Abstract
A 75-year-old woman presented with severe abdominal pain and diarrhoea. Symptoms started 10 years earlier but multiple investigations failed to offer a clear diagnosis. On recent admission, blood tests, endoscopies and CT scans indicated chronic colonic inflammation. Treatment strategies for bowel inflammation were unsuccessful and the patient was subsequently discussed at a multidisciplinary team meeting with surgeons for consideration of colectomy. A drug review highlighted that the patient was taking an antiangina drug, nicorandil, thought to cause bowel ulceration. This was discontinued, which dramatically improved symptoms and avoided surgery and the patient was discharged within days. Follow-up colonoscopy showed much improved colitis, and the diarrhoea had resolved. It is important that clinicians are aware of the link between pharmacotherapy, specifically nicorandil and gastrointestinal ulceration and inflammation causing severe diarrhoea. Drug cessation is the only necessary and immediately effective treatment. Awareness of this will become more clinically relevant as nicorandil use increases.
Background
The patient was clerked on the day she was admitted. She was exhausted and emotional from chronic debilitating symptoms. The diarrhoea and abdominal pain had progressed to the point where she could no longer live her life normally. She had recently missed her sister's birthday because she feared she would not complete the journey without soiling herself. She had undergone numerous investigations over the years, to no avail. It was not until nicorandil was stopped, that she could hope to live a normal life again.
The patient marginally escaped an invasive, surgical intervention that would have incurred substantial morbidity with not entirely predictable positive outcomes. Drug cessation is the only cure and she would still be taking this medication had it not been for a surgeon in a multidisciplinary team meeting highlighting the link between nicorandil and gastrointestinal ulceration.
Intestinal ulceration is listed in the British National Formulary (BNF) for nicorandil as a rare side effect. The increasing polypharmacy clouded our eventual ability to spot nicorandil and discontinue the drug. There is particular focus on increasing the awareness and understanding of this link among clinicians who may come across similar cases in practice.
Case presentation
A 75-year-old woman with a 10-year history of abdominal pain and diarrhoea was admitted because her symptoms had become unbearable. Although haemodynamically stable, she was passing stools almost 15 times a day and her quality of life was being adversely affected. She had little sleep, and could not leave the house for fear of incontinence. CT scans and endoscopies from previous similar admissions had revealed atheromatous disease but little else to explain these severe symptoms. She was reviewed in gastroenterology as well as in colorectal outpatient clinics; a definitive diagnosis for her symptoms could not be provided.
She lives alone and has no significant family history. Her comorbidities include: irritable bowel syndrome, ruptured and repaired abdominal aortic aneurysm, anxiety disorder and asthma. She also has chronic ischaemic heart disease, previous myocardial infarction and coronary artery bypass grafting and a ventricular tachycardia that required DC cardioversion.
Investigations
On this admission, there was non-specific abdominal tenderness and inflammatory markers were raised (C reactive protein (CRP) 100, white cell count 17.3). The abdominal CT showed thickened mucosa, suggestive of pancolitis (figure 1), so intravenous steroids and nasogastric enteral nutritional support were started. Sigmoidoscopy showed granular mucosa suggestive of ulcerative colitis as well as linear ulceration more characteristic of Crohn's disease (figure 2). The biopsy confirmed evidence of chronic inflammation with focal ulceration, but could not identify a precise diagnosis.
Figure 1.
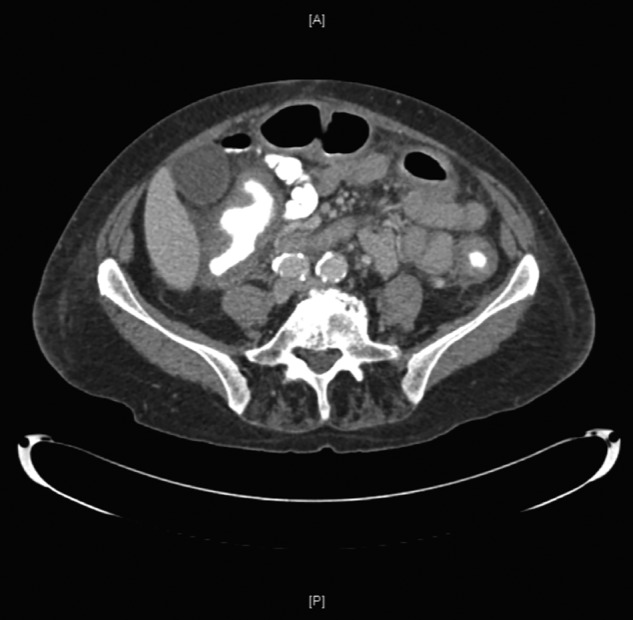
CT of the abdomen (22 February 2013) showing thickened mucosa and appearances consistent with inappropriate, excessive inflammation. The report identifies features suggestive of pancolitis, most marked in the ascending colon extending to the ileocaecal region.
Figure 2.
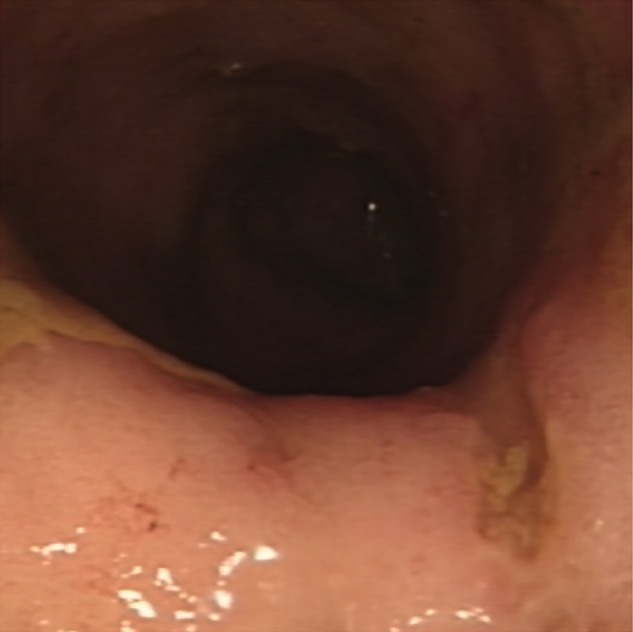
Flexible sigmoidoscopy (28 February 2013) showing there is loss of the vascular pattern, and the mucosa appears granular. A linear ulcer is identified in the proximal sigmoid.
Given the patient's history of ischaemic heart disease and a previously ruptured and repaired abdominal aortic aneurysm, a CT angiogram was performed to exclude mesenteric ischaemia. This showed no evidence of major vessel occlusion. The patient continued to symptomatically deteriorate, becoming tachycardic with ongoing diarrhoea. Inflammatory markers rose (CRP 102), and albumin and haemoglobin levels fell further. A colonoscopy was requested, which showed severe colitis features suggestive of Crohn's disease (figure 3), so mesalazine and 6-MP were started. As the biopsy showed the patient to be cytomegalovirus (CMV) positive, she was also started on valganciclovir to treat CMV colitis. However, she responded poorly to all these treatment regimens.
Figure 3.

Colonoscopy (12 March 2013) showing severe colitis, with macroscopic characteristics consistent with Crohn's disease.
Differential diagnosis
There was some discussion that the patient's symptoms may be attributed to inflammatory bowel disease, namely Crohn's disease, and CMV colitis. Treatment started for these conditions but she failed to respond. An investigation was also conducted for mesenteric ischaemia, but the CT angiogram was unremarkable.
Treatment
Surgical opinion was sought and the case was discussed at a multidisciplinary meeting for consideration of possible colectomy. A Surgeon noticed the patient was taking the drug nicorandil, a third-line agent used to prevent recurrent angina. The uncommonly recognised association between nicorandil and ulceration in the gastrointestinal tract was brought to the attention of the clinicians, and supported by a repeat sigmoidoscopy that revealed punched out ulcers (figure 4).
Figure 4.
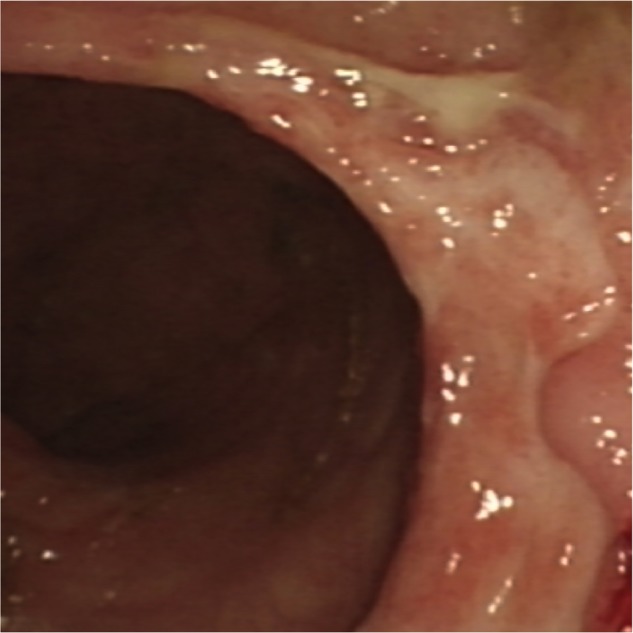
Sigmoidoscopy (2 April 2013) showing punched out ulcers.
The patient had been taking nicorandil 20 mg twice daily for over 10 years for ischaemic heart disease, with bowel symptoms starting shortly thereafter. Once this drug was discontinued, symptoms improved dramatically. She started passing formed stools and her CRP decreased from 100 to 40, and albumin rose from 27 to 35.
On detailed retrospective review of all her clinical notes, it is relevant to mention that stopping nicorandil had been suggested by the otorhinolaryngology team earlier that year after a clinic attendance for a painful tongue ulcer.
Outcome and follow-up
Two months later, symptoms were much improved, although there was some ongoing diarrhoea. A follow-up sigmoidoscopy confirmed improvement: ‘very minimal colitis with granularity and patches of mild inflammation’. The patient was on no medication to treat ulcerative colitis or Crohn's disease.
Discussion
Nicorandil is a nicotinamide ester that opens ATP-sensitive potassium ion channels and has a nitric oxide-like action.1 It venodilates and vasodilates, reducing cardiac load and increasing coronary perfusion. First introduced in Japan in 1984, it is a third-line agent used to prevent angina-associated chest pain, as well as an alternative medication for patients intolerant to nitrates and β-blockers.2 Its cardioprotective effects were proven and identified by the Impact of Nicorandil in Angina Study in 2001.3
A year prior to this study, a paper commending the use of nicorandil for angina pectoris sufferers mentioned case reports of mouth ulcers in patients receiving the drug, but that ‘causality had not been conclusively established’.4 Common side effects noted in the BNF include nausea and vomiting, and rectal bleeding and tachycardia at high doses. A less common side effect is oral ulceration, and rare side effects include intestinal, anal and skin ulceration.5 The ulceration attributed to nicorandil use gives a punched out appearance, with ulcers that are large, deep and persistent. Histology samples from previous cases have shown granulation tissue with acute inflammatory changes,6 that is, non-specific inflammation or ulceration.2
Up until 2004, only 49 cases of nicorandil-associated oral ulceration had been reported, 34 of which were in France.6 Nonetheless, cases of aphthous, and anal and perianal ulceration, secondary to nicorandil, were well reported compared to nicorandil-induced ulceration distal to the mucosa, such as penile7 or corneal. In fact, a study conducted in Cumbria found that 4 in every 1000 patients prescribed nicorandil per annum, suffered anal ulceration.8 A literature review on conjunctival and corneal ulceration associated with nicorandil concluded that increasing age and the accumulation of nicotinic acid in tissues made certain individuals susceptible to ocular ulceration.9 Whether this applies to ulceration elsewhere has yet to be explored. There has also been a case of a rectovaginal fistula10 that healed completely within 6 months of nicorandil cessation. However, the association with nicorandil had only been made after the patient had undergone a colostomy that was then complicated by peristomal ulceration.10 11 Moreover, a study of patients with diverticulitis who received nicorandil demonstrated significant complications including: nicorandil-associated perforation, fistulation and abscess formation.12
The pathophysiology of ulcers associated with nicorandil use is unknown, but various theories have been considered. The literature includes a possible hypersensitivity reaction, or an idiosyncratic effect. As aforementioned, abnormal accumulation of nicotinic acid within mucosa may increase the likelihood of ulceration.10 Another theory is the ‘vascular steal phenomenon’:2 since nicorandil redistributes arterial and venous blood flow, some watershed areas, in particular in the colon, may ulcerate in response to a lack of blood supply to those areas. This, however, fails to explain oral and anal mucosal ulceration, as these areas are well perfused.
Since patients who take nicorandil tend to be vasculopaths on anticoagulants and antiplatelet agents for ischaemic heart disease, it is difficult to differentiate whether nicorandil or vascular disease causes the increased risk of bleeding and symptomatic ulceration.6 What is known is that nicorandil-induced colitis is dose-dependent; the higher the dose of nicorandil, the more severe the side effects are likely to be.2 Ulceration has also been reported with doses as low as 10 mg daily.6 The effect of duration of treatment on the extent of associated colitis, if any, is not yet known. The variability may be attributed to worsening cardiac function impairing perfusion precipitating more rapid ulceration.2
In this patient, had the likely association between nicorandil and colitis been recognised, earlier extensive investigations and consideration for colectomy, are likely to have been avoided. Complete healing of ulcers after stopping nicorandil can take between 2 weeks and 10 months. With over 1 000 000 prescriptions of nicorandil per year in the UK, and the numbers increasing, there are likely to be more presentations of these non-conventional ulcers. It is important for clinicians to recognise this condition as part of the differential diagnoses, and understand its reversibility on stopping the offending drug. Drug cessation is the only definitive treatment, and was indeed the only cure for this patient's colitis.
Learning points.
The link between nicorandil use and the development of colitis secondary to intestinal ulceration is becoming increasingly recognised. The report highlights how a lack of awareness of this association can lead to multiple unnecessary, potentially invasive investigations and procedures.
Nicorandil-induced ulceration poses a diagnostic challenge to today's clinicians. However, other causes of ulceration should first be excluded before nicorandil is stopped.
Discussions with the cardiologist should be held in deciding on alternative antiangina treatment if nicorandil is stopped.
Footnotes
Contributors: KS wrote the article and performed the literature search with assistance from LM. SP is the guarantor who retrieved the endoscopy images in the figures and had the original idea for the case report. TP suggested the association of nicorandil with ulceration, which led to colitis in this patient.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Opie LH, Gersh BJ. Drugs for the heart. 8th edn Saunders, 2013. [Google Scholar]
- 2.Lee BC, Allen PB, Mainie I et al. . Nicorandil associated colonic ulceration: case series of an increasingly recognised complication. Dig Dis Sci 2011;56:2404–8. 10.1007/s10620-011-1634-x [DOI] [PubMed] [Google Scholar]
- 3.IONA Study Group. Effect of nicorandil on coronary events in patients with stable angina: the Impact of Nicorandil in Angina (IONA) randomised trial. Lancet 2002;359:1269–75. 10.1016/S0140-6736(02)08265-X [DOI] [PubMed] [Google Scholar]
- 4.Markham A, Plosker GL, Goa KL. Nicorandil. An updated review of its use in ischaemic heart disease with emphasis on its cardioprotective effects. Drugs 2000;60:955–74. 10.2165/00003495-200060040-00007 [DOI] [PubMed] [Google Scholar]
- 5.Joint Formulary Committee. British national formulary. March—September 2013 edn London: BMJ Group and Pharmaceutical Press, 2013. [Google Scholar]
- 6.Healy CM, Smyth Y, Flint SR. Persistent nicorandil induced oral ulceration. Heart 2004;90:e38 10.1136/hrt.2003.031831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birnie A, Dearing N, Littlewood S et al. . Nicorandil-induced ulceration of the penis. Clin Exp Dermatol 2008;33:215–16. 10.1111/j.1365-2230.2007.02650.x [DOI] [PubMed] [Google Scholar]
- 8.Colvin HS, Barakat T, Moussa O et al. . Nicorandil associated anal ulcers: an estimate of incidence. Ann R Coll Surg Engl 2012;94:170–2. 10.1308/003588412X13171221501573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraunfelder FW, Fraunfelder FT. Conjunctival and corneal ulceration associated with nicorandil. Cutan Ocul Toxicol 2014;33:120–1. 10.3109/15569527.2013.811248 [DOI] [PubMed] [Google Scholar]
- 10.Neely DT, Minford EJ. Nicorandil-induced rectovaginal fistula. Am J Obstet Gynecol 2011;204:e5–6. 10.1016/j.ajog.2010.12.035 [DOI] [PubMed] [Google Scholar]
- 11.Kidd L, Dixon C. Nicorandil-induced peristomal ulceration. Int Wound J 2010;7:541 10.1111/j.1742-481X.2010.00720.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Brien A, Young A, Duthie F. Nicorandil is associated with complicated diverticulitis. GUT 2014;63:A123–4. 10.1136/gutjnl-2014-307263.266 [DOI] [Google Scholar]


