Abstract
AIM
To find the risk factors related to the reproliferation of the pterygial tissue after excision and graft surgery.
METHODS
Charts of 130 eyes of 130 patients who had pterygial excision from March 2006 to April 2011 were reviewed. Preoperative pterygium morphology, surgical methods, and adjunctive treatments were statistically analyzed for their relationship with recurrence.
RESULTS
During the follow-up period, recurrence was observed in 20 eyes (15.4%). None of the preoperative morphologic features were affected the rate of the recurrence. However, an age < 40y [P =0.085, odds ratio (OR) 3.609, 95% confidence interval (CI) 0.838-15.540] and amniotic membrane graft instead of conjunctival autograft (P =0.002, OR 9.093, 95% CI 2.316-35.698) were statistically significant risk factors for recurrence. Multivariate analysis revealed that intraoperative mitomycin C (MMC) (P=0.072, OR 0.298, 95% CI 0.080-1.115) decreased the rate of recurrence.
CONCLUSION
Younger age is a risk factor for reproliferation of pterygial tissue after excision and amniotic membrane transplantation (AMT) are less effective in preventing recurrence of pterygium after excision based on the comparison between conjunctival autograft and AMT. Intraoperative MMC application and conjunctival autograft reduce recurrence.
Keywords: pterygium recurrence, conjunctival autograft, amniotic membrane graft, mitomycin C
INTRODUCTION
Pterygium is a limbal disorder characterized by the growth of fibrovascular tissue from the bulbar conjunctiva onto the cornea. Histologically, it is composed of the head of the conjunctival epithelium and the hyperproliferative vessels along with the body of the degenerated connective tissue, which show elastotic degeneration[1]. Due to these characteristics pterygium was traditionally considered as a chronic degenerative disease. However, it also has proliferative histologic and clinical characteristics. Among them are mild dysplasia, local invasiveness, abnormal p53 expression, and the clinical characteristic of high recurrence rate. Furthermore, treatment modalities of pterygia, such as wide excision, use of antimetabolites, and irradiation, mimic those used to treat neoplastic diseases[2],[3]. However, the mechanisms or causes for the tendency to proliferate are unknown. The tendency for the pterygium to proliferate is most prominent when it recurs after excision. Fibrovascular proliferation in recurrent pterygium occurs much more aggressively compared to primary pterygium. Several studies have addressed the recurrence and its incidence after pterygial tissue excision[4]–[6]. The results varied greatly with preoperative demographics, surgical techniques, and adjunctive treatments. However, all of the studies were comparative case-control studies with few variables. A comparative study would directly reveal the specific superiority between compared variables, such as the comparison of surgical techniques and the efficacy of adjunctive treatment. However, since pterygium can recur after surgery, like cancerous diseases, knowledge of the risk factors related with recurrence and proliferation are necessary when the treatment strategy is formulated. However, a risk factor analysis for postoperative recurrence of pterygium has not been done.
Presently, we clinically analyzed the conditions inducing proliferation from the bulbar conjunctiva. Rather than comparing the procedures causing the recurrence, we studied the factors that caused the pterygium to proliferate and what eventually caused postsurgical recurrence after surgery with univariate and multivariate analyses of the risk factors. To perform a controlled, objective retrospective study, we developed a grading system for the morphologic severity of pterygial tissue. Patients who were treated with pterygium excision with grafting were analyzed retrospectively considering preoperative demographic variables, morphologic severities, surgical techniques, and adjuvant procedures influencing fibrovascular tissue proliferation.
SUBJECTS AND METHODS
Subjects
This study was conducted according to the principles of the Declaration of Helsinki and was approved by Institutional Review Board of Kyungpook National University Hospital. Written informed consent was obtained from all subjects after an explanation of the research purpose. Totally 130 eyes of 130 patients with primary or recurrent pterygium who had surgery for pterygium from March 2006 to April 2011 in Kyungpook National University Hospital were reviewed in this retrospective study.
Patients have pterygium extending at least to the limbus, prominent vascularization, thickened fibrotic proliferation that obscured the episcleral vessels, and diplopia due to fibrous adhesion were indicated for surgery. Patients with a follow-up period of less than 6mo, pseudopterygium, which is fibrovascular proliferation of conjunctiva secondary to injury, a severe ocular surface disease like blepharitis, infection of the ocular surface, or a systemic pathology that might be a contraindication to ocular surgery were excluded.
Methods
Standard grading system of pterygium
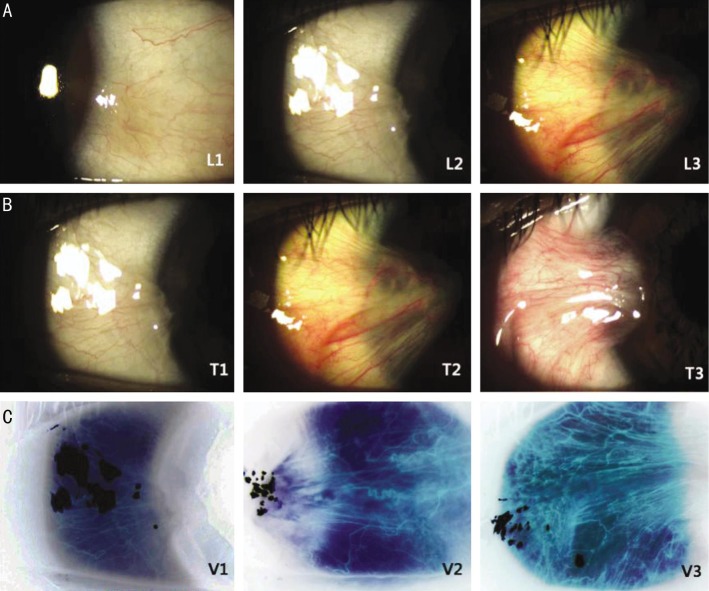
Through a preoperative examination, the examiners analyzed the morphologic severity of the pterygium and captured images using a slit lamp system (Emedio®, Korea). We reviewed all preoperative images and classified the morphologic severity based on the previously developed pterygium grading system[7]. The system classifies the appearance of pterygial relative thickness, increased vascularity, and the anatomical position of the abnormal fibrovasular head (Table 1). For grading the degree of tissue vascularization, the digital image files were equalized by Photoshop CS2® (Adobe System Inc., USA) using each pixels and RGB value; the colors were then inverted. This method of image processing emphasized the vessels that were analyzed (Figure 1). With this definition, standard photos of each category were created (Figure 2). The preoperative slit lamp photographs of patients were compared with the standard photos and graded. All photos were graded twice by different examiners. Disagreements prompted comparison with the standard photographs until consensus was reached. The agreement between readers was compared by using the kappa statistic to assess the validity of photo-documentation (SPSS Ver. 19.0 for Windows).
Table 1. The standard grading system of pterygium based on the photography captured by image capture system.
| Grade | Location of abnormal fibrovacular head (L) | Thickness (T) | Vascularity (V) |
| 0 | No abnormal fibrovascular growth (L0) | No elevation (T0) | No directional vascular pattern (V0) |
| 1 | Abnormal fibrovascular tissue confined to the conjunctival area (L1) | Minimal elevation with definite confirmation of episcleral vessel in most of the elevated area (T1) | Minimal vascularization with unidirectional pattern (V1) |
| 2 | Abnormal fibrovascular tissue located in the limbal area (L2) | Moderate elevation, episcleral vessel can be found in some of the elevated area (T2) | Moderate vascularization with unidirectional and enlarged vessels (V2) |
| 3 | Abnormal fibrovascular tissues encroach over the limbal area (> 1.0 mm from limbus) (L3) | Marked elevation, episcleral vessel cannot be found because of the pterygial fleshiness (T3) | Marked vascularization with unidirectional, engorged vessels (V3) |
Figure 1. Slit lamp phtography.

A: Representative preoperative slit lamp photograph; B: Converted image by photoshop CS2® (Adobe) to compare the vascularity of each pterygial tissue.
Figure 2. Standard photographs classified by the pterygial thickness.
Location of abnormal fibrovascular head (A), thickness (B) and vascularity (C) of abnormal tissue.
Surgical treatment
Sixteen eyes of the patients received additional 0.15 mg/0.1 mL mitomycin C (MMC) subconjunctival injection as a preoperative adjunctive treatment at 4wk before surgical procedure. All surgical procedures were performed by one operator (Kim HK). In the procedure, 2% lidocane was injected into the subtenon to achieve anesthesia, then the pterygium tissue and the subconjunctivalfibrovascular tissue was removed as broadly as possible. During the surgery, in 68 eyes, a small surgical sponge soaked with 0.04% MMC solution was placed in contact with the upper and lower exposed scleral surface for 2min. The bare sclera after the excision was covered with a conjunctivalautograft (CAG) or amniotic membrane transplantation (AMT). Each graft was sutured by 10-0 nylon. Prednisolone acetate (1%, Pred Forte®; Allergan), 0.5% levofloxacin (Cravit®, Santen) and preservative-free 0.1% hyaluronic acid (Hyalein Mini®, Santen) were applied four times daily for 1mo after the surgery. The sutures were removed 10d postoperatively.
Postoperative examination and definition of pterygium recurrence
Patients were observed at least 2y postoperatively. Slit lamp examination was performed to check for recurrence at every visit, and the last image taken for the observation was used to decide whether there was a recurrence. Recurrence of pterygium was defined as any fibrovascular proliferation from the original pterygium site and classified by standardized methods.
Statistical Analysis
Statistical analysis was performed considering each risk factor for the recurrence and no recurrence group (Table 2). Fisher's exact tests were used to analyze categoric variables such as gender and recurrence. A P<0.05 was considered significant. To evaluate the strength of association, logistic regression analysis was conducted and odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. In multivariate data analysis, binary multiple logistic regression using backward conditional elimination was performed to identify significant independent factors associated with recurrence. In a backward elimination model, P<0.1 was considered significant. SPSS version 19.0 was used for data analysis.
Table 2. List of variables for the risk assessment of pterygial recurrence.
| Categories | Variables for the risk assessment of pterygial recurrence |
| Demographics | Age (under 40 years old group/over 40 years old group) |
| Gender (M/F) | |
| Preoperativemorphologic feature | Primary/Recurrent |
| Preoperative location of abnormal fibrovascular head (L2/L3) | |
| Preoperative fibrous thickness (T2/T3) | |
| Preoperative vascularity (V2/V3) | |
| Symblepharous fibrous traction | |
| Operation technique | Type of graft (amniotic membrane/CAG) |
| Adjunctive treatment | Application of preoperative MMC injection (Yes/No) |
| Application of intraoperative 0.04% MMC soaking (Yes/No) |
RESULTS
Treatment Results
Of the 130 patients, 62 were male (47.7%) and 68 were female (52.3%). The mean age was 55.24±11.65y (range, 23-82y). Ninety (69.2%) of 130 eyes were primary pterygium and 40 (30.8%) of 130 eyes were recurrent pterygium. The investigator classified identical images twice and the index of coincidence that classified the same group into the same grade was statically significant in this classification (Kappa 0.86, P<0.001). One hundred and ten eyes (84.6%) of all pterygia were graded L3 (fibrovascular proliferation invading >1 mm over the limbus) and 20 eyes (15.4%) were graded L2 (fibrovascular head advanced within 1 mm of the limbus). Ninety-four eyes (72.3%) were grade T3 and 36 eyes (27.7%) were grade T2. 100 eyes (76.9%) were grade V3 (marked vascularization) and 30 eyes (23.1%) were grade V2 (moderate vascularization). 16 eyes (12.4%) showed symblepharous fibrous adhesion to palpebral conjunctiva. Sixteen eyes (12.4%) received additional 0.15 mg/0.1 mL MMC via a subconjunctival injection 4wk before surgical procedure and in 68 eyes (52.3%), a small surgical sponge soaked with 0.04% MMC was placed in contact with the upper and lower exposed scleral surface for 2min during surgery. The bare sclera was covered with graft after pterygium excision. Forty-two patients had AMT and 88 patients had CAG.
Pterygium Recurrence and Risk Factors
The mean postoperative follow-up period was 35.12±11.05mo (range, 24-48mo). During the follow-up, recurrence was observed in 20 eyes (15.4%). The association between clinicopathologic variables and recurrence in the study group is reported in Table 3. Of the analyzed variables, an age <40y (P=0.009; OR 5.464; 95% CI 1.651-18.085) and AMT (P<0.0001; OR 12.923; 95% CI 3.968-42.084) were significantly associated with recurrence in univariate analysis. None of the preoperative morphologic features and adjuvant treatments affected the rate of the recurrence (Table 3). In multivariate logistic regression analysis to control confounding factors, AMT (P=0.002, OR 9.093, 95% CI 2.316-35.698) and an age <40y (P=0.085, OR 3.609, 95% CI 0.838-15.540) were still significantly associated with recurrence. Furthermore, unlike univariate analysis, patients group who were applied intraoperative MMC showed statistically significant decreased recurrence rate (P=0.017, OR 5.032, 95% CI 1.341-18.892;P=0.072, OR 0.298, 95% CI 0.080-1.115; Table 4).
Table 3. Association between clinicopathologic variables and recurrence in the study group.
| Risk factors | No. of patients | P | Odds ratio (95% CI) |
| Age (a) | |||
| ≤40 | 14 | 0.009 | 5.464 (1.651-18.085) |
| ≥41 | 116 | ||
| Gender | |||
| F | 68 | 0.478 | 1.446 (0.549-3.814) |
| M | 62 | ||
| Eyes | |||
| OS | 70 | 1.000 | 1.056 (0.406-2.752) |
| OD | 60 | ||
| Primary/recurrence | |||
| Recurrent | 40 | 0.793 | 1.256 (0.460-3.433) |
| Primary | 90 | ||
| Advancement off ibrovascular head | |||
| L3 | 110 | 0.309 | 3.967 (0.500-31.462) |
| ≤L2 | 20 | ||
| Preoperative fibrous thickness | |||
| T3 | 94 | 0.588 | 1.641 (0.509-5.289) |
| ≤T2 | 36 | ||
| Preoperative vascularity | |||
| V3 | 100 | 0.564 | 1.843 (0.501-6.777) |
| ≤V2 | 30 | ||
| Adhesive traction | |||
| N | 114 | 0.270 | 2.042 (0.586-7.118) |
| Y | 16 | ||
| Preoperative MMC | |||
| N | 114 | 1.000 | 1.313 (0.274-6.276) |
| Y | 16 | ||
| Type of graft | |||
| AMT | 42 | <0.0001 | 12.923 (3.968-42.084) |
| CAG | 88 | ||
| Intraoperative MMC | |||
| N | 62 | 0.627 | 1.414 (0.543-3.683) |
| Y | 68 | ||
OD: Right eye; OS: Left eye; AMT: Aminotic membrane transplantation; CAG: Conjunctivalautograft.
Table 4. Independent predictors of recurrence (logistic regression analysis).
| Variables | β | SE | P | Odds ratio |
|
| Value | 95% CI | ||||
| Age 40 years old | 1.284 | 0.745 | 0.085 | 3.609 | (0.838, 15.540) |
| AMT | 2.208 | 0.698 | 0.002 | 9.093 | (2.316, 35.698) |
| MMC | -1.210 | 0.672 | 0.072 | 0.298 | (0.080, 1.115) |
| Constant | -1.703 | 0.387 | 0.000 | 0.182 | |
AMT: Amniotic membrane transplantation; Model: Z=-1.703+1.284 age 40 + 2.208 AMT-1.210MMC.
DISCUSSION
For proliferative diseases like malignant tumors, risk factor analysis has been important in setting the treatment protocol. When we treat clinically high-risk patients, we need a more aggressive and intensive treatment modality. Low-risk patients would not need the same treatment strategy. Likewise, the treatment modality for patients with pterygium should be considered along with the analyzed risk factors. In this study, to evaluate the factors, we retrospectively analyzed the variables that might affect proliferation. Demographic variables, preoperative clinical severity, preoperative or intraoperative adjunctive treatments, and surgical variations were considered.
The most widely recognized etiologic factor of pterygium is ultraviolet (UV) radiation. Therefore, populations residing in tropical regions and those living closest to the equator are thought to be at greatest risk for developing this condition[8],[9]. However, in this study, we did not consider UV. Since re-proliferation after pterygial excision is usually fast and aggressive when compared to growth of primary pterygium, the effect of environmental factors like UV exposure is very limited.
Age and gender can influence wound healing after excision. We included these variables in the risk analysis for recurrence after pterygial excision[10]. Although gender had no relationship with recurrence, younger age was associated with high recurrent cases and was statistically significant. Several reports have described age-related wound healing changes. Rapid re-epithelialization, aggressive collagen synthesis, and angiogenesis have been observed in young subjects as compared with aged[11]. After pterygial excision, we usually observed aggressive wound healing response and inflammation during the early postoperative period. The aggressiveness might be related with the postoperative recurrence, and age-related wound healing response was more important than any other preoperative demographic factor or clinical severity.
The preoperative morphologic severity of pterygium varied widely in the patients. We defined its severity in three morphologic categories: thickness, degree of vascularization, and advancement of fibrovascular head. As pterygial growth is characterized by proliferating fibrous tissue and vascular neovascularization, we additionally included the presence of symblepharous adhesion as more aggressive and severe morphologic variables. We assumed that the preoperative morphologic severity would relate with the aggressive fibrovascular proliferative character[6],[12],[13]. Similarly, like other tumorous growing, the advancement of fibrovascular head and the frequency of recurrence that patients can be related with the invasiveness and high recurrence rate. Sandra et al[6] reported that pterygium recurrence is related to the fleshiness of the pterygium after conjunctival autografting with fibrin glue. However, we found that these morphologic features and the frequency of recurrence did not have any statistical impact on postoperative reproliferation. We have presumed that the difference might be related with the inflammatory response caused by fibrin glue.
Adjuvant treatment, such as MMC, 5-fluorouracil, bevacizumab and the use of expanded polytetrafluoroethylene (PTFE) to pterygium have significant effectiveness, especially recurrent pterygium with symblepharous adherence [14]–[18]. Unfortunately, most of these adjunctive variables were not included in this study. However, the effectiveness of MMC as an adjunctive treatment could be analysed. Sixteen eyes received additional 0.15 mg/0.1 mL MMC subconjunctival injection 4wk before surgical procedure. In 68 eyes, an intraoperative application of 0.04% MMC was done after the excision. These adjuvant procedures is purposed to suppress the fibroblastic activation of remained adjacent tissue after surgery[14]. While preoperative MMC subconjuctival injection did not show any significant effect on the recurrence rate, intraoperative MMC statistically significantly lowered the risk of recurrence in a multivariate analysis. Interestingly, in the univariate analysis for the intraoperative MMC variable, it did not show the statistical significance. The different result might be caused by non-randomized patient selection for the application of intraoperative MMC. In this study, after the excision of pterygial tissue, the surgeon decided whether to apply intraoperative MMC, which might have produced selection bias. With the multivariate analysis, we could minimize the interference.
The pterygium surgical technique, which is a very important factor that can influence the recurrence rate, can be classified by simple excision, such as the bare sclera method, and also by graft after excision methods, such as CAG or AMT. Sanchez-Thorin et al[19] reported that the pooled OR for pterygium recurrence in patients who had only bare sclera resection was 6.1 (95%CI 1.8 to 18.8) compared with patients who had CAG. Considering the high recurrence rate of bare sclera method, all patients were operated on using CAG or AMT. Since Prabhasawat et al[20] reported that AMT reduced the recurrence rates, there have been many reports about AMT in pterygium excision. However, in this study, AMT are less effective in preventing recurrence of pterygium after excision based on the comparison between CAG and AMT in pterygium surgery. When AMT is compared to CAG, another aggressive wound healing response is required; epithelial regeneration and many growth factors and cytokine products might be produced. These are considered as resulting in epithelial proliferation as well as the proliferation of fibroblasts[21],[22]. On the contrary, CAG can provide healthy epithelial sheet over the exposed sclera without any activation of epithelial regeneration. Nevertheless, CAG is a technically demanding procedure, and surgeon-related factors play a role in the recurrence rates. Furthermore, it is not feasible to cover large defects created in double-headed primary or large recurrent pterygia, and concern has been raised for those who may require future glaucoma filtering surgeries[23]. In such cases, a substitute tissue like amniotic membrane should be considered. However, a surgeon should also understand the proliferative postoperative character and consider the patient's age. When we have to use amniotic membrane instead of conjunctival graft, intraoperative MMC application would be very helpful.
In this study, we standardized the preoperative clinical grading and evaluated the risk factors using multivariate logistic regression analysis to minimize the interference of the correlating factors. Although our study design could not show a definitive comparative decision, we could get important information in various clinical situations. When deciding on the surgical indication of the pterygium, we should consider patient's age, not morphologic severity.
Conclusively, the factors related to aggressive wound healing like young age and the absence of healthy conjunctival epithelium were strongly related with postoperative proliferative characteristics of pterygium. In addition, AMT are less effective in preventing recurrence of pterygium after excision based on the comparison between CAG and AMT in pterygium surgery. To minimize the recurrences, we should consider patient age, surgical technique, and the use of intraoperative MMC.
Acknowledgments
Foundation: Supported by Biomedical Research Institute grant, Kyungpook National University Hospital at 2013.
Conflicts of Interest: Ha SW, None; Park JH, None; Shin IH, None; Kim HK, None.
REFERENCES
- 1.Mauro J, Foster CS. Pterygia: pathogenesis and the role of subconjunctival bevacizumab in treatment. Semin Ophthalmol. 2009;24(3):130–134. doi: 10.1080/08820530902801106. [DOI] [PubMed] [Google Scholar]
- 2.Nubile M, Curcio C, Lanzini M, Calienno R, Iezzi M, Mastropasqua A, Di Nicola M, Mastropasqua L. Expression of CREB in primary pterygium and correlation with cyclin D1, ki-67, MMP7, p53, p63, Survivin and Vimentin. Ophthalmic Res. 2013;50(2):99–107. doi: 10.1159/000347124. [DOI] [PubMed] [Google Scholar]
- 3.Young AL, Ho M, Jhanji V, Cheng LL. Ten-year results of a randomized controlled trial comparing 0.02% mitomycin C and limbal conjunctival autograft in pterygium surgery. Ophthalmology. 2013;120(12):2390–2395. doi: 10.1016/j.ophtha.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 4.Kim SH, Oh JH, Do JR, Chuck RS, Park CY. A comparison of anchored conjunctival rotation flap and conjunctival autograft techniques in pterygium surgery. Cornea. 2013;32(12):1578–1581. doi: 10.1097/ICO.0b013e3182a73a48. [DOI] [PubMed] [Google Scholar]
- 5.Ozer A, Yildirim N, Erol N, Yurdakul S. Long-term results of bare sclera, limbal-conjunctival autograft and amniotic membrane graft techniques in primary pterygium excisions. Ophthalmologica. 2009;223(4):269–273. doi: 10.1159/000210444. [DOI] [PubMed] [Google Scholar]
- 6.Sandra S, Zeljka J, Zeljka VA, Kristian S, Ivana A. The influence of pterygium morphology on fibrin glue conjunctival autografting pterygium surgery. Int Ophthalmol. 2014;34(1):75–79. doi: 10.1007/s10792-013-9799-2. [DOI] [PubMed] [Google Scholar]
- 7.Oh JH, Kim HK. The effect of preoperative subconjunctival injection of mitomycin C and triamcinolone in recurrent pterygium. J Korean Ophthalmol Soc. 2009;50(7):1005–1014. [Google Scholar]
- 8.Chao SC, Hu DN, Yang PY, Lin CY, Nien CW, Yang SF, Roberts JE. Ultraviolet-A irradiation upregulated urokinase-type plasminogen activator in pterygium fibroblasts through ERK and JNK pathways. Invest Ophthalmol Vis Sci. 2013;54(2):999–1007. doi: 10.1167/iovs.12-10469. [DOI] [PubMed] [Google Scholar]
- 9.Sacca SC, Roszkowska AM, Izzotti A. Environmental light and endogenous antioxidants as the main determinants of non-cancer ocular diseases. Mutat Res. 2013;752(2):153–171. doi: 10.1016/j.mrrev.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Engeland CG, Bosch JA, Cacioppo JT, Marucha PT. Mucosal wound healing: The roles of age and sex. Archives of Surgery. 2006;141(12):1193–1197. doi: 10.1001/archsurg.141.12.1193. [DOI] [PubMed] [Google Scholar]
- 11.Gosain A, DiPietro LA. Review aging and wound healing. World J Surg. 2004;28(3):321–326. doi: 10.1007/s00268-003-7397-6. [DOI] [PubMed] [Google Scholar]
- 12.Kim KW, Park SH, Wee SW, Kim JC. Overexpression of angiogenin in pterygium body fibroblasts and its association with proliferative potency. Invest Ophthalmol Vis Sci. 2013;54(9):6355–6362. doi: 10.1167/iovs.13-12141. [DOI] [PubMed] [Google Scholar]
- 13.Di Girolamo N, Coroneo MT, Wakefield D. Active matrilysin (MMP-7) in human pterygia: potential role in angiogenesis. Invest Ophthalmol Vis Sci. 2001;42(9):1963–1968. [PubMed] [Google Scholar]
- 14.Kheirkhah A, Hashemi H, Adelpour M, Nikdel M, Rajabi MB, Behrouz MJ. Randomized trial of pterygium surgery with mitomycin C application using conjunctival autograft versus conjunctival-limbal autograft. Ophthalmology. 2012;119(2):227–232. doi: 10.1016/j.ophtha.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Prabhasawat P, Tesavibul N, Leelapatranura K, Phonjan T. Efficacy of subconjunctival 5-fluorouracil and triamcinolone injection in impending recurrent pterygium. Ophthalmology. 2006;113(7):1102–1109. doi: 10.1016/j.ophtha.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Said DG, Faraj LA, Elalfy MS, Yeung A, Miri A, Fares U, Otri AM, Rahman I, Maharajan S, Dua HS. Intra-lesional 5 fluorouracil for the management of recurrent pterygium. Eye (Lond) 2013;27(10):1123–1129. doi: 10.1038/eye.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Q, Qiao Y, Nie X, Cheng X, Ma Y. Bevacizumab in the treatment of pterygium: a meta-analysis. Cornea. 2014;33(2):154–160. doi: 10.1097/ICO.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 18.Kim KW, Kim JC, Moon JH, Koo H, Kim TH, Moon NJ. Management of complicated multirecurrent pterygia using multimicroporous expanded polytetrafluoroethylene. Br J Ophthalmol. 2013;97(6):694–700. doi: 10.1136/bjophthalmol-2012-302784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sánchez-Thorin JC, Rocha G, Yelin JB. Meta-analysis on the recurrence rates after bare sclera resection with and without MMC use and conjunctival autograft placement in surgery for primary pterygium. Br J Ophthalmol. 1998;82(6):661–665. doi: 10.1136/bjo.82.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prabhasawat P, Barton K, Burkett G, Tseng SC. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997;104(6):974–985. doi: 10.1016/s0161-6420(97)30197-3. [DOI] [PubMed] [Google Scholar]
- 21.Govinden R, Bhoola K. Genealogy, expression, and cellular function of transforming growth factor-beta. Pharmacol Ther. 2003;98(2):257–265. doi: 10.1016/s0163-7258(03)00035-4. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura K, Kurosaka D, Yoshino M, Oshima T, Kurosaka H. Injured corneal epithelial cells promote myodifferentiation of corneal fibroblasts. Invest Ophthalmol Vis Sci. 2002;43(8):2603–2608. [PubMed] [Google Scholar]
- 23.Sekundo W, Droutsas K, Cursiefen C. Operative techniques for surgical treatment of primary and recurrent pterygia. Ophthalmologe. 2010;107(6):525–528. doi: 10.1007/s00347-009-2099-6. [DOI] [PubMed] [Google Scholar]