Abstract
AIM: To determine the prevalence and incidence of diabetic nephropathy in Africa.
METHODS: We performed a systematic narrative review of published literature following the MOOSE Guidelines for Meta-Analysis and Systematic Reviews of Observational Studies. We searched PubMed-MEDLINE for all articles published in English and French languages between January 1994 and July 2014 using a predefined strategy based on the combination of relevant terms and the names of each of the 54 African countries and African sub-regions to capture the largest number of studies, and hand-searched the reference lists of retrieved articles. Included studies reported on the prevalence, incidence or determinants of chronic kidney disease (CKD) in people with diabetes within African countries.
RESULTS: Overall, we included 32 studies from 16 countries; two being population-based studies and the remaining being clinic-based surveys. Most of the studies (90.6%) were conducted in urban settings. Methods for assessing and classifying CKD varied widely. Measurement of urine protein was the most common method of assessing kidney damage (62.5% of studies). The overall prevalence of CKD varied from 11% to 83.7%. Incident event rates were 94.9% for proteinuria at 10 years of follow-up, 34.7% for end-stage renal disease at 5 years of follow-up and 18.4% for mortality from nephropathy at 20 years of follow-up. Duration of diabetes, blood pressure, advancing age, obesity and glucose control were the common determinants of kidney disease.
CONCLUSION: The burden of CKD is important among people with diabetes in Africa. High quality data from large population-based studies with validated measures of kidney function are still needed to better capture the magnitude and characteristics of diabetic nephropathy in Africa.
Keywords: Diabetes, Diabetes nephropathy, Chronic kidney disease, Epidemiology, Prevalence, Incidence, Mortality, Africa, Systematic review
Core tip: Chronic kidney disease is a serious health threat for people with diabetes in Africa, with prevalence figures ranging from 11% to 83.7%. The incidence estimates suggest that 95% of people with diabetes may have proteinuria after 10 years from diabetes diagnosis; about 35% may develop end-stage renal disease after 5 years and 18% die from nephropathy after 20 years of disease duration. Hypertension, obesity, poor glycemic control and diabetes duration are the main risk factors of chronic kidney disease among diabetic patients in Africa. High quality data are needed to refine the epidemiology of diabetic nephropathy on the continent.
INTRODUCTION
Africa, like the rest of the world, is experiencing an increasing prevalence of diabetes alongside other non-communicable diseases, mainly as a result of urbanization, sedentary lifestyles, obesity and population growth and ageing[1]. Estimates for 2013 by the International Diabetes Federation (IDF) indicate that the number of adults with diabetes in the world will expand by 55%, from 381.8 million in 2013 to 591.9 million in 2035[2]. The largest increase of the population with diabetes will occur in sub-Saharan Africa, with a projected growth of 109.6%, from 19.8 million in 2013 to 41.5 million in 2035[2].
Diabetes causes significant morbidity, disability and early mortality. Diabetes has been identified as a major contributor in several other important diseases, both non-communicable diseases such as cardiovascular disease and renal disease[3,4], and communicable diseases such as invasive bacterial infections[5,6]. Mortality attributable to diabetes in sub-Saharan Africa was estimated to account for 8.6% of the total death in 2013[7]. Diabetic nephropathy (DN) is one of the most common complications of diabetes. The prevalence of DN is increasing steeply along with the diabetes epidemic[8]. Approximately one third to half of patients with diabetes develops renal manifestations[8-11]. DN is associated with increased premature mortality, end-stage renal disease and need to renal replacement therapy, cardiovascular diseases, and escalating health-care costs[8].
DN has been suggested to be more frequent among patients with diabetes in Africa as compared to those in the developed world due to delayed diagnosis, limited screening and diagnostic resources, poor control of blood sugar and other risk factors, and inadequate treatment at an early stage[7,12,13]. However, evidence to support the burden of kidney diseases in people with diabetes in Africa remains very patchy, and we are not aware of any effort to synthesize existing data on the occurrence of kidney disease in African populations with diabetes. Accordingly, the aim of this review is to provide a comprehensive overview of the published evidence on the occurrence of nephropathy in African people with diabetes.
MATERIALS AND METHODS
Data sources and search strategy
A systematic narrative review of published literature was performed following the MOOSE Guidelines for Meta-Analyses and Systematic Reviews of Observational Studies[14]. We searched MEDLINE via PubMed for articles published in English and French on DN in Africa between January 1994 and July 2014, using a predefined strategy based on the combination of relevant terms and the names of each of the 54 African countries and African sub-regions to capture the largest number of studies. The data search was limited to human studies. The last search date was October 22, 2014. Search histories are provided in Table 1. Once duplicate references were removed the titles and abstracts of the references were screened. The references of included articles were scanned to identify additional articles of interest.
Table 1.
Search history PubMed
| Search | Search terms | Hits |
| 1 | Diabetes[tw] OR Diabetes mellitus[tw] OR Type 1 diabetes[tw] OR Type 1 diabetes mellitus[tw] OR T1DM[tw] OR Type 2 diabetes[tw] OR Type 2 diabetes mellitus[tw] OR T2DM[tw] OR Hyperglycemia[tw] OR Glucose intolerance[tw] | 445204 |
| 2 | Renal insufficiency[tw] OR Renal failure[tw] OR Renal injury[tw] OR Renal disease[tw] Kidney insufficiency[tw] OR Kidney failure[tw] OR Kidney injury[tw] OR Kidney disease[tw] OR End-stage renal disease[tw] OR End-stage renal failure[tw] OR End-stage kidney disease[tw] OR End-stage kidney failure [tw] OR End stage renal disease[tw] OR End stage renal failure[tw] OR End stage kidney disease[tw] OR End stage kidney failure [tw] OR Microalbuminuria [tw] OR Micro-albuminuria OR Macroalbuminuria [tw] or Macro-albuminuria [tw] | 154354 |
| 3 | # 1 AND # 2 | 20388 |
| 4 | Diabetic nephropathy [MeSH Terms] | 19406 |
| 5 | # 3 OR # 4 | 34221 |
| 6 | ((((("Africa"[MeSH] OR Africa*[tw] OR Algeria[tw] OR Angola[tw] OR Benin[tw] OR Botswana[tw] OR "Burkina Faso"[tw] OR Burundi[tw] OR Cameroon[tw] OR "Canary Islands"[tw] OR "Cape Verde"[tw] OR "Central African Republic"[tw] OR Chad[tw] OR Comoros[tw] OR Congo[tw] OR "Democratic Republic of Congo"[tw] OR Djibouti[tw] OR Egypt[tw] OR "Equatorial Guinea"[tw] OR Eritrea[tw] OR Ethiopia[tw] OR Gabon[tw] OR Gambia[tw] OR Ghana[tw] OR Guinea[tw] OR "Guinea Bissau"[tw] OR "Ivory Coast"[tw] OR "Cote d'Ivoire"[tw] OR Jamahiriya[tw] OR Jamahiryia[tw] OR Kenya[tw] OR Lesotho[tw] OR Liberia[tw] OR Libya[tw] OR Libia[tw] OR Madagascar[tw] OR Malawi[tw] OR Mali[tw] OR Mauritania[tw] OR Mauritius[tw] OR Mayote[tw] OR Morocco[tw] OR Mozambique[tw] OR Mocambique[tw] OR Namibia[tw] OR Niger[tw] OR Nigeria[tw] OR Principe[tw] OR Reunion[tw] OR Rwanda[tw] OR "Sao Tome"[tw] OR Senegal[tw] OR Seychelles[tw] OR "Sierra Leone"[tw] OR Somalia[tw] OR "South Africa"[tw] OR "St Helena"[tw] OR Sudan[tw] OR Swaziland[tw] OR Tanzania[tw] OR Togo[tw] OR Tunisia[tw] OR Uganda[tw] OR "Western Sahara"[tw] OR Zaire[tw] OR Zambia[tw] OR Zimbabwe[tw] OR "Central Africa"[tw] OR "Central African"[tw] OR "West Africa"[tw] OR "West African"[tw] OR "Western Africa"[tw] OR "Western African"[tw] OR "East Africa"[tw] OR "East African"[tw] OR "Eastern Africa"[tw] OR "Eastern African"[tw] OR "North Africa"[tw] OR "North African"[tw] OR "Northern Africa"[tw] OR "Northern African"[tw] OR "South African"[tw] OR "Southern Africa"[tw] OR "Southern African"[tw] OR "sub Saharan Africa"[tw] OR "sub Saharan African"[tw] OR "subSaharan Africa"[tw] OR "subSaharan African"[tw]) NOT ("guinea pig"[tw] OR "guinea pigs"[tw] OR "aspergillus niger"[tw]))))) | 354928 |
| 7 | # 5 AND # 6 | 1065 |
| 8 | #4 Limits: 1994/01/01 to 2014/10/22 and studies done in Humans | 918 |
Study selection and data extraction
We included cross-sectional, case-control or cohort studies of subjects with diabetes mellitus resident in African countries reporting the prevalence or incidence or progression of DN. We excluded studies of populations of African origin residing outside Africa; case series (sample size less than 50 subjects), letters, comments and editorials; studies not published in English or French. Two investigators (JJNN, APK) independently identified articles and sequentially screened them for inclusion (Figure 1). Disagreements were solved by a third investigator (JN). Full text articles were reviewed by two investigators (JJNN and APK) who independently extracted data regarding study setting and design, study population characteristics and prevalence or incidence of DN.
Figure 1.
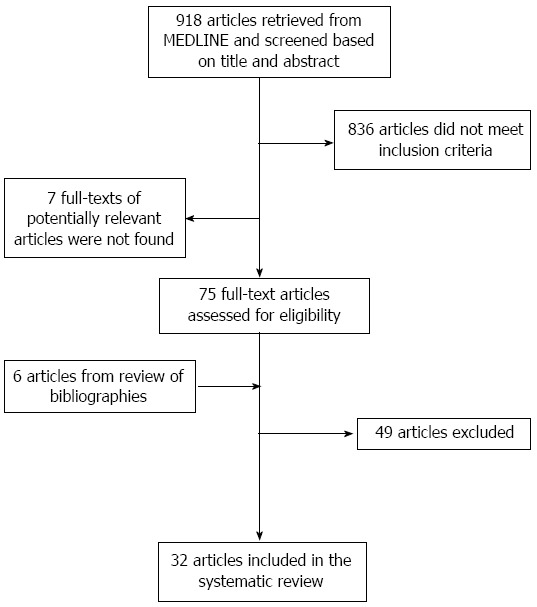
Flow diagram of study selection.
RESULTS
We identified 730 articles, of which 73 were reviewed in full-text; 32 met the inclusion criteria (Figure 1)[15-46].
Characteristics of included studies
Characteristics of the included studies are summarized in Table 2. The 32 studies were performed in 16 countries, with a geographical distribution covering all the African regions. However, more than half the studies [18 (56.3%)] were from South Africa (five), Nigeria (four), DR Congo (three) and Ethiopia (three).
Table 2.
General characteristics of studies of chronic kidney disease in people with diabetes in Africa
| Ref. | Country | Period | Design | Setting | Sample size | Mean or median age (yr) | Male (%) | Type and duration of diabetes (yr) | Duration FUP |
Method for CKD assessment |
|||
| Proteinuria | MDRD | Urine ACR | Cockroft-Gault | ||||||||||
| Motala et al[37], 2001 | South Africa | Not precised | Retrospective cohort study | Clinic, urban | 219 | 39.5 T1DM; 58.4 T2DM | 19.6 | 16.10 T1DM; 18.6 T2DM | At least 10 yr | persistent proteinuria (Dipstick) | |||
| Elbagir et al[26], 1995 | Sudan | Jan-July 1992 | Cross-sectional, self-selected sampling | Clinic, urban | 128 | 31.5 (15-75) | 48.4 | Insulin-treated; 9 (1-40) | NA | Proteinuria (Dipstick) | |||
| Sobngwi et al[44], 1999 | Cameroon | Not precised | Cross-sectional, self-selected sampling | Clinic, urban | 64 | 37.4 normotensive T1DM; 51.7 normotensive T2DM; 57.9 hypertensive T1DM | 57.8 | 6.7 normotensive T1DM; 4.7 normotensive T2DM; 4.8 hypertensive T1DM | NA | Proteinuria | |||
| Katchunga et al[30], 2010 | DR congo | 2005-2007 | Cross-sectional, self-selected sampling | Clinic, urban | 98 | 58 (10.4) | 35.7 | 7.3 T2DM | NA | MDRD (corrected for Blacks) | |||
| Choukem et al[22], 2012 | Cameroon | Jan 2008- Oct 2010 | Cross-sectional, self-selected sampling | Clinic, urban | 420 | 56.7 | 49 | 4 (1-9) T2DM | NA | Proteinuria (Dipstick) | |||
| Keeton et al[31], 2004 | South Africa | Not precised | Prospective cohort, self-selected sampling | Clinic, urban | 59 | 62 | 35.6 | 17.8 T2DM | 12 yr | Urine ACR | |||
| Pruijm et al[39], 2008 | Seychelles | 2004 | Cross-sectional; random sex and age-stratified sample | Population | 1218 (whole sample, including diabetic patients) | Not precised | 45.9 | Newly diagnosed patients | NA | Urine ACR | |||
| Alebiosu[16], 2003 | Nigeria | Jan 2000 June 2001 | Cross-sectional, self-selected sampling | Clinic, urban | 342 | 6.5 T1DM; 9.4 T2DM | 53.8 | 26 T1DM; 53.4 T2DM | NA | Persistent proteinuria | |||
| Bouaziz et al[20], 2012 | Tunisia | Jan 2008Dec 2010 | Cross-sectional, self-selected sampling | Clinic, urban | 73 | 59.3 | 23.3 | T2DM 10.6 | NA | Proteinuria | |||
| Ajayi et al[15], 2014 | Nigeria | Not precised | Retrospective cross-sectional | Clinic, urban | 65 | Not available | Not available | T2DM | NA | MDRD | |||
| Levitt et al[32], 1997 | South Africa | July-December 1992 | Cross-sectional, stratified random sampling | Clinic, urban | 243 | 56.4 | 38.3 | 8 T2DM and T1DM | NA | Persistent proteinuria | Urine ACR | ||
| Majaliwa et al[34], 2007 | Tanzania | June 2005- Feb 2006 | Cross-sectional, self-selected sampling | Clinic, urban | 99 | 12.6 | 42.4 | 4.76 T1DM | NA | Proteinuria | |||
| Marshall et al[36], 2013 | Rwanda | June 2009-Nov 2010 | Cross-sectional, self-selected sampling | Clinic, urban | 286 | 18.6 | 46.5 | 3.4 T1DM | NA | Proteinuria | Urine ACR | ||
| Alebiosu et al[18], 2003 | Nigeria | Sept 1999- August 2002 | Cross-sectional, self-selected sampling | Clinic, urban | 465 | Not precised | Not precised | T2DM | NA | Proteinuria | |||
| Gill et al[28], 2005 | South Africa | From 1982 to 2002 | Prospective cohort, self-selected sampling | Clinic, urban | 88 | 22 at onset | 52 | T1DM | 20 yr | ||||
| Djrolo et al[24], 2001 | Benin | Not indicated | Cross-sectional | Clinic, urban | 152 | 53.3 | 65.8 | T1DM and T2DM | NA | Proteinuria | |||
| Rotchford et al[43], 2002 | South Africa | 1999 | Cross-sectional, self-selected sampling | Clinic, rural | 253 | 56.5 | 26.9 | 42.2; T1DM and T2DM | NA | Urine ACR | |||
| Rissassi et al[42], 2009 | DR congo | 11 june 2008 to 30 july 2008 | Cross-sectional, self-selected sampling | Clinic, urban | 181 | 19.1 | 38.7 | 57.6 T1DM | NA | Urine ACR | |||
| Rahlenbeck et al[40], 1997 | Ethiopia | January - April 1995 | Cross-sectional, self-selected sampling | Clinic, urban | 170 | 31.4 T1DM; 56.7 T2DM | 60 | 5.9 T1DM; 6.0 T2DM | NA | Proteinuria | |||
| Wanjohi et al[45], 2002 | Kenya | June 2000 - January 2001 | cross-sectional, self-selected sampling | Clinic, urban | 100 | 53.7 | 37 | 10.3 T2DM | NA | Albuminuria | |||
| Nambuya et al[38], 1996 | Uganda | 1 January 1993 - 10 August 1994 | Cross-sectional, self-selected sampling | Clinic, urban/urban (origin of participants) | 252 | Not precised | 46.4 | 45 (range 30-69)T2DM and T1DM | NA | Proteinuria | |||
| Rasmussen et al[41], 2013 | Zambia | February - April 2011 | Cross-sectional, self-selected sampling | Clinic, rural | 101 | 50 (range 50-68) | 37.3 | T2DM and T1DM | NA | Urine ACR | |||
| Bentata et al[19], 2013 | Morocco | From September 2006 | Prospective cohort study | Clinic, urban | 72 | 29.5 | 69.4 | 17 (11-20) T1DM | 5 yr | Proteinuria | MDRD | ||
| Gill et al[27], 2008 | Ethiopia | Not precised | Cross-sectional, self-selected sampling | Clinic, rural | 105 | 41 | 70.5 | 7 T1DM and T2DM | NA | Urine ACR | |||
| Bouzid et al[21], 2011 | Tunisia | June 2006 - July 2008 | Cross-sectional, self-selected sampling | Clinic, urban | 689 | 60 | 39.3 | 11 T2DM | NA | Proteinuria | Cockroft-Gault | ||
| Janmohamed et al[29], 2013 | Tanzania | October 2011 - March 2012 | Cross-sectional, self-selected sampling | Clinic, urban | 369 | 54 (IQR 45-62) | 46.6 | 6 (3-11)T1DM (6.2%) and T2DM (93.8%) | NA | Cockroft-Gault | |||
| Danquah et al[23], 2012 | Ghana | August 2007 - June 2008 | Cross-sectional, self-selected sampling | Clinic, urban | 675 | 54.7 | 25 | T2DM | NA | Proteinuria | |||
| Lutale et al[33], 2007 | Tanzania | July 2003 - March 2004 | Cross-sectional, self-selected sampling | Clinic, urban | 244 | T1DM 21(range 4-44.8)T2DM 53 (range 23.5-85) | 46.3 | T1DM 3 (0-17)T2DM 4 (range 0-25) | NA | Proteinuria | Cockroft-Gault | ||
| Worku et al[46], 2010 | Ethiopia | October 2008 | Cross-sectional, self-selected sampling | Clinic, urban | 305 | 44.4 | 62.9 | T1DM and T2DM; 53.4% less than 5 yr and 33.8% 5-9 yr | NA | Proteinuria | |||
| Makulo et al[35], 2010 | DR Congo | 30 March - 24 April 2007 | Cross-sectional, self-selected sampling | Population-based, Urban | 81 | Not precised | Not precised | No precision | NA | MDRD | Urine ACR | ||
| Eghan et al[25], 2007 | Ghana | January - July 2005 | Cross-sectional, self-selected sampling | Clinic, urban | 109 | 54.1 | 28 | T1DM and T2DM 10.7 | NA | Proteinuria | |||
| Alebiosu et al[17], 2004 | Nigeria | January 2000 - June 2001 | Case (T2DM with persistent proteinuria-control (T2DM patients nephropathy) | Clinic, urban | 162 | 53.4 | 50 | T2DM9.4 cases, 5.5 controls | NA | ||||
ACR: Albumin-to-Creatinine Ratio; FUP: Follow-up; MDRD: Modification of diet renal disease; NA: Not applicable.
Only two population-based studies were identified. In Democratic Republic of Congo, between March and April 2007, Makulo et al[35] studied pathologic albuminuria among 81 diabetic patients identified through a population-based survey on the prevalence of diabetes involving 1898 participants[35]. Pruijm et al[39] in Seychelles in 2004, conducted a large-scale population-based estimate of the prevalence of microalbuminuria among 1218 adults. All other studies were clinic-based surveys conducted mostly in diabetic clinics. There were three cohort studies (two prospective and one retrospective), one case-control study and the other 28 studies were cross-sectional with non-random sampling. Only three (9.4%) studies were conducted in rural settings.
Methods of assessment and classification of chronic kidney disease (CKD) varied widely. The studies assessed kidney function by urine protein [20 (62.5%) studies], urine albumin-to-creatinine ration (ACR) [9 (28.1%) studies], and estimation of glomerular filtration rate (GFR) by Cockcroft-Gault formula [3 (9.4%) studies] or by MDRD formula [4 (12.4%) studies]. Six studies (18.8%) measured kidney function by two methods, and renal biopsy was not performed in any study.
Prevalence of CKD
As depicted in Table 3, the overall prevalence of CKD varied from 11% in Tunisia to 83.7% in Tanzania[20,29]. In studies where proteinuria was used to assess CKD, the prevalence varied from 5.3% in South Africa to 53.1% in Cameroon (study with a small sample size)[32,44]. When considering the estimation of the GFR, the prevalence ranged from 4.6% in Tanzania to 43.1% in Nigeria (study with a small sample size)[15,33].
Table 3.
Prevalence and incidence of chronic kidney disease in people with diabetes across studies in Africa
| Ref. | Country | Sample size | Type of diabetes | Duration of follow-up | Diagnostic criteria for CKD | Prevalence | Incidence | Comments |
| Motala et al[37], 2001 | South Africa | 219 | T1DM and T2DM | 16.10 (4.9) T1DM; 18.6 (5.7) T2DM; at least 10 yr | Persistent proteinuria (dipstix proteinuria on three or more consecutive occasions over 18 mo in the at absence of infection or cardiac failure) | Not applicable | 24.6% | |
| Elbagir et al[26], 1995 | Sudan | 128 | Insulin-treated | Not applicable | Proteinuria (≥ 30 mg/dL) | 22% | Not applicable | |
| Sobngwi et al[44], 1999 | Cameroon | 64 | T1DM and T2DM | Not applicable | Proteinuria | 53.1% | Not applicable | |
| Katchunga et al[30], 2010 | DR Congo | 98 | T2DM | Not applicable | MDRD: CKD stage ≥ 2 according to the National Kidneyfoundation | 18.1% | Not applicable | |
| Choukem et al[22], 2012 | Cameroon | 420 | T2DM | Not applicable | Proteinuria (30 mg/24 h) | 31% | Not applicable | |
| Keeton et al[31], 2004 | South Africa | 59 | T2DM | 12 yr | Urine Albumin-to-Creatinine Ratio (no detail) | After 12 yr of follow-up or death, 94.9% (56/59) had a proteinuria with a mean duration from diabetes onset to proteinuria of 9.7 (5.9) yr | 83% (49/59) had an elevated SCr atthe end of the study and in 66.1% (39/59) the SCr level had doubled during the study | |
| Pruijm et al[39], 2008 | Seychelles | 1218 | All types | Not applicable | Microalbuminuria: Urine Albumin-to-Creatinine Ratio 3.4-33.9 mg albumin/mmol creatinine | 36.1% | Not applicable | |
| Alebiosu[16], 2003 | Nigeria | 342 | T1DM and T2DM | Not applicable | Persistent proteinuria | 28.4% | Not applicable | |
| Bouaziz et al[20], 2012 | Tunisia | 73 | T2DM | Not applicable | Microalbuminuria: < 2.8 g/mol for women and < 2.3 g/mol for men | 11% | Not applicable | |
| Ajayi et al[15], 2014 | Nigeria | 65 | T2DM | Not applicable | MDRD: eGFR ≤ 60 mL/min per 1.73 m2 | 43.1% | Not applicable | |
| Levitt et al[32], 1997 | South Africa | 243 | T2DM and T1DM | Not applicable | Urine Albumin-to-Creatinine Ratio > 3.4 mm/mmol | 36.7% | Not applicable | |
| Persistent proteinuria (for at least 3 consecutive visits) | 5.3% | |||||||
| Majaliwa et al[34], 2007 | Tanzania | 99 | T1DM | Not applicable | Proteinuria (no detail) | 29.3% | Not applicable | |
| Marshall et al[36], 2013 | Rwanda | 286 | T1DM | Not applicable | Microalbuminuria: Urine Albumin-to-Creatinine Ratio = 30-299 mg/gMacroalbuminuria or overt nephropathy: Urine Albumin-to-Creatinine Ratio ≥ 300 mg/g | Microalbuminuria: 21%; Macroalbuminuria: 5% | Not applicable | |
| Alebiosu et al[18], 2003 | Nigeria | 465 | T2DM | Not applicable | Proteinuria and eGFR | 41.1% | Not applicable | The method for the estimation of the GFR is not indicated |
| Gill et al[28], 2005 | South Africa | 88 | T1DM | 20 yr | Persistent dipstick proteinuria | Death of renal cause after 20 yr = 18.4% (9/49) | Death due to chronic renal failure after 20 yr of follow-up was 9/49 (after exclusion of lost to follow) | |
| Djrolo et al[24], 2001 | Benin | 152 | T1DM and T2DM | Not applicable | Proteinuria (no detail) | 20% | Not applicable | |
| Rotchford et al[43], 2002 | South Africa | 253 | T1DM and T2DM | Not applicable | Microalbuminuria > 2.5 mg/mmol in men or 3.5 mg/mmol in women | 46.4% | Not applicable | |
| Rissassi et al[42], 2009 | DR congo | 181 | T1DM | Not applicable | Microalbuminuria: Urine Albumin-to-Creatinine Ratio = 30-299 mg/gMacroalbuminuria: Urine Albumin-to-Creatinine Ratio ≥ 300 mg/g | 21.9% (microalbuminuria) and 7.3% (macroalbuminuria) | Not applicable | |
| Rahlenbeck et al[40], 1997 | Ethiopia | 170 | T1DM and T2DM | Not applicable | Microalbuminuria: > 30 mg/LMacroalbuminuria: > 300 mg/L | T1DM: 32% (microalbuminuria) and 15% (macroalbuminuria)T2DM: 37% (microalbuminuria) and 20% (macroalbuminuria) | Not applicable | |
| Wanjohi et al[45], 2002 | Kenya | 100 | T2DM | Not applicable | Proteinuria ≥ 20 mg | 26% | Not applicable | |
| Nambuya et al[38], 1996 | Uganda | 252 | T1DM and T2DM | Not applicable | Proteinuria (no detail) | 17.1% | Not applicable | Newly diagnosed patients |
| Rasmussen et al[41], 2013 | Zambia | 101 | T1DM and T2DM | Not applicable | Microalbuminuria: ACR = 3.5-35.0 for women and 2.5-25.0 mg/mmol for menMacroalbuminuria were ACR> 35.0 for women and > 25.0 for men | Microalbuminuria: 23.8%Macroalbuminuria: 8.9% | Not applicable | There were 33 patients with diabetes alone, and 68 patients with diabetes and hypertension |
| Bentata et al[19], 2013 | Morocco | 72 | T1DM | 5 yr | Microalbuminuria: albumin excretion rate 30-300 mg/24 hMacroalbuminuria: albumin excretion rate > 300 mg/24 hNephrotic proteinuria: albumin excretion rate ≥ 3000 mg/24 hRenal failure: eGFR < 60 mL/min (MDRD) | At the time of enrollementMicroalbuminuria: 48.6%Macroalbuminuria: 36.1%Nephrotic proteinuria: 15.3% | The incidence of end stage renal disease after 5 yr: 34.7% | Urinary assays done onadmission were repeated on three specimens atthree-monthly intervals |
| Gill et al[27], 2008 | Ethiopia | 105 | T1DM and T2DM | Not applicable | Nephropathy: ACR> 25.0 mg/mmol and retinopathy presentMicroalbuminuria: ACR> 2.5 and < 25.0 mg/mmol in men and > 3.5 and< 25.0 mg/mmol in women | Nephropathy: 2%Microalbuminuria: 51% | Urinary ACR levels (toassess microalbuminuria and nephropathy) were done on59 patients, as those with haematuria and/or urinary infection were excluded | |
| Bouzid et al[21], 2011 | Tunisia | 689 | T2DM | Not applicable | CKD: eGFR < 60 mL/min per 1.73 m2 (Cockroft-Gault)Microalbuminuria: albumin excretion rate 30-300 mg/24 hMacroalbuminuria: albumin excretion rate > 300 mg/24 h | CKD: 19.8%Microalbuminuria: 13%Macroalbuminuria: 10.1% | Not applicable | Macroalbuminuria was significantly associated with CKD (P < 0.00001) |
| Janmohamed et al[29], 2013 | Tanzania | 369 | T1DM and T2DM | Not applicable | CKD: eGFR < 60 mL/min per 1.73 m2 (Cockroft-Gault) or microalbuminuria (> 20 mg/L) or overt protienuria | CKD: 83.7%eGFR < 60 mL/min per 1.73 m2: 24.7%Microalbuminuria: 45.8%Overt proteinuria: 34.1% | Not applicable | |
| Danquah et al[23], 2012 | Ghana | 671 | T2DM | Not applicable | Proteinuria ≥ 20 mg/L | 43% | Not applicable | |
| Lutale et al[33], 2007 | Tanzania | 244 | T1DM and T2DM | Not applicable | Microalbuminuria: AER 20-200 μg/minMacroalbuminuria: AER > 200 μg/minRenal failure: eGFR < 60 mL/min per 1.73 m2: | Microalbuminuria: 12.1% (T1DM); 9.8% (T2DM)Macroalbuminuria: 1.1% (T1DM); 7.2% (T2DM)Renal failure: 4.6% (T1DM); 22% (T2DM) | Not applicable | |
| Worku et al[46], 2010 | Ethiopia | 305 | T1DM (38%) and T2DM (62%) | Not applicable | Proteinuria (no detail) | 15.7% | Not applicable | |
| Makulo et al[35], 2010 | DR Congo | 81 | No precision | Not applicable | Microalbuminuria: ACR 30-299 mg/gMacroalbuminuria: ACR ≥ 300 mg/gRenal failure: eGFR < 60 mL/min per 1.73 m2 | Microalbuminuria: 43.5%Macroalbuminuria: 12%Renal failure: 21.4% | Not applicable | |
| Eghan et al[25], 2007 | Ghana | 109 | T1DM and T2DM | Not applicable | Microalbuminuria: ACR 30-300 mg/g | 43.1% | Not applicable | |
| Alebiosu et al[17], 2004 | Nigeria | 162 | T2DM | Not applicable | Not applicable | Not applicable | Not applicable | The study did not assess the prevalence or incidence of diabetic nephropathy, but its predictors |
ACR: Albumin-to-Creatinine Ratio; T1DM: Type 1 diabetes mellitus; T2DM: Type 2 diabetes mellitus; CKD: Chronic kidney disease; eGFR: Epidermal growth factor receptor.
Incidence of CKD
A study in South Africa investigated the long-term incidence of proteinuria among T2DM patients. After 12 years of follow-up or death, 94.9% (56/59) had a proteinuria with a mean duration from diabetes onset to proteinuria of 9.7 (5.9) years[31]. In another study in South Africa, found that 18.4% of T1DM patients had died from renal nephropathy after 20 years of follow-up[28]. In a recent study in Morocco, the incidence of end-stage renal disease after 5 years was 34.7%[19].
Risk factors of CKD
Twenty studies (62.5%) reported factors associated with CKD in diabetic patients (Table 4). However, in most studies the method to assess this association was imprecise. In cross-sectional studies, correlates of CKD included systolic and diastolic high blood pressure, long duration of diabetes, older age, dyslipidemia, obesity[16-22,25,26,29-31,33,36,40,42-44,46]. In a study in Cameroon, T2DM patients with systolic hypertension and diastolic hypertension were respectively 1.45 (95%CI: 1.15-1.84; P = 0.006) and 1.33 (95%CI: 1.06-1.66; P = 0.026) times more likely to have nephropathy[22]. Two studies in Rwanda and South Africa respectively showed that a one year increase in the duration of T1DM increased by 0.86 (95%CI: 0.77-0.96; P = 0.008) the odds of microalbuminuria[36], and that T1DM and T2DM patients with a duration of diabetes greater than 10 years were 4.19 times (95%CI: 1.93-9.10; P < 0.001) more likely to have microalbuminuria[43]. Poor glycemic control as measured by HbA1c was also a strong predictor of nephropathy. For instance, HbA1c level greater than 10% and 14% were respectively associated with a 2.6 fold (95%CI: 1.1-6.4) and a 4.69 (95%CI: 1.65-13.3; P = 0.004)[42,43]. A 1 g/dL decrease in hemoglobin level has been found to be associated with end-stage renal disease (OR 3.18, 95%CI: 1.47-6.87; P = 0.003)[19]. Studies in Nigeria showed that left ventricular hypertrophy, stroke, myocardial infarction and peripheral arterial disease were more frequent in T2DM patients with nephropathy, especially those with advanced stages[17,18].
Table 4.
Risk factors for chronic kidney disease in people with diabetes
| Ref. | Country | Sample size | Type of diabetes | Diagnostic criteria for CKD | Risk factor |
Measure of association |
Factors adjusted for | Comments | |
| Effect size | P-value | ||||||||
| Motala et al[37], 2001 | South Africa | 219 | T1DM and T2DM | Persistent proteinuria | Not assessed | ||||
| Elbagir et al[26], 1995 | Sudan | 128 | Insulin-treated | Proteinuria | Age | P = 0.006 | |||
| Duration of diabetes | P = 0.003 | ||||||||
| Systolic BP | P = 0.0001 | ||||||||
| Diastolic BP | P = 0.001 | ||||||||
| Serum cholesterol | P < 0.05 | ||||||||
| Sobngwi et al[44], 1999 | Cameroon | 64 | T1DM and T2DM | Proteinuria | Duration of diabetes | P = 0.04 | |||
| Diastolic BP | P = 0.01 | ||||||||
| Katchunga et al[30], 2010 | DR Congo | 98 | T2DM | MDRD (corrected for Blacks); CKD stage ≥ 1 according to the National Kidneyfoundation | Hypertension | aOR: 2.49 (0.98-6.34) | P = 0.04 | Age, duration of diabetes, BMI | |
| Choukem et al[22], 2012 | Cameroon | 420 | T2DM | Proteinuria (30 mg/24 h) | Systolic BP | aOR: 1.45 (1.15-1.84) | P = 0.006 | ||
| Diastolic BP | aOR: 1.33 (1.06-1.66) | P = 0.026 | |||||||
| Pulse pressure | aOR: 1.35 (1.06-1.71) | P = 0.0007 | |||||||
| Mean arterial pressure | aOR: 1.42 (1.13-1.78) | P = 0.006 | |||||||
| Keeton et al[31], 2004 | South Africa | 59 | T2DM | Urine Albumin-to-Creatinine Ratio (no detail) | High entry serum creatinine | P < 0.006 | These are risk factors for death from chronic renal failure (compared with the patients who were still alive at follow-up)By the end of study 47 of the 59 patients had died; the cause of death not established in 2 patients. Death was due to chronic renal failure in 17 cases | ||
| BMI < 28 | P < 0.003 | ||||||||
| Severe retinopathy | P < 0.002 | ||||||||
| Mean glucose level of > 14 mmol/L | P < 0.035 | ||||||||
| Pruijm et al[39], 2008 | Seychelles | 1218 | All types | Microalbuminuria: Urine Albumin-to-Creatinine Ratio 3.4-33.9 mg albumin/mmol creatinine | Not assessed | Risk factors were investigated in the whole study population in both diabetics and non-diabetics | |||
| Alebiosu[16], 2003 | Nigeria | 342 | T1DM and T2DM | Persistent proteinuria | Not assessed | ||||
| Bouaziz et al[20], 2012 | Tunisia | 73 | T2DM | Microalbuminuria: < 2.8 g/mol for women and < 2.3 g/mol for men | Family history of nephropathy | P = 0.0289 | Comparison of T2DM patients with nephropathy with those without nephropathy | ||
| Smoking | P = 0.0056 | ||||||||
| Insulin therapy | P = 0.0310 | ||||||||
| Glitazones therapy | P = 0.0115 | ||||||||
| Anti-hypertensives (not ACE inhibitor) | P < 0.0001 | ||||||||
| Lipid-lowering agents | P < 0.0001 | ||||||||
| Ajayi et al[15], 2014 | Nigeria | 65 | T2DM | MDRD: eGFR ≤ 60 mL/min per 1.73 m2 | Not assessed | ||||
| Levitt et al[32], 1997 | South Africa | 243 | T2DM and T1DM | Urine Albumin-to-Creatinine Ratio > 3.4 mm/mmol | Not assessed | ||||
| and Persistent proteinuria (for at least 3 consecutive visits) | |||||||||
| Majaliwa et al[34], 2007 | Tanzania | 99 | T1DM | Proteinuria (no detail) | Missing insulin doses | P = 0.045 | Not available | ||
| Marshall et al[36], 2013 | Rwanda | 286 | T1DM | Microalbuminuria: Urine Albumin-to-Creatinine Ratio = 30-299 mg/g | Age (increase) | aOR: 0.86, 95%CI: 0.77-0.96 | P = 0.009 | Each variable is adjusted for the others | These are risk factors of microalbuminuria. There was no factor associated to macroalbuminuria |
| Duration of diabetes (one year increase) | aOR: 0.86, 95%CI: 0.77-0.96 | P = 0.008 | |||||||
| Diastolic BP (increase) | aOR: 0.86, 95%CI: 0.77-0.96 | P = 0.004 | |||||||
| HBA1c (increase) | aOR: 0.86, 95%CI: 0.77-0.96 | P = 0.047 | |||||||
| Alebiosu et al[18], 2003 | Nigeria | 465 | T2DM | Proteinuria and eGFR (no detail) | Hypertension, left ventricular hypertrophy, stroke and myocardial infarction were more frequent in advanced stages of nephropathy | Not available | P < 0.05 | Not available | Patients with advanced stages of nephropathy (IV and V) were compared with those with stages ≤ III |
| Gill et al[28], 2005 | South Africa | 88 | T1DM | Persistent dipstick proteinuria | Not assessed | ||||
| Djrolo et al[24], 2001 | Benin | 152 | T1DM and T2DM | Proteinuria (no detail) | Not available | Not available | Not available | Proteinuria was more frequent in insulin-treated patients compared those on oral antidiabetic treatment. The prevalence of proteinuria also increased with the duration of diabetes | |
| Rotchford et al[43], 2002 | South Africa | 253 | T1DM and T2DM | Microalbuminuria > 2.5 mg/mmol in men or 3.5 mg/mmol in women | Duration of diabetes > 10 yr | 4.19 (1.93-9.10) | < 0.001 | Model contains duration of diabetes, BMI, HbA1c, age andhypertension | |
| BMI > 33 | 0.27 (0.08-0.48) | 0.002 | |||||||
| HbA1c > 14% | 4.69 (1.65-13.3) | 0.004 | |||||||
| Hypertension | 2.11 (1.07-4.17) | 0.031 | |||||||
| Rissassi et al[42], 2009 | DR congo | 181 | T1DM | Microalbuminuria: Urine Albumin-to-Creatinine Ratio = 30-299 mg/gMacroalbuminuria: Urine Albumin-to-Creatinine Ratio ≥ 300 mg/g | Duration of diabetes > 5 yr | 4.1 (1.9-8.4) | No precision | ||
| Age > 18 yr | 2.9 (1.3-6.2) | ||||||||
| HbA1c > 10% | 2.6 (1.1-6.4) | ||||||||
| Rahlenbeck et al[40], 1997 | Ethiopia | 170 | T1DM and T2DM | albuminuria: > 30 mg/L | Duration of diabetes | Beta = 0.061, SE = 0.018 for T1DM | < 0.001 | Hypertensive patients excluded | |
| Systolic blood pressure | Beta = 0.027, SE = 0.005 for T1DM | < 0.001 | |||||||
| Wanjohi et al[45], 2002 | Kenya | 100 | T2DM | Proteinuria ≥ 20mg | None identified | ||||
| Nambuya et al[38], 1996 | Uganda | 252 | T1DM and T2DM | Proteinuria (no detail) | None assessed | ||||
| Rasmussen et al[41], 2013 | Zambia | 101 | T1DM and T2DM | Microalbuminuria: ACR = 3.5-35.0 for women and 2.5-25.0 mg/mmol for menMacroalbuminuria were ACR> 35.0 for women and > 25.0 for men | None assessed | ||||
| Bentata et al[19], 2013 | Maroc | 72 | T1DM | End-stage renal disease: eGFR < 15 mL/min | Hemoglobin blood (per 1 g/dL decrease) | 3.18 (1.47-6.87) | 0.003 | No precision | These are independent risk factors for ESRD in type-1 diabetes patients with diabetic nephropathy |
| Diastolic blood pressure (per 1 mmHg increase) | 1.15 (1.04-1.27) | 0.006 | |||||||
| Gill et al[27], 2008 | Ethiopia | 105 | T1DM and T2DM | Nephropathy: ACR> 25.0 mg/mmol and retinopathy presentMicroalbuminuria: ACR> 2.5 and < 25.0 mg/mmol in men and > 3.5 and< 25.0 mg/mmol in women | None assessed | ||||
| Bouzid et al[21], 2011 | Tunisia | 689 | T2DM | Renal failure: creatinine clearance < 60 mL/min (Cockroft-Gault) | Older age | Not provided | < 0.00001 | ||
| Hypertension | < 0.00001 | ||||||||
| Long duration of diabetes | < 0.001 | ||||||||
| Higher BMI | 0.02 | ||||||||
| Dyslipidemia | 0.01 | ||||||||
| Janmohamed et al[29], 2013 | Tanzania | 369 | T1DM and T2DM | CKD: eGFR < 60 mL/min per 1.73 m2 (Cockroft-Gault) or microalbuminuria (> 20 mg/L) or overt proteinuria | Older age | 1.03 (1.00-1.05) | 0.03 | Adjustment made, but no precision | |
| Danquah et al[23], 2012 | Ghana | 671 | T2DM | Proteinuria ≥ 20mg/l | Not assessed | ||||
| Lutale et al[33], 2007 | Tanzania | 244 | T1DM and T2DM | Abnormal proteinuria: AER > 20 μg/min | Duration of diabetes | 0.090 (0.049- 0.131) | < 0.0001 | Predictors in the model: diabetes duration, Systolic BP, age, serum creatinine | Measure of association is β |
| Elevated systolic blood pressure | 0.012 (0.003-0.021) | 0.010 | |||||||
| Elevated serum creatinine | 0.011 (0.002- 0.020) | 0.016 | |||||||
| Worku et al[46], 2010 | Ethiopia | 305 | T1DM and T2DM | Proteinuria (no detail) | Duration of diabetes | Not provided | 0.001 | ||
| T2DM on insulin | 0.018 | ||||||||
| Makulo et al[35], 2010 | DR Congo | 81 | No precision | Microalbuminuria: ACR 30-299 mg/gMacroalbuminuria: ACR ≥ 300 mg/gRenal failure: eGFR < 60 mL/min per 1.73 m2 | Not assessed | ||||
| Eghan et al[25], 2007 | Ghana | 109 | T1DM and T2DM | Microalbuminuria: ACR 30-300 mg/g | Duration of diabetes | 0.04 | The associations were assessed by comparing patients with and without microalbuminuria | ||
| Serum creatinine | 0.05 | ||||||||
| Blood urea nitrogen | 0.01 | ||||||||
| Urine potassium | 0.0061 | ||||||||
| Alebiosu et al[17], 2004 | Nigeria | 162 | T2DM | No precision | Duration of diabetes | < 0.05 | The study assessed the predictors of diabetic nephropathy comparing T2DM patients with and without nephropathy | ||
| Serum total cholesterol | < 0.05 | ||||||||
| Alcohol > 30 mg/d | < 0.05 | ||||||||
| Peripheral vascular disease | < 0.05 | ||||||||
| Stroke | < 0.05 | ||||||||
CKD: Chronic kidney disease; BMI: Body mass index; ACR: Albumin-to-Creatinine Ratio; T1DM: Type 1 diabetes mellitus; T2DM: Type 2 diabetes mellitus; eGFR: Epidermal growth factor receptor.
DISCUSSION
Diabetic nephropathy is a common and morbid complication of diabetes and the leading cause of CKD in the developed world. The lack of renal registries means that there are no reliable statistics about the burden of CKD in people with diabetes in the majority of African countries. The current systematic review identified 32 relevant studies published over the last 20 years on kidney diseases in people with diabetes residing in Africa. Prevalence rates ranged from 11% to 83.7% for the overall CKD, 5.3% to 53.1% for CKD based on proteinuria, and 4.6% to 43.1% for CKD based on eGFR. Incident event rates were 94.9% for proteinuria at 10 years for follow-up, 34.7% for ERSD at 5 years of follow-up and 18.4% for mortality from nephropathy at 20 years of follow-up. Diagnosed duration of diabetes, blood pressure variables, advancing age, obesity and to some extend glucose control were the common determinants of kidney disease in people with diabetes. Studies were overwhelmingly hospital-based studies; half of them originated from four countries while variable definitions and methods for assessing nephropathy had been used across studies.
The most recent overview of CKD in populations within Africa was completed in 2012, and was restricted to sub-Saharan African Countries[47]. This review identified 90 articles representing data from 21 countries, with over half of the studies originating from South Africa, Nigeria and Ethiopia alones. Across 21 studies deemed to be of medium to high quality by the investigators, the pooled prevalence of CKD was 13.9% (95%CI: 12.2-15.7), with substantial heterogeneity across studies. The prevalence in people with diabetes ranged from 4% to 24% based essentially on proteinuria defined CKD[47]. In our review without applying quality criteria, we found much higher prevalence of CKD, regardless of the definition. In four studies published in 2013 for instance, the prevalence of microalbuminuria ranged between 21% and 45%. Although issues with the quality of the studies preclude direct comparisons, it is likely that nephropathy is more frequent in population with diabetes within Africa than in developed countries. The review by Stanifer et al[47] also identified many challenges and limitations, which largely apply to the current study.
The most important aspect in assessing incidence and prevalence of diabetic nephropathy in Africa is currently different diagnostic criteria for CKD. There are no clear definitions on DN. The 2012 KDIGO CKD classification assesses diabetes related kidney changes according to urinary albumin-to-creatinine ratio based on early morning spot urine samples[48]. Quantification of proteinuria in assessing CKD is controversial as no optimal test exists. The National Institute for Health and Clinical Excellence (NICE) guidance has recommended that an early morning urinary ACR should be preferred to other tests of proteinuria, because ACR offers greater sensitivity for the detecting lower, but clinically significant, levels of proteinuria[49]. Almost all the studies included in our review utilized urine tests to diagnose CKD, but only nine studies used ACR. Inconsistencies in the way and manner of reaching a diagnosis of DN in Africans are explained at least in part by issues relating to availability and accessibility of screening or diagnostic tools. Swanepoel et al[50] have reviewed in detail some of the problems associated with nephrology in Africa and discussed the role of lack of amenities in diagnosing renal diseases. Another challenge to making the diagnosis of diabetic nephropathy in Africa is the degree to which other causes of chronic kidney disease have been excluded. A standard armamentarium of tests would include tests looking for HIV, hepatitis B and C, brief collagen screen, syphilis exclusion and other tests would have to be based on history and physical exam.
The classification of CKD is important in the definition of DN and has a few limitations that are universally acknowledged: eGFR underestimates kidney function and there is discordance in the estimates across different estimators[51]; isolated microalbuminuria is a normal feature of aging, inflammation, vascular pathologies, smoking, diet and obesity which are all frequent in diabetes; decline in kidney function is an expected phenomenon with advanced age, just like diabetes risk increases with age. Further considerations to CKD classifications and DN definition limitations is that current guidelines take no notice of the single most important risk factor associated with CKD namely hypertension, which is present in over 50% of people with type 2 diabetes.
Risk factor association was not assessed in 12 of the 32 studies, however common risk factors included were hypertension, raised BMI, HbA1c and duration of diabetes. Despite advances in management over the last three decades, many people with diabetes still develop CKD. This may be partly explained by the poor achievement of blood pressure and blood glucose targets. Recently the JNC 8 guidelines have added to the controversy of various blood pressure targets needed for diabetic patients that would assist in preventing progression to CKD. Optimal targets when reached, however have shown to aid in progression to progression. Another risk factor pertinent to the developing world is the socioeconomic status of individuals in the causative role of diabetic nephropathy. Weil et al[52], in 2010 reviewed factors associated with disadvantage that may increase the risk of diabetic kidney disease, and the barriers to care that hinder attempts to provide an adequate therapeutic response[52].
Several mechanisms underlying the pathogenesis of diabetic nephropathy have been suggested and include glomerular hyperfiltration; hyperglycemia and the increased production of advanced glycation end products; hypoxia-inflammation and the activation of cytokines. Hyperfiltration commonly occur in early in the course of diabetes and involves glucose-dependent dilation of the afferent arteriolar dilation, and the enhanced filtration area secondary to the increase in the number of mesangial cells and capillary loops. Molecular level action involves vasoactive mediators like insulin-like growth factor 1, transforming growth factor beta, nitric oxide, prostaglandin, glucagon and vascular endothelial growth factor[53]. Other hallmarks of diabetic nephropathy include nodular diabetic glomerulosclerosis and diffuse glomerulosclerosis, mediated at least in part by inflammatory processes and immune cells activity[53]. Interstitial fibrosis and tubular atrophy are also seen early in DN, with the underlying pathogenetic mechanism being similar to those in progressive non diabetic renal disease[54].
Diabetic nephropathy ultimately occurs only in susceptible individuals with diabetes; which susceptibility is determined by the combined effect of genetic predisposition and non-genetic factors. Genetic susceptibility to diabetic nephropathy is by nature polygenetic. Whole-genome scanning studies have identified several chromosomal regions linked with diabetic nephropathy; however, the pathophysiologic function of such genetic regions has yet to be fully elucidated. Genetic polymorphisms may explain the familial clustering of diabetic nephropathy[55]. Some studies have suggested some detrimental effect of the double-deletion (DD) polymorphism of the angiotensin-converting enzyme (ACE) genotype on disease progression[56]. Non-genetic determinants of diabetic nephropathy include among others socioeconomic factors, dietary factors, poor hyperglycemic control, hypertension, obesity and early life factors[57,58]. Hypertension appears to be a strong correlate of disease progression in Black people[59,60].
The current review has some limitations. Included studies were mostly based on small samples, with different study designs and most of the studies were cross sectional with only two being retrospective cohorts and one case-control. A large proportion were based in urban clinics with and most of the populations studied were that attending a general diabetic clinic and the results may not be generalizable to primary care populations. Ideally chronic kidney disease should not be diagnosed on the basis of single measurements of serum creatinine and albuminuria, and standard baseline investigations are needed to exclude other causative kidney disease, although there is precedence for this in other studies in the West as well. Finally, detection of microalbuminuria was one the most frequent method to assess the presence of diabetic nephropathy. As microalbuminuria is more a quantitative estimate of endothelial/vascular dysfunction than of diabetic nephropathy, the incidence and prevalence rate of diabetic nephropathy have probably been overestimated when assessing kidney function by urine protein.
In conclusion, the current review gives a small glimpse of the larger numbers of CKD in diabetics in Africa compared to Western society. CKD is a substantial health burden among diabetic patients on the African continent, with prevalence varying from 11% to 83.7% depending on the method of assessment. Estimates suggest that 95% of diabetics may have proteinuria after a 10 years duration of diabetes, about 35% may have an end-stage renal disease after 5 years and 18% die from nephropathy after 20 years of disease duration. Risk factors of CKD include mainly hypertension, obesity, poor glycemic control and disease duration. Better surveillance of diabetes is a necessary first step toward its prevention and control, which is now recognized as an urgent priority. An electronic database in African regions would be ideal to assist in this entity although it is presumed that we are light years away from that. At a primary care level it is very plausible that with early detection, proper screening, and management, the impact of diabetic nephropathy may be better mitigated to lessen its impact on society and healthcare.
COMMENTS
Background
African countries are experiencing an epidemics of diabetes mellitus. Diabetic nephropathy is one the most frequent complications of diabetes mellitus. Several studies on the epidemiology of diabetic nephropathy have been conducted in Africa, but there is no previous published work which synthesizes evidences from this study to provide an overview of the disease on the continent.
Research frontiers
Epidemiological data on diabetic nephropathy in Africa are sparse. These data are important to quantify the magnitude of the disease and assist the formulation of strategies to reduce the impact of nephropathy on people with diabetes in Africa.
Innovations and breakthroughs
This review is the first to synthesize relevant data on diabetic nephropathy in Africa. The authors performed extensive electronic and manual bibliographic searches to determine the prevalence and incidence of diabetic nephropathy on the continent. Although the quality of data was not optimal, estimates suggest that the prevalence of diabetic nephropathy vary between 11%-83.7%. About one third of diabetic patients have end-stage renal disease after 5 years and about one fifth die from nephropathy after 20 years of disease duration. Hypertension, obesity, poor glycemic control and disease duration are the main risk factors of chronic kidney disease among diabetic patients in Africa.
Applications
This review shows that the burden of chronic kidney disease is important among people with diabetes in Africa. The findings will have implications for policy, practice and future research on diabetic nephropathy on the continent.
Terminology
Diabetic nephropathy is an alteration of the function of the kidneys due to diabetes mellitus. It is associated with substantial morbidity and mortality.
Peer-review
The authors of the present manuscript performed extensive electronic and manual bibliographic research to determine the prevalence and incidence of kidney disease in people with diabetes mellitus within countries in Africa. Overall the review is well written.
Footnotes
Conflict-of-interest: None for all co-authors.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 9, 2014
First decision: January 20, 2015
Article in press: March 18, 2015
P- Reviewer: de Oliveira JMF, Laghmani K S- Editor: Ji FF L- Editor: A E- Editor: Zhang DN
References
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.Aguiree F, Brown A, Cho NH, Dahlquist G, Dodd S, Dunning T, Hirst M, Hwang C, Magliano D, Patterson C, et al. IDF Diabetes Atlas: sixth edition. 6th ed. Basel, Switzerland: International Diabetes Federation; 2013. [Google Scholar]
- 3.Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662–1673. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller LM, Gorter KJ, Hak E, Goudzwaard WL, Schellevis FG, Hoepelman AI, Rutten GE. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41:281–288. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 7.Kengne AP, Echouffo-Tcheugui JB, Sobngwi E, Mbanya JC. New insights on diabetes mellitus and obesity in Africa-part 1: prevalence, pathogenesis and comorbidities. Heart. 2013;99:979–983. doi: 10.1136/heartjnl-2012-303316. [DOI] [PubMed] [Google Scholar]
- 8.Harjutsalo V, Groop PH. Epidemiology and risk factors for diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21:260–266. doi: 10.1053/j.ackd.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Assogba GF, Couchoud C, Roudier C, Pornet C, Fosse S, Romon I, Druet C, Stengel B, Fagot-Campagna A. Prevalence, screening and treatment of chronic kidney disease in people with type 2 diabetes in France: the ENTRED surveys (2001 and 2007) Diabetes Metab. 2012;38:558–566. doi: 10.1016/j.diabet.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Bakris GL. Recognition, pathogenesis, and treatment of different stages of nephropathy in patients with type 2 diabetes mellitus. Mayo Clin Proc. 2011;86:444–456. doi: 10.4065/mcp.2010.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas MC, Weekes AJ, Broadley OJ, Cooper ME, Mathew TH. The burden of chronic kidney disease in Australian patients with type 2 diabetes (the NEFRON study) Med J Aust. 2006;185:140–144. doi: 10.5694/j.1326-5377.2006.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 12.Kengne AP, Sobngwi E, Echouffo-Tcheugui JB, Mbanya JC. New insights on diabetes mellitus and obesity in Africa-Part 2: prevention, screening and economic burden. Heart. 2013;99:1072–1077. doi: 10.1136/heartjnl-2013-303773. [DOI] [PubMed] [Google Scholar]
- 13.Mbanya JC, Motala AA, Sobngwi E, Assah FK, Enoru ST. Diabetes in sub-Saharan Africa. Lancet. 2010;375:2254–2266. doi: 10.1016/S0140-6736(10)60550-8. [DOI] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Ajayi S, Mamven M, Ojji D. eGFR and chronic kidney disease stages among newly diagnosed asymptomatic hypertensives and diabetics seen in a tertiary health center in Nigeria. Ethn Dis. 2014;24:220–225. [PubMed] [Google Scholar]
- 16.Alebiosu CO. Clinical diabetic nephropathy in a tropical African population. West Afr J Med. 2003;22:152–155. doi: 10.4314/wajm.v22i2.27938. [DOI] [PubMed] [Google Scholar]
- 17.Alebiosu CO, Odusan O, Familoni OB, Jaiyesimi AE. Cardiovascular risk factors in type 2 diabetic Nigerians with clinical diabetic nephropathy. Cardiovasc J S Afr. 2004;15:124–128. [PubMed] [Google Scholar]
- 18.Alebiosu CO, Odusan O, Jaiyesimi A. Morbidity in relation to stage of diabetic nephropathy in type-2 diabetic patients. J Natl Med Assoc. 2003;95:1042–1047. [PMC free article] [PubMed] [Google Scholar]
- 19.Bentata Y, Haddiya I, Latrech H, Serraj K, Abouqal R. Progression of diabetic nephropathy, risk of end-stage renal disease and mortality in patients with type-1 diabetes. Saudi J Kidney Dis Transpl. 2013;24:392–402. doi: 10.4103/1319-2442.109617. [DOI] [PubMed] [Google Scholar]
- 20.Bouaziz A, Zidi I, Zidi N, Mnif W, Zinelabidine HT. Nephropathy following type 2 diabetes mellitus in Tunisian population. West Indian Med J. 2012;61:881–889. doi: 10.7727/wimj.2012.053. [DOI] [PubMed] [Google Scholar]
- 21.Bouzid C, Smida H, Kacem A, Turki Z, Ben Salem L, Ben Rayana C, Slama BC. [Renal failure in Tunisian patients with type 2 diabetes: frequency and related factors] Tunis Med. 2011;89:10–15. [PubMed] [Google Scholar]
- 22.Choukem SP, Dzudie A, Dehayem M, Halle MP, Doualla MS, Luma H, Kengne AP. Comparison of different blood pressure indices for the prediction of prevalent diabetic nephropathy in a sub-Saharan African population with type 2 diabetes. Pan Afr Med J. 2012;11:67. [PMC free article] [PubMed] [Google Scholar]
- 23.Danquah I, Bedu-Addo G, Terpe KJ, Micah F, Amoako YA, Awuku YA, Dietz E, van der Giet M, Spranger J, Mockenhaupt FP. Diabetes mellitus type 2 in urban Ghana: characteristics and associated factors. BMC Public Health. 2012;12:210. doi: 10.1186/1471-2458-12-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djrolo F, Attolou VG, Avode DG, Houngbe F, Akpona S, Addra B, Kodjoh N. [Diabetic nephropathy: an epidemiological study based on proteinuria in a population of black African diabetics in Cotonou, Benin] Sante. 2001;11:105–109. [PubMed] [Google Scholar]
- 25.Eghan BA, Frempong MT, Adjei-Poku M. Prevalence and predictors of microalbuminuria in patients with diabetes mellitus: a cross-sectional observational study in Kumasi, Ghana. Ethn Dis. 2007;17:726–730. [PubMed] [Google Scholar]
- 26.Elbagir MN, Eltom MA, Mahadi EO, Berne C. Pattern of long-term complications in Sudanese insulin-treated diabetic patients. Diabetes Res Clin Pract. 1995;30:59–67. doi: 10.1016/0168-8227(95)01146-3. [DOI] [PubMed] [Google Scholar]
- 27.Gill G, Gebrekidan A, English P, Wile D, Tesfaye S. Diabetic complications and glycaemic control in remote North Africa. QJM. 2008;101:793–798. doi: 10.1093/qjmed/hcn096. [DOI] [PubMed] [Google Scholar]
- 28.Gill GV, Huddle KR, Monkoe G. Long-term (20 years) outcome and mortality of Type 1 diabetic patients in Soweto, South Africa. Diabet Med. 2005;22:1642–1646. doi: 10.1111/j.1464-5491.2005.01712.x. [DOI] [PubMed] [Google Scholar]
- 29.Janmohamed MN, Kalluvya SE, Mueller A, Kabangila R, Smart LR, Downs JA, Peck RN. Prevalence of chronic kidney disease in diabetic adult out-patients in Tanzania. BMC Nephrol. 2013;14:183. doi: 10.1186/1471-2369-14-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katchunga P, Hermans MP, Manwa B, Lepira F, Kashongwe Z, M’Buyamba-Kabangu JR. [Hypertension, insulin resistance and chronic kidney disease in type 2 diabetes patients from South Kivu, DR Congo] Nephrol Ther. 2010;6:520–525. doi: 10.1016/j.nephro.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Keeton GR, Smit Rv, Bryer A. Renal outcome of type 2 diabetes in South Africa--a 12-year follow-up study. S Afr Med J. 2004;94:771–775. [PubMed] [Google Scholar]
- 32.Levitt NS, Bradshaw D, Zwarenstein MF, Bawa AA, Maphumolo S. Audit of public sector primary diabetes care in Cape Town, South Africa: high prevalence of complications, uncontrolled hyperglycaemia, and hypertension. Diabet Med. 1997;14:1073–1077. doi: 10.1002/(SICI)1096-9136(199712)14:12<1073::AID-DIA498>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 33.Lutale JJ, Thordarson H, Abbas ZG, Vetvik K. Microalbuminuria among Type 1 and Type 2 diabetic patients of African origin in Dar Es Salaam, Tanzania. BMC Nephrol. 2007;8:2. doi: 10.1186/1471-2369-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majaliwa ES, Munubhi E, Ramaiya K, Mpembeni R, Sanyiwa A, Mohn A, Chiarelli F. Survey on acute and chronic complications in children and adolescents with type 1 diabetes at Muhimbili National Hospital in Dar es Salaam, Tanzania. Diabetes Care. 2007;30:2187–2192. doi: 10.2337/dc07-0594. [DOI] [PubMed] [Google Scholar]
- 35.Makulo R, Nseka MN, Jadoul M, Mvitu M, Muyer MT, Kimenyembo W, Mandja M, Bieleli E, Mapatano MA, Epira FB, et al. [Albuminuria during the screening for diabetes in a semi-rural area (Kisantu City, DR Congo)] Nephrol Ther. 2010;6:513–519. doi: 10.1016/j.nephro.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Marshall SL, Edidin D, Sharma V, Ogle G, Arena VC, Orchard T. Current clinical status, glucose control, and complication rates of children and youth with type 1 diabetes in Rwanda. Pediatr Diabetes. 2013;14:217–226. doi: 10.1111/pedi.12007. [DOI] [PubMed] [Google Scholar]
- 37.Motala AA, Pirie FJ, Gouws E, Amod A, Omar MA. Microvascular complications in South African patients with long-duration diabetes mellitus. S Afr Med J. 2001;91:987–992. [PubMed] [Google Scholar]
- 38.Nambuya AP, Otim MA, Whitehead H, Mulvany D, Kennedy R, Hadden DR. The presentation of newly-diagnosed diabetic patients in Uganda. QJM. 1996;89:705–711. doi: 10.1093/qjmed/89.9.705. [DOI] [PubMed] [Google Scholar]
- 39.Pruijm MT, Madeleine G, Riesen WF, Burnier M, Bovet P. Prevalence of microalbuminuria in the general population of Seychelles and strong association with diabetes and hypertension independent of renal markers. J Hypertens. 2008;26:871–877. doi: 10.1097/HJH.0b013e3282f624d9. [DOI] [PubMed] [Google Scholar]
- 40.Rahlenbeck SI, Gebre-Yohannes A. Prevalence and epidemiology of micro- and macroalbuminuria in Ethiopian diabetic patients. J Diabetes Complications. 1997;11:343–349. doi: 10.1016/s1056-8727(96)00122-5. [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen JB, Thomsen JA, Rossing P, Parkinson S, Christensen DL, Bygbjerg IC. Diabetes mellitus, hypertension and albuminuria in rural Zambia: a hospital-based survey. Trop Med Int Health. 2013;18:1080–1084. doi: 10.1111/tmi.12139. [DOI] [PubMed] [Google Scholar]
- 42.Rissassi JR, Nseka M, Jadoul M, Lepira FB, Mvitu M, Mbenza G, Yekoladio D, Aloni M, Nge OO. [Prevalence and determinants of microalbuminuria and macroalbuminuria in children and young adults with type 1 diabetes in Kinshasa] Nephrol Ther. 2010;6:40–46. doi: 10.1016/j.nephro.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Rotchford AP, Rotchford KM. Diabetes in rural South Africa--an assessment of care and complications. S Afr Med J. 2002;92:536–541. [PubMed] [Google Scholar]
- 44.Sobngwi E, Mbanya JC, Moukouri EN, Ngu KB. Microalbuminuria and retinopathy in a diabetic population of Cameroon. Diabetes Res Clin Pract. 1999;44:191–196. doi: 10.1016/s0168-8227(99)00052-2. [DOI] [PubMed] [Google Scholar]
- 45.Wanjohi FW, Otieno FC, Ogola EN, Amayo EO. Nephropathy in patients with recently diagnosed type 2 diabetes mellitus in black Africans. East Afr Med J. 2002;79:399–404. doi: 10.4314/eamj.v79i8.8824. [DOI] [PubMed] [Google Scholar]
- 46.Worku D, Hamza L, Woldemichael K. Patterns of diabetic complications at jimma university specialized hospital, southwest ethiopia. Ethiop J Health Sci. 2010;20:33–39. doi: 10.4314/ejhs.v20i1.69424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanifer JW, Jing B, Tolan S, Helmke N, Mukerjee R, Naicker S, Patel U. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e174–e181. doi: 10.1016/S2214-109X(14)70002-6. [DOI] [PubMed] [Google Scholar]
- 48.Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85:49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 49.National Collaborating Centre for Chronic Conditions. Chronic kidney disease: Early identification and management of chronic kidney disease in adults in primary and secondary care. London: NICE; 2008. Available from: http://www.nice.org.uk/nicemedia/live/12069/42117/42117.pdf. [Google Scholar]
- 50.Swanepoel CR, Wearne N, Okpechi IG. Nephrology in Africa--not yet uhuru. Nat Rev Nephrol. 2013;9:610–622. doi: 10.1038/nrneph.2013.168. [DOI] [PubMed] [Google Scholar]
- 51.Matsha TE, Yako YY, Rensburg MA, Hassan MS, Kengne AP, Erasmus RT. Chronic kidney diseases in mixed ancestry south African populations: prevalence, determinants and concordance between kidney function estimators. BMC Nephrol. 2013;14:75. doi: 10.1186/1471-2369-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weil EJ, Curtis JM, Hanson RL, Knowler WC, Nelson RG. The impact of disadvantage on the development and progression of diabetic kidney disease. Clin Nephrol. 2010;74 Suppl 1:S32–S38. doi: 10.5414/cnp74s032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruster C, Wolf G. The role of chemokines and chemokine receptors in diabetic nephropathy. Front Biosci. 2008;13:944–955. doi: 10.2741/2734. [DOI] [PubMed] [Google Scholar]
- 54.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seaquist ER, Goetz FC, Rich S, Barbosa J. Familial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med. 1989;320:1161–1165. doi: 10.1056/NEJM198905043201801. [DOI] [PubMed] [Google Scholar]
- 56.Marre M, Jeunemaitre X, Gallois Y, Rodier M, Chatellier G, Sert C, Dusselier L, Kahal Z, Chaillous L, Halimi S, et al. Contribution of genetic polymorphism in the renin-angiotensin system to the development of renal complications in insulin-dependent diabetes: Genetique de la Nephropathie Diabetique (GENEDIAB) study group. J Clin Invest. 1997;99:1585–1595. doi: 10.1172/JCI119321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nelson RG, Morgenstern H, Bennett PH. Intrauterine diabetes exposure and the risk of renal disease in diabetic Pima Indians. Diabetes. 1998;47:1489–1493. doi: 10.2337/diabetes.47.9.1489. [DOI] [PubMed] [Google Scholar]
- 58.Zandi-Nejad K, Luyckx VA, Brenner BM. Adult hypertension and kidney disease: the role of fetal programming. Hypertension. 2006;47:502–508. doi: 10.1161/01.HYP.0000198544.09909.1a. [DOI] [PubMed] [Google Scholar]
- 59.Brancati FL, Whittle JC, Whelton PK, Seidler AJ, Klag MJ. The excess incidence of diabetic end-stage renal disease among blacks. A population-based study of potential explanatory factors. JAMA. 1992;268:3079–3084. [PubMed] [Google Scholar]
- 60.Chaiken RL, Palmisano J, Norton ME, Banerji MA, Bard M, Sachimechi I, Behzadi H, Lebovitz HE. Interaction of hypertension and diabetes on renal function in black NIDDM subjects. Kidney Int. 1995;47:1697–1702. doi: 10.1038/ki.1995.235. [DOI] [PubMed] [Google Scholar]


