Abstract
AIM
To identify whether Aspergillus fumigatus (A. fumigatus) hyphae antigens induced the release of interleukin-8 (IL-8) in anti-fungal innate immunity of cultured human corneal epithelial cells (HCECs) and determine the involvement of intracellular signalling pathways.
METHODS
HCECs were treated with A. fumigatus hyphae antigens with different concentrations and time. The cytoplasmic calcium of HCECs were assessed by fluorescence imaging. Western blot was used to detect the expression of Ca2+-dependent protein kinase C (PKC). The IL-8 levels were determined by specific human IL-8 enzyme-linked immunosorbent assay (ELISA) and reverse transcriptase polymerase chain reaction (RT-PCR). Using a series of pharmacological inhibitors, we examined the upstream signalling pathway responsible for IL-8 expression in response to A. fumigatus hyphae antigens.
RESULTS
Cells exposed to A. fumigatus hyphae antigens showed higher level of IL-8 mRNA expression and protein production. We demonstrated here that stimulation of HCECs with A. fumigatus hyphae triggers an intracellular Ca2+ flux and results in the activation of Ca2+-dependent PKC (α, βI and βII) which can be attenuated by pre-treatment of cells with laminarin, suggesting that Dectin-1 signals pathway induced cytoplasmic calcium and influence the activation of PKC in HCECs. Inhibitors of Ca2+-dependent PKC (Ro-31-8220 and Go6976) significantly abolished hyphae-induced expression of IL-8.
CONCLUSION
Our findings suggest that A. fumigatus hyphae-induced IL-8 expression was regulated by the activation of Dectin-1-mediated Ca2+-dependent PKC in HCECs.
Keywords: Dectin-1, Ca2+, protein kinase C, interleukin-8, corneal epithelium, Aspergillus fumigatus
INTRODUCTION
Fungal keratitis (FK) is one of the most serious infectious corneal disease and can more easily lead to blindness than some other corneal diseases. Occurrence of FK is caused by the interaction between fungal inflammatory factors and host defense factors. Innate immune system which participates in the defence against fungal infections through pattern-recognition receptors (PRRs) is the first line to resist the microbial infection. Aspergillus fumigatus (A. fumigatus) is one of the major pathogens of FK[1]. Cell surface receptor Dectin-1 played crucial role in the process of recognition and response to A. fumigatus in human corneal epithelial cells (HCECs)[2]. Dectin-1, a C-type lectin receptor (CLR) which can identify β-glucan in the cell walls of fungi, mediates a variety of fungal innate immune response and triggers signal transduction[3]. This initiates a cascade of inflammatory signal transduction events, including infiltration of inflammatory cells (polymorphonuclear neutrophils and monocytes/macrophages)[4], production of cytokines [interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β)] and chemokine (IL-8)[5],[6], even triggers for the intracellular Ca2+ flux[7]. Ca2+ mobilization is one of the most important signaling events after these antigen receptors engagement[8],[9]. Stimulation of dendritic cells (DCs) with particulate curdlan, which specifically engages Dectin-1, triggers an intracellular Ca2+ flux that can be attenuated by a blocking anti-Dectin-1 antibody, suggesting that Dectin-1 signals induce Ca2+ flux in DCs[7]. However, it is largely unknown that the effects of Dectin-1 have on intracellular Ca2+ flux with the stimulation of fungi in HCECs.
The inflammatory response is necessary for corneal epithelial cells to initiate host defense, kill and clear the invading pathogens, such as fungi[10]. In response to fungal infections, the expression and secretion of cytokines and chemokines, including IL-8 which is a member of the CXC chemokine family have been enhanced in HCECs[11]. IL-8 has been suggested to act as an important mediator in FK, and high level expression of IL-8 has been found in the FK patients[11]. These results indicate that IL-8 plays a major role in the inflammation of corneal epithelium and innate immunity.
Protein kinase C (PKC) is composed of a family of serine-threonine kinases that modulate the function of a variety of signal transduction pathways controlling cell growth, cell differentiation, and wound healing. Some researchers have been reported that PKC participate in mitogen-activated protein kinase (MAPK) cascade activation and cytokine secretion after stimulation in various cell types[12]–[14]. Furthermore, PKC are important signalling intermediates in keratitis[15]. PKC, as a serine/threonine protein kinase, can be classified into three groups based on their regulatory domains: the classical PKC (α, β and γ) which are Ca2+ dependent and activated by diacylgylcerol (DAG), the novel PKC (δ, ε, η, µ and θ) which are Ca2+ independent but regulated by DAG and the atypical PKC (ξ and λ/ι) which are Ca2+ independent and do not require DAG[12],[16]–[18]. Previous studies have shown that the activation of PKC intermediates IL-6, IL-8 and TNF-α production in human bronchial epithelial cells and works as a critical component of Dectin-1 signaling in different types of cells[19]- [21]. But in HCECs, only six different PKC isoforms (PKCα, PKCβI, PKCβII, PKCδ, PKCε, and PKCµ) have been identified[16]. However, little is known about the role of Ca2+-dependent PKC(α, βI, βII) inducing the release and gene expression of IL-8 in the innate immunity of HCECs to A. fumigatus hyphae.
In the present study, we investigated that the participation of Dectin-1 induced the intracellular Ca2+ flux in HCECs and played a crucial role in the activation of the Ca2+-dependent PKC (α, βI, βII) in the innate immune response of HCECs to A. fumigatus hyphae. In addition, our results indicated that Dectin-1-mediated Ca2+-dependent PKC signalling pathways induced the expression and secretion of IL-8 after A. fumigatus stimulation in HCECs.
MATERIALS AND METHODS
Materials and reagents HCECs were given as a gift by Zhongshan university. A. fumigatus strains (NO3.0772) was purchased from China General Microbiological Culture Collection Center; Sabouroud liquid culture was from American Sigma company; Dulbecco modified Eagle medium (DMEM), Ham F-12, fetal bovine serum (FBS), insulin, human epidermal growth factor (EGF) and penicillin and streptomycin were purchased from Solarbio (China); RNAiso Plus was from TaKaRa (Dalian, Liaoning Province, China); PrimeScript RT reagent Kit With gDNA Eraser (Perfect Real Time) was from TaKaRa; Primers and SYBR Premix Ex TaqTM (Tli RNaseH Plus) were from TaKaRa; The Dectin-1 inhibitor-Laminarin was purchased from Sigma. Ro-31-8220 (a pan inhibitor of PKC), Gottlerin6976 (Go6976) (a specific inhibitor of PKCα/β) and calphostin C (a specific inhibitor of PKC-γ isoform) were purchased from Calbiochem (San Diego, CA, USA). Antibodies used for confocal microscope were from AAT Bioquest. Antibodies used for Western blot were as follows: from Abcam: anti-PKCα, anti-phospho-PKCα; from Santa Cruz: anti-PKCβI and anti-PKCβII, anti-phospho-PKCβI and anti-phospho-PKCβII.
The preparation of A. fumigatus suspension conidia of A. fumigatus were inoculated in Sabouroud liquid culture at 37°C and 200 rpm for 2-3d. The mycelia recovered by grinded were washed twice by sterile phosphate buffer saline (PBS) and sterilized by 70% ethanol at 4 °C for 12h. Inactive A. fumigatus mycelia was washed three times and added in PBS. The mycelia suspensions were quantified using a haemacytometer, and stored at -20°C[22],[23].
Cell Culture
HCECs were cultured and maintained in TCECs growth medium [1:1 DMEM/Hams F12 supplemented with 5% FBS, 5 mg/mL insulin, 10 ng/mL human EGF and 50 mg/mL penicillin and streptomycin in a humidified 5% CO2 incubator at 37°C][24]. The medium was replaced every 2d before experiments. THCE cell suspensions of 1×105/mL were seeded in 12 or 6-well tissue culture plates and when ninety percent of the cells were attaehed, the medium was replaced.
Human Corneal Epithelial Cell Stimulation Assay
HCECs untreated were set as controls, anothers were added with A. fumigatus hyphea (5×106/mL). Or HCECs were treated with 0.3 mg/mL laminarin or the different concentrations Ca2+-dependent PKC inhibitors for 30min prior to A. fumigatus hyphae antigens stimulation in order to block Dectin-1 and Ca2+-dependent PKC. After 30min or 8h incubation, HCECs were harvested to detect the protein and mRNA expression by western blot and reverse transcriptase polymerase chain reaction (RT-PCR).
Calcium Imaging
For analysis of cytoplasmic calcium, HCECs which seeded on the glass-bottom culture dishes (NEST) were labeled with Fluo-3 AM (5µmol/L; AAT Bioquest) for 60min. To block Dectin-1, Fluo-3-loaded HCECs were preincubated with laminarin for 30min at room temperature. After resting for 30min, cells were stimulated with A. fumigatus hyphea (5×106/mL) and cytoplasmic calcium was monitored on a Leica TCS SPE confocal microscope in real time for 6-8min. The images were acquired using Leica LAS software before and after A. fumigatus hyphea were added for each condition. The fluoresecence intensity were measured using Image J software.
Western Blot
Cells were lysed in RIPA buffer for 1h, and then were centrifuged. After estimation of protein content, addition of sodium dodecyl sulfonate (SDS) sample buffer, and boiling, total protein was separated on 10% acrylamide sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene difluoride membrane. The membranes were blocked with 5% bovine serum albumin (BSA) liquid, and then were incubated with a monoclonal antibody to human β-actin, and a monoclonal antibody to human Primary antibody at 4°C overnight. After washed in PBST for three times, the membranes were incubated with corresponding peroxidase-conjugated secondary antibodies at 37°C for 1h. Then the blots were developed using chemiluminescence (ECL; Thermo Scientific).
Real-time Polymerase Chain Reaction
RNAiso plus reagent were used to extracte total RNA from samples according to the manufacturer's protocol, and the RNA was quantified by sprctrophotometry. The first strand cDNA was synthesized by RT from total RNA. The real-time PCR was performed in a Mx3005PTM system (Stratagene) with 20 µL reaction volume containing 2 µL of cDNA. cDNA was amplified by PCR using primers. β-actin was used as the endogenous control. The thermocycler parameters were 95°C for 30s, followed by 40 cycles of 95°C for 5s and 60°C for 30s. A melting curve was used to confirm the specificity of the PCR products following each reaction. The ΔΔCT method was used for quantization of target gene products of stimulated and unstimulated group. Data are expressed as fold of increase in mRNA expression. Each experiment was performed in triplicate. The double-stranded probes used are as follow: the following primers were used TTTCAGAGACAGCAGAGCACACAA (forward) and CACACAGAGCTGCAGAAATCAGG (reverse) for IL-8 (human); TGGCACCCAGCACAATGAA (forward) and CTAAGTCATAGTCCGCCTAGAAGCA (reverse) for β-actin (human) as housekeeping gene.
Enzyme-linked Immunosorbent Assay
Double-sandwich enzyme-linked immunosorbent assay (ELISA) for human IL-6 and IL-8 was performed, according to the manufacturer's protocol, to determine the concentration of IL-6 and IL-8 protein in conditioned media and culture cell lysates from different treatments. Absorbance was read at 450 nm with a reference wavelength of 570 nm by a VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Statistical Analysis
All data were presented as mean±SD from independent experiments. The data were analyzed using SPSS19.0 statistical package. One-way ANOVA test was used to make comparison among three or more groups, and least-significant difference (LSD) was used to identify between each two groups. P<0.05 was considered to be statistically significant.
RESULTS
IL-8 Production Induced by A. fumigatus Hyphae in a Time- and Dose-dependent Manner
To investigate A. fumigatus hyphae-induced IL-8 protein secretion and mRNA expression, hyphae-treated cells and supernatants were analyzed by RT-PCR and ELISA. Cells exposed to A. fumigatus hyphae (5×105, 5×106 and 5×107/mL) followed by incubation secreted IL-8, which was assessed at multiple time points (2, 4, 8 and 16h) (Figure 1A). The level of IL-8 mRNA expression was elevated and peaked at 8h, and then returned to decrease after hyphae stimulation (5×106/mL) significantly. The maximal protein production was recorded at 24h (Figure 1B). The data demonstrates that IL-8 protein secretion and mRNA expression were induced by hyphae in HCECs in a time- and dose-dependent manner.
Figure 1. The effect of hyphae on IL-8 production and mRNA expression.
A: The RNAs from cells treated with different concentrations of A. fumigatus hyphae (5×105, 5×106 and 5×107/mL) were harvested and used for RT-PCR; B: Cells were stimulated with A. fumigatus hyphae at 37°C. The supernatants were collected and assayed for IL-8 by ELISA. The data represented the mean±SEM of three separate experiments. aP<0.05 versus control alone; bP<0.01 versus control alone.
Cytoplasmic Calcium with the Stimulation of A. fumigatus hyphae Induced by Dectin-1 Engagement in HCECs
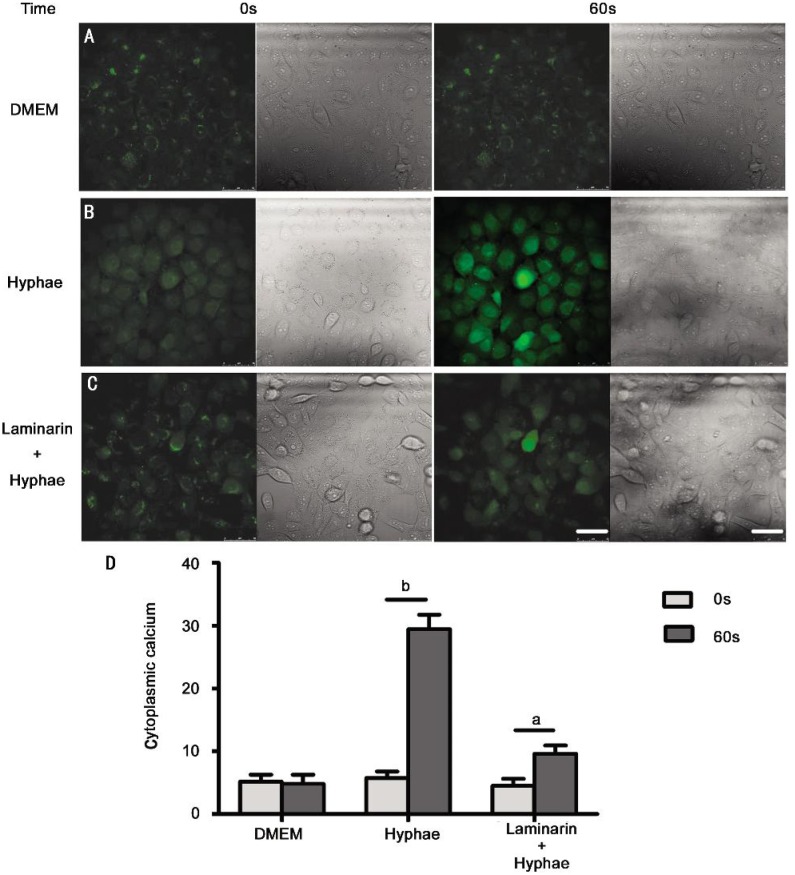
It has been proved that Dectin-1 expressed in HCECs, and A. fumigatus hyphae stimulation elevated the expression of Dectin-1[25]. Since Ca2+ mobilization is one of the most important signaling events in various types of cells, we first investigated if the stimulation of A. fumigatus hyphae could induce Ca2+ flux in HCECs. The stimulation of HCECs by hyphae, which consist of β-glucan structure, led to an induction of intracellular Ca2+ flux in these cells, even the image of Ca2+ flux elicited by hyphae stimulation is transient (Figure 2B). But someone might argue that the nature of the Ca2+ flux is not elicited by the engagement of Dectin-1 stimulation with A. fumigatus hyphae, because the Ca2+ flux could be elicited by engagement of other pattern recognition receptors (PRRs) such as toll-like receptor 2 (TLR2) and TLR4[26],[27]. To confirm whether the engagement of Dectin-1 could induce Ca2+ flux in HCECs, we preincubated the cells with laminarin, a soluble β-glucan that could effectively inhibit the binding of fungi to Dectin-1[28], prior to stimulating them with A. fumigatus hyphae. Laminarin could dampen the Ca2+ flux elicited by hyphae treatment in HCECs, implying that stimulation through Dectin-1 could indeed trigger Ca2+ signaling in these cells (Figure 2C).
Figure 2. Engagement of Dectin-1 elicits cytoplasmic calcium in HCECs. HCECs were loaded with Fluo-3 and stimulated with 5×106/mL A. fumigatus hyphae.
A: DMEM; B: 5×106/mL A. fumigatus hyphae; C: 5×106/mL A. fumigatus hyphae in the presence of 0.3 mg/mL laminarin. Confocal image of HCECs showed cytoplasmic calcium expression (green stain). D: The corresponding results of fluoresecence intensity were measured by Image J software. Magnifications 400×. Calibration bar is 50 µm. aP<0.05 versus control alone; bP<0.01 versus control alone.
Ca2+-dependent PKC Activated by the Engagement of Dectin-1
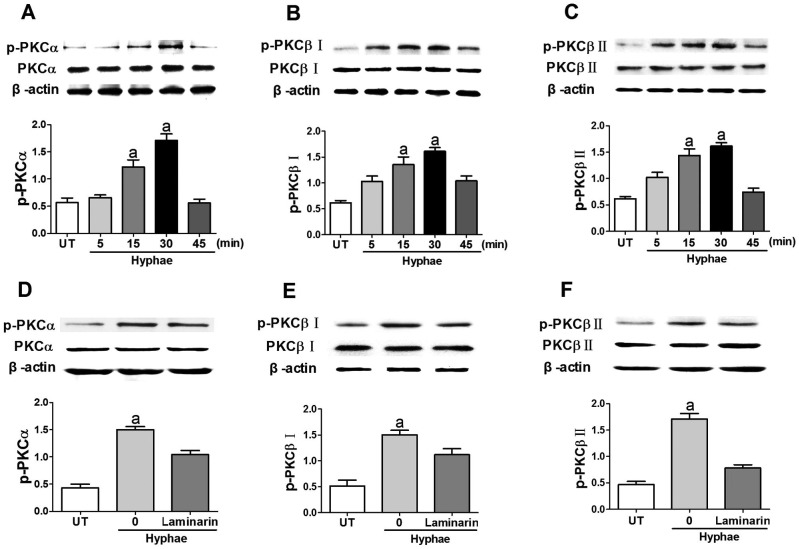
It has been reported that expression of Ca2+-dependent PKC (α, βI and βII) are identified in HCECs[16]. But we wondered whether the engagement of Dectin-1 could increase the activition of Ca2+-dependent PKC in HCECs. To address this question, we stimulated HCECs with A. fumigatus hyphae, and the data showed that HCECs express Ca2+-dependent PKC, include PKCα, PKCβI and PKCβII. We found that the stimulation of HCECs with hyphae led to the activation of PKCα, PKCβI and PKCβII as indicated by their phosphorylation status. The phosphorylation of them was significantly increased after A. fumigatus hyphae infection with a time-dependent manner, and peaked at 30min (Figure 3A). In order to prove that Dectin-1 take part in the activation of these enzymes by treating HCECs with hyphae, we preincubated HCECs with the Dectin-1 inhibitor laminarin before the stimulation and data showed that this prior treatment could decrease the expression of p-PKCα, p-PKCβI and p-PKCβII (Figure 3B). Thus, the data suggested that the engagement of Dectin-1 could activate Ca2+-dependent PKC with the stimulaton of hyphae in HCECs.
Figure 3. Engagement of Dectin-1 activates Ca2+-dependent PKC with stimulation of A. fumigates hyphae in HCECs.
A, B and C: HCECs were treated with 5×106/mL A. fumigatus hyphae for various times. The activation status of PKCα, PKCβI and PKCβII were determined by Western blot (WB), respectively. D, E and F: HCECs were preincubated for 30min with Dectin-1 inhibitor laminarin, followed by treatment with hyphae for 30min. The activation of PKCα, PKCβI and PKCβII was assayed by WB. Data shown were representative of more than three independent sets of experiments. aP<0.05.
Important Role of Ca2+-dependent PKC in the Production of IL-8
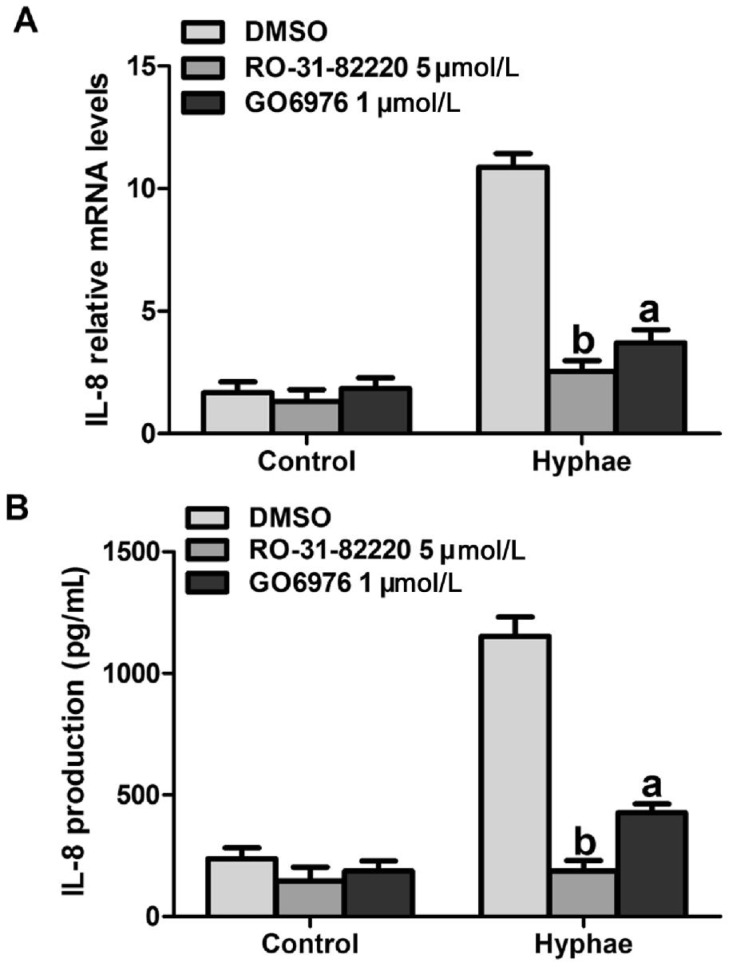
It has been shown that PKC plays a key role in the regulation of IL-8 production in lung fibroblasts[29]. To examine the impact of Ca2+-dependent PKC in regulating hyphae-induced IL-8 production, HCECs were pre-treated with pan-PKC inhibitor Ro-31-8220 (5 µmol/L) and the specific PKC-α/β inhibitor Go6976 (1µmol/L) for 30min, and then stimulated with hyphae to evaluate their contribution to specific PKC isoform on IL-8 production. Following pre-treatment with Ro-31-8220 and Go6976, IL-8 production was significantly decreased (Figure 4A, 4B). These results suggested that Ca2+-dependent PKC isoforms could induce IL-8 production with the stimulation of A. fumigatus hyphae.
Figure 4. The effect of Ca2+-dependent PKC inhibitors on A. fumigatus hyphae induce IL-8 production. Cells were pre-treated with indicated concentrations of DMSO, Ro-31-8220 and Go6976 for 30min prior to stimulation with 5×106/mL A. fumigatus hyphae.
A: The RNAs from cells treated with A. fumigatus hyphae were harvested and used for RT-PCR. B: The supernatants were collected and evaluated for IL-8 presence. The data represented the mean±SEM of three separate experiments. aP<0.05 versus hyphae alone; bP<0.01 versus hyphae alone.
DISCUSSION
IL-8 is a potent proinflammatory cytokine that plays a major role in infective corneal diseases such as FK. Patients with FK tend to have higher expression of IL-8[30]. Moreover, various allergens such as cockroach, fungus, mite and bacterium can stimulate HCECs to produce IL-8, following a series of intracellular signalling pathways. Cytosolic Ca2+ release induced by chitinase and β-glucan in the cell walls of fungi led to the up-regulation of IL-8 through the series of PAR-2, phospholipase C/inositol 1,4,5-trisphosphate (IP3) pathway in human airway epithelial cells[31]. In this study, we investigated the effect of A. fumigates hyphae exposure on HCECs, defined a role for activation of Dectin-1-mediated Ca2+-dependent PKC as well as confirmed higher IL-8 production.
A. fumigatus hyphae elevated both mRNA expression and protein secretion of IL-8 (Figure 1A, 1B). Our earlier study suggested that A. fumigates hyphae could induce the engagement of Dectin-1 in human corneal epithelial cells[25], and expression of IL-8 could be elevated via Dectin-1 signalling which can identify β-glucan in the cell walls of fungi and mediate a variety of fungal innate immune response and trigger signal transduction[3],[30]. Another interesting finding that arises from the current study is the discovery that stimulation of HCECs with A. fumigatus hyphae triggers an intracellular Ca2+ flux that can be attenuated by pre-treatment of cells with the Dectin-1-inhibitor laminarin, suggesting that Dectin-1 signals induce intracellular Ca2+ flux in HCECs. It has been reported that innate PRRs such as Dectin-1 and TLR2 could elicit intracellular Ca2+ flux in DCs[7]. Our data indicate that intracellular Ca2+ mobilization, as a mechanism of cellular signaling in the immune system, is not confined to lymphocytes, DCs and their antigen receptors. However, we thought that only chemical inhibitor experiment is not enough to explain the detailed mechanism of A. fumigates hyphae triggered intracellular Ca2+ flux. It is possible that A. fumigates hyphae triggering of Dectin-1 and other PRRs (TLR2 or TLR4) together contribute to a more synergistic activation of Ca2+ flux in HCECs. Thus, we are planning further investigation of the related mechanism of cellular signaling contribute to the induction of Ca2+ flux in HCECs through additional experiments.
PKC which participate in MAPK cascade activation and IL-8 production after stimulation are vital signalling intermediates in various cell types[12]–[14],[19]. We prefered to study on the Ca2+-dependent PKC(α, βI, βII) from the three categories based on above conclusion. Our studies have shown that A. fumigatus hyphae exposure can result in the activation of Ca2+-dependent PKC (Figure 3A, 3B, 3C). Treatment with laminarin results in the expression of Ca2+-dependent PKC decrease as does exposure with A. fumigatus hyphae (Figure 3D, 3E, 3F). It showed that A. fumigatus hyphae-induced activation of Ca2+-dependent PKC was regulated by the Dectin-1 pathway in HCECs.
Using pharmacological inhibitors against PKC, we demonstrated that activation of Dectin-1-mediated Ca2+-dependent PKC (α, βI, βII) induced IL-8 production after A. fumigatus hyphae challenged HCECs (Figure 4A, 4B). It is well known that the participation of PKC after stimulation is an important signalling intermediate in infective corneal diseases like FK. Many studies have demonstrated that PKC are potential candidates for the upstream activators of MAPK/extracellular signal-regulated kinase (MEK) and ERK[13],[14],[31]–[33]. But the entire related role of PKC in the inflammatory response is little known. Thus, we are intending to investigate the downstream mechanism of PKC signalling pathways in follow-up experiments.
In conclusion, we have demonstrated that in HCECs A. fumigatus hyphae-induced IL-8 production is regulated by Dectin-1-mediated Ca2+-dependent PKC signalling pathways in most extent. Further, we showed that A. fumigatus hyphae could play as a potent trigger in signal transmission for the production of proinflammatory chemokine IL-8 in HCECs.
Acknowledgments
Foundation: Supported by National Natural Science Foundation of China (No. 81470609; No. 81170825); the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20123706110003); the Key Project of Natural Science Foundation of Shandong Province (No. ZR2012FZ001); the Youth Project of Natural Science Foundation of Shandong Province (No. ZR2013HQ007).
Conflicts of Interest: Peng XD, None; Zhao GQ, None; Lin J, None; Jiang N, None; Xu Q, None; Zhu CC, None; Qu JQ, None; Cong L, None; Li H, None.
REFERENCES
- 1.Leal SM, Jr, Cowden S, Hsia YC, Ghannoum MA, Momany M, Pearlman E. Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS Pathog. 2010;6:e1000976. doi: 10.1371/journal.ppat.1000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Che CY, Jia WY, Xu Q, Li N, Hu LT, Jiang N, Lin J, Wang Q, Zhao GQ. The roles of surfactant protein D during Aspergillus fumigatus infection in human corneal epithelial cells. Int J Ophthalmol. 2012;5(1):13–17. doi: 10.3980/j.issn.2222-3959.2012.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu S, Huo J, Gunawan M, Su IH, Lam KP. Activated dectin-1 localizes to lipid raft microdomains for signaling and activation of phagocytosis and cytokine production in dendritic cells. J Biol Chem. 2009;284(33):22005–22011. doi: 10.1074/jbc.M109.009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClellan SA, Huang X, Barrett RP, van Rooijen N, Hazlett LD. Macrophages restrict pseudomonas aeruginosa growth, regulate polymorphonuclear neutrophil influx, and balance pro- and anti-inflammatory cytokines in BALB/c Mice. J Immunol. 2003;170(10):5219–5227. doi: 10.4049/jimmunol.170.10.5219. [DOI] [PubMed] [Google Scholar]
- 5.Hazlett LD. Corneal response to pseudomonas aeruginosa infection. Prog Retin Eye Res. 2004;23(1):1–30. doi: 10.1016/j.preteyeres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Kernacki KA, Goebel DJ, Poosch MS, Hazlett LD. Early cytokine and chemokine gene expression during pseudomonas aeruginosa corneal infection in mice. Infect Immun. 1998;66(1):376–379. doi: 10.1128/iai.66.1.376-379.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu S, Huo J, Lee KG, Kurosaki T, Lam KP. Phospholipase Cgamma2 is critical for Dectin-1-mediated Ca2+ flux and cytokine production in dendritic cells. J Biol Chem. 2009;284(11):7038–7046. doi: 10.1074/jbc.M806650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cambier JC, Pleiman CM, Clark MR. Signal transduction by the B cell antigen receptor and its coreceptors. Annu Rev Immunol. 1994;12:457–486. doi: 10.1146/annurev.iy.12.040194.002325. [DOI] [PubMed] [Google Scholar]
- 9.Weiss A, Irving BA, Tan LK, Koretzky GA. Signal transduction by the T cell antigen receptor. Semin Immunol. 1991;3(5):313–324. [PubMed] [Google Scholar]
- 10.Sun M, Zhu M, Chen K, Nie X, Deng Q, Hazlett LD, Wu Y, Li M, Wu M, Huang X. TREM-2 promotes host resistance against pseudomonas aeruginosa infection by suppressing corneal inflammation via a PI3K/Akt signaling pathway. Invest Ophthalmol Vis Sci. 2013;54(5):3451–3462. doi: 10.1167/iovs.12-10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Wu J, Xin Z, Wu X. Aspergillus fumigatus triggers innate immune response via NOD1 signaling in human corneal epithelial cells. Exp Eye Res. 2014;127:170–178. doi: 10.1016/j.exer.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Dempsey EC, Cool CD, Littler CM. Lung disease and PKCs. Pharmacol Res. 2007;55(6):545–559. doi: 10.1016/j.phrs.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann M, Zaper J, Bernd A, Bereiter-Hahn J, Kaufmann R, Kippenberger S. Mechanical pressure-induced phosphorylation of p38 mitogen-activated protein kinase in epithelial cells via src and protein kinase C. Biochem Biophys Res Commun. 2004;316(3):673–679. doi: 10.1016/j.bbrc.2004.02.101. [DOI] [PubMed] [Google Scholar]
- 14.Hewson CA, Edbrooke MR, Johnston SL. PMA induces the MUC5AC respiratory mucin in human bronchial epithelial cells, via PKC, EGF/TGF-alpha, Ras/Raf, MEK, ERK and Sp1-dependent mechanisms. J Mol Biol. 2004;344(3):683–695. doi: 10.1016/j.jmb.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 15.Griffith GL, Russell RA, Kasus-Jacobi A, Thavathiru E, Gonzalez ML, Logan S, Pereira HA. CAP37 activation of PKC promotes human corneal epithelial cell chemotaxis. Invest Ophthalmol Vis Sci. 2013;54(10):6712–6723. doi: 10.1167/iovs.13-12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang F, Wen Q, Mergler S, Yang H, Wang Z, Bildin VN, Reinach PS. PKC isoform-specific enhancement of capacitative calcium entry in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2006;47(9):3989–4000. doi: 10.1167/iovs.06-0253. [DOI] [PubMed] [Google Scholar]
- 17.Liu WS, Heckman CA. The sevenfold way of PKC regulation. Cell Signal. 1998;10(8):529–542. doi: 10.1016/s0898-6568(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 18.Lin WN, Luo SF, Lin CC, Hsiao LD, Yang CM. Differential involvement of PKC-dependent mapks activation in lipopolysaccharide-induced ap-1 expression in human tracheal smooth muscle cells. Cell Signal. 2009;21(9):1385–1395. doi: 10.1016/j.cellsig.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Kim H, Zamel R, Bai XH, Liu M. PKC activation induces inflammatory response and cell death in human bronchial epithelial cells. PLoS One. 2013;8(5):e64182. doi: 10.1371/journal.pone.0064182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elsori DH, Yakubenko VP, Roome T, Thiagarajan PS, Bhattacharjee A, Yadav SP, Cathcart MK. Protein kinase Cdelta is a critical component of dectin-1 signaling in primary human monocytes. J Leukoc Biol. 2011;90(3):599–611. doi: 10.1189/jlb.0610376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strasser D, Neumann K, Bergmann H, Marakalala MJ, Guler R, Rojowska A, Hopfner KP, Brombacher F, Urlaub H, Baier G, Brown GD, Leitges M, Ruland J. Syk kinase-coupled C-type lectin receptors engage protein kinase C-sigma to elicit Card9 adaptor-mediated innate immunity. Immunity. 2012;36(1):32–42. doi: 10.1016/j.immuni.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jie Z, Wu XY, Yu FS. Activation of Toll-like receptors 2 and 4 in Aspergillus fumigatus keratitis. Innate Immun. 2009;15(3):155–168. doi: 10.1177/1753425908101521. [DOI] [PubMed] [Google Scholar]
- 23.Meier A, Kirschning CJ, Nikolaus T, Wagner H, Heesemann J, Ebel F. Toll-like receptor (TLR) 2 and TLR4 are essential for aspergillus-induced activation of murine macrophages. Cell Microbiol. 2003;5(8):561–570. doi: 10.1046/j.1462-5822.2003.00301.x. [DOI] [PubMed] [Google Scholar]
- 24.Wen L, Zhu M, Madigan MC, You J, King NJ, Billson FA, McClellan K, Sutton G, Petsoglou C. Immunomodulatory effects of bone marrow-derived mesenchymal stem cells on pro-inflammatory cytokine-stimulated human corneal epithelial cells. PLoS One. 2014;9(7):e101841. doi: 10.1371/journal.pone.0101841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Zhao GQ, Che CY, Li N, Lin J, Xu Q, Wang Q, Liu Y, Qiu S. Expression of Dectin-1 during fungus infection in human corneal epithelial cells. Int J Ophthalmol. 2014;7(1):34–37. doi: 10.3980/j.issn.2222-3959.2014.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by Dectin-1 and toll-like receptor 2. J Exp Med. 2003;197(9):1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197(9):1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413(6851):36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 29.Ludwicka-Bradley A, Tourkina E, Suzuki S, Tyson E, Bonner M, Fenton JW, 2nd, Hoffman S, Silver RM. Thrombin upregulates interleukin-8 in lung fibroblasts via cleavage of proteolytically activated receptor-i and protein kinase c-gamma activation. Am J Respir Cell Mol Biol. 2000;22(2):235–243. doi: 10.1165/ajrcmb.22.2.3642. [DOI] [PubMed] [Google Scholar]
- 30.Karthikeyan RS, Leal SM, Jr, Prajna NV, Dharmalingam K, Geiser DM, Pearlman E, Lalitha P. Expression of innate and adaptive immune mediators in human corneal tissue infected with Aspergillus or fusarium. J Infect Dis. 2011;204(6):942–950. doi: 10.1093/infdis/jir426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong JY, Lee KE, Kim KW, Sohn MH, Kim KE. Chitinase induce the release of IL-8 in human airway epithelial cells, via Ca2+-dependent PKC and ERK pathways. Scand J Immunol. 2010;72(1):15–21. doi: 10.1111/j.1365-3083.2010.02404.x. [DOI] [PubMed] [Google Scholar]
- 32.Cohen-Kedar S, Baram L, Elad H, Brazowski E, Guzner-Gur H, Dotan I. Human intestinal epithelial cells respond to beta-glucans via Dectin-1 and Syk. Eur J Immunol. 2014;44(12):3729–3740. doi: 10.1002/eji.201444876. [DOI] [PubMed] [Google Scholar]
- 33.Lee JS, Kim IS, Ryu JS, Yun CY. House dust mite, Dermatophagoides pteronissinus increases expression of MCP-1, IL-6, and IL-8 in human monocytic THP-1 cells. Cytokine. 2008;42(3):365–371. doi: 10.1016/j.cyto.2008.03.010. [DOI] [PubMed] [Google Scholar]