Abstract
AIM
To investigate the expression and role of nuclear factor κB (NF-κB) in diabetic retinopathy (DR) and its relationship with neovascularization and retinal cell apoptosis.
METHODS
A total of 80 male Wistar rats were randomly assigned to control (4, 8, 12 and 16wk, n=10 in each group) and diabetes mellitus (DM) groups (4, 8, 12 and 16wk, n=10 in each group). A diabetic rat model was established by intraperitoneal injection of streptozotocin (60 mg/kg). After 4, 8, 12 and 16wk, rats were sacrificed. Retinal layers and retinal neovascularization growth were stained with hematoxylin-eosin and examined under light microscopy. Cell apoptosis in the retina was detected by TdT-mediated dUTP nick end labeling, and NF-κB distribution and expression in the retina was determined using immunohistochemistry.
RESULTS
DM model success rate up to 100%. Diabetes model at each time point after the experimental groupcompared with the control group, the blood glucose was significantly increased, decreased body weight, each time point showed significant differences compared with the control group (P<0.01). After 12wk other pathological changes in the retina of diabetic rats were observed; after 16wk, neovascularization were observed. After 1mo, retinal cell apoptosis was observed. Compared with the control group, NF-κB expression in the DM group significantly increased with disease duration.
CONCLUSION
With the prolonging of DM progression, the expression NF-κB increases. NF-κB may be related to retinal cell apoptosis and neovascularization.
Keywords: nuclear factor κB, retinal neovascularization, cell apoptosis, diabetic retinopathy
INTRODUCTION
Diabetic retinopathy (DR) is a common, serious microvascular complication of diabetes mellitus (DM) and is a major blindness-causing oculopathy[1]. Because the pathogenesis of DR is very complex and has not been thoroughly clarified, it is important to study DR. Cytokines play a key role in the occurrence and development of DR. Nuclear factor κB (NF-κB), an important polyphenic nuclear factor, is present in many cell types and participates in cell apoptosis and neovascularization. A large number of studies have shown that NF-κB is closely related to inflammation, tumor occurrence, cell apoptosis and other pathological processes. Major pathological changes in DR include retinal cell apoptosis and neovascularization[2]. It has been inferred that NF-κB plays a role in DR. This study explored NF-κB expression in the retina of a diabetic rat model and its relationship with retinal cell apoptosis and neovascularization.
MATERIALS AND METHODS
Animal Model
A total of 80 male Wistar rats specific pathogen free (SPF) grade, body weight: 250-280 g were provided by the Animal Laboratory at Shengjing Hospital of China Medical University. The study was carried out with approval from the Animal Experiment Committee of the China Institutes for Biological Sciences. Rats were randomly assigned to control (4, 8, 12 and 16wk, n=10 in each group) and DM groups (4, 8, 12 and 16wk, n=10 in each group). After a 12h fast, rats in the DM group were intraperitoneally injected with 1% streptozotocin (STZ, Sigma, USA) at 60 mg/kg, and rats in the control group were injected with an equivalent amount of citrate buffer solution. At 72h after intraperitoneal injection, blood glucose was measured via the caudal vein using a blood glucose tester (Breeze, USA) and urine glucose was measured with a urine glucose test strip. Urine glucose (+++) and random blood glucose >16.7 mmol/L indicated successful establishment of the diabetic rat model. Urine glucose was measured weekly, blood glucose was measured monthly, and body weight was recorded monthly.
Histological Treatment
At 4, 8, 12 and 16wk, rats were sacrificed by intraperitoneal injection of excessive 10% chloral hydrate (>0.3 mL/100 g). Rat eyeballs were removed rapidly, fully fixed by intravitreal injection of 4% paraformaldehyde (PFA) solution, cut into pathological sections, and stained with hematoxylin-eosin (HE). Retinal cell apoptosis was analyzed by immunohistochemistry.
HE Staining
Circular shearing parallel to the direction of the corneal limbus 0.5 mm behind the corneal limbus was performed to gently remove the lens and vitreum. The cupula oculi was fixed again, dehydrated, embedded with soft and hard paraffin, and successively cut into 4.0 µm sections. The sections were conventionally stained with HE, dehydrated, vitrified, mounted, observed and imaged under a light microscope.
Detection of Retinal Cell Apoptosis by TUNEL
Paraffin sections were dewaxed and dehydrated. Detection was performed using a cell apoptosis test kit (POD, Roche, Switzerland). Sections were treated with proteinase K and 50 µL TdT-mediated dUTP nick end labeling (TUNEL) reaction solution added dropwise and incubated in a wet box at 37°C. After 1h, sections were labeled with POD, developed with 3,3N-diaminobenzidine (DAB), counterstained with hematoxylin, dehydrated, vitrified, mounted, observed and imaged under a light microscope.
Immunohistochemistry
Paraffin sections were dewaxed with dimethyl benzene, hydrated with ethanol at a gradient, and rinsed with PBS for 3×5min. A total of 30 mmol/L H2O2 was added dropwise and sections were blocked at room temperature for 10min to interrupt endogenous peroxidase activity. Sections were subsequently treated with antigen retrieval solution for 10min for microwave heat retrieval, rinsed with PBS, treated with 1:100 diluted NF-κB primary antibody (Abcam, USA), incubated at 37°C for 30min, and preserved overnight at 4°C. Sections were then rinsed with PBS for 3×3min, treated with 1:200 diluted goat anti-mouse IgG secondary antibody, and incubated at 37°C for 20min. They were washed with PBS, treated with SABC working solution, incubated at 37°C for 30min, washed again with PBS, developed with DAB, washed with tap water, counterstained with hematoxylin, dehydrated with alcohol, vitrified with dimethyl benzene, and mounted with neutral gum. Nuclei with NF-κB positive expression were stained pale brown, while those with NF-κB negative expression were not stained. Observation and imaging were performed under a light microscope: 5 sequential fields were selected for each section and the percentage of NF-κB-positive cells was calculated (i.e. the number of positive cells/the total number of cells).
Statistical Analysis
All quantitative data are presented as the mean±standard deviation (x±s). Data were analyzed with SPSS 19.0 statistical software. Pair-wise comparison was performed using the t-test. P≤0.05 indicated statistically significant differences.
RESULTS
Diabetes Mellitus Rat Model of Diabetes
There were no significant differences in body weight and blood glucose between rats in the control and DM groups at baseline (P>0.05, Table 1). At 72h after STZ intraperitoneal injection, urine glucose was tested as +++ and blood glucose was significantly increased to >16.7 mmol/L, suggesting a DM model success rate of up to 100%. In the control group, rats had no changes in blood and urine glucose and a stably increasing trend in body weight during breeding. After successful establishment of the diabetic rat model, blood glucose in the DM group was significantly increased at various time points compared with controls (t=23.58, 25.94, 28.43, 20.96, P<0.01), while body weight decreased significantly (t=13.25, 17.74, 26.36, 14.12, P<0.01).
Table 1. Body weight and blood glucose at different time points.
| Course of disease (wk) | Body weight (g) |
Blood glucose (mmol/L) |
||
| Control group | DM group | Control group | DM group | |
| 0 | 269.23±8.06 | 269.53±8.43 | 8.81±1.29 | 8.89±1.25 |
| 4 | 297.95±8.43 | 253.60±12.37 | 8.96±0.94 | 26.26±3.14 |
| 8 | 388.25±8.88 | 294.45±21.92 | 8.685±1.012 | 26.79±2.95 |
| 12 | 420.15±12.60 | 281.75±19.81 | 8.94±1.11 | 28.12±2.80 |
| 16 | 456.20±14.44 | 323.85±39.37 | 9.01±1.43 | 26.95±3.55 |
DM: Diabetes mellitus.
HE Staining of Retinal Tissues
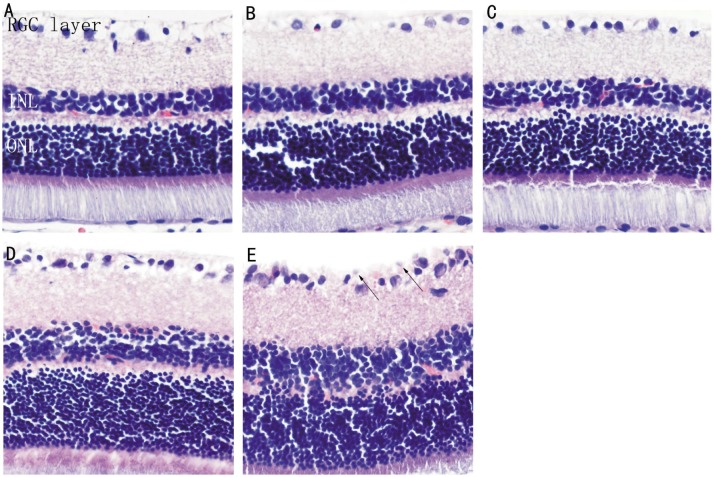
In the control group, the retina had a smooth surface, and retinal ganglion cells (RGCs), cells at the inner and outer nuclear layers, cone cells and rod cells were all regularly arranged. In the DM group, the rats had no significant morphological changes at 4 and 8wk compared with the control group. At ≥12wk, RGCs were irregularly arranged. At 16wk, the cell gap was further widened, cells were irregularly arranged, the inner limiting membrane was slightly swollen and had a non-smooth surface, the RGC layer was edematus, and capillary endothelial cells were observed to protrude through the inner limiting membrane (Figure 1).
Figure 1. Histopathological examination of the retina (HE×400).
A: Normal retina in the control group; B: DM 4wk; C: DM 8wk; D: DM 12wk; E: DM 16wk. Arrows show vascular endothelial cell nuclei protruding through the inner limiting membrane.
Retinal Cell Apoptosis
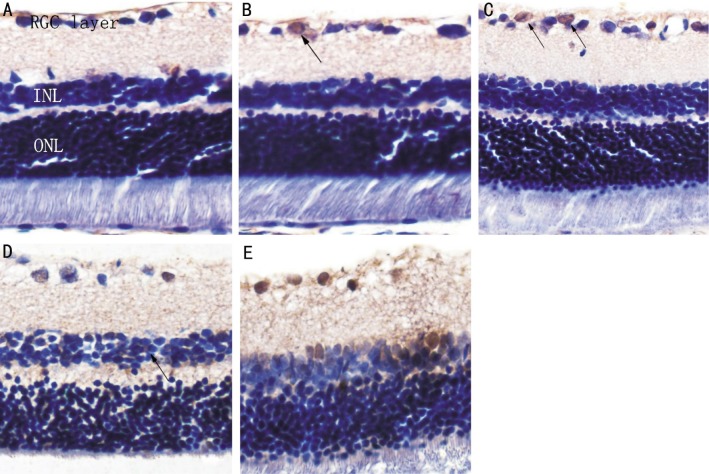
Apoptotic positive cells had nuclei and cytoplasm that were stained brown or pale brown. No apoptotic cells were found in the normal rat retina. In the DM group, several TUNEL-positive cells were located at the RGC layer, and no obviously apoptotic cells were observed in the inner and outer nuclear layers at 4wk. At 8wk, the number of positive cells was increased, and at ≥12wk, positive staining was found in the inner nuclear layer. At 16wk, the number of positive cells had increased and apoptosis of vascular endothelial cells and perithelial cells was observed (Figure 2).
Figure 2. TUNEL staining shows apoptotic cells in the retina of rats (colored brown indicated by arrows).
A: Control group; B: DM 4wk; C: DM 8wk; D: DM 12wk; E: DM 16wk (× 400).
Immunohistochemical Results
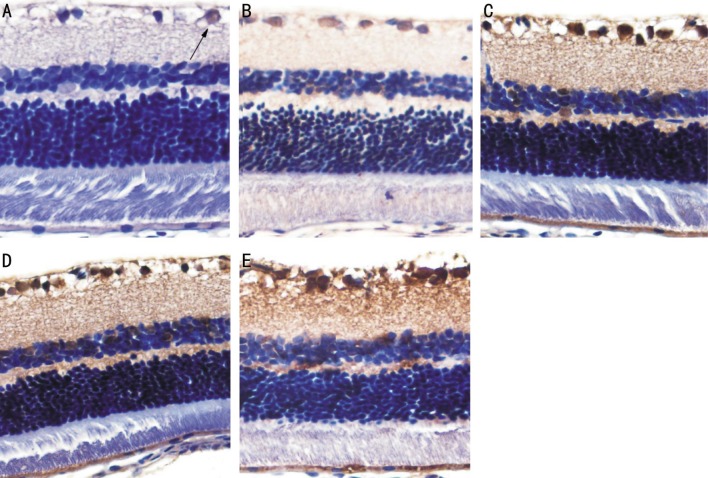
In the control group, cells with NF-κB positive expression were found in the RGC layer with the cytoplasm stained pale brown and nuclei stained blue. At ≥4wk, NF-κB was found in the RGC layer, and cells with NF-κB positive expression were observed in the inner nuclear layer with nuclei stained pale brown. The number of NF-κB positive cells increased gradually over time. In the control group, there was no significant difference in the percentage of NF-κB positive cells at different time points (P>0.05). Compared with the control group, the percentage of NF-κB positive cells in the retina in the DM group significantly increased at various time points (P<0.05; Figure 3, Table 2).
Figure 3. Staining in the retinal cell layer in control and diabetic retinas for expression of NF-κB.
A: Control group; B: DM 4wk; C: DM 8wk; D: DM 12wk; E: DM 16wk (×400).
Table 2. Percentage of NF-κB positive cells in the control and DM groups at different time points.
| Course of disease (wk) | Control group | DM group |
| 0 | 1.12±0.36 | 1.89±0.28 |
| 4 | 1.62±0.65 | 20.39±7.24 |
| 8 | 2.43±0.51 | 27.84±8.79 |
| 12 | 3.53±0.48 | 34.12±9.37 |
| 16 | 3.91±0.70 | 38.66±6.54 |
DM: Diabetes mellitus.
DISCUSSION
DR is a microvascular complication of DM and can cause blindness in severe cases[3]. Research on the roles of cytokines in DR development can better clarify DR pathogenesis from a molecular biology perspective and provide new ideas for DR prevention and treatment.
NF-κB is a protein molecule with a polyphenic regulation effect that is widely present in cells. NF-κB is present in nearly all cell types and has wide biological activity, including promotion of gene transcription of several cytokines[4],[5].
Based on immunohistochemical results, low levels of NF-κB were expressed only in the cytoplasm of the RGC layer in the control group. In the DM group, the nuclei were stained pale brown at various time points, indicating that NF-κB was mainly expressed in the nuclei, with expression extending to inner and outer nuclear RGC layers. Several studies[6],[7] have suggested that NF-κB is present in the cytoplasm at a resting state and that a NF-κB dimer binds with the inhibitory protein of NF-κB (IκB) to form a trimer, which is in a non-active state. When cells are stimulated by hypoxia, hyperglycemia and some inflammatory cytokines, the specific inhibitory protein of NF-κB (IκB-α) is phosphorylated and rapidly degraded to release NF-κB, resulting in NF-κB activation, which enters the nucleus to regulate gene transcription. This is consistent with the immunohistochemical findings presented in this study.
According to immunohistochemical results, NF-κB expression gradually increased with duration of disease. In addition to the abovementioned stimulation factors that cause NF-κB activation, Medeiros et al[8] reported that advanced glycation end products (AGE) could result in continuous NF-κB activation by binding with its receptor RAGE in a hyperglycemic environment. Other studies[9],[10] have confirmed that NF-κB is associated with hyperglycemia.
In this experiment, HE staining of retinal tissues indicated that the vascular structure of the retina in the DM group underwent a significant change at 12wk and at 16wk that vascular endothelial cells protruding through the inner limiting membrane were observed. Immunohistochemical results showed that NF-κB was activated and entered the nucleus at 4wk. NF-κB may become activated prior to pathological changes in retinal vessels, or ultrastructural changes in retinal vessels could occur after NF-κB activation. This is consistent with results from brain tissues obtained by Yoritsune et al[11].
Vascular endothelial growth factor (VEGF) is a cytokine that promotes vascularization and is closely correlated with late pathological changes in DR, particularly neovascularization[12]. In late stage DR, ischemia and hypoxia become increasingly serious, and various vascular growth factors are successively activated and highly expressed, leading to neovascularization and fibrous tissue proliferation[13]. Correlation between continuous NF-κB activation and increased VEGF expression has been studied. Lukiw et al[14] found that VEGF expression was activated in a hypoxic state and that activated NF-κB up-regulated VEGF mRNA expression via COX-2 to promote neovascularization. The underlying mechanism may be positive regulation of VEGF mRNA and protein expression by NF-κB, which strengthens the binding capacity of NF-κB and joint contributions to neovascularization[15].
Current studies have shown that cell apoptosis plays an important role in the occurrence and development of DR. In STZ rats, RGC apoptosis occurs at 4wk[16], followed by apoptosis of photoreceptor and nerve cells[17]. A recent study revealed that NF-κB in perithelial cells is activated in a hyperglycemic environment, causing excessive expression of pro-apoptosis factor Bax, which results in perithelial cell apoptosis; this process can be interrupted by NF-κB inhibitors[18]. The theory that an inflammatory reaction is involved in the pathological process of DR has been recognized by scholars. The release of various inflammatory factors, such as IL-1 and TNF-α, can activate NF-κB, and activated NF-κB can promote expression of these inflammatory factors to form a vicious cycle that ultimately causes retinal injury[19],[20].
The present study confirms that NF-κB plays an important role in the occurrence and developmental processes of DR. Research on the relationship between NF-κB and DR is relatively new, and further exploration will be needed. Studying the relationship between NF-κB and DR and use of NF-κB inhibitors can provide new strategies for DR prevention and treatment.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81371045); Shenyang Science and Technology Plan Project (No.F12-193-9-49)
Conflicts of Interest: Jiang N, None; Chen XL, None; Yang HW, None; Ma YR, None.
REFERENCES
- 1.Agarwal A, Soliman MK, Sepah YJ, Do DV, Nguyen QD. Diabetic retinopathy: variations in patient therapeutic outcomes and pharmacogenomics. Pharmgenomics Pers Med. 2014;7:399–409. doi: 10.2147/PGPM.S52821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30(5):343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sasongko MB, Wong TY, Nguyen TT, Shaw JE, Jenkins AJ, Wang JJ. Novel versus traditional risk markers for diabetic retinopathy. Diabetologia. 2012;55(3):666–670. doi: 10.1007/s00125-011-2424-x. [DOI] [PubMed] [Google Scholar]
- 4.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12(8):695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 5.Shin HC, Seo J, Kang BW, Moon JH, Chae YS, Lee SJ, Lee YJ, Han S, Seo SK, Kim JG, Sohn SK, Park TI. Clinical significance of nuclear factor κB and chemokine receptor CXCR4 expression in patients with diffuse large B-cell lymphoma who received rituximab-based therapy. Korean J Intern Med. 2014;29(6):785–792. doi: 10.3904/kjim.2014.29.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salles A, Romano A, Freudenthal R. Synaptic NF-kappa B pathway in neuronal plasticity and memory. J Physiol Paris. 2014;108(4–6):256–262. doi: 10.1016/j.jphysparis.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Sung YH, Shin MS, Ko IG, Kim SE, Kim CJ, Ahn HJ, Yoon HS, Lee BJ. Ulinastatin suppresses lipopolysaccharide-induced prostaglandin E2 synthesis and nitric oxide produc-tion through the downregulation of nuclear factor-κB in BV2 mouse microglial cells. Int J Mol Med. 2013;31(5):1030–1036. doi: 10.3892/ijmm.2013.1322. [DOI] [PubMed] [Google Scholar]
- 8.Medeiros MC, Frasnelli SC, Bastos Ade S, Orrico SR, Rossa C., Jr Modulation of cell proliferation, survival and gene expression by RAGE and TLR signaling in cells of the innate and adaptive immune response: role of p38 MAPK and NF-κB. J Appl Oral Sci. 2014;22(3):185–193. doi: 10.1590/1678-775720130593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madhyastha R, Madhyastha H, Pengjam Y, Nakajima Y, Omura S, Maruyama M. NF-kappaB activation is essential for miR-21 induction by TGFβ1 in high glucose conditions. Biochem Biophys Res Commun. 2014;451(4):615–621. doi: 10.1016/j.bbrc.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 10.Liu P, Li F, Qiu M, He L. Expression and cellular distribution of TLR4, MyD88, and NF-κB in diabetic renal tubulointerstitial fibrosis, in vitro and in vivo. Diabetes Res Clin Pract. 2014;105(2):206–216. doi: 10.1016/j.diabres.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 11.Yoritsune E, Furuse M, Kuwabara H, Miyata T, Nonoguchi N, Kawabata S, Hayasaki H, Kuroiwa T, Ono K, Shibayama Y, Miyatake S. Inflammation as well as angiogenesis may participate in the pathophysiology of brain radiation necrosis. J Radiat Res. 2014;55(4):803–811. doi: 10.1093/jrr/rru017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stagos D, Apostolou A, Poulios E, Kermeliotou E, Mpatzilioti A, Kreatsouli K, Koulocheri SD, Haroutounian SA, Kouretas D. Antiangiogenic potential of grape stem extract through inhibition of vascular endothelial growth factor expression. J Physiol Pharmacol. 2014;65(6):843–852. [PubMed] [Google Scholar]
- 13.Agarwal P, Jindal A, Saini VK, Jindal S. Advances in diabetic retinopathy. Indian J Endocrinol Metab. 2014;18(6):772–777. doi: 10.4103/2230-8210.140225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukiw WJ, Ottlecz A, Lambrou G, Grueninger M, Finley J, Thompson HW, Bazan NG. Coordinate activation of HIF-1 and NF-kappaB DNA binding and COX-2 and VEGF expression in retinal cells by hypoxia. Invest Ophthalmol Vis Sci. 2003;44(10):4163–4170. doi: 10.1167/iovs.02-0655. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Lu Y, Ma L, Cao X, Xiao J, Chen J, Jiao S, Gao Y, Liu C, Duan Z, Li D, He Y, Wei B, Wang H. Activation of vascular endothelial growth factor receptor-3 in macrophages restrains TLR4-NF-κB signaling and protects against endotoxin shock. Immunity. 2014;40(4):501–514. doi: 10.1016/j.immuni.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Tatsumi Y, Kanamori A, Nagai-Kusuhara A, Nakanishi Y, Agarwal N, Negi A, Nakamura M. Nipradilol protects rat retinal ganglion cells from apoptosis induced by serum deprivation in vitro and by diabetes in vivo. Curr Eye Res. 2008;33(8):683–692. doi: 10.1080/02713680802323157. [DOI] [PubMed] [Google Scholar]
- 17.Kohzaki K, Vingrys AJ, Bui BV. Early inner retina dysfunction in streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 2008;49(8):3595–3604. doi: 10.1167/iovs.08-1679. [DOI] [PubMed] [Google Scholar]
- 18.Kumar N, Gangappa D, Gupta G, Karnati R. Chebulagic acid from Terminalia chebula causes G1 arrest, inhibits NFκB and induces apoptosis in retinoblastoma cells. BMC Complement Altern Med. 2014;14:319. doi: 10.1186/1472-6882-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajab HA, Baker NL, Hunt KJ, Klein R, Cleary PA, Lachin J, Virella G, Lopes-Virella MF, DCCT/EDIC Group of Investigators The predictive role of markers of Inflammation and endothelial dysfunction on the course ofdiabetic retinopathy in type 1 diabetes. J Diabetes Complications. 2015;29(1):108–114. doi: 10.1016/j.jdiacomp.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei W, Yuan YH, Gao YN, Yan WF, Li CJ, Zhang DM, Chen NH. Polygalasaponin F inhibits secretion of inflammatory cytokines via NF-κB pathway regulation. J Asian Nat Prod Res. 2014;16(8):865–875. doi: 10.1080/10286020.2014.918962. [DOI] [PubMed] [Google Scholar]