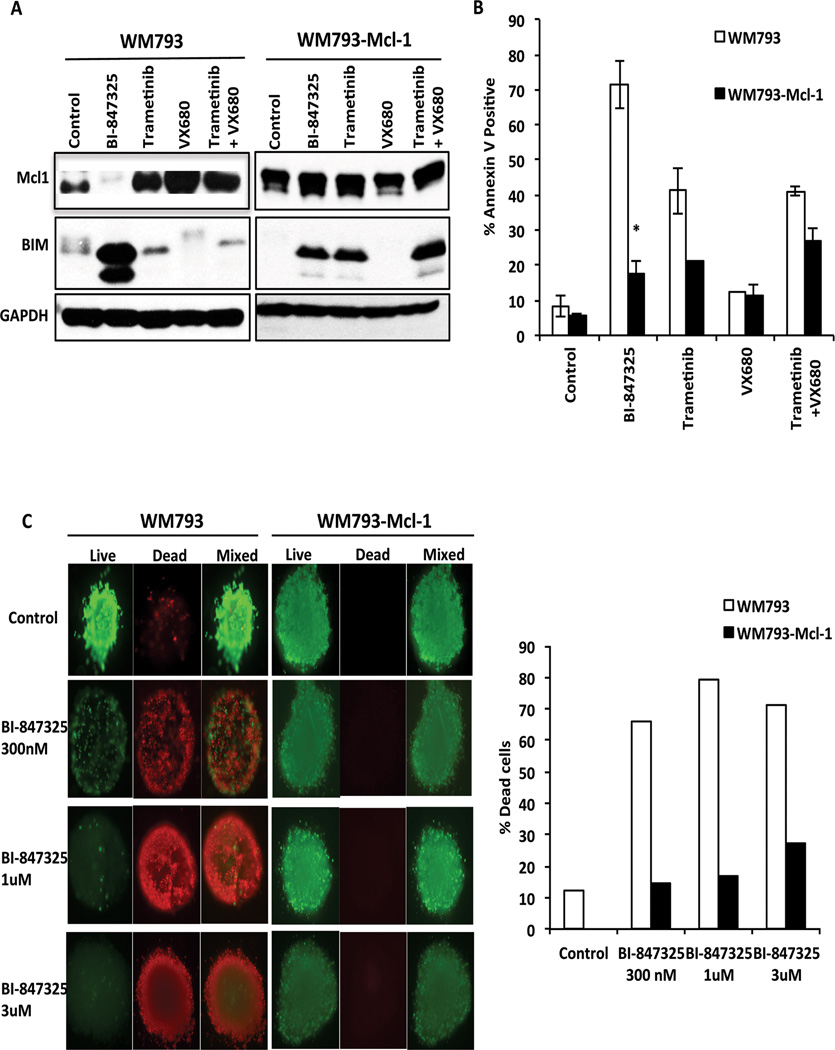
Western blot studies showed BI-847325 to inhibit both MEK and Aurora Kinase as shown by decreased expression of both phospho-ERK and phospho-histone-3 (Figure 3A). BI-847325 treatment was also associated with upregulated expression of the pro-apoptotic protein BIM and decreased expression of Mcl-1 (Figure 3A). Pharmacodynamic studies revealed BI-847325 to mediate concentration and time-dependent inhibition of MEK and Aurora kinase. Although it was noted that the inhibition of Aurora kinase and MEK was equipotent, a time-dependent recovery of phospho-ERK signaling was seen following treatment with 100nM, but not 1µM, BI-847325 (Figures 3B,C). No recovery of Aurora Kinase signaling was noted at either concentration of BI-847325. We next determined whether the effects of BI-847325 could be recapitulated through the dual inhibition of MEK and Aurora kinase. We began by determining concentrations of both the MEK inhibitor trametinib and the Aurora kinase inhibitor VX680 that supramaximally inhibited MEK and Histone H3 phosphorylation, respectively (Supplemental Figure 2A). It was noted that 300nM of each drug inhibited MEK and Aurora kinase, respectively, to the same degree as that seen to 1 µM of BI-847325, and that neither the Aurora Kinase inhibitor alone, nor its combination with trametinib, inhibited Mcl-1 expression (Supplemental Figure 2B). A partial role for BIM induction in the cytotoxic activity of BI-847325 was confirmed through siRNA studies in which the knockdown of BIM was associated with a significant (but minor) decrease in apoptosis (Figure 3D).
Figure 3. BI-847325 induces apoptosis altering the expression of pro and anti-apoptotic proteins.
A, Western blot analysis reveals that treatment with 1µM BI-847325 for 48h inhibits phospho-ERK, phospho-Histone3, decreases Mcl-1 expression and upregulates BIM expression in 4 BRAF-mutant cell lines. B, BI-847325 inhibits MEK and Aurora kinase in a dose dependent manner. Two BRAF-mutant melanoma cell lines were treated with BI-847325 (10nM–3µM: 48h) followed by Western Blotting for pERK and pHistone-H3. C, ERK signaling recovers at lower concentrations of BI-847325. Treatment with 100nM and 1µM BI-847325 showed a time-dependent decrease in the phosphorylation of Histone-H3 and ERK. D, Top; Western blot showing siRNA-mediated knockdown of BIM in 1205Lu and 1205LuR cells. Cells were transfected with 25 nM siRNA overnight, followed by lipofectamine washout and treatment with 1µM BI-847325 for 48h. Cells transfected with non-targeting siRNA were used as control. Bottom; siRNA knockdown of BI-847325 mediated BIM significantly reduces apoptosis in 1205Lu and 1205LuR cell lines. Cells were stained with Annexin V and analyzed by flow cytometry. * P<0.05
BI-847325-mediated apoptosis is associated with reduced Mcl-1 expression
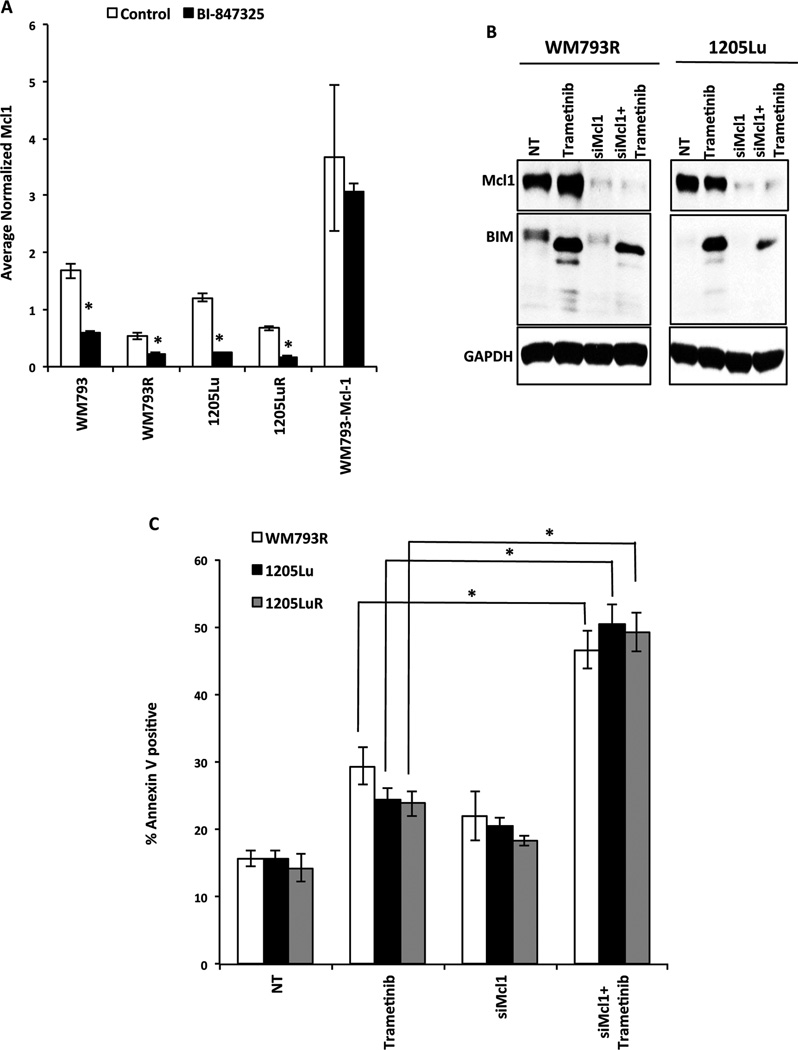
We next investigated the role of Mcl-1 downregulation in the cytotoxic activity of BI-847325. Quantitative RT-PCR experiments showed BI-847325 treatment to be associated with a significant reduction in Mcl-1 mRNA in 4 BRAF mutant melanoma cell lines, including 2 with acquired BRAF inhibitor resistance (WM793R and 1205LuR) (Figure 4A). In contrast, no downregulation of mRNA was seen in the WM793-Mcl-1 cell line where overexpression of Mcl-1 was driven under a doxycycline-inducible promoter (the WM793-Mcl-1 cell line). The decreased expression of Mcl-1 appeared to be required for the pro-apoptotic activity of BI-847325, with studies showing Mcl-1 knockdown through siRNA to significantly enhance trametinib-induced apoptosis (Figures 4B,C).
Figure 4. BI-847325-mediated apoptosis is induced following downregulation of Mcl-1.
A, qRT-PCR showing that treatment with 1µM BI-847325 (48h) significantly decreases Mcl-1 mRNA expression. * P<0.05. B, Western blots demonstrating siRNA-mediated downregulation of Mcl-1. Cells were transfected with 25 nM siRNA overnight, followed by lipofectamine washout and treatment with 30 nM Trametinib for 48h. Cells transfected with non-targeting siRNA were used as control. C, siRNA mediated knockdown of Mcl-1 in combination with MEK inhibitor trametinib (30nM, 48h) leads to significant induction of apoptosis in 1205Lu and 1205LuR cell lines. Cells were stained with Annexin V to measure apoptosis. The results were analyzed by flow cytometry. * P<0.05.
Induction of Mcl-1 expression protects from BI-847325-mediated cell death
We next used a BRAF-mutant melanoma cell line with inducible Mcl-1 expression (WM793-Mcl-1) to determine whether forced Mcl-1 expression protected melanoma cells from BI-847325-mediated cell death. Initial studies showed that BI-847325 was unable to decrease the expression of induced Mcl-1 in the WM793-Mcl-1 cell line (Figure 5A). We next used Annexin-V binding/flow cytometry assays to show that induction of Mcl-1 was associated with a significant reduction of BI-847325-mediated apoptosis (Figure 5B). The combination of the MEK inhibitor trametinib with the Aurora kinase inhibitor VX680 did not recapitulate the induction of apoptosis by BI-847325 (Figure 5A,B). Mcl-1 overexpression also enhanced survival of the cells in a 3D collagen-implanted spheroid model treatment with little cell death seen at BI-847325 concentrations as high as 3µM (Figure 5C).
Figure 5. Decreased expression of Mcl-1 is required for BI-847325-mediated apoptosis.
A, Western blot showing overexpression of Mcl-1 in WM793-Mcl-1 cell lines following treatment with 100 ng/ml of doxycycline (72h). WM793 and WM793-Mcl-1 cells were treated with vehicle, BI-847325, trametinib, VX680 or trametinib+VX680 for 48h, before being blotted for Mcl-1 and BIM. B, BI-847325-mediated apoptosis is significantly reduced in Mcl-1 overexpressed cells. Cells were treated with vehicle, BI-847325, trametinib, VX680 or trametinib+VX680 for 48h, before being stained with Annexin-V. Apoptosis was measured by flow cytometry. * P<0.05. C, Mcl-1 overexpression prevents BI-847325 induced cell death in 3D organotypic cell cultures of BRAF-mutant melanoma cell lines. (Left) Cultures were treated with BI-847325 (1 µM, 48h) before being stained with ethidium bromide and calcein-AM for dead and live cells respectively. (Right) Quantitative analysis of the percentage dead cells from the 3D spheroid model following BI-847325 treatment.
BI-847325 mediated cytotoxicity in vemurafenib-naïve and resistant melanoma cell lines is associated with decreased MEK expression
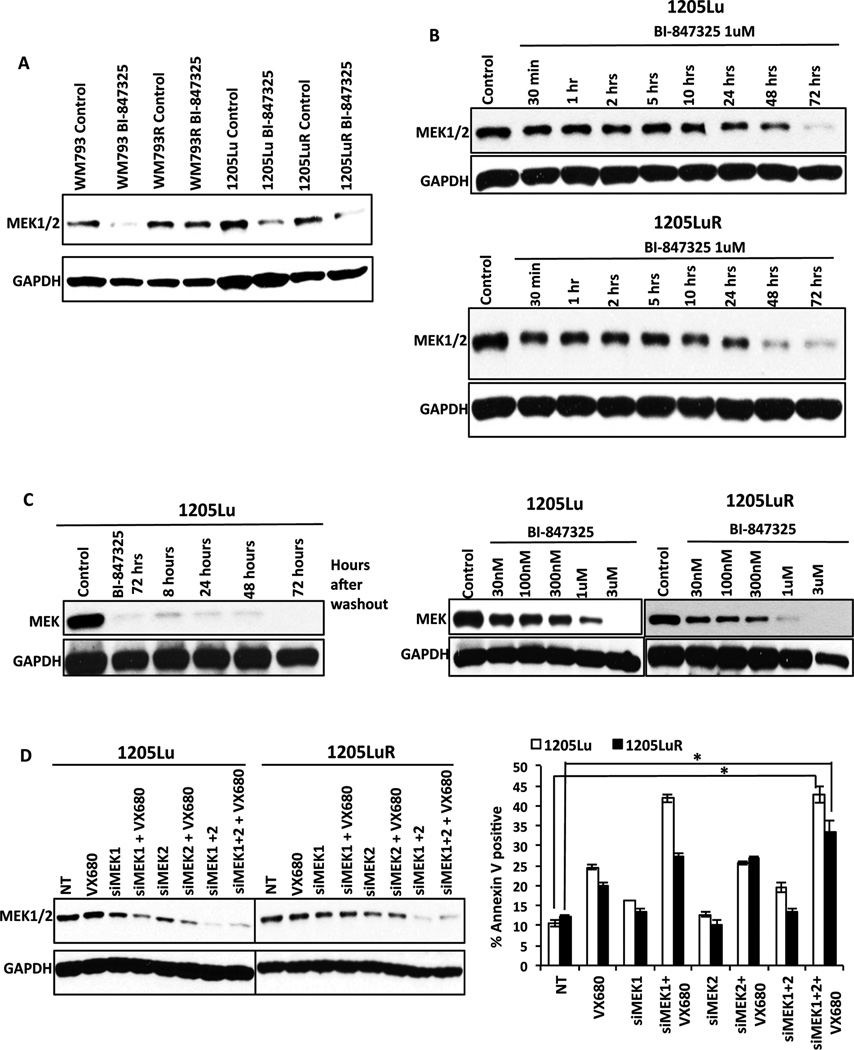
Acquired resistance to BRAF inhibitors typically leads to the reactivation of MAPK signaling. As treatment with BI-847325 was highly effective at overcoming acquired BRAF inhibitor resistance we next determined the effects the drug had upon components of the MAPK signaling pathway. Unexpectedly it was found that BI-847325 treatment decreased total MEK expression in 3/4 melanoma cell lines tested (Figure 6A). The decrease in MEK expression seen was slow in onset (>24 h) (Figure 6B) but was durable, with no recovery of expression seen 72 h after washout of the drug (Figure 6C; left). These effects were also concentration-dependent, with differences in sensitivity observed between the naïve and resistant cell lines. In the vemurafenib-naïve 1205Lu cell lines, decreased MEK expression was seen at 1 µM, the same concentration required to block reactivation of ERK signaling (Figures 3C and 6C). We confirmed that the decrease in MEK expression following BI-847325 treatment was not mimicked by MEK inhibition (trametinib), Aurora kinase inhibition (VX680) or the MEK/Aurora kinase inhibitor combination in BRAF mutant melanoma cell lines (Supplemental Figure 2B).
Figure 6. BI-847325 leads to decreased MEK expression in BRAF-mutant naïve and vemurafenib-resistant cells.
A, Western blot analysis showing BI-847325 (1µM, 48h) to significantly reduce the expression of MEK. B, BI-847325 leads to the time-dependent decreases in MEK expression. Cells were treated with BI-847325 (1µM) for increasing periods of time before being probed for MEK expression by Western blot. C, BI-847325 treatment leads to a durable, concentration-dependent decrease in MEK expression. Left, MEK expression does not recover following washout. 1205Lu cells were treated with BI-847325 (1µM, 72h) followed by washout for 8–72h. No recovery of MEK expression was seen following 72h washout. Right, 1205Lu and 1205LuR cells were treated with increasing concentrations of BI-847325 followed by Western blotting for MEK. D, siRNA knockdown of MEK1/2 enhances aurora kinase inhibitor mediated apoptosis. Left; Western blot confirming siRNA knockdown of MEK1/2. Right; siRNA mediated knockdown of MEK1 and MEK2 enhances the level of apoptosis seen following treatment with the aurora kinase inhibitor VX680. Graph shows levels of apoptosis following treatment with non-targeting siRNA (NT), VX680, siMEK1, siMEK2 and their combination with VX680. * P<0.05
We next performed siRNA knockdown of MEK1/2 to determine if suppression of MEK enhanced the activity of Aurora kinase inhibitors. Knockdown of MEK1 and MEK2 was confirmed through Western Blot analysis (Figure 6D). In this instance, the downregulation of MEK1 expression enhanced the pro-apoptotic effects of VX680 in the 1205Lu cell line. Additionally, a significant enhancement of the response was also seen in the 1205LuR cell line when both MEK1 and MEK2 were knocked down (Figure 6D). The effects of BI-847325 upon MEK expression were not mediated through increased targeting of MEK to the proteasome, as these were not reversed following treatment with the proteasome inhibitor MG-132 (Supplemental Figure 3A). There was also little evidence that BI-847325 consistently altered mRNA levels of MEK (Supplemental Figure 3B).
BI-847325 treatment suppresses the growth of BRAF-mutant and vemurafenib-resistant human melanoma xenografts in mice
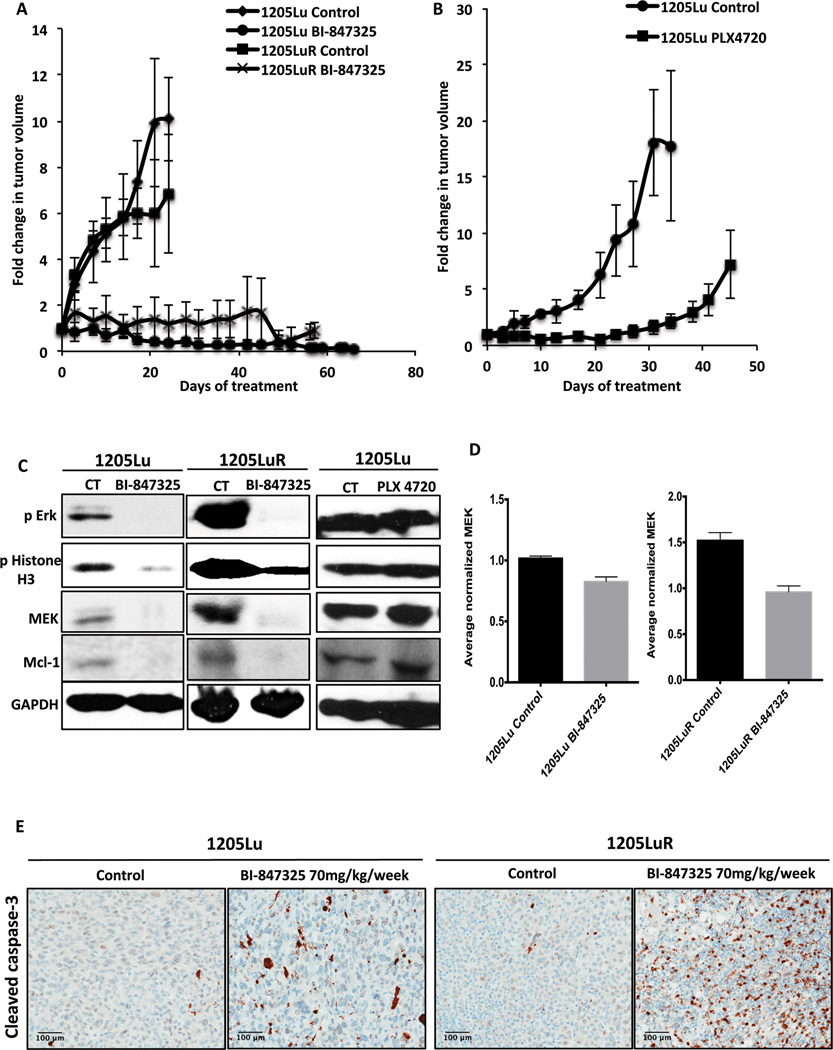
We next assessed the anti-melanoma activity of BI-847325 and its ability to overcome vemurafenib-resistance in vivo. Of the 4 BRAF-mutant melanoma cell lines tested, the 1205Lu and 1205LuR formed xenografts in SCID mice. A 70 mg/kg/week dose schedule of BI-847325 was evaluated for efficacy against established 1205Lu and 1205LuR tumors, with significant tumor suppression being seen in the drug-naïve 1205Lu xenografts that lasted >65 days (Figure 7A). Long-term suppression of tumor growth was also observed in the vemurafenib-resistant 1205LuR xenograft model throughout the 55-day treatment period (Figure 7A). No significant alteration in the body weights of the mice was observed during the study period, indicating that the BI-847325 was well tolerated (data not shown).
Figure 7. BI-847325 inhibits growth of BRAF-mutant melanoma xenografts.
A, BI-847325 treatment was associated with a significant and durable tumor regression. Animals bearing 1205Lu and 1205LuR xenografts were treated with BI-847325 70 mg/kg/week for 65 days. The data shows the fold-change in tumor volume normalized to starting tumor volumes. B, animals bearing 1205Lu xenografts were kept on a diet with 412 mg PLX 4720/kg for 53 days. A slow progression of tumor growth was observed with a relapse after 30 days of treatment. The data represents fold-change in tumor volume normalized to starting tumor volumes. C, Western blots showing the inhibition of intratumoral pERK and pHistone H3, and decreased Mcl-1 and MEK expression following BI-847325 treatment but not PLX4720 treatment. Animals were dosed with either vehicle (CT) or drug for 30 days before tumors were collected and analyzed. D, qRT-PCR results show a minor decrease in MEK mRNA levels following BI-847325 treatment. E, Images showing IHC staining for cleaved caspase-3 in 1205Lu and 1205LuR xenografts treated with 70mg/ kg/ week BI-847325.
PLX4720 is a BRAF inhibitor that has a similar potency and pharmacological profile to vemurafenib, but was not selected for clinical development (25). In contrast to the long-term suppression of growth seen in response to BI-847325, treatment of 1205Lu tumors with PLX4720 was only associated with a short-term inhibition of tumor growth and the onset of resistance by day 30 (Figure 7B). A pharmacodynamic analysis of 1205Lu and 1205LuR xenograft specimens dosed with BI-847325 (70mg/kg/week) showed significant decreases in intratumoral Mcl-1 and MEK and expression after 55 days of treatment (Figure 7C). Decreases were also seen in phospho-ERK and phospho-Histone H3, indicating that both the MAPK and Aurora kinase were being inhibited (Figure 7C). Together, our results indicate that BI-847325 is effective in overcoming vemurafenib resistance in vivo. It was further found that PLX4720 had little effect upon phospho-ERK, phospho-Histone H3, MEK or Mcl-1 expression (Figure 7C). Treatment of 1205Lu and 1205LuR xenografts with BI-847325 was also associated with decreased expression of MEK at the RNA level (Figure 7D). Increased intratumoral levels of apoptosis were also seen following BI-847325 treatment as shown by the increased staining for cleaved caspase-3 (Figure 7E).
Discussion
Despite the improvement in survival rates of patients with BRAF-mutant melanoma after vemurafenib treatment responses still remain transient with relapse and resistance being common in most cases (7, 8). Most of the mechanisms of acquired BRAF inhibitor resistance identified thus far lead to restoration of signaling through the MAPK pathway (26). Studies have shown the MAPK pathway to be reactivated in 70–79% of melanomas with acquired vemurafenib and dabrafenib resistance (26, 27). The recovery of MAPK signaling can be driven through the acquisition of mutations in NRAS, MEK1/2, as well as through BRAF-splice form mutants (11, 20, 28). Although there is good evidence that vertical targeting of the MAPK pathway through combined BRAF/MEK inhibition delays therapeutic escape, this seems largely ineffective after resistance is acquired (29). In the current study, we provide evidence that the novel ATP-competitive MEK/Aurora kinase inhibitor BI-847325 overcomes acquired BRAF inhibitor resistance through a novel mechanism involving the suppression of both MEK and Mcl-1. This strategy was effective against multiple cellular mechanisms of acquired BRAF inhibitor resistance and reduced tumor growth in in vivo models of BRAF inhibitor naïve and resistant melanoma.
Pharmacological studies showed BI-847325 to abrogate BRAF inhibitor resistance mediated through increased PDGFRβ expression (M229R), cyclin D1 amplification (WM39), acquisition of a RAS mutation (A375R, M249R) and COT amplification (RPMI7951). It was also active in a number of resistance models for which the mechanism has not yet been determined (1205LuR, WM793R, WM164R). In all cases, BI-847325 durably suppressed the growth of multiple BRAF-mutant melanoma cell lines with little evidence of colonies emerging after 4 weeks of in vitro treatment. By contrast it was noted that the same cell lines when treated with vemurafenib, gave rise to resistant colonies in every case (22). BI-847325 was also highly cytotoxic; inducing apoptosis in 2D cell culture as well as high levels of cell death in more physiologically relevant 3D cell cultures (23) and in in vivo xenograft models. In some of the cell lines, BI-847325 showed a greater potency on the resistant cell line than its drug-naïve counterpart.
In BRAF-mutant melanoma cells the MAPK pathway is an important regulator of cell survival through the suppression of expression of pro-apoptotic BH3 family proteins as well as by increasing levels of important pro-survival proteins (30–32). BIM is a BH3-only protein that induces cell death through antagonizing the pro-survival effects of Bcl-2, Bcl-XL, Bcl-w and Mcl-1 (33). In melanoma cell lines, levels of BIM are kept low through the MAPK-mediated phosphorylation of BIM at Ser69, leading to its proteasomal targeting (30, 34). Treatment with inhibitors of MEK and BRAF prevent the destruction of BIM by the proteasome leading to apoptosis (35, 36). Acquired BRAF inhibitor resistance is associated with increased basal phosphorylation of BIM at Ser69, as well as a suppression of its expression through epigenetic means - a state that can be reversed through treatment with inhibitors of HSP90 and epigenetic modifiers such as HDAC inhibitors (21, 37). Treatment of both BRAF inhibitor-naïve and resistant melanoma cell lines with BI-847325 increased expression of BIM and there was evidence through siRNA knockdown that BIM induction was at least partly required for the BI-847325 mediated apoptotic response.
The anti-apoptotic protein myeloid cell leukemia-1 (Mcl-1) is a member of the Bcl-2 family of proteins (38). It is known to play a critical role in cell survival and is overexpressed in many cancers (39). The pro-survival effects of Mcl-1 are mediated through it binding to and neutralizing the death-inducing effects of BIM, as well as Bak and Bax (40). Previous studies have shown Mcl-1 to be overexpressed in melanoma cells and that its expression is subject to regulation by the BRAF/MEK signaling cascade (39). Downregulation of Mcl-1 by RNA interference increases the susceptibility of BRAF mutant melanoma cell lines to anoikis, a form of apoptosis induced by inappropriate adhesion (39).
BI-847325 treatment decreased the expression of Mcl-1 at both the protein and mRNA levels. In this regard, BI-847325 differed from other Aurora Kinase inhibitors such as VX680, which had little effect upon Mcl-1 expression in the BRAF mutant cell lines. There is evidence in other systems that Aurora Kinase inhibitors alter the dependency on the expression of pro-survival BH3-only family proteins. In carcinoma cell lines, the Aurora kinase inhibitor VX680 induced apoptosis following the entry into polyploidy (41). In this context, the polyploid state was associated with neutralization of Mcl-1 function and an increased dependency upon Bcl-XL for survival (41). Downregulation of Mcl-1 expression in the context of MEK inhibition was implicated in the pro-apoptotic effects of BI-847325. It was noted that the inducible overexpression of Mcl-1 conveyed complete protection to BI-847325-mediated death in both 2D cell culture assays and 3D collagen implanted spheroids. siRNA knockdown of Mcl-1 in combination with the MEK inhibitor trametinib recapitulated the cytotoxic effects of BI-847325. These findings confirm earlier studies showing Mcl-1 to be a key regulator of sensitivity to MEK inhibition in BRAF-mutant melanoma cells (42).
BI-847325 also appeared to have unique MEK inhibitory activity through the regulation of its expression. The decrease in MEK levels seen following BI-847325 treatment was slow in onset; occurring between 24 h and 72 h of treatment and was durable - with little recovery seen 72 h after washout. This activity was in marked contrast to allosteric inhibitors of MEK such as trametinib, which inhibited MEK kinase function without altering protein expression. Mechanistically, the effects of BI-847325 upon MEK downregulation did not seem to be dependent upon its increased targeting to the proteasome, as they were not reversed upon treatment with the proteasome inhibitor MG-132. There was little evidence that BI-847325 altered MEK expression at the mRNA level, with a q-RT-PCR analysis showing differing results across the cell lines. Together these data suggested that the decrease in MEK protein expression following BI-847325 treatment was unlikely to be a consequence of its downregulation at the RNA level.
The long-term suppression of MEK expression appeared to underlie the durable anti-tumor responses seen in vivo when BI-847325 was administered weekly. Although concentrations >1 µM were required to achieve this, this can be readily achieved in vivo, with the once weekly dose of BI-847325 (70 mg/kg) being associated with peak plasma concentrations of 2700nM in mice (P. Sini, unpublished observations). As the vast majority of therapeutic escape mechanisms involve reactivation of MAPK signaling, the direct suppression of MEK expression appears to be an excellent strategy to delay and abrogate the onset of resistance. Our group and others have already provided evidence that the combined decreased of CRAF and Mcl-1 expression and the inhibition of MAPK signaling through the use of HSP90 inhibitors may be a viable strategy to overcome and prevent acquired BRAF inhibitor resistance (21, 43, 44).
Although our study confirms the utility of combined MEK and Aurora kinase inhibition, not all the activity of BI-847325 could be recapitulated by the inhibition of Aurora Kinase and MEK. An analysis of BI-847325 against a panel of 30 kinases, showed only Lck and p38 MAPK to have an IC50 <100 nM (P. Sini, unpublished observations). As yet the potential role of these kinases in the anti-melanoma activity seen to BI-847325 remains to be determined. A more interesting explanation may be based on the fact that BI-847325 interacts with the ATP binding site of the target protein, whereas trametinib (as well as all other MEK inhibitors currently in clinical development) are allosteric inhibitors that bind to a distant site on MEK. It is conceivable that these distinct protein-ligand interactions result in differences in MEK conformation and, consequently, its interaction with other proteins and perhaps its stability.
In BRAF-mutant melanoma cell lines two groups have already demonstrated that the MAPK pathway directly regulates Aurora kinase B, with one group implicating the transcription factor FOXM1 (15). The Aurora protein family consists of three members Aurora A, Aurora B and Aurora C all of which are frequently overexpressed in cancer. Of these, overexpression of Aurora A in NIH-3T3 cells is known to enhanced colony formation and solid tumor formation, as well as being implicated in the emergence of centrosome abnormalities and aneuploidy in breast cancer (45, 46). Previous studies have reported that overexpression of Aurora kinase B contributes to melanoma progression by causing genomic instability and aneuploidy (15, 47). Other work has implicated Aurora kinase in the escape from vemurafenib therapy, with 2 BRAF inhibitor resistant cell lines showing sensitivity to the Aurora kinase B inhibitor AZD1152 (15). There is also some limited evidence in 3D cell culture models that the BRAF/MEK/Aurora kinase A inhibitor combination is more effective at reducing melanoma cell growth than any of these agents alone (48).
At this time there is much focus upon pathways that can be targeted in combination with the MAPK pathway in melanoma. Although there has been interest in the dual targeting of BRAF with the PI3K/AKT/mTOR pathway, this has been associated with significant toxicity and little clinical efficacy. Here we show for the first time that the dual targeting of Aurora kinase and MEK gives more durable effects than BRAF inhibition alone. Our study demonstrates that drugs like BI-847325 that suppress expression of both MEK and Mcl-1, are highly effective at both abrogating the onset and overcoming acquired BRAF inhibitor resistance. We suggest that strategies such as these are worthy of further clinical investigation in BRAF-mutant melanoma.
Supplementary Material
Acknowledgments
Grant support: Work in the Smalley lab is supported by SPORE grant 1P50CA168536-01A1 and R01 CA161107-01 from the National Institutes of Health and through support from Boehringer Ingelheim.
Footnotes
Conflict of interest: Patrizia Sini is an employee of Boehringer-Ingelheim. Keiran Smalley received grant support from Boehringer-Ingelheim. No other conflicts of interest are declared.
References
- 1.Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist. 2011;16:5–24. doi: 10.1634/theoncologist.2010-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 4.Fedorenko IV, Paraiso KH, Smalley KS. Acquired and intrinsic BRAF inhibitor resistance in BRAF V600E mutant melanoma. Biochem Pharmacol. 2011;82:201–209. doi: 10.1016/j.bcp.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein RM, Aplin AE. Rnd3 regulation of the actin cytoskeleton promotes melanoma migration and invasive outgrowth in three dimensions. Cancer Res. 2009;69:2224–2233. doi: 10.1158/0008-5472.CAN-08-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arozarena I, Sanchez-Laorden B, Packer L, Hidalgo-Carcedo C, Hayward R, Viros A, et al. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell. 2011;19:45–57. doi: 10.1016/j.ccr.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 8.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. The New England journal of medicine. 2012;367:1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 11.Van Allen EM, Wagle N, Sucker A, Treacy DJ, Johannessen CM, Goetz EM, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4:94–109. doi: 10.1158/2159-8290.CD-13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagle N, Van Allen EM, Treacy DJ, Frederick DT, Cooper ZA, Taylor-Weiner A, et al. MAP kinase pathway alterations in BRAF-mutant melanoma patients with acquired resistance to combined RAF/MEK inhibition. Cancer Discov. 2014;4:61–68. doi: 10.1158/2159-8290.CD-13-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 14.Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nature reviews Molecular cell biology. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- 15.Bonet C, Giuliano S, Ohanna M, Bille K, Allegra M, Lacour JP, et al. Aurora B is regulated by the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling pathway and is a valuable potential target in melanoma cells. The Journal of biological chemistry. 2012;287:29887–29898. doi: 10.1074/jbc.M112.371682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma A, Madhunapantula SV, Gowda R, Berg A, Neves RI, Robertson GP. Identification of aurora kinase B and Wee1-like protein kinase as downstream targets of (V600E)B-RAF in melanoma. The American journal of pathology. 2013;182:1151–1162. doi: 10.1016/j.ajpath.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lens SM, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nature reviews Cancer. 2010;10:825–841. doi: 10.1038/nrc2964. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Hawkins OE, Su Y, Vilgelm AE, Sobolik T, Thu YM, et al. Targeting aurora kinases limits tumour growth through DNA damage-mediated senescence and blockade of NF-kappaB impairs this drug-induced senescence. EMBO molecular medicine. 2013;5:149–166. doi: 10.1002/emmm.201201378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smalley KS, Contractor R, Haass NK, Lee JT, Nathanson KL, Medina CA, et al. Ki67 expression levels are a better marker of reduced melanoma growth following MEK inhibitor treatment than phospho-ERK levels. Br J Cancer. 2007;96:445–449. doi: 10.1038/sj.bjc.6603596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paraiso KH, Haarberg HE, Wood E, Rebecca VW, Chen YA, Xiang Y, et al. The HSP90 inhibitor XL888 overcomes BRAF inhibitor resistance mediated through diverse mechanisms. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:2502–2514. doi: 10.1158/1078-0432.CCR-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paraiso KH, Fedorenko IV, Cantini LP, Munko AC, Hall M, Sondak VK, et al. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer. 2010;102:1724–1730. doi: 10.1038/sj.bjc.6605714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smalley KS, Haass NK, Brafford PA, Lioni M, Flaherty KT, Herlyn M. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol Cancer Ther. 2006;5:1136–1144. doi: 10.1158/1535-7163.MCT-06-0084. [DOI] [PubMed] [Google Scholar]
- 24.Smalley KS, Brafford P, Haass NK, Brandner JM, Brown E, Herlyn M. Up-regulated expression of zonula occludens protein-1 in human melanoma associates with N-cadherin and contributes to invasion and adhesion. Am J Pathol. 2005;166:1541–1554. doi: 10.1016/S0002-9440(10)62370-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi H, Hugo W, Kong X, Hong A, Koya RC, Moriceau G, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4:80–93. doi: 10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizos H, Menzies AM, Pupo GM, Carlino MS, Fung C, Hyman J, et al. BRAF Inhibitor Resistance Mechanisms in Metastatic Melanoma: Spectrum and Clinical Impact. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:1965–1977. doi: 10.1158/1078-0432.CCR-13-3122. [DOI] [PubMed] [Google Scholar]
- 28.Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim KB, Kefford R, Pavlick AC, Infante JR, Ribas A, Sosman JA, et al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:482–489. doi: 10.1200/JCO.2012.43.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cartlidge RA, Thomas GR, Cagnol S, Jong KA, Molton SA, Finch AJ, et al. Oncogenic BRAF(V600E) inhibits BIM expression to promote melanoma cell survival. Pigment Cell Melanoma Res. 2008;21:534–544. doi: 10.1111/j.1755-148X.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang CC, Lucas K, Avery-Kiejda KA, Wade M, deBock CE, Thorne RF, et al. Up-regulation of Mcl-1 is critical for survival of human melanoma cells upon endoplasmic reticulum stress. Cancer Res. 2008;68:6708–6717. doi: 10.1158/0008-5472.CAN-08-0349. [DOI] [PubMed] [Google Scholar]
- 32.Boisvert-Adamo K, Aplin AE. Mutant B-RAF mediates resistance to anoikis via Bad and Bim. Oncogene. 2008;27:3301–3312. doi: 10.1038/sj.onc.1211003. [DOI] [PubMed] [Google Scholar]
- 33.Ley R, Ewings KE, Hadfield K, Cook SJ. Regulatory phosphorylation of Bim: sorting out the ERK from the JNK. Cell Death Differ. 2005;12:1008–1014. doi: 10.1038/sj.cdd.4401688. [DOI] [PubMed] [Google Scholar]
- 34.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278:18811–18816. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 35.Lucas KM, Mohana-Kumaran N, Lau D, Zhang XD, Hersey P, Huang DC, et al. Modulation of NOXA and MCL-1 as a strategy for sensitizing melanoma cells to the BH3-mimetic ABT-737. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:783–795. doi: 10.1158/1078-0432.CCR-11-1166. [DOI] [PubMed] [Google Scholar]
- 36.Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, et al. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22:6785–6793. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- 37.Shao Y, Aplin AE. BH3-only protein silencing contributes to acquired resistance to PLX4720 in human melanoma. Cell death and differentiation. 2012;19:2029–2039. doi: 10.1038/cdd.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boisvert-Adamo K, Longmate W, Abel EV, Aplin AE. Mcl-1 is required for melanoma cell resistance to anoikis. Mol Cancer Res. 2009;7:549–556. doi: 10.1158/1541-7786.MCR-08-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morales AA, Kurtoglu M, Matulis SM, Liu J, Siefker D, Gutman DM, et al. Distribution of Bim determines Mcl-1 dependence or codependence with Bcl-xL/Bcl-2 in Mcl-1-expressing myeloma cells. Blood. 2011;118:1329–1339. doi: 10.1182/blood-2011-01-327197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah OJ, Lin X, Li L, Huang X, Li J, Anderson MG, et al. Bcl-XL represents a druggable molecular vulnerability during aurora B inhibitor-mediated polyploidization. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12634–12639. doi: 10.1073/pnas.0913615107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang YF, Jiang CC, Kiejda KA, Gillespie S, Zhang XD, Hersey P. Apoptosis induction in human melanoma cells by inhibition of MEK is caspase-independent and mediated by the Bcl-2 family members PUMA, Bim, and Mcl-1. Clin Cancer Res. 2007;13:4934–4942. doi: 10.1158/1078-0432.CCR-07-0665. [DOI] [PubMed] [Google Scholar]
- 43.Acquaviva J, Smith DL, Jimenez JP, Zhang C, Sequeira M, He S, et al. Overcoming acquired BRAF inhibitor resistance in melanoma via targeted inhibition of Hsp90 with ganetespib. Molecular Cancer Therapeutics. 2014;13:353–363. doi: 10.1158/1535-7163.MCT-13-0481. [DOI] [PubMed] [Google Scholar]
- 44.Smyth T, Paraiso KH, Hearn K, Rodriguez-Lopez AM, Munck JM, Haarberg HE, et al. Inhibition of HSP90 by AT13387 Delays the Emergence of Resistance to BRAF Inhibitors and Overcomes Resistance to Dual BRAF and MEK Inhibition in Melanoma Models. Mol Cancer Ther. 2014;13:2793–2804. doi: 10.1158/1535-7163.MCT-14-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomonaga T, Nomura F. Chromosome instability and kinetochore dysfunction. Histology and Histopathology. 2007;22:191–197. doi: 10.14670/HH-22.191. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Zhou YX, Qiao W, Tominaga Y, Ouchi M, Ouchi T, et al. Overexpression of aurora kinase A in mouse mammary epithelium induces genetic instability preceding mammary tumor formation. Oncogene. 2006;25:7148–7158. doi: 10.1038/sj.onc.1209707. [DOI] [PubMed] [Google Scholar]
- 47.Pirker C, Lotsch D, Spiegl-Kreinecker S, Jantscher F, Sutterluty H, Micksche M, et al. Response of experimental malignant melanoma models to the pan-Aurora kinase inhibitor VE-465. Experimental Dermatology. 2010;19:1040–1047. doi: 10.1111/j.1600-0625.2010.01182.x. [DOI] [PubMed] [Google Scholar]
- 48.Caputo E, Miceli R, Motti M, Tate R, Fratangelo F, Botti G, et al. AurkA inhibitors enhance the effects of B-RAF and MEK inhibitors in melanoma treatment. Journal of translational medicine. 2014;12:216. doi: 10.1186/s12967-014-0216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.