Abstract
AIM
To determine whether red blood cell (RBC) membrane and plasma lipids, particularly long-chain polyunsaturated fatty acids such as eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), arachidonic acid (AA) are significantly correlated with severity of normal tension glaucoma (NTG).
METHODS
This study included 35 patients with NTG and 12 healthy normal control subjects, matched for age and sex with the study group. The stage of glaucoma was determined according to the Hodapp-Parrish-Anderson classification. Lipids were extracted from RBC membranes and plasma, and fatty acid methyl esters prepared and analyzed by gas chromatography-mass spectrometry (GC-MS).
RESULTS
When RBC lipids were analyzed, the levels of EPA, the levels of DHA and the ratio of n3 to n6 were positively associated with the Humphrey Perimetry mean deviation (MD) score (r=0.617, P<0.001; r=0.727, P<0.001 and r=0.720, P<0.001, respectively), while the level of AA was negatively associated with the MD score (r=-0.427, P=0.001). When plasma lipids were analyzed, there was a significant positive relationship between the levels of EPA and the MD score (r=0.648, P<0.001), and the levels of AA were inversely correlated with the MD score (r=-0.638, P<0.001).
CONCLUSION
The levels of n3 and n6 polyunsaturated fatty acids in RBC membrane and plasma lipids were associated with severity of NTG.
Keywords: normal tension glaucoma, polyunsaturated fatty acid, erythrocytes, plasma
INTRODUCTION
The pathogenesis of glaucoma, which is the second leading cause of blindness and visual field loss, is usually associated with elevated intraocular pressure (IOP). Primary open angle glaucoma (POAG) is the most common form of glaucoma among the Caucasians. It was once believed that POAG was rare among Chinese people. However, recent surveys reported POAG affects 0.71%-2.1% of Chinese people and the increase of POAG prevalence in China is expected in the future[1],[2]. Patients with statistically normal IOP who develop the characteristic changes such as progressive retinal ganglion cell loss and defects in the retinal nerve fiber layer (RNFL) are grouped as normal tension glaucoma (NTG), an important subset of POAG. Vascular dysregulation is thought to be a principal risk factor for NTG[3]. Even newly diagnosed NTG patients showed signs of subclinical vascular abnormalities at both macro- and micro-vascular levels, indicating the need to consider multi-level circulation-related pathologies in the development and progression of this type of glaucoma[4].
The n3 and n6 polyunsaturated fatty acids (PUFAs), including linoleic acid (LA, 18:2n6), α-linolenic acid (ALA, 18:3n3), arachidonic acid (AA, 20:4n6), docosahexaenoic acid (DHA, 22:6n3), and eicosapentanoic acid (EPA, 20:5n3) are essential nutrients, which cannot be synthesized de novo by mammalians and have to be ingested in the diet. AA is only considered a conditionally essential fatty acid as it can be synthesized in the body as long as LA is present. The n3 PUFAs, especially DHA and EPA, have been shown to have neuroprotective actions, and play a role in the pathogenesis of some degenerative retinal diseases and neurodegerative disease[5],[6]. Evidence has been accumulating that the changes in eyes with POAG are associated with the levels of PUFA. Ren et al[7] reported reduction of levels of n3 PUFAs in the erythrocyte membranes of patients with POAG, which may partly explain their increased rigidity and aggregability. This reasoning was further supported by a study in which the stage of POAG patients was determined according to the Hodapp and Parrish classification and lipids were extracted from red blood cell (RBC) membranes. Reduced levels of choline plasmalogens carrying PUFAs have been displayed in POAG patients. These differences were greater as the severity of the disease increased. Furthermore, the levels of phosphatidyl-choline carrying DHA were linearly correlated to visual field loss[8].
Given the alteration of the levels of PUFAs in POAG patients described above, we hypothesized that NTG, as a subset of POAG, may elicit similar changes in the lipid profile of RBC membrane. PUFAs and particularly those from the n3 family, such as DHA, have been confirmed to enhance cell membrane flexibility owing to their multiple double bonds[9]. Hence, reduced levels of PUFAs result in vascular disorders and/or altered hemorheology, which may occur in NTG patients. In this study, we examined fatty acid composition in phospholipids of both plasma and RBC membrane in NTG patients. We also measured the levels of plasma fatty acids on account of a brief report from Japan indicating that no significant association was found in the levels of serum free fatty acids between NTG patients and controls. However, only the newly diagnosed NTG patients were recruited in their study[10]. A causal relationship need to be determined in the present study including NTG patients with mild, moderate and severe glaucoma.
SUBJECTS AND METHODS
Normal Tension Glaucoma Subject Selection
NTG patients (n=35) and control subjects (n=12) were enrolled in the study at the Department of Ophthalmology of the Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital. None of the subjects were blood related. We excluded the patients with the history of documented dyslipidemia and/or medication treatment affecting lipid metabolism. We did not exclude the patients who had undergone cataract. The study followed the tenets of the Declaration of Helsinki, and it was approved by the ethics committee of the Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital, Chengdu City, Sichuan Province, China. Informed consent was obtained from the subjects after explanation. All participants in this study underwent an ophthalmologic examination including visual acuity assessment, refractometry, measurement of central corneal thickness, slit-lamp-assisted biomicroscopy of the anterior and posterior segment of the eye, applanation tonometry, gonioscopy, photography of the optic nerve head and fundus, measurement of the RNFL thickness by optical coherence tomography (Optovue, Inc., Fremont, CA, USA) and a systematic body examination. Perimetry was performed with Humphery field analysis using the 30-2 SITA standard strategy (Carl-Zeiss Meditec, Dublin, CA, USA). The reliability was assessed by fixation losses, false-positive results, and false negative results (below 20%).
The diagnosis of NTG was based on criteria as previously proposed[11]. Briefly, NTG patients presented with open angle glaucoma, but they never have a recorded IOP higher than 21 mm Hg in their history. All criteria were confirmed by at least a second visual field examination. Angle closure glaucoma and secondary glaucoma were excluded. The glaucoma stages were defined according to the classification of Hoddap, Parrish, and Anderson (H-P-A)[12].
Control subjects were age- and gender-matched (Table 1) and recruited among patients who came to the clinic for annual ophthalmic check-up and were volunteers to participate in the study. They had an IOP lower than 21 mm Hg, no evidence of glaucoma on optic disc examination (vertical cup-disc ratio of less than 0.6, symmetry disc cupping less than 0.2, normal neuroretinal rim followed by the Inferior-Superior-Nasal-Temporal rule without notching and undermining, and normal blood vessels on disc without bayoneting), no evidence of RNFL damage and a normal SITA visual field (glaucoma hemifield test within normal limits).
Table 1. Summary of clinical data.
| Parameters | Control | Normal tension glaucoma |
||
| Early | Moderate | Severe | ||
| M/F (n) | 5/7 | 7/5 | 6/9 | 4/4 |
| Total (n) | 12 | 12 | 15 | 8 |
| Mean age (a) | 61±8.6 | 59±9.5 | 58±9.0 | 63±10.5 |
| MD in worse eye (dB) | -0.85±0.46 | -3.74±1.33 | -9.12±1.34 | -18.90±5.22 |
MD: Mean deviation. No statistical difference was found among groups for gender (P=0.78) or age (P=0.76).
x±s
Blood samples were collected in an ethylene diamine tetraacetic acid (EDTA) tube from the forearm vein of all participants after overnight fasting. Plasma and erythrocytes were separated by centrifugation at 3000 rpm for 10min at 4°C. RBC membranes were obtained by hemolysis with distilled water and centrifugation and were washed with an isotonic saline solution. Subsequent to separation, plasma and RBC membranes were stored at -80°C for a maximum of 1wk before the lipid analyses.
Fatty Acid Extraction
For plasma, total lipids were extracted following the method of Bligh and Dyer[13]. The plasma were extracted in chloroform:methanol:water (1:1:1) and the chloroform phase collected. The remaining aqueous phase was extracted once again with chloroform, keeping the chloroform:methanol:water ratios at 1:1:1. As before, the chloroform phase was collected and combined with the chloroform phase from the initial extraction. The combined chloroform phases were then extracted with chloroform:methanol:water (3:48:47) and the aqueous phase discarded. The remaining purified lipid extract was stored under nitrogen. For RBC membrane, total lipids were extracted in chiloroform-methanol solution (2:1) according to the method of Folch et al[14]. Proteins in the homogenate were pelleted by centrifugation at 1000×g for 10min and the lipid extract was removed. The pellet was washed twice with 1 mL of chloroform:methanol (1:1) and the washes were combined with the lipid extract. The lipid extract was then washed with 0.2 volumes of 1 mmol/L DTPA (aq) followed by 0.2 volumes of chloroform:methanol:water (3:48:47), each time discarding the aqueous phase. The resulting purified lipid extract was dried under nitrogen and re-suspended in a known volume of toluene. Purified lipid extracts from plasma and RBC membrane were resolved into individual lipid classes using one-dimensional thin-layer chromatography. To the purified lipid extracts, 50 nmol of 15:0, 17:0, 23:0 each were added as internal standards. One milliliter of 16.6% concentrated HCl in methanol was then added and the tubes were sealed under nitrogen with Teflon-lined caps and heated at 100°C overnight. The tubes were cooled on ice and fatty acid methyl esters (FAMEs) were extracted and processed as previously reported[15].
Fatty Acid Analysis by Gas Chromatography-mass Spectrometry and Gas Chromatography-Flame Ionization Detector
FAMEs were identified using an Agilent Technologies 7890A gas chromatograph (GC) with a 5975C inert XL mass spectrometer (MS) detector (Agilent Technologies; Santa Clara, CA, USA). The GC-MS was operated in the electron impact total-ion and single-ion monitoring (SIM) modes. The injection volume was 1 µL and the inlet, held at 280°C, was set to pulsed splitless mode. An Agilent Technologies DB-23 column (60 m×0.32 mm×0.25 µm) was used with a helium carrier gas flow rate of 1.9 mL/min. The oven temperature began at 130°C for 0.96min, was ramped to 170°C at 6.8°C /min, and then ramped to 215°C at 2.9°C /min. After holding at 215°C for 11.4min, the oven was ramped to 230°C at 42°C /min and held for 9.6min. The oven was then ramped to 290°C at 10°C /min and held for 14.4min. The MS transfer line, ion source, and quadrupole temperatures were 290°C, 230°C, and 150°C, respectively. The PUFAs were identified by using the m/z ratios 79.1, 108.1, and 150.1 in SIM mode and the full scan mass spectra in total-ion mode.
FAMEs were quantified using an Agilent Technologies 6890N GC with flame ionization detector (FID). Sample concentrations were determined by comparison to internal standards 15:0, 17:0, 23:0. The injection volume was 1 µL and the inlet, held at 280°C, was set to pulsed split mode (10:1 ratio). An Agilent Technologies DB-23 column (60 m×0.32 mm×0.25 µm) was used with a hydrogen carrier gas constant pressure of 13.1 psi. The oven temperature began at 130°C for 0.8min, was ramped to 170°C at 8.2°C /min then ramped to 215°C at 3.5°C /min. After holding at 215°C for 9.5min, the oven was ramped to 230°C at 50°C /min and held for 8min. The oven was then ramped to 290°C at 12.0°C /min and held for 12min. The FID was held at 290°C.
Statistical Analysis
Statistical analyses were done using StatSoft Inc., Statistica 2000 and GraphPad Prism 5 software. A Kruskal-Wallis test with a Dunn multiple comparison test was used to compare the data from the different groups. All relative mol%s of n3 and n6 fatty acid are from the total lipids extracted from sample homogenates equivalent to 2.0 mg of protein and were converted to FAMEs and quantified using GC-FID. Pearson correlation coefficients were calculated to determine associations between the MD score and the levels of EPA, DHA, AA and the ratio of n3 to n6. Results are expressed as the mean±SD. Statistically significant differences are indicated as (a) for P<0.05, (b) for P<0.01, and (c) for P<0.001.
RESULTS
Clinical Characteristics of Normal Tension Glaucoma Patients and Control Subjects
The clinical characteristics of the NTG patients and control subjects are shown in Table 1. No significant difference was found among the four groups for gender (P=0.78, Chi-square test) or age (P=0.76, Kruskal-Wallis test). Visual field defects are classified by H-P-A classification system that considers two criteria: the overall extent of damage using both MD value and the number of defective points in the Humphrey Statpac-2 pattern deviation probability map of the 24-2, SITA-Standard test.
n3 and n6 Polyunsaturated Fatty Acids Levels of Red Blood Cell Membrane and Plasma
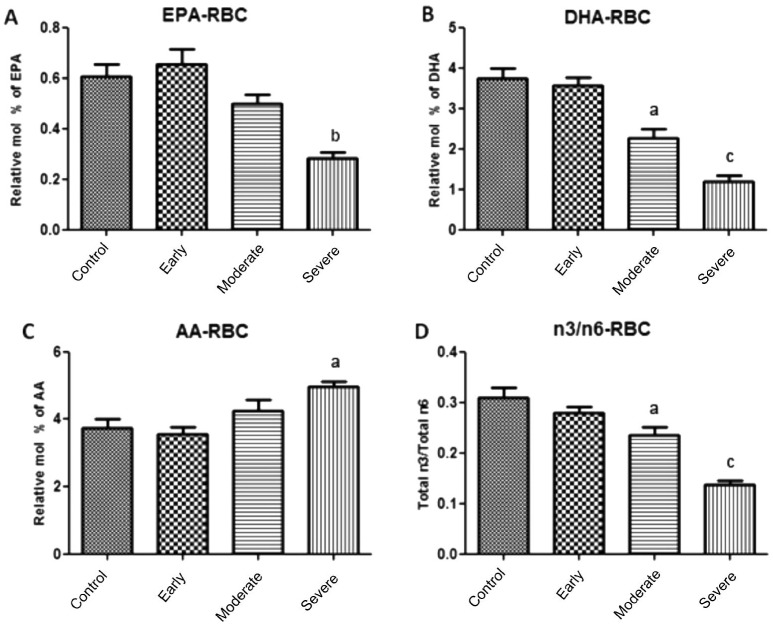
In the present study, we were trying to understand possible physiological role of EPA, DHA, AA and the ratio of n3 to n6 PUFAs in RBC membrane and plasma which may lead to the onset of optic nerve atrophy in NTG patients. As shown in Figure 1 (also see Table 2), the ratio of total n3 to total n6 in RBC membrane of moderate and severe NTG patients was significantly lower than that of control group (P<0.05 and P<0.001, respectively). In RBC membrane of severe group, the levels of both EPA and DHA were also significantly lower than that of control group (P<0.01 and P<0.001, respectively). However, higher level of AA was observed in the severe group (P<0.05). There was no significant different at the levels of n3 or n6 PUFAs between early and control group in RBC membrane.
Figure 1. Association of fatty acid levels in RBC membrane and NTG severity.
A: Lower level of EPA was observed in severe NTG patients; B: Lower level of DHA was observed in moderate and severe groups; C: Higher level of AA was observed in severe NTG group; D: The ratios of n3 to n6 in moderate and severe groups were significantly lower than that of control group. aP<0.05, bP<0.01, cP<0.001.
Table 2. n3 and n6 PUFA composition of RBC membrane.
| Fatty acids | Control (n=12) | Normal tension glaucoma |
||
| Early (n=12) | Moderate (n=15) | Severe (n=8) | ||
| 18:2n6 (linoleic acid) | 18.14±0.62 | 21.15±1.45 | 22.15±2.73 | 20.69±3.11 |
| 18:3n6 (γ-linolenic acid) | 0.11±0.07 | 0.16±0.03 | 0.15±0.02 | 0.13±0.04 |
| 18:3n3 (α-linolenic acid) | 1.45±0.49 | 2.21±0.83 | 2.23±0.70 | 1.44±0.34 |
| 20:4n3 (eicosatetraenoic acid) | 1.49±0.86 | 0.93±0.37 | 0.98±0.26 | 0.68±0.12 |
| 20:5n3 (eicosapentanoic acid) | 0.61±0.17 | 0.65±0.21 | 0.50±0.14 | 0.28±0.08 |
| 20:2n6 (eicosadienoic acid) | 1.44±0.14 | 1.27±0.39 | 1.34±0.38 | 1.18±0.24 |
| 20:3n6 (eicosatrienoic acid) | 1.52±0.74 | 2.22±0.33 | 2.57±0.68 | 2.23±0.72 |
| 20:4n6 (arachidonic acid) | 3.75±0.87 | 3.56±0.77 | 4.26±1.17 | 4.96±0.42 |
| 22:4n6 (docosatetraenoic acid ) | 0.32±0.02 | 0.34±0.16 | 0.37±0.17 | 0.46±0.10 |
| 22:5n6 (docosapentaenoic acid) | 0.24±0.16 | 0.31±0.08 | 0.39±0.11 | 0.27±0.09 |
| 22:5n3 (docosapentaenoic acid) | 1.09±0.31 | 1.05±0.83 | 1.28±0.75 | 0.83±0.22 |
| 22:6n3 (docosahexaenoic acid) | 3.10±0.94 | 3.22±0.86 | 2.26±0.93 | 1.20±0.40 |
| n3/n6 ratio | 0.31±0.06 | 0.28±0.04 | 0.23±0.06 | 0.14±0.02 |
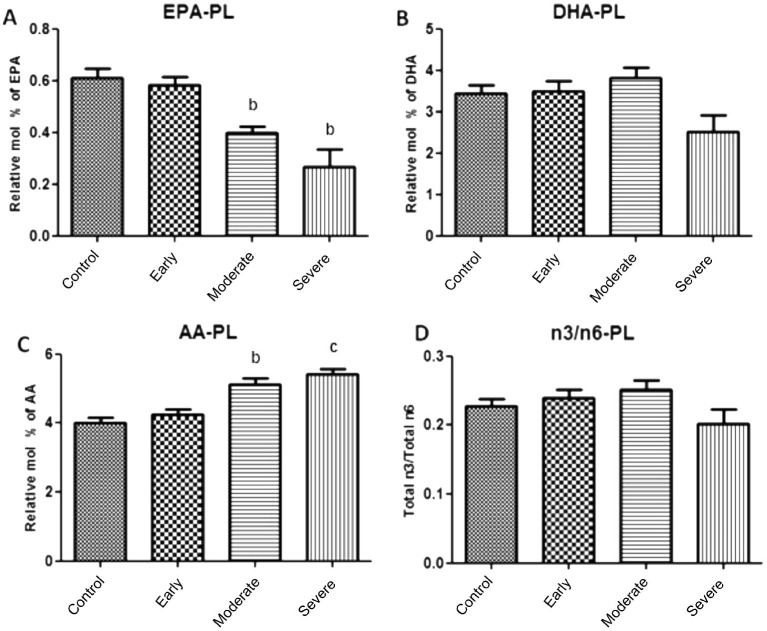
Figure 2 demonstrated the effects of NTG severity on the levels of EPA, DHA, AA and the ratio of n3 to n6 in plasma (Table 3). The ratio of total n3 to total n6 PUFAs and the level of DHA were not statistically different between NTG patients and healthy controls. However, lower level of EPA and higher level of AA were observed in plasma of moderate and severe NTG group (P<0.01).
Figure 2. Association of fatty acid levels in plasma and NTG severity.
A: Lower level of EPA was observed in both moderate and severe NTG patients; B: There was no significant different at the levels of DHA between NTG patients and healthy controls; C: Higher level of AA was observed in both moderate and severe groups; D: There was no significant different at the ratios of n3 to n6 between NTG patients and healthy controls. aP<0.05, bP<0.01, cP<0.001.
Table 3. n3 and n6 PUFA composition of plasma.
| Fatty acids | Control (n=12) | Normal tension glaucoma |
||
| Early (n=12) | Moderate (n=15) | Severe (n=8) | ||
| 18:2n6 (linoleic acid) | 20.33±3.59 | 19.75±4.12 | 20.11±6.21 | 22.31±7.33 |
| 18:3n6 (γ-linolenic acid) | 0.08±0.02 | 0.08±0.02 | 0.09±0.02 | 0.12±0.02 |
| 18:3n3 (α-linolenic acid) | 2.19±0.27 | 2.46±0.28 | 2.15±1.01 | 2.64±0.42 |
| 20:4n3 (eicosatetraenoic acid) | 0.21±0.04 | 0.21±0.03 | 0.15±0.02 | 0.12±0.03 |
| 20:5n3 (eicosapentanoic acid) | 0.61±0.12 | 0.58±0.11 | 0.40±0.08* | 0.27±0.20* |
| 20:2n6 (eicosadienoic acid) | 2.39±1.21 | 2.16±1.03 | 2.03±0.90 | 1.71±0.37 |
| 20:3n6 (eicosatrienoic acid) | 1.30±0.19 | 1.39±0.29 | 1.55±0.45 | 1.62±0.28 |
| 20:4n6 (arachidonic acid) | 4.00±0.58 | 4.26±0.51 | 5.13±0.65* | 5.42±0.43* |
| 22:4n6 (docosatetraenoic acid ) | 0.16±0.04 | 0.16±0.03 | 0.15±0.02 | 0.14±0.01 |
| 22:5n6 (docosapentaenoic acid) | 0.16±0.09 | 0.15±0.06 | 0.14±0.08 | 0.19±0.05 |
| 22:5n3 (docosapentaenoic acid) | 0.81±0.11 | 0.79±0.11 | 0.60±0.09 | 0.58±0.10 |
| 22:6n3 (docosahexaenoic acid) | 3.43±0.78 | 3.50±0.82 | 3.80±1.01 | 2.53±1.11 |
| n3/n6 ratio | 0.23±0.04 | 0.24±0.04 | 0.25±0.05 | 0.20±0.06 |
Levels of n3 and n6 Polyunsaturated Fatty Acids in Red Blood Cell Membrane and Plasma Associated with Normal Tension Glaucoma Stage
We performed linear regression analyses using the mean levels of EPA, DHA, AA and the ratio of total n3 to total n6 in each of the NTG group and the visual field MD score in order to investigate whether the levels of lipids in RBC membrane and plasma are associated with advanced NTG. Since the worse eye was the initial eye injured and would better denote the time of disease onset, the MD score in the worse eye was used in the analysis.
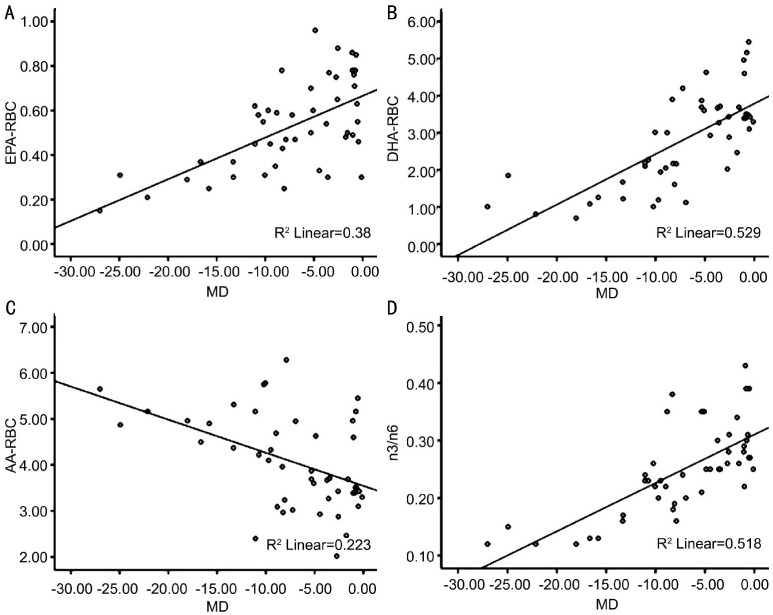
In RBC membrane, a high association was observed between the MD score and the levels of EPA, DHA, AA and the ratio of n3 to n6. The levels of EPA, DHA and the ratio of n3 to n6 were positively associated with the Humphrey Perimetry MD score (Figure 3A, 3B, 3D), while the level of AA was negatively associated with the MD score (Figure 3C).
Figure 3. Scatter plots showing correlations between MD and the levels of EPA, DHA, AA and n3/n6 in RBC membrane.
Pearson correlations between A: MD and the level of EPA in RBC membrane (r=0.617, P<0.001); B: MD and the level of DHA in RBC membrane (r=0.727, P<0.001); C: MD and the level of AA in RBC membrane (r=-0.472, P=0.001); D: MD and the ratio of n3 to n6 in RBC membrane (r=0.720, P<0.001).
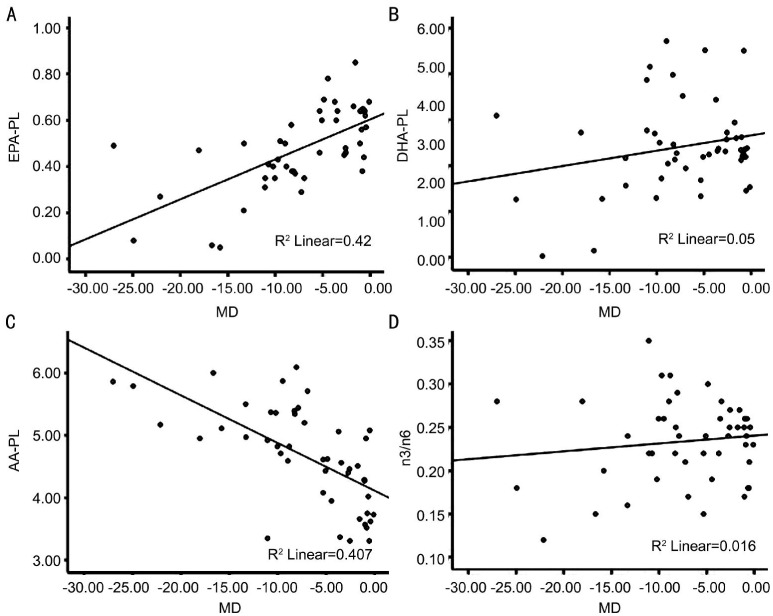
In plasma, the MD score in the worse eye and the levels of EPA and AA were moderately correlated (Figure 4A, 4C) although there was no association between the MD score and the levels of DHA and the ratio of n3 to n6 (Figure 4B, 4D).
Figure 4. Scatter plots showing correlations between MD and the levels of EPA, DHA, AA and n3/n6 in plasma.
Pearson correlations between A: MD and the level of EPA in plasma (r=0.648, P<0.001); B: MD and the level of DHA in plasma (r=0.223, P=0.132); C: MD and the level of AA in plasma (r=-0.638, P<0.001); D: MD and the ratio of n3 to n6 in plasma (r=0.126, P=0.40).
DISCUSSION
To date, the pathology of glaucomatous optic neuropathy has been investigated extensively at the level of the retina, optic nerve and central nervous system[16],[17]. The prevailing mechanical theory considers that increased IOP leads to stretching of the lamina cribosa and damage to the retinal ganglion axon cells[18]. However, a large body of evidence in the literature supports the possible role in glaucoma played by the vascular system, related vascular cellular mediators other than IOP[19]. The optic neuropathy of glaucoma, especially of NTG might be a consequence of insufficient blood supply and other risk factors influencing hemorheology. Both n3 PUFA and n6 PUFA, as the important constituents of all cell membranes, affect cell membrane properties such as the fluidity, flexibility, permeability, and activity of membrane-bound enzymes when they incorporated into phospholipids[9]. Ren et al[7] have pointed out patients with POAG had reduced EPA, DHA and n3 PUFA in their erythrocyte membrane and plasma lipid composition. Therefore, alteration of the lipid composition of blood may also lead to NTG development.
RBC membranes have been known to consist mainly of phospholipids[20]. Fatty acid composition of RBC membrane has been shown to reflect the fatty acid composition of other cell membrane[21]. In the present study, only severe group has reduced EPA in RBC membrane. Both moderate and severe NTG patients have reduced DHA and ratio of n3 to n6. The significant positive correlation was found between the MD score and the levels of EPA, DHA in RBC membrane. A similar positive trend between the MD score and the ratio of n3 to n6 was noted. Our data supports that reduced EPA, DHA or n3 PUFAs may play a role in the pathology of apoptosis of retinal ganglion cells. Actually, the findings in this study are consistent with that in POAG study by Ren et al[7] described above. However, the lipids in choline phosphoglycerides, ethanolamine phosphoglycerides, serine phosphoglycerides were analyzed respectively in their study. Here, we showed the total lipid composition in erythrocyte membrane and plasma. Over the decades, a lot of work has been done which shows that n3 long chain-PUFAs including EPA and DHA are necessary for normal neural development and function[22],[23]. They also play significant roles in maintaining cell structure and physiological function by modulating cell differentiation and growth through signal transduction and cellular metabolism[23].
Furthermore, the higher level of AA in RBC membrane lipids was noted in severe group. The inverse relationship was found between the MD score and level of AA. Metabolites of n6 PUFA can be more inflammatory (especially AA) than those of n3 PUFA. Since both n3 and n6 PUFA are essential fatty acid and they compete for the same metabolic enzymes, the alteration of their ratio will influence the ratio of the following eicosanoids (hormones), e.g. prostaglandins, leukotrienes, and thromboxanes, and will alter cell membrane composition and fluidity as well as organ function[24]. This result indicates that the potential detrimental effects from the increase in the levels of AA or n6 PUFA on the development of NTG.
Interestingly, we found reduced level of EPA and the higher level of AA in plasma of both moderate and severe group, but there was no alteration in the levels of DHA and the ratio of n3 to n6 between NTG patients and healthy controls. Likewise, the positive correlation was found between the MD score and the level of EPA, and the inverse correlation was found between the MD score and the level of AA. The fatty acids composition of plasma, as the only transporters of circulating fatty acids, has been believed to reflect short-term dietary n3 PUFA consumption[21],[25]. Thus, the results of plasma lipids of NTG patients may be affected by their diet.
In conclusion, our study detected the association n3 and n6 PUFAs in RBC membrane and plasma with severity of NTG. However, there are some limitations for this study. Firstly, the small sample size makes the conclusions in this study less strong. Secondly, a recent study showed that visual field index (VFI) was associated with glaucoma severity in terms of quality of life scores[26], which indicated VFI may be more accurate than MD for monitoring glaucoma progression. Therefore, a causal relationship needs to be established in a large well-designed prospective study. Further study would benefit from even more patients with NTG to demonstrate that EPA, DHA and/or n3 PUFA are protective against the development of NTG in a more compelling manner.
Acknowledgments
The authors thank Martin-Paul Agbaga for reviewing and editing the original manuscript for this paper.
Foundation: Partly supported by National Natural Science Foundation of China (NO. 81000370).
Conflicts of Interest: Yu M, None; Chen B, None; Gong B, None; Shuai P, None; Wu ZZ, None; Lin W, None.
REFERENCES
- 1.Kong X, Zhu W, Chen X, Chen Y, Sun X. Familial aggregation of primary open angle glaucoma in Shanghai, China. Mol Vis. 2013;19:1859–1865. [PMC free article] [PubMed] [Google Scholar]
- 2.Wang YX, Xu L, Yang H, Jonas JB. Prevalence of glaucoma in North China: the Beijing Eye Study. Am J Ophthalmol. 2010;150(6):917–924. doi: 10.1016/j.ajo.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarieh M, Flammer J. New insights in the pathogenesis and treatment of normal tension glaucoma. Curr Opin Pharmacol. 2013;13(1):43–49. doi: 10.1016/j.coph.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Mroczkowska S, Ekart A, Sung V, Negi A, Qin L, Patel SR, Jacob S, Atkins C, Benavente-Perez A, Gherghel D. Coexistence of macro- and micro-vascular abnormalities in newly diagnosed normal tension glaucoma patients. Acta Ophthalmol. 2012;90(7):e553–e559. doi: 10.1111/j.1755-3768.2012.02494.x. [DOI] [PubMed] [Google Scholar]
- 5.German OL, Insua MF, Gentili C, Rotstein NP, Politi LE. Docosahexaenoic acid prevents apoptosis of retina photoreceptors by activating the ERK/MAPK pathway. J Neurochem. 2006;98(5):1507–1520. doi: 10.1111/j.1471-4159.2006.04061.x. [DOI] [PubMed] [Google Scholar]
- 6.Lynch AM, Loane DJ, Minogue AM, Clarke RM, Kilroy D, Nally RE, Roche OJ, O'Connell F, Lynch MA. Eicosapentaenoic acid confers neuroprotection in the amyloid-beta challenged aged hippocampus. Neurobiol Aging. 2007;28(6):845–855. doi: 10.1016/j.neurobiolaging.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Ren H, Magulike N, Ghebremeskel K, Crawford M. Primary open-angle glaucoma patients have reduced levels of blood docosahexaenoic and eicosapentaenoic acids. Prostaglandins Leukot Essent Fatty Acids. 2006;74(3):157–163. doi: 10.1016/j.plefa.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Acar N, Berdeaux O, Juaneda P, Grégoire S, Cabaret S, Joffre C, Creuzot-Garcher CP, Bretillon L, Bron AM. Red blood cell plasmalogens and docosahexaenoic acid are independently reduced in primary open-angle glaucoma. Exp Eye Res. 2009;89(6):840–853. doi: 10.1016/j.exer.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126(1):1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 10.Yuki K, Kimura I, Tsubota K. Serum free fatty acids levels not associated with normal tension glaucoma. Clin Ophthalmol. 2010;4:91–94. doi: 10.2147/opth.s9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shibuya E, Meguro A, Ota M, Kashiwagi K, Mabuchi F, Iijima H, Kawase K, Yamamoto T, Nakamura M, Negi A, Sagara T, Nishida T, Inatani M, Tanihara H, Aihara M, Araie M, Fukuchi T, Abe H, Higashide T, Sugiyama K, Kanamoto T, Kiuchi Y, Iwase A, Ohno S, Inoko H, Mizuki N. Association of Toll-like receptor 4 gene polymorphisms with normal tension glaucoma. Invest Ophthalmol Vis Sci. 2008;49(10):4453–4457. doi: 10.1167/iovs.07-1575. [DOI] [PubMed] [Google Scholar]
- 12.Susanna R, Jr, Vessani RM. Staging glaucoma patient: why and how? Open Ophthalmol J. 2009;3:59–64. doi: 10.2174/1874364100903010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 14.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 15.Agbaga MP, Brush RS, Mandal MN, Henry K, Elliott MH, Anderson RE. Role of Stargardt-3 macular dystrophy protein (ELOVL4) in the biosynthesis of very long chain fatty acids. Proc Natl Acad Sci U S A. 2008;105(35):12843–12848. doi: 10.1073/pnas.0802607105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta N, Ly T, Zhang Q, Kaufman PL, Weinreb RN, Yücel YH. Chronic ocular hypertension induces dendrite pathology in the lateral geniculate nucleus of the brain. Exp Eye Res. 2007;84(1):176–184. doi: 10.1016/j.exer.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Sasaoka M, Nakamura K, Shimazawa M, Ito Y, Araie M, Hara H. Changes in visual fields and lateral geniculate nucleus in monkey laser-induced high intraocular pressure model. Exp Eye Res. 2008;86(5):770–782. doi: 10.1016/j.exer.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24(1):39–73. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Flammer J, Orgül S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, Renard JP, Stefánsson E. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21(4):359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 20.Ways P, Reed CF, Hanahan DJ. Red-cell and plasma lipids in acanthocytosis. J Clin Invest. 1963;42:1248–1260. doi: 10.1172/JCI104810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjorgvinsdottir L, Indridason OS, Heidarsdottir R, Skogstrand K, Arnar DO, Torfason B, Hougaard DM, Palsson R, Skuladottir GV. Inflammatory response following heart surgery and association with n-3 and n-6 long-chain polyunsaturated fatty acids in plasma and red blood cell membrane lipids. Prostaglandins Leukot Essent Fatty Acids. 2013;89(4):189–194. doi: 10.1016/j.plefa.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 22.SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24(1):87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Agbaga MP, Mandal MN, Anderson RE. Retinal very long-chain PUFAs: new insights from studies on ELOVL4 protein. J Lipid Res. 2010;51(7):1624–1642. doi: 10.1194/jlr.R005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adkins Y, Kelley DS. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem. 2010;21(9):781–792. doi: 10.1016/j.jnutbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res. 1997;38(10):2012–2022. [PubMed] [Google Scholar]
- 26.Lee JW, Chan CW, Chan JC, Li Q, Lai JS. The association between clinical parameters and glaucoma-specific quality of life in Chinese primary open-angle glaucoma patients. Hong Kong Med J. 2014;20(4):274–278. doi: 10.12809/hkmj134062. [DOI] [PubMed] [Google Scholar]