Abstract
Müller cells are macroglia and play many essential roles as supporting cells in the retina. To respond to pathological changes in diabetic retinopathy (DR), a major complication in the eye of diabetic patients, retinal Müller glia produce a high level of vascular endothelial growth factor (VEGF or VEGF-A). As VEGF is expressed by multiple retinal cell-types and Müller glia comprise only a small portion of cells in the retina, it has been a great challenge to reveal the function of VEGF or other globally expressed proteins produced by Müller cells. With the development of conditional gene targeting tools, it is now possible to dissect the function of Müller cell-derived VEGF in vivo. By using conditional gene targeting approach, we demonstrate that Müller glia are a major source of retinal VEGF in diabetic mice and Müller cell-derived VEGF plays a significant role in the alteration of protein expression and peroxynitration, which leads to retinal inflammation, neovascularization, vascular leakage, and vascular lesion, key pathological changes in DR. Therefore, Müller glia are a potential cellular target for the treatment of DR, a leading cause of blindness.
Keywords: Müller glia, Vascular endothelial growth factor, Protein modification, Inflammation, Blood-retina barriers, Diabetic retinopathy
Core tip: Diabetic retinopathy is a disorder of blood-retina barriers (BRBs) and neurons. Anti-vascular endothelial growth factor (VEGF) drugs are explored for treating BRB breakdown in the disease. As VEGF is also potentially beneficial, it is essential to understand the cellular and molecular mechanisms of VEGF action in the retina. Discussion is centered on the usefulness of conditional gene targeting mice in dissecting the function of globally expressed VEGF and in identifying significant roles for Müller glia-derived VEGF in diabetes-induced changes in protein expression/modification, inflammation, and BRB lesions and leakage.
INTRODUCTION
Müller cells, the principal macroglia of mammalian retina, span from the vitreal surface to subretinal space and cover all retinal layers. This structural arrangement is ideal for them to serve as major supporting cells in regulating physiological and pathological responses in retinal vasculature and neurons. In the retina, Müller glia play many essential roles in metabolism, functions, maintenance, and protection by providing trophic factors, removing metabolic wastes, controlling extracellular space volumes and ion and water homeostasis, participating visual cycles, releasing neurotransmitters, regulating blood-retina barrier (BRB) function, and modulating innate immunity[1]. Müller glia is also a major respondent to various stresses by reactive gliosis which involves in morphological, biochemical, and physiological changes. Under some pathological conditions, uncontrollable alteration in growth factor production, such as overexpression of vascular endothelial growth factor (VEGF-A or VEGF) in diabetic retinopathy (DR), results in a detrimental effect and causes vision loss. In this mini-review, we will discuss the general role of VEGF in DR and the function of Müller cell-derived-VEGF (MCD-VEGF) in protein expression/modification, inflammation, BRB function, and vascular and neuronal integrity in diabetic animals.
DIABETIC RETINOPATHY
DR is a leading cause of blindness in working-age people in industrialized countries and is traditionally regarded as a microvascular complication in the eye of diabetic patients due to an apparent breakdown of the endothelial barrier, which is formed by orderly arranged tight junction proteins. The structural interactions between these tight junction proteins control the fluid flow through the barrier[2]. Patients with endothelial barrier breakdown demonstrate the following key clinical characteristics: retinal hemorrhages, microaneurysms, cotton-wool spots, lipid exudates, macular edema, capillary occlusion, and retinal neovascularization[2]. Studies with retinal pigment epithelium (RPE) barrier by others and us suggest that the breakdown of the tight junctions in the RPE barrier may contribute substantially to diabetes-induced blood-content leakage[3-5], which is responsible for at least some form of macular edema[6]. Therefore, DR is not just a microvascular disease, but rather a disorder of BRB. Macular edema resulted from BRB breakdown and retinal neovascularization are two most devastating causes of vision loss in diabetic patients. On the other hand, it is increasingly recognized that the loss of retinal neuronal function and viability occurs before the onset of BRB abnormalities in diabetic patients and in experimental animals[7-11]. Perhaps neuronal and BRB disorder is a more appropriate description for DR.
VEGF IN REGULATING BLOOD-RETINA BARRIER FUNCTION
VEGF-A or VEGF, a heparin-binding homodimeric glycoprotein[12,13], belongs to a family of seven members, including VEGF-A to -F and placental growth factor and (PlGF). Each of them may also have several isoforms due to alternative splicing, which affects their solubility, and thus is responsible for their cellular localization. The most intensively studied and predominant isoform of VEGF-A in humans is VEGF-A165. VEGFs exert their function through complicated receptor- and co-receptor-mediated signaling cascades, which involves VEGF receptor-1 (VEGFR1), VEGFR2, VEGFR3, neuropilin-1, neuropilin-2, vascular endothelial cadherin, and integrin[14]. Much of the literature information regarding VEGFs in the eye is centered on the pathobiology of VEGF-A due to its high relevance to the pathogenesis of DR, retinopathy of prematurity (ROP), and age-related macular degeneration (AMD), leading causes of blindness.
VEGF is a potent mitogenic factor for endothelial proliferation and migration and tube formation during vessel development and is a major stimulator for embryogenesis, vasculogenesis, and angiogenesis[13,15]. Disruption of a single VEGF allele is lethal in mice at embryonic day 11-12[16,17]. VEGF is a major regulator of pathological neovascularization in proliferative DR[18]. VEGF blockade has been shown to inhibit hypoxia-induced retinal angiogenesis[19-21]. Due to its potent activity in inducing blood barrier hyper-permeability[22-25], VEGF is regarded as a major contributor to the high level of blood-content leakage in DR[26,27]. Overexpression of VEGF or its receptors, which causes disorganization of endothelial and RPE tight junctions[25,28,29], is associated with diabetic macular edema[30].
A major regulator for VEGF signaling is oxygen tension and VEGF expression is induced by hypoxia, a pathological condition occurrs after early stages of DR[31]. While hypoxia-inducible factor-1 (HIF-1) is a critical regulator in this response[32], its degradation is controlled by von-Hippel-Lindhal (VHL) suppressor protein[33]. Therefore, HIF-1 (perhaps other HIFs) and VHL are key upstream regulators for VEGF-induced BRB breakdown in DR through VEGFR2[13,15,34]. Although VEGF signaling may be a major pathogenic mechanism for DR, various growth factors, inflammatory cytokines, and prostaglandins may also affect the disease through VEGF signaling-dependent or independent mechanisms[35-38]. As a result of intensive effort in VEGF pathobiology and pharmacology, anti-VEGF agents are utilized as a major strategy for the treatment of retinal neovascularization, BRB breakdown, and macula edema in DR.
CONDITIONAL VEGF DISRUPTION IN MÜLLER GLIA
VEGF is produced by several retinal cell-types. Müller glia and RPE cells are thought to produce high levels of VEGF[39]. Indirect evidences obtained from in vivo localization and molecular biology approach with cell cultures suggest a significant role for VEGF in regulating BRB function in DR[40,41]. However, the importance of MCD-VEGF in DR remained unclear for a long time after the initial discovery of VEGF as a potential pathogenic factor in DR. A major reason for this is that Müller glia only comprise a small portion of retinal cells and VEGF is expressed by multiple cell-types, which makes it difficult to detect Müller cell-specific VEGF expression by immunohistochemistry (IHC) or immunoblotting (IB). Additionally, more and more “new” VEGF functions have been identified since the discovery of its involvement in BRB function in the mid-1990s[27,42]. While other retinal cells may not produce a high level of VEGF at a given time, it is almost impossible to pinpoint the local effect of VEGF produced by these cells, if VEGF action is blocked globally by genetic, immunological, biochemical, or pharmacological approaches. Therefore, cell-specific approach may be the “only” way to delineate the precise function of Müller cell-derived VEGF (MCD-VEGF). As Müller glia play such a critical role in general health and functions of the retina, a better understanding of their biology is paramount to the prevention and treatment of retinal diseases[43]. For this purpose, several laboratories developed cell-specific genetic tools for Müller glia[44], which is very helpful for dissecting the specific function of globally expressed proteins, such as VEGF, in Müller cells.
In a serendipity fashion while developing inducible Cre/lox system for the RPE using the promoter of human vitelliform macular dystrophy-2 (Vmd2) gene[45,46], we identified one transgenic founder mouse that was capable of carrying out productive Cre-mediated excisive recombination in Müller glia. This transgenic Cre-drive line provides an opportunity to generate conditional VEGF knockout (CVKO) mice that disrupt VEGF expression mainly in Müller glia. The CVKO mice were generated by breeding this Müller glial Cre-drive line with a mouse line carrying loxP-flanked VEGF gene (VEGFff), called floxed VEGF mice[45,47,48]. The degree of VEGF disruption in the Müller glia was assessed by IB with primary Müller cell cultures derived from the CVKO mice, which reduced VEGF production by 66%. This degree of VEGF knockout (KO) caused a near 50% reduction of total retinal VEGF in CVKO mice under normal conditions[48,49]. To ascertain whether the production of MCD-VEGF was substantially decreased in CVKO mice in disease models[49], we examined VEGF expression in diabetic and hypoxic models (see detail below). While diabetes and hypoxia doubled retinal VEGF in WT mice, the deletion of MCD-VEGF caused a near 50% decrease of VEGF overexpression in the retina under hypoxic or diabetic conditions (Table 1). These data were corroborated by IHC with CVKO mice[48,49]. Considering the fact that Müller cells only comprise a small portion of retinal cells, our data undisputedly suggest that Müller glia are a major cellular origin of VEGF in mouse retinas.
Table 1.
Alteration in protein expression/modification in diabetic or hypoxic Müller cell-specific KO mice
| Model/time | Proteins/modification | Alteration |
| STZ-induced diabetes/6 mo | Albumin | Decrease (59%) |
| STZ-induced diabetes/2 mo | ICAM1 | Decrease (62%) |
| STZ-induced diabetes/2 mo | Nitrotyrosine | Decrease (19%) |
| STZ-induced diabetes/6 mo | Occludin | Increase (60%) |
| STZ-induced diabetes/2 mo | pNF-κB p65 | Decrease (48%) |
| STZ-induced diabetes/2 mo | TNF1α | Decrease (53%) |
| STZ-induced diabetes/6 mo | VEGF | Decrease (51%) |
| STZ-induced diabetes/6 mo | ZO1 | Increase (130%) |
| Oxygen-induced retinopathy | Albumin | Decrease (56%) |
| Oxygen-induced retinopathy | VEGF | Decrease (45%) |
| Oxygen-induced retinopathy | Occludin | Increase (35%) |
STZ: Streptozotocin; VEGF: Vascular endothelial growth factor; ICAM1: Intercellular adhesion molecule-1; TNFα: Tumor necrosis factor-α; NF: Nuclear factor; ZO1: Zonula occludens-1.
MÜLLER CELL-DERIVED VEGF IN PROTEIN EXPRESSION/MODIFICATION AND RETINAL INFLAMMATION
To determine whether deletion of MCD-VEGF resulted in any significant changes in DR-associated gene expression, we performed IB analysis with retinal extracts from CVKO mice after diabetes was induced with streptozotocin (STZ). HIF1α is a major parameter for oxygen tension and the induction of HIF1α contributes to the increase in VEGF[50]. To delineate the regulatory mechanisms between HIF1α and VEGF, we examined the expression level of HIF1α in hypoxic and diabetic CVKO mice. Although HIF1α was up-regulated significantly in hypoxic retinas at P14 (see detail below) and diabetic retinas (two month post-STZ injection) in WT controls, there was no apparent difference in the levels of retinal HIF1α between the CVKO mice and WT controls under hypoxic and diabetic conditions[48,49], suggesting that hypoxia/HIF1α is an upstream regulator of VEGF produced by Müller glia.
Nuclear factor-kappa-B (NF-κB) is a transcription factor and a major player in inducing early pathological changes, such as inflammation, in DR[51,52]. To explore if MCD-VEGF regulated NF-κB in diabetic retina, we examined the expression/phosphorylation of NF-κB p65 subunit. While there was no detectable change in the total NF-κB p65 level between the CVKO mice and WT controls, the loss of MCD-VEGF caused a dramatic decrease (48%) of phosphorylated (activated) NF-κB p65 in the retina 2 mo after STZ-injection (Table 1). This result suggests that activated p65 is downstream of MCD-VEGF in DR[49]. Nitric oxide (NO) is an important inflammatory mediator and its level is a representation of oxidative stress in the retina of diabetic patients and animals[53-55]. Peroxynitrite is a highly reactive oxidant, which is formed by the rapid combination of NO with superoxide. Increased peroxynitrite formation may be directly linked to diabetes-induced VEGF overexpression and there is a possible loop effect of VEGF signaling and peroxynitrite formation[56,57]. To determine the role of MCD-VEGF in protein nitration, we examined the level of nitrotyrosine, a biomarker of peroxynitrite, in retinal protein extracts from CVKO and control mice 2 mo after STZ-injection. The retinal extracts from diabetic CVKO mice demonstrated a 19% decrease of proteins with nitrotyrosine (Table 1), indicating that the disruption of MCD-VEGF reduced oxidative stress.
Inflammation is an early pathological response in DR. To identify the role of MCD-VEGF in retinal inflammation in DR, we examined the levels of pro-inflammatory markers in CVKO mice by IB analysis for intercellular adhesion molecule-1 (ICAM1) and tumor necrosis factor-α (TNFα), 2 mo after STZ-injection. Compared with controls, the CVKO mice showed 62.3% and 52.9% reduction of ICAM1 and TNFα (Table 1), respectively. These results suggest that the deletion of MCD-VEGF substantially inhibits inflammation in diabetic retinas[49]. This notion is reinforced by the result from the leukostasis assay showing a 75.0% reduction of adherent leukocytes, a cardinal feature of retinal inflammation in DR, in the retinal microvasculature of the CVKO mice 2 mo after STZ injection (Table 2). Collectively, these data point to a major role for MCD-VEGF in developing inflammation in DR.
Table 2.
Pathological changes in diabetic or hypoxic Müller cell-specific KO mice
| Model | Pathological changes | Alteration |
| STZ-induced diabetes | Leukocytosis | Decrease (75%) |
| STZ-induced diabetes | Vascular leakage | Decrease (60%) |
| STZ-induced diabetes | Acellular capillaries | Decrease (45%) |
| STZ-induced diabetes | Vascular leakage | Decrease (60.0%) |
| Oxygen-induced retinopathy | Pre-retinal neovascularization | Decrease (34%) |
| Oxygen-induced retinopathy | Neovascularization area | Decrease (40%) |
| Oxygen-induced retinopathy | Vaso-obliteration area | Decrease (30%) |
STZ: Streptozotocin.
MÜLLER CELL-DERIVED VEGF IN BRB FUNCTION AND INTEGRITY
As diabetic rodents usually do not develop retinal neovascularization[58], the closest way to investigate this is to utilize oxygen induced retinopathy (OIR), a model mimicking an infant blinding disease, ROP. To examine the effect of MCD-VEGF on BRB function, the severity of retinal neovascularization was examined in CVKO mice subjected to OIR. The CVKO mice were placed in hyperoxia (75% oxygen) from postnatal day 7 (P7) to P12 and were then kept under normoxia for up to 5 d. In OIR model, the retina was relatively hypoxic at P14 (compared with that at P7-12), as judged by the abundance of HIF1α. The level of retinal VEGF in hypoxic CVKO mice was decreased by 45% (Table 1). At P17, fluorescein angiography was performed and OIR-treated CVKO mice demonstrated 40% and 30% reductions in areas of retinal neovascularization and of vaso-obliteration, respectively (Table 2). Retinal sections from OIR-treated CVKO mice also showed a 34% reduction in the number of pre-retinal neovascular endothelia (Table 2). As a consequence of decreasing retinal neovascularization and unhealthy microvasculature, we observed a 56% reduction of OIR-induced vascular leakage in CVKO mice (Table 1), judged by IB for albumin. This observation was supported by IHC data[48].
IB analysis with retinal and vitreous extracts prepared from PBS-perfused diabetic WT mice demonstrated a 1.5-fold increase in extravascular albumin 6 mo after STZ-injection. However, the disruption of MCD-VEGF caused a near 60% reduction of albumin leakage in age-matched diabetic mice (Table 1). Such a reduction can be visualized in the retinal flat-mounts of diabetic CVKO mice by intravenously injected fluorescein isothiocyanate-labeled albumin[49].
To delineate the mechanistic insights of MCD-VEGF in diabetes-induced vascular leakage, we analyzed the levels of occludin and Zonula occludens-1 (ZO1), two major tight junction proteins, in the retina[49]. While diabetes resulted in 39% and 58% decreases of occludin and ZO1, respectively, in WT animals, no alteration in occludin and ZO1 expression was observed in diabetic CVKO mice. As a result, the diabetic CVKO mice had 60% and 130% upregulation of occludin and ZO1 (Table 1), compared with that of diabetic WT controls. Our data indicated that the disruption of MCD-VEGF resulted in a significant reduction of diabetes-induced retinal vascular leakage by attenuating the depletion of occludin and ZO1. This result was supported by a 36% increase in the level of occludin in the retina (Table 1) and by qualitative evidence from IHC in hypoxic CVKO mice generated with OIR[48]. Collectively, our data suggest that MCD-VEGF was a major inducer of vascular leakage in OIR-treated hypoxic mice and in diabetic mice through VEGF signaling-induced decrease of tight junction integrity.
To assess retinal vascular lesions in diabetic CVKO mice, the number of acellular capillaries in trypsin digested retinal flat-mounts was examined 6 mo after diabetes was induced[49]. The diabetic CVKO mice had 45% fewer acellular capillaries than that in controls (Table 2), suggesting that the loss of MCD-VEGF had a protective effect on retinal microvasculatures, which reduced vascular leakage through the endothelial barrier in DR.
MÜLLER CELL-DERIVED VEGF IN RETINAL DEVELOPMENT AND INTEGRITY
Disruption of MCD-VEGF in the CVKO mice did not affect the development of retinal and choroidal vasculatures and overall retina[48], as determined by morphological analysis in retinal sections with light microscopy, functional test with electroretinography, and retinal and choroidal vascular density and morphological examination in retinal and RPE/choroidal flat-mounts with IHC and fluorescein angiography. Although negative results from conditional gene KO studies are not conclusive, our observation is somewhat expected as Müller glia are one of the last few cell-types to develop and KO of VEGF in neural retina during embryonic development results in abnormal retinal vessels[59]. In our study, the loss of MCD-VEGF did not affect retinal integrity in the aging CVKO mice under normal and diabetic mice[49]. This result is seemly contradictory to the observation that VEGF is a survival factor for retinal ganglion cells, photoreceptors, and Müller glia[60,61]. The following may account for the “discrepancy”: the disruption of MCD-VEGF in the CVKO mice did not remove VEGF completely as several types of retinal cells produce permeable VEGF and a basal level of VEGF may be sufficient for physiological VEGF function, such as neuronal function and integrity in the retina. In addition, our studies did not blocked VEGF signaling in any retinal cells. However, the result that MCD-VEGF had no apparent role in retinal development and integrity provides an advantage to investigating the role of MCD-VEGF in CVKO mice. Since there was no distinguishable defect in the animals under normal conditions, any phenotypical difference between the CVKO and control mice can be attributed to the defects from deleting MCD-VEGF.
CONCLUSION
Work from others and our laboratories demonstrated a major role for MCD-VEGF in DR, as summarized in Figure 1. Our data clearly pinpoint that MCD-VEGF plays a major role in protein alteration/modification, inflammation, neovascularization, vascular leakage, and vascular lesion in DR. Our study also suggests that MCD-VEGF may be a downstream regulator of DR-related master regulator, such as HIF1α/hypoxia, but upstream regulator for others, such as NF-κB and peroxynitrite. We also need to keep in mind that DR is a multifactorial disease. Other growth factors and pro-inflammatory mediators may be involved in developing DR in a VEGF-independent manner. A better understanding of VEGF-dependent and -independent pathways is the key to new therapeutic strategies for intervening multiple drug targets simultaneously, since anti-VEGF strategy alone cannot prevent DR completely. The CVKO mice provide an excellent animal model in this new endeavor.
Figure 1.
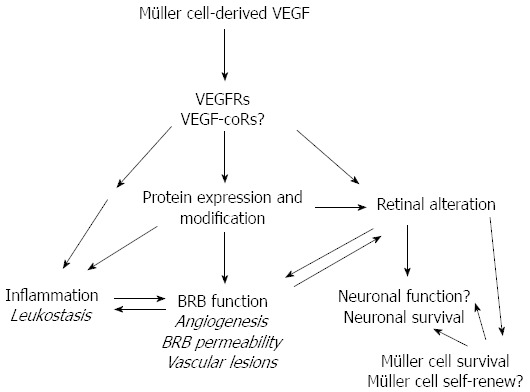
A simplified schematic diagram for the potential roles of Müller cell-derived-vascular endothelial growth factor in diabetic retinopathy. Alteration in pathological characteristics caused by MCD-VEGF is indicated in italic. Potential functions without direct proof by experimental and clinical data are indicated with a question marker. MCD-VEGF plays a significant role in causing retinal inflammation, neovascularization, vascular leakage, vascular lesion, and protein alteration and modification in the pathogenesis of DR. MCD-VEGF: Müller cell-derived-VEGF; DR: Diabetic retinopathy; VEGF: Vascular endothelial growth factor.
VEGF is a neural protectant and it has been shown to modulate neuronal function in the brain[62]. Potentially, MCD-VEGF may also be involved in regulating neuronal integrity. With the development of tools in studying Müller glia and their relationship with neuronal survival in diabetes and hypoxia[44,63,64], the role of MCD-VEGF in neuroprotection in the retina should be sorted out shortly. Current literature suggests that MCD-VEGF may have a critical role in maintaining Müller glia through autocrine in hypoxia and diabetes. However, it is not clear whether MCD-VEGF acts solely as trophic factor in the maintenance of Müller glia. Other mechanism, such as proliferation (Müller cell self-renew), may also be potentially important to the maintenance of retinal integrity. In principles, mammalian Müller cells are capable of dedifferentiation, proliferation, and differentiation into various retinal neurons under various conditions and are considered as a major retinal stem cell[65]. It will be fascinating if MCD-VEGF can act similarly as other growth factors in differentiating Müller cells to neurons for neuro-protection[66].
Although there are many publications on VEGF or MCD-VEGF in DR, little is known about its actually signaling pathways. As discussed earlier, the presence of at least seven VEGF receptors and co-receptors accounts for the difficulties in revealing their mechanisms. Additionally, VEGF is a secreted protein and loss of VEGF produced by a single cell-type can be compensated by others. Delineating detailed mechanisms may greatly enhance our understanding to the pathogenesis, treatment, and neuronal function in DR, which is critical to the improvement and safety of current anti-VEGF strategy and to the design of new treatments for the disease.
ACKNOWLEDGMENTS
We thank Dr. Gennadiy P Moiseyev for critical reading of this manuscript and members of our laboratories for providing data and helpful discussions.
Footnotes
Supported by The NIH grants, Nos. GM104934, EY020900 and EY021725 (NEI Core); Chinese National Natural Science Foundation grant, No. 81200699; grants from Presbyterian Health Foundation and Oklahoma Center for Adult Stem Cell Research, and an endowment from Choctaw Nation (to Le YZ).
Conflict-of-interest: None.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 18, 2014
First decision: January 8, 2015
Article in press: March 18, 2015
P- Reviewer: Das UN, García Mayor R, McNally RJQ, Saleh J, Swierczynski J S- Editor: Tian YL L- Editor: A E- Editor: Zhang DN
References
- 1.Reichenbach A, Bringmann A. New functions of Müller cells. Glia. 2013;61:651–678. doi: 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]
- 2.Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002;47 Suppl 2:S253–S262. doi: 10.1016/s0039-6257(02)00387-9. [DOI] [PubMed] [Google Scholar]
- 3.Xu HZ, Le YZ. Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Invest Ophthalmol Vis Sci. 2011;52:2160–2164. doi: 10.1167/iovs.10-6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu HZ, Song Z, Fu S, Zhu M, Le YZ. RPE barrier breakdown in diabetic retinopathy: seeing is believing. J Ocul Biol Dis Infor. 2011;4:83–92. doi: 10.1007/s12177-011-9068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ablonczy Z, Prakasam A, Fant J, Fauq A, Crosson C, Sambamurti K. Pigment epithelium-derived factor maintains retinal pigment epithelium function by inhibiting vascular endothelial growth factor-R2 signaling through gamma-secretase. J Biol Chem. 2009;284:30177–30186. doi: 10.1074/jbc.M109.032391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozdemir H, Karacorlu M, Karacorlu S. Serous macular detachment in diabetic cystoid macular oedema. Acta Ophthalmol Scand. 2005;83:63–66. doi: 10.1111/j.1600-0420.2005.00387.x. [DOI] [PubMed] [Google Scholar]
- 7.Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004;45:3330–3336. doi: 10.1167/iovs.04-0247. [DOI] [PubMed] [Google Scholar]
- 8.Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Nakamura M. New insights into the pathophysiology of diabetic retinopathy: potential cell-specific therapeutic targets. Diabetes Technol Ther. 2000;2:601–608. doi: 10.1089/15209150050502023. [DOI] [PubMed] [Google Scholar]
- 9.Terasaki H, Hirose H, Miyake Y. S-cone pathway sensitivity in diabetes measured with threshold versus intensity curves on flashed backgrounds. Invest Ophthalmol Vis Sci. 1996;37:680–684. [PubMed] [Google Scholar]
- 10.Aspinall PA. Rod-cone interaction: some indirect evidence. Acta Ophthalmol (Copenh) 1977;55:294–302. doi: 10.1111/j.1755-3768.1977.tb01311.x. [DOI] [PubMed] [Google Scholar]
- 11.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 14.Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27:331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibuya M. Structure and dual function of vascular endothelial growth factor receptor-1 (Flt-1) Int J Biochem Cell Biol. 2001;33:409–420. doi: 10.1016/s1357-2725(01)00026-7. [DOI] [PubMed] [Google Scholar]
- 16.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 18.Adamis AP, Miller JW, Bernal MT, D’Amico DJ, Folkman J, Yeo TK, Yeo KT. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 19.Adamis AP, Shima DT, Tolentino MJ, Gragoudas ES, Ferrara N, Folkman J, D’Amore PA, Miller JW. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a nonhuman primate. Arch Ophthalmol. 1996;114:66–71. doi: 10.1001/archopht.1996.01100130062010. [DOI] [PubMed] [Google Scholar]
- 20.Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozaki H, Seo MS, Ozaki K, Yamada H, Yamada E, Okamoto N, Hofmann F, Wood JM, Campochiaro PA. Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol. 2000;156:697–707. doi: 10.1016/S0002-9440(10)64773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senger DR, Connolly DT, Van de Water L, Feder J, Dvorak HF. Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Res. 1990;50:1774–1778. [PubMed] [Google Scholar]
- 23.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 24.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 25.Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C, Mori F, Ciulla TA, Ways K, Jirousek M, et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997;46:1473–1480. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- 26.Miller JW, Adamis AP, Shima DT, D’Amore PA, Moulton RS, O’Reilly MS, Folkman J, Dvorak HF, Brown LF, Berse B. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol. 1994;145:574–584. [PMC free article] [PubMed] [Google Scholar]
- 27.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 28.Hartnett ME, Lappas A, Darland D, McColm JR, Lovejoy S, D’Amore PA. Retinal pigment epithelium and endothelial cell interaction causes retinal pigment epithelial barrier dysfunction via a soluble VEGF-dependent mechanism. Exp Eye Res. 2003;77:593–599. doi: 10.1016/s0014-4835(03)00189-1. [DOI] [PubMed] [Google Scholar]
- 29.Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42:2408–2413. [PubMed] [Google Scholar]
- 30.Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002;133:70–77. doi: 10.1016/s0002-9394(01)01269-7. [DOI] [PubMed] [Google Scholar]
- 31.Dor Y, Porat R, Keshet E. Vascular endothelial growth factor and vascular adjustments to perturbations in oxygen homeostasis. Am J Physiol Cell Physiol. 2001;280:C1367–C1374. doi: 10.1152/ajpcell.2001.280.6.C1367. [DOI] [PubMed] [Google Scholar]
- 32.Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- 33.Mole DR, Maxwell PH, Pugh CW, Ratcliffe PJ. Regulation of HIF by the von Hippel-Lindau tumour suppressor: implications for cellular oxygen sensing. IUBMB Life. 2001;52:43–47. doi: 10.1080/15216540252774757. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto T, Claesson-Welsh L. VEGF receptor signal transduction. Sci STKE. 2001;2001:re21. doi: 10.1126/stke.2001.112.re21. [DOI] [PubMed] [Google Scholar]
- 35.Kolch W, Martiny-Baron G, Kieser A, Marmé D. Regulation of the expression of the VEGF/VPS and its receptors: role in tumor angiogenesis. Breast Cancer Res Treat. 1995;36:139–155. doi: 10.1007/BF00666036. [DOI] [PubMed] [Google Scholar]
- 36.Michiels C, Minet E, Michel G, Mottet D, Piret JP, Raes M. HIF-1 and AP-1 cooperate to increase gene expression in hypoxia: role of MAP kinases. IUBMB Life. 2001;52:49–53. doi: 10.1080/15216540252774766. [DOI] [PubMed] [Google Scholar]
- 37.Berra E, Milanini J, Richard DE, Le Gall M, Viñals F, Gothié E, Roux D, Pagès G, Pouysségur J. Signaling angiogenesis via p42/p44 MAP kinase and hypoxia. Biochem Pharmacol. 2000;60:1171–1178. doi: 10.1016/s0006-2952(00)00423-8. [DOI] [PubMed] [Google Scholar]
- 38.Pagès G, Milanini J, Richard DE, Berra E, Gothié E, Viñals F, Pouysségur J. Signaling angiogenesis via p42/p44 MAP kinase cascade. Ann N Y Acad Sci. 2000;902:187–200. doi: 10.1111/j.1749-6632.2000.tb06313.x. [DOI] [PubMed] [Google Scholar]
- 39.Blaauwgeers HG, Holtkamp GM, Rutten H, Witmer AN, Koolwijk P, Partanen TA, Alitalo K, Kroon ME, Kijlstra A, van Hinsbergh VW, et al. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol. 1999;155:421–428. doi: 10.1016/S0002-9440(10)65138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jingjing L, Xue Y, Agarwal N, Roque RS. Human Müller cells express VEGF183, a novel spliced variant of vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1999;40:752–759. [PubMed] [Google Scholar]
- 42.Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 43.Koury CB. Genetic system offer option for studying the role of retinal müller cells. Retina Today. 2007;I:31. [Google Scholar]
- 44.Le YZ. Conditional gene targeting: dissecting the cellular mechanisms of retinal degenerations. J Ophthalmol. 2011;2011:806783. doi: 10.1155/2011/806783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ueki Y, Ash JD, Zhu M, Zheng L, Le YZ. Expression of Cre recombinase in retinal Müller cells. Vision Res. 2009;49:615–621. doi: 10.1016/j.visres.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu M, Zheng L, Ueki Y, Ash JD, Le YZ. Unexpected transcriptional activity of the human VMD2 promoter in retinal development. Adv Exp Med Biol. 2010;664:211–216. doi: 10.1007/978-1-4419-1399-9_24. [DOI] [PubMed] [Google Scholar]
- 47.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 48.Bai Y, Ma JX, Guo J, Wang J, Zhu M, Chen Y, Le YZ. Müller cell-derived VEGF is a significant contributor to retinal neovascularization. J Pathol. 2009;219:446–454. doi: 10.1002/path.2611. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Xu X, Elliott MH, Zhu M, Le YZ. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59:2297–2305. doi: 10.2337/db09-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kondo T, Kahn CR. Altered insulin signaling in retinal tissue in diabetic states. J Biol Chem. 2004;279:37997–38006. doi: 10.1074/jbc.M401339200. [DOI] [PubMed] [Google Scholar]
- 51.Kowluru RA, Koppolu P, Chakrabarti S, Chen S. Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic Res. 2003;37:1169–1180. doi: 10.1080/10715760310001604189. [DOI] [PubMed] [Google Scholar]
- 52.Kowluru RA, Engerman RL, Kern TS. Abnormalities of retinal metabolism in diabetes or experimental galactosemia VIII. Prevention by aminoguanidine. Curr Eye Res. 2000;21:814–819. doi: 10.1076/ceyr.21.4.814.5545. [DOI] [PubMed] [Google Scholar]
- 53.Du Y, Smith MA, Miller CM, Kern TS. Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. J Neurochem. 2002;80:771–779. doi: 10.1046/j.0022-3042.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 54.Abu El-Asrar AM, Desmet S, Meersschaert A, Dralands L, Missotten L, Geboes K. Expression of the inducible isoform of nitric oxide synthase in the retinas of human subjects with diabetes mellitus. Am J Ophthalmol. 2001;132:551–556. doi: 10.1016/s0002-9394(01)01127-8. [DOI] [PubMed] [Google Scholar]
- 55.Leal EC, Manivannan A, Hosoya K, Terasaki T, Cunha-Vaz J, Ambrósio AF, Forrester JV. Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2007;48:5257–5265. doi: 10.1167/iovs.07-0112. [DOI] [PubMed] [Google Scholar]
- 56.El-Remessy AB, Al-Shabrawey M, Platt DH, Bartoli M, Behzadian MA, Ghaly N, Tsai N, Motamed K, Caldwell RB. Peroxynitrite mediates VEGF’s angiogenic signal and function via a nitration-independent mechanism in endothelial cells. FASEB J. 2007;21:2528–2539. doi: 10.1096/fj.06-7854com. [DOI] [PubMed] [Google Scholar]
- 57.El-Remessy AB, Behzadian MA, Abou-Mohamed G, Franklin T, Caldwell RW, Caldwell RB. Experimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor. Am J Pathol. 2003;162:1995–2004. doi: 10.1016/S0002-9440(10)64332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 59.Haigh JJ, Morelli PI, Gerhardt H, Haigh K, Tsien J, Damert A, Miquerol L, Muhlner U, Klein R, Ferrara N, et al. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev Biol. 2003;262:225–241. doi: 10.1016/s0012-1606(03)00356-7. [DOI] [PubMed] [Google Scholar]
- 60.Nishijima K, Ng YS, Zhong L, Bradley J, Schubert W, Jo N, Akita J, Samuelsson SJ, Robinson GS, Adamis AP, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saint-Geniez M, Maharaj AS, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, Darland DC, Young MJ, D’Amore PA. Endogenous VEGF is required for visual function: evidence for a survival role on müller cells and photoreceptors. PLoS One. 2008;3:e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCloskey DP, Croll SD, Scharfman HE. Depression of synaptic transmission by vascular endothelial growth factor in adult rat hippocampus and evidence for increased efficacy after chronic seizures. J Neurosci. 2005;25:8889–8897. doi: 10.1523/JNEUROSCI.2577-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong S, Liu Y, Zhu M, Xu X, Le YZ. Simplified system to investigate alteration of retinal neurons in diabetes. Adv Exp Med Biol. 2014;801:139–143. doi: 10.1007/978-1-4614-3209-8_18. [DOI] [PubMed] [Google Scholar]
- 64.Fu S, Zhu M, Ash JD, Wang Y, Le YZ. Investigating the role of retinal Müller cells with approaches in genetics and cell biology. Adv Exp Med Biol. 2014;801:401–405. doi: 10.1007/978-1-4614-3209-8_51. [DOI] [PubMed] [Google Scholar]
- 65.Karl MO, Hayes S, Nelson BR, Tan K, Buckingham B, Reh TA. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci USA. 2008;105:19508–19513. doi: 10.1073/pnas.0807453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karl MO, Reh TA. Studying the generation of regenerated retinal neuron from Müller glia in the mouse eye. Methods Mol Biol. 2012;884:213–227. doi: 10.1007/978-1-61779-848-1_15. [DOI] [PubMed] [Google Scholar]


