Abstract
While dengue virus infection leads to a mild to moderate elevation of liver transaminases in almost all cases, hepatic failure rarely dominates the clinical picture in adults. We present a case of dengue haemorrhagic fever in a young adult, leading to the rare complication of acute liver failure. He was managed with supportive care and discharged after 5 days. At follow-up after 1 week, he had complete recovery and no residual symptoms.
Background
Dengue fever, an arboviral disease that is endemic in Southeast Asia, poses a persistent public health concern and threat in the region. Dengue outbreaks have been reported from almost all states in India, and the disease is endemic in various parts of the country.1 A prospective, observational study conducted at our institution found that dengue fever accounted for 7% of the cases of acute undifferentiated febrile illness in adults.2 The spectrum of dengue infection ranges from mild undifferentiated viral fever to severe dengue (inclusive of dengue shock syndrome and dengue haemorrhagic fever). Liver involvement in dengue can be quite varied, with mild to moderate elevation of serum transaminases seen in up to 97% of cases. Other manifestations such as hypoproteinaemia, hypoalbuminaemia and hyperbilirubinaemia, and deranged coagulation parameters, have also been reported in varying rates depending on the population studied and disease severity.3 4 Occasionally, severe dengue leads to acute hepatic failure; however, the majority of these cases reported are among children. We report an unusual case of a young adult with dengue fever leading to acute liver failure.
Case presentation
A 22-year-old farmer presented with symptoms of high-grade intermittent fever, generalised myalgia and diffuse abdominal pain with non-bilious, non-projectile vomiting for 5 days. He also had symptoms of mucosal bleeding in the form of scanty haemoptysis, haematuria, melena and haematemesis 2 days prior to presentation. There were no significant past medical conditions.
Clinical examination revealed a temperature of 100°F, pulse rate of 96/min, blood pressure 100/60 mm Hg and respiratory rate of 18/min. The patient was noted to have icterus, bilateral subconjunctival haemorrhages (figure 1) and a petechial rash over the trunk. He also had mild gum bleeding, while the rest of the general examination was unremarkable; there was no eschar. Examination of the chest showed a right-sided pleural effusion. Abdominal examination was remarkable for right upper quadrant tenderness, hepatomegaly and minimal free fluid.
Figure 1.
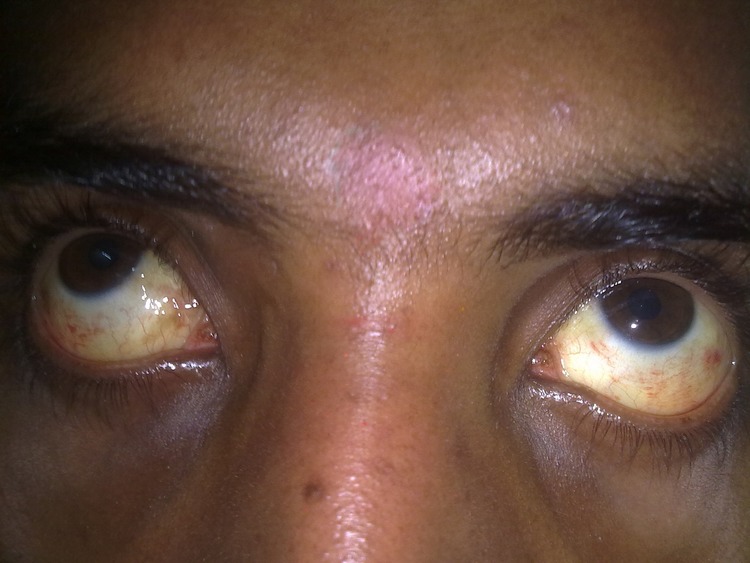
Image showing icterus and bilateral subconjunctival haemorrhages.
The patient's haematological and biochemical investigations at admission, during hospital stay and at follow-up, are listed in table 1. Chest radiograph revealed a small right-sided pleural effusion while ultrasonography of the abdomen was significant for hepatomegaly (liver span 15.1 cm) with minimal ascites.
Table 1:
Laboratory profile of the patient during the course of illness
| Admission | Discharge | Follow-up | |||||
|---|---|---|---|---|---|---|---|
| Day of illness | 6 | 7 | 8 | 9 | 10 | 11 | 18 |
| Haemoglobin (g/dL) | 14.2 | 12.8 | 12.4 | 11.6 | 11.1 | 11.9 | 11.3 |
| Total white cell count (per cu mm) | 5800 | 11 600 | – | – | – | – | 6100 |
| Platelet count (per cu mm) | 8000 | 60 000 | 93 000 | 71 000 | 60 000 | 46 000 | 309 000 |
| Total bilirubin (mg/dL) | 1.5 | 4.8 | 5.19 | 6.6 | 7.4 | 6.2 | 2.6 |
| Direct bilirubin (mg/dL) | 0.6 | 3.0 | 4.1 | 4.6 | 5.6 | 4.0 | 2.4 |
| Total protein (g/dL) | 5.8 | 5.8 | – | 5.2 | 5.9 | – | 8.1 |
| Albumin (g/dL) | 2.9 | 2.7 | – | 2.4 | 2.4 | – | 3.6 |
| Aspartate aminotransferase (U/L) | 1351 | 8110 | 1662 | 2317 | 1535 | 1042 | 186 |
| Alanine aminotransferase (U/L) | 517 | 2496 | 4875 | 1006 | 805 | 720 | 204 |
| Alkaline phosphatase (U/L) | 191 | 165 | – | 148 | 159 | – | 205 |
| Creatinine (mg/dL) | 0.47 | 0.79 | – | – | 0.61 | – | – |
| Lactate dehydrogenase (U/L) | 5095 | 11 320 | – | 2982 | – | – | – |
| Prothrombin time (seconds)/international normalised ratio | 12.5/1.14 | 17.7/1.58 | 16.6/1.48 | 15.6/1.4 | – | 14.3/1.29 | 12.0/1.09 |
| Activated partial thromboplastin time (seconds) | 50.9 | 33.6 | 32.2 | – | – | 27.6 | 27.3 |
| Fibrinogen (mg/dL) | 150.7 | – | – | – | – | – | – |
| Creatine phosphokinase (U/L) | 1556 | 433 | – | – | – | – | – |
On day 2 of hospitalisation, the patient developed deep icterus and worsening of the right upper quadrant abdominal pain with associated lethargy and a disordered sleep pattern. At this stage, a diagnosis of acute liver failure was considered, with aetiological possibilities of ischaemic hepatitis, and drug-induced, viral-induced and alcohol-induced hepatitis.
Investigations
The patient's serology was positive for IgM and IgG antibodies, suggestive of secondary dengue infection.5 Serological tests for leptospirosis, scrub typhus, hepatitis A (IgM), hepatitis B surface antigen, hepatitis C and hepatitis E (IgM) were negative. The patient's liver function tests on the second day of admission showed an unusual increase in transaminases, with a relatively smaller increment in serum bilirubin.
Treatment
The patient was managed conservatively with supportive care. Since he had features of grade I hepatic encephalopathy, he was managed in the ward with oral lactulose and other supportive care. He required transfusion of 3 units of platelet-rich concentrate, 15 mL/kg body weight (total 6 units) of FFP and 30 mL/10 kg body weight (total 5 units) of cryoprecipitate, as he had deranged coagulation parameters with an ongoing haemorrhage. Subsequently, he remained haemodynamically stable and his blood parameters were closely monitored; no further transfusions were deemed necessary.
Outcome and follow-up
The patient made good clinical improvement and was discharged after 5 days of hospital stay. He was in good health at a follow-up visit 1 week later.
Discussion
Although elevation of serum transaminases is invariably seen in patients with dengue, incidence of acute liver failure in adult dengue patients is less than 1%, as indicated by various large studies.6 7 The largest series to date, studying the pattern of liver involvement in 1585 dengue cases, reported no case of acute liver failure or hepatic encephalopathy.8 In the present case, features of hepatic failure, coinciding with the peak of serum transaminases and prothrombin time, developed on day 7 of the illness, which is consistent with the findings in other cases reported in the literature.6 9 10
In dengue-associated hepatic dysfunction, aspartate aminotransferase (AST) levels are more elevated than alanine transaminase (ALT) levels—a pattern that is easily distinguishable from viral hepatitis, where ALT levels are typically higher than or equal to those of AST. The higher AST values are presumably due to release from injured myocytes.6 The pathogenesis of hepatic injury in dengue is poorly elucidated. A direct cytopathic effect has been suggested by the identification of dengue virus within hepatocytes and Kupffer cells of infected individuals.11 An alternate theory postulates an immune-mediated liver cell injury brought about by rapid induction of cross-reactive NS-3 specific memory T-cells during a secondary infection, where Th2 cells release proinflammatory cytokines.12
Management of acute liver failure in severe dengue is similar to hepatic failure from any other cause. Patients require nursing in a quiet environment with head-end elevated and serial monitoring of serum aminotransferases, coagulation parameters, plasma glucose and electrolytes. Periodic surveillance for infection and prompt initiation of antibiotics at any sign of systemic inflammatory response syndrome are crucial. Attention should be paid to maintain adequate hydration and haemodynamic stability. Fresh frozen plasma (FFP) and platelet transfusion is reserved only for active bleeding and invasive procedures.13 N-acetyl cysteine (NAC) therapy, though not routinely indicated in non-acetaminophen related acute liver failure, may benefit patients with other aetiologies.14 A retrospective analysis in dengue-associated liver failure showed survival advantage if NAC therapy was instituted in early (grade I or II coma) liver failure stage.15 In patients with worsening parameters, a prognostic model such as King's College criteria or Model for End-Stage Liver Disease (MELD) score may be used to determine the likelihood of spontaneous recovery and identify patients who will require orthotopic liver transplantation. However, to the best of our knowledge, there are no cases in the current literature of dengue-related acute liver failure managed with liver transplantation.
In contrast to other aetiologies of acute liver failure in adults, case fatality in dengue-related hepatic failure is considerably lower. For instance, eight patients managed with standard medical therapy alone in one series had 100% survival (10). On the contrary, dengue infection in the paediatric population results in a relatively higher rate of acute liver failure16 and up to 50% mortality.16–18
Learning points.
Though uncommon, dengue infection can lead to acute liver failure in a minority of cases.
The treatment is largely supportive.
Footnotes
Contributors: SA and JCP collected the data, drafted and revised the manuscript. SDN and SGH critically reviewed and approved the final version of the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Chaturvedi UC, Nagar R. Dengue and dengue haemorrhagic fever: Indian perspective. J Biosci 2008;33:429–41. 10.1007/s12038-008-0062-3 [DOI] [PubMed] [Google Scholar]
- 2.Chrispal A, Boorugu H, Gopinath KG et al. Acute undifferentiated febrile illness in adult hospitalized patients: the disease spectrum and diagnostic predictors—an experience from a tertiary care hospital in South India. Trop Doct 2010;40:230–4. 10.1258/td.2010.100132 [DOI] [PubMed] [Google Scholar]
- 3.Lee LK, Gan VC, Lee VJ et al. Clinical relevance and discriminatory value of elevated liver aminotransferase levels for dengue severity. PLoS Negl Trop Dis 2012;6:e1676 10.1371/journal.pntd.0001676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samanta J, Sharma V. Dengue and its effects on liver. World J Clin Cases 2015;3:125–31. 10.12998/wjcc.v3.i2.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guzman MG, Halstead SB, Artsob H et al. Dengue: a continuing global threat. Nat Rev Microbiol 2010;8:S7–16. 10.1038/nrmicro2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo CH, Tai DI, Chang-Chien CS et al. Liver biochemical tests and dengue fever. Am J Trop Med Hyg 1992;47:265–70. [DOI] [PubMed] [Google Scholar]
- 7.Trung DT, Thao LTT, Hien TT et al. Liver involvement associated with dengue infection in adults in Vietnam. Am J Trop Med Hyg 2010;83:774–80. 10.4269/ajtmh.2010.10-0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Souza LJ, Alves JG, Nogueira RMR et al. Aminotransferase changes and acute hepatitis in patients with dengue fever: analysis of 1,585 cases. Braz J Infect Dis 2004;8:156–63. 10.1590/S1413-86702004000200006 [DOI] [PubMed] [Google Scholar]
- 9.Soundravally R, Narayanan P, Bhat BV et al. Fulminant hepatic failure in an infant with severe dengue infection. Indian J Pediatr 2010;77:435–7. 10.1007/s12098-010-0027-z [DOI] [PubMed] [Google Scholar]
- 10.Tan S-S, Bujang MA. The clinical features and outcomes of acute liver failure associated with dengue infection in adults: a case series. Braz J Infect Dis Off Publ Braz Soc Infect Dis 2013;17:164–9. 10.1016/j.bjid.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huerre MR, Lan NT, Marianneau P et al. Liver histopathology and biological correlates in five cases of fatal dengue fever in Vietnamese children. Virchows Arch 2001;438:107–15. [DOI] [PubMed] [Google Scholar]
- 12.Kurane I, Innis BL, Nimmannitya S et al. Human immune responses to dengue viruses. Southeast Asian J Trop Med Public Health 1990;21:658–62. [PubMed] [Google Scholar]
- 13. AASLD Position Paper: The Management of Acute Liver Failure: Update 2011—alfenhanced.pdf [Internet]. [cited 2015 Mar 12]. http://aasld.org/sites/default/files/guideline_documents/alfenhanced.pdf.
- 14.Lee WM, Hynan LS, Rossaro L et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology 2009;137:856–64, 864.e1 10.1053/j.gastro.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumarasena RS, Mananjala Senanayake S, Sivaraman K et al. Intravenous N-acetylcysteine in dengue-associated acute liver failure. Hepatol Int 2010;4:533–4. 10.1007/s12072-010-9176-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poovorawan Y, Hutagalung Y, Chongsrisawat V et al. Dengue virus infection: a major cause of acute hepatic failure in Thai children. Ann Trop Paediatr 2006;26:17–23. 10.1179/146532806X90565 [DOI] [PubMed] [Google Scholar]
- 17.Seneviratne SL, Malavige GN, de Silva HJ. Pathogenesis of liver involvement during dengue viral infections. Trans R Soc Trop Med Hyg 2006;100:608–14. 10.1016/j.trstmh.2005.10.007 [DOI] [PubMed] [Google Scholar]
- 18.Shah I. Dengue and liver disease. Scand J Infect Dis 2008;40:993–4. 10.1080/00365540802209027 [DOI] [PubMed] [Google Scholar]


