Abstract
We present a rare case of Schizophyllum commune causing allergic fungal rhinosinusitis (AFRS) in a 56-year-old immunocompetent woman. In our case, diagnosis of AFRS was based on the history of illness, CT scan findings, culture and PCR. The PCR product was further analysed by sequencing to confirm S. commune. The patient was treated by functional endoscopic sinus surgery (FESS) and antiallergic drugs.
Background
In recent years, fungal infection of the nose and paranasal sinus has been increasing in immunocompetent and immunocompromised patients.1 2 Saprobes are the most common fungi causing sinusitis and commonly include Aspergillus, Alternaria, Bipolaris and Curvularia. Allergic fungal rhinosinusitis (AFRS) is mostly caused by Aspergillus.3 However, nowadays, rarely encountered species, for example, Schizophyllum commune, are also being reported in diseases such as pulmonary disorders, ulcerative lesions of the palate, cerebral abscess, onychomycosis and chronic and allergic sinusitis.4 5 Early diagnosis of AFRS prevents repeat courses of antibiotics and surgical procedures, however, criteria for the diagnosis are still evolving.
Conventional diagnosis of allergic fungal sinusitis is still predominantly based on a combination of radiological, histopathological and culture techniques. There is a tendency to define histopathological features as being sufficient criteria for the diagnosis of AFRS, however, these should not be the sole basis of diagnosis. There is extensive expanding literature on allergic fungal sinusitis and its aetiological agents, histopathological correlates, diagnostic criteria and therapeutic modalities over the past several decades. Currently, in addition, there is focus on the use of molecular and culture methods in the diagnosis of AFRS. However, the use of molecular techniques is still debatable.
In this case study, we present a rare strain of fungus, S. commune, rather than common fungi, causing AFRS, and also emphasise the importance of culture and molecular methods in diagnosing fungus in patients of AFRS.
Case presentation
A 56-year-old woman presented with nasal obstruction and mucopurulent nasal discharge of 3-month and headache of 1-month duration. The patient's clinical manifestations persisted in spite of her using various antibiotics. She also had a family history of asthma. She was a non-smoker, without diabetes and non-hypertensive.
On examination, the patient was found to have facial congestion on the right. A nasal polyp was found in the right nasal cavity.
Investigations
A CT scan of paranasal sinuses revealed mucosal thickening in maxilla, ethmoid, sphenoid, left frontal sinus and a deviated nasal septum, pneumatisation of right middle turbinate and bilateral obstruction of osteomeatal units of bilateral sinuses (figure 1). Other laboratory investigations revealed white cell count count of 8200 with 1% eosinophils. Functional endoscopic sinus surgery (FESS) was performed and tissue was sent for histopathological and microbiological examination. Potassium hydroxide (KOH) microscopy of the tissue was negative for fungal elements. No bacterium was isolated after aerobic culture. After 2 weeks of incubation at 25°C, the culture on sabouraud dextrose agar grew from white to a pale buff, densely woolly fungus with a white to cream reverse. A lactophenol cotton blue (LCB) mount of the colony revealed septate fungal hyphae with clamp connection. Histopathological sections of the nasal tissue were negative for fungal hyphae. They showed a polypoidal structure lined by respiratory epithelium. The subepithelium had oedema with mild mixed inflammatory cell infiltrate consisting of a few eosinophils, lymphocytes and plasma cells.
Figure 1.
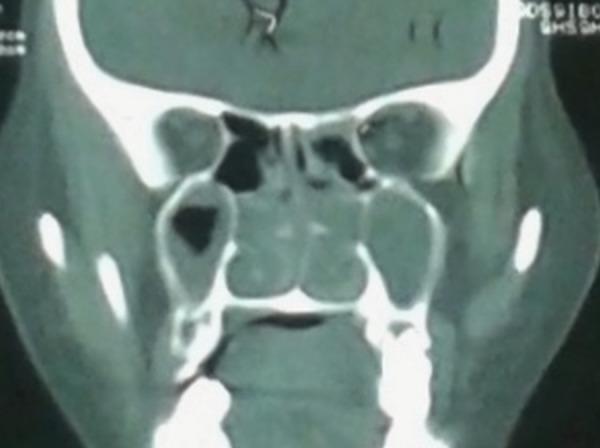
CT scan of the sinus showing mucosal thickening and polypoidal growth in nasal cavities, maxillary, ethmoidal and frontal sinuses.
Fungal tissue DNA was extracted employing a Zymo research fungal/bacterial DNA extraction kit using a spin bead column method. Universal primers for the 28 S rDNA were used to amplify a DNA sequence of 269 bp (primer U1- GTG AAA TTG TTG AAA GGG AA and primer U2- GAC TTG GTC CGT GTT).6
PCR was carried out in a PCR thermal cycler (T100, Bio-rad, USA) with the following steps: one cycle of denaturation at 94°C for 7 min, 35 cycles of denaturation at 94°C for 60 s, primer annealing at 45°C for 60 s, primer extension at 72°C for 60 s; and final extension 10 min at 72°C. A PCR product approximately 269 base pair was found in gel electrophoresis. The 269 base pair PCR product was sequenced by automated nucleotide sequencing using a BigDye-Terminator-Cycle-Sequencing Ready Reaction kit (Applied Biosystems) as per the manufacturer's protocol. The sequence determined was submitted to NCBI Gen Bank (accession number KM576 799). The obtained sequence was analysed using a Basic Local Alignment Search Tool (BLAST) and was found to have 100% homology with S. commune.
Differential diagnosis
Chronic rhinosinusitis.
Treatment
The patient was treated by tissue debridement with FESS.
Outcome and follow-up
The patient was followed post surgically for 6 months and was found to be asymptomatic.
Discussion
AFRS constitutes 6–10% of rhinosinusitis cases requiring surgical intervention.3 7 8 AFRS is non-invasive fungal rhinosinusitis caused mostly by Aspergillus, Penicillium, Alternaria and, rarely, by S. commune. S. commune belongs to phylum basidiomycota and family Schizophyllaceae, which has been reported to cause allergic fungal rhinosinusitis, invasive fungal sinusitis, allergic bronchopulmonary mycosis and onychomycosis.9 Infections caused by S. commune in immunocompetent and in immunocompromised hosts are on the rise. In immunocompromised patients, S. commune can also cause invasive infections such as brain abscesses, which can be fatal.
Histopathological, radiological and immunological characteristics of the disease form the basis of AFRS diagnostic criteria. The criteria given by Bent and Kuhn is usually considered as the standard for diagnosis of AFRS today. Patients must meet all the major criteria, which include a history of type I hypersensitivity, skin testing or in vitro testing; nasal polyposis; characteristic CT scan findings; the presence of eosinophilic mucin without invasion; and a positive fungal stain of sinus contents removed at the time of surgery for diagnosis. Whereas, features such as history of asthma, unilateral predominance of disease, radiographic evidence of bone erosion, fungal cultures, presence of Charcot-Leyden crystals in surgical specimens and serum eosinophilia, form the minor criteria, which can serve as supporting evidence for diagnosis.10
We based our diagnosis on the history of illness, CT scan findings, culture and PCR directly from the tissue followed by sequencing. Thereafter, a final diagnosis of allergic fungal rhinosinusitis was made. Histopathology of the tissue was negative for fungal hyphae. The diagnosis could be confirmed only after PCR and sequencing of the fungal DNA isolated from the nasal tissue. Though it grew in culture, initially it was considered a contaminant, since it is an ubiquitous saprophyte. Histopathology may be negative in non-invasive cases, therefore, molecular identification directly from the tissue or culture becomes more important. It has been suggested that since hyphae of these basidiomycetes appear similar to those of the Aspergillus species, for accurate diagnosis of S. commune, culture or sequencing should be carried out.11
In this case, the patient was treated by surgical debridement and drainage of the paranasal sinuses. Antifungal treatment in this patient was not advised because of the limited role of antifungals in allergic fungal disease. The optimal approach to the management of infections caused by S. commune is uncertain. The utility of azoles in treating S. commune infections is not known. Previous studies have shown non-efficacy of itraconazole treatment.12 13 Contradicting these, another study, by Denning et al,14 observed decrease in total IgE when patients with allergic bronchopulmonary aspergillosis were treated with itraconazole. It has been pointed out that the limited literature on it, along with the cost and drug-related morbidity of systemic antifungal therapy, limits its worth for treatment of non-invasive fungal disease.15
In our study, the sequence based identification of the fungal isolates revealed S. commune as the causative agent of AFRS. In fact, given the excellent specificity of this technique, gene sequencing has been recognised as the gold standard for fungal identification. The fungi also resembled S. communemorphologically (figure 2A, B). Occasionally, the fungi may not show typical morphological and microscopic features resembling S. commune. In such cases, molecular techniques can be more specific and accurate. Therefore, it is advised to compare morphological findings and molecular data to ensure reliability.16
Figure 2.
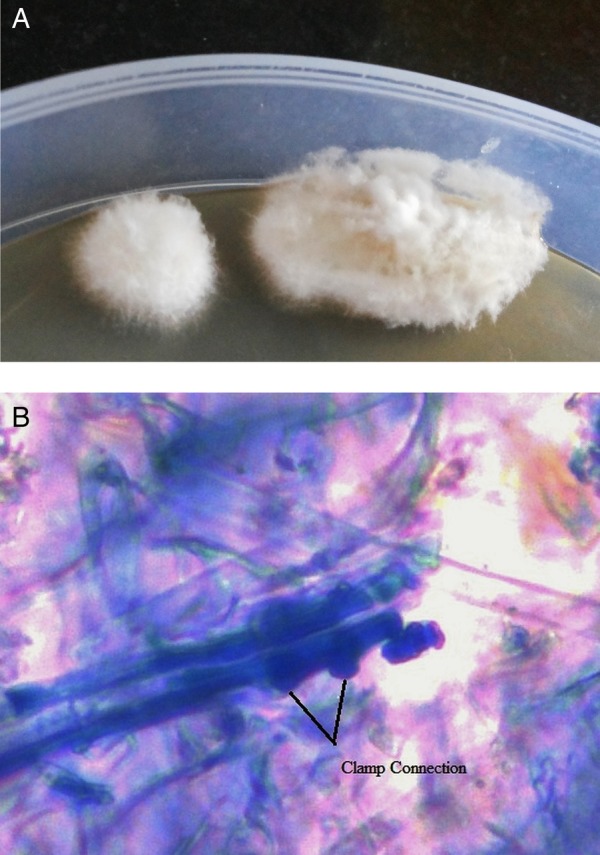
(A) White and densely woolly fungus growing on sabouraud dextrose agar media. (B) Hyphae of Schizophyllum commune with clamp connections.
Learning points.
Schizophyllum commune in cultures must not be considered a contaminant as it can be a potential cause of allergic fungal rhinosinusitis (AFRS).
Early diagnosis of FRS is best made by a combination of tests, microscopy and PCR.
As it is difficult to identify S. commune in culture, PCR followed by sequencing is the most advisable method.
Footnotes
Contributors: The patient was clinically evaluated and treated by SPA. Laboratory investigations were performed by PG, NV and AKS. All authors contributed to writing this article.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sarti EJ, Blaugrund SM, Lin PT et al. Paranasal sinus disease with intracranial extension: aspergillosis versus malignancy. Laryngoscope 1998;98(6 Pt 1):632–63. [PubMed] [Google Scholar]
- 2.Sarti EJ, Laucente FE. Aspergillosis of the paranasal sinuses. ENT J 1998;67:824–31. [PubMed] [Google Scholar]
- 3.Schubertz MS. Allergic fungal sinusitis pathophysiology, diagnosis and management . Med Mycol 2009;47:S324–30. 10.1080/13693780802314809 [DOI] [PubMed] [Google Scholar]
- 4.Sigler L, Bartley JR, Parr DH et al. Maxillary sinusitis caused by medusoid form of Schizophyllum commune. J Clin Microbiol 1999;37:3395–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taguchi K, Oharaseki T, Yokouchi Y et al. Allergic fungal sinusitis caused by Bipolaris spicifera and Schizophyllum commune. Med Mycol 2007;45:559–64. 10.1080/13693780701487813 [DOI] [PubMed] [Google Scholar]
- 6.Sandhu G, Kline BC, Atockman L et al. Molecular probes for diagnosis of fungal infections. J Clin Microbiol 1995;33:2913–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fokkens W, Lund V, Mullol J: European Position Paper on Rhinosinusitis and Nasal Ployps Group. EP3OS 2007: European position paper on rhinosinusitis and nasal polyps. A summary for otorhinolaryngologist. Rhinology 2007;45:97–101. [PubMed] [Google Scholar]
- 8.Ponikau JU, Sherris DA, Kern EB et al. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc 1999;74:877–84. 10.4065/74.9.877 [DOI] [PubMed] [Google Scholar]
- 9.Nazeri M, Mohammadi M, Moazeni M et al. A case of fungus ball type pansinusitis caused by Schizophillum commune. Med Mycol Case Rep 2012;1:115–18. 10.1016/j.mmcr.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bent JP, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg 1994;111:580–8. 10.1016/S0194-5998(94)70525-9 [DOI] [PubMed] [Google Scholar]
- 11.Swain B, Panigrahy R, Panigrahi D. Schizophyllum commune sinusitis in an immunocompetent host. Indian J Med Microbiol 2011;29:439–42. 10.4103/0255-0857.90194 [DOI] [PubMed] [Google Scholar]
- 12.Kamai K, Unno H, Nagao K et al. Allergic bronchopulmonary mycosis caused by the basidiomycetous fungus Schizophyllum commune. Clin Infect Dis 1994;18:305–9. 10.1093/clinids/18.3.305 [DOI] [PubMed] [Google Scholar]
- 13.Premamalini T, Ambujavalli BT, Anitha S et al. Schizophyllum commune a causative agent of fungal sinusitis: a case report. Case Rep Infect Dis 2011;2011:821259 10.1155/2011/821259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denning DW, Van Wye JE, Lewiston NJ et al. Adjunctive therapy of allergic bronchopulmonary aspergillosis with itraconazole. Chest 1991;100:813–19. 10.1378/chest.100.3.813 [DOI] [PubMed] [Google Scholar]
- 15.Ferguson BJ. What role do systemic corticosteroids, immunotherapy, and antifungal drugs play in the therapy of allergic fungal rhinosinusitis. Arch Otolaryngol Head Neck Surg 1998;124:1174–8. 10.1001/archotol.124.10.1174 [DOI] [PubMed] [Google Scholar]
- 16.Balajee SA, Singler L, Brandit ME. DNA and the classical way: identification of medically important molds in the 21st century. Med Mycol 2007;45:475–90. 10.1080/13693780701449425 [DOI] [PubMed] [Google Scholar]


