Abstract
Antidepressants can cause a variety of gastrointestinal effects, including nausea, dyspepsia, anorexia, constipation, and, rarely, diarrhoea and adynamic ileus. There is a lack of cases associating antidepressants to adynamic ileus and diarrhoea. We report a case of an elderly woman in whom the use of several antidepressants apparently induced adynamic ileus with diarrhoea.
Background
Adynamic ileus (AI) is a non-mechanic intestinal obstruction resulting from the impairment of the peristalsis, characterised by a massive bowel dilation. It can be associated to drugs, such as narcotics, phenothiazines, anticholinergics and, rarely, to antidepressants (AD).1–3 AI's symptoms include abdominal pain and distension, constipation and, sometimes, vomiting, with diarrhoea being an uncommon symptom.
AD are widely used in order to regulate the flow of neurotransmitters (NT) into the nervous system, including noradrenalin, serotonin, dopamine and acetylcholine.4 Their adverse effects are associated with the increased activity of each NT. Gastrointestinal effects—such as nausea, dyspepsia, anorexia, constipation, diarrhoea and AI5—mainly derive from increasing serotonergic and antimuscarinic activity.1 6
Although diarrhoea or constipation have been reported as side effects of AD,5 to date, no cases of AD associated with AI and diarrhoea have been described. Our study reports the case of an elderly woman in whom the use of several AD appeared to induce ileus with diarrhoea.
Case presentation
We present the clinical case of a 76-year-old woman with a history of fibromyalgia, treated with maprotiline, amitriptyline, cyclobenzaprine, flupirtine, naproxen and acetaminophen. The patient had been admitted to Hospital da Luz (Lisbon, Portugal) 3 years before, reporting watery diarrhoea (with over 10 stools/day, without blood or mucus) and weight loss (about 44 pounds), within 6 months of evolution. No other associated symptoms were present then. Blood and stool tests were performed, resulting inconclusively (tables 1 and 2), as well as a colonoscopy with biopsy. The colonoscopy revealed ileocolitis with a few erosions in the terminal ileum, and soft hyperaemia and oedema in the descending and sigmoid colon. The histology revealed villous atrophy with lymphocytic and plasmacytic infiltration. The patient was started on mesalazine 3 g/day and budesonide 9 mg/day (for 1 year), since her condition was hypothesised to be an ulcerative colitis. There was a partial improvement of symptoms. Nevertheless, the patient had recurrent diarrhoea, which was controlled with loperamide.
Table 1.
Performed blood tests
| Blood test | Patient values |
Reference values | |
|---|---|---|---|
| 2011 | 2013 | ||
| TSH, mUI/L | 1.35 | 0.65 | 0.5–8.90 |
| Free T3, pmol/L | 3.5 | 4.0 | 3.5–6.5 |
| Free T4, pmol/L | 13.26 | 15.43 | 10.4–22.7 |
| ACTH, pg/mL | 15.1 | <46 | |
| Gastrin, pg/mL | 13.0 | 13.0–115.0 | |
| Calcitonin, pg/mL | <4.0 | <4.0 | <4.8 |
| Serotonin, µg/L | 46 | 45–193 | |
| Vasoactive intestinal peptide, pmol/L | 22 | 20 | <30 |
| HAV antibody (IgM) | Negative | – | |
| HBs | Negative | – | |
| HBs antibodies (IgM/IgG) | Negative/positive | ||
| HBc antibodies (IgM/IgG) | Negative | ||
| HCV antibodies | Negative | – | |
| HIV 1–2 antibodies | Negative | – | |
| CMV antigen | Negative | – | |
| Antiendomysial antibodies | |||
| IgA | Negative | – | |
| Antigliadin antibodies | |||
| IgM, UA/mL | <0.1 | – | <7.0 |
| IgG, UA/mL | <0.4 | <7.0 | |
| Antitransglutaminase antibodies | |||
| IgA, UA/mL | <0.1 | – | |
| IgG, UA/mL | <0.6 | ||
| Antinuclear antibodies | Negative | ||
| RA test, UI/mL | <20 | – | <20 |
ACTH, adrenocorticotropic hormone; CMV, cytomegalovirus; HAV, hepatitis A virus; HBc, hepatitis B core antigen; HBs, hepatitis B surface antigen; HCV, hepatitis C virus; RA, rheumatoid arthritis; T3L, tri-iodothyronine; T4L, thyroxine; TSH, thyroid stimulating hormone.
Table 2.
Performed stool tests
| Stool test | Patient values |
Reference values | |
|---|---|---|---|
| 2011 | 2013 | ||
| Parasitological test | Negative | Negative | |
| Bacteriological test | Negative | Negative | |
| Clostridium difficile toxin | Negative | Negative | |
| Giardia’s antigen | Negative | Negative | |
| Cryptosporidium’s antigen | Negative | Negative | |
| Pancreatic elastase, µg/g stool | 402 | 388 | >200 |
| Reducing substances | Negative | Negative | |
Two years later, there was a new clinical worsening, including diarrhoea, of the same clinical features, occurring predominantly at night, and severe dehydration. No metabolic–electrolyte abnormalities were found, except for elevated serum urea nitrogen (130 mg/dL) and serum creatinine (1.54 mg/dL). The patient was admitted to the internal medicine ward for further aetiological research and acute renal failure treatment. New blood and stool tests (table 1 and 2) were performed and, once more, revealed no changes. The abdomen X-ray and CT scan showed marked colonic and small bowel distension (figures 1 and 2). Sigmoidoscopy demonstrated pseudomembranous colitis (figure 3), and endoscopy and videoenteroscopy presented no abnormalities. The patient was started on therapy with metronidazole and vancomycin (14 days), with parenteral nutrition, which resulted in no improvement. Later, a gluten-free and lactose-free diet was introduced, but with an ileus and diarrhoea maintenance. On the 14th day of hospitalisation, for a suspected iatrogenic cause, all AD were suspended, with a complete resolution of diarrhoea and AI. The colonoscopy performed at discharge was normal.
Figure 1.

An exuberant adynamic ileus in abdomen X-ray.
Figure 2.

An exuberant adynamic ileus in abdomen CT scan.
Figure 3.

A pseudomembranous colitis.
Six months later, the depressive symptoms worsened. The patient underwent sertraline therapy; the diarrhoea and AI, though less exuberantly (figure 4), reappeared. After the discontinuation of the drug, a complete resolution of the clinical condition was established. The patient is currently treated with agomelatine, flurazepam and alprazolam, with normal bowel movements.
Figure 4.
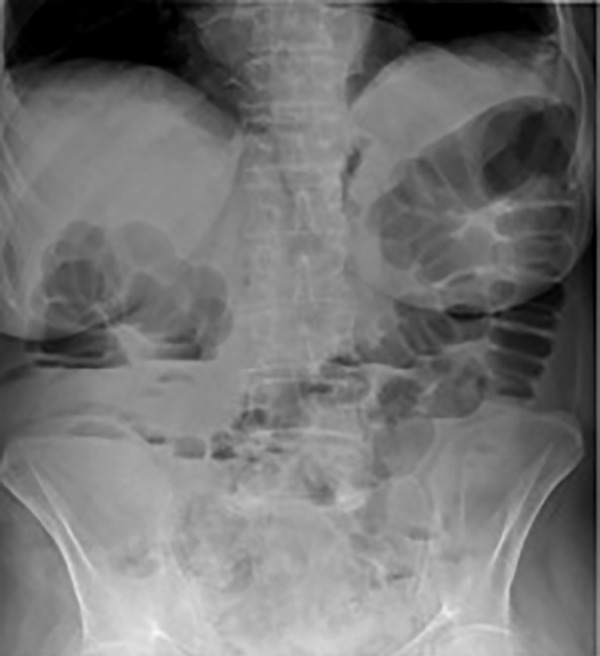
A slight adynamic ileus.
Discussion
AD are often associated with constipation, but AI is a rare outcome of their use, due to their antimuscarinic effect.1 The reviewed literature only presented two cases of AI associated with tricyclic AD, but no cases of ileus and diarrhoea resulting from AD administration. The case here reported can be, first, explained by the effect of serotonin and acetylcholine on the intestine, leading, on the one hand, to a quick change of small and large bowel movements6 due to a decrease of the magnitude of contractile waves with consequent hypomotility. Abdominal pain and nausea are rare, but patients often present diarrhoea caused by colonic stasis and bacterial overgrowth. On the other hand, rectum dilation leads to faecal incontinence by changing the wall sensitivity.7 Second, we can postulate that there was a greater vulnerability of the patient to this adverse effect, considering the possibility of a functional colonopathy in the context of her depressive/anxious syndrome. Blood and stool tests were both useful in ruling out other causes of chronic diarrhoea. The colonoscopy was important to preclude intestinal obstruction, whereas abdomen CT was important to exclude retroperitoneal and pelvic pathology.
In conclusion, the relationship between AI and AD was demonstrated and, although improved after drug withdrawal, we admit a chronic pseudo-obstruction. Despite the selective serotonin reuptake inhibitors being considered safer than tricyclic AD, both are well tolerated in the elderly.5 It is important to know this adverse effect in order to identify it early and to provide adequate treatment, avoiding unnecessary interventions and therapies.
Learning points.
Antidepressants are widely used drugs with multiple adverse effects, but, nevertheless, well tolerated in the elderly.
Antidepressants can lead to adynamic ileus and constipation, with a possible occurrence of diarrhoea, due to the intestinal effect of serotonin.
It is important to know these side effects to identify and treat them appropriately.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sood A, Kumar R. Imipramine induced an acute colonic pseudo-obstruction (Ogilvie's syndrome): a report of two cases. Indian J Gastroenterol 1996;15:70–1. [PubMed] [Google Scholar]
- 2.Kales H, Mellow A. Ileus as a possible result of bupropion in an elderly woman. J Clin Psychiatric 1999;60:337 10.4088/JCP.v60n0512c [DOI] [PubMed] [Google Scholar]
- 3.Milner G, Hills NF. Adynamic ileus and nortriptyline. BMJ 1966;5500:1421 10.1136/bmj.1.5500.1421-b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blier P. Neurotransmitter targeting in the treatment of depression. J Clin Psychiatry 2013;74:19–2. 10.4088/JCP.12084su1c.04 [DOI] [PubMed] [Google Scholar]
- 5.Uher R, Farmer A, Henigsberg N et al. Adverse reactions of antidepressants. Br J Psychiatry 2009;195:202–10. 10.1192/bjp.bp.108.061960 [DOI] [PubMed] [Google Scholar]
- 6.Hasler W. Serotonin and the GI tract. Curr Gastroenterol Rep 2009;11:383–91. 10.1007/s11894-009-0058-7 [DOI] [PubMed] [Google Scholar]
- 7.Benlloch S. Pseudoobstrucción colónica crónica secundaria a neurolépticos. Gastroenterol Hepatol 2001;24:500–2. 10.1016/S0210-5705(01)70222-1 [DOI] [PubMed] [Google Scholar]


